Abstract
The sagittal orientation and osteoarthritis of facet joints, paravertebral muscular dystrophy and loss of ligament strength represent mechanical factors leading to degenerative spondylolisthesis. The importance of sagittal spinopelvic imbalance has been described for the developmental spondylolisthesis with isthmic lysis. However, it remains unclear if these mechanisms play a role in the pathogenesis of degenerative spondylolisthesis. The purpose of this study was to analyze the sagittal spinopelvic alignment, the body mass index (BMI) and facet joint degeneration in degenerative spondylolisthesis. A group of 49 patients with L4–L5 degenerative spondylolisthesis (12 males, 37 females, average age 65.9 years) was compared to a reference group of 77 patients with low back pain without spondylolisthesis (41 males, 36 females, average age 65.5 years). The patient’s height and weight were assessed to calculate the BMI. The following parameters were measured on lateral lumbar radiographs in standing position: L1–S1 lordosis, segmental lordosis from L1–L2 to L5–S1, pelvic tilt, pelvic incidence and sacral slope. The sagittal orientation and the presence of osteoarthritis of the facet joints were determined from transversal plane computed tomography (CT). The average BMI was significantly higher (P = 0.030) in the spondylolisthesis group compared to the reference group (28.2 vs. 24.8) and 71.4% of the spondylolisthesis patients had a BMI > 25. The radiographic analysis showed a significant increase of the following parameters in spondylolisthesis: pelvic tilt (25.6° vs. 21.0°; P = 0.046), sacral slope (42.3° vs. 33.4°; P = 0.002), pelvic incidence (66.2° vs. 54.2°; P = 0.001), L1–S1 lordosis (57.2° vs. 49.6°; P = 0.045). The segmental lumbar lordosis was significantly higher (P < 0.05) at L1–L2 and L2–L3 in spondylolisthesis. The CT analysis of L4–L5 facet joints showed a sagittal orientation in the spondylolisthesis group (36.5° vs. 44.4°; P = 0.001). The anatomic orientation of the pelvis with a high incidence and sacral slope seems to represent a predisposing factor for degenerative spondylolisthesis. Although the L1–S1 lordosis keeps comparable to the reference group, the increase of pelvic tilt suggests a posterior tilt of the pelvis as a compensation mechanism in patients with high pelvic incidence. The detailed analysis of segmental lordosis revealed that the lordosis increased at the levels above the spondylolisthesis, which might subsequently increase posterior stress on facet joints. The association of overweight and a relatively vertical inclination of the S1 endplate is predisposing for an anterior translation of L4 on L5. Furthermore, the sagittally oriented facet joints do not retain this anterior vertebral displacement.
Keywords: Degenerative spondylolisthesis, Sagittal spinopelvic balance, Body mass index, Facet joints
Introduction
Degenerative spondylolisthesis was initially described by Junghanns in 1930 [1]. It is defined as slipping of a lumbar vertebra with an intact neural arch and occurs mostly at L4–L5 in adults older than 40 years [2]. Several predisposing factors have been advocated in the physiopathology of this vertebral displacement: the sagittal orientation and osteoarthritis of the facet joints [3–7], insufficiency of ligaments and paravertebral muscles [2, 8], increased body mass index (BMI) [9] and female sexual hormones (sex ratio 4 females/1 male) [2]. The possible influence of pregnancy remains unclear [9, 10].
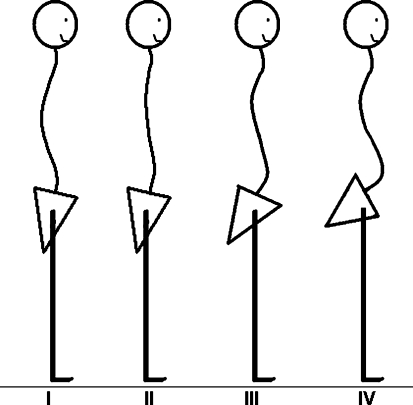
The importance of sagittal spinopelvic imbalance has been described for developmental spondylolisthesis with isthmic lysis [11]. However, the spinopelvic alignment might also play a role in the pathogenesis of degenerative spondylolisthesis [12, 13]. The sagittal orientation of the lumbar spine and pelvis, defined as type IV in the Roussouly classification [11], is characterized by a high pelvic incidence and sacral slope, associated with an important lumbar lordosis (Fig. 1). With this spinopelvic configuration, the sagittal S1 endplate orientation is relatively vertical, which could represent a predisposing factor for slippage of lumbar vertebrae. An anterior displacement of the center of gravity is usually compensated by a posterior tilt of the pelvis [14]. This mechanism leads to a posterior shift of load which increases the stress on facet joints which might enhance the development of posterior facet joint osteoarthritis.
Fig. 1.
Physiologic types of sagittal spinopelvic balance according to Roussouly. Type I: low pelvic incidence and low sacral slope with a short caudal lumbar lordosis and a thoracolumbar kyphosis. Type II: low pelvic incidence and low sacral slope with a flat back. Type III: normal pelvic incidence and normal sacral slope with a balanced lumbar lordosis and thoracic kyphosis. Type IV: high pelvic incidence and high sacral slope with an increased lumbar lordosis and a thoracic kyphosis
The purpose of this study was to analyze the sagittal spinopelvic alignment, the BMI and facet joint degeneration in degenerative spondylolisthesis. These parameters were compared to a reference population to verify if morphological parameters (high pelvic incidence and sacral slope) as well as overweight were more frequent in spondylolisthesis patients.
Materials and methods
Patients
Institutional review board approval was obtained for this retrospective study. Two patient groups were compared. The first group consisted of 49 patients with degenerative spondylolisthesis at L4–L5 (Meyerding stages I or II) operated on from January 2003 until December 2006 at our institution. There were 12 male and 37 female patients. Their average age at the time of surgery was 65.9 years and ranged from 44 to 81 years. The reference group consisted of 77 patients with low back pain due to moderate degenerative disc disease, but without spondylolisthesis. There were 41 male and 36 female patients. Their average age was 65.5 years and ranged from 40 to 81 years. Both patient populations were comparable in terms of age. Exclusion criteria were: age less than 40 years, idiopathic or degenerative scoliosis, developmental spondylolisthesis with isthmic lysis, and degenerative spondylolisthesis at a different level than L4–L5, previous spinal fractures, tumors or previous surgery. The patients’ clinical and radiographic charts were reviewed by an independent spine surgeon (S.S.).
Clinical measurements
The patient’s height and weight were measured preoperatively at the clinics. These data allowed determining the anthropometric characteristics of patients in spondylolisthesis and reference groups by calculating the BMI: weight (kg)/height (m)2. The following categories according to the World Health Organization were considered: underweight < 18.5, normal weight 18.5–24.9, overweight 25–29.9, obesity ≥ 30.
Radiographic measurements
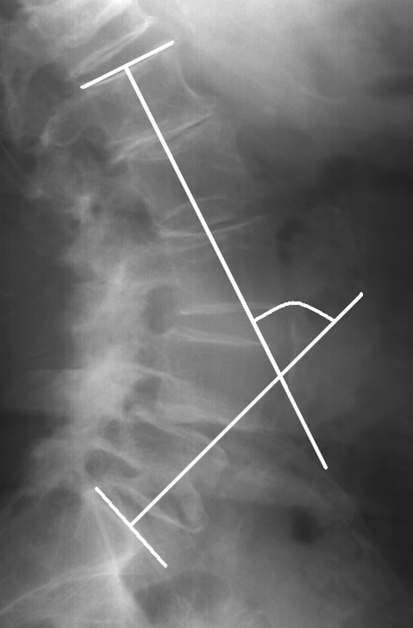
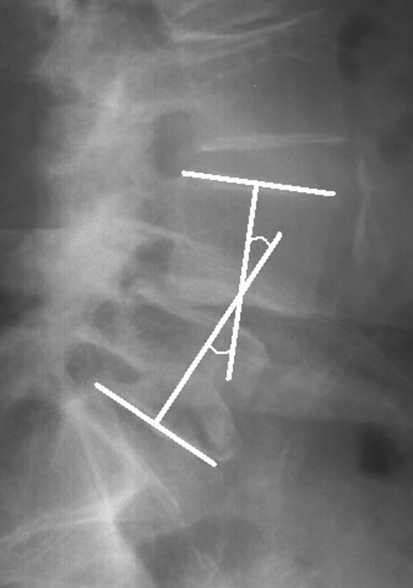
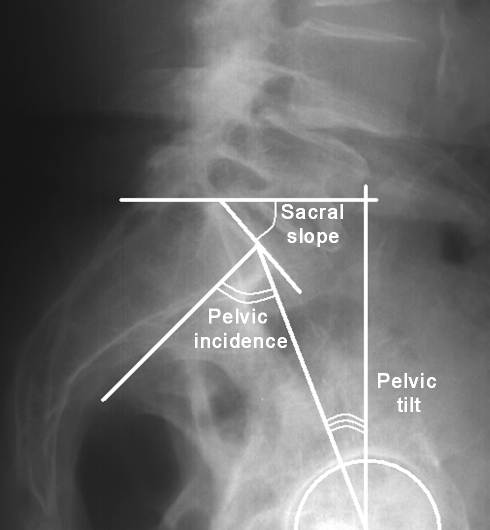
Lateral lumbar radiographs were taken for each patient using a standardized procedure. A constant distance was maintained between the subject and the X-ray source. Each patient was placed in the same comfortable standing position with the upper limbs raised forward on two arms support. With this protocol, T12, the lumbar spine, the sacrum and both femoral heads were clearly visible for subsequent radiographic evaluation. Computer-assisted measurements were made using Spineview software (Surgiview, Paris, France) [15]. After being digitalized, the superior and inferior endplate lines of each vertebra from T12 to S1 as well as the contours of both femoral heads were defined manually using the software. The following radiographic parameters were then obtained automatically with the computer program: lumbar lordosis between the superior endplate of L1 and S1 (Fig. 2), segmental lordosis from L1–L2 to L4–L5, respectively, between the superior endplate of the upper vertebra and the inferior endplate of the lower vertebra (Fig. 3), segmental lordosis of L5–S1 between the superior endplate of L5 and S1, pelvic tilt, pelvic incidence and sacral slope (Fig. 4). The degree of slippage between L4 and L5 was assessed from the same radiographs using the classification of Meyerding [16].
Fig. 2.
Lumbar lordosis between superior endplates of L1 and S1
Fig. 3.
Segmental lordosis between superior endplate of L4 and inferior endplate of L5
Fig. 4.
Spinopelvic parameters: pelvic tilt, pelvic incidence, sacral slope
Computed tomography (CT) measurements
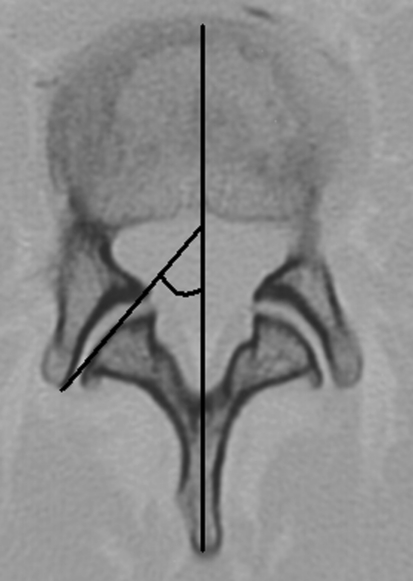
The sagittal orientation of the L3–L4, L4–L5 and L5–S1 facet joints was measured on axial planes of CT images. The level of measurement corresponded to the plane of the upper vertebral endplate of L4 for L3–L4 facet joints, L5 for the level L4–L5 and S1 for L5–S1 joints. The measurements were performed using the OsiriX imaging software version 4.19 (Open Source, Geneva, Switzerland) [17]. A sagittal line was drawn through the center of the vertebral body and the spinous process. A second line was made from the anterior to the posterior tip of the facet joint. The angle formed by these lines was then measured for the right and the left facet joint, respectively (Fig. 5). Both values were summed and divided by two for further statistical evaluation, in order to consider an average facet joint angle for each level. Furthermore, the presence of osteophytes was considered to define the presence of facet joint osteoarthritis.
Fig. 5.
Sagittal facet joint orientation: angle measurement on axial computed tomography plane
Statistics
Statistical evaluation was performed with SPSS software version 16.0 (SPSS Inc, Chicago, IL). The Wilcoxon test was used for the analysis of quantitative data in the evaluation of radiographic and CT measurements. The Fisher exact test was used for two-tailed qualitative data in the analysis of anthropometric data. The significance level was set at P < 0.05.
Results
Body mass index
Values of height, weight and BMI for both patient groups are detailed in Table 1. The average BMI was 28.2 in patients with degenerative spondylolisthesis and 24.8 in the reference group. This difference was significantly higher in the spondylolisthesis group (P = 0.030). In the spondylolisthesis group, 71.4% of the patients presented with overweight and obesity versus 50.6% in the reference group (P = 0.004).
Table 1.
Anthropometric characteristics of patients in spondylolisthesis and reference groups
| Spondylolisthesis | Reference | Significance | |
|---|---|---|---|
| Number of patients | 49 | 77 | |
| Weight (kg) | 72.7 ± 13 | 76.2 ± 13.8 | 0.046 |
| Height (m) | 1.62 ± 0 | 1.69 ± 0.08 | 0.638 |
| Body mass index (kg/m2) | 28.2 | 24.8 | 0.030 |
| Morphotype | |||
| Underweight | 1 | 0 | 0.004 |
| Normal range | 13 | 38 | |
| Overweight | 24 | 23 | |
| Obese class I | 8 | 12 | |
| Obese class II | 3 | 4 | |
Sagittal spinopelvic alignment
The radiographic parameters describing the spinopelvic alignment are detailed in Table 2. The analysis showed a significant increase of the following parameters in spondylolisthesis compared to the reference group: pelvic tilt (25.6° vs. 21.0°; P = 0.046), sacral slope (42.3° vs. 33.4°; P = 0.002), pelvic incidence (66.2° vs. 54.2°; P = 0.001), and L1–S1 lordosis (57.2° vs. 49.6°; P = 0.045).
Table 2.
Sagittal spinopelvic alignment of patients in spondylolisthesis and reference groups
| Spondylolisthesis | Reference | Significance | |
|---|---|---|---|
| L1–S1 lordosis | 57.2 ± 13.4 | 49.6 ± 18.1 | 0.045 |
| Pelvic tilt | 25.6 ± 8.7 | 21.0 ± 7.5 | 0.046 |
| Pelvic incidence | 66.2 ± 12.6 | 54.2 ± 15.9 | 0.001 |
| Sacral slope | 42.3 ± 13.1 | 33.4 ± 11.3 | 0.002 |
The segmental lumbar lordosis of each segment from L1–L2 to L5–S1 is demonstrated in Table 3. At the spondylolisthesis level L4–L5, no significant difference was observed when compared to the reference group (11.9° vs. 11.5°; P = 0.535). The same applied to the caudal adjacent segment L5–S1 (18.8° vs. 19.5°; P = 0.865). However, at the cranial adjacent segment L3–L4, the segmental lordosis was higher in the spondylolisthesis group although not statistically clearly significant (12.7° vs. 9.7°; P = 0.068). The segmental lordosis was significantly higher in patients with spondylolisthesis at the levels L1–L2 (4.4° vs. 2.5°; P = 0.038) and L2–L3 (9.5° vs. 6.4°; P = 0.024).
Table 3.
Segmental lumbar lordosis of patients in spondylolisthesis and reference groups
| Spondylolisthesis | Reference | Significance | |
|---|---|---|---|
| L1–L2 lordosis | 4.4 ± 5.4 | 2.5 ± 3.4 | 0.038 |
| L2–L3 lordosis | 9.5 ± 4.6 | 6.4 ± 5.6 | 0.024 |
| L3–L4 lordosis | 12.7 ± 5.1 | 9.7 ± 5.6 | 0.068 |
| L4–L5 lordosis | 11.9 ± 6.0 | 11.5 ± 5.7 | 0.535 |
| L5–S1 lordosis | 18.8 ± 8.2 | 19.5 ± 6.5 | 0.865 |
Facet joint orientation
As detailed in Table 4, the CT analysis evidenced a significantly more sagittal orientation of the L4–L5 facet joints in the spondylolisthesis group compared to the reference group (36.5° vs. 44.4°; P = 0.001). At the adjacent segments, however, the facet joint orientation was similar in both groups at L3–L4 (33.8° vs. 33.3°; P = 0.946) and L5–S1 (46.3° vs. 48.9°; P = 0.217). At L4–L5, osteoarthritis was present in all spondylolisthesis patients, but only in 20 out of 77 patients (26.7%) in the reference group.
Table 4.
Facet joint orientation of patients in spondylolisthesis and reference groups
| Spondylolisthesis | Reference | Significance | |
|---|---|---|---|
| L3–L4 facet joint | 33.8 ± 9.9 | 33.3 ± 10.1 | 0.946 |
| L4–L5 facet joint | 36.5 ± 11.2 | 44.4 ± 8.2 | 0.001 |
| L5–S1 facet joint | 46.3 ± 7.9 | 48.9 ± 9.9 | 0.217 |
Discussion
Several predisposing factors such as sagittal orientation and osteoarthritis of lumbar facet joints, hormonal factors as well as ligament insufficiency play a role in the pathogenesis of degenerative spondylolisthesis [2–8]. Sagittal spinopelvic imbalance recently appeared to be essential in the management of lumbar degenerative disorders [13]. However, the exact interaction mechanism of sagittal imbalance and aforementioned factors remains unclear. Roussouly et al. [11] described four typical subtypes of sagittal orientation of the lumbar spine and pelvis. Type IV of this classification is characterized by a high pelvic incidence and sacral slope, associated with an important lumbar lordosis. This spinopelvic configuration is associated with a relatively vertical S1 endplate in the sagittal plane. This orientation of the pelvis and sacrum might lead to an anterior displacement of lumbar vertebrae above. Morel et al. [18] found an increased average pelvic incidence of 62.6° in patients with spondylolisthesis versus 54.7° in a reference population. Barrey et al. [12] also measured a high pelvic incidence of 60.1° in degenerative spondylolisthesis compared to 52.0° in a control group. The results of our study are consistent with these previous findings. The average pelvic incidence was 66.2° in spondylolisthesis versus 54.2° in the reference group. Pelvic incidence represents a constitutional anatomic parameter in each individual. An increased pelvic incidence is usually associated with a high sacral slope, although variations are possible, because this parameter depends on the standing position. In spondylolisthesis patients, Morel et al. [18] measured a sacral slope of 37.0°, Barrey et al. [12] found an average value of 40.1°, and it was 42.3° in the present study compared to 33.4° in the control group. Pelvic incidence and sacral slope were found to be significantly higher in these three populations of spondylolisthesis patients compared to reference groups.
Further than high pelvic incidence and sacral slope that might facilitate an anterior slippage of L4 on L5, the ventral shift of the trunk and the center of gravity have been advocated as another possible aggravating factor in spondylolisthesis [13]. Full spine lateral standing radiographs were not systematically available for our patients, which might represent a certain limitation of our study because the C7 plumbline could not be systematically determined. However, anthropometric data aimed to characterize the morphology of the patient and the occurrence of overweight were assessed. The analysis of their height and weight revealed that 71.4% of the spondylolisthesis patients had a BMI > 25 compared to 50.6% in the reference population. Jacobsen et al. [9] analyzed the cross-sectional epidemiological data of the Copenhagen Osteoarthritis Study and demonstrated a strong correlation between overweight and the incidence of degenerative spondylolisthesis. The BMI remains a global anthropometric parameter describing a spondylolisthesis population which is often characterized by overweight. This overweight might lead to an increased axial load of the L4–L5 disc and facet joints, but it might also lead to an anterior displacement of the trunk and enhance the risk of degenerative spondylolisthesis (Fig. 6c).
Fig. 6.
Influence of spinopelvic alignment and overweight on degenerative spondylolisthesis. a Normal pelvic incidence and normal lumbar lordosis; b high pelvic incidence and high lumbar lordosis; c high pelvic incidence and high lumbar lordosis + overweight; d high pelvic incidence and high lumbar lordosis + overweight + decrease of pelvic tilt (compensation mechanism); e decompensation of spinopelvic balance resulting in degenerative spondylolisthesis
An anterior displacement of the center of gravity is usually compensated by a posterior tilt of the pelvis [11, 14]. Guigui et al. [19] reported average values for pelvic tilt in healthy young individuals: 13.7° in females and 12.7° in males. Reference values reported on by Skalli et al. [14] range between 0° and 25°, the average pelvic tilt is around 12° with a standard deviation from 7° to 20°. Compared to these values, in our reference group made of patients from 40 to 81 years with low back pain, the average pelvic tilt of 21.0° was relatively high. This might be due to the fact that lumbar degenerative disorders other than spondylolisthesis were frequent in these patients. Barrey et al. [13] found an increased pelvic tilt in patients with degenerative disc disease. They emphasized that the pelvis tilts backward to decrease intradiscal pressure and lumbar pain. This theory possibly explains the occurrence of elevated values in our reference group with a range of ages comparable to the spondylolisthesis group. However, patients with spondylolisthesis had a significantly higher pelvic tilt of 25.6° on average, which exceeds the upper limit of normal range. This clear increase of pelvic tilt describes a population of patients with overweight and a posterior tilt of the pelvis, which possibly occurs to counterbalance an anterior displacement of the trunk (Fig. 6d).
Lumbar lordosis between L1 and S1 ranges around 60° on average, with a standard deviation from 50° to 70°, and extreme values between 40° and 80° [14]. Guigui et al. [19] found an average L1–S1 lordosis of 61.0° in young healthy subjects. In comparison, patients in our reference group presented a relatively low lordosis of 49.6°, although they had a normal pelvic incidence of 54.2°. This loss of global lumbar lordosis is possibly explained by lower lumbar disc degeneration. In our patients with spondylolisthesis, the L1–S1 lordosis of 57.2° was comparable to normal values from the literature above, although their pelvic incidence was high at 66.2°. Their standing position with a posteriorly tilted pelvis might decrease their lumbar lordosis which would theoretically be higher according to the classification of Roussouly et al. [11].
The detailed analysis of segmental lordosis showed that the levels L4–L5 and L5–S1 were comparable in patients with spondylolisthesis and in those who had low back pain without slippage of L4 on L5. When comparing our results to a population of young volunteers analyzed by Guigui et al. [19], the loss of segmental lordosis becomes evident in older patients. The average L4–L5 segment measured 24.6° and the L5–S1 segment measured 24.0° in their study. The L4–L5 segment measured 11.9° in the spondylolisthesis group and 11.5° in the reference group, and the segmental lordosis of L5–S1 was 18.8° and 19.5°, respectively. These lower values might be explained by the presence of degenerative disc disease in our patients which leads to a decrease of the intersomatic height compared to a young population. Chen and Wei [20] defined an index which describes the disc height of L4–L5 and which was found to be significantly decreased in patients with degenerative spondylolisthesis. When analyzing the cranial levels, it became evident that the segmental lordosis of L1–L2 and L2–L3 was significantly increased in spondylolisthesis patients compared to the reference group (P = 0.038 and P = 0.024, respectively). An increase of L3–L4 was also noted although not clearly statistically significant (P = 0.068). These findings describe that the levels above the spondylolisthesis hyperextend and that the lordosis increases in the cranial zone of the lumbar spine. Morel et al. [18] found that the loss of lordosis occurred in the sector L3–S1 in patients with degenerative spondylolisthesis, which is probably due to disc degeneration of lower lumbar levels and the posterior pelvic tilt. On the other hand, our results demonstrate that the segmental hyperlordosis occurs above L4–L5, as a possible compensation mechanism of lower lordosis decrease, posterior tilt of the pelvis and overweight leading to an anterior trunk displacement. Further than overweight, hyperlordosis of cranial lumbar segments increases posterior stress on L4–L5 facet joints, as suggested by Beckers and Bekaert [21].
The sagittal orientation of lumbar facet joints has been incriminated as a cause of degenerative spondylolisthesis by several authors [5, 22, 23]. Biomechanically, the facet joints share load in compression and extension [24], but they also control excessive axial rotation and anterior shear forces due to their anatomical orientation [25]. It, therefore, has been argued that a more sagittal alignment of the facet joints leads to anterior gliding due to a reduced resistance to anterior shear forces [23, 26]. As demonstrated by Boden et al. [23] and Toyone et al. [27], the results of our study showed a significant increase (P = 0.001) of sagittal facet joint orientation at the level L4–L5 in spondylolisthesis patients compared to the reference group. Contrarily to the findings of those authors, who described a greater sagittal orientation of the facet joints also at adjacent levels, the L3–L4 and L5–S1 facet orientation was comparable in both of our patient groups. Fujiwara et al. [4] and Chaput et al. [6] considered the sagittal alignment as the result of osteoarthritis and facet joint effusion. On the other hand, Berlemann et al. [28] regarded the presence of osteoarthritis as a secondary remodeling of the joint orientation rather than a pre-existing morphological feature. Further factors, such as overweight and hyperlordosis of segments cranial to L4–L5, may increase the stress on facet joints. These factors, added to osteoarthritis and sagittal alignment of facet joints, lead to segmental instability of L4–L5. Shear forces may not sufficiently be compensated and an anterior slippage of L4 on L5 occurs (Fig. 6e).
Conclusion
In patients with degenerative spondylolisthesis, the anatomic orientation of the pelvis with a high incidence and sacral slope seems to represent a predisposing factor for degenerative spondylolisthesis. The association of overweight and a relatively vertical inclination of the S1 endplate is predisposing for an anterior translation of L4 on L5. The posterior tilt of the pelvis suggests a compensation mechanism in patients with high pelvic incidence and a normal lumbar lordosis. Segmental lordosis is decreased at L4–L5 and L5–S1 as a result of degenerative disc disease and posterior pelvic tilt. Lordosis increases at the levels above L4-L5, as a compensation of decreased caudal lordosis and overweight, which might enhance posterior stress on facet joints. Furthermore, the sagittally oriented and osteoarthritic facet joints do not retain anterior shear forces and the subsequent vertebral displacement.
Acknowledgments
We would like to thank Julien Godet, MSc, for statistical evaluation, Département de Santé Publique, Université de Strasbourg, France.
References
- 1.Junghanns H. Spondylolisthesis ohne Spalt im Zwischengelenkstück. Arch Orthop Unfall Chir. 1930;29:118–127. doi: 10.1007/BF02561886. [DOI] [Google Scholar]
- 2.Pedram M, Dupuy R, Vital JM (2003) Spondylolisthésis lombaire dégénératif. Encyclopédie Médico-Chirurgicale. Elsevier, Paris, Chapter 15-835-B-10
- 3.Schaik JP, Verbiest H, Schaik FD. The orientation of laminae and facet joints in the lower lumbar spine. Spine. 1985;10:59–63. doi: 10.1097/00007632-198501000-00009. [DOI] [PubMed] [Google Scholar]
- 4.Fujiwara A, Tamai K, An HS, Lim TH, Yoshida H, Kurihashi A, Saotome K. Orientation and osteoarthritis of the lumbar facet joint. Clin Orthop Relat Res. 2001;385:88–94. doi: 10.1097/00003086-200104000-00015. [DOI] [PubMed] [Google Scholar]
- 5.Grobler LJ, Robertson PA, Novotny JE, Pope MH. Etiology of spondylolisthesis. Assessment of the role played by lumbar facet joint morphology. Spine. 1993;18:80–91. doi: 10.1097/00007632-199301000-00013. [DOI] [PubMed] [Google Scholar]
- 6.Chaput C, Padon D, Rush J, Lenehan E, Rahm M. The significance of increased fluid signal on magnetic resonance imaging in lumbar facets in relationship to degenerative spondylolisthesis. Spine. 2007;32:1883–1887. doi: 10.1097/BRS.0b013e318113271a. [DOI] [PubMed] [Google Scholar]
- 7.Nagaosa Y, Kikuchi S, Hasue M, Sato S (1998) Pathoanatomic mechanisms of degenerative spondylolisthesis: a radiographic study. Spine 23:1447–1451 [DOI] [PubMed]
- 8.Butler D, Trafimow JH, Andersson GB, McNeill TW, Huckman MS. Discs degenerate before facets. Spine. 1990;15:111–113. doi: 10.1097/00007632-199002000-00012. [DOI] [PubMed] [Google Scholar]
- 9.Jacobsen S, Sonne-Holm S, Rovsing H, Monrad H, Gebuhr P. Degenerative lumbar spondylolisthesis: an epidemiological perspective: the Copenhagen Osteoarthritis Study. Spine. 2007;32:120–125. doi: 10.1097/01.brs.0000250979.12398.96. [DOI] [PubMed] [Google Scholar]
- 10.Sanderson PL, Fraser RD. The influence of pregnancy on the development of degenerative spondylolisthesis. J Bone Joint Surg Br. 1996;78:951–954. doi: 10.1302/0301-620X78B6.1291. [DOI] [PubMed] [Google Scholar]
- 11.Roussouly P (2007) Influence de l’organisation morphologique sagittale de l’ensemble rachis-bassin sur l’évolution des pathologies dégénératives rachidiennes. Cahiers d’enseignement de la SOFCOT (Elsevier, Paris). Alternatives à l’arthrodèse lombaire et lombosacrée 96:27–35
- 12.Barrey C, Jund J, Perrin G, Roussouly P. Spinopelvic alignment of patients with degenerative spondylolisthesis. Neurosurgery. 2007;61:981–986. doi: 10.1227/01.neu.0000303194.02921.30. [DOI] [PubMed] [Google Scholar]
- 13.Barrey C, Jund J, Noseda O, Roussouly P. Sagittal balance of the pelvis–spine complex and lumbar degenerative diseases. A comparative study about 85 cases. Eur Spine J. 2007;16:1469–1470. doi: 10.1007/s00586-006-0294-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Skalli W, Champain S, Mosnier T (2007) Biomécanique du rachis. Cahiers d’enseignement de la SOFCOT (Elsevier, Paris). Alternatives à l’arthrodèse lombaire et lombosacrée 96:8–18
- 15.Vialle R, Ilharreborde B, Dauzac C, Guigui P. Intra and inter-observer reliability of determining degree of pelvic incidence in high-grade spondylolisthesis using a computer assisted method. Eur Spine J. 2006;15:1449–1453. doi: 10.1007/s00586-006-0096-x. [DOI] [PubMed] [Google Scholar]
- 16.Meyerding HW. Spondylolisthesis. Surg Gynecol Obstet. 1932;54:371–377. [Google Scholar]
- 17.Rosset C, Rosset A, Ratib O. General consumer communication tools for improved image management and communication in medicine. J Digit Imaging. 2005;18:270–279. doi: 10.1007/s10278-005-6703-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morel E, Ilharreborde B, Lenoir T, Hoffmann E, Vialle R, Rillardon L, Guigui P. Analyse de l’équilibre sagittal du rachis dans les spondylolisthésis dégénératifs. Rev Chir Orthop Reparatrice Appart Mot. 2005;91:615–626. doi: 10.1016/s0035-1040(05)84465-4. [DOI] [PubMed] [Google Scholar]
- 19.Guigui P, Levassor N, Rillardon L, Wodecki P, Cardinne L. Valeur physiologique des paramètres pelviens et rachidiens de l’équilibre sagittal du rachis. Analyse d’une série de 250 volontaires. Rev Chir Orthop Reparatrice Appart Mot. 2003;89:496–506. [PubMed] [Google Scholar]
- 20.Chen IR, Wei TS. Disc height and lumbar index as independent predictors of degenerative spondylolisthesis in middle-aged women with low back pain. Spine. 2009;34:1402–1409. doi: 10.1097/BRS.0b013e31817b8fbd. [DOI] [PubMed] [Google Scholar]
- 21.Beckers L, Bekaert J. The role of lordosis. Acta Orthop Belg. 1991;57:198–202. [PubMed] [Google Scholar]
- 22.Sato K, Wakamatsu E, Yoshizumi A, Watanabe N, Irei O. The configuration of the laminas and the lumbar facet joints in degenerative spondylolisthesis: a clinicoradiologic study. Spine. 1989;14:1265–1271. doi: 10.1097/00007632-198911000-00022. [DOI] [PubMed] [Google Scholar]
- 23.Boden SD, Riew KD, Yamaguchi K, Branch TP, Schellinger D, Wiesel SW. Orientation of the lumbar facet joints: association with degenerative disc disease. J Bone Joint Surg Am. 1996;78:403–411. doi: 10.2106/00004623-199603000-00012. [DOI] [PubMed] [Google Scholar]
- 24.Adams MA, Hutton WC (1980) The effect of posture on the role of the apophysial joints in resisting intervertebral compressive forces. J Bone Joint Surg Br 62:358–362 [DOI] [PubMed]
- 25.Adams MA, Hutton WC. The relevance of torsion to the mechanical derangement of the lumbar spine. Spine. 1981;6:241–248. doi: 10.1097/00007632-198105000-00006. [DOI] [PubMed] [Google Scholar]
- 26.Sharma M, Langrana NA, Rodriguez J. Role of ligaments and facets in lumbar spinal stability. Spine. 1995;20:887–900. doi: 10.1097/00007632-199504150-00003. [DOI] [PubMed] [Google Scholar]
- 27.Toyone T, Ozawa T, Kamikawa K, Watanabe A, Matsuki K, Yamashita T, Wada Y. Facet joint orientation difference between cephalad and caudal portions. A possible cause of degenerative spondylolisthesis. Spine. 2009;34:2259–2262. doi: 10.1097/BRS.0b013e3181b20158. [DOI] [PubMed] [Google Scholar]
- 28.Berlemann U, Jeszenszky DJ, Bühler DW, Harms J. Facet joint remodeling in degenerative spondylolisthesis: an investigation of joint orientation and tropism. Eur Spine J. 1998;7:376–380. doi: 10.1007/s005860050093. [DOI] [PMC free article] [PubMed] [Google Scholar]