Abstract
Most animals experience marked changes in reproductive status across development that are regulated by changes in the hypothalamo-pituitary-gonadal (HPG) axis. The upstream mechanisms regulating this axis, however, remain less well understood. The neuropeptide kisspeptin serves as a positive regulator of reproduction; the precise actions of kisspeptin on the HPG axis in animals of differing developmental and seasonal reproductive states, however, remain unresolved. Further, sex differences in response to kisspeptin have not been fully explored. In Experiment 1, we investigated whether sensitivity to a broad range of kisspeptin doses differed in adult male and female Siberian hamsters held on reproductively inhibitory or stimulatory photoperiods. In Experiment 2, we asked whether the response to kisspeptin differed across different stages of reproductive development. Males and females displayed elevated luteinizing hormone LH) in response to kisspeptin; however, the sexes differed in this response, with males showing greater LH responses to kisspeptin than females. Hamsters responded to kisspeptin across all stages of reproductive development, although the magnitude of this response differed between animals of different ages and between the sexes. Males showed significant increases in LH at an earlier development age than females; females also showed blunted LH responses during early adulthood whereas males remained relatively constant in their response to kisspeptin. These findings suggest that reproductively active and inactive hamsters are responsive to kisspeptin, but that the sexes differ in their responsiveness. Collectively, these data provide further insight into the basic actions of kisspeptin in the regulation of reproduction, and provide a potential mechanism for the regulation of differential reproductive responses between the sexes.
Keywords: metastin, GPR54, Kiss1, seasonal reproduction, puberty
Introduction
All vertebrates experience marked changes in reproductive physiology during the developmental transitions from a sexually immature, pre-pubertal state to a post-pubertal, reproductively-active state. In addition to these relatively permanent developmental changes in reproductive status, seasonally breeding animals must also transition between reproductively active and inactive states on an annual basis. These seasonal changes in reproduction have previously been likened to a form of “seasonal puberty” [20].
Changes in the activity of hypothalamo-pituitary-gonadal (HPG) axis are responsible for regulating both pubertal and seasonal transitions in reproductive function. The fundamental role that the hypothalamic hormone gonadotropin-releasing hormone (GnRH) plays in regulating reproduction and fertility has been unequivocally established [33, 43]. Most animals do not display continuous reproduction, however, and must encode and integrate salient internal and environmental signals (e.g., energy stores, photoperiod), and subsequently adjust GnRH release to regulate seasonal reproductive timing [2, 8, 19, 67]. Specifically, most rodents respond to short “winter-like” day lengths by down-regulating HPG activity and inhibiting reproduction; reproductive function is restored after prolonged exposure to long “summer-like” day lengths. The upstream mechanisms that integrate these signals to regulate HPG activity, however, are less well understood. Further, it remains unclear whether such regulatory mechanisms act in a similar manner during both developmental and seasonal reproductive transitions [11, 20, 42]. Seasonally breeding animals, therefore, serve as excellent model system to address these questions [18, 20].
Recently, the neuropeptide kisspeptin has been identified as a potential regulator of both developmental and photoperiodic changes in reproduction [9, 16, 25, 31, 45, 53, 55, 56, 59]. Kisspeptin acts as a potent positive regulator of gonadotropin-releasing hormone (GnRH) release in all mammals studied to date, and is the endogenous ligand for the kisspeptin receptor (Kiss1R)(previously called GPR54) [28]. The importance of kisspeptin in normal reproductive maturation has been demonstrated by the observation that mutations in either the Kiss1 gene or the gene for its cognate receptor Kiss1R render the animal unable to reach puberty [15, 16, 25]; animals expressing these mutations display pre-pubertal reproductive morphology and physiology for the remainder of their lives. Additionally, in non-human primates and rodents, Kiss1 gene expression and kisspeptin protein are up-regulated in the hypothalamus during reproductive pubertal development [13, 32, 39, 50, 56]. Further, kisspeptin content in the hypothalamus of rodents changes in response to manipulations of sex steroids as well as changes in photoperiod in seasonally breeding rodents [29, 31, 45, 53, 59–62]. These findings highlight a potential role for kisspeptin to serve as an integrative signal to the HPG axis; kisspeptin responds to relevant internal and environmental signals and alters activity of the HPG axis.
Although kisspeptin has been shown to regulate GnRH release in mammals [12, 14, 23, 30, 34, 40], its basic functions are still being determined. For example, it remains unclear whether kisspeptin activates the HPG axis similarly in developmentally non-reproductive (i.e., pre-pubertal) animals as it does in seasonally non-reproductive (i.e., short-day) animals [9, 31, 48], and whether kisspeptin acts in a similar fashion in both sexes, where the cost of activating or maintaining reproductive physiology may differ substantially [3]. Interestingly, Kiss1 gene expression differs between male and female rats in one hypothalamic nucleus, the anteroventral periventricular nucleus (AVPV)[38], and female Siberian hamsters display reduced levels of the pituitary gonadotropin luteinizing hormone (LH) compared with males following repeated injections of a single dose of kisspeptin [31, 45]. These findings support the idea that kisspeptin and its downstream effects may differ between the sexes in certain contexts.
In the current study, we examined the ability of the HPG axis of male and female Siberian hamsters (Phodopus sungorus) to respond to an injection of exogenous kisspeptin in differing photoperiod-induced reproductive states (Experiment 1) and at different time points across reproductive development (Experiment 2). Additionally, in both experiments we investigated potential sex differences in sensitivity to kisspeptin. By comparing the actions of kisspeptin across reproductive conditions, this study will help elucidate the potential role of kisspeptin as a key mechanism regulating activity of the GnRH neuronal system.
Materials and Methods
Animals and Housing
All animals were obtained from a breeding colony maintained at Indiana University. All animals were group-housed at weaning with same-sex siblings in a long-day photoperiod (L:D 16:8). Breeders and pre-weaned offspring were housed together in large polypropylene cages (45 × 23 × 15 cm) until weaning at 18 days of age; weaned and adult individually housed animals were housed in smaller polypropylene cages (27.8 × 17.5 × 13.0 cm). Temperature was kept constant at 20 ± 2°C and relative humidity was maintained at 50 ± 5%. Food (Purina Rat Chow) and tap water were available ad libitum throughout the experiments. All experimental procedures follow NIH guidelines for the Care and Use of Experimental Animals and were approved by the Bloomington Institutional Animal Care and Use Committee (BIACUC).
Experiment 1: Effect of photoperiod on HPG axis sensitivity to exogenous kisspeptin
The aims of this experiment were to: 1) test the sensitivity of the HPG axis to a range of kisspeptin doses, 2) investigate potential sex differences in sensitivity to kisspeptin, and 3) determine whether photoperiod alters endocrine response to kisspeptin. To accomplish this, both males and females were housed individually in long days (L:D 16:8) and allowed to acclimate for 5–7 days. The hamsters were then weighed to the nearest 0.1g and placed in either long-day (L:D 16:8) or short-day (8:16) photoperiods for > 8 weeks. Due to space limitations, males and females were run separately and each sex was run in cohorts; all other procedures were identical between sexes and cohorts. Short-day hamsters that did not respond to photoperiod, so called “non-responders” [44], were indentified by a lack of body mass loss and pelage coloration (males and females) and bye non-regressed testes (males). These animals were excluded from further analysis.
Kisspeptin injections and blood sampling
A baseline blood sample was collected from the retro-orbital sinus prior to the animals receiving a single i.p. injection of 100µl of a 0.1, 1, 5, or 10 µM kisspeptin-10 [KiSS-1 (112–121)/ metastin (45–54)(human); Phoenix Pharmaceuticals, Inc. Belmont, CA], or PBS vehicle, creating 20 distinct treatments: long-day males receiving a 100µl injection of vehicle (n = 9), 0.1µM (n = 9), 1µM (n = 10), 5µM (n = 10), or 10µM kisspeptin (n = 10); short-day males receiving vehicle (n = 8), 0.1µM (n = 8), 1µM (n = 6), 5µM (n = 7), or 10µM kisspeptin (n = 6); long-day females receiving vehicle (n = 10), 0.1µM (n = 10), 1µM (n = 10), 5µM (n = 9), or 10µM kisspeptin (n = 10); and short-day females receiving vehicle (n = 8), 0.1µM (n = 8), 1µM (n = 10), 5µM (n = 10), or 10µM kisspeptin (n = 7). A second blood sample was obtained 30 minutes after this injection for luteinizing hormone (LH) analysis. A subset of long-day males that had sufficient serum left after LH analysis were combined with additional long-day males following the same procedures and assayed only for testosterone (Final sample sizes: vehicle, n = 8; 0.1µM, n = 8; 1µM, n = 11; 5µM, n = 11; 10µM n = 8). Blood samples were left at room temperature to allow clots to form. Clots were then removed, samples were centrifuged at 2500 RPM for 30 min., and serum was collected and stored at −80°C until assayed for reproductive hormones (see below for assay details). Following blood sampling, necropsies were performed and reproductive tissues were extracted, cleaned of fat and connective tissue and weighed to the nearest 0.1g.
Experiment 2: Effect of development on HPG axis sensitivity to exogenous kisspeptin
To capture any dynamic changes in the ability of the HPG axis to respond to exogenous kisspeptin over the course of reproductive development, pups from a given litter were assigned pseudo-randomly (controlling for initial body mass) on the day of their birth to be sampled either prior to entering puberty (on Day 15 [D15]), during pubertal development (D30), as sub-adults with developed gonads but undeveloped accessory organs (D45), during young adulthood when animals are fully reproductively capable (D60), and during adulthood (D 75) [1, 35, 63]. Pups were obtained from 7 breeding pairs producing multiple litters, and pups from at least 4 different breeding pairs were represented at each of the designated sampling periods (e.g., D15, D30).
Kisspeptin injections and blood sampling
To assess HPG axis activation in response to kisspeptin, animals were injected with kisspeptin-10, or a 0.1 M PBS vehicle injection. Specifically, hamsters received a single i.p. injection of either 100 µl PBS or 100 µl of a PBS solution containing 10 µM kisspeptin-10 (Phoenix Pharmaceuticals, Inc.), yielding 20 treatment groups: males injected with vehicle on D15 (n = 7), D30 (n = 6), D45 (n= 7), D60 (n = 5), D75 (n = 7); males injected with kisspeptin on D15 (n = 7), D30 (n = 12), D45 (n= 8), D60 (n = 6), D75 (n = 7); females injected with vehicle on D15 (n = 10), D30 (n = 5), D45 (n= 7), D60 (n = 10), D75 (n = 6); and females injected with kisspeptin on D15 (n = 7), D30 (n = 7), D45 (n= 8), D60 (n = 8), D75 (n = 8). Thirty minutes after the injection, a blood sample was collected via the retro-orbital sinus. Blood was left at room temperature to allow clots to form, the clots were removed, the sample was centrifuged at 2500 RPM for 30 min., and serum was collected and stored at −80°C until assayed for LH (see below for assay details). Following blood sampling, the animals were killed and necropsies were performed. Gonads were removed, to confirm sex and reproductive status, cleaned of fat and connective tissue, and weighed.
Hormone Assays
Serum LH concentrations were measured in duplicate via a radioimmunoassay (RIA) with reagents obtained from the National Institutes of Health based on a previous protocol [10]. The antiserum was rLH-S-11 and the standard was rLH-RP3. The sensitivity was 0.01ng/tube and the intra-assay coefficient of variation (CV) was 5.87% for the low pool and 5.25% for the high pool, the inter-assay CV was 6.13% for the low pool and 5.91% for the high pool; samples from both sexes were run in each assay. Serum testosterone was measured via a commercial EIA kit (Correlate-EIA Kit #900-065; Assay Designs, Ann Arbor, MI). Serum samples were diluted 1:20 and run in duplicate for each sample. The sensitivity of the assay was 3.82 pg/ml and the intra-assay coefficient of variation for the assays was < 8.1%; the inter-assay of variation was 5.24%. The antisera used in both assays were highly specific for the hormones measured, with low cross-reactivity with other hormones. Both the LH and T assays have been previously validated for use in Siberian hamsters [17, 68].
Statistical Analyses
In Experiment 1, the effects of differing doses of kisspeptin or vehicle injection on testosterone in long-day males and luteinizing hormone in all animals held in either long- or short-day photoperiods were analyzed using a General Linear Model (GLM). For testosterone analysis, injection dose was the dependent variable, post-injection testosterone was the independent variable, and baseline testosterone values were included as a covariate in the model. For LH analysis, injection dose, sex, and photoperiod and all interactions were set as the dependant variables, post-injection LH as the independent variable, and baseline LH levels were included as a covariate in the model. Significant effects were subsequently probed with separate ANOVAs combined with Tukey HSD post-hoc tests.
In Experiment 2, the effects of kisspeptin or vehicle injection on serum luteinizing hormone levels across development were analyzed using a GLM with injection type, sex, and age as main effects and including all interaction effects. To meet the assumptions of normality and equality of variances, LH values were log-transformed prior to analysis. As in Experiment 1, significant interactions were probed with further ANOVAs. Differences in body and gonad mass between the age groups within each sex were analyzed separately using a one-way ANOVA. To meet the assumption of normality of the residuals, female gonad masses were log transformed prior to analysis. Tukey’s HSD post-hoc tests were employed to probe pair-wise differences between the age groups.
Results
Experiment 1: Effect of photoperiod on HPG axis sensitivity to exogenous kisspeptin
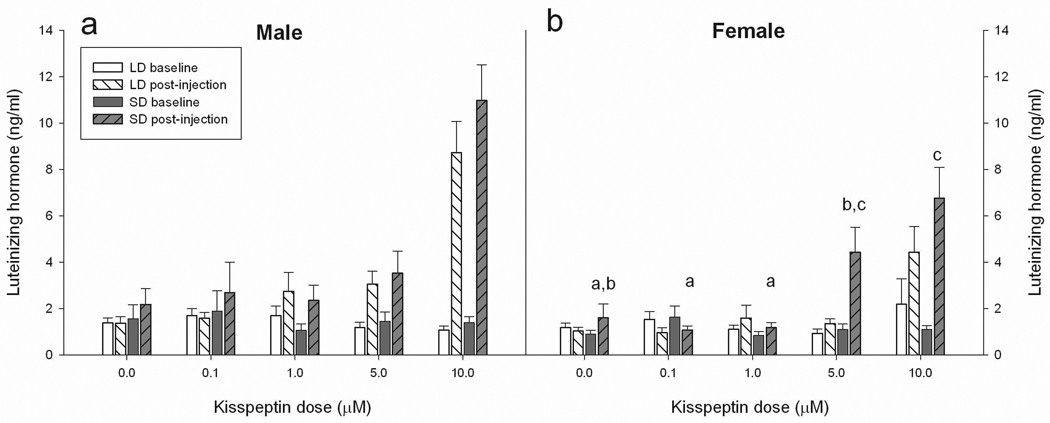
The GLM revealed significant main effects of sex (F1,154 = 15.80, p ≤ 0.001), injection dose (F4,154 = 40.95, p ≤ 0.001) and photoperiod (F1,154 = 8.51, p ≤ 0.01). A significant interaction between injection dose and sex was also revealed (F4,154 = 4.35, p ≤ 0.01); all other interactions were not significant (P > 0.05 in all cases)(Figure 1). To probe the above effects, separate ANOVAs were performed with the sexes split to investigate the effect of differing doses and photoperiod, and their interaction on post-injection LH levels in these groups. Males displayed a significant response to differing doses (F4, 73 = 24.95, p ≤ 0.001), but neither photoperiod (p > 0.05), nor the interaction of photoperiod and injection dose significantly affected post-injection LH levels, (p > 0.05). Post-hoc analysis revealed that males receiving an injection with 10µM kisspeptin had significantly higher LH levels compared with all injection doses. Female post-injection LH levels were significantly affected by injection dose (F4, 82 = 14.31, p ≤ 0.001), photoperiod (F1, 82 = 6.94, p ≤ 0.01) and the interaction between dose × photoperiod (F4, 82 = 2.47, p ≤ 0.05). To probe the dose × photoperiod interaction in the females, the data were further split by photoperiod to investigate how the responses to differing doses of kisspeptin varied in animals held in differing photoperiod treatment. In separate ANOVAs, the dose of kisspeptin significantly affected post-injection LH levels in both long-day (F4, 44 = 6.37, p ≤ 0.001) and short-day females (F4, 38 = 9.27, p ≤ 0.001). Tukey HSD post-hoc analysis revealed that long-day females receiving the 10µM dose significantly elevated LH levels compared with all other long-day treatment groups (p < 0.01 in all cases). Post-hoc analysis of short-day females revealed that females receiving a 10µM dose did not differ from females receiving a 5µM dose (p > 0.05), while females receiving the 10µM kisspeptin dose had significantly higher LH values compared with the 1.0 and 0.1µM dose and higher values compared with vehicle treated animals (p ≤ 0.001 in all cases). Short-day females receiving a 5µM dose of kisspeptin did not differ from females receiving a 10µM dose (p > 0.05), but had significantly higher post-injection LH levels when compared with short-day females receiving 1.0 and 0.1µM doses (p ≤ 0.05 in all cases). Short-day females receiving vehicle injection tended to have lower post-injection LH values compared with those receiving the 5µM dose (p < 0.1).
Figure 1.
Effect of differing doses of kisspeptin injections on serum levels of luteinizing hormone (LH). Male (a) and female (b) hamsters housed in either long-day (white bars) or short-day (dark-bars) photoperiods were injected with 100µl of vehicle, 0.1µM, 1µM, 5µM or 10µM kisspeptin. Baseline (solid bars) and post-injection (striped bars) blood samples were assayed for LH. A significant main effect of injection dose was revealed in both sexes. Differing letters indicate significant differences in post-injection LH in the females revealed by post-hoc analysis probing the significant dose × photoperiod interaction in this sex. Figure legend: LD = long-day, SD = short-day. Significance is set at p < 0.05
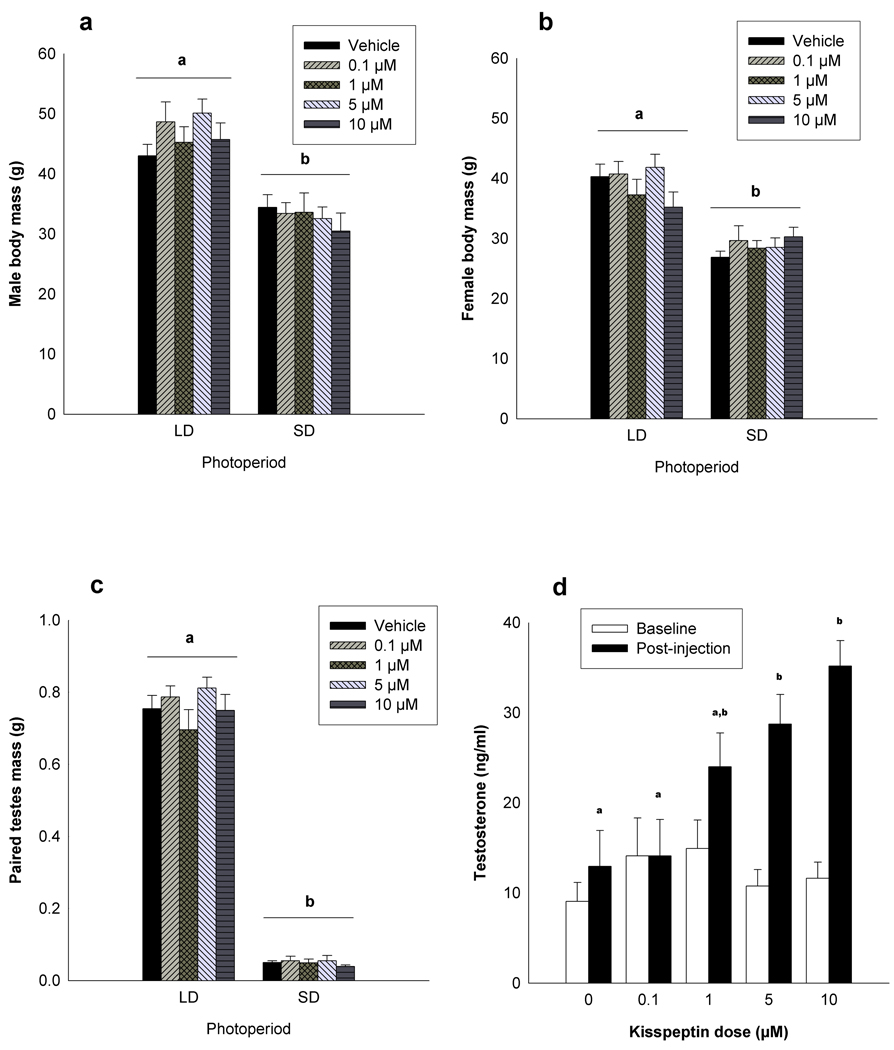
A significant main effect of injection dose on serum testosterone levels was observed (F4,40 = 10.63, p ≤ 0.001)(Figure 2). Post-hoc analysis revealed that males injected with 10 or 5µM of kisspeptin had significantly elevated post-injection LH levels compared with vehicle injected animals and animals injected with a dose of 0.1µM (p ≤ 0.05 in all cases). No other significant pair-wise comparisons were revealed (p > 0.05 in all cases).
Figure 2.
Photoperiod treatment affects body mass and paired testes masses. Body masses of long-day male (a) and female (b) as well as paired testes masses of male (c) Siberian hamsters that received an injection of 100µl of vehicle, 0.1µM, 1µM, 5µM or 10µM of kisspeptin were significantly affected by photoperiod treatment. Different letters indicates that groups differed significantly. Long-day hamsters injected with 100µl of vehicle, 0.1µM, 1µM, 5µM or 10µM of kisspeptin displayed differences in post-injection testosterone titers compared with baseline titers. Post-injection serum testosterone concentrations differed significantly between injection dose, and is indicated by differing letters. Significance is set at p < 0.05
Photoperiod had a significant effect on both male (F1,79 = 65.36, p < 0.001) and female body masses (F1,79 = 65.28, p < 0.001) (Figure 2). No differences in body mass were observed between injection treatment groups within each photoperiod (p > 0.05 in all cases). Photoperiod had a significant effect on paired-testes masses (F1,78 = 963.23, p < 0.001)(Figure 2). No differences in paired-testes mass were observed between injection treatment groups within each photoperiod (p > 0.05 in all cases).
Experiment 2: Effect of development on HPG axis sensitivity to exogenous kisspeptin
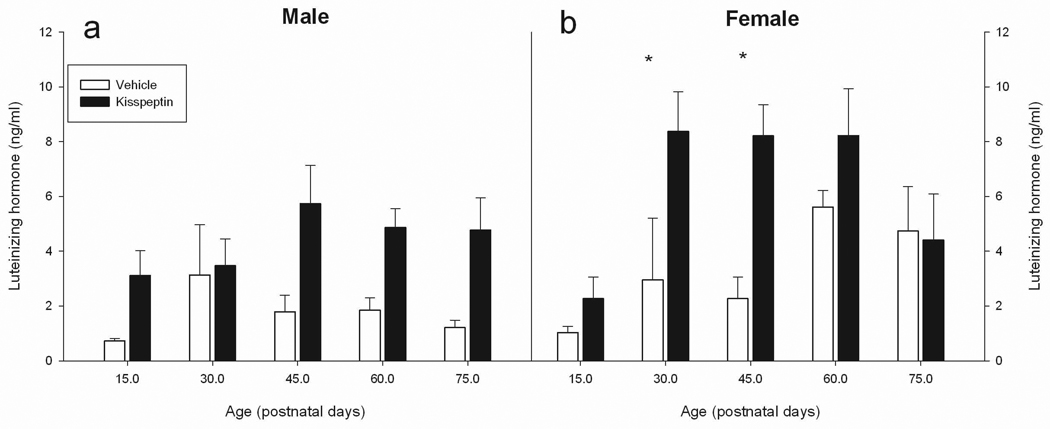
The GLM revealed significant main effects of injection treatment (F1,128 = 45.09, p ≤ 0.001), age (F4,128 = 7.96, p ≤ 0.001), and sex (F1,128 = 6.85, p ≤ 0.01) as well as a sex × age × injection interaction (F4,128 = 3.50, p ≤ 0.01) on serum LH levels (Figure 3); all two-way interactions were not significant (p > 0.05). To probe the 3-way interaction, the data was split by sex and then run in separate ANOVAs to investigate the effects of age and injection and the age × injection interaction to uncover the nature of the sex × age × injection interaction. These analyses revealed that in males, a main effect of injection type (kisspeptin vs. vehicle) was significant (F1,62 = 31.92, p ≤ 0.001), while age and injection × age interactions were not significant (p > 0.05). In females, significant main effects of both injection type (F1,66 = 15.48, p ≤ 0.001) and age (F4,66 = 6.89, p ≤ 0.001) were revealed, as well as a significant interaction between age and injection (F4,66 = 3.99, p ≤ 0.01).
Figure 3.
Kisspeptin affects serum luteinizing hormone (LH) concentrations in Siberian hamsters at different stages of reproductive development. Male (a) and female (b) Siberian hamsters were injected with either 100µl of vehicle or kisspeptin (10µM) before puberty (D15), during puberty (D30), as sub-adults (d45), young adults (D60) or adults (D75). A significant main effect of kisspeptin in males was observed. In females a significant main effect of kisspeptin was also found, as well as an injection × age interaction. Post-hox analysis in females revealed significant effects of kisspetpin compared with vehicle on circulating LH levels (p < 0.05); these effects are denoted by an asterisk (*).
The significant age × injection interaction was further probed by splitting the data by age and investigating the effect of the injection treatment (kisspeptin vs. vehicle) on differing aged females. These data revealed significant differences between kisspeptin and vehicle treated females at D30 (F1,12 = 11.00, p ≤ 0.01), and D45 (F1,13 = 20.20, p ≤ 0.001); 15 day old females tended to have higher LH levels in kisspeptin injected females compared with vehicle injected females (p ≤ 0.1). No significant effect of kisspeptin injection on circulating LH was found in adult (D60 and D75) females (p > 0.05 in both cases).
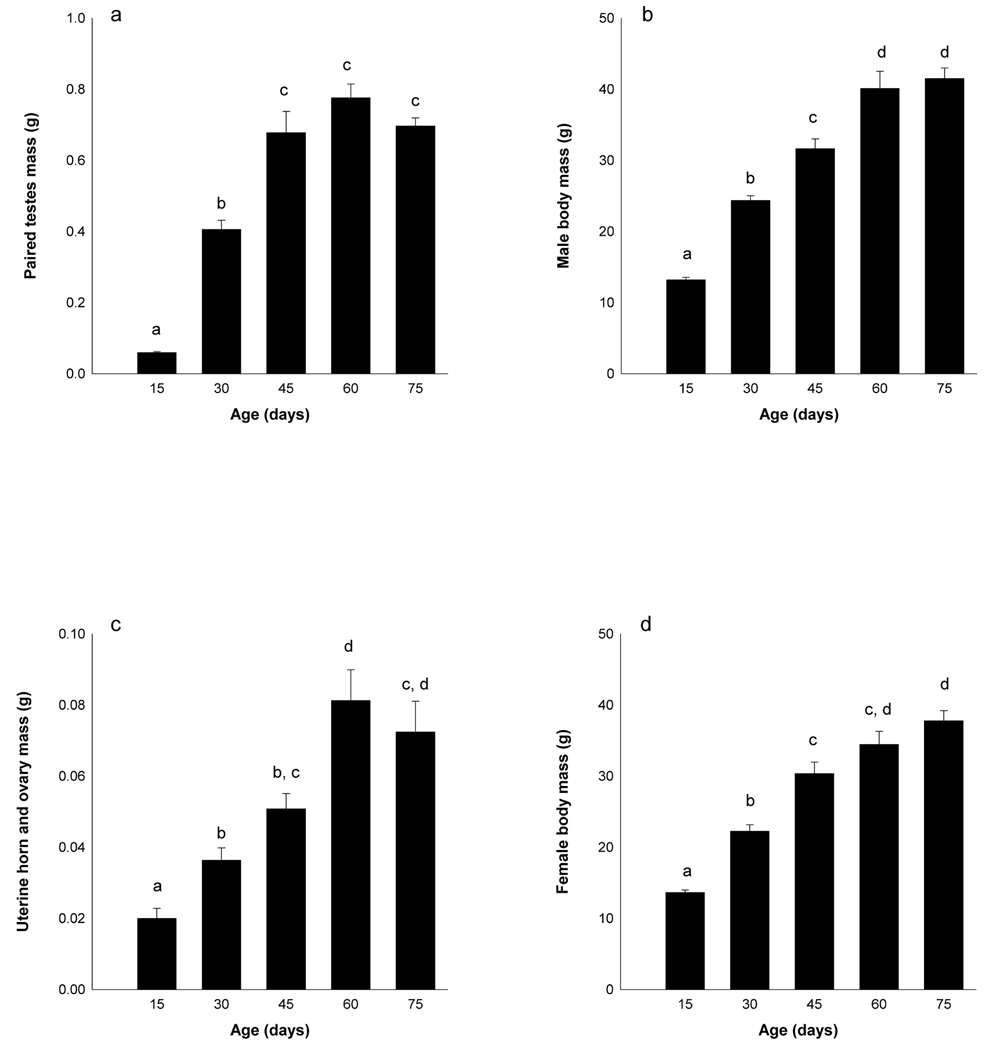
In male hamsters, there was a significant effect of age on body mass (F4,62 = 84.05, p < 0.001) and paired testes mass (F4,65 = 65.03, p < 0.001)(Figure 4). Pre-pubertal (D15) males weighed less than all other groups (p < 0.05 in all cases) and had smaller paired testes masses. Pubertal males (D30) weighed less and had smaller testes than sub-adult (D45), young adult (D60) and adult males (D75) (p < 0.05 in all cases). Sub-adult males (D45) weighed less than young adult and adult males (p < 0.05 in all cases); paired testes masses did not differ between sub-adult males and young adult and adult males (p > 0.05 in all cases). The body mass and paired testes masses of young adult (D60) and adult (D75) males did not differ (p > 0.05), and both groups were heavier than all other groups (p < 0.05 in all cases).
Figure 4.
Observed changes in body mass and reproductive organs across reproductive development. Male paired testes mass (a) and body mass (b), and female uterine and ovarian masses (c) and body masses (d) were recorded from individuals before puberty (D15), during puberty (D30), as sub-adults (d45), young adults (D60) or adults (D75). Different letters indicate that the groups significantly differed from each other (p < 0.05).
In female hamsters, there was a significant effect of age on body masses (F4,65 = 52.03, p < 0.001) and uterine horn and ovarian mass (F4,68 = 37.38, p < 0.001)(Figure 4). Pre-pubertal (D15) females weighed significantly less and had smaller gonads than all other groups (p < 0.05 in all cases). Pubertal females (D30) were significantly lighter than sub-adult (D45), young adult (D60) and adult (D75) females, and had lighter gonads than young adult and adult females (p < 0.05 in all cases); pubertal and sub-adult gonad masses did not differ (p > 0.05). Sub-adult (D45) females were heavier than pre-pubertal and pubertal females, and weighed less than adult females (p < 0.05 in all cases). Young-adult (D60) weights were not significantly different than sub-adults (D45) or adults (D75)(p > 0.05). Gonadal masses did not differ between sub-adult (D45) and adult (D75) females, nor between young adult (D60) and adult (D75) females (p > 0.05 in all cases); young-adult females had heavier gonads than all other groups (p < 0.05 in all cases).
Discussion
Overall, significant activation of the reproductive neuroendocrine axis (as measured by serum LH) in response to kisspeptin injections was observed in both long- and short-day hamsters and across all stages of reproductive development; however, the magnitude of this response differed depending on the age of the animals. Additionally, sex differences were observed in response to kisspeptin; males and females displayed different patterns of LH responses to an intermediate dose of kisspeptin depending on reproductive status. Sex differences were also observed across pubertal development; kisspeptin injected females tended to have higher LH levels compared with males, and responded more robustly to kisspeptin at 30 days of age. Taken together, these data demonstrate a modest sex difference in the sensitivity to exogenous kisspeptin depending on the reproductive status of the animals. Differences in kisspeptin sensitivity during periods of reproductive transition may serve as one potential mechanism for the differential regulation of reproductive responses between male and female animals.
Potential differences in the sensitivity of the HPG axis of hamsters housed either in reproductively inhibitory short-day photoperiods or stimulatory long-day photoperiods were investigated both within and between the sexes in Experiment 1. The results of this experiment demonstrated that the sensitivities to kisspeptin differed between the sexes. Specifically, male hamsters demonstrated dose-dependent increases in LH in both photoperiods, with both long- and short-day males injected with 10µM kisspeptin elevating LH levels over baseline; long-day males injected with 5µM kisspeptin also significantly elevated LH levels over baseline whereas short-day males injected with 5µM displayed a non-significant elevation. These observations are consistent with a previous finding indicating a similar capability for kisspeptin to activate the HPG axis in both reproductive and non-reproductive male hamsters [31].
Both long-day reproductive and short-day non-reproductive female hamsters displayed a significant increase in serum LH over baseline in response to an injection of 10µM kisspeptin. However, female hamsters differed in their response to an injection with an intermediate dose of 5µM kisspeptin. Whereas long-day reproductive male hamsters displayed a robust increase in LH over baseline in response to kisspeptin, long-day reproductive females failed to elevate LH levels, while short-day non-reproductive females significantly increased serum LH levels over baseline. These observations suggest the possibility that males and females differ in sensitivity to differing concentrations of kisspeptin depending on their reproductive status.
The source of the observed sex differences in sensitivity to kisspeptin reported here remains unknown and most studies to date have focused on sex differences in Kiss1 gene expression. In female mice, for example, treatment of neonatal mice with testosterone grossly reduces AVPV Kiss1 expression (Kauffman et al., 2007). Likewise, neonatal castration of male mice leads to markedly enhanced Kiss1 gene expression in the AVPV (Kauffman et al., 2007). Interestingly, this sex difference remains in gonadectomized animals, indicating the importance of early sex steroid exposure in the extent of AVPV Kiss1 expression, although estrogen generally up-regulates Kiss1 expression in adult females. Given these differences, even in the absence of gonadal steroids, it is likely that receptor numbers are similarly sexually differentiated. Additionally, photoperiod may differentially alter the number of receptors for kisspeptin, Kiss1R, between the sexes, although this idea remained to be tested. Sex differences in expression of the Kiss1R gene have been observed in rhesus monkeys of differing developmental reproductive states; Kiss1R mRNA increased during puberty in female but not male monkeys [56]. Whether or not differences in gene expression translate to actual differences in receptor number, and whether sex differences in seasonal changes in receptor number are observed in seasonally breeding animals requires further exploration. Photoperiod-induced differences in inhibitory neuropeptides, such as gonadotropin-inhibitory hormone (avian GnIH)(called RF-amide related peptide [RFRP] in mammals)[4, 41, 65] may also act as a potential mechanism regulating sex differences in the ability of the HPG axis to respond to the stimulatory cue of kisspeptin [30, 40].
In a previous study, female hamsters receiving repeated injections of 10µM kisspeptin, a protocol that had been found to significantly elevate LH levels in adult wild-type male mice and adult male hamsters [31, 48], displayed significant differences in LH response based on their photoperiodically-induced reproductive condition; long-day reproductive females displayed significantly elevated serum LH over baseline in response to injections of kisspeptin, whereas serum LH levels in short-day females did not differ from baseline or from animals injected with vehicle [45]. In the current study both long-day reproductive and short-day non-reproductive female hamsters receiving one injection of 10µM kisspeptin displayed a significant increase in serum LH, demonstrating that, while short-day females are capable of responding to kisspeptin, other factors are capable of altering the ability of the axis to respond to subsequent presentations of kisspeptin. One likely candidate for the observed differences in LH responses to differing kisspeptin regimes is an increase in short-day induced steroidal negative feedback. Negative feedback to gonadal steroids is more pronounced in photo-inhibited seasonal breeders [7, 22, 37, 49]. Thus, the up-regulation of this axis induced by a single injection of kisspeptin combined with short-day induced increases in negative feedback of the axis could facilitate the previously reported basal levels of LH measured in female hamsters after 4 total injections, 2 hours after the initial injection [45]. It remains unclear, however, whether the ovaries of photo-inhibited females are capable of elevating sex steroid levels in response to kisspeptin, and this question warrants further study. If photo-inhibited females do not alter circulating levels of sex steroids in response to kisspeptin, a down-regulation of this axis in response to multiple kisspeptin injections may be regulated via steroidal independent mechanisms acting on the kisspeptin-HPG system [5, 6, 29, 46, 60, 66, 69].
Experiment 2 documents for the first time the effects of single injection of kisspeptin on serum LH levels in male and female seasonally breeding rodents in different developmental reproductive states. Specifically, male hamsters injected with kisspeptin displayed higher LH levels compared with vehicle-injected animals. This observed sensitivity to kisspeptin, regardless of developmental reproductive status, is similar to previous observations in adult male hamsters and female sheep in differing reproductive conditions; both reproductive and non-reproductive animals display significant elevations in LH in response to kisspeptin [9, 31]. Female hamsters also demonstrated significantly elevated LH levels in kisspeptin-injected animals compared with those injected with vehicle, this elevation, however, was only significantly different in pubertal (D30) and sub-adult (D45) females; neither pre-pubertal (D15) nor adult (D60 and D75) females injected with kisspeptin displayed elevated LH levels compared with vehicle injected controls. The lack of a detectable kiss-induced LH surge in adult females may be due to naturally occurring increased variation in LH levels in control females that are now cycling adults.
Because many of the physiological processes experienced during developmental and photoperiodic transition from a non-reproductive state to a reproductive state are similar, seasonally breeding animals have served as useful models for the study of the neuroendocrine mechanisms regulating reproductive status [18, 20]. For example, GnRH secretion is reduced during the pre-pubertal and seasonal non-reproductive period, while the ability of the pituitary to respond to GnRH remains [24, 27, 47, 52, 57, 58]. The reduction of GnRH release and subsequent inhibition of LH surges in both pre-pubertal and seasonal non-reproductive animals is regulated by increased steroidal negative feedback; ovariectomy or castration increases LH pulse frequency and circulating LH in both pre-pubertal and seasonally non-reproductive animals and sex steroid replacement suppresses circulating LH [21, 26, 36, 37, 51, 54, 64]. The current findings are consistent with this idea; significant similarities in the actions of kisspeptin on the HPG axis in hamsters of differing developmental and photoperiod-induced reproductive states were observed.
Additionally, significant sex differences in the responsiveness to kisspeptin were observed. The mechanisms regulating these differences, however, remain unresolved. Sex differences in the maintenance and timing of changes of reproductive function often differ between the sexes, and these differences likely reflect differing selective pressures between the sexes [3]. The observed difference in sensitivity to kisspeptin may act as one such sexually differentiated mechanism that has been shaped by differing selective pressures. Collectively, these results provide further insight into the role of kisspeptin in the developmental and seasonal regulation of reproductive physiology.
Acknowledgements
The authors thank Drs. Melissa-Ann Scotti and Lance Kriegsfeld for assistance and feedback on the study design and interpretation. The authors also wish to thank Drs. Ellen Ketterson and Devin Zysling, and Emily Chester and Jacqueline Ho for comments and discussion on earlier presentations of the data, and Nick Garcia, Stefanie Frommeyer, and Jill Lodde for assistance. This work was supported by a Society for Integrative and Comparative Biology Grant-in-Aid and NIH/T32 training grant HD049336-0 (T.J.G.), an Eli Lilly Endowment METACyt grant, Indiana University Faculty Research Support Program and NSF IOB 0543798 (G.E.D).
References
- 1.Adam CL, Moar KM, Logie TJ, Ross AW, Barrett P, Morgan PJ, Mercer JG. Photoperiod regulates growth, puberty and hypothalamic neuropeptide and receptor gene expression in female Siberian hamsters. Endocrinology. 2000;141:4349–4356. doi: 10.1210/endo.141.12.7807. [DOI] [PubMed] [Google Scholar]
- 2.Baker JR. The evolution of breeding seasons. In: DeBeer GB, editor. Evolution: essays on aspects of evolutionary biology. Oxford, UK: Clarendon Press; 1938. pp. 161–177. [Google Scholar]
- 3.Ball GF, Ketterson ED. Sex differences in the response to environmental cues regulating seasonal reproduction in birds. Philosophical Transactions of the Royal Society B-Biological Sciences. 2008;363:231–246. doi: 10.1098/rstb.2007.2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bentley GE, Perfito N, Ukena K, Tsutsui K, Wingfield JC. Gonadotropin-inhibitory peptide in song sparrows (Melospiza melodia) in different reproductive conditions, and in house sparrows (Passer domesticus) relative to chicken-gonadotropin-releasing hormone. Journal of Neuroendocrinology. 2003;15:794–802. doi: 10.1046/j.1365-2826.2003.01062.x. [DOI] [PubMed] [Google Scholar]
- 5.Bittman E, Goldman B. Serum levels of gonadotrophins in hamsters exposed to short photoperiods: effects of adrenalectomy and ovariectomy. Journal of Endocrinology. 1979;83:113–118. doi: 10.1677/joe.0.0830113. [DOI] [PubMed] [Google Scholar]
- 6.Bittman EL, Jonassen JA, Hegarty CM. Photoperiodic Regulation of Pulsatile Luteinizing-Hormone Secretion and Adenohypophyseal Gene-Expression in Female Golden-Hamsters. Biology of Reproduction. 1992;47:66–71. doi: 10.1095/biolreprod47.1.66. [DOI] [PubMed] [Google Scholar]
- 7.Bittman EL, Karsch FJ, Hopkins JW. Role of the pineal gland in ovine photoperiodism: regulation of seasonal breeding and negative feedback effects of estradiol upon luteinizing hormone secretion. Endocrinology. 1983;113:329–336. doi: 10.1210/endo-113-1-329. [DOI] [PubMed] [Google Scholar]
- 8.Bronson FH. Mammalian Reproductive Biology. Chicago: University of Chicago Press; 1989. [Google Scholar]
- 9.Caraty A, Smith JT, Lomet D, Ben Said S, Morrissey A, Cognie J, Doughton B, Baril G, Briant C, Clarke IJ. Kisspeptin synchronizes Preovulatory surges in cyclical ewes and causes ovulation in seasonally acyclic ewes. Endocrinology. 2007;148:5258–5267. doi: 10.1210/en.2007-0554. [DOI] [PubMed] [Google Scholar]
- 10.Chappell PE, Lydon JP, Conneely OM, Malley BWO, Levine JE. Endocrine defects in mice carrying a null mutation for the progesterone receptor gene 1. Endocrinology. 1997;138:4147–4152. doi: 10.1210/endo.138.10.5456. [DOI] [PubMed] [Google Scholar]
- 11.Clarke IJ, Pompolo S. Synthesis and secretion of GnRH. Anim Reprod Sci. 2005;88:29–55. doi: 10.1016/j.anireprosci.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 12.Clarke IJ, Smith JT, Caraty A, Goodman RL, Lehman MN. Kisspeptin and seasonality in sheep. Peptides. 2009;30:154–163. doi: 10.1016/j.peptides.2008.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clarkson J, Herbison AE. Postnatal development of kisspeptin neurons in mouse hypothalamus; Sexual dimorphism and projections to gonadotropin-releasing hormone neurons. Endocrinology. 2006;147:5817–5825. doi: 10.1210/en.2006-0787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crown A, Clifton DK, Steiner RA. Neuropeptide signaling in the integration of metabolism and reproduction. Neuroendocrinology. 2007;86:175–182. doi: 10.1159/000109095. [DOI] [PubMed] [Google Scholar]
- 15.d'Anglemont de Tassigny X, Fagg LA, Dixon JPC, Day K, Leitch HG, Hendrick AG, Zahn D, Franceschini I, Caraty A, Carlton MBL, Aparicio SAJR, Colledge WH. Hypogonadotropic hypogonadism in mice lacking a functional Kiss1 gene. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:10714–10719. doi: 10.1073/pnas.0704114104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:10972–10976. doi: 10.1073/pnas.1834399100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Demas GE, Johnson C, Polacek KM. Social interactions differentially affect reproductive and immune responses of Siberian hamsters. Physiology & Behavior. 2004;83:73–79. doi: 10.1016/j.physbeh.2004.06.025. [DOI] [PubMed] [Google Scholar]
- 18.Ebling F, Cronin A. The neurobiology of reproductive development. Neuroreport. 2000;11:R23. doi: 10.1097/00001756-200011090-00002. [DOI] [PubMed] [Google Scholar]
- 19.Ebling FJ. The neuroendocrine timing of puberty. Reproduction. 2005;129:675–683. doi: 10.1530/rep.1.00367. [DOI] [PubMed] [Google Scholar]
- 20.Ebling FJ, Foster DL. Seasonal Breeding - a model for puberty? In: Delamarre-van de Wall HA, Plant TM, van Rees GP, Shoemaker J, editors. Control of the Onset of Puberty III. Amsterdam: Excerpta Medica; 1990. pp. 253–264. [Google Scholar]
- 21.Ebling FJ, Schwartz ML, Foster DL. Endogenous opioid regulation of pulsatile luteinizing hormone secretion during sexual maturation in the female sheep. Endocrinology. 1989;125:369–383. doi: 10.1210/endo-125-1-369. [DOI] [PubMed] [Google Scholar]
- 22.Ellis GB, Turek FW. Time course of the photoperiod-induced change in sensitivity of the hypothalamic-pituitary axis to testosterone feedback in castrated male hamsters. Endocrinology. 1979;104:625–630. doi: 10.1210/endo-104-3-625. [DOI] [PubMed] [Google Scholar]
- 23.Fernandez-Fernandez R, Martini AC, Navarro VM, Castellano JM, Dieguez C, Aguilar E, Pinilla L, Tena-Sempere M. Novel signals for the integration of energy balance and reproduction. Molecular and Cellular Endocrinology. 2006;254:127–132. doi: 10.1016/j.mce.2006.04.026. [DOI] [PubMed] [Google Scholar]
- 24.Foster DL, Ryan KD. Endocrine mechanisms governing transition into adulthood: a marked decrease in inhibitory feedback action of estradiol on tonic secretion of luteinizing hormone in the lamb during puberty. Endocrinology. 1979;105:896–904. doi: 10.1210/endo-105-4-896. [DOI] [PubMed] [Google Scholar]
- 25.Funes S, Hedrick JA, Vassileva G, Markowitz L, Abbondanzo S, Golovko A, Yang SJ, Monsma FJ, Gustafson EL. The KiSS-1 receptor GPR54 is essential for the development of the murine reproductive system. Biochemical and Biophysical Research Communications. 2003;312:1357–1363. doi: 10.1016/j.bbrc.2003.11.066. [DOI] [PubMed] [Google Scholar]
- 26.Glass JD, Dolan PL. Melatonin acts in the brain to mediate seasonal steroid inhibition of luteinizing hormone secretion in the white-footed mouse (Peromyscus leucopus) Proc Soc Exp Biol Med. 1988;188:375–380. doi: 10.3181/00379727-188-42750. [DOI] [PubMed] [Google Scholar]
- 27.Goodman R, Bittman E, Foster D, Karsch F. Alterations in the control of luteinizing hormone pulse frequency underlie the seasonal variation in estradiol negative feedback in the ewe. Biology of Reproduction. 1982;27:580–589. doi: 10.1095/biolreprod27.3.580. [DOI] [PubMed] [Google Scholar]
- 28.Gottsch ML, Clifton DK, Steiner RA. From KISS1 to kisspeptins: An historical perspective and suggested nomenclature. Peptides. 2009;30:4–9. doi: 10.1016/j.peptides.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Greives TJ, Humber SA, Goldstein AN, Scotti MAL, Demas GE, Kriegsfeld LJ. Photoperiod and Testosterone Interact to Drive Seasonal Changes in Kisspeptin Expression in Siberian Hamsters (Phodopus sungorus) Journal of Neuroendocrinology. 2008;20:1339–1347. doi: 10.1111/j.1365-2826.2008.01790.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Greives TJ, Kriegsfeld LJ, Bentley GE, Tsutsui K, Demas GE. Recent advances in reproductive neuroendocrinology: a role for RFamide peptides in seasonal reproduction? Proceedings of the Royal Society B-Biological Sciences. 2008;275:1943–1951. doi: 10.1098/rspb.2008.0433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Greives TJ, Mason AO, Scotti MA, Levine J, Ketterson ED, Kriegsfeld LJ, Demas GE. Environmental control of kisspeptin: implications for seasonal reproduction. Endocrinology. 2007;148:1158–1166. doi: 10.1210/en.2006-1249. [DOI] [PubMed] [Google Scholar]
- 32.Han SK, Gottsch ML, Lee KJ, Popa SM, Smith JT, Jakawich SK, Clifton DK, Steiner RA, Herbison AE. Activation of gonadotropin-releasing hormone neurons by kisspeptin as a neuroendocrine switch for the onset of puberty. Journal of Neuroscience. 2005;25:11349–11356. doi: 10.1523/JNEUROSCI.3328-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Herbison AE. Physiology of the GnRH neuronal networks. In: Knobil E, Neill JD, editors. Physiology of Reproduction. San Diego: Elsevier; 2005. pp. 1415–1482. [Google Scholar]
- 34.Herbison AE. Genetics of puberty. Hormone Research. 2007;68:75–79. doi: 10.1159/000110583. [DOI] [PubMed] [Google Scholar]
- 35.Hoffmann K. Effects of short photoperiods on puberty, growth and moult in the Djungarian hamster (Phodopus sungorus) J Reprod Fertil. 1978;54:29–35. doi: 10.1530/jrf.0.0540029. [DOI] [PubMed] [Google Scholar]
- 36.Karsch FJ, Bittman EL, Foster DL, Goodman RL, Legan SJ, Robinson JE. Neuroendocrine basis of seasonal reproduction. Recent Prog Horm Res. 1984;40:185–232. doi: 10.1016/b978-0-12-571140-1.50010-4. [DOI] [PubMed] [Google Scholar]
- 37.Karsch FJ, Dahl GE, Evans NP, Manning JM, Mayfield KP, Moenter SM, Foster DL. Seasonal changes in gonadotropin-releasing hormone secretion in the ewe: alteration in response to the negative feedback action of estradiol. Biol Reprod. 1993;49:1377–1383. doi: 10.1095/biolreprod49.6.1377. [DOI] [PubMed] [Google Scholar]
- 38.Kauffman AS, Gottsch ML, Roa J, Byquist AC, Crown A, Clifton DK, Hoffman GE, Steiner RA, Tena-Sempere M. Sexual differentiation of Kiss1 gene expression in the brain of the rat. Endocrinology. 2007;148:1774–1783. doi: 10.1210/en.2006-1540. [DOI] [PubMed] [Google Scholar]
- 39.Keen KL, Wegner FH, Bloom SR, Ghatei MA, Terasawa E. An increase in kisspeptin-54 release occurs with the pubertal increase in luteinizing hormone-releasing hormone-1 release in the stalk-median eminence of female rhesus monkeys in vivo. Endocrinology. 2008;149:4151–4157. doi: 10.1210/en.2008-0231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kriegsfeld LJ. Driving reproduction: RFamide peptides behind the wheel. Hormones and Behavior. 2006;50:655–666. doi: 10.1016/j.yhbeh.2006.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kriegsfeld LJ, Mei DF, Bentley GE, Ubuka T, Mason AO, Inoue K, Ukena K, Tsutsui K, Silver R. Identification and characterization of a gonadotropin-inhibitory system in the brains of mammals. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:2410–2415. doi: 10.1073/pnas.0511003103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lehman MN, Goodman RL, Karsch FJ, Jackson GL, Berriman SJ, Jansen HT. The GnRH system of seasonal breeders: anatomy and plasticity. Brain Res Bull. 1997;44:445–457. doi: 10.1016/s0361-9230(97)00225-6. [DOI] [PubMed] [Google Scholar]
- 43.Levine JE. Gonadotropin-releasing hormone (GnRH) In: Henry H, Normona A, editors. Encycopedia of Hormones. San Diego: Academic Press; 2003. pp. 157–165. [Google Scholar]
- 44.Lynch GR, Lynch CB. Seasonal photoperiodism in the Djungarian hamster - a genetic component influences photoresponsiveness. Behavior Genetics. 1986;16:625–626. [Google Scholar]
- 45.Mason AO, Greives TJ, Scotti MAL, Levine J, Frommeyer S, Ketterson ED, Demas GE, Kriegsfeld LJ. Suppression of kisspeptin expression and gonadotropic axis sensitivity following exposure to inhibitory day lengths in female Siberian hamsters. Hormones and Behavior. 2007;52:492–498. doi: 10.1016/j.yhbeh.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Meikle LM, Fisher MW. Regulation of reproductive seasonality in the red deer hind: oestradiol-dependent and -independent influences on the patterns of LH concentrations. J Reprod Fertil. 1996;106:213–220. doi: 10.1530/jrf.0.1060213. [DOI] [PubMed] [Google Scholar]
- 47.Meredith J, Turek F, Levine J. Effects of Gonadotropin-Releasing Hormone Pulse Frequency Modulation on the Reproductive Axis of Photoinhibited Male Siberian Hamsters 1. Biology of Reproduction. 1998;59:813–819. doi: 10.1095/biolreprod59.4.813. [DOI] [PubMed] [Google Scholar]
- 48.Messager S, Chatzidaki EE, Ma D, Hendrick AG, Zahn D, Dixon J, Thresher RR, Malinge I, Lomet D, Carlton MBL, Colledge WH, Caraty A, Aparicio S. Kisspeptin directly stimulates gonadotropin-releasing hormone release via G protein-coupled receptor 54. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:1761–1766. doi: 10.1073/pnas.0409330102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moffatt CA, Gerber JM, Blom JMC, Kriegsfeld LJ, Nelson RJ. Photoperiodic Effects on Steroid Negative Feedback in Female Prairie Voles (Microtus-Ochrogaster) General and Comparative Endocrinology. 1995;100:92–95. doi: 10.1006/gcen.1995.1137. [DOI] [PubMed] [Google Scholar]
- 50.Navarro VM, Castellano JM, Fernandez-Fernandez R, Barreiro ML, Roa J, Sanchez-Criado JE, Aguilar E, Dieguez C, Pinilla L, Tena-Sempere M. Developmental and hormonally regulated messenger ribonucleic acid expression of KiSS-1 and its putative receptor, GPR54, in rat hypothalamus and potent luteinizing hormone-releasing activity of KiSS-1 peptide. Endocrinology. 2004;145:4565–4574. doi: 10.1210/en.2004-0413. [DOI] [PubMed] [Google Scholar]
- 51.Olster D, Foster D. Control of gonadotrophin secretion during the pubertal and seasonal transitions in the male sheep. Reproduction. 1988;82:179–191. doi: 10.1530/jrf.0.0820179. [DOI] [PubMed] [Google Scholar]
- 52.Pickard GE, Silverman AJ. Effects of photoperiod on hypothalamic luteinizing hormone releasing hormone in the male hamster. J Endocrinol. 1979;83:421–428. doi: 10.1677/joe.0.0830421. [DOI] [PubMed] [Google Scholar]
- 53.Revel FG, Saboureau M, Masson-Pevet M, Pevet P, Mikkelsen JD, Simonneaux V. Kisspeptin mediates the photoperiodic control of reproduction in hamsters. Current Biology. 2006;16:1730–1735. doi: 10.1016/j.cub.2006.07.025. [DOI] [PubMed] [Google Scholar]
- 54.Richardson HN, Gore AC, Venier J, Romeo RD, Sisk CL. Increased expression of forebrain GnRH mRNA and changes in testosterone negative feedback following pubertal maturation. Mol Cell Endocrinol. 2004;214:63–70. doi: 10.1016/j.mce.2003.11.013. [DOI] [PubMed] [Google Scholar]
- 55.Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS, Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG, Zahn D, Dixon J, Kaiser UB, Slaugenhaupt SA, Gusella JF, O'Rahilly S, Carlton MBL, Crowley WF, Aparicio S, Colledge WH. The GPR54 gene as a regulator of puberty. New England Journal of Medicine. 2003;349:1614–U8. doi: 10.1056/NEJMoa035322. [DOI] [PubMed] [Google Scholar]
- 56.Shahab M, Mastronardi C, Seminara SB, Crowley WF, Ojeda SR, Plant TM. Increased hypothalamic GPR54 signaling: A potential mechanism for initiation of puberty in primates. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:2129–2134. doi: 10.1073/pnas.0409822102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sisk C. Evidence that a decrease in testosterone negative feedback mediates the pubertal increase in luteinizing hormone pulse frequency in male ferrets. Biology of Reproduction. 1987;37:73. doi: 10.1095/biolreprod37.1.73. [DOI] [PubMed] [Google Scholar]
- 58.Sisk C, Foster D. The neural basis of puberty and adolescence. Nature neuroscience. 2004;7:1040–1047. doi: 10.1038/nn1326. [DOI] [PubMed] [Google Scholar]
- 59.Smith JT, Clay CM, Caraty A, Clarke IJ. KiSS-1 messenger ribonucleic acid expression in the hypothalamus of the ewe is regulated by sex steroids and season. Endocrinology. 2007;148:1150–1157. doi: 10.1210/en.2006-1435. [DOI] [PubMed] [Google Scholar]
- 60.Smith JT, Coolen LM, Kriegsfeld LJ, Sari IP, Jaafarzadehshirazi MR, Maltby M, Bateman K, Goodman RL, Tilbrook AJ, Ubuka T, Bentley GE, Clarke IJ, Lehman MN. Variation in kisspeptin and RFamide-related peptide (RFRP) expression and terminal connections to gonadotropin-releasing hormone neurons in the brain: a novel medium for seasonal breeding in the sheep. Endocrinology. 2008;149:5770–5782. doi: 10.1210/en.2008-0581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Smith JT, Cunningham MJ, Rissman EF, Clifton DK, Steiner RA. Regulation of Kiss1 gene expression in the brain of the female mouse. Endocrinology. 2005;146:3686–3692. doi: 10.1210/en.2005-0488. [DOI] [PubMed] [Google Scholar]
- 62.Smith JT, Dungan HM, Stoll EA, Gottsch ML, Braun RE, Eacker SM, Clifton DK, Steiner RA. Differential regulation of KiSS-1 mRNA expression by sex steroids in the brain of the male mouse. Endocrinology. 2005;146:2976–2984. doi: 10.1210/en.2005-0323. [DOI] [PubMed] [Google Scholar]
- 63.Stetson M, Elliott J, Goldman B. Maternal transfer of photoperiodic information influences the photoperiodic response of prepubertal Djungarian hamsters (Phodopus sungorus sungorus) Biology of Reproduction. 1986;34:664–669. doi: 10.1095/biolreprod34.4.664. [DOI] [PubMed] [Google Scholar]
- 64.Tilbrook AJ, de Kretser DM, Clarke IJ. Seasonal changes in the negative feedback regulation of the secretion of the gonadotrophins by testosterone and inhibin in rams. Journal of Endocrinology. 1999;160:155–167. doi: 10.1677/joe.0.1600155. [DOI] [PubMed] [Google Scholar]
- 65.Tsutsui K, Saigoh E, Ukena K, Teranishi H, Fujisawa Y, Kikuchi M, Ishii S, Sharp JP. A novel avian hypothalamic peptide inhibiting gonadotropin release. Biochemical and Biophysical Research Communications. 2000;275:661–667. doi: 10.1006/bbrc.2000.3350. [DOI] [PubMed] [Google Scholar]
- 66.Turek FW, Ellis GB. Steroid-dependent and steroid-independent aspects of the photoperiodic control of seasonal reproductive cycles in male hamsters. In: Follett BK, Follett DE, editors. Biological Clocks in Seasonal Reproductive Cycles. Bristol, UK: Wright, Bristol; 1981. pp. 251–260. [Google Scholar]
- 67.Wingfield JC. Organization of vertebrate annual cycles: implications for control mechanisms. Philosophical Transactions of the Royal Society B-Biological Sciences. 2008;363:425–441. doi: 10.1098/rstb.2007.2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wolfe AM, Turek FW, Levine JE. Blockade of Singular Follicle-Stimulating-Hormone Secretion and Testicular Development in Photostimulated Djungarian Hamsters (Phodopus-Sungorus) by a Gonadotropin-Releasing-Hormone Antagonist. Biology of Reproduction. 1995;53:724–731. doi: 10.1095/biolreprod53.3.724. [DOI] [PubMed] [Google Scholar]
- 69.Zucker I, Licht P. Seasonal variations in plasma luteinizing hormone levels of gonadectomized male ground squirrels (Spermophilus lateralis) Biol Reprod. 1983;29:278–285. doi: 10.1095/biolreprod29.2.278. [DOI] [PubMed] [Google Scholar]