Abstract
Despite all the therapeutic advances in the field of cardiology, cardiovascular diseases, and in particular coronary artery disease, remain the leading cause of death and disability worldwide, thereby underlining the importance of acquiring new therapeutic options in this field. A reduction in elevated resting heart rate (HR) has long been postulated as a therapeutic approach in the management of cardiovascular disease. An increased HR has been shown to be associated with increased progression of coronary atherosclerosis in animal models and patients. A high HR has also been associated with a greatly increased risk of plaque rupture in patients with coronary atherosclerosis. Endothelial function may be an important link between HR and atherosclerosis. An increased HR has been shown experimentally to cause endothelial dysfunction. Inflammation plays a significant role in the pathogenesis and progression of atherosclerosis. In the literature, there is data that shows an association between HR and circulating markers of vascular inflammation. In addition, HR reduction by pharmacological intervention with ivabradine (a selective HR-lowering agent that acts by inhibiting the pacemaker ionic current If in sinoatrial node cells) reduces the formation of atherosclerotic plaques in animal models of lipid-induced atherosclerosis. The aim of this editorial is to review the possible role of ivabradine on atherosclerosis.
Keywords: Ivabradine, Heart rate, Atherosclerosis, Inflammation
INTRODUCTION
Resting heart rate (HR) is an easily accessible clinical parameter. The initiation of the HR by spontaneous sinoatrial node depolarization is determined by voltage-sensitive membrane currents, particularly the hyperpolarization activated pacemaker current If, and by calcium release from the sarcoplasmic reticulum, leading to diastolic depolarization through activation of the sodium-calcium exchanger current[1].
Experimental data and clinical observations support the notion of the importance of HR in the pathophysiology of atherosclerosis and plaque rupture[2]. An elevated HR enhances mechanical arterial wall stress and it prolongs the exposure of the coronary endothelium to systolic low and oscillatory shear stress. All these processes induce structural and functional changes in endothelial cells, which accumulate over time in atherosclerosis-prone regions, promoting atherosclerosis[2]. Furthermore, elevated HR caused by mechanical stress may promote weakening of the fibrous cap, ultimately increasing the risk of plaque disruption and the onset of an acute coronary syndrome[3].
Ivabradine, a selective inhibitor of the If channel, reduces resting and exercise HR without affecting cardiac contractility or blood pressure[4-6]. Clinical trials have revealed an improved exercise tolerance, an increased time to exercise-induced ischemia, and a reduced frequency of ambient angina attacks after If channel inhibition[7,8]. This editorial summarizes the possible role of ivabradine on atherosclerosis.
THE ROLE OF HR IN CARDIOVASCULAR DISEASE
A large number of studies in healthy and asymptomatic subjects as well as in patients with already established coronary artery disease (CAD) have demonstrated that HR is a very important and major independent cardiovascular risk factor for prognosis[9]. In the general population, life expectancy is associated inversely with elevated HR[10-12]. This association is independent of gender and genetic background. An increase in risk is derived from data comparing individuals with HR < 60 beats per minute with those with HR of 90-99 beats per minute[13]. In particular, there is an increase in CAD mortality and there is also an increase in sudden cardiac death[11]. The contribution of HR reduction to the clinical effects of β-blockers and calcium-channel blockers has been analyzed in several studies[14,15].
In the Framingham study, cardiovascular and coronary mortality increased progressively with resting HR in a cohort of 5070 subjects free from cardiovascular disease at the time of entry into the study. The effect of HR on mortality was independent of traditional cardiovascular risk factors[2,16-18].
The analysis of a pre-specified subgroup of the BEAUTIFUL (morbidity-mortality evaluation of the If inhibitor ivabradine in patients with coronary disease and left-ventricular dysfunction) trial demonstrated that, in patients with CAD and left ventricular systolic dysfunction, a resting HR > 70 beats per minute is associated with an increased cardiovascular mortality as well as increased risk for hospitalization due to heart failure, myocardial infarction, or the need for coronary revascularization[19]. Recently, the systolic heart failure treatment with If inhibitor ivabradine trial, demonstrated that patients with HR ≥ 87 beats per minute, had a two-fold higher risk of the primary composite endpoint (cardiovascular death or hospital admission for worsening heart failure) than patients with the lowest HR (70 to < 72 beats per minute). The risk of primary composite endpoint events increased by 3% with every beat increase from baseline HR, and 16% for every 5 beats per minute increase. Thus, the authors conclude that high HR is a risk factor in heart failure and therefore, it should be an important target for treatment of heart failure[20]. Taken together, there is compelling epidemiologic evidence that elevated resting HR is predictive of cardiovascular risk, independently of the other currently accepted risk factors.
ATHEROSCLEROSIS, INCREASED HR AND IVABRADINE
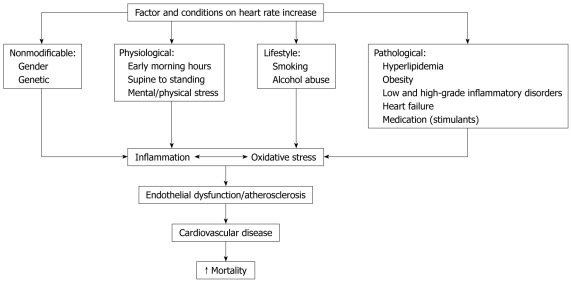
HR is influenced by a variety of physiological processes mainly via effects on the balance of sympathetic and vagal tone. The factors and conditions which influence HR are summarized in Figure 1. The importance of an increased HR in cardiovascular prognosis can be explained by its relationship with major pathophysiological determinants: (1) greater myocardial oxygen consumption; (2) decreased myocardial perfusion; (3) increased severity and progression of coronary atherosclerosis; (4) less development of collaterals; (5) increased risk of coronary plaque disruption; (6) increased arterial rigidity; and (7) a marker and possible mediator of sympathetic overactivity[21].
Figure 1.
The role of heart rate in the pathophysiology of cardiovascular disease.
Experimental and clinical evidence also suggests that sustained elevations in HR may also play a direct role in the pathogenesis of coronary atherosclerosis and its complications[2]. Accelerated atherogenesis resulting from increased HR may be due to both mechanical and metabolic factors. Increased vascular wall stress may contribute to endothelial injury, potentially promoting the complex cascade of events leading to increased atherosclerosis[21]. Experimental data also show that a reduction in HR can delay the progression of coronary atherosclerosis in monkeys[22]. Additionally, in young patients with myocardial infarction, there is a strong positive relationship between higher HR and the extent of atherosclerotic coronary lesions[23].
In apolipoprotein E knockout mice, cholesterol-induced atherosclerosis was inhibited by HR reduction with ivabradine[24]. In this study, ivabradine also markedly reduced vascular oxidative stress, nicotinamide adenine dinucleotide phosphate oxidase activity, superoxide production, and lipid peroxidation[24]. Ivabradine also prevented atherogenesis when given simultaneously with a high cholesterol diet, but it was also effective in reducing plaques size when given to animals 4 wk after initiation of a high-cholesterol diet[25]. Presumably, therefore, mechanical load on the vessel wall caused by higher HR might lead to endothelial dysfunction, increased oxidative stress, and enhanced plaque formation, which can be reversed or prevented by the inhibition of If channels and consequent HR reduction with ivabradine[26].
Subclinical inflammation and the concentration of inflammatory markers have shown in many studies to correlate strongly with cardiovascular mortality and morbidity in both healthy subjects and in subjects with known CAD[27,28]. Two population-based studies reported a positive correlation between increased resting HR and markers of inflammation in apparently healthy subjects[29,30]. Thus, increased HR may contribute to endothelial dysfunction by upregulation of inflammatory cytokines[2]. In summary, the data show an association of HR with circulating markers of vascular inflammation.
These observations support the rationale for HR reduction with ivabradine as an intervention to improve endothelial function and to attenuate the progression of atherosclerosis and cardiovascular event prevention[2]. In this respect, the RIVIERA study (randomized, double-blind, placebo-controlled trial of ivabradine in patients with acute coronary syndrome: effects of the If current inhibitor ivabradine on reduction of inflammation markers in patients with acute coronary syndrome), is the first opportunity to investigate whether a pure HR-lowering agent reduces vascular inflammatory stress in patients with acute coronary syndrome[31]. The importance of this study will explore potentially new cardiovascular effects of ivabradine that may be useful for management of these patients. In addition, the use of ivabradine can be further expanded by investigating its mechanism of action in high grade inflammatory disorders in models of inflammation-induced accelerated atherosclerosis leading to a substantial cardiovascular burden[32].
Likewise, the effect of ivabradine had been demonstrated in various clinical trials. In the international trial on the treatment of angina with ivabradine vs atenolol (INITIATIVE), ivabradine was compared with atenolol in a double-blind trial in 939 patients with stable angina randomized to receive ivabradine 5 mg bid for 4 wk and then either 7.5 or 10 mg bid for 12 wk or atenolol 50 mg od for 4 wk and then 100 od for 12 wk. Patients underwent treadmill exercise tests at randomization and after 4 and 16 wk of treatment. Increases in total exercise duration and other exercise test parameters at trough of drug activity were not inferior with ivabradine, suggesting that ivabradine is as effective as atenolol in patients with stable angina[7].
The ASSOCIATE (evaluation of the antianginal efficacy and safety of the association of the If current inhibitor ivabradine with a β-blocker) study was an international, double blind, placebo-controlled trial which investigated the effects of ivabradine in patients with stable angina receiving atenolol[33]. This study clearly demonstrates that ivabradine in patients with stable angina receiving the β-blocker atenolol had a significant long-term improvement in total exercise duration in standardized Bruce protocol exercise testing.
Regarding ivabradine-associated adverse effects, the most frequently encountered (sinus bradycardia and visual disturbances) are related to the drug’s mechanism of action; e.g. inhibition of sinus node If channels and inhibition of h channels in retinal rods and cones, though their density is low[19]. In BEAUTIFUL, the incidence of symptomatic sinus bradycardia was 3%. The rate of visual symptoms (phosphenes, blurred vision, and visual disturbances) was also very low and led to discontinuation in only 0.5% of patients receiving ivabradine vs 0.2% of patients receiving placebo[19].
CONCLUSION
HR should become a significant cardiovascular parameter for predicting complications because it is an integral sign of cardiovascular function and is related to several complications at different stages of the cardiovascular continuum. Therefore, in all future cardiovascular studies, HR should be carefully monitored in order to improve our knowledge of this important physiological risk marker, or even risk factor. HR reduction by the HR-lowering agent, ivabradine, should prevent atherosclerosis and hence cardiovascular events. All observations commented here constitute a strong rationale for further clinical investigation of the cardioprotective effects of pure HR reduction.
Footnotes
Peer reviewers: Moses S Elisaf, Professor of Medicine, Department of Internal Medicine, Medical School, University of Ioannina, Ioannina 45110, Greece; Armen Yuri Gasparyan, MD, PhD, FESC, Associate Professor of Medicine, Postdoctoral Research Fellow, Clinical Research Unit, Dudley Group of Hospitals NHS Foundation Trust, Russell’s Hall Hospital, Pensnett Road, Dudley, West Midlands, DY1 2HQ, United Kingdom; Mustafa Yildiz, MD, PhD, Associate Professor, EC, Cardiologist, Internal Medicine Specialist and Physiologist, Department of Cardiology, Kartal Kosuyolu Yuksek Ihtisas Educational and Research Hospital, Istanbul 81410, Turkey
S- Editor Cheng JX L- Editor Cant MR E- Editor Zheng XM
References
- 1.Brown HF, DiFrancesco D, Noble SJ. How does adrenaline accelerate the heart? Nature. 1979;280:235–236. doi: 10.1038/280235a0. [DOI] [PubMed] [Google Scholar]
- 2.Custodis F, Schirmer SH, Baumhäkel M, Heusch G, Böhm M, Laufs U. Vascular pathophysiology in response to increased heart rate. J Am Coll Cardiol. 2010;56:1973–1983. doi: 10.1016/j.jacc.2010.09.014. [DOI] [PubMed] [Google Scholar]
- 3.Giannoglou GD, Chatzizisis YS, Zamboulis C, Parcharidis GE, Mikhailidis DP, Louridas GE. Elevated heart rate and atherosclerosis: an overview of the pathogenetic mechanisms. Int J Cardiol. 2008;126:302–312. doi: 10.1016/j.ijcard.2007.08.077. [DOI] [PubMed] [Google Scholar]
- 4.Bois P, Bescond J, Renaudon B, Lenfant J. Mode of action of bradycardic agent, S 16257, on ionic currents of rabbit sinoatrial node cells. Br J Pharmacol. 1996;118:1051–1057. doi: 10.1111/j.1476-5381.1996.tb15505.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gardiner SM, Kemp PA, March JE, Bennett T. Acute and chronic cardiac and regional haemodynamic effects of the novel bradycardic agent, S16257, in conscious rats. Br J Pharmacol. 1995;115:579–586. doi: 10.1111/j.1476-5381.1995.tb14971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Riccioni G. Ivabradine: recent and potential applications in clinical practice. Expert Opin Pharmacother. 2011;12:443–450. doi: 10.1517/14656566.2011.548321. [DOI] [PubMed] [Google Scholar]
- 7.Tardif JC, Ford I, Tendera M, Bourassa MG, Fox K. Efficacy of ivabradine, a new selective I(f) inhibitor, compared with atenolol in patients with chronic stable angina. Eur Heart J. 2005;26:2529–2536. doi: 10.1093/eurheartj/ehi586. [DOI] [PubMed] [Google Scholar]
- 8.Borer JS, Fox K, Jaillon P, Lerebours G. Antianginal and antiischemic effects of ivabradine, an I(f) inhibitor, in stable angina: a randomized, double-blind, multicentered, placebo-controlled trial. Circulation. 2003;107:817–823. doi: 10.1161/01.cir.0000048143.25023.87. [DOI] [PubMed] [Google Scholar]
- 9.Aboyans V, Criqui MH. Can we improve cardiovascular risk prediction beyond risk equations in the physician's office? J Clin Epidemiol. 2006;59:547–558. doi: 10.1016/j.jclinepi.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 10.Singh BN. Morbidity and mortality in cardiovascular disorders: impact of reduced heart rate. J Cardiovasc Pharmacol Ther. 2001;6:313–331. doi: 10.1177/107424840100600401. [DOI] [PubMed] [Google Scholar]
- 11.Kannel WB, Wilson P, Blair SN. Epidemiological assessment of the role of physical activity and fitness in development of cardiovascular disease. Am Heart J. 1985;109:876–885. doi: 10.1016/0002-8703(85)90653-2. [DOI] [PubMed] [Google Scholar]
- 12.Gillum RF, Makuc DM, Feldman JJ. Pulse rate, coronary heart disease, and death: the NHANES I Epidemiologic Follow-up Study. Am Heart J. 1991;121:172–177. doi: 10.1016/0002-8703(91)90970-s. [DOI] [PubMed] [Google Scholar]
- 13.Wilhelmsen L, Berglund G, Elmfeldt D, Tibblin G, Wedel H, Pennert K, Vedin A, Wilhelmsson C, Werkö L. The multifactor primary prevention trial in Göteborg, Sweden. Eur Heart J. 1986;7:279–288. doi: 10.1093/oxfordjournals.eurheartj.a062065. [DOI] [PubMed] [Google Scholar]
- 14.Cucherat M. Quantitative relationship between resting heart rate reduction and magnitude of clinical benefits in post-myocardial infarction: a meta-regression of randomized clinical trials. Eur Heart J. 2007;28:3012–3019. doi: 10.1093/eurheartj/ehm489. [DOI] [PubMed] [Google Scholar]
- 15.Bangalore S, Sawhney S, Messerli FH. Relation of beta-blocker-induced heart rate lowering and cardioprotection in hypertension. J Am Coll Cardiol. 2008;52:1482–1489. doi: 10.1016/j.jacc.2008.06.048. [DOI] [PubMed] [Google Scholar]
- 16.Kannel WB, Kannel C, Paffenbarger RS Jr, Cupples LA. Heart rate and cardiovascular mortality: the Framingham Study. Am Heart J. 1987;113:1489–1494. doi: 10.1016/0002-8703(87)90666-1. [DOI] [PubMed] [Google Scholar]
- 17.Dyer AR, Persky V, Stamler J, Paul O, Shekelle RB, Berkson DM, Lepper M, Schoenberger JA, Lindberg HA. Heart rate as a prognostic factor for coronary heart disease and mortality: findings in three Chicago epidemiologic studies. Am J Epidemiol. 1980;112:736–749. doi: 10.1093/oxfordjournals.aje.a113046. [DOI] [PubMed] [Google Scholar]
- 18.Jouven X, Empana JP, Schwartz PJ, Desnos M, Courbon D, Ducimetière P. Heart-rate profile during exercise as a predictor of sudden death. N Engl J Med. 2005;352:1951–1958. doi: 10.1056/NEJMoa043012. [DOI] [PubMed] [Google Scholar]
- 19.Fox K, Ford I, Steg PG, Tendera M, Robertson M, Ferrari R. Heart rate as a prognostic risk factor in patients with coronary artery disease and left-ventricular systolic dysfunction (BEAUTIFUL): a subgroup analysis of a randomised controlled trial. Lancet. 2008;372:817–821. doi: 10.1016/S0140-6736(08)61171-X. [DOI] [PubMed] [Google Scholar]
- 20.Böhm M, Swedberg K, Komajda M, Borer JS, Ford I, Dubost-Brama A, Lerebours G, Tavazzi L. Heart rate as a risk factor in chronic heart failure (SHIFT): the association between heart rate and outcomes in a randomised placebo-controlled trial. Lancet. 2010;376:886–894. doi: 10.1016/S0140-6736(10)61259-7. [DOI] [PubMed] [Google Scholar]
- 21.Tardif JC. Heart rate as a treatable cardiovascular risk factor. Br Med Bull. 2009;90:71–84. doi: 10.1093/bmb/ldp016. [DOI] [PubMed] [Google Scholar]
- 22.Beere PA, Glagov S, Zarins CK. Retarding effect of lowered heart rate on coronary atherosclerosis. Science. 1984;226:180–182. doi: 10.1126/science.6484569. [DOI] [PubMed] [Google Scholar]
- 23.Perski A, Hamsten A, Lindvall K, Theorell T. Heart rate correlates with severity of coronary atherosclerosis in young postinfarction patients. Am Heart J. 1988;116:1369–1373. doi: 10.1016/0002-8703(88)90469-3. [DOI] [PubMed] [Google Scholar]
- 24.Custodis F, Baumhäkel M, Schlimmer N, List F, Gensch C, Böhm M, Laufs U. Heart rate reduction by ivabradine reduces oxidative stress, improves endothelial function, and prevents atherosclerosis in apolipoprotein E-deficient mice. Circulation. 2008;117:2377–2387. doi: 10.1161/CIRCULATIONAHA.107.746537. [DOI] [PubMed] [Google Scholar]
- 25.Baumhäkel M, Custodis F, Schlimmer N, Laufs U, Böhm M. Heart rate reduction with ivabradine improves erectile dysfunction in parallel to decrease in atherosclerotic plaque load in ApoE-knockout mice. Atherosclerosis. 2010;212:55–62. doi: 10.1016/j.atherosclerosis.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 26.Drouin A, Gendron ME, Thorin E, Gillis MA, Mahlberg-Gaudin F, Tardif JC. Chronic heart rate reduction by ivabradine prevents endothelial dysfunction in dyslipidaemic mice. Br J Pharmacol. 2008;154:749–757. doi: 10.1038/bjp.2008.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morrow DA, Rifai N, Antman EM, Weiner DL, McCabe CH, Cannon CP, Braunwald E. C-reactive protein is a potent predictor of mortality independently of and in combination with troponin T in acute coronary syndromes: a TIMI 11A substudy. Thrombolysis in Myocardial Infarction. J Am Coll Cardiol. 1998;31:1460–1465. doi: 10.1016/s0735-1097(98)00136-3. [DOI] [PubMed] [Google Scholar]
- 28.Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med. 1997;336:973–979. doi: 10.1056/NEJM199704033361401. [DOI] [PubMed] [Google Scholar]
- 29.Rogowski O, Shapira I, Shirom A, Melamed S, Toker S, Berliner S. Heart rate and microinflammation in men: a relevant atherothrombotic link. Heart. 2007;93:940–944. doi: 10.1136/hrt.2006.101949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sajadieh A, Nielsen OW, Rasmussen V, Hein HO, Abedini S, Hansen JF. Increased heart rate and reduced heart-rate variability are associated with subclinical inflammation in middle-aged and elderly subjects with no apparent heart disease. Eur Heart J. 2004;25:363–370. doi: 10.1016/j.ehj.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 31.Dominguez-Rodriguez A, Fard SS, Abreu-Gonzalez P, Bosa-Ojeda F, Consuegra-Sanchez L, Jiménez-Sosa A, Grande AS, Kaski JC. Randomised, double-blind, placebo-controlled trial of ivabradine in patients with acute coronary syndrome: effects of the If current inhibitor ivabradine on reduction of inflammation markers in patients with acute coronary syndrome--RIVIERA trial study design and rationale. Cardiovasc Drugs Ther. 2009;23:243–247. doi: 10.1007/s10557-009-6164-9. [DOI] [PubMed] [Google Scholar]
- 32.Gasparyan AY, Stavropoulos-Kalinoglou A, Mikhailidis DP, Toms TE, Douglas KM, Kitas GD. The rationale for comparative studies of accelerated atherosclerosis in rheumatic diseases. Curr Vasc Pharmacol. 2010;8:437–449. doi: 10.2174/157016110791330852. [DOI] [PubMed] [Google Scholar]
- 33.Tardif JC, Ponikowski P, Kahan T. Efficacy of the I(f) current inhibitor ivabradine in patients with chronic stable angina receiving beta-blocker therapy: a 4-month, randomized, placebo-controlled trial. Eur Heart J. 2009;30:540–548. doi: 10.1093/eurheartj/ehn571. [DOI] [PMC free article] [PubMed] [Google Scholar]