Abstract
Coronary heart disease (CHD) that is due to atherosclerosis is associated with low-grade systemic inflammation. Congestive cardiac failure and arrhythmias that are responsible for mortality in CHD can be suppressed by appropriate vagal stimulation that is anti-inflammatory in nature. Acetylcholine, the principal vagal neurotransmitter, is a potent anti-inflammatory molecule. Polyunsaturated fatty acids (PUFAs) augment acetylcholine release, while acetylcholine can enhance the formation of prostacyclin, lipoxins, resolvins, protectins and maresins from PUFAs, which are anti-inflammatory and anti-arrhythmic molecules. Furthermore, plasma and tissue levels of PUFAs are low in those with CHD and atherosclerosis. Hence, vagal nerve stimulation is beneficial in the prevention of CHD and cardiac arrhythmias. Thus, measurement of catecholamines, acetylcholine, various PUFAs, and their products lipoxins, resolvins, protectins and maresins in the plasma and peripheral leukocytes, and vagal tone by heart rate variation could be useful in the prediction, prevention and management of CHD and cardiac arrhythmias.
Keywords: Vagal nerve stimulation, Acetylcholine, Coronary heart disease, Cardiac arrhythmias, Heart failure
INTRODUCTION
Coronary heart disease (CHD) is usually due to underlying atherosclerosis and is the leading cause of death in the United States and elsewhere. Both heart failure and cardiac arrhythmias are the common causes of death due to CHD. It is interesting to note that both CHD and atherosclerosis are associated with low-grade systemic inflammation[1,2]. Diseases that predispose to the development of atherosclerosis and CHD, such as obesity, hypertension, type 2 diabetes mellitus and the metabolic syndrome, are also considered as low-grade systemic inflammatory conditions[3-8]. Thus, inflammation plays a significant role in CHD and cardiac arrhythmias and conditions that predispose to its development. Vagal stimulation has an antifibrillatory effect and is beneficial in animal models of heart failure[9]. I propose that inflammation plays a role in cardiac arrhythmias, and that vagal stimulation is beneficial because of its anti-inflammatory actions.
Previously, it has been reported that increasing heart rate within a physiological range by diminishing vagal tone during myocardial ischemia decreases ventricular electrical stability by increasing ischemia consequent to the rate-induced increase in myocardial oxygen requirements, and a direct electrophysiological action of the vagus on the ventricular myocardium[10]. The incidence of ventricular arrhythmias is significantly higher and ventricular fibrillation tends to occur more frequently in the atropine-treated group, while vagally mediated bradycardia exerts a protective effect, which indicates that vagal stimulation per se (independent of heart rate) increases ventricular electrical stability in non-ischemic and ischemic hearts[11]. In addition, acetylcholine, the principle vagal neurotransmitter, depresses the slope of diastolic depolarization, and increases the rise time, amplitude, and conduction velocity of action potentials recorded in the proximal portion of the His-Purkinje system of the canine ventricle[12], and thus, in addition to its heart-rate-mediated effects, atropine increases the incidence of arrhythmia by attenuating a stabilizing vagal influence. These and other studies have suggested that vagal nerve stimulation (VNS) could prevent or even abrogate cardiac arrhythmias[13-15].
VNS FAVORABLY INFLUENCES ENERGY PROVISION TO THE ISCHEMIC MYOCARDIUM
Vagal stimulation increases coronary resistance and decreases regional myocardial blood flow (RMBF) in non-ischemic myocardium, while increasing endocardial RMBF, endo/epicardial ratio and ischemic/non-ischemic areas flow ratio, thus inducing a “reverse coronary steal phenomenon” in the ischemic myocardium. These effects are independent of the induced bradycardia because they persist during atrial pacing, but result from muscarinic receptor activation because they are abolished by atropine[16]. Vagal stimulation results in decreased collateral resistance in the ischemic area and a marked reduction of myocardial oxygen requirement in non-ischemic and border zone myocardium, when myocardial ischemia produced by acute coronary occlusion during β-receptor blockade is examined[17]. This suggests that the provision of energy to the ischemic myocardium is favorably balanced with its actual demand during vagal stimulation.
Low-frequency electroneurostimulation (ENS) of the efferent vagus endings and brainstem structures via transauricular electroacupuncture increase the parasympathetic tone of the autonomic nervous system. ENS has a central vagotonic/sympatholytic influence on the heart, which leads to relief of anginal symptoms, diminution of biochemical myocardial signs of disease, in the form of a decrease in heat shock protein 70 and myocardial ATP content, and an increase in cardiac tolerance of operative reperfusion damage in patients with coronary artery disease (CAD) who underwent coronary artery bypass grafting[18]. These results are supported by the observation that CAD is characterized by overactivity of sympathetic cardiac tone, whereas vagal stimulation reduces sympathetic inflow to the heart via inhibition of norepinephrine release from sympathetic nerves. It has been noted that VNS induces sympatholytic/vagotonic dilation of cardiac microcirculatory vessels and improves left ventricular (LV) contractility in patients with severe CAD[19].
VNS PREVENTS CARDIAC ARRHYTHMIAS
VNS exerts anti-arrhythmogenic effects by preventing the loss of phosphorylated connexin (CX)43 during acute myocardial infarction[20], and ameliorates LV remodeling in heart failure by inducing tissue inhibitor of matrix metalloproteinase (TIMP) expression and reducing matrix metalloproteinase (MMP)-9 in cardiomyocytes[21]. Cardiac microdialysis has demonstrated that topical perfusion of acetylcholine has similar actions on CX43, TIMP expression and MMP-9 protein level, which is suppressed by co-perfusion of atropine. The protective action of VNS in CAD appears to be mediated by a vagus-nerve-mediated, brain cholinergic protective mechanism that is activated by melanocortin peptides[22], which suggests that melanocortins and pertinent compounds able to activate such a pathway may form a novel approach to management of ischemic heart disease.
CARDIAC ARRHYTHMIAS ARE DUE TO LOCAL INFLAMMATION
Recent studies have suggested that cardiac arrhythmias are due to local inflammation, oxidative injury, altered myocyte metabolism, extracellular matrix remodeling, and fibrosis. This is because myeloperoxidase (MPO)-deficient mice pretreated with angiotensin-II have lower atrial tissue MPO, reduced activity of MMPs, blunted myocardial fibrosis, and markedly reduced incidence of arrhythmias. Patients with cardiac arrhythmias had higher plasma concentrations of MPO and larger MPO content in the myocardial tissue compared to the controls. These data support the mechanistic involvement of MPO in the pathogenesis of cardiac arrhythmias and suggest a strong association between cardiac arrhythmias and inflammation[22-24], and have led to the suggestion that the activation state of leukocytes[25] (activation of leukocytes leads to excess production of MPO) could be secondary to a deficiency of lipoxin A4 (LXA4), a potent anti-inflammatory, organ-protective and antifibrotic molecule[24,25] and prostacyclin (PGI2), another anti-arrhythmic and anti-inflammatory molecule[24,26].
POLYUNSATURATED FATTY ACIDS, PGI2 AND LIPOXINS HAVE ANTIARRHYTHMIC ACTIVITY
Free polyunsaturated fatty acids (PUFAs) (10-15 μmol/L) eicosapentaenoic acid (EPA, 20:5 n-3), docosahexaenoic acid (DHA, 22:6 n-3), α-linolenic acid (18:3 n-3), arachidonic acid (AA, 20:4 n-6) and linoleic acid (18:2 n-6) effectively prevent and terminate lysophosphatidylcholine- or acylcarnitine-induced arrhythmias of cultured, spontaneously beating, neonatal rat cardiomyocytes, while monounsaturated oleic acid (18:1 n-9) and saturated stearic acid (18:0) are not effective[27]. Such antiarrhythmic actions of n-3 PUFAs have been described in experimental animals and humans[28-31]. Among elderly adults, consumption of EPA/DHA-rich, fish-based diet lowers the incidence of cardiac arrhythmias[32,33].
In adult dogs, intravenous infusion of n-3 PUFAs (EPA 1.25-2.82g/100mL, DHA 1.44-3.09g/100mL) significantly reduces cardiac arrhythmias[34]. It is particularly interesting that CX40 and CX43 levels are lower[35] in n-3 PUFA-treated dogs, which suggests that n-3 PUFAs reduce vulnerability to induction of cardiac arrhythmias by modulating cardiac CXs. Supplementation with n-3 PUFAs not only reduces all-cause mortality and cardiac arrhythmias in patients with post-myocardial infarction[36], but also downregulates protein kinase B (Akt), epidermal growth factor, JAM3, myosin heavy chain α and CD99, and significantly decreases levels of Smad6 compared with controls. This suggests that PUFA-mediated prevention of cardiac arrhythmias is due to attenuation of fibrosis, hypertrophy, and inflammation-related genes in response to mechanical stress[37].
MPO could mediate cardiac arrhythmias by augmenting myocardial fibrosis[23]. It is noteworthy that AA, EPA and DHA form precursors to anti-inflammatory products PGI2, lipoxins, resolvins, protectins and maresins[24], which stop leukocyte entry into the exudates as well as counter-regulate the signs of inflammation. Leukocytes that enter an exudate interact with other cells such as monocytes, platelets, endothelial cells, mucosal epithelial cells, fibroblasts and myocardial cells in their immediate vicinity, and are able to perform transcellular biosynthesis of these anti-inflammatory compounds, especially lipoxins. During the course of inflammation and resolution, mediator switching occurs between families of lipid mediators, namely from eicosanoids to lipoxins, resolvins as well as protectins; a process that depends on the availability of substrate within the evolving exudates. Thus, resolution of inflammation involves the appearance of EPA and DHA, which follows the appearance of unesterified AA that is transformed via enzymatic mechanisms to bioactive compounds such as lipoxins, resolvins and protectins that regulate the duration and magnitude of inflammation. Lipoxins, resolvins and protectins also increase the expression of CCR5 receptors on T cells and aging leukocytes, which help clear local chemokine depots from the inflammatory site[24]. Lipoxins stimulate PGI2 generation by endothelial cells and nitric oxide production by vascular endothelial cells[38]; lipoxins and resolvins reduce neutrophil transendothelial migration, interleukin (IL)-12 production, block tumor necrosis factor (TNF)-α, IL-8, interferon-γ, and IL-6 production, signal transduction by nuclear factor-κB, as well as intercellular adhesion molecule-1 expression[39-41]. Intravenous, oral and topical application of LXA4, lipoxin B4 and their synthetic analogs suppresses inflammation and lung and leukocyte MPO activity[42,43]. Furthermore, statins and thiazolidinediones that have anti-inflammatory properties increase the myocardial content of LXA4 and 15-epi-LXA4, which demonstrates that myocardial cells are capable of producing anti-inflammatory and antiarrhythmic lipid mediators[44].
These results suggest that inflammation plays a key role in the pathobiology of cardiac arrhythmias. VNS also suppresses cardiac arrhythmias, therefore, it is likely that it has anti-inflammatory actions.
VAGUS STIMULATION SUPPRESSES INFLAMMATION
Wang et al[45] have shown that the vagus nerve can inhibit significantly and rapidly the release of macrophage TNF, and attenuate systemic inflammatory responses, and have termed it as the “cholinergic anti-inflammatory pathway”. The essential macrophage acetylcholine-mediated (cholinergic) receptor that responds to vagus nerve signals has been identified as the nicotinic acetylcholine receptor α7 subunit that is required for acetylcholine inhibition of macrophage TNF release. Electrical stimulation of the vagus nerve inhibits TNF synthesis in wild-type mice, but fails to do so in α7-deficient mice. It has been reported that stimulation of cholecystokinin receptors leads to attenuation of the inflammatory response by way of the efferent vagus nerve and nicotinic receptors[46]. Even the functional relationship between the cholinergic anti-inflammatory pathway and the reticuloendothelial system has been found to be mediated via the vagus nerve. VNS fails to inhibit TNF production in splenectomized animals during lethal endotoxemia, whereas selective lesioning of the common celiac nerve abolishes TNF suppression by VNS, which suggests that the cholinergic pathway is functionally hard wired to the spleen via this branch of the vagus nerve[47]. These results indicate that electrical VNS or administration of α7 agonists inhibits proinflammatory cytokine production. VNS strongly inhibits lipopolysaccharide (LPS)-induced procoagulant responses, attenuates the fibrinolytic response, and LPS-induced increases in plasma and splenic concentrations of the proinflammatory cytokines TNF-α and IL-6, while not influencing the release of the anti-inflammatory cytokine IL-10[48]. On the other hand, spleen vagal denervation inhibits the production of antibodies to circulating antigens[49]. Transcutaneous VNS dose-dependently reduces systemic TNF levels, inhibits high mobility group protein B1 (HMGB1) levels, and improves survival in mice with polymicrobial sepsis[50]. These observations attest to the fact that VNS suppresses inflammation.
CONCLUSION
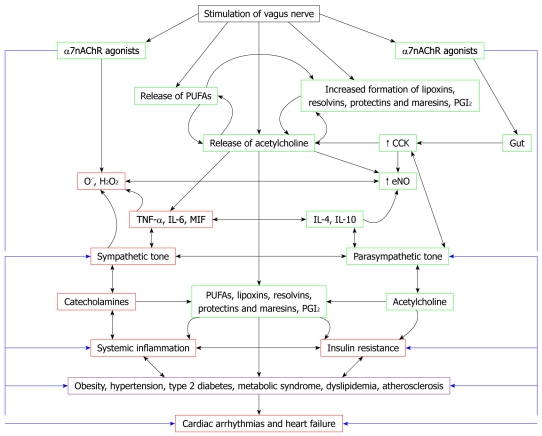
The observation that vagal tone is decreased, sympathetic tone is enhanced, production of IL-6, TNF-α, migration inhibitory factor and HMGB1 is increased, and plasma and tissue concentrations of AA and DHA and their products PGI2, lipoxins, resolvins, protectins and maresins are decreased in CHD, atherosclerosis and cardiac arrhythmias has important therapeutic implications. If this is true, it implies that blockade of α2-adrenoreceptors (blocking these receptor inhibits inflammation injury due to catecholamines[51]), stimulation of the vagus nerve[52] and the nicotinic acetylcholine receptor α7 subunit[45], and administration of AA, DHA, PGI2, lipoxins, resolvins, protectins and maresins, or their stable synthetic analogs, could be of significant benefit in the prevention and management of CHD and cardiac arrhythmias (Figure 1). It is also likely that acetylcholine and VNS enhance the production of anti-inflammatory molecules such as lipoxins, resolvins, protectins and maresins by inducing the release of PUFAs (such as AA, EPA and DHA) from the cell membrane lipid pool.
Figure 1.
Scheme showing relationship between vagal nerve stimulation, autonomic nervous system, inflammation, insulin resistance, metabolic syndrome and cardiac arrhythmias and cardiac failure. Inflammation plays a significant role in coronary heart disease (CHD) and cardiac arrhythmias and conditions that predispose to its development such as atherosclerosis. In contrast, vagal nerve stimulation (VNS) has anti-inflammatory activity, an antifibrillatory effect, and is beneficial in heart failure. Cardiac arrhythmias are due to local inflammation, oxidative injury, altered myocyte metabolism, extracellular matrix remodeling, and fibrosis. Myeloperoxidase (MPO)-deficient mice show reduced activity of matrix metalloproteinases (MMPs), blunted myocardial fibrosis, and markedly reduced incidence of arrhythmias. Patients with cardiac arrhythmias have higher plasma and myocardial concentrations of MPO compared to controls. Activation of leukocytes occurs in cardiac arrhythmias and congestive heart failure (CHF) that leads to excess production of MPO. Increased production of leukocyte MPO could be secondary to a deficiency of lipoxin A4 (LXA4) and prostacyclin (PGI2), which are potent anti-inflammatory, organ-protective, antifibrotic, and antiarrhythmic molecules. Thus, under normal physiological conditions, a delicate balance exists between proinflammatory molecules such as interleukin (IL)-6, tumor necrosis factor (TNF)-α, macrophage migration inhibitory factor (MIF), MPO and anti-inflammatory molecules such as IL-4, IL-10, NO, lipoxins, resolvins, protectins and maresins. Similarly, a balance exists between sympathetic tone and parasympathetic tone. Catecholamines (the neurotransmitters of the sympathetic nervous system) have proinflammatory actions, whereas acetylcholine (the principal neurotransmitter of the vagus nerve) has anti-inflammatory actions. Acetylcholine and VNS might augment the production of anti-inflammatory molecules lipoxins, resolvins, protectins and maresins. Thus, VNS is beneficial in cardiac arrhythmias, CHF and in other low-grade systemic inflammatory conditions such as obesity, hypertension, type 2 diabetes, metabolic syndrome, dyslipidemia, atherosclerosis and insulin resistance. Hence, measurement of plasma/leukocyte content of acetylcholine, catecholamines, IL-6, TNF-α, MIF, IL-4, IL-10, various PUFAs, lipoxins, resolvins, protectins, maresins and vagal tone could be used for prediction of disease progression, and assessing prognosis and response to treatment of CHF and cardiac arrhythmias.
VNS is already in clinical use as an adjunctive treatment for certain types of intractable epilepsy and major depression[53-56]. VNS uses an implanted stimulator that sends electric impulses to the left vagus nerve in the neck via a lead wire implanted under the skin. The advantage of VNS is that it can be performed as an outpatient procedure. It is possible to target pharmacologically the nicotinic acetylcholine receptor α7 subunit-dependent control of cytokine release in CHD, cardiac arrhythmias and atherosclerosis. It is also likely that in future, the currently available treatment regimens for CHD, cardiac arrhythmias and atherosclerosis could be combined with VNS and nicotinic acetylcholine receptor α7 subunit agonists. Another exciting possibility is that VNS might potentially enhance myocardial stem cell (progenitor cell) proliferation and thus augment myocardial healing and function in patients with CHD, as has been shown for hippocampal progenitor proliferation[57].
Acknowledgments
Das UN was in receipt of Ramalingaswami Fellowship of the Department of Biotechnology, India during tenure of this study.
Footnotes
Peer reviewers: Paul Erne, MD, Professor, Head, Department of Cardiology, Luzerner Kantonsspital, CH-6000 Luzern 16, Switzerland; Cristina Vassalle, PhD, G. Monasterio Foundation and Institute of Clinical Physiology, Via Moruzzi 1, I-56124, Pisa, Italy
S- Editor Cheng JX L- Editor Kerr C E- Editor Zheng XM
References
- 1.Koenig W, Meisinger C, Baumert J, Khuseyinova N, Löwel H. Systemic low-grade inflammation and risk of coronary heart disease: results from the MONICA/KORA Augsburg cohort studies. Gesundheitswesen. 2005;67 Suppl 1:S62–S67. doi: 10.1055/s-2005-858246. [DOI] [PubMed] [Google Scholar]
- 2.Schwartz CJ, Valente AJ, Sprague EA, Kelley JL, Suenram CA, Rozek MM. Atherosclerosis as an inflammatory process. The roles of the monocyte-macrophage. Ann N Y Acad Sci. 1985;454:115–120. doi: 10.1111/j.1749-6632.1985.tb11849.x. [DOI] [PubMed] [Google Scholar]
- 3.Das UN. Is obesity an inflammatory condition? Nutrition. 2001;17:953–966. doi: 10.1016/s0899-9007(01)00672-4. [DOI] [PubMed] [Google Scholar]
- 4.Das UN. Hypertension as a low-grade systemic inflammatory condition that has its origins in the perinatal period. J Assoc Physicians India. 2006;54:133–142. [PubMed] [Google Scholar]
- 5.Das UN. Is metabolic syndrome X a disorder of the brain with the initiation of low-grade systemic inflammatory events during the perinatal period? J Nutr Biochem. 2007;18:701–713. doi: 10.1016/j.jnutbio.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 6.Das UN. Metabolic syndrome is a low-grade systemic inflammatory condition. Expert Rev Endocrinol Metab. 2010;5:577–592. doi: 10.1586/eem.10.19. [DOI] [PubMed] [Google Scholar]
- 7.Das UN. Is metabolic syndrome X an inflammatory condition? Exp Biol Med (Maywood) 2002;227:989–997. doi: 10.1177/153537020222701106. [DOI] [PubMed] [Google Scholar]
- 8.Monteiro R, Azevedo I. Chronic inflammation in obesity and the metabolic syndrome. Mediators Inflamm. 2010;2010:289645. doi: 10.1155/2010/289645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schwartz PJ. Vagal stimulation for heart diseases: From animals to men - an example of translational cardiology. Circ J. 2011;75:20–27. doi: 10.1253/circj.cj-10-1019. [DOI] [PubMed] [Google Scholar]
- 10.Kent KM, Smith ER, Redwood DR, Epstein SE. Electrical stability of acutely ischemic myocardium. Influences of heart rate and vagal stimulation. Circulation. 1973;47:291–298. doi: 10.1161/01.cir.47.2.291. [DOI] [PubMed] [Google Scholar]
- 11.Goldstein RE, Karsh RB, Smith ER, Orlando M, Norman D, Farnham G, Redwood DR, Epstein SE. Influence of atropine and of vagally mediated bradycardia on the occurrence of ventricular arrhythmias following acute coronary occlusion in closed-chest dogs. Circulation. 1973;47:1180–1190. doi: 10.1161/01.cir.47.6.1180. [DOI] [PubMed] [Google Scholar]
- 12.Bailey JC, Greenspan K, Elizari MV, Anderson GJ, Fisch C. Effects of acetylcholine on automaticity and conduction in the proximal portion of the His-Purkinje specialized conduction system of the dog. Circ Res. 1972;30:210–216. doi: 10.1161/01.res.30.2.210. [DOI] [PubMed] [Google Scholar]
- 13.Zuanetti G, De Ferrari GM, Priori SG, Schwartz PJ. Protective effect of vagal stimulation on reperfusion arrhythmias in cats. Circ Res. 1987;61:429–435. doi: 10.1161/01.res.61.3.429. [DOI] [PubMed] [Google Scholar]
- 14.Rosenshtraukh L, Danilo P Jr, Anyukhovsky EP, Steinberg SF, Rybin V, Brittain-Valenti K, Molina-Viamonte V, Rosen MR. Mechanisms for vagal modulation of ventricular repolarization and of coronary occlusion-induced lethal arrhythmias in cats. Circ Res. 1994;75:722–732. doi: 10.1161/01.res.75.4.722. [DOI] [PubMed] [Google Scholar]
- 15.Del Rio CL, Dawson TA, Clymer BD, Paterson DJ, Billman GE. Effects of acute vagal nerve stimulation on the early passive electrical changes induced by myocardial ischaemia in dogs: heart rate-mediated attenuation. Exp Physiol. 2008;93:931–944. doi: 10.1113/expphysiol.2007.041558. [DOI] [PubMed] [Google Scholar]
- 16.Vilaine JP, Berdeaux A, Giudicelli JF. Effects of vagal stimulation on regional myocardial flows and ischemic injury in dogs. Eur J Pharmacol. 1980;66:243–247. doi: 10.1016/0014-2999(80)90148-x. [DOI] [PubMed] [Google Scholar]
- 17.Kjekshus JK, Blix AS, Grøttum P, Aasen AO. Beneficial effects of vagal stimulation on the ischaemic myocardium during beta-receptor blockade. Scand J Clin Lab Invest. 1981;41:383–389. doi: 10.3109/00365518109092060. [DOI] [PubMed] [Google Scholar]
- 18.Zamotrinsky A, Afanasiev S, Karpov RS, Cherniavsky A. Effects of electrostimulation of the vagus afferent endings in patients with coronary artery disease. Coron Artery Dis. 1997;8:551–557. [PubMed] [Google Scholar]
- 19.Zamotrinsky AV, Kondratiev B, de Jong JW. Vagal neurostimulation in patients with coronary artery disease. Auton Neurosci. 2001;88:109–116. doi: 10.1016/S1566-0702(01)00227-2. [DOI] [PubMed] [Google Scholar]
- 20.Ando M, Katare RG, Kakinuma Y, Zhang D, Yamasaki F, Muramoto K, Sato T. Efferent vagal nerve stimulation protects heart against ischemia-induced arrhythmias by preserving connexin43 protein. Circulation. 2005;112:164–170. doi: 10.1161/CIRCULATIONAHA.104.525493. [DOI] [PubMed] [Google Scholar]
- 21.Uemura K, Li M, Tsutsumi T, Yamazaki T, Kawada T, Kamiya A, Inagaki M, Sunagawa K, Sugimachi M. Efferent vagal nerve stimulation induces tissue inhibitor of metalloproteinase-1 in myocardial ischemia-reperfusion injury in rabbit. Am J Physiol Heart Circ Physiol. 2007;293:H2254–H2261. doi: 10.1152/ajpheart.00490.2007. [DOI] [PubMed] [Google Scholar]
- 22.Issac TT, Dokainish H, Lakkis NM. Role of inflammation in initiation and perpetuation of atrial fibrillation: a systematic review of the published data. J Am Coll Cardiol. 2007;50:2021–2028. doi: 10.1016/j.jacc.2007.06.054. [DOI] [PubMed] [Google Scholar]
- 23.Rudolph V, Andrié RP, Rudolph TK, Friedrichs K, Klinke A, Hirsch-Hoffmann B, Schwoerer AP, Lau D, Fu X, Klingel K, et al. Myeloperoxidase acts as a profibrotic mediator of atrial fibrillation. Nat Med. 2010;16:470–474. doi: 10.1038/nm.2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Das UN. Lipoxin A4 may function as an endogenous anti-arrhythmic molecule. Med Hypotheses. 2011;76:14–16. doi: 10.1016/j.mehy.2010.08.019. [DOI] [PubMed] [Google Scholar]
- 25.Fontes ML, Mathew JP, Rinder HM, Zelterman D, Smith BR, Rinder CS. Atrial fibrillation after cardiac surgery/cardiopulmonary bypass is associated with monocyte activation. Anesth Analg. 2005;101:17–23, table of contents. doi: 10.1213/01.ANE.0000155260.93406.29. [DOI] [PubMed] [Google Scholar]
- 26.Das UN. Prostacyclin as an endogenous anti-arrhythmic agent. Basic Res Cardiol. 1983;78:716–718. doi: 10.1007/BF01907219. [DOI] [PubMed] [Google Scholar]
- 27.Kang JX, Leaf A. Protective effects of free polyunsaturated fatty acids on arrhythmias induced by lysophosphatidylcholine or palmitoylcarnitine in neonatal rat cardiac myocytes. Eur J Pharmacol. 1996;297:97–106. doi: 10.1016/0014-2999(95)00701-6. [DOI] [PubMed] [Google Scholar]
- 28.London B, Albert C, Anderson ME, Giles WR, Van Wagoner DR, Balk E, Billman GE, Chung M, Lands W, Leaf A, et al. Omega-3 fatty acids and cardiac arrhythmias: prior studies and recommendations for future research: a report from the National Heart, Lung, and Blood Institute and Office Of Dietary Supplements Omega-3 Fatty Acids and their Role in Cardiac Arrhythmogenesis Workshop. Circulation. 2007;116:e320–e335. doi: 10.1161/CIRCULATIONAHA.107.712984. [DOI] [PubMed] [Google Scholar]
- 29.Xiao YF, Sigg DC, Leaf A. The antiarrhythmic effect of n-3 polyunsaturated fatty acids: modulation of cardiac ion channels as a potential mechanism. J Membr Biol. 2005;206:141–154. doi: 10.1007/s00232-005-0786-z. [DOI] [PubMed] [Google Scholar]
- 30.Leaf A, Albert CM, Josephson M, Steinhaus D, Kluger J, Kang JX, Cox B, Zhang H, Schoenfeld D. Prevention of fatal arrhythmias in high-risk subjects by fish oil n-3 fatty acid intake. Circulation. 2005;112:2762–2768. doi: 10.1161/CIRCULATIONAHA.105.549527. [DOI] [PubMed] [Google Scholar]
- 31.Xiao YF, Ke Q, Chen Y, Morgan JP, Leaf A. Inhibitory effect of n-3 fish oil fatty acids on cardiac Na+/Ca2+ exchange currents in HEK293t cells. Biochem Biophys Res Commun. 2004;321:116–123. doi: 10.1016/j.bbrc.2004.06.114. [DOI] [PubMed] [Google Scholar]
- 32.Mozaffarian D, Psaty BM, Rimm EB, Lemaitre RN, Burke GL, Lyles MF, Lefkowitz D, Siscovick DS. Fish intake and risk of incident atrial fibrillation. Circulation. 2004;110:368–373. doi: 10.1161/01.CIR.0000138154.00779.A5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Biscione F, Totteri A, De Vita A, Lo Bianco F, Altamura G. [Effect of omega-3 fatty acids on the prevention of atrial arrhythmias] Ital Heart J Suppl. 2005;6:53–59. [PubMed] [Google Scholar]
- 34.da Cunha DN, Hamlin RL, Billman GE, Carnes CA. n-3 (omega-3) polyunsaturated fatty acids prevent acute atrial electrophysiological remodeling. Br J Pharmacol. 2007;150:281–285. doi: 10.1038/sj.bjp.0706977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sarrazin JF, Comeau G, Daleau P, Kingma J, Plante I, Fournier D, Molin F. Reduced incidence of vagally induced atrial fibrillation and expression levels of connexins by n-3 polyunsaturated fatty acids in dogs. J Am Coll Cardiol. 2007;50:1505–1512. doi: 10.1016/j.jacc.2007.05.046. [DOI] [PubMed] [Google Scholar]
- 36.Macchia A, Monte S, Pellegrini F, Romero M, Ferrante D, Doval H, D'Ettorre A, Maggioni AP, Tognoni G. Omega-3 fatty acid supplementation reduces one-year risk of atrial fibrillation in patients hospitalized with myocardial infarction. Eur J Clin Pharmacol. 2008;64:627–634. doi: 10.1007/s00228-008-0464-z. [DOI] [PubMed] [Google Scholar]
- 37.Ramadeen A, Laurent G, dos Santos CC, Hu X, Connelly KA, Holub BJ, Mangat I, Dorian P. n-3 Polyunsaturated fatty acids alter expression of fibrotic and hypertrophic genes in a dog model of atrial cardiomyopathy. Heart Rhythm. 2010;7:520–528. doi: 10.1016/j.hrthm.2009.12.016. [DOI] [PubMed] [Google Scholar]
- 38.Das UN. Essential fatty acids and their metabolites could function as endogenous HMG-CoA reductase and ACE enzyme inhibitors, anti-arrhythmic, anti-hypertensive, anti-atherosclerotic, anti-inflammatory, cytoprotective, and cardioprotective molecules. Lipids Health Dis. 2008;7:37. doi: 10.1186/1476-511X-7-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baker N, O'Meara SJ, Scannell M, Maderna P, Godson C. Lipoxin A4: anti-inflammatory and anti-angiogenic impact on endothelial cells. J Immunol. 2009;182:3819–3826. doi: 10.4049/jimmunol.0803175. [DOI] [PubMed] [Google Scholar]
- 40.Wu SH, Lu C, Dong L, Zhou GP, He ZG, Chen ZQ. Lipoxin A4 inhibits TNF-alpha-induced production of interleukins and proliferation of rat mesangial cells. Kidney Int. 2005;68:35–46. doi: 10.1111/j.1523-1755.2005.00379.x. [DOI] [PubMed] [Google Scholar]
- 41.Ariel A, Chiang N, Arita M, Petasis NA, Serhan CN. Aspirin-triggered lipoxin A4 and B4 analogs block extracellular signal-regulated kinase-dependent TNF-alpha secretion from human T cells. J Immunol. 2003;170:6266–6272. doi: 10.4049/jimmunol.170.12.6266. [DOI] [PubMed] [Google Scholar]
- 42.Bannenberg G, Moussignac RL, Gronert K, Devchand PR, Schmidt BA, Guilford WJ, Bauman JG, Subramanyam B, Perez HD, Parkinson JF, et al. Lipoxins and novel 15-epi-lipoxin analogs display potent anti-inflammatory actions after oral administration. Br J Pharmacol. 2004;143:43–52. doi: 10.1038/sj.bjp.0705912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weinberger B, Quizon C, Vetrano AM, Archer F, Laskin JD, Laskin DL. Mechanisms mediating reduced responsiveness of neonatal neutrophils to lipoxin A4. Pediatr Res. 2008;64:393–398. doi: 10.1203/PDR.0b013e318180e4af. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Birnbaum Y, Ye Y, Lin Y, Freeberg SY, Nishi SP, Martinez JD, Huang MH, Uretsky BF, Perez-Polo JR. Augmentation of myocardial production of 15-epi-lipoxin-a4 by pioglitazone and atorvastatin in the rat. Circulation. 2006;114:929–935. doi: 10.1161/CIRCULATIONAHA.106.629907. [DOI] [PubMed] [Google Scholar]
- 45.Wang H, Yu M, Ochani M, Amella CA, Tanovic M, Susarla S, Li JH, Wang H, Yang H, Ulloa L, et al. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature. 2003;421:384–388. doi: 10.1038/nature01339. [DOI] [PubMed] [Google Scholar]
- 46.Luyer MD, Greve JW, Hadfoune M, Jacobs JA, Dejong CH, Buurman WA. Nutritional stimulation of cholecystokinin receptors inhibits inflammation via the vagus nerve. J Exp Med. 2005;202:1023–1029. doi: 10.1084/jem.20042397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huston JM, Ochani M, Rosas-Ballina M, Liao H, Ochani K, Pavlov VA, Gallowitsch-Puerta M, Ashok M, Czura CJ, Foxwell B, et al. Splenectomy inactivates the cholinergic antiinflammatory pathway during lethal endotoxemia and polymicrobial sepsis. J Exp Med. 2006;203:1623–1628. doi: 10.1084/jem.20052362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van Westerloo DJ, Giebelen IA, Meijers JC, Daalhuisen J, de Vos AF, Levi M, van der Poll T. Vagus nerve stimulation inhibits activation of coagulation and fibrinolysis during endotoxemia in rats. J Thromb Haemost. 2006;4:1997–2002. doi: 10.1111/j.1538-7836.2006.02112.x. [DOI] [PubMed] [Google Scholar]
- 49.Buijs RM, van der Vliet J, Garidou ML, Huitinga I, Escobar C. Spleen vagal denervation inhibits the production of antibodies to circulating antigens. PLoS One. 2008;3:e3152. doi: 10.1371/journal.pone.0003152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huston JM, Gallowitsch-Puerta M, Ochani M, Ochani K, Yuan R, Rosas-Ballina M, Ashok M, Goldstein RS, Chavan S, Pavlov VA, et al. Transcutaneous vagus nerve stimulation reduces serum high mobility group box 1 levels and improves survival in murine sepsis. Crit Care Med. 2007;35:2762–2768. doi: 10.1097/01.CCM.0000288102.15975.BA. [DOI] [PubMed] [Google Scholar]
- 51.Flierl MA, Rittirsch D, Nadeau BA, Chen AJ, Sarma JV, Zetoune FS, McGuire SR, List RP, Day DE, Hoesel LM, et al. Phagocyte-derived catecholamines enhance acute inflammatory injury. Nature. 2007;449:721–725. doi: 10.1038/nature06185. [DOI] [PubMed] [Google Scholar]
- 52.Mioni C, Bazzani C, Giuliani D, Altavilla D, Leone S, Ferrari A, Minutoli L, Bitto A, Marini H, Zaffe D, et al. Activation of an efferent cholinergic pathway produces strong protection against myocardial ischemia/reperfusion injury in rats. Crit Care Med. 2005;33:2621–2628. doi: 10.1097/01.ccm.0000186762.05301.13. [DOI] [PubMed] [Google Scholar]
- 53.Das UN. Vagus nerve stimulation, depression, and inflammation. Neuropsychopharmacology. 2007;32:2053–2054. doi: 10.1038/sj.npp.1301286. [DOI] [PubMed] [Google Scholar]
- 54.Nemeroff CB, Mayberg HS, Krahl SE, McNamara J, Frazer A, Henry TR, George MS, Charney DS, Brannan SK. VNS therapy in treatment-resistant depression: clinical evidence and putative neurobiological mechanisms. Neuropsychopharmacology. 2006;31:1345–1355. doi: 10.1038/sj.npp.1301082. [DOI] [PubMed] [Google Scholar]
- 55.Follesa P, Biggio F, Gorini G, Caria S, Talani G, Dazzi L, Puligheddu M, Marrosu F, Biggio G. Vagus nerve stimulation increases norepinephrine concentration and the gene expression of BDNF and bFGF in the rat brain. Brain Res. 2007;1179:28–34. doi: 10.1016/j.brainres.2007.08.045. [DOI] [PubMed] [Google Scholar]
- 56.Elliott RE, Morsi A, Tanweer O, Grobelny B, Geller E, Carlson C, Devinsky O, Doyle WK. Efficacy of vagus nerve stimulation over time: Review of 65 consecutive patients with treatment-resistant epilepsy treated with VNS >10years. Epilepsy Behav. 2011;20:478–483. doi: 10.1016/j.yebeh.2010.12.042. [DOI] [PubMed] [Google Scholar]
- 57.Revesz D, Tjernstrom M, Ben-Menachem E, Thorlin T. Effects of vagus nerve stimulation on rat hippocampal progenitor proliferation. Exp Neurol. 2008;214:259–265. doi: 10.1016/j.expneurol.2008.08.012. [DOI] [PubMed] [Google Scholar]