Abstract
The enthusiasm surrounding the clinical potential of embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs) is tempered by the fact that key issues regarding their safety, efficacy, and long-term benefits have thus far been suboptimal. Small molecules can potentially relieve these problems at major junctions of stem cell biology and regenerative therapy. In this review, we will introduce recent advances in these important areas and the first-generation of small molecules used in the regenerative context. Current chemical biology studies will provide the archetype for future interdisciplinary collaborations, and improve clinical benefits of cell-based therapies.
Promise and Challenges for Regenerative Medicine
Life expectancy has increased dramatically in the modern era. Along with it, there is the observed increase in chronic diseases such as heart disease, neurodegenerative disorders, and diabetes. These progressively degenerative conditions are largely irreversible and incurable, except for rare cases where organ transplantation is an option. The recognizable need to correct or replace defective and failing tissues has led to a surging interest in cell-based regenerative therapy. The main goal is to produce a reliable source of replacement biomaterials and tissues ex vivo and supplant the current donor-based system, which is always in limited supply. Furthermore, an ex vivo source can potentially be tailored to specific individuals, which may prevent rejection due to donor-recipient incompatibility and the accompanying risks of immunosuppressive drugs, which are necessary components of organ and tissue transplant procedures (Teo and Vallier, 2010; Rolletschek and Wobus, 2009).
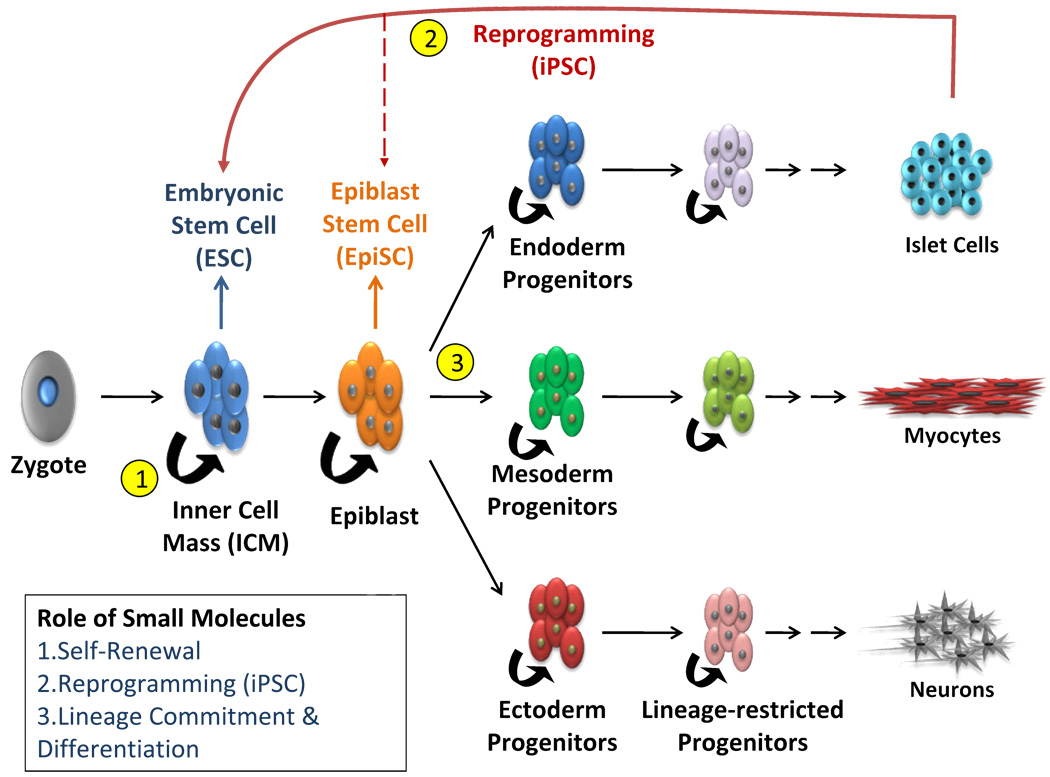
Cell-based therapy has been used as a blanket term that encompasses the usage of significantly different varieties of pluripotent cells. Each variation possesses unique properties that are not fully characterized, and has different implications under each therapeutic context. The establishment of in vitro cultures of mouse embryonic stem cells (mESCs) (Evans and Kaufman, 1981), human embryonic stem cells (hESCs) (Thomson et al., 1998), inducible pluripotent stem cells (iPSCs) (Takahashi and Yamanaka, 2006), and the discovery of adult somatic stem cells in various tissues initiated a flurry of studies into their respective therapeutic potential for basic research and for cell replacement (Figure 1). Recent characterization of different pluripotency states put forth intriguing possibilities for further refining lineage specification and increasing their utility (Brons et al., 2007; Bao et al., 2009).
Figure 1. Applications of small molecules in cell-based therapies.
Small molecules can intervene at many junctions of the stepwise differentiation process. They can 1) promote self-renewal in culture, 2) enhance reprogramming of adult somatic cells, and 3) direct differentiation of pluripotent or lineage-committed progenitor cells. These features will be valuable for harnessing the full potential of pluripotent cells in regenerative therapy.
The initial body of research revealed a number of technical obstacles against the practical usage of embryonic and induced pluripotent cell types. The most pressing challenges are: developing a stable and renewable source of pluripotent cells, reliably maintaining pluripotency without compromising genomic integrity, and efficiently directing differentiation to eliminate cellular heterogeneity. Guiding cell fate determination is especially important as it relates directly to the feasibility and safety of exogenous cell transplants. This is because undifferentiated cells can result in tumor formation as they spontaneously differentiate (Cooke et al., 2006; Blum and Benvenisty, 2008). It is also obvious that established methods are inadequate due to the inconsistent and haphazard nature of current maintenance and directed differentiation approaches (Nagy et al., 1993; Reubinoff et al., 2000). For example, mESCs require the addition of Leukemia Inhibitory Factor (LIF) in the medium to maintain pluripotency and cell proliferation. By contrast, hESCs do not respond to LIF, but instead require Transforming Growth Factor-β (TGF-β)/Nodal and Basic Fibroblast Growth Factor (bFGF) in the medium to sustain pluripotency (Vallier et al., 2005). Another problem is the reliance of growth factors from feeder layers or animal-derived serums in culture protocols, which inevitably introduces batch variability. In addition, the high costs of growth factor additives are prohibitive to the large scale production of pluripotent cells and further limit clinical applications.
A potential alternative source of pluripotent cells is to reprogram differentiated somatic cell types to a pluripotent state. There are two practical advantages to this approach: 1) it circumvents ethical concerns of using embryo-derived stem cells, and 2) it employs a patient’s own cells and would limit immune rejection. A landmark study from Yamanaka’s group identified four transcription factors (Sox2, Oct4, Klf4 and c-Myc) that, when introduced via viral-mediated transduction, re-established pluripotency in adult fibroblasts (Takahashi and Yamanaka, 2006). The resulted iPSCs were shown to closely resemble ESCs, as they were pluripotent and could be induced to differentiate into every cell type (Takahashi and Yamanaka, 2006; Yu et al., 2007). It has since been reported that another combination of genetic factors (Sox2, Oct4, Lin28 and Nanog) can also induce pluripotency (Yu et al., 2007), and that c-Myc can be omitted (Nakagawa et al., 2008). While these results are exciting, some major issues must be resolved before iPSCs become a viable option for cell replacement therapy. The first is the introduction of reprogramming factors using viral transduction systems, which raise reasonable concerns for oncogenic risks in patients. This issue has been partly addressed with the availability of plasmid-based, protein-based, and modified RNA-based strategies that have resulted in successful virus-free cellular reprogramming (Cho et al., 2010; Okita et al., 2008; Warren et al., 2010). The second issue is the extremely low reprogramming efficiency of 0.001 to 0.005% (Hasegawa et al., 2010), which remains unresolved because reprogramming mechanisms are imperfectly understood.
Endogenous somatic stem cells have been scrutinized as an alternative to ex vivo sources. They are resident pools of lineage-restricted multipotent cells that are responsible for tissue turnover, and have been identified in the brain (Doetsch, 2003), skin (Jones, 1993), heart (Messina et al., 2004), skeletal muscle (Martin et al., 2006), and intestines (Casali and Batlle, 2009). They are a tempting source because it is theoretically possible to direct them towards tissue repair, all without risking immunological incompatibility problems. However, they are currently on the fringes of therapeutic options as their isolation, propagation and usage are unrefined and may be restricted to local niches.
Another concern for cell-based therapies is post-transplantation events. The capacity of exogenous cells to integrate with host tissue and restore normal physiological functions is low using current methods. While free floating, suspension cells like bone marrow-derived hematopoietic stem cells (HSCs) are less resistant to integration, the actual integration rate is highly dependent on the stage of HSC differentiation and its affinity for the local microenvironment (Lo Celso et al., 2009). The situation is more complicated when stem cells are introduced to highly-structured organs like the heart, where synchronous contractions are crucial for function. Implanted cells either die from the initial onslaught of inflammatory cytokines, or fail to integrate into the host tissue; instead establishing themselves as a separate or hybrid entity at the transplant site (Reinecke et al., 1999; Alvarez-Dolado et al., 2003). Furthermore, the impact of paracrine cell signaling cannot be understated. Initial benefits described from transplantation studies using various sources of stem cells including bone marrow, mesenchymal, and neural stem cells offered temporary functional improvements. These benefits were subsequently characterized as the result of paracrine factors secreted by the stem cells rather than true cell autonomous repair (Kim et al., 2010a; Perez-Ilzarbe et al., 2008).
Future technologies must take advantage of the synergistic interactions between the transplanted cells and the local microenvironment in order to harness the full potential of regenerative therapy. Advances in chemical biology can conceivably serve first as a tool to dissect the complicated pathways regulating self-renewal and cell fate decisions, and second as the means to manipulate those same pathways for the desired therapeutic outcome.
Chemical Biology: Advantages of Small Molecules
The guiding principal in chemical biology is to discover and develop synthetic bioactive molecules. It presents several advantages over traditional protein- or gene-based tools. The usage of traditional biomolecules is limited as they are difficult to produce and manipulate. Their effects are unstable and cannot be fine tuned since genetic switches are generally all-or-none. They can modulate only a single target at a time and introduction of multiple biomolecules into a specific tissue is technically challenging. In contrast, small molecules can be delivered efficiently into the cell, can be targeted to specific tissues and their effects are reversible. The dosage of the compounds can be modulated for maximum benefit, and individual molecules can be further modified via medicinal chemistry to increase potency, safety, or stability. Small molecules can target the biology of a desired phenotype by stimulating multiple druggable categories via intersecting signaling nodes. Small molecules are also relatively inexpensive to produce, and can be scaled to particular needs. These attributes place small molecules on a favorable position for regenerative medicine developments.
Small molecules can participate at several junctions of regenerative medicine as many key cellular events utilize the same pathways and form druggable nodes. These key pathways are Hedgehog (Hh), Wnt, Transforming Growth Factor -β (TGF-β), Notch, and Fibroblast Growth Factor (FGF) (Jiang and Hui, 2008; MacDonald et al., 2009; Wu and Hill, 2009; Bolós et al., 2007; Beenken and Mohammadi, 2009). Specific information on how these key pathways function in pluripotency and differentiation is discussed in other excellent reviews (Loebel et al., 2003; Pera and Tam, 2010). Furthermore, small molecules have been identified that target these pathways singly and in combination with other small molecules or genetic factors. As an emerging area of study, small molecules have provided early proof that cell fate is dynamic and can be manipulated using artificial means. The attributes of pluripotent and lineage-restricted progenitor cells, when combined with the practical utility of small molecules, are powerful assets for realizing the full potential of regenerative therapy.
Small Molecules in Regenerative Medicine
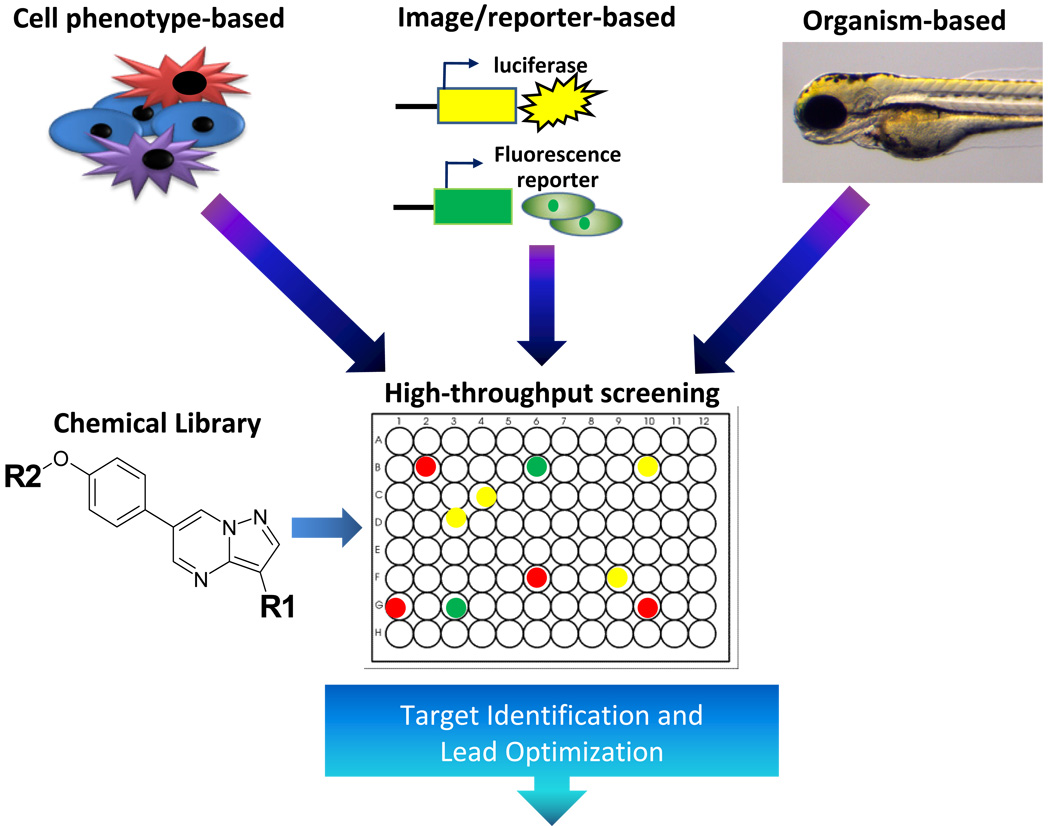
Many small molecules relevant to stem cell biology were identified from chemical libraries using high-throughput cell phenotype-based, reporter-based, or organism-based screens (Figure 2). Lead compounds were further examined to identify associated targets and relevant pathways, which then guide future optimization based on biochemical, pharmacological, and physiological requirements (Ding and Schultz, 2004). Compounds that promote self-renewal, facilitate reprogramming, and direct differentiation pathways have been identified using the above methods. These chemical biology discoveries, detailed below, are shaping the future of regenerative medicine.
Figure 2. Strategies for high-throughput chemical screening.
Small molecules from a chemical library are identified in high-throughput screening assays using stem cells. Compounds are evaluated for the desired effects using cellular images, specific promoter driven reporters, or organism phenotypes as readout.
Self renewal and Pluripotency
A major obstacle for the practical usage of stem cells has been maintaining their pluripotent state in culture. Spontaneous differentiation occurs due to constant bombardment from undefined and varied amounts of growth factors found in traditional culture protocols that use animal serum and feeder cells. For this reason, a number of serum- and feeder-free protocols have been developed that use commercially available supplements. Many of these supplements still use animal-derived and recombinant growth factors, but they are optimized for pluripotent cell culture and reduce batch variability. They include KnockOut (Cheng et al., 2004) or N2 and B27 (N2B27) (Ying and Smith, 2003) that consist of essential recombinant growth factors for mESCs and iPSCs using GIBCO’s proprietary formula. Another formulation is to add LIF and bone morphogenetic protein (BMP) to N2B27 media, which also drive continuous self-renewal in serum-free conditions (Nichols and Ying, 2006). A feeder-free, serum-free method has successfully maintained hESCs and iPSCs in culture (Ludwig et al., 2006). It replaces the feeder layer with an animal-derived extracellular matrix called Matrigel (BD Bioscience), and a serum-free medium called mTeSR1 (STEMCELL Tech) supplemented with recombinant proteins (Ludwig et al., 2006). As promising as these new feeder-free, serum-free approaches are, they are not yet compatible with wide-scale clinical applications since they still require complex animal-derived products or recombinant protein modulators, which can be expensive and unstable.
The above-mentioned issues motivate the development of synthetic media formulations that minimizes or eliminates animal-derived or recombinant bioactive products. The first-generation collection of small molecules has been identified for this purpose. Pluripotin (Table 1) was the first compound identified in a chemical screen that propagates mESCs in an undifferentiated state (Chen et al., 2006). This discovery was especially remarkable because it showed that pluripotent cells can indeed be maintained in chemically defined conditions, without the use of animal-derived products or LIF. Furthermore, pluripotin did not stimulate the predicted pluripotency pathways, i.e. LIF-STAT3 (Niwa et al., 1998), BMP4-Smad-Id (Ying et al., 2003) or Wnt signaling (Sato et al., 2004). Instead, pluripotin blocked two major differentiation-inducing pathways, i.e. MEK-ERK and Ras-GAP signaling (Johnson and Lapadat, 2002; Lypowy et al., 2005). This was significant because it demonstrated the existence of a basal, self-renewing stem cell state that can be maintained by inhibiting differentiation.
Table 1.
Small molecules for self-renewal in stem cells and iPSCs
| Molecule | Name | Target | Reference |
|---|---|---|---|
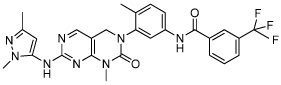 |
Pluripotin/SC1 | Dual Inhibitor RasGAP/ERK1 |
Chen (2006) |
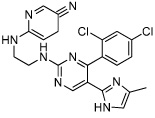 |
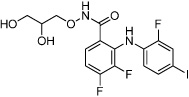
CHIR99021 | GSK3 inhibitor |
Ying (2008) Li (2009a) Li (2009b) |
 |
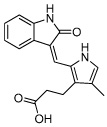
PD0325901 | MEK inhibitor |
Lin (2009) Zhu (2010) |
 |
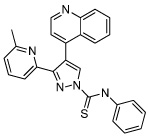
SU5402 | FGF inhibitor | Ying (2008) |
 |
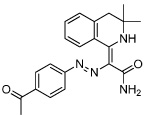
A-83-01 | TGF-β Inhibitor |
Li (2009a) Zhu (2010) |
 |
IQ-1 | Phosphatase PP2A Inhibitor (Wnt modulator) |
Miyabayashi (2007) |
A different approach to boost pluripotency is to upregulate known pluripotency pathways. 6-Bromoindirubin-3’-oxime (BIO, Table 3), which activates the canonical Wnt signaling by inhibiting GSK3, could maintain pluripotency in human and mouse ESCs (Sato et al., 2004). However, this compound is strictly a stabilizer of pluripotency signals, and requires LIF to initiate pluripotency. Another Wnt signal modulator called IQ-1 (Table 1) can replace exogenous LIF and feeder cell requirements in mouse stem cell culture (Miyabayashi et al., 2007). While these studies suggest Wnt signaling is a fundamental component in regulating pluripotency, one must caution against generalization since the role of any single signaling pathway may be highly dependent on cellular context (Sato et al., 2004; Dravid et al., 2005).
Table 3.
Small molecules for directed differentiation
| Molecule | Name | Target | Reference |
|---|---|---|---|
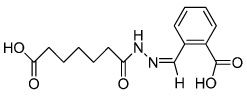 |
IDE1 | Unknown | Borowiak (2009) |
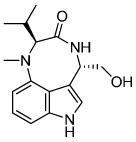 |
Indolactam V(ILV) | PKC Activator |
Borowiak (2009) Chen (2009) |
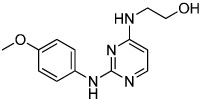 |
Cardiogenol C | Unknown | Wu (2004) |
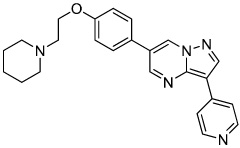 |
Dorsomorphin | BMP Type 1 receptor Inhibitor |
Hao (2008) |
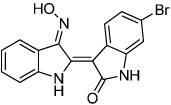 |
6-Bromoindirubin-3'-oxime (BIO) | GSK3 Inhibitor (Wnt activator) |
Sato(2004) Naito (2006) Tseng (2006) Qyang(2007) |
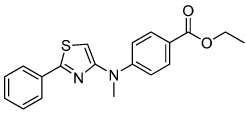 |
Neuropathiazol | Unknown | Warashina (2006) |
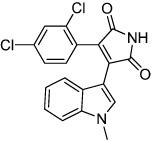 |
SB216763 | GSK3 Inhibitor (Wnt activator) |
Lange (2011) |
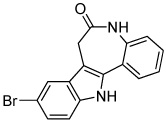 |
Kenpaullone | GSK3 Inhibitor (Wnt activator) |
Lange (2011) |
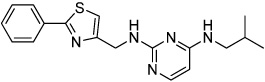 |
KHS101 | TACC3 (Mitotic spindle) |
Wurdak(2010) |
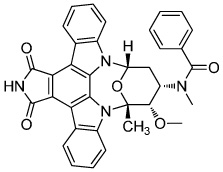 |
Stauprimide | NME2(metastatic factor) |
Zhu (2009) |
Combining Wnt activation with inhibition of the differentiation-inducing signals, particularly the FGF (fibroblast growth factor)/MEK-ERK pathway, has proven to be highly effective for maintaining pluripotency of ESCs. For example, a cocktail of small molecule inhibitors, dubbed “3i,” composed of GSK3 inhibitor CHIR99021 (Table 1), MEK inhibitor PD0325901 (Table 1) and FGF receptor inhibitor SU5402 (Table 1) added to the basal N2B27 media effectively inhibited spontaneous differentiation and ensured homogeneous Nanog expression, a key component for pluripotency maintenance (Mitsui et al., 2003; Ying et al., 2008). A similar strategy combining CHIR99021 (Table 1), PD0325901 (Table 1) and TGF-β receptor inhibitor A-83-01 (Table 1) was also capable of maintaining rat and human iPSCs (Li et al., 2009a).
Although significant progress has been made in understanding the basic mechanisms and characteristics of pluripotency, current understanding is far from complete and basal pluripotency requirements remain undefined. Overcoming these obstacles through the discovery of additional pluripotency pathways and their chemical modulators could enable large-scale production of pluripotent cells and finally provide a stable source of starting materials for tissue regeneration.
Reprogramming
Major hurdles against the practical usage of iPSCs include low reprogramming efficiency, and safety concerns raised by the use of viral transduction in the process. The fast moving pace of the reprogramming and iPSC field has yielded numerous reports of chemicals that either improve reprogramming efficiency or can substitute for specific reprogramming factors. Mechanistically, the chemicals generally function by altering signal transduction pathways, or modify chromatin structure to remove epigenetic barriers. These properties, when known, will be described below.
Several small molecules improve the reprogramming process by lowering the epigenetic barrier to initiate pluripotency. The accompanying side effect is eliminating the need for one or two Yamanaka factors (c-Myc or Sox2) in the reprogramming cocktail. This is because those two factors are not necessary to initiate pluripotency, but are responsible for its maintenance (Masui et al., 2007). These initial experiments focused on mouse embryonic fibroblast (MEF) reprogramming. Valproic acid (VPA, Table 2) is a histone deacetylase (HDAC) inhibitor that dramatically improved reprogramming efficiency by 100-fold without the use of c-Myc (Huangfu et al., 2008). A G9a histone methyltransferase (HMTase) inhibitor, BIX01294 (Table 2), substantially increased reprogramming efficiency of Sox2-expressing mouse neural progenitor cells (NPCs) transduced with Oct4 and Klf4 (OK) to levels obtained with canonical Yamanaka factors (Shi et al., 2008b). A similar example used a combination of DNA methyltransferase (DNMT) inhibitor called RG108 (Table 2), an L-type calcium channel agonist called BayK8644 (Table 2), and BIX01294 to promote reprogramming in MEFs transduced with OK (Shi et al., 2008a). Another chemical screen identified a TGF-β signaling inhibitor called RepSox (Table 2) as a potent Sox2 replacement (Ichida et al., 2009). This molecule was shown to promote reprogramming by inducing Nanog expression, which is a transcription factor known to drive self-renewal in ESCs (Pan and Thomson, 2007).
Table 2.
Small molecules for enhanced reprogramming efficiency
| Molecule | Name | Target | Reference |
|---|---|---|---|
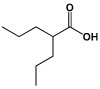 |
Valproic Acid (VPA) | Histone deacetylase (HDAC) Inhibitor |
Huangfu (2008) |
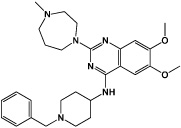 |
BIX01294 | AG9a histone methyltransferase (HMTase) inhibitor |
Shi (2008b) |
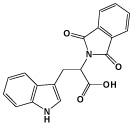 |
RG108 | DNA methyltransferase (DNMT) Inhibitor |
Shi (2008a) |
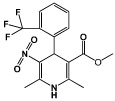 |
BayK8644 | L-type calcium channel agonist |
Shi (2008a) |
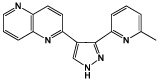 |
RepSox | TGFβ inhibitor | Ichida (2009) |
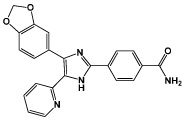 |
SB431542 | TGFβ inhibitor |
Lin (2009) Chambers (2009) |
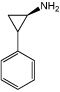 |
Parnate | lysine-specific demethylase 1 (LSD1) inhibitor |
Li (2009a) |
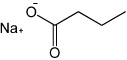 |
Sodium Butyrate (NaB) | Histone Deacetylase (HDAC) inhibitor |
Zhu (2010) |
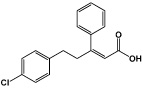 |
PS48 | PDK1 activator | Zhu (2010) |
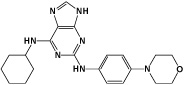 |
Reversine | Aurora B Kinase Inhibitor |
Chen (2004) D'Alise (2008) |
The chemical cocktails perfected in MEFs were quickly adapted for human somatic cell reprogramming. A combination of SB431542 (Table 2) and PD0323901 (Table 1), which inhibit TGF-β and MAPK/ERK pathways respectively, and thiazovivin, which improves the survival of hESCs upon trypsinization, increased reprogramming efficiency in human fibroblasts by 200-fold (Lin et al., 2009). The GSK3 inhibitor, CHIR99021 (Table 1), when combined with a lysine-specific demethylase 1 (LSD1) inhibitor called Parnate (also called tranylcypromine) (Table 2) enhanced reprogramming of human keratinocytes transduced with OK (Li et al., 2009b). A more recent report described a stepwise chemical treatment protocol that required only OCT4 transduction (Zhu et al., 2010). This protocol used a combination of TGF-β receptor inhibitor A-83-01 (Table 1), HDAC inhibitor sodium butyrate (NaB, Table 2), and PDK1 activator called PS48 (Table 2) for the first four weeks. For the next four weeks, PD0323901 (Table 1) was combined with the aforementioned mixture to complete reprogramming. The report identified a switch in metabolic state from mitochondrial oxidation to glycolysis, known in oncology as the “Warburg Effect” (Robey et al., 2008), which may be critical during reprogramming (Zhu et al., 2010). The reprogramming efficiency using this protocol was extremely low, as only 4 to 6 iPSC colonies formed for every 1 × 106 cells seeded (Zhu et al., 2010). Nevertheless, this initial report demonstrates the power chemical biology can hold for the future of human somatic cell reprogramming.
There is another class of compounds that functions by de-differentiating lineage-committed cells to a more primitive, multipotent state. Multiple studies have described reversine (Table 2) as a potent dedifferentiation agent, which facilitated transformation of differentiated cells to other lineages via a lineage-restricted progenitor (Chen et al., 2004; Lee et al., 2009). Reversine was characterized as an Aurora kinase inhibitor (D'Alise et al., 2008), possibly specific for Aurora kinase B (Amabile et al., 2009). Another Aurora kinase inhibitor, VX-608, was also found to dedifferentiate mouse myoblasts (Amabile et al., 2009). The partially reprogrammed state induced by these compounds confirmed that cell fate commitment is a stepwise process, and more importantly, some of the intermediate steps can be manipulated chemically and may even be reversible.
These chemical biology studies suggest that somatic cells are highly plastic and can be induced to assume multiple de-differentiated states. This notion has parallels in the emerging concept of distinct “naïve” and “primed” pluripotent states, which has gained serious consideration since the recent identification of murine epiblast stem cells (EpiSCs) (Nichols and Smith, 2009). EpiSCs derived from postimplantation epiblast represent a more mature stage than mESCs, derived from the inner cell mass of preimplantation blastocysts (Tesar et al., 2007; Bao et al., 2009; Chou et al., 2008; Brons et al., 2007). EpiSCs resemble hESCs in many ways, including their dependence on bFGF/Activin A signaling instead of LIF/STAT signaling, flattened colony morphology, their X-inactivation status, and their inability to be passaged as single cells (Tesar et al., 2007; Brons et al., 2007). These similarities suggest that hESCs are analogous to the more mature, primed pluripotent state of EpiSCs. Recent chemical biology studies demonstrated that EpiSCs, as well as hESCs and hiPSCs, can be converted to the earlier, naïve pluripotent state. The converted cells become functionally, transcriptionally and epigenetically similar to mESCs. Taking cues from the 3i strategy described above, Ding and colleagues successfully converted EpiSCs to the mESC phenotype using a cocktail of small molecule inhibitors of TGF-β receptor (A-83-01), FGF receptor (PD173074), MEK (PD0325901), GSK3 (CHIR99021) and LSD1 (Parnate) added to the LIF-containing media (Zhou et al., 2010). Using a similar chemical biology approach, Jaensich and colleagues demonstrated that the combination of 3 compounds PD173074, CHIR99021 and forskolin, an activator of the enzyme adenylate cyclase, added to the LIF-containing basal N2B27 media induced the naïve pluripotent state in both hESCs and hiPSCs (Hanna et al., 2010). Distinction between the naïve and primed pluripotent states is important because human iPSCs derived using current protocols are thought to represent the later, characterized by significant biases in differentiation potential (Nichols and Smith, 2009). Thus conversion of hiPSCs to the naïve pluripotent state may be necessary for efficient generation of diverse patient-specific tissues (Hanna et al., 2010).
The naïve and primed pluripotent states have important implications for reprogramming and directed differentiation especially in light of the recent discovery of the epigenetic memory in iPSCs (Kim et al., 2010b; Ji et al., 2010). Epigenetic memory is a phenomenon in which iPSCs derived from adult tissues retain DNA methylation signatures characteristic of their somatic tissue of origin, unlike the classical ESCs. Furthermore, it may sustain a residual gene expression signature from the parental cell and account for gene expression differences between iPSCs and ESCs (Ghosh et al., 2010; Chin et al., 2009). Consequently, iPSCs preferentially differentiation along lineages related to the parental cell, with restricted potential for alternative cell fates (Kim et al., 2010b). While the epigenetic memory of parental tissues could at least partially be reset by treatment with chromatin-modifying drugs Trichostatin A (TSA), an inhibitor of histone deacetylase, and 5-azacytidine (AZA), a cytosine analog resistant to methylation (Kim et al., 2010b), it represents a significant barrier against full reprogramming and directed differentiation toward many desired cell types.
There are now many examples of chemical modulators facilitating cellular reprogramming by increasing reprogramming efficiency, eliminating potentially oncogenic factors in the reprogramming cocktail, or increasing cell fate plasticity. These observations have also fueled new questions and challenges. In most cases, the precise mechanisms by which synthetic chemicals influence reprogramming are yet to be discovered. Additional chemical biology advances are needed to completely erase epigenetic memory, and to eliminate the need for exogenous reprogramming factors altogether. Given tremendous advances in the past few years, we anticipate that future developments will substantially improve the process of iPSC generation and provide better understanding of reprogramming.
Directed Differentiation
Achieving and maintaining a pluripotent stem cell population is one element in regenerative therapy improvement. Another requirement is to produce large quantities of stage-specific cells in a controlled manner in vivo. A pluripotent cell population cannot be used directly in patients because they can form tumors. Therefore, it is necessary to pre-differentiate pluripotent cells to a desired cell type prior to transplantation. Compounds for this purpose have been identified in high-throughput assays based on lineage-specific gene expression profiles (Figure 2), and will be discussed below in relations to the three germ layers: endoderm, mesoderm, and ectoderm. The net effects of these chemicals have been to functionally promote lineage commitment, or to block self-renewal maintenance and spur differentiation.
The endoderm lineage has been the least characterized of the three lineages due to the lack of early endoderm markers, but imperfect markers, like Sox17 and Foxa2, exist for later endoderm commitment (Iwamuro et al., 2010). Two chemical compounds named IDE1 (Table 3) and IDE2 were identified from a library of putative HDAC inhibitors based on Sox17 induction (Borowiak et al., 2009). IDE1 and IDE2 activated close to 80% endoderm progenitor production in mouse and human stem cells, which is above that of known TGF-β modulators Activin A and Nodal (Borowiak et al., 2009). The compounds upregulated Nodal signaling, and increased endoderm lineage commitment and developmental competence (Borowiak et al., 2009). Another compound called Indolactum V (ILV, Table 3) increased development of Pdx-1 expressing pancreatic progenitors from endoderm-biased progenitors (Borowiak et al., 2009; Chen et al., 2009). Proliferation studies showed ILV did not drive proliferation of existing Pdx-1 expressing cells, but rather committed other cell types of the heterogeneous endoderm-restricted pool towards the pancreatic progenitor lineage via activation of protein kinase C (PKC) (Chen et al., 2009). However, there was no mechanism proposed on how PKC activation increases pancreatic progenitors.
Similarly, there are examples of chemical cocktails that direct mesoderm lineage commitment but the mechanisms are unknown. Cardiogenol C (Table 3) was identified in a high-throughput, cardiac-specific ANF reporter assay (Wu et al., 2004). It specifically induces cardiomyocyte formation in mESC culture after 3 days of treatment (Wu et al., 2004). However, the compound appears to only be effective in certain cell types (Jasmin et al., 2010), which would suggest that it is a non-specific compound that requires a specific cellular context to support pro-cardiac effects.
Modulation of BMP signaling can direct ectoderm and mesoderm formation during embryonic differentiation (Winnier et al., 1995; Finley et al., 1999). A BMP selective inhibitor identified using an unbiased, high-throughput in vivo screen in zebrafish, called dorsomorphin (Table 3), induced beating cardiomyocyte formation in mouse ESC culture (Yu et al., 2008; Hao et al., 2008). Unlike cardiogenol C, dorsomorphin is required only during the first 24 hours of induction to specify cardiomyocyte commitment. This timeframe makes the pro-cardiomyogenic mechanism of dorsomorphin especially interesting as it occurs prior to the expression of known early mesoderm markers like BryT and Mesp1 (Kubo et al., 2004; Bondue et al., 2008). This strongly suggests that dorsomorphin increases cardiomyogenesis by way of an unknown progenitor.
Chemical modulation of the Wnt signaling also has profound effects on mesoderm specification and cardiomyocyte induction. GSK3 inhibitor BIO (Table 3), which activates Wnt signaling, significantly induced cardiomyocyte formation in mESCs when introduced during the first three days of ESC differentiation (Naito et al., 2006). BIO was also reported to markedly expand the specific subset of ESC-derived, embryonic and postnatal cardiovascular progenitor cells which express the Isl1 marker (Qyang et al., 2007). These results outlined the stage-specific role of Wnt signaling in cardiac progenitor specification and proliferation. Paradoxically, cardiomyocyte differentiation was suppressed when mESCs were exposed to BIO after day 5 of differentiation, following the formation of cardiovascular progenitor cells (Naito et al., 2006). Similar biphasic role of Wnt signaling was demonstrated in hESCs (Paige et al., 2010), whereby inhibiting Wnt signaling in multipotent mesodermal progenitor cells promoted cardiac differentiation (Kattman et al., 2011). Consistent with these results, XAV939, a selective small molecule inhibitor of Tankyrase required for Wnt signaling, markedly induced cardiomyogenesis in mESCs when introduced after mesoderm formation (Wang et al., 2011). In summary, these studies suggest that stage-specific chemical modulation of Wnt signaling is a promising strategy for directed differentiation of cardiac cell types in human pluripotent stem cells.
In contrast to the endoderm and mesoderm lineages, ectoderm differentiation of ESCs is commonly considered a default developmental pathway (Reubinoff et al., 2000). Ectoderm commitment can be further promoted by inhibiting BMP (Winnier et al., 1995; Finley et al., 1999) and downstream SMAD signaling (Sirard et al., 1998) that are required for mesoderm formation (Finley et al., 1999). This signaling pathway can be targeted using chemical means in conjunction with other protein modulators. The TGF-β inhibitor SB431542 (Table 2), when used in combination with BMP inhibitor Noggin, induced neural differentiation in human ESC and human iPSC (Chambers et al., 2009). There are other compounds that can generate the neurogenic phenotype, but the mechanism is not well characterized. Neuropathiazol (Table 3) can induce differentiation of human hippocampus progenitors into neurons (Warashina et al., 2006), which may hold therapeutic potential as a treatment for Parkinson’s disease. An additional pro-neurogenesis cocktail was described recently; GSK3 inhibitors SB216763 and kenpaullone (Table 3) were found to stimulate human neural progenitor cells commitment by upregulating Wnt signaling without changing cell cycle progression or proliferation (Lange et al., 2011). This observation would suggest that the neurogenic effect of SB216763 and kenpaullone may be to increase lineage commitment rather than to expand existing neural cells.
A study performed in rats showed the compound KHS101 (Table 3) can induce neural differentiation by inhibiting neural progenitor cell maintenance, while also stabilizing pro-neurogenesis transcriptional pathways (Wurdak et al., 2010). KHS101 was shown to negatively affect cell cycle exit and proliferation, thereby blocking the maintenance of the undifferentiated neural progenitor cell phenotype. Concurrently, KHS101 physically interacted with TACC3 (a structural component of the centrosome and mitotic spindle) and prevents its ability to sequester ARNT2 (a pro-neurogenesis transcription factor) in the cytoplasm (Wurdak et al., 2010). The net effect was KHS101 accelerated neural differentiation from the progenitor pool by blocking self-renewal.
It would be extremely useful if protein modulators could be excluded completely from the cell fate decision process, and stepwise lineage commitment was achieved chemically. In the event that such compounds or protocols failed to arise, ESCs can be chemically manipulated to increase their sensitivity to exogenous differentiation signals. Stauprimide (Table 3) was reported to prime mouse and human ESCs for differentiation via interaction with NME2, a metastatic factor with a possible role in promoting pluripotency (Zhu et al., 2009). The initial report did not demonstrate that the compound stimulates differentiation towards any particular lineage, but rather amplifies induction efficiency (Zhu et al., 2009). The mechanism is unclear at this time, but it may be that NME2 inhibition limits self-renewal maintenance by reducing c-Myc expression (Thakur et al., 2009). In this case, stauprimide may function by making self-renewal an unfavorable condition for the cell and thus lowering the energy requirements for extracellular differentiation signals. It would be interesting to see whether modulating self-renewal can be a general approach to promote directed differentiation.
The above examples introduced some of the potential advantages of using small molecules for directed differentiation. Not only do they help uncover novel differentiation mechanisms and interact with known development pathways, but they also offer fine temporal control to the investigator. Given our limited knowledge of the temporal regulation of individual pathways, chemical biology has yielded intriguing insights into the dynamic interactions that drive cell fate commitment, and continues to be integrated with developmental and cellular biology. The foreseeable trajectory for all these areas is to promote basic understanding of developmental mechanisms and to apply this knowledge towards improving clinical outcomes.
The Future of Bioactive Compounds in Cell-based Therapy
An emerging area of interest for both regenerative medicine and chemical biology is the manipulation of the local tissue microenvironment following transplantation. Transplanted cells are generally introduced to a hostile environment in injured or diseased tissues, resulting in massive cell loss and depleting much of their therapeutic potential. Identification of signaling pathways that improve the survival of newly introduced cells either by inhibiting apoptosis or sustaining proliferation would be of special interest. In addition, transplanted cells will likely require extracellular cues to fully mature and undertake their functional role in regeneration or repair. Therefore, understanding and controlling these extracellular factors is required to extract long-term benefits from any cell-based system.
A paradigm shift has occurred in regenerative medicine that will require a multifaceted approach to cell-based therapies. It would require focus not only on robust pluripotency maintenance and appropriate cell differentiation, but also on their interactions with the extracellular milieu. The future of this field will require a fully coordinated view that will match specific pluripotent /progenitor states to the desired differentiated population, and their incorporation into the optimal extracellular support matrices for downstream applications. At this point, the usage of bioactive compound is in the early stages and questions remain concerning their cellular effects compared to that produced by natural processes. Additional studies are required to better correlate in vitro benefits to in vivo applications as well as their pharmacological properties. Nevertheless, small molecules have shown themselves as enablers of regenerative therapy and have reduced many of its challenges. They will undoubtedly play a critical role in many areas of stem cell research and help mobilize those discoveries towards therapeutic options.
Acknowledgements
We thank Malathi Narayan for editing the manuscript. This work was supported by the Veterans Affairs CDTA and Merit Awards, the Developmental Grant from the Center for Research in Fibrodysplasia Ossificans Progressiva and Related Disorders, and the NIH grants 5U01HL100398 and 1R01HL104040.
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alvarez-Dolado M, Pardal R, Garcia-Verdugo JM, Fike JR, Lee HO, Pfeffer K, Lois C, Morrison SJ, Alvarez-Buylla A. Fusion of bone-marrow-derived cells with Purkinje neurons, cardiomyocytes and hepatocytes. Nature. 2003;425:968–973. doi: 10.1038/nature02069. [DOI] [PubMed] [Google Scholar]
- Amabile G, D'Alise AM, Iovino M, Jones P, Santaguida S, Musacchio A, Taylor S, Cortese R. The Aurora B kinase activity is required for the maintenance of the differentiated state of murine myoblasts. Cell Death Differ. 2009;16:321–330. doi: 10.1038/cdd.2008.156. [DOI] [PubMed] [Google Scholar]
- Bao S, Tang F, Li X, Hayashi K, Gillich A, Lao K, Surani MA. Epigenetic reversion of post-implantation epiblast to pluripotent embryonic stem cells. Nature. 2009;461:1292–1295. doi: 10.1038/nature08534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beenken A, Mohammadi M. The FGF family: biology, pathophysiology and therapy. Nat Rev Drug Discov. 2009;8:235–253. doi: 10.1038/nrd2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum B, Benvenisty N. The tumorigenicity of human embryonic stem cells. Adv. Cancer Res. 2008;100:133–158. doi: 10.1016/S0065-230X(08)00005-5. [DOI] [PubMed] [Google Scholar]
- Bolós V, Grego-Bessa J, de la Pompa JL. Notch signaling in development and cancer. Endocr. Rev. 2007;28:339–363. doi: 10.1210/er.2006-0046. [DOI] [PubMed] [Google Scholar]
- Bondue A, Lapouge G, Paulissen C, Semeraro C, Iacovino M, Kyba M, Blanpain C. Mesp1 acts as a master regulator of multipotent cardiovascular progenitor specification. Cell Stem Cell. 2008;3:69–84. doi: 10.1016/j.stem.2008.06.009. [DOI] [PubMed] [Google Scholar]
- Borowiak M, Maehr R, Chen S, Chen AE, Tang W, Fox JL, Schreiber SL, Melton DA. Small molecules efficiently direct endodermal differentiation of mouse and human embryonic stem cells. Cell Stem Cell. 2009;4:348–358. doi: 10.1016/j.stem.2009.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brons IG, Smithers LE, Trotter MW, Rugg-Gunn P, Sun B, Chuva de Sousa Lopes SM, Howlett SK, Clarkson A, Ahrlund-Richter L, Pedersen RA, et al. Derivation of pluripotent epiblast stem cells from mammalian embryos. Nature. 2007;448:191–195. doi: 10.1038/nature05950. [DOI] [PubMed] [Google Scholar]
- Casali A, Batlle E. Intestinal stem cells in mammals and Drosophila. Cell Stem Cell. 2009;4:124–127. doi: 10.1016/j.stem.2009.01.009. [DOI] [PubMed] [Google Scholar]
- Chambers SM, Fasano CA, Papapetrou EP, Tomishima M, Sadelain M, Studer L. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat. Biotechnol. 2009;27:275–280. doi: 10.1038/nbt.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Do JT, Zhang Q, Yao S, Yan F, Peters EC, Scholer HR, Schultz PG, Ding S. Self-renewal of embryonic stem cells by a small molecule. Proc Natl Acad Sci U S A. 2006;103:17266–17271. doi: 10.1073/pnas.0608156103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Borowiak M, Fox JL, Maehr R, Osafune K, Davidow L, Lam K, Peng LF, Schreiber SL, Rubin LL, et al. A small molecule that directs differentiation of human ESCs into the pancreatic lineage. Nat. Chem. Biol. 2009;5:258–265. doi: 10.1038/nchembio.154. [DOI] [PubMed] [Google Scholar]
- Chen S, Zhang Q, Wu X, Schultz PG, Ding S. Dedifferentiation of lineage-committed cells by a small molecule. J. Am. Chem. Soc. 2004;126:410–411. doi: 10.1021/ja037390k. [DOI] [PubMed] [Google Scholar]
- Cheng J, Dutra A, Takesono A, Garrett-Beal L, Schwartzberg PL. Improved generation of C57BL/6J mouse embryonic stem cells in a defined serum-free media. Genesis. 2004;39:100–104. doi: 10.1002/gene.20031. [DOI] [PubMed] [Google Scholar]
- Chin MH, Mason MJ, Xie W, Volinia S, Singer M, Peterson C, Ambartsumyan G, Aimiuwu O, Richter L, Zhang J, et al. Induced pluripotent stem cells and embryonic stem cells are distinguished by gene expression signatures. Cell Stem Cell. 2009;5:111–123. doi: 10.1016/j.stem.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho H, Lee C, Kwon Y, Paek JS, Lee S, Hur J, Lee EJ, Roh T, Chu I, Leem S, et al. Induction of pluripotent stem cells from adult somatic cells by protein-based reprogramming without genetic manipulation. Blood. 2010;116:386–395. doi: 10.1182/blood-2010-02-269589. [DOI] [PubMed] [Google Scholar]
- Chou Y, Chen H, Eijpe M, Yabuuchi A, Chenoweth JG, Tesar P, Lu J, McKay RDG, Geijsen N. The growth factor environment defines distinct pluripotent ground states in novel blastocyst-derived stem cells. Cell. 2008;135:449–461. doi: 10.1016/j.cell.2008.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke MJ, Stojkovic M, Przyborski SA. Growth of teratomas derived from human pluripotent stem cells is influenced by the graft site. Stem Cells Dev. 2006;15:254–259. doi: 10.1089/scd.2006.15.254. [DOI] [PubMed] [Google Scholar]
- D'Alise AM, Amabile G, Iovino M, Di Giorgio FP, Bartiromo M, Sessa F, Villa F, Musacchio A, Cortese R. Reversine, a novel Aurora kinases inhibitor, inhibits colony formation of human acute myeloid leukemia cells. Mol. Cancer Ther. 2008;7:1140–1149. doi: 10.1158/1535-7163.MCT-07-2051. [DOI] [PubMed] [Google Scholar]
- Ding S, Schultz PG. A role for chemistry in stem cell biology. Nat. Biotechnol. 2004;22:833–840. doi: 10.1038/nbt987. [DOI] [PubMed] [Google Scholar]
- Doetsch F. A niche for adult neural stem cells. Curr. Opin. Genet. Dev. 2003;13:543–550. doi: 10.1016/j.gde.2003.08.012. [DOI] [PubMed] [Google Scholar]
- Dravid G, Ye Z, Hammond H, Chen G, Pyle A, Donovan P, Yu X, Cheng L. Defining the role of Wnt/beta-catenin signaling in the survival, proliferation, and self-renewal of human embryonic stem cells. Stem Cells. 2005;23:1489–1501. doi: 10.1634/stemcells.2005-0034. [DOI] [PubMed] [Google Scholar]
- Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154–156. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- Finley MF, Devata S, Huettner JE. BMP-4 inhibits neural differentiation of murine embryonic stem cells. J. Neurobiol. 1999;40:271–287. [PubMed] [Google Scholar]
- Ghosh Z, Wilson KD, Wu Y, Hu S, Quertermous T, Wu JC. Persistent donor cell gene expression among human induced pluripotent stem cells contributes to differences with human embryonic stem cells. PLoS ONE. 2010;5:e8975. doi: 10.1371/journal.pone.0008975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna J, Cheng AW, Saha K, Kim J, Lengner CJ, Soldner F, Cassady JP, Muffat J, Carey BW, Jaenisch R. Human embryonic stem cells with biological and epigenetic characteristics similar to those of mouse ESCs. Proc. Natl. Acad. Sci. U.S.A. 2010;107:9222–9227. doi: 10.1073/pnas.1004584107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao J, Daleo MA, Murphy CK, Yu PB, Ho JN, Hu J, Peterson RT, Hatzopoulos AK, Hong CC. Dorsomorphin, a selective small molecule inhibitor of BMP signaling, promotes cardiomyogenesis in embryonic stem cells. PLoS One. 2008;3:e2904. doi: 10.1371/journal.pone.0002904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa K, Zhang P, Wei Z, Pomeroy JE, Lu W, Pera MF. Comparison of reprogramming efficiency between transduction of reprogramming factors, cell-cell fusion, and cytoplast fusion. Stem Cells. 2010;28:1338–1348. doi: 10.1002/stem.466. [DOI] [PubMed] [Google Scholar]
- Huangfu D, Maehr R, Guo W, Eijkelenboom A, Snitow M, Chen AE, Melton DA. Induction of pluripotent stem cells by defined factors is greatly improved by small-molecule compounds. Nat. Biotechnol. 2008;26:795–797. doi: 10.1038/nbt1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichida JK, Blanchard J, Lam K, Son EY, Chung JE, Egli D, Loh KM, Carter AC, Di Giorgio FP, Koszka K, et al. A small-molecule inhibitor of tgf-Beta signaling replaces sox2 in reprogramming by inducing nanog. Cell Stem Cell. 2009;5:491–503. doi: 10.1016/j.stem.2009.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamuro M, Komaki T, Kubota Y, Seita M, Kawamoto H, Yuasa T, Shahid JM, Hassan RARA, Hassan WARA, Nakaji S, et al. Comparative analysis of endoderm formation efficiency between mouse ES cells and iPS cells. Cell Transplant. 2010;19:831–839. doi: 10.3727/096368910X508951. [DOI] [PubMed] [Google Scholar]
- Jasmin Spray DC, Campos de Carvalho AC, Mendez-Otero R. Chemical induction of cardiac differentiation in p19 embryonal carcinoma stem cells. Stem Cells Dev. 2010;19:403–412. doi: 10.1089/scd.2009.0234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji H, Ehrlich LIR, Seita J, Murakami P, Doi A, Lindau P, Lee H, Aryee MJ, Irizarry RA, Kim K, et al. Comprehensive methylome map of lineage commitment from haematopoietic progenitors. Nature. 2010;467:338–342. doi: 10.1038/nature09367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J, Hui C. Hedgehog signaling in development and cancer. Dev. Cell. 2008;15:801–812. doi: 10.1016/j.devcel.2008.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson GL, Lapadat R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science. 2002;298:1911–1912. doi: 10.1126/science.1072682. [DOI] [PubMed] [Google Scholar]
- Jones P. Separation of human epidermal stem cells from transit amplifying cells on the basis of differences in integrin function and expression. Cell. 1993;73:713–724. doi: 10.1016/0092-8674(93)90251-k. [DOI] [PubMed] [Google Scholar]
- Kattman SJ, Witty AD, Gagliardi M, Dubois NC, Niapour M, Hotta A, Ellis J, Keller G. Stage-specific optimization of activin/nodal and BMP signaling promotes cardiac differentiation of mouse and human pluripotent stem cell lines. Cell Stem Cell. 2011;8:228–240. doi: 10.1016/j.stem.2010.12.008. [DOI] [PubMed] [Google Scholar]
- Kim H, Lee J, Kim S. Therapeutic effects of human mesenchymal stem cells on traumatic brain injury in rats: secretion of neurotrophic factors and inhibition of apoptosis. J. Neurotrauma. 2010a;27:131–138. doi: 10.1089/neu.2008.0818. [DOI] [PubMed] [Google Scholar]
- Kim K, Doi A, Wen B, Ng K, Zhao R, Cahan P, Kim J, Aryee MJ, Ji H, Ehrlich LIR, et al. Epigenetic memory in induced pluripotent stem cells. Nature. 2010b;467:285–290. doi: 10.1038/nature09342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo A, Shinozaki K, Shannon JM, Kouskoff V, Kennedy M, Woo S, Fehling HJ, Keller G. Development of definitive endoderm from embryonic stem cells in culture. Development. 2004;131:1651–1662. doi: 10.1242/dev.01044. [DOI] [PubMed] [Google Scholar]
- Lange C, Mix E, Frahm J, Glass Ä, Müller J, Schmitt O, Schmöle A, Klemm K, Ortinau S, Hübner R, et al. Small molecule GSK-3 inhibitors increase neurogenesis of human neural progenitor cells. Neuroscience Letters. 2011;488:36–40. doi: 10.1016/j.neulet.2010.10.076. [DOI] [PubMed] [Google Scholar]
- Lee EK, Bae G, You JS, Lee JC, Jeon YJ, Park JW, Park JH, Ahn SH, Kim YK, Choi WS, et al. Reversine increases the plasticity of lineage-committed cells toward neuroectodermal lineage. J. Biol. Chem. 2009;284:2891–2901. doi: 10.1074/jbc.M804055200. [DOI] [PubMed] [Google Scholar]
- Li W, Wei W, Zhu S, Zhu J, Shi Y, Lin T, Hao E, Hayek A, Deng H, Ding S. Generation of rat and human induced pluripotent stem cells by combining genetic reprogramming and chemical inhibitors. Cell Stem Cell. 2009a;4:16–19. doi: 10.1016/j.stem.2008.11.014. [DOI] [PubMed] [Google Scholar]
- Li W, Zhou H, Abujarour R, Zhu S, Young Joo J, Lin T, Hao E, Schöler HR, Hayek A, Ding S. Generation of human-induced pluripotent stem cells in the absence of exogenous Sox2. Stem Cells. 2009b;27:2992–3000. doi: 10.1002/stem.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin T, Ambasudhan R, Yuan X, Li W, Hilcove S, Abujarour R, Lin X, Hahm HS, Hao E, Hayek A, et al. A chemical platform for improved induction of human iPSCs. Nat. Methods. 2009;6:805–808. doi: 10.1038/nmeth.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo Celso C, Fleming HE, Wu JW, Zhao CX, Miake-Lye S, Fujisaki J, Côté D, Rowe DW, Lin CP, Scadden DT. Live-animal tracking of individual haematopoietic stem/progenitor cells in their niche. Nature. 2009;457:92. doi: 10.1038/nature07434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loebel DAF, Watson CM, De Young RA, Tam PPL. Lineage choice and differentiation in mouse embryos and embryonic stem cells. Dev. Biol. 2003;264:1–14. doi: 10.1016/s0012-1606(03)00390-7. [DOI] [PubMed] [Google Scholar]
- Ludwig TE, Levenstein ME, Jones JM, Berggren WT, Mitchen ER, Frane JL, Crandall LJ, Daigh CA, Conard KR, Piekarczyk MS, et al. Derivation of human embryonic stem cells in defined conditions. Nat Biotechnol. 2006;24:185–187. doi: 10.1038/nbt1177. [DOI] [PubMed] [Google Scholar]
- Lypowy J, Chen I, Abdellatif M. An alliance between Ras GTPase-activating protein, filamin C, and Ras GTPase-activating protein SH3 domain-binding protein regulates myocyte growth. J. Biol. Chem. 2005;280:25717–25728. doi: 10.1074/jbc.M414266200. [DOI] [PubMed] [Google Scholar]
- MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev. Cell. 2009;17:9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin CM, Russell JL, Ferdous A, Garry DJ. Molecular signatures define myogenic stem cell populations. Stem Cell Rev. 2006;2:37–42. doi: 10.1007/s12015-006-0007-x. [DOI] [PubMed] [Google Scholar]
- Masui S, Nakatake Y, Toyooka Y, Shimosato D, Yagi R, Takahashi K, Okochi H, Okuda A, Matoba R, Sharov AA, et al. Pluripotency governed by Sox2 via regulation of Oct3/4 expression in mouse embryonic stem cells. Nat. Cell Biol. 2007;9:625–635. doi: 10.1038/ncb1589. [DOI] [PubMed] [Google Scholar]
- Messina E, De Angelis L, Frati G, Morrone S, Chimenti S, Fiordaliso F, Salio M, Battaglia M, Latronico MVG, Coletta M, et al. Isolation and expansion of adult cardiac stem cells from human and murine heart. Circ. Res. 2004;95:911–921. doi: 10.1161/01.RES.0000147315.71699.51. [DOI] [PubMed] [Google Scholar]
- Mitsui K, Tokuzawa Y, Itoh H, Segawa K, Murakami M, Takahashi K, Maruyama M, Maeda M, Yamanaka S. The homeoprotein Nanog is required for maintenance of pluripotency in mouse epiblast and ES cells. Cell. 2003;113:631–642. doi: 10.1016/s0092-8674(03)00393-3. [DOI] [PubMed] [Google Scholar]
- Miyabayashi T, Teo JL, Yamamoto M, McMillan M, Nguyen C, Kahn M. Wnt/beta-catenin/CBP signaling maintains long-term murine embryonic stem cell pluripotency. Proc Natl Acad Sci U S A. 2007;104:5668–5673. doi: 10.1073/pnas.0701331104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy A, Rossant J, Nagy R, Abramow-Newerly W, Roder JC. Derivation of completely cell culture-derived mice from early-passage embryonic stem cells. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:8424–8428. doi: 10.1073/pnas.90.18.8424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naito AT, Shiojima I, Akazawa H, Hidaka K, Morisaki T, Kikuchi A, Komuro I. Developmental stage-specific biphasic roles of Wnt/beta-catenin signaling in cardiomyogenesis and hematopoiesis. Proc. Natl. Acad. Sci. U.S.A. 2006;103:19812–19817. doi: 10.1073/pnas.0605768103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa M, Koyanagi M, Tanabe K, Takahashi K, Ichisaka T, Aoi T, Okita K, Mochiduki Y, Takizawa N, Yamanaka S. Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nat Biotechnol. 2008;26:101–106. doi: 10.1038/nbt1374. [DOI] [PubMed] [Google Scholar]
- Nichols J, Smith A. Naive and primed pluripotent states. Cell Stem Cell. 2009;4:487–492. doi: 10.1016/j.stem.2009.05.015. [DOI] [PubMed] [Google Scholar]
- Nichols J, Ying Q. Derivation and propagation of embryonic stem cells in serum- and feeder-free culture. Methods Mol. Biol. 2006;329:91–98. doi: 10.1385/1-59745-037-5:91. [DOI] [PubMed] [Google Scholar]
- Niwa H, Burdon T, Chambers I, Smith A. Self-renewal of pluripotent embryonic stem cells is mediated via activation of STAT3. Genes Dev. 1998;12:2048–2060. doi: 10.1101/gad.12.13.2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okita K, Nakagawa M, Hyenjong H, Ichisaka T, Yamanaka S. Generation of mouse induced pluripotent stem cells without viral vectors. Science. 2008;322:949–953. doi: 10.1126/science.1164270. [DOI] [PubMed] [Google Scholar]
- Paige SL, Osugi T, Afanasiev OK, Pabon L, Reinecke H, Murry CE. Endogenous Wnt/beta-catenin signaling is required for cardiac differentiation in human embryonic stem cells. PLoS ONE. 2010;5:e11134. doi: 10.1371/journal.pone.0011134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan G, Thomson JA. Nanog and transcriptional networks in embryonic stem cell pluripotency. Cell Res. 2007;17:42–49. doi: 10.1038/sj.cr.7310125. [DOI] [PubMed] [Google Scholar]
- Pera MF, Tam PPL. Extrinsic regulation of pluripotent stem cells. Nature. 2010;465:713–720. doi: 10.1038/nature09228. [DOI] [PubMed] [Google Scholar]
- Perez-Ilzarbe M, Agbulut O, Pelacho B, Ciorba C, San Jose-Eneriz E, Desnos M, Hagège AA, Aranda P, Andreu EJ, Menasché P, et al. Characterization of the paracrine effects of human skeletal myoblasts transplanted in infarcted myocardium. Eur. J. Heart Fail. 2008;10:1065–1072. doi: 10.1016/j.ejheart.2008.08.002. [DOI] [PubMed] [Google Scholar]
- Qyang Y, Martin-Puig S, Chiravuri M, Chen S, Xu H, Bu L, Jiang X, Lin L, Granger A, Moretti A, et al. The renewal and differentiation of Isl1+ cardiovascular progenitors are controlled by a Wnt/beta-catenin pathway. Cell Stem Cell. 2007;1:165–179. doi: 10.1016/j.stem.2007.05.018. [DOI] [PubMed] [Google Scholar]
- Reinecke H, Zhang M, Bartosek T, Murry CE. Survival, integration, and differentiation of cardiomyocyte grafts: a study in normal and injured rat hearts. Circulation. 1999;100:193–202. doi: 10.1161/01.cir.100.2.193. [DOI] [PubMed] [Google Scholar]
- Reubinoff BE, Pera MF, Fong CY, Trounson A, Bongso A. Embryonic stem cell lines from human blastocysts: somatic differentiation in vitro. Nat. Biotechnol. 2000;18:399–404. doi: 10.1038/74447. [DOI] [PubMed] [Google Scholar]
- Robey IF, Stephen RM, Brown KS, Baggett BK, Gatenby RA, Gillies RJ. Regulation of the Warburg effect in early-passage breast cancer cells. Neoplasia. 2008;10:745–756. doi: 10.1593/neo.07724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolletschek A, Wobus AM. Induced human pluripotent stem cells: promises and open questions. Biol. Chem. 2009;390:845–849. doi: 10.1515/BC.2009.103. [DOI] [PubMed] [Google Scholar]
- Sato N, Meijer L, Skaltsounis L, Greengard P, Brivanlou AH. Maintenance of pluripotency in human and mouse embryonic stem cells through activation of Wnt signaling by a pharmacological GSK-3-specific inhibitor. Nat Med. 2004;10:55–63. doi: 10.1038/nm979. [DOI] [PubMed] [Google Scholar]
- Shi Y, Desponts C, Do JT, Hahm HS, Scholer HR, Ding S. Induction of pluripotent stem cells from mouse embryonic fibroblasts by Oct4 and Klf4 with small-molecule compounds. Cell Stem Cell. 2008a;3:568–574. doi: 10.1016/j.stem.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Shi Y, Do JT, Desponts C, Hahm HS, Schöler HR, Ding S. A combined chemical and genetic approach for the generation of induced pluripotent stem cells. Cell Stem Cell. 2008b;2:525–528. doi: 10.1016/j.stem.2008.05.011. [DOI] [PubMed] [Google Scholar]
- Sirard C, de la Pompa JL, Elia A, Itie A, Mirtsos C, Cheung A, Hahn S, Wakeham A, Schwartz L, Kern SE, et al. The tumor suppressor gene Smad4/Dpc4 is required for gastrulation and later for anterior development of the mouse embryo. Genes Dev. 1998;12:107–119. doi: 10.1101/gad.12.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Teo AKK, Vallier L. Emerging use of stem cells in regenerative medicine. Biochem. J. 2010;428:11–23. doi: 10.1042/BJ20100102. [DOI] [PubMed] [Google Scholar]
- Tesar PJ, Chenoweth JG, Brook FA, Davies TJ, Evans EP, Mack DL, Gardner RL, McKay RD. New cell lines from mouse epiblast share defining features with human embryonic stem cells. Nature. 2007;448:196–199. doi: 10.1038/nature05972. [DOI] [PubMed] [Google Scholar]
- Thakur RK, Kumar P, Halder K, Verma A, Kar A, Parent J, Basundra R, Kumar A, Chowdhury S. Metastases suppressor NM23-H2 interaction with G-quadruplex DNA within c-MYC promoter nuclease hypersensitive element induces c-MYC expression. Nucleic Acids Res. 2009;37:172–183. doi: 10.1093/nar/gkn919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- Vallier L, Alexander M, Pedersen RA. Activin/Nodal and FGF pathways cooperate to maintain pluripotency of human embryonic stem cells. J. Cell. Sci. 2005;118:4495–4509. doi: 10.1242/jcs.02553. [DOI] [PubMed] [Google Scholar]
- Wang H, Hao J, Hong CC. Cardiac Induction of Embryonic Stem Cells by a Small Molecule Inhibitor of Wnt/β-Catenin Signaling. ACS Chem. Biol. 2011;6:192–197. doi: 10.1021/cb100323z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warashina M, Min KH, Kuwabara T, Huynh A, Gage FH, Schultz PG, Ding S. A synthetic small molecule that induces neuronal differentiation of adult hippocampal neural progenitor cells. Angew. Chem. Int. Ed. Engl. 2006;45:591–593. doi: 10.1002/anie.200503089. [DOI] [PubMed] [Google Scholar]
- Warren L, Manos PD, Ahfeldt T, Loh Y, Li H, Lau F, Ebina W, Mandal PK, Smith ZD, Meissner A, et al. Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell Stem Cell. 2010;7:618–630. doi: 10.1016/j.stem.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winnier G, Blessing M, Labosky PA, Hogan BL. Bone morphogenetic protein-4 is required for mesoderm formation and patterning in the mouse. Genes Dev. 1995;9:2105–2116. doi: 10.1101/gad.9.17.2105. [DOI] [PubMed] [Google Scholar]
- Wu MY, Hill CS. Tgf-beta superfamily signaling in embryonic development and homeostasis. Dev. Cell. 2009;16:329–343. doi: 10.1016/j.devcel.2009.02.012. [DOI] [PubMed] [Google Scholar]
- Wu X, Ding S, Ding Q, Gray NS, Schultz PG. Small molecules that induce cardiomyogenesis in embryonic stem cells. J. Am. Chem. Soc. 2004;126:1590–1591. doi: 10.1021/ja038950i. [DOI] [PubMed] [Google Scholar]
- Wurdak H, Zhu S, Min KH, Aimone L, Lairson LL, Watson J, Chopiuk G, Demas J, Charette B, Halder R, et al. A small molecule accelerates neuronal differentiation in the adult rat. Proceedings of the National Academy of Sciences. 2010;107:16542–16547. doi: 10.1073/pnas.1010300107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying QL, Nichols J, Chambers I, Smith A. BMP induction of Id proteins suppresses differentiation and sustains embryonic stem cell self-renewal in collaboration with STAT3. Cell. 2003;115:281–292. doi: 10.1016/s0092-8674(03)00847-x. [DOI] [PubMed] [Google Scholar]
- Ying QL, Wray J, Nichols J, Batlle-Morera L, Doble B, Woodgett J, Cohen P, Smith A. The ground state of embryonic stem cell self-renewal. Nature. 2008;453:519–523. doi: 10.1038/nature06968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying Q, Smith AG. Defined conditions for neural commitment and differentiation. Meth. Enzymol. 2003;365:327–341. doi: 10.1016/s0076-6879(03)65023-8. [DOI] [PubMed] [Google Scholar]
- Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- Yu PB, Hong CC, Sachidanandan C, Babitt JL, Deng DY, Hoyng SA, Lin HY, Bloch KD, Peterson RT. Dorsomorphin inhibits BMP signals required for embryogenesis and iron metabolism. Nat Chem Biol. 2008;4:33–41. doi: 10.1038/nchembio.2007.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Li W, Zhu S, Joo JY, Do JT, Xiong W, Kim JB, Zhang K, Schöler HR, Ding S. Conversion of mouse epiblast stem cells to an earlier pluripotency state by small molecules. J. Biol. Chem. 2010;285:29676–29680. doi: 10.1074/jbc.C110.150599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu S, Li W, Zhou H, Wei W, Ambasudhan R, Lin T, Kim J, Zhang K, Ding S. Reprogramming of Human Primary Somatic Cells by OCT4 and Chemical Compounds. Cell Stem Cell. 2010;7:651–655. doi: 10.1016/j.stem.2010.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu S, Wurdak H, Wang J, Lyssiotis CA, Peters EC, Cho CY, Wu X, Schultz PG. A small molecule primes embryonic stem cells for differentiation. Cell Stem Cell. 2009;4:416–426. doi: 10.1016/j.stem.2009.04.001. [DOI] [PubMed] [Google Scholar]