Abstract
Objective
Comparative studies of social responsiveness, an ability that is impaired in autistic spectrum disorders, can inform our understanding of both autism and the cognitive architecture of social behavior. Because there is no existing quantitative measure of social responsiveness in chimpanzees, we generated a quantitative, cross-species (human-chimpanzee) social responsiveness measure.
Method
We translated the Social Responsiveness Scale (SRS), an instrument that quantifies human social responsiveness, into an analogous instrument for chimpanzees. We then retranslated this "Chimp SRS" into a human "Cross-Species SRS" (XSRS). We evaluated three groups of chimpanzees (n=29) with the Chimp SRS and typical and autistic spectrum disorder (ASD) human children (n=20) with the XSRS.
Results
The Chimp SRS demonstrated strong inter-rater reliability at the three sites (ranges for individual ICCs: .534–.866 and mean ICCs: .851–.970). As has been observed in humans, exploratory principal components analysis of Chimp SRS scores supports a single factor underlying chimpanzee social responsiveness. Human subjects' XSRS scores were fully concordant with their SRS scores (r=.976, p=.001) and distinguished appropriately between typical and ASD subjects. One chimpanzee known for inappropriate social behavior displayed a significantly higher score than all other chimpanzees at its site, demonstrating the scale's ability to detect impaired social responsiveness in chimpanzees.
Conclusion
Our initial cross-species social responsiveness scale proved reliable and discriminated differences in social responsiveness across (in a relative sense) and within (in a more objectively quantifiable manner) humans and chimpanzees.
Keywords: comparative cognition, autism, social responsiveness scale, chimpanzee, cross-species
Introduction
Comparative social cognition research is a valuable approach for studying childhood social development. By determining whether or not certain cognitive functions are conserved across species, comparative social cognition research can elucidate the mechanisms of social behavior that are unique to humans.1 Comparative studies of chimpanzees and humans have identified key causal reasoning abilities -- involving unobservable variables2 -- which we believe are uniquely human and necessary for the emergence of higher social cognitive abilities, such as Theory of Mind (ToM). Cross-species investigations can thus provide novel insight into human neurodevelopmental disorders such as autism, in which both evolutionarily conserved aspects of social relatedness and human-unique abilities, such as ToM, may be impaired. More precise quantitative characterization of highly evolved aspects of non-human primate social behavior may further our understanding of the developmental trajectory of core autistic symptoms, which could improve our diagnostic proficiency, particularly at early ages, and guide future therapies.
In turn, autism, with its characteristic profile of social deficits, provides a model to explore the cognitive architecture underlying social behavior. We and others have proposed that effective social behavior requires a hierarchy of interdependent, social domain-specific and domain-general cognitive abilities.3, 4 These span from evolutionarily conserved cognitive functions not unique to humans, such as gaze following,5 to human-unique cognitive functions, such as higher-order relational reasoning, the ability to simultaneously recognize similarities between the relationships of multiple distinct entities (e.g., as tested with Raven’s Progressive Matrices6). We hypothesize4 that higher-order relational reasoning is necessary but not sufficient for certain human-unique, social domain-specific aspects of cognition, such as theory of mind (ToM),7 which has consistently been shown to be disrupted in autism.8, 9
The occurrence of complex social interactions in many non-human species suggests that a significant degree of social functioning is mediated by evolutionarily conserved cognition that is not unique to humans. Studies examining specific aspects of social behavior across species are thus uniquely suited to tease apart the roles of human-unique and evolutionarily conserved cognitive abilities that are interdependent in humans and that contribute to social function. Clarifying the relationship between domain-general cognition, ToM, and evolutionarily conserved elements of social behavior may provide novel insights for the field of social cognition as well as autism, in which social impairment entails a range of capacities in addition to ToM.
As a first step in this process, we must know which of the variations in social function that characterize autism and that are quantitatively distributed in the entire human population can be measured in non-human primates. Studies of other constructs of social behavior in chimpanzees have demonstrated that individual differences in personality can be reliably detected by surveying human raters10 and that surveys can be used to detect quantitative differences in normal characteristics, such as subjective well-being,11 as well as pathological characteristics, such as psychopathy.12 We therefore designed an initial experiment to test whether quantitative variation in this specific aspect of social function could be reliably captured in chimpanzees, ideal candidates given their close phylogenetic relationship to humans and their sophisticated, well-studied social behavior.
We developed a cross-species measure of social function based on the Social Responsiveness Scale (SRS), a well-validated instrument designed to quantify the severity of social impairment related to characteristic symptoms of autistic spectrum disorders.13–15 SRS scores are continuously distributed in the general human population, demonstrating that the SRS measures quantitative variation in traits that comprise autism.16 Analysis of the factor structure of autistic traits represented by the SRS suggests that the autistic phenotype stems from a heritable, unitary dimension of social function which maps to all three categories of autistic symptomatology, namely reciprocal social behavior, the ability to engage in emotionally appropriate, turn-taking interactions; language development; and stereotypic behaviors, including repetitive mannerisms and/or restricted interests.17, 18 Hence, the SRS operationalizes “social responsiveness” as a behavioral domain encompassing social deficits, communication deficits, and stereotyped behaviors characteristic of autistic spectrum disorders.
To test whether this construct of social responsiveness could be measured in chimpanzees, we translated the human SRS into an initial version applicable to chimpanzees. We distributed this “Chimpanzee SRS” to raters associated with chimpanzee populations at a three distinct sites to evaluate the reliability of our measurements. We hypothesized that chimpanzee social responsiveness would parallel human social responsiveness, both in regard to the distribution of levels of social responsiveness in the chimpanzee population and its factor structure. Further, we asked whether deviance in chimpanzee social behavior would be ascertainable with the Chimpanzee SRS. Our results suggest that our initial cross-species measure is reliable and can detect meaningful variation in social behavior. The development of a cross-species measure of social responsiveness has implications not only for improving understanding of the core features of autism throughout development, but also for understanding the evolutionary conservation of brain systems related to social function.
Method
Subjects
We invited consecutive subjects participating in ongoing studies in the Cognitive & Perceptual Development Lab (Pruett). These children were aged 9–12 (table 1) and carried either a diagnosis of an autistic spectrum disorder (ASD) or no Axis I diagnosis (Typical). Assessments included a brief history, pedigree, the Child Behavior Checklist,19 IQ measures, and the SRS.13 A research ASD diagnosis was confirmed using the Autism Diagnostic Interview-Revised 20 and the Autism Diagnostic Observation Schedule.21 Male controls were over-recruited to approximate the 80% male prevalence in ASDs.
Table 1.
Participant characteristics
| Subjects | ||||
|---|---|---|---|---|
| Species | Group | N | Age (years +/− standard deviation) | % male |
| Chimpanzee | Site 1 | 11 | 22.6 +/− 12.0 | 36.4 |
| Chimpanzee | Site 2 | 7 | 20.3 +/− 0.5 | 14.3 |
| Chimpanzee | Site 3 | 11 | 18.9 +/− 12.8 | 27.3 |
| Human | Typical | 10 | 10.6 +/− 1.2 | 90 |
| Human | ASD | 10 | 10.5 +/− 1.4 | 90 |
| Raters | ||||
| Site | Roles | N | Time at site (years) | |
| 1 | Caretakers, researcher | 5 | 0.25 –5 | |
| 2 | Caretakers, research assistants | 3 | 5–15 | |
| 3 | Zoological manager, caretakers | 5 | 1–8 |
Note: ASD = Autism Spectrum Disorder (human subjects).
29 chimpanzees, aged 6 to 40, were included from three sites: 1) a chimpanzee sanctuary, 2) a laboratory setting, and 3) a public zoo (table 1). Chimpanzees were evaluated by all raters associated with each site. Raters were asked to rate the chimpanzees according to their overall impression of the subjects in their time working with them and were instructed not to share their ratings with each other. Assessments included brief descriptive histories and dominance rankings. Time spent mother-reared in months was noted at Site 3, where six chimpanzees were identified as mother-reared and five as human-reared. Chimpanzees were considered mother-reared if their mothers had demonstrated sufficient maternal competency to hold and feed the infant for at least one year.
Animal work was approved by the Washington University Animal Studies Committee and the Institutional Animal Care and Use Committees at the University of Kentucky and the University of Louisiana at Lafayette. The study was also approved by the Washington University Human Research Protection Office.
General
We reworded questions from the standard SRS,13 a 65-item rating scale that ascertains autistic symptoms as quantitative traits based on social impairments observed by parents or teachers in naturalistic social settings. The majority of SRS questions inquire about patterns of behavior as opposed to simply prompting for the selection of adjective-based descriptions. In translating the human SRS for chimpanzees, we attempted to preserve the original wording as much as possible. Changes included substituting the word “child” with “chimpanzee,” or, in some cases, adding a brief phrase for clarification (e.g., for the item “Is too tense in social situations,” we added, “walks stiff, stiffens or freezes when others approach”). We excluded questions involving verbal language and behaviors not observed or difficult to interpret in chimpanzees, such that thirty-four questions were excluded from the original SRS. Questions 11 and 31 were based on the preschool SRS.22 Two items relating to specific chimpanzee social behaviors were added for a total of thirty-six questions. One item pertained to grooming variability, which is observed in wild and captive chimpanzee communities and which facilitates chimpanzees’ relationships with conspecifics.23, 24 The second item queried whether a chimpanzee showed a species-typical reaction to the loss of a valued resource, as such responses influence social interactions with conspecifics.25 We also retranslated this “Chimpanzee SRS” into a “Cross-Species SRS” (XSRS), in which questions were reworded to apply to humans. One item, question 28 (“Knows when he/she is making too much noise yet continues being noisy”), was removed from the Chimpanzee SRS and XSRS due to concerns for poor face validity. Like the original SRS, scores on the Chimpanzee SRS and XSRS are inversely related to degree of social responsiveness. We distributed the Chimpanzee SRS to raters who worked closely with the chimpanzees (table 1). All raters at each site rated each chimpanzee. We distributed the XSRS to the primary parent of human subjects, either a mother or father, who had also completed a standard SRS. Human scores on the SRS and XSRS were strongly correlated (r=.976, p<.001).
Object handling, a proxy for object intelligence26, was assessed at Site 1 by adapting a previously described protocol of focal animal sampling.27 Chimpanzees were observed handling a variety of objects (e.g. cups, a straw, a tire, shoes) that were introduced every morning and removed every afternoon. A given chimpanzee was observed for a 15 minute period, three times daily for five days. The order of the focal chimpanzee was randomized. The observer recorded the frequency of each object handled and the number of objects handled for the focal chimpanzee. These observations were designed to serve as a preliminary investigation of the relationship between physical and social intelligence. While time spent manipulating objects and frequency of objects handled may not directly measure physical intelligence, those individuals who interact more with objects are more likely to demonstrate advanced physical intelligence, as such behavior allows them to gather potentially important information about their environment. For example, Takeshita and Walraven discuss that object manipulation may be correlated with tool use.26 To test whether variation in Chimpanzee SRS score is independent from variation in chimpanzee object intelligence, we calculated the frequency and number of objects handled during observation periods at Site 1. We found that neither object handling frequency nor number of objects handled correlated with SRS score (frequency: r=.293, p<.382; number: r= −.110, p<.748).
Analyses
We calculated intraclass correlation coefficients (ICC), including both ICC(3,1) which reflects the reliability of individual ratings, as well as ICC(3,k), which reflects the reliability of mean ratings averaged across all raters.28
To explore preliminarily whether observations of specific chimpanzee traits exhibited informative factorial tendencies, we incorporated data from all rater observations for the 35-question Chimpanzee SRS into an initial exploratory principal components factor analysis (PCFA). These thirty-five questions represented all aspects of social responsiveness assessed in the human SRS which could be extrapolated to chimpanzees. This preliminary analysis optimized statistical power, although it employed non-independent observations, an issue resolved in the formal analysis described in the results. The Kaiser-Meyer-Olkin measure29 (KMO) verified good sampling adequacy for the analysis, as it was greater than .7 (KMO=.823). The factor structures in this and the subsequent PCFA were determined by eliminating factors below large inflections in the scree plot. In this analysis, we observed strong evidence for a unitary factor structure, with a first factor accounting for 27% of the variance and remaining factors each explaining less than 10% of the variance.
This result supported the utility of examining survey total scores as an index of social deficiency in a more formal factor analysis. We, therefore, conducted a second PCFA restricted to the twelve items on the Chimpanzee SRS whose endorsement, from the viewpoint of the research team, would best capture the parameters of social variation most specifically related to abnormalities characterizing autism in humans. This 12-question subset reflected a balanced composition of questions addressing all three domains of dysfunction (social, communicative, and stereotyped behaviors). The twelve items displayed good internal consistency (Cronbach’s alpha=.768). We also obtained scores for the analogous twelve items from human SRS data previously collected in a clinical sample of autistic subjects enrolled in a voluntary, national, Internet-based database through the Interactive Autism Network.30 Average scores on the 12-item subset (scores were scaled to match the 65-item human SRS) were significantly different for unaffected versus affected individuals (males: unaffected 21.3+/− 25.4 (standard deviation: SD), affected 110.3+/− 35.0 (SD) [t(1645)=61.7, p<.0000001]; females: unaffected 16.6 +/− 22.5 (SD), affected 109.1+/− 35.5 (SD) [t(272)=36.4, p<.0000001]), supporting the discriminant validity of the 12-item subset.
For the second PCFA, each chimpanzee’s mean item score across all raters constituted a fully-independent case, resulting in a case-to-item ratio of 29:12. KMO was equal to .740. SRS items were considered to load robustly on a factor if their loadings were greater than or equal to an absolute value of 0.6 in both the rotated and unrotated solutions.
Pearson and Spearman correlations explored the relationships between raw total Chimpanzee SRS scores and other variables. For analyses related to age, our sample size was sufficient to detect a moderate effect size, r=0.45, where effect sizes of .1, .3, and .5 are considered small, moderate, and large, respectively.31 For correlations and t-tests related to sex, dominance, and mother-rearing, our sample sizes had sufficient power to detect a large effect size. A student’s t-test was used to compare Chimpanzee SRS scores between different sexes. Analysis of variance (ANOVA) evaluated differences across subject groups and between chimpanzees at individual sites. Tukey HSD test corrected post-hoc analyses for multiple comparisons.
Results
The Chimpanzee SRS demonstrates strong inter-rater reliability at multiple sites
We observed generally strong inter-rater reliability for Chimpanzee SRS measurements at all sites [Site 1: ICC(3,1)=.534, ICC(3,k)=.851; Site 2: ICC(3,1)=.810, ICC(3,k)=.927; Site 3: ICC(3,1)=.866, ICC(3,k)=.970]. On average, raters at Sites 2 and 3 had been acquainted the longest with their chimpanzees, which may have accounted for the lower reliability estimates at Site 1.
We also determined inter-rater reliability for individual items, since questions with negative ICCs at multiple sites would be candidates for modification in future versions of the Chimpanzee SRS. 74% of the items demonstrated positive ICCs at all three sites. At Site 3, no questions obtained a negative ICC. Four items (10, 29, 35, and 36) obtained negative ICCs at Site 1, while five items (9, 15, 24, 26, 17, and 32) obtained negative ICCs at Site 2. No item displayed a negative ICC at multiple sites. Visual inspection of frequency distributions of ratings for each question showed that no items were dichotomously endorsed. These findings suggest that the initial Chimpanzee SRS is reliable in multiple settings both at the level of the entire survey as well as its component questions.
Chimpanzee social responsiveness displays a continuous distribution
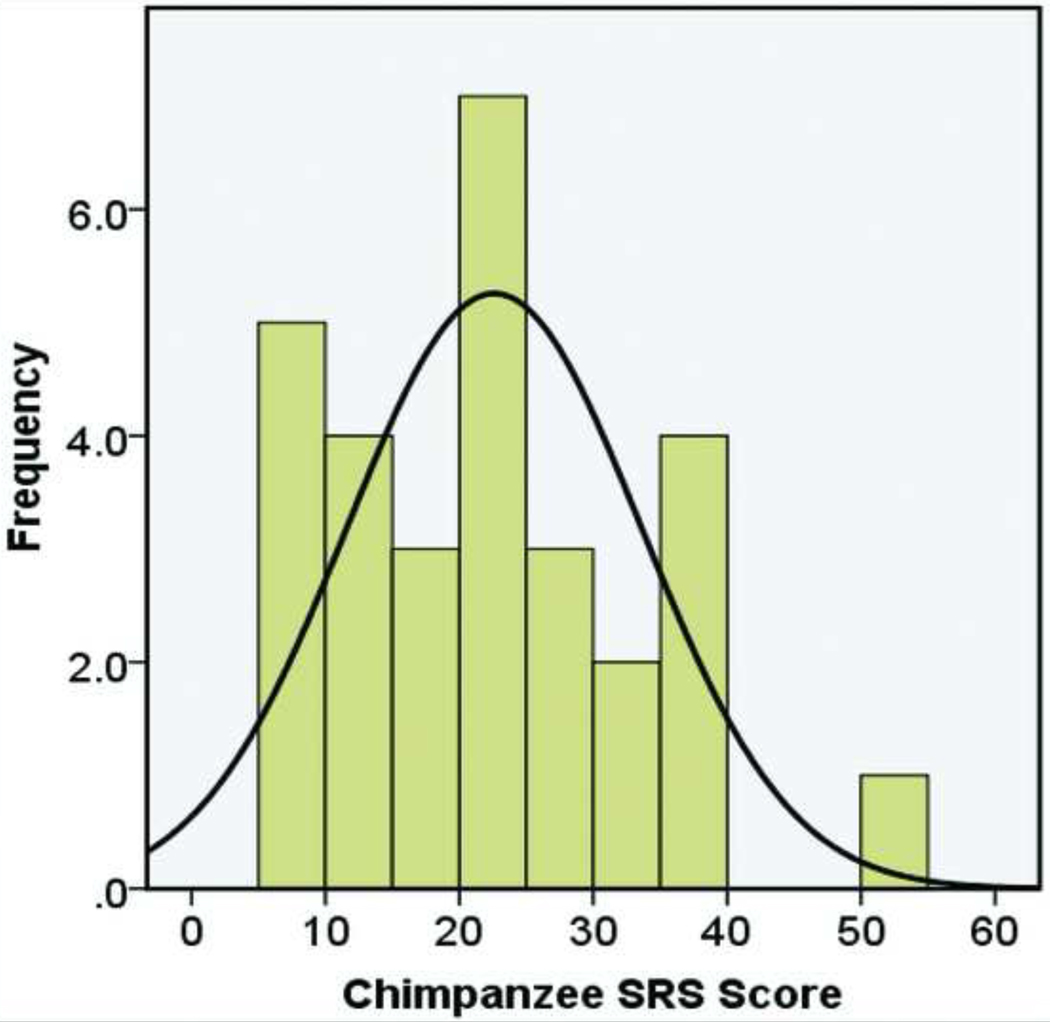
We next explored whether social responsiveness in chimpanzees demonstrated a continuous distribution of scores, as seen in humans. Chimpanzee SRS scores, computed from the average total scores across raters for each chimpanzee, are continuously distributed (Figure 1). The highest score is separated by a small gap, likely secondary to our small sample size. A Kolmogorov-Smirnov analysis of the distribution did not differ significantly from a normal distribution (p<.200). Skew and kurtosis were close to zero (skew=0.523, kurtosis=−0.48) and z-scores for skew and kurtosis were not significantly different from a normal distribution (skew z-score=1.205, kurtosis z-score=−0.568, p>.05 for both).
Figure 1.
Chimpanzee Social Responsiveness Scale scores are continuously distributed. Note: The histogram displays Chimpanzee Social Responsiveness Scale (Chimpanzee SRS) scores pooled from all three groups (n=29). Each chimpanzee’s score represents the averaged score from all raters. Scores range between 7 and 51, with a mean of 22.6 and a median of 22.8. Possible scores range between 0 and 105 for the thirty-five items. Higher scores indicate decreased social responsiveness. The scores are normally distributed, with a normal curve overlaid for illustration.
Chimpanzee social responsiveness displays a unitary factor structure
We performed two exploratory factor analyses using 1) Chimpanzee SRS scores from the full instrument and 2) a 12-question subset of the SRS. Our PCFA on both the full instrument and the 12-item version demonstrated a single factor solution, recapitulating prior results from large scale studies of human social responsiveness using the human SRS. Here, we discuss the analysis of the 12-item version, since it incorporates only independent observations and is therefore more statistically reliable. In this analysis, a first factor explained a majority of the variance, 52%, with the second factor accounting for a much lower percentage of the variance, 13%, and the remaining factors each accounting for less than 10% of the variance (table 2). This result bears striking similarity to published PCFAs for the human SRS.18
Table 2.
Principal Components Factor Analysis of the 12-question subset of the Chimpanzee Social Responsiveness Scale
| Component | Eigenvalue | % of variance |
Cumulative % |
|---|---|---|---|
| 1 | 6.273 | 52.272 | 52.272 |
| 2 | 1.531 | 12.755 | 65.027 |
| 3 | 1.116 | 9.298 | 74.324 |
| 4 | 0.970 | 8.083 | 82.407 |
| 5 | 0.669 | 5.572 | 87.979 |
Four items loaded robustly on the first factor (table 3). As with human data, these items represented symptoms from all three criterion domains of autism, including social behavior (item #8 ‘responds appropriately to other chimpanzees’ vocalizations and facial expressions’), communication (item #19 ‘is socially awkward’), and odd, repetitive behaviors (item #4 ‘behaves in ways which seems strange for his/her age’ and item #27 ‘has repetitive odd behaviors such as flapping or rocking/swaying’). We examined items strongly loading on factor 2 and observed only one item (item #11 ‘when in the playroom, does not attempt to interact’) that has a robust loading both in the unrotated and varimax-rotated solutions. Thus, our analysis supports that chimpanzee and human social responsiveness share a unitary factor structure that is organized along similar dimensions.
Table 3.
First factor loadings for the 12-question subset of the Chimpanzee Social Responsiveness Scale
| Items | Loading without rotation |
Loading with varimax rotation |
|---|---|---|
| Behaves in ways which seem strange for his/her age | 0.875 | 0.813 |
| Is able to communicate feelings to others with gestures | −0.875 | −0.574 |
| Responds appropriately to other chimpanzees' vocalizations and facial expressions | −0.888 | −0.751 |
| Avoids eye contact, or has unusual eye contact | 0.674 | 0.221 |
| When in a group with other chimpanzees, the chimpanzee does not attempt to interact with other chimpanzees | 0.515 | 0.001 |
| Has more difficulty than other chimpanzees with changes in routine | 0.640 | 0.558 |
| Offers comfort to others when they are "sad" (grooming, offers reassurance) | −0.713 | −0.303 |
| Is socially awkward | 0.922 | 0.750 |
| Focuses attention to where others are looking or listening | −0.730 | −0.412 |
| Is too silly or makes a lot of inappropriate noise | 0.377 | −0.143 |
| Has repetitive odd behaviors such as flapping or rocking/swaying | 0.616 | 0.906 |
| Is emotionally distant, doesn't show emotions | 0.641 | −0.206 |
Note: These twelve Chimpanzee Social Responsiveness Scale questions are analogous to human Social Responsiveness Scale questions with high face validity for assessing social responsiveness. Highlighted items load robustly on the first factor in both unrotated and varimax rotated solutions.
Chimpanzee social responsiveness appears independent from several intrinsic and environmental factors
Our sample included a broad age range of chimpanzees, spanning from childhood to old age. Chimpanzee age did not correlate with Chimpanzee SRS scores (r= −.059, p<.761), similar to what is observed in humans.14, 16 We also analyzed the potential effect of sex on social responsiveness. In contrast to humans,16 scores were not significantly different in males versus females [t(27)=−.906, p=.373)].
We also evaluated whether dominance rank correlated with Chimpanzee SRS scores. Our results showed no significant correlation between dominance rank at each of the three sites and social responsiveness [ρ=−.200, p<.555 (Site 1); ρ=−.162, p<.728 (Site 2); ρ=−.032, p<.926 (Site 3)].
Since catastrophic aberrancies in rearing can lead to deficits in human social behavior,32 we computed correlations between “time mother-reared” in months vs. Chimpanzee SRS scores at Site 3; there was no significant correlation (r=−.080, p<.816). We also compared average mean SRS scores of chimpanzees at Site 3 that were mother-reared for at least one year versus chimpanzees that were human-reared. There was no significant difference in mean Chimpanzee SRS scores between the two groups [t(9)=.865, p= .410)].
The Cross-Species SRS Measures Social Responsiveness Within and Across Species
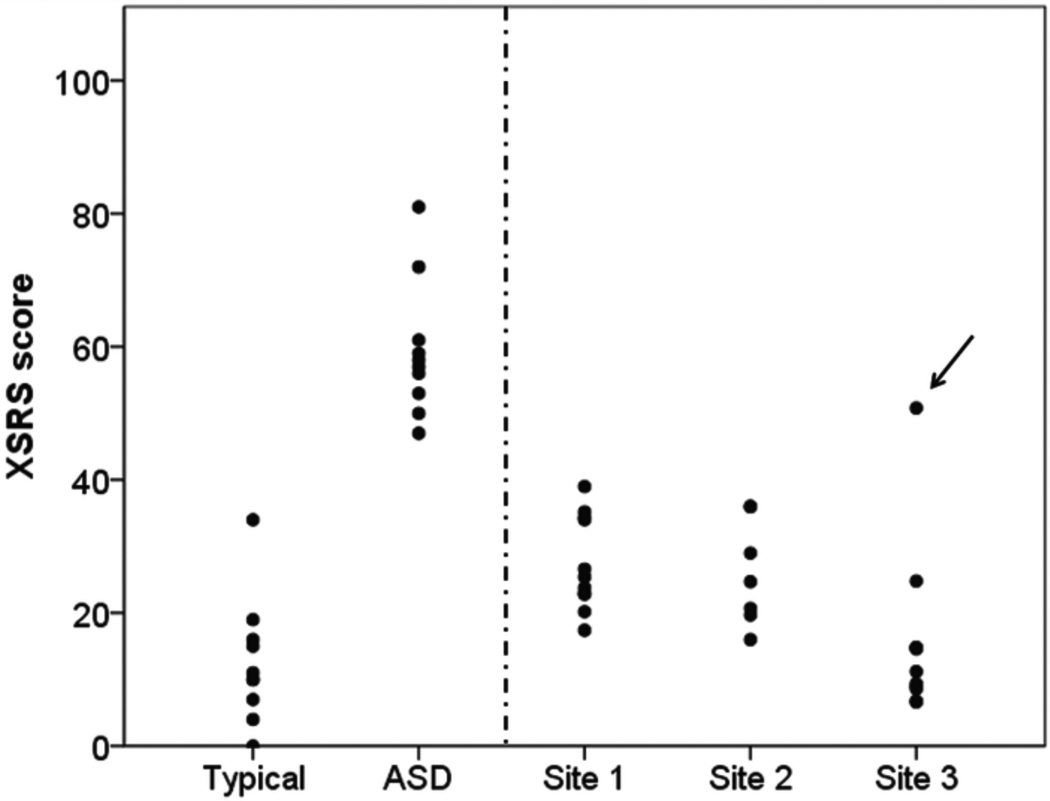
We compared social responsiveness across chimpanzee and human subjects using their total scores on the “Chimpanzee SRS” and “Cross-Species SRS,” both referred to as ‘XSRS’ for simplicity (Figure 2). The human control group has the lowest average score of 12.60 +/− 2.98 (standard error: SE), while the human ASD group has the highest mean score, 59.40 +/− 3.22 (SE), underscoring the ability of the XSRS to distinguish appropriately between typical and ASD children.
Figure 2.
Distribution of Cross-Species Social Responsiveness Scale scores across chimpanzee and human groups. Note: For chimpanzees, each dot represents the Cross-Species Social Responsiveness Scale (XSRS) score for an individual averaged across raters. Dots for human subjects represent the score from a single parent rater. A dotted line separates human and chimpanzee scores, indicating that XSRS scores allow relative but not absolute comparisons across species. The arrow indicates an outlier chimpanzee at Site 3, whose score was significantly higher than all other group members. ASD = autism spectrum disorder.
XSRS scores differed significantly across the five groups [F(4, 44)=36.231; p =.000]. On post-hoc testing, we observed three homogenous subsets whose members were not significantly different. Human controls and Site 3 chimpanzees comprised the subset with the lowest SRS scores (Typical=12.60, Site 3=15.58), followed by a second subset containing the three chimpanzee groups (Site 1=27.44, Site 2=26.01, Site 3=15.58). Unlike Site 3, Sites 1 and 2 showed significantly higher XSRS scores compared to typical humans (p<.05). Finally, ASD subjects occupied a unique subset (ASD=59.40) and differed significantly from all groups (p<.05).
We also examined whether there were significant differences between individual chimpanzees at each site based on individual rater scores. All three sites demonstrated significant differences among their chimpanzees (Site 1: [F(10,44) =2.276; p=.030], Site 2: [F(6,14)=10.454; p=.000], and Site 3: [F(10,44)=23.887; p=.000]). Sites 2 and 3 both exhibited post-hoc homogenous subsets. At site 2, three homogenous subsets contained four chimpanzees each. Site 3 subgroups included: 1) a group with nine chimpanzees, 2) a group with four chimpanzees, and 3) an outlier group with one chimpanzee. These significant differences and post-hoc groupings demonstrate that the Chimpanzee SRS, even in its initial form, has sufficient sensitivity to detect distinct levels of social responsiveness within small chimpanzee communities.
As mentioned, Site 3 had an outlier that was significantly different from all the other chimpanzees on post-hoc analysis (p<.05; figure 2, arrow). Prior to our involvement, staff at that site had noted odd social and repetitive behaviors in this chimpanzee. We repeated our group-wise comparisons without the outlier chimpanzee, again obtaining significant differences across groups [F(4,43)=55.349; p=.000]. In the absence of this outlier, Site 3 mean XSRS scores were the lowest among all the groups, 12.06 +/− 1.73 (SE), although not significantly different from the human controls. Without the outlier, Site 3 no longer fell into a homogenous subset with the other chimpanzee groups, suggesting greater social responsiveness than Sites 1 and 2.
Discussion
We translated the human SRS, which operationalizes social responsiveness as behaviors compromised in autism, into a Chimpanzee SRS. While other rating scales exist for measuring aspects of social behavior in chimpanzees and other non-human primates,10–12 to our knowledge, this is the first example of a scale that reliably quantifies social responsiveness in chimpanzees. We observed similarities between human and chimpanzee social responsiveness that supported the construct validity of the Chimpanzee SRS. Combined use of our Chimpanzee SRS and a human XSRS demonstrated a range of social responsiveness in chimpanzees and allowed preliminary comparisons of social responsiveness within and across species.
Quantification of social responsiveness in chimpanzees
We administered the Chimpanzee SRS to twenty-nine chimpanzees at three sites with a diversity of experiences and living conditions. We were challenged by our small sample, which reflects the relative rarity of accessible chimpanzee communities. Nevertheless, the favorable inter-rater reliability obtained at all three sites suggests that the Chimpanzee SRS quantifies a measurable aspect of social behavior that can be generalized across distinct chimpanzee communities. The lowest ICC(3,1) of .534 at Site 1, fair by common standards33, was still within the range of what is considered good reliability in other chimpanzee research involving human raters, where reliabilities can typically be somewhat lower.10
Even in our small sample, chimpanzee social responsiveness displayed a continuous distribution, as observed for human social responsiveness quantified by the SRS. The existence of a continuous distribution of SRS scores in both species is consistent with an evolutionarily conserved basis for social responsiveness in humans and chimpanzees. By extension, the ability of the Chimpanzee SRS to detect a continuous range of social responsiveness can be interpreted to support the construct validity of the Chimpanzee SRS.
An exploratory factor analysis of a subset of Chimpanzee SRS questions produced a unitary factor structure, similar to that observed in humans. Intriguingly, although autism is a human diagnosis, questions pertaining to all three criterion autistic symptom domains loaded strongly on the predominant first factor, showing that these symptom categories likewise cluster within the primary dimension of social responsiveness in chimpanzees. This finding further supports that our scale is measuring an evolutionarily conserved domain of social behavior in chimpanzees and humans. Deconstructing behavioral dimensions impaired in autism may therefore inform our understanding of social functioning in humans and other species.
Our conclusions regarding construct and discriminant validity of the Chimpanzee SRS will require replication in larger samples to substantiate our results. The same point holds for the 35-question human XSRS and the 12-question subset, which accurately distinguished ASD from typical children in different samples. This result, as well as the conserved factor structure found with the 12-item Chimpanzee SRS, supports the possible utility of a shortened SRS version for both humans and chimpanzees. These observations were unexpected given prior data showing that removing single questions from the original 65-item SRS reduced specificity for detecting children with an ASD.14 Because our human sample enriched for extremes of social responsiveness, replication in a larger, community-wide sample is required to determine whether an abbreviated SRS could be routinely used to evaluate for an ASD.
The relationship of intrinsic and environmental characteristics to social responsiveness in chimpanzees
In humans, social responsiveness emerges early in childhood, as evidenced by social referencing between mothers and infants34 and the reliability and validity of the preschool SRS.22 If elements of social responsiveness are evolutionarily conserved, the most parsimonious model would involve a conserved developmental trajectory as well. Our Chimpanzee SRS data showed no correlation between age and SRS scores, a finding supporting a model in which social responsiveness emerges early in development in species other than humans.
Little data exist about the relationship between chimpanzee dominance and social responsiveness, although a related social characteristic, affiliativeness, is not correlated with dominance.35 Our correlation analyses showed no relationship between dominance rank and Chimpanzee SRS score at any of the three sites, a finding consistent with that study. To the contrary, however, the outlier chimpanzee at Site 3 had the second lowest dominance ranking in its group. Although this represents a single observation, it suggests that significant impairment in social responsiveness may affect dominance rank. Further study is needed with a larger sample to clarify the relationship of dominance rank and social responsiveness.
Surprisingly, we found no correlation between time mother-reared and social responsiveness. Several researchers have explored the impact of depriving young chimpanzees of parenting by their mothers,36, 37 and these groups have repeatedly found that the disruption of mother-rearing provokes abnormal social behavior in chimpanzees. It is worth noting that while such research has explored a variety of social behaviors, our study is the first to analyze a relationship between mother-rearing and social responsiveness, a subset of social behavior. It remains possible that 1) limited mother rearing is not catastrophic for chimpanzee social responsiveness although it disrupts other aspects of chimpanzee social behavior and/or 2) limited mother rearing can be compensated by excellent animal husbandry. It is also worth noting that our small sample size was powered to detect large effect sizes, so important but smaller effects might not have been detected. Substantiation of our preliminary findings will require a larger study sample involving chimpanzees raised in a variety of environments.
One goal in developing the Chimpanzee SRS is to explore the relationship between social responsiveness and domain-general reasoning abilities. We thus measured object handling as a proxy for object intelligence, a non-social form of intelligence. We hypothesized that Chimpanzee SRS scores should be unrelated to object handling, as human SRS scores and IQ have been shown to be unrelated,14, 15 a hypothesis confirmed by our exploratory analysis at Site 1. Although this interpretation is based on a small sample and a crude measure, our data support the concept that social responsiveness, as measured by the Chimpanzee SRS, is independent of higher-order, domain-general reasoning.
Cross-species comparisons of social responsiveness
Our analysis of rater scores revealed clusters of chimpanzees with significantly different levels of social responsiveness. The most striking cluster contained the outlier chimpanzee at Site 3, which had a history of dysfunctional social behavior and the highest Chimpanzee SRS score. In spite of its elevated SRS score, we do not believe that this chimpanzee has autism. First, we do not believe that autism can exist in chimpanzees (see below). Second, conditions other than autism, such as attention-deficit/hyperactivity disorder, are associated with impaired social responsiveness,38 although it is not clear that any human psychiatric disorder fully explains this chimpanzee’s behavior. Nevertheless, the existence of an outlier with observable social deficits demonstrates that our initial scale has sufficient sensitivity to detect abnormal social behavior in chimpanzees.
Social Responsiveness—A specific aspect of chimpanzee social behavior
The entity of “social responsiveness” is derived from social impairment fundamental to autism, a human disorder. While one of our central hypotheses is that cognitive abilities supporting social responsiveness are evolutionarily conserved, we cannot generalize our measure of chimpanzee “social responsiveness” to other classifications of non-human primate social behavior. For example, although several questions on the SRS are related to reciprocal social behavior, defined as “the extent to which a child engages in emotionally appropriate turn-taking social interaction”,14 our Chimpanzee SRS is not designed to measure social reciprocity in chimpanzees, an entity that covers a broad range of exchange behaviors, such as food sharing, and whose existence remains controversial.23, 39, 40
Comparative approaches and anthropomorphism
Another limitation is the potential influence of anthropomorphism, which in theory could misleadingly account for similarities between chimpanzee and human social responsiveness. According to Penn et al.’s “relational reinterpretation hypothesis”,4 recently evolved, human-unique brain systems will tend to attribute higher-order mental states to other animals – regardless of whether the animal possesses such mental states.41 Human raters may be inherently biased to infer certain patterns of social behavior in other species based on their human experience. Such a process could have led raters to make inappropriate attributions to some chimpanzee behaviors, so that the results could reflect a human behavioral construct rather than true behavioral traits in chimpanzees. This issue represents a fundamental dilemma in comparative research involving human rating scales, which nevertheless remain useful research tools due to their efficiency, reliability and reported construct validity.42 In the future, we plan to examine correlations between Chimpanzee SRS scores and behavioral observations as a means to explore further the validity of the scale.
The translation of the human SRS into a Chimpanzee SRS represents a “top-down” approach, as most of the questions on the Chimpanzee SRS were originally derived from behaviors found to have face validity for human social responsiveness. One common critique of top-down approaches is that they may not reflect species-specific expressions of the trait under study.43 Hence, it is possible that the Chimpanzee SRS does not fully account for expressions of social responsiveness unique to chimpanzees. As the current Chimpanzee SRS is a pilot instrument, our primary goal was to determine first whether social responsiveness generalized from humans to a closely related species. One of the goals of our research program is to generate revised versions of this measure with additional questions related to chimpanzee-specific examples of social behavior.
Through our initial attempts to quantify social responsiveness across species, we are in no way suggesting that there is anything less than human about individuals with autism. Likewise, we do not want to be construed as having developed an animal model of autism, another potential misinterpretation. Rather, the long-term aim of our comparative approach is to determine the degree to which social deficits in autism result from compromises to brain systems that are unique to humans, that are conserved across species, or that link conserved and human-unique systems. If autism requires a hit to both unique and conserved systems and/or the “hook-up” between these systems, then autism could only exist in humans. A chimpanzee with deficits in social responsiveness, such as the outlier at Site 3, could appear socially impaired but would not be autistic in the same sense as the human disorder.
Future Directions
Our operational definition of social responsiveness, encapsulated by the XSRS, is preliminary and is intended to evolve. We are aware that current XSRS questions could mean different things for different species, thereby permitting only relative and not absolute comparisons of social responsiveness across species. Two approaches may allow us to address this issue. First, in a follow-up study, chimpanzee experts could rate human children according to chimpanzee social norms, while human experts could rate chimpanzees according to human social norms (we credit colleague Derek Penn with this idea). This procedure would help establish equivalent standards between different species. Second, through intensive research, we could generate species-specific norms for individual questions. For the item “Avoids eye contact,” we could ask, “What are norms for the duration of eye-eye gaze during specific dyadic interactions in humans and chimpanzees?” A recent study 44 showed that human observers can reliably classify and estimate the frequencies of different types of gaze in chimpanzees. Parallel studies in chimpanzees and humans could calibrate norms for eye gaze duration across species. Iterative versions of the XSRS based on research-derived, species-specific norms would allow absolute quantification social responsiveness across species, effectively erasing the dotted line in figure 2.
Conclusions
Our initial efforts to develop a quantitative cross-species social responsiveness scale resulted in a reliable instrument that measured social responsiveness both within and, in a relative sense, across chimpanzees and humans. This approach could be extended to other species. Future versions of the XSRS may help clarify the contributions that evolutionarily conserved versus more recently evolved brain systems make to social functioning, thereby providing insight into the developmental progression of autistic symptoms. An absolute measure of social responsiveness in different species will enable both comparative studies of cognitive factors that contribute to social behavior and comparative neuroimaging studies of systems that sub-serve social behavior, as others have begun to do for other cognitive domains.45 A better understanding of the cognitive architecture and neural basis of complex social behavior may lead to assessments and interventions for autism and other disorders affecting social relatedness that we cannot yet imagine.
Acknowledgments
This research was supported by a James S. McDonnell Foundation Centennial Fellowship to Dr. Povinelli and K12 EY16336 to Dr. Pruett.
We thank Kelly McVey, Emma Squire, and Yi Zhang of Washington University School of Medicine for excellent technical assistance and Derek Penn, affiliate scientist for the University of California Los Angeles and the University of Louisiana, for insightful discussions that contributed to the interpretation of these experiments. We also thank the Primate Rescue Center, the St. Louis Zoo, including Ingrid Porton, and Terri Hunnicutt of the Center for Great Apes.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure: Dr. Constantino receives royalties on the Social Responsiveness Scale, which is published and distributed by Western Psychological Services. Drs. Marrus, Petersen, Povinelli, and Pruett, and Ms. Faughn and Mr. Shuman, report no biomedical financial interest or potential conflicts of interest.
References
- 1.Gosling SD, Graybeal A. Tree thinking: a new paradigm for integrating comparative data in psychology. J Gen Psychol. 2007 Apr;134(2):259–277. doi: 10.3200/GENP.134.2.259-278. [DOI] [PubMed] [Google Scholar]
- 2.Povinelli DJ. Folk Physics for Apes: the chimpanzee's theory of how the world works. Oxford: Oxford University Press; 2000. [Google Scholar]
- 3.Stone VE, Gerrans P. What's domain-specific about theory of mind? Soc Neurosci. 2006;1(3–4):309–319. doi: 10.1080/17470910601029221. [DOI] [PubMed] [Google Scholar]
- 4.Penn DC, Holyoak KJ, Povinelli DJ. Darwin's mistake: Explaining the discontinuity between human and nonhuman minds. Behavioral and Brain Sciences. 2008 Apr;31(2):109–130. doi: 10.1017/S0140525X08003543. discussion 130–178. [DOI] [PubMed] [Google Scholar]
- 5.Povinelli DJ, Eddy TJ. What young chimpanzees know about seeing. Monogr Soc Res Child Dev. 1996;61(3):i–vi. 1–152. discussion 153–191. [PubMed] [Google Scholar]
- 6.Raven JC. Standard Progressive Matrices. New York: Psychological Corporation; Manual. 1958
- 7.Penn DC, Povinelli DJ. On the lack of evidence that non-human animals possess anything remotely resembling a 'theory of mind'. Philos Trans R Soc Lond B Biol Sci. 2007 Apr 29;362(1480):731–744. doi: 10.1098/rstb.2006.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baron-Cohen S, Leslie AM, Frith U. Does the autistic child have a "theory of mind"? Cognition. 1985 Oct;21(1):37–46. doi: 10.1016/0010-0277(85)90022-8. [DOI] [PubMed] [Google Scholar]
- 9.Hill EL, Frith U. Understanding autism: insights from mind and brain. Philos Trans R Soc Lond B Biol Sci. 2003 Feb 28;358(1430):281–289. doi: 10.1098/rstb.2002.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.King JE, Figueredo AJ. The Five-Factor Model plus Dominance in Chimpanzee Personality. Journal of Research in Personality. 1997;31(2):257–271. [Google Scholar]
- 11.King JE, Landau VI. Can chimpanzee (Pan troglodytes) happiness be estimated by human raters? Journal of Research in Personality. 2003;37(1):1–15. [Google Scholar]
- 12.Lilienfeld SO, Gershon J, Duke M, Marino L, de Waal FB. A preliminary investigation of the construct of psychopathic personality (psychopathy) in chimpanzees (Pan troglodytes) J Comp Psychol. 1999 Dec;113(4):365–375. doi: 10.1037/0735-7036.113.4.365. [DOI] [PubMed] [Google Scholar]
- 13.Constantino J, Gruber C. Social responsiveness scale. Los Angeles: Western Psychological Services; 2005. [Google Scholar]
- 14.Constantino JN, Przybeck T, Friesen D, Todd RD. Reciprocal social behavior in children with and without pervasive developmental disorders. J Dev Behav Pediatr. 2000 Feb;21(1):2–11. doi: 10.1097/00004703-200002000-00002. [DOI] [PubMed] [Google Scholar]
- 15.Constantino JN, Davis SA, Todd RD, et al. Validation of a brief quantitative measure of autistic traits: comparison of the social responsiveness scale with the autism diagnostic interview-revised. J Autism Dev Disord. 2003 Aug;33(4):427–433. doi: 10.1023/a:1025014929212. [DOI] [PubMed] [Google Scholar]
- 16.Constantino JN, Todd RD. Autistic traits in the general population: a twin study. Arch Gen Psychiatry. 2003 May;60(5):524–530. doi: 10.1001/archpsyc.60.5.524. [DOI] [PubMed] [Google Scholar]
- 17.Constantino JN, Todd RD. Intergenerational transmission of subthreshold autistic traits in the general population. Biol Psychiatry. 2005 Mar 15;57(6):655–660. doi: 10.1016/j.biopsych.2004.12.014. [DOI] [PubMed] [Google Scholar]
- 18.Constantino JN, Gruber CP, Davis S, Hayes S, Passanante N, Przybeck T. The factor structure of autistic traits. J Child Psychol Psychiatry. 2004 May;45(4):719–726. doi: 10.1111/j.1469-7610.2004.00266.x. [DOI] [PubMed] [Google Scholar]
- 19.Achenbach T. CBLC/6–18 profile for boys - syndrome scales. ASEBA. 2001 [Google Scholar]
- 20.Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. 1994 Oct;24(5):659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- 21.Lord C, Risi S, Lambrecht L, et al. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord. 2000 Jun;30(3):205–223. [PubMed] [Google Scholar]
- 22.Pine E, Luby J, Abbacchi A, Constantino JN. Quantitative assessment of autistic symptomatology in preschoolers. Autism. 2006 Jul;10(4):344–352. doi: 10.1177/1362361306064434. [DOI] [PubMed] [Google Scholar]
- 23.Watts DP. Reciprocity and interchange in the social relationships of wild male chimpazees. Behaviour. 2002;139:343–370. [Google Scholar]
- 24.Watts DP. Male Chimpanzees at Ngogo, Kibale National Park. I. Partner Number and Diversity and Grooming Reciprocity. International Journal of Primatology. 2000;21(2):189–210. [Google Scholar]
- 25.Jensen K, Call J, Tomasello M. Chimpanzees are vengeful but not spiteful. Proc Natl Acad Sci U S A. 2007 Aug 7;104(32):13046–13050. doi: 10.1073/pnas.0705555104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takeshita H, Walraven V. A Comparative Study of the Variety and Complexity of Object Manipulation in Captive Chimpanzees (Pan troglodytes) and Bonobos (Pan paniscus) Primates. 1996 October;37(4):423–441. 1996. [Google Scholar]
- 27.Altman J. Observational study of behavior: sampling methods. Behaviour. 1974;49(17):227–266. doi: 10.1163/156853974x00534. [DOI] [PubMed] [Google Scholar]
- 28.Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychol Bull. 1979 Mar;86(2):420–428. doi: 10.1037//0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- 29.Kaiser HF. An index of factorial simplicity. Psychometrika. 1974;39:31–36. [Google Scholar]
- 30.Constantino JN, Zhang Y, Frazier T, Abbacchi AM, Law P. Sibling recurrence and the genetic epidemiology of autism. Am J Psychiatry. 2010 Nov;167(11):1349–1356. doi: 10.1176/appi.ajp.2010.09101470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cohen J. A power primer. Psychological Bulletin. 1992;112(1):155–159. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- 32.Rutter M, Andersen-Wood L, Beckett C, et al. Quasi-autistic patterns following severe early global privation. English and Romanian Adoptees (ERA) Study Team. J Child Psychol Psychiatry. 1999 May;40(4):537–549. [PubMed] [Google Scholar]
- 33.Fleiss JL. The measurement of interrater agreement. In: Fleiss JL, editor. Statistical methods for rates and proportions. Vol 2. New York: Wiley; 1981. pp. 221–225. [Google Scholar]
- 34.Walden TA, Ogan TA. The development of social referencing. Child Dev. 1988 Oct;59(5):1230–1240. doi: 10.1111/j.1467-8624.1988.tb01492.x. [DOI] [PubMed] [Google Scholar]
- 35.Anestis SF. Behavioral style, dominance rank, and urinary cortisol in young chimpanzees (Pan troglodytes) Behaviour. 2005;142:1245–1268. [Google Scholar]
- 36.Turner CH, Davenport RK, Rogers CM. The effect of early deprivation on the social behavior of adolescent chimpanzees. Am J Psychiatry. 1969 May;125(11):1531–1536. doi: 10.1176/ajp.125.11.1531. [DOI] [PubMed] [Google Scholar]
- 37.Mason WA, Berkson G. Conditions Influencing Vocal Responsiveness of Infant Chimpanzees. Science. 1962 Jul 13;137(3524):127–128. doi: 10.1126/science.137.3524.127. [DOI] [PubMed] [Google Scholar]
- 38.Reiersen AM, Constantino JN, Volk HE, Todd RD. Autistic traits in a population-based ADHD twin sample. J Child Psychol Psychiatry. 2007 May;48(5):464–472. doi: 10.1111/j.1469-7610.2006.01720.x. [DOI] [PubMed] [Google Scholar]
- 39.Brosnan SF, Silk JB, Henrich J, Mareno MC, Lambeth SP, Schapiro SJ. Chimpanzees (Pan troglodytes) do not develop contingent reciprocity in an experimental task. Anim Cogn. 2009 Jul;12(4):587–597. doi: 10.1007/s10071-009-0218-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Melis AP, Hare B, Tomasello M. Do chimpanzees reciprocate received favours? Animal Behaviour. 2008;76:951–962. [Google Scholar]
- 41.Eddy TJ, Gallup GG, Povinelli DJ. Attribution of cognitive states to animals: Anthropomorphism in comparative perspective. Journal of Social Issues. 1993;49(1):87–101. [Google Scholar]
- 42.Gosling SD. From mice to men: what can we learn about personality from animal research? Psychol Bull. 2001 Jan;127(1):45–86. doi: 10.1037/0033-2909.127.1.45. [DOI] [PubMed] [Google Scholar]
- 43.Uher J. Comparative personality research: methodological approaches. European Journal of Personality. 2008;22:427–455. [Google Scholar]
- 44.Bethell EJ, Vick SJ, Bard KA. Measurement of eye-gaze in chimpanzees (Pan troglodytes) Am J Primatol. 2007 May;69(5):562–575. doi: 10.1002/ajp.20376. [DOI] [PubMed] [Google Scholar]
- 45.Vincent JL, Patel GH, Fox MD, et al. Intrinsic functional architecture in the anesthetized monkey brain. Nature. 2007;447(7140):46–47. doi: 10.1038/nature05758. [DOI] [PubMed] [Google Scholar]