Abstract
AIM: To document unusual findings in appendectomy specimens.
METHODS: The clinicopathological data of 5262 patients who underwent appendectomies for presumed acute appendicitis from January 2006 to October 2010 were reviewed retrospectively. Appendectomies performed as incidental procedures during some other operation were excluded. We focused on 54 patients who had unusual findings in their appendectomy specimens. We conducted a literature review via the PubMed and Google Scholar databases of English language studies published between 2000 and 2010 on unusual findings in appendectomy specimens.
RESULTS: Unusual findings were determined in 54 (1%) cases by histopathology. Thirty were male and 24 were female with ages ranging from 15 to 84 years (median, 32.2 ± 15.1 years). Final pathology revealed 37 cases of enterobiasis, five cases of carcinoids, four mucinous cystadenomas, two eosinophilic infiltrations, two mucoceles, two tuberculosis, one goblet-cell carcinoid, and one neurogenic hyperplasia. While 52 patients underwent a standard appendectomy, two patients who were diagnosed with tuberculous appendicitis underwent a right hemicolectomy. All tumors were located at the distal part of the appendix with a mean diameter of 6.8 mm (range, 4-10 mm). All patients with tumors were alive and disease-free during a mean follow-up of 17.8 mo. A review of 1366 cases reported in the English literature is also discussed.
CONCLUSION: Although unusual pathological findings are seldom seen during an appendectomy, all appendectomy specimens should be sent for routine histopathological examination.
Keywords: Appendicitis, Carcinoid, Unusual findings, Goblet cell carcinoid, Enterobius vermicularis, Mucocele
INTRODUCTION
Appendicitis is one of most common acute surgical conditions of the abdomen, and an appendectomy is one of the most frequently performed operations worldwide. The incidence of acute appendicitis roughly parallels that of lymphoid development, with peak incidence in the late teens and twenties. Obstruction of the lumen is the dominant factor in acute appendicitis, and although fecoliths and lymphoid hyperplasia are the usual cause of obstructions, some unusual factors could also be involved[1-128]. Obstruction may be due to enterobiasis[1,4,7,29], ascariasis[57,92-94], balantidiasis[2,92], taeniasis[14,18], actinomycosis[32-38], schistosomiasis[2,8,42-51,57], amebiasis[7,84-86,90], trichuriasis[52,57], Blastocystis hominis[20], tuberculosis (TB)[8,23,53-55,57], carcinoid tumor[1-3,5,9,12,26,28,31,95], goblet-cell carcinoid (GCC)[5,12,21,25], primary or secondary adenocarcinoma[16,31], cystadenocarcinoma[31], lymphoma[2], dysplastic changes[2], endometriosis[1,16,58-69], granulomatous diseases[31,32], gastrointestinal stromal tumor (GIST)[71,72,103], mucocele[1-3,52], villous adenoma[24,39,56], tubulovillous adenoma[24], tubular adenoma[24,31], leiomyoma[2], eosinophilic granuloma[32,52], or neurogenic appendicopathy[30].
MATERIALS AND METHODS
Between January 2006 and October 2010, 5262 patients with presumed acute appendicitis underwent surgical treatment at Diyarbakir Education and Research Hospital, Turkey. Appendectomies performed as an incidental procedure during some other operation were excluded. The data of 54 (1%) patients who were pathologically reported to have unusual appendix findings were retrospectively collected. The original pathology specimens with unusual findings were evaluated again by an experienced pathologist. The records analysis was composed of the patient’s age, gender, clinical presentation, operative reports, radiological tools, pathological report, and follow-up. The length of follow-up was calculated by months from the date of diagnosis until the last clinical information available on the patient up to November 2010.
English medical language PubMed and Google Scholar database searches were conducted for case reports, retrospective and prospective studies, and literature reviews relating to “unusual causes of appendicitis”. Keywords used were parasites, enterobiasis, schistosomiasis, amebiasis, yersiniosis, strongyloidiasis, actinomycosis, TB, idiopathic granulomatous appendicitis, Crohn’s disease, endometriosis, appendicular adenocarcinoma, carcinoid, GCC, mucocele, mucinous cystadenoma, lymphoma, polypoid lesion, appendectomy, and appendicitis. The search included all articles from 2000 until November 2010. Patients who had undergone an operation for presumed acute appendicitis and had “unusual findings” pathology were included in the study, whereas articles that provided inconclusive information about patients and those in which the patients could not be reached were excluded. Additionally, appendicitis cases that developed due to foreign bodies were also excluded[1-128].
RESULTS
In total, 5262 appendectomies were performed with a diagnosis of acute appendicitis at Diyarbakir Education and Research Hospital from January 2006 through October 2010. All patients were diagnosed clinically with acute appendicitis on the basis of physical and laboratory examinations. Of all appendectomies performed, 54 (1%) specimens revealed incidental abnormal histopathological diagnoses. The general characteristics of these 54 patients are summarized in Table 1. Thirty of the patients were male and 24 were female with ages ranging from 15 to 84 years (median, 32.2 ± 15.1 years). Thirty-seven of the 54 patients revealed Enterobius vermicularis, five a carcinoid tumor, six a mucinous cystadenoma (two were mucoceles), two TB, and two eosinophilic infiltration, and two each were diagnosed with GCCs and neurogenic hyperplasia (Figure 1). While 52 patients underwent a standard appendectomy, two patients, who were preoperatively diagnosed with tuberculous appendicitis, had a right hemicolectomy. All patients with malignant tumors were diagnosed clinically with acute appendicitis, and none of them had symptoms of carcinoid syndrome or were preoperatively diagnosed with an appendicular tumor. After pathological confirmation of the diagnosis, the patients were referred to our clinic for staging. Staging included abdominal ultrasonography (US), computed tomography (CT), and 24-h urinary 5-hydroxyindoleacetic acid levels. After staging, all patients were followed up at the outpatient clinic every 3 mo for the first year. All patients with tumors were alive and disease-free during a mean follow-up of 17.8 mo. The clinicopathological characteristics of six patients with tumors are summarized in Table 2.
Table 1.
General characteristics of the 54 patients with abnormal pathological findings
| Patients’ characteristics | Results | Rate (%) |
| Age (yr) (range) | 32.2 ± 15.1 (15-84) | |
| Sex | ||
| Male | 30 | 55.50 |
| Female | 24 | 44.50 |
| WBC (K/UL) (range) | 11.7 ± 4.9 (4.5-26.7) | |
| Histopathologic findings | 54 | |
| E.vermicularis | 37 | 68.50 |
| Tuberculosis | 2 | 3.70 |
| Carcinoid | 5 | 9.20 |
| Goblet-cell carcinoid | 1 | |
| Mucocele | 2 | 3.70 |
| Mucinous cystadenoma | 4 | 7.40 |
| Eosinophilic infiltration | 2 | 3.70 |
| Neurogenic hyperplasia | 1 | |
| Follow-up (mo) (range) | 10.4 ± 12.4 (1-54) | |
| Surgical Approach | ||
| Appendectomy | 52 | 96.30 |
| Right hemicolectomy | 2 | 3.70 |
| Recurrence | 0 | |
Figure 1.
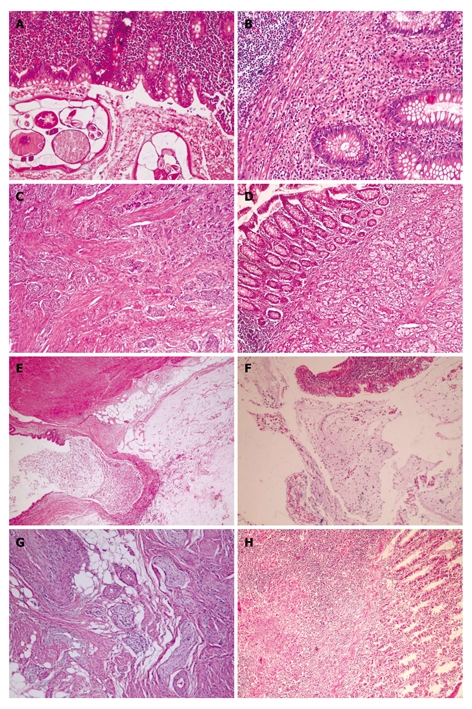
Unusual histopathologic findings. A: Adult of E.Vermicularis in appendices (HE, × 200); B: Eosinophilic appendicitis: diffuse eosinophilic infiltrate in lamina propria (HE, × 200); C: Carcinoid tumor of classic type is formed by solid nest of small monotonous cells with occasional acinar formation (HE, × 100); D: Microglandular goblet cell carcinoma. Acute appendicitis with a diffusely infiltrating goblet cell neoplasm. tumor cells infiltrated muscularis propria (HE, × 200); E: Mucosel. Dilatation of lumen by mucinous secretion, thin appendiceal wall. Mucin is protruding into surrounding fatty tissue (HE, × 40); F: Mucinous cystadenoma of appendix. Typical epithelium of a cystadenoma with pseudostratified, columnar cells containing elonged, crowded, hyperchromatic nuclei and scattered goblet cells with mucus in cavity (HE, × 100); G: Neurogenous hyperplasia of appendix. The proliferating spindle cells shown in this photography (HE, × 200); H: Tuberculous appendicitis. Granuloma which contain a caseating center surrounded by epithelioid cells, lymphocytes and histiocytes. A giant cell is present in the granuloma (HE, × 20).
Table 2.
Clinicopathological characteristics of the six patients with primary appendicular tumors
| Age | Sex | Tumor size (mm) | Location | Treatment | Pathology | Parietal spread | Follow-up (mo) |
| 43 | F | 5 | Distal | Appendectomy | Carcinoid | Serosa | 54 |
| 42 | F | 10 | Distal | Appendectomy | Carcinoid | Serosa | 33 |
| 23 | F | 6 | Distal | Appendectomy | Carcinoid | Subserosa | 15 |
| 39 | M | 4 | Distal | Appendectomy | Carcinoid | Submucosa | 1 |
| 36 | M | 10 | Distal | Appendectomy | Goblet cell | M.Propria | 3 |
| 26 | M | 6 | Distal | Appendectomy | Carcinoid | Subserosa | 1 |
A histopathological examination of patients with E. vermicularis revealed 12 with acute inflammation and 25 with no evidence of any pathological change. After obtaining the pathology reports, the patients with oxyuris were prescribed a single oral dose of 100 mg mebendazole, which was repeated 7-10 d later. All patients with oxyuris were asymptomatic on follow-up (mean, 7.2 mo; range, 1-54 mo).
Two female patients (18 and 48 years old, respectively) with tuberculous appendicitis received antitubercular therapy during the preoperative period. A right hemicolectomy was performed in patients with an acute abdomen in the follow-up, considering the intraoperative findings. We have presented the details of these two cases in a previous article[53].
Results of the literature review
Using the PubMed and Google Scholar databases, 128 studies published between January 2000 and November 2010 were compatible with our criteria. Fifty-one of these were written as original articles (50 retrospective and 1 prospective), 67 as case reports, eight as letters to the editor, and two as case series. When we looked at the countries in which the articles were prepared, 59 were from Europe, 40 from Asia, 19 from the Americas, six from Africa, and four were from Australia. In total, 80 698 cases were discussed in these articles, and all patients who were operated on had presumed acute appendicitis. Unusual findings were detected in 1366 (1.7%) of the cases with or without histopathologically acute appendicitis in their appendectomy specimens. We have summarized the causes that we qualified as “unusual findings in appendectomy specimens” in Table 3. As shown in the table, causes such as enterobiasis, schistosomiasis, amebiasis, and carcinoid tumor comprised 75.7% of all cases. The etiological (tumoral and non-tumoral causes) distribution of the 1366 patients by continent is summarized in Figure 2 to demonstrate the effects of geographic and sociocultural differences.
Table 3.
Distribution of the 1366 cases defined as “unusual findings” according to etiological causes
| Total patients | 1366 (1366/80 698 = 1.7%) | |
| Unusual findings | 1366 | 1.7% |
| Enterobius vermicularis | 389 | 28.4% |
| Carcinoid | 287 | 21.0% |
| Schistosomiasis | 174 | 12.7% |
| Amoebic appendicitis | 118 | 8.6% |
| Mucinous cystadenoma (+mucocele) | 72 | 5.2% |
| Ascaris lumbricoides | 39 | 2.8% |
| Tuberculous appendicitis | 34 | 2.5% |
| Endometriosis | 41 | 3.0% |
| Goblet-cell carcinoid | 28 | 2.0% |
| Trichuris trichiura | 22 | 1.6% |
| Idiopathic granulomatous appendicitis | 35 | 2.5% |
| Crohn disease | 18 | |
| Lymphoma | 14 | |
| Primary adenocarcinoma | 11 | |
| Mucinous cystadenocarcinoma | 9 | |
| Actinomycosis | 8 | |
| Melanosis | 8 | |
| Secondary adenocarcinoma | 7 | |
| Dysplastic change | 7 | |
| Villous adenoma | 6 | |
| Hyperplastic polyp | 5 | |
| Taeniasis | 8 | |
| GIST (+leiomyoma) | 5 | |
| Balantidium coli | 3 | |
| Tubulovillous adenoma | 3 | |
| Eosinophilic granuloma | 3 | |
| Neurogenic hyperplasia | 2 | |
| Tubular adenoma | 2 | |
| Leukaemia | 4 | |
| Blastocystis hominis | 1 | |
| Adenovirus | 1 | |
| Strongyloides stercoralis | 1 | |
| Yersinia enterocolitica | 1 | |
Figure 2.
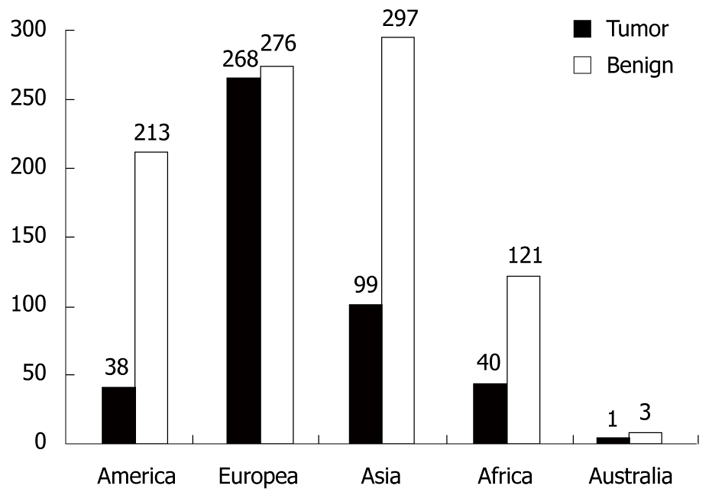
Worldwide distribution of the 1366 cases defined as ''unusual findings''. Tumor: Carcinoid, goblet cell carcinoid, mucocele, appendix adenocarcinoma, lymphoma, mucinous cystadenoma and adenocarcinoma, polypoid lesions, leukemia, gastrointestinal stromal tumor, dysplastic change; Benign: Non-tumoral causes.
DISCUSSION
Acute appendicitis is the most common general surgical emergency, and obstruction of the appendiceal lumen seems to be essential for developing an appendiceal infection. Although fecaliths and lymphoid hyperplasia are the usual causes of the obstruction, some unusual factors could also be involved[2,8,16,57].
Appendiceal tumors, occurring in less than 3% of all appendectomies, are rarely associated with clinical manifestations; they are frequently recognized either during an operation or the pathological examination. Malignant tumors of the appendix include carcinoids, GCCs, lymphomas, mucoceles, primary adenocarcinomas, and mucinous cystadenocarcinomas. Benign tumors of the appendix consist of tubular adenomas, villous adenomas, leiomyomas, neuromas, and lipomas[2,5,31].
An appendiceal carcinoid tumor is considered the most common type of appendiceal primary malignant lesion and accounts for almost 60% of all appendiceal tumors[28]. An appendiceal carcinoid tumor is found in 0.3%-2.27% of patients undergoing an appendectomy. Characteristics of all appendiceal carcinoids predicting aggressive behavior include tumor size, histological subtype, and mesoappendiceal involvement. The tumors are smaller than 1 cm in 70%-95% of cases[26-28]. The calculated risk of metastasis from tumors 1 cm or smaller is nearly zero and therefore may be managed with a simple appendectomy. An increase in metastasis risk of up to 85% occurs with a tumor of 2 cm or larger. An appendiceal carcinoid tumor larger than 2 cm should be managed with a formal right hemicolectomy[1,3,5,6,9-13,16,26,28].
GCCs, also known as adenocarcinomas and first described by Gagne in 1969, are uncommon primary tumors of the vermiform appendix characterized by dual endocrine and glandular differentiation[129]. Whether GCCs represent a morphological variant of appendiceal classical carcinoid or a mucin-producing adenocarcinoma is a matter of conjecture[12]. GCCs account for 2% of primary appendiceal malignancies. Most tumors are less than 2 cm in diameter and 20% metastasize to the ovaries. Recent studies suggest that GCCs have biological and immunohistochemical profiles more similar to adenocarcinomas than to classical carcinoids, which may explain their aggressive behavior and therefore requirement for more extensive treatment[12]. A right hemicolectomy is generally advised if any of the following features are present: tumors greater than 2 cm, involvement of resected margins greater than 2 mitoses/10 high-power fields, extension of the tumor beyond the serosa, lymphovascular invasion, or lymph node metastases[5,12,21,25]. In our series, one patient had a GCC tumor located distally in the appendix that measured 1 cm in diameter. The patient was advised to undergo a right hemicolectomy, but he refused the procedure.
Mucinous cystadenoma is a rare tumor of the appendix associated with cystic dilatation, to which the more general term of mucocele has been applied. A mucocele of the appendix denotes an obstructive dilatation of the appendiceal lumen due to abnormal accumulation of mucus, which may be caused by a retention cyst, endometriosis, mucosal hyperplasia, cystadenoma, or a cystadenocarcinoma. The incidence of mucocele ranges from 0.2% to 0.3% of all appendectomy specimens. Mucoceles are often asymptomatic and discovered as incidental findings at appendicectomy, or during laparotomy for another indication or at histological examination of an operative specimen. However, mucoceles may be diagnosed clinically from features of acute appendicitis. Appendectomy is the standard of care for mucinous cystadenoma, whereas a cystadenocarcinoma requires a right hemicolectomy. Because of the high association of mucinous cystadenoma with colon and ovarian malignancy, follow-up CT, US, and colonoscopy examinations must be performed during the postoperative period[1-3,5,16,25,27,31,52,106,107].
Mucinous cystadenocarcinoma of the appendix, also known as a mucinous adenocarcinoma or malignant mucocele, constitutes a rare malignancy of the appendix and is often associated with a second malignancy of the gastrointestinal (GI) tract. The most common type of presentation is that of acute appendicitis. The diagnosis of mucinous adenocarcinoma of the appendix is usually given after an appendectomy, or other explorative surgical procedure, and consequent pathological evaluation of the appendiceal specimen[5,25,31,108].
Primary adenocarcinoma of the appendix is an extraordinarily rare tumor, and its incidence was 0.01% (11 of 80 698 cases) in our literature review. Adenocarcinomas behave aggressively and in a fashion similar to that of colonic adenocarcinomas, so in the case of an appendicular adenocarcinoma, oncologic resection with right hemicolectomy is the treatment of choice[2,16,23,31,105].
The GI tract is the most common site for extranodal lymphomas and accounts for 30%-45% of all extranodal cases. The stomach is the most commonly involved organ followed by the small intestine, colon, and esophagus. The incidence of primary appendiceal lymphoma has been estimated at 0.015%-0.022% of all appendiceal specimens. An appendiceal lymphoma usually presents in the second and third decades of life, usually manifests as acute appendicitis, and is often diagnosed postoperatively by histopathology. Therapy guidelines for primary appendiceal lymphomas are unclear because of their rarity. Our literature review revealed 14 lymphoma cases with clinical evidence of acute appendicitis; 12 of these were of B-cell origin, whereas two were of T-cell origin[2,8,97-101,109-112].
Leukemia can involve the GI tract but rarely involves the appendix. Although appendicitis is a known complication in patients with leukemia, leukemic cell involvement in the appendix is extremely rare. When the leukemia involves soft tissue including the appendix, it is called granulocytic sarcoma. The incidence of leukemic appendicitis was 0.005% (4 of 80 698 cases) in our literature review. Surgical management of patients with leukemia and acute abdomen has not been advocated because of the high rate of operative mortality. However, some support exists for surgically managing appendicitis as the most effective method of therapy in acute leukemia cases. Systemic chemotherapy is necessary prior to additional surgery in patients with leukemia[113-115].
GISTs, which occur most commonly in the stomach (60%) and the small bowel (30%), are the most common primary mesenchymal neoplasms of the GI tract. GISTs, known as leiomyoma or leiomyosarcoma before 1983, primary to the vermiform appendix are exceptionally rare, with only eight cases reported so far[2,71,72,103]. Five out of eight patients were operated due to acute appendicitis symptoms. The size of the mass and degree of mitotic activity play a crucial role in tumor behavior and recurrence development. Therefore, when approaching the appendix for GIST tumors, tumor location should be evaluated along with tumor size and mitotic activity.
Enterobius vermicularis, also known as pinworm or oxyuris, is a widespread parasitic infection estimated to affect up to 200 million people worldwide. The association of oxyuris and appendicitis was first made in the late 19 century, when Still initially documented this organism in the appendix lumen. While the reported incidence of pinworm in appendectomy specimens of patients with presumed appendicitis ranged from 0.2% to 41.8%, the reported rates of inflammation in specimens from appendices infested with pinworm ranged from 13% to 37%[4,7,14,29]. Patients must receive antihelminthic treatment because the appendectomy treats only the consequence and not the cause of the disease. An E. vermicularis infestation is treated with an oral dose of mebendazole, which is repeated in 1-2 wk[1,2,4,7,11,14,16-20,22,29,52,57,92,93].
TB may affect all tissues and organs in the body, but it most frequently involves the lungs. The GI system is ranked sixth among all extrapulmonary involvements. TB may affect all of the segments of the GI system, from the mouth to anus. However, the ileum and ileocecal region are the sites most commonly involved, followed by the colon and vermiform appendix. The appendix may be affected secondarily to ileocecal TB, but appendicular TB may occur in an even rarer primary form without any evidence of the disease elsewhere. The reported incidence of appendicular TB varies from 0.1% to 3.0% among all appendectomies performed. An accurate diagnosis is usually established after histopathological examination of a specimen. Classic histopathological analysis of an appendectomy specimen usually reveals the presence of caseating granulomas and Langhans giant cells, suggesting TB of the appendix. Although some studies have reported that treatment is not necessary for the primary disease and that appendectomy alone is sufficient, no consensus has been reached. When we reviewed the literature, 34 cases of patients undergoing an appendectomy with presumed appendicitis have been published in the last decade, including our own two cases[23,32,53-55,57].
Actinomycosis is an uncommon chronic infectious disease. Common sites of involvement include the cervicofacial, thoracic, and abdominopelvic regions. In abdominal actinomycosis, the ileocecal region including the appendix is the most commonly involved site. A correct diagnosis can be made by culture or histopathological examination, although a definitive diagnosis of actinomycosis requires microscopic proof of either the pathogen itself or the presence of specific sulfur granules. After the diagnosis has been confirmed, the general therapeutic recommendation is to initiate treatment with intravenous antibiotic therapy for 2-12 mo. Eight cases of patients undergoing an appendectomy with presumed appendicitis have been published in the last decade[32-38].
Taeniasis, a well-known worm infection, is characterized by the presence of the helminth in the intestine. Infection is generally recognized when a segment of the parasite appears in the stool. The occurrence of Taenia spp. in the appendix is so rare that the situation invites a case report. In our literature review, Taenia was found in only five of the cases operated on for presumed acute appendicitis. In cases of taeniasis, specific species identification is not required for treatment, as patients are treated with a single dose of praziquantel[14,18,93,127,128].
Amebiasis is an infection of the large intestine caused by Entamoeba histolytica, which affects 10% of the world population and has a worldwide distribution. This parasite is occasionally found in the appendix, usually in the lumen without accompanying inflammation, but is rarely associated with acute appendicitis. A preoperative diagnosis of amebic appendicitis is almost impossible because no clinical features or diagnostic laboratory tests distinguish amebic from bacterial appendicitis, other than a stool examination. The clinical picture presented in this report represents a typical case of amebic appendicitis with a good outcome after surgical resection and treatment with metronidazole[84,85,87-93].
Schistosomiasis, also known as bilharziasis and most commonly caused by Schistosoma haematobium, only rarely leads to appendicitis, even in nations in which schistosomiasis is endemic. The pathogenesis is most probably due to a periappendicular granulomatous reaction of the host against the schistosome. Inflammation and repair causes scarring and strictural deformation of the appendiceal wall, leading to luminal obstruction and acute appendicitis. Histologically, appendices may show transmural inflammation rich in eosinophils, with a granulomatous reaction to ova. Treatment for schistosomal appendicitis consists of an appendectomy and administration of praziquantel[2,8,13,16,32,40-52,57,93,125,126].
Ascaris lumbricoides, also known as roundworm, is one of the most common human helminthic diseases worldwide. The highest prevalence of ascariasis occurs in tropical and semitropical countries. The domain of the worm extends from the stomach to the ileocecal valve; 99% of worms inhabit the jejunum and proximal ileum, and it is rarely seen in the appendix. Appendicitis due to migration of roundworm into the appendix is still debatable because the symptoms of this migration may simulate appendicitis but rarely cause it[57,92,94].
Because parasites such as Balantidium coli[2,92], Blastocystis hominis[20], Trichuris trichiura[52,57,92], and Strongyloides stercoralis[121] have few causative roles, interpreting their pathogenesis is difficult. A final diagnosis should be established with a histopathological evaluation of all three parasites, and antihelminthic treatment should be administered after the appendectomy.
Endometriosis is defined as the presence of ectopic endometrial tissue outside the lining of the uterine cavity. Many women of reproductive age suffer from this disease, but its occurrence in the GI tract is rare. Intestinal endometriosis is classified as external endometriosis and occurs in only about 10% of women with endometriosis. Most intestinal endometriosis occurs in the rectum and sigmoid colon but rarely in the appendix. Appendiceal endometriosis is usually asymptomatic, but it occasionally causes appendicitis, perforation, and intussusception. The diagnosis of appendiceal endometriosis is based on the histological presence of endometrial tissue in the specimen. The treatment strategy consists mainly of surgery and hormone therapy[1,16,27,57-69,102].
The incidence of granulomatous appendicitis (GA), a rare condition that may be discovered incidentally in a patient with a clinical presentation of acute appendicitis, ranges from 0.31% to 0.95%. Various infectious and noninfectious factors cause GA. Systemic conditions, such as Crohn’s disease and sarcoidosis, may also be associated with granulomatous inflammation of the appendix. The initial belief that it represented a manifestation of Crohn’s disease is incorrect in the great majority of cases, as only 5%-10% of patients with GA develop Crohn’s disease elsewhere in their GI tract. Distinguishing idiopathic granulomatous appendicitis from early Crohn’s disease, which affects only the appendix, is difficult. A definitive diagnosis can only be made after long-term follow-up, and sometimes further investigations are required[31,52,116-120,122,123].
Crohn’s disease is a chronic transmural inflammation characterized by epithelioid granulation formation in the intestinal wall. The clinical presentation is always variable, and patients often present with findings consistent with acute appendicitis such as right-lower quadrant pain, fever, nausea, and anorexia. The diagnosis of appendiceal Crohn’s disease requires exclusion of multiple entities. Infectious causes of granulomatous appendicitis include Yersinia, Mycobacterium tuberculosis, blastomycosis, Schistosoma, Actinomyces, Campylobacter, Histoplasma capsulatum, and some parasites. An appendectomy is a routine surgical procedure when the Crohn’s disease is limited to the appendix with no postoperative or intraoperative mortality and a low rate of fistula formation[16,32,123].
In summary, although fecaliths and lymphoid hyperplasia are the usual causes of acute appendicitis, some unusual factors may also cause appendicitis. The most common unusual findings in appendectomy specimens are parasites and benign or malignant tumors. A simple appendectomy or right hemicolectomy can be performed depending on the localization, size, and histopathological structure of the tumor in the primary malignant appendiceal tumor, whereas an appendectomy alone is sufficient for benign tumors. Administering the appropriate antibacterial or antiparasitic treatment after the appendectomy is the proper approach for parasitic and bacterial infections that cause chronic inflammation. We emphasize and strongly recommend that all appendectomy specimens be examined histopathologically regardless of whether the specimens are macroscopically normal.
COMMENTS
Background
Appendicitis is one of most common acute surgical conditions of the abdomen, and an appendectomy is one of the most frequently performed operations worldwide. Obstruction of the lumen is the dominant factor in acute appendicitis, and although fecoliths and lymphoid hyperplasia are the usual causes of obstructions, some unusual factors could also be involved.
Research frontiers
The authors conducted a literature review via the PubMed and Google Scholar databases of English language studies published between 2000 and 2010 on unusual findings in appendectomy specimens. Also, we presented 54 patients who had unusual findings in their appendectomy specimens.
Innovations and breakthroughs
The authors emphasize and strongly recommend that all appendectomy specimens be examined histopathologically regardless of whether the specimens are macroscopically normal.
Peer review
This is a very interesting paper. It will be cited many times in the future and this is good for our journal.
Footnotes
Peer reviewer: Jean-Luc Faucheron, MD, Professor, Colorectal Unit, Department of Surgery, Michallon Hospital, BP 217, Grenoble cedex 9, 38043, France
S- Editor Sun H L- Editor Logan S E- Editor Ma WH
References
- 1.Agarwala N, Liu CY. Laparoscopic appendectomy. J Am Assoc Gynecol Laparosc. 2003;10:166–168. doi: 10.1016/s1074-3804(05)60292-7. [DOI] [PubMed] [Google Scholar]
- 2.Duzgun AP, Moran M, Uzun S, Ozmen MM, Ozer VM, Seckin S, Coskun F. Unusual findings in appendicectomy specimens: Evaluation of 2458 cases and review of the literature. Indian J Surg. 2004;66:221–226. [Google Scholar]
- 3.Machado NO, Chopra P, Pande G. Appendiceal tumour--retrospective clinicopathological analysis. Trop Gastroenterol. 2004;25:36–39. [PubMed] [Google Scholar]
- 4.Arca MJ, Gates RL, Groner JI, Hammond S, Caniano DA. Clinical manifestations of appendiceal pinworms in children: an institutional experience and a review of the literature. Pediatr Surg Int. 2004;20:372–375. doi: 10.1007/s00383-004-1151-5. [DOI] [PubMed] [Google Scholar]
- 5.Bucher P, Mathe Z, Demirag A, Morel P. Appendix tumors in the era of laparoscopic appendectomy. Surg Endosc. 2004;18:1063–1066. doi: 10.1007/s00464-003-9255-x. [DOI] [PubMed] [Google Scholar]
- 6.Guraya SY, Khairy GA, Ghallab A, Al-Saigh A. Carcinoid tumors of the appendix. Our experience in a university hospital. Saudi Med J. 2005;26:434–437. [PubMed] [Google Scholar]
- 7.Yildirim S, Nursal TZ, Tarim A, Kayaselcuk F, Noyan T. A rare cause of acute appendicitis: parasitic infection. Scand J Infect Dis. 2005;37:757–759. doi: 10.1080/00365540510012161. [DOI] [PubMed] [Google Scholar]
- 8.Al-Jaradi M, Sallam A, Saqran N, Petrucci MD, Burger N. Is appendiceal pathology important? Morphological study of 745 appendectomies: Sana Yemen. Pak J Pathol. 2006;17:105–108. [Google Scholar]
- 9.Tchana-Sato V, Detry O, Polus M, Thiry A, Detroz B, Maweja S, Hamoir E, Defechereux T, Coimbra C, De Roover A, et al. Carcinoid tumor of the appendix: a consecutive series from 1237 appendectomies. World J Gastroenterol. 2006;12:6699–6701. doi: 10.3748/wjg.v12.i41.6699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bucher P, Gervaz P, Ris F, Oulhaci W, Inan I, Morel P. Laparoscopic versus open resection for appendix carcinoid. Surg Endosc. 2006;20:967–970. doi: 10.1007/s00464-005-0468-z. [DOI] [PubMed] [Google Scholar]
- 11.Sah SP, Bhadani PP. Enterobius vermicularis causing symptoms of appendicitis in Nepal. Trop Doct. 2006;36:160–162. doi: 10.1258/004947506777978361. [DOI] [PubMed] [Google Scholar]
- 12.Coşkun H, Bostanci O, Dilege ME, Mihmanli M, Yilmaz B, Akgün I, Yildirim S. Carcinoid tumors of appendix: treatment and outcome. Ulus Travma Acil Cerrahi Derg. 2006;12:150–154. [PubMed] [Google Scholar]
- 13.Gali BM, Nggada HA, Eni EU. Schistosomiasis of the appendix in Maiduguri. Trop Doct. 2006;36:162–163. doi: 10.1258/004947506777978073. [DOI] [PubMed] [Google Scholar]
- 14.Aydin O. Incidental parasitic infestations in surgically removed appendices: a retrospective analysis. Diagn Pathol. 2007;2:16. doi: 10.1186/1746-1596-2-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van Gompel JJ, Stoddard E, Chen H. Incidental carcinoid tumors of the appendix: do they affect presentation or prognosis? Int Surg. 2007;92:331–334. [PubMed] [Google Scholar]
- 16.Jones AE, Phillips AW, Jarvis JR, Sargen K. The value of routine histopathological examination of appendicectomy specimens. BMC Surg. 2007;7:17. doi: 10.1186/1471-2482-7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ramezani MA, Dehghani MR. Relationship between Enterobius vermicularis and the incidence of acute appendicitis. Southeast Asian J Trop Med Public Health. 2007;38:20–23. [PubMed] [Google Scholar]
- 18.da Silva DF, da Silva RJ, da Silva MG, Sartorelli AC, Rodrigues MA. Parasitic infection of the appendix as a cause of acute appendicitis. Parasitol Res. 2007;102:99–102. doi: 10.1007/s00436-007-0735-0. [DOI] [PubMed] [Google Scholar]
- 19.Isik B, Yilmaz M, Karadag N, Kahraman L, Sogutlu G, Yilmaz S, Kirimlioglu V. Appendiceal Enterobius vermicularis infestation in adults. Int Surg. 2007;92:221–225. [PubMed] [Google Scholar]
- 20.Deniz K, Sökmensüer LK, Sökmensüer C, Patiroğlu TE. Significance of intraepithelial lymphocytes in appendix. Pathol Res Pract. 2007;203:731–735. doi: 10.1016/j.prp.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 21.In't Hof KH, van der Wal HC, Kazemier G, Lange JF. Carcinoid tumour of the appendix: an analysis of 1,485 consecutive emergency appendectomies. J Gastrointest Surg. 2008;12:1436–1438. doi: 10.1007/s11605-008-0545-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sodergren MH, Jethwa P, Wilkinson S, Kerwat R. Presenting features of Enterobius vermicularis in the vermiform appendix. Scand J Gastroenterol. 2009;44:457–461. doi: 10.1080/00365520802624227. [DOI] [PubMed] [Google Scholar]
- 23.Zulfıkar I, Khanzada TW, Sushel C, Samad A. Review of the Pathologic Diagnoses of Appendectomy Specimens Annals. Ann King Edward Med Univ. 2009;15:168–178. [Google Scholar]
- 24.Terada T. Schistosomal appendicitis: incidence in Japan and a case report. World J Gastroenterol. 2009;15:1648–1649. doi: 10.3748/wjg.15.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Graham RP, Williams NP, West KA. Primary epithelial tumours of the appendix in a black population: a review of cases. World J Gastroenterol. 2009;15:1472–1474. doi: 10.3748/wjg.15.1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hatzipantelis E, Panagopoulou P, Sidi-Fragandrea V, Fragandrea I, Koliouskas DE. Carcinoid tumors of the appendix in children: experience from a tertiary center in northern Greece. J Pediatr Gastroenterol Nutr. 2010;51:622–625. doi: 10.1097/MPG.0b013e3181e05358. [DOI] [PubMed] [Google Scholar]
- 27.Sieren LM, Collins JN, Weireter LJ, Britt RC, Reed SF, Novosel TJ, Britt LD. The incidence of benign and malignant neoplasia presenting as acute appendicitis. Am Surg. 2010;76:808–811. [PubMed] [Google Scholar]
- 28.Shapiro R, Eldar S, Sadot E, Venturero M, Papa MZ, Zippel DB. The significance of occult carcinoids in the era of laparoscopic appendectomies. Surg Endosc. 2010;24:2197–2199. doi: 10.1007/s00464-010-0926-0. [DOI] [PubMed] [Google Scholar]
- 29.Ariyarathenam AV, Nachimuthu S, Tang TY, Courtney ED, Harris SA, Harris AM. Enterobius vermicularis infestation of the appendix and management at the time of laparoscopic appendectomy: case series and literature review. Int J Surg. 2010;8:466–469. doi: 10.1016/j.ijsu.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 30.Petnehazy T, Saxena AK, Ainoedhofer H, Hoellwarth ME, Schalamon J. Single-port appendectomy in obese children: an optimal alternative? Acta Paediatr. 2010;99:1370–1373. doi: 10.1111/j.1651-2227.2010.01791.x. [DOI] [PubMed] [Google Scholar]
- 31.Ma KW, Chia NH, Yeung HW, Cheung MT. If not appendicitis, then what else can it be? A retrospective review of 1492 appendectomies. Hong Kong Med J. 2010;16:12–17. [PubMed] [Google Scholar]
- 32.AbdullGaffar B. Granulomatous diseases and granulomas of the appendix. Int J Surg Pathol. 2010;18:14–20. doi: 10.1177/1066896909349246. [DOI] [PubMed] [Google Scholar]
- 33.Karagulle E, Turan H, Turk E, Kiyici H, Yildirim E, Moray G. Abdominal actinomycosis mimicking acute appendicitis. Can J Surg. 2008;51:E109–E110. [PMC free article] [PubMed] [Google Scholar]
- 34.Liu V, Val S, Kang K, Velcek F. Case report: actinomycosis of the appendix--an unusual cause of acute appendicitis in children. J Pediatr Surg. 2010;45:2050–2052. doi: 10.1016/j.jpedsurg.2010.06.015. [DOI] [PubMed] [Google Scholar]
- 35.Peitsidis P, Papadimitriou C, Rodolakis A, Peitsidou A. Actinomycosis of the appendix and pelvis: a case report. J Reprod Med. 2008;53:711–713. [PubMed] [Google Scholar]
- 36.Maternini M, Saucy F, Sandmeier D, Vuilleumier H. Simple appendicitis? Can J Surg. 2008;51:E54–E55. [PMC free article] [PubMed] [Google Scholar]
- 37.Nissotakis C, Sakorafas GH, Koureta T, Revelos K, Kassaras G, Peros G. Actinomycosis of the appendix: diagnostic and therapeutic considerations. Int J Infect Dis. 2008;12:562–564. doi: 10.1016/j.ijid.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 38.Yiğiter M, Kiyici H, Arda IS, Hiçsönmez A. Actinomycosis: a differential diagnosis for appendicitis. A case report and review of the literature. J Pediatr Surg. 2007;42:E23–E26. doi: 10.1016/j.jpedsurg.2007.03.057. [DOI] [PubMed] [Google Scholar]
- 39.Karmarkar P, Joshi A, Wilkinson A, Mahore S, Bothale K. Villous adenoma of the appendix with dysplasia. Saudi J Gastroenterol. 2008;14:38–39. doi: 10.4103/1319-3767.37807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gabbi C, Bertolotti M, Iori R, Rivasi F, Stanzani C, Maurantonio M, Carulli N. Acute abdomen associated with schistosomiasis of the appendix. Dig Dis Sci. 2006;51:215–217. doi: 10.1007/s10620-006-3111-5. [DOI] [PubMed] [Google Scholar]
- 41.Halkic N, Abdelmoumene A, Gintzburger D, Mosimann F. Schistosomal appendicitis in pregnancy. Swiss Surg. 2002;8:121–122. doi: 10.1024/1023-9332.8.3.121. [DOI] [PubMed] [Google Scholar]
- 42.Kanoksil W, Larbcharoensub N, Soontrapa P, Phongkitkarun S, Sriphojanart S, Nitiyanant P. Eosinophilic appendicitis caused by Schistosoma japonicum: a case report and review of the literature. Southeast Asian J Trop Med Public Health. 2010;41:1065–1070. [PubMed] [Google Scholar]
- 43.Adisa AO, Omonisi AE, Osasan SA, Alatise OI. Clinicopathological review of schistosomal appendicitis in south western Nigeria. Trop Gastroenterol. 2009;30:230–232. [PubMed] [Google Scholar]
- 44.Webb JK, Thompson G. Schistosomal appendicitis in a Sudanese immigrant. Med J Aust. 2009;190:716–717. doi: 10.5694/j.1326-5377.2009.tb02654.x. [DOI] [PubMed] [Google Scholar]
- 45.Al-Waheeb S, Al-Murshed M, Dashti F, Hira PR, Al-Sarraf L. Disseminated peritoneal Schistosoma japonicum: a case report and review of the pathological manifestations of the helminth. Ann Saudi Med. 2009;29:149–152. doi: 10.4103/0256-4947.51800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Konstantinidou E, Alexiou C, Demonakou M, Sakellaridis T, Fotopoulos A, Antsaklis G. Schistosomal peritonitis: a rare cause of acute abdomen. Trans R Soc Trop Med Hyg. 2009;103:1068–1070. doi: 10.1016/j.trstmh.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 47.Nandipati K, Parithivel V, Niazi M. Schistosomiasis: a rare cause of acute appendicitis in the African American population in the United States. Am Surg. 2008;74:221–223. [PubMed] [Google Scholar]
- 48.Garg J, Bakhtar OR, Ramamoorthy S. Image of the month. Perforated schistosomal appendicitis. Arch Surg. 2007;142:487–488. doi: 10.1001/archsurg.142.5.487. [DOI] [PubMed] [Google Scholar]
- 49.Badmos KB, Komolafe AO, Rotimi O. Schistosomiasis presenting as acute appendicitis. East Afr Med J. 2006;83:528–532. doi: 10.4314/eamj.v83i10.9464. [DOI] [PubMed] [Google Scholar]
- 50.Elazary R, Maly A, Khalaileh A, Rubinstein C, Olstain-Pops K, Almogy G, Rivkind AI, Mintz Y. Schistosomiasis and acute appendicitis. Isr Med Assoc J. 2005;7:533–534. [PubMed] [Google Scholar]
- 51.Adehossi E, Parola P. Schistosomal appendicitis. Lancet Infect Dis. 2004;4:498. doi: 10.1016/S1473-3099(04)01104-1. [DOI] [PubMed] [Google Scholar]
- 52.Khan GM, Grillo IA, Abu-Eshy SA, Khan AR, Mubarak J, Jastaniah S. Pathology of the appendix. J Natl Med Assoc. 2000;92:533–535. [PMC free article] [PubMed] [Google Scholar]
- 53.Akbulut S, Yagmur Y, Bakir S, Sogutcu N, Yilmaz D, Senol A, Bahadir MV. Appendicular tuberculosis: review of 155 published cases and a report of two cases. Eur J Trauma Emerg Surg. 2010;36:579–585. doi: 10.1007/s00068-010-0040-y. [DOI] [PubMed] [Google Scholar]
- 54.Ito N, Kawamoto S, Inada K, Nagao S, Kanemaru T, Noda N, Ochiai R. Primary tuberculosis of the appendix in a young male patient: report of a case. Surg Today. 2010;40:668–671. doi: 10.1007/s00595-009-4111-9. [DOI] [PubMed] [Google Scholar]
- 55.Chowdhury FR, Amin MR, Khan KH, Alam MB, Ahasan HA. Isolated appendicular tuberculosis (TB) presented as peritonitis. Nepal Med Coll J. 2010;12:51–52. [PubMed] [Google Scholar]
- 56.Salemis NS, Nisotakis K, Nazos K, Stavrinou P, Tsohataridis E. Perforated appendix and periappendicular abscess within an inguinal hernia. Hernia. 2006;10:528–530. doi: 10.1007/s10029-006-0132-0. [DOI] [PubMed] [Google Scholar]
- 57.Chamisa I. A clinicopathological review of 324 appendices removed for acute appendicitis in Durban, South Africa: a retrospective analysis. Ann R Coll Surg Engl. 2009;91:688–692. doi: 10.1308/003588409X12486167521677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Akbulut S, Dursun P, Kocbiyik A, Harman A, Sevmis S. Appendiceal endometriosis presenting as perforated appendicitis: report of a case and review of the literature. Arch Gynecol Obstet. 2009;280:495–497. doi: 10.1007/s00404-008-0922-y. [DOI] [PubMed] [Google Scholar]
- 59.Astroza G, Faundes V, Nanjarí R, Fleiderman M, Rodríguez C. Appendiceal endometriosis differentially diagnosed from acute appendicitis. Chin Med J (Engl) 2010;123:1610–1611. [PubMed] [Google Scholar]
- 60.Faucheron JL, Pasquier D, Voirin D. Endometriosis of the vermiform appendix as an exceptional cause of acute perforated appendicitis during pregnancy. Colorectal Dis. 2008;10:518–519. doi: 10.1111/j.1463-1318.2007.01428.x. [DOI] [PubMed] [Google Scholar]
- 61.Hasegawaa T, Yoshidab K, Matsuic K. Endometriosis of the Appendix Resulting in Perforated Appendicitis. Case Rep Gastroenterol. 2007;1:27–31. doi: 10.1159/000104223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Idetsu A, Ojima H, Saito K, Yamauchi H, Yamaki E, Hosouchi Y, Nishida Y, Kuwano H. Laparoscopic appendectomy for appendiceal endometriosis presenting as acute appendicitis: report of a case. Surg Today. 2007;37:510–513. doi: 10.1007/s00595-006-3440-1. [DOI] [PubMed] [Google Scholar]
- 63.Khoo JJ, Ismail MS, Tiu CC. Endometriosis of the appendix presenting as acute appendicitis. Singapore Med J. 2004;45:435–436. [PubMed] [Google Scholar]
- 64.Ruiz Marín M, Parra Baños PA, González Valverde FM, Rodenas Moncada J, Candel Arenas MF, Méndez Martínez M, Terol Garaulet E, Tamayo Rodríguez ME, Benavides Buleje JA, Escamilla Segade C, et al. Appendiceal intussusception resulting from endometriosis presenting as acute appendicitis. Am Surg. 2010;76:906–908. [PubMed] [Google Scholar]
- 65.Naghshvar F, Torabizadeh Zh, Haghgoo A, Ghahremani M. Ovarian pregnancy: a case report. Pak J Biol Sci. 2008;11:151–152. doi: 10.3923/pjbs.2008.151.152. [DOI] [PubMed] [Google Scholar]
- 66.Alexiou VG, Ierodiakonou V, Peppas G, Falagas ME. Antimicrobial prophylaxis in surgery: an international survey. Surg Infect (Larchmt) 2010;11:343–348. doi: 10.1089/sur.2009.023. [DOI] [PubMed] [Google Scholar]
- 67.Tazaki T, Oue N, Ichikawa T, Tsumura H, Hino H, Yamaoka H, Kanehiro T, Yasui W. A case of endometriosis of the appendix. Hiroshima J Med Sci. 2010;59:39–42. [PubMed] [Google Scholar]
- 68.Tumay V, Ozturk E, Ozturk H, Yilmazlar T. Appendiceal endometriosis mimicking acute appendicitis. Acta Chir Belg. 2006;106:712–713. doi: 10.1080/00015458.2006.11679989. [DOI] [PubMed] [Google Scholar]
- 69.Uncu H, Taner D. Appendiceal endometriosis: two case reports. Arch Gynecol Obstet. 2008;278:273–275. doi: 10.1007/s00404-008-0570-2. [DOI] [PubMed] [Google Scholar]
- 70.D'Aleo C, Lazzareschi I, Ruggiero A, Riccardi R. Carcinoid tumors of the appendix in children: two case reports and review of the literature. Pediatr Hematol Oncol. 2001;18:347–351. doi: 10.1080/088800101300312627. [DOI] [PubMed] [Google Scholar]
- 71.Yap WM, Tan HW, Goh SG, Chuah KL. Appendiceal gastrointestinal stromal tumor. Am J Surg Pathol. 2005;29:1545–1547. doi: 10.1097/01.pas.0000180445.79398.d4. [DOI] [PubMed] [Google Scholar]
- 72.Agaimy A, Pelz AF, Wieacker P, Roessner A, Wünsch PH, Schneider-Stock R. Gastrointestinal stromal tumors of the vermiform appendix: clinicopathologic, immunohistochemical, and molecular study of 2 cases with literature review. Hum Pathol. 2008;39:1252–1257. doi: 10.1016/j.humpath.2007.12.016. [DOI] [PubMed] [Google Scholar]
- 73.Prommegger R, Obrist P, Ensinger C, Profanter C, Mittermair R, Hager J. Retrospective evaluation of carcinoid tumors of the appendix in children. World J Surg. 2002;26:1489–1492. doi: 10.1007/s00268-002-6440-3. [DOI] [PubMed] [Google Scholar]
- 74.Li CC, Hirowaka M, Qian ZR, Xu B, Sano T. Expression of E-cadherin, b-catenin, and Ki-67 in goblet cell carcinoids of the appendix: an immunohistochemical study with clinical correlation. Endocr Pathol. 2002;13:47–58. doi: 10.1385/ep:13:1:47. [DOI] [PubMed] [Google Scholar]
- 75.Aizawa M, Watanabe O, Naritaka Y, Katsube T, Imamura H, Kinoshita J, Shimakawa T, Kobayashi S, Asaka S, Haga S, et al. Adenocarcinoid of the appendix: report of two cases. Surg Today. 2003;33:375–378. doi: 10.1007/s005950300085. [DOI] [PubMed] [Google Scholar]
- 76.Pelizzo G, La Riccia A, Bouvier R, Chappuis JP, Franchella A. Carcinoid tumors of the appendix in children. Pediatr Surg Int. 2001;17:399–402. doi: 10.1007/s003830000559. [DOI] [PubMed] [Google Scholar]
- 77.Barakat AJ, Reese D, Menezes G. Carcinoid tumors of the appendix in children: a reminder. Case Rep Clin Pract Rev. 2003;4:69–72. [Google Scholar]
- 78.Dall'Igna P, Ferrari A, Luzzatto C, Bisogno G, Casanova M, Alaggio R, Terenziani M, Cecchetto G. Carcinoid tumor of the appendix in childhood: the experience of two Italian institutions. J Pediatr Gastroenterol Nutr. 2005;40:216–219. doi: 10.1097/00005176-200502000-00025. [DOI] [PubMed] [Google Scholar]
- 79.Bucher P, Gervaz P, Ris F, Oulhaci W, Egger JF, Morel P. Surgical treatment of appendiceal adenocarcinoid (goblet cell carcinoid) World J Surg. 2005;29:1436–1439. doi: 10.1007/s00268-005-7958-y. [DOI] [PubMed] [Google Scholar]
- 80.Suzuki O, Ono K, Sekishita Y, Fujimori M, Shiono T, Kondo S. Laparoscopic two-stage surgery for goblet cell carcinoid of the appendix: report of a case and review of the Japanese literature. Surg Laparosc Endosc Percutan Tech. 2006;16:106–108. doi: 10.1097/00129689-200604000-00012. [DOI] [PubMed] [Google Scholar]
- 81.O'Donnell ME, Carson J, Garstin WI. Surgical treatment of malignant carcinoid tumours of the appendix. Int J Clin Pract. 2007;61:431–437. doi: 10.1111/j.1742-1241.2006.00875.x. [DOI] [PubMed] [Google Scholar]
- 82.Christianakis E, Paschalidis N, Chorti M, Filippou G, Rizos S, Filippou D. Carcinoid tumour of the appendix in children: a case report. Cases J. 2008;1:136. doi: 10.1186/1757-1626-1-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pitiakoudis M, Kirmanidis M, Tsaroucha A, Christianakis E, Filippou D, Sivridis E, Simopoulos C. Carcinoid tumor of the appendix during pregnancy. A rare case and a review of the literature. J BUON. 2008;13:271–275. [PubMed] [Google Scholar]
- 84.Singh NG, Mannan AA, Kahvic M. Acute amebic appendicitis: report of a rare case. Indian J Pathol Microbiol. 2010;53:767–768. doi: 10.4103/0377-4929.72080. [DOI] [PubMed] [Google Scholar]
- 85.Andrade JE, Mederos R, Rivero H, Sendzischew MA, Soaita M, Robinson MJ, Sendzischew H, Danielpour P. Amebiasis presenting as acute appendicitis. South Med J. 2007;100:1140–1142. doi: 10.1097/SMJ.0b013e318158326f. [DOI] [PubMed] [Google Scholar]
- 86.Gilboa Y, Fridman E, Ofir K, Achiron R. Carcinoid tumor of the appendix: ultrasound findings in early pregnancy. Ultrasound Obstet Gynecol. 2008;31:576–578. doi: 10.1002/uog.5313. [DOI] [PubMed] [Google Scholar]
- 87.Pervez S, Raza AN. A child with acute appendicitis. Eur J Pediatr. 2008;167:127–128. doi: 10.1007/s00431-007-0431-1. [DOI] [PubMed] [Google Scholar]
- 88.Guzmán-Valdivia G. Acute amebic appendicitis. World J Surg. 2006;30:1038–1042. doi: 10.1007/s00268-005-0104-z. [DOI] [PubMed] [Google Scholar]
- 89.Zardawi IM, Kattampallil JS, Rode JW. Amoebic appendicitis. Med J Aust. 2003;178:523–524. doi: 10.5694/j.1326-5377.2003.tb05335.x. [DOI] [PubMed] [Google Scholar]
- 90.Ramdial PK, Madiba TE, Kharwa S, Clarke B, Zulu B. Isolated amoebic appendicitis. Virchows Arch. 2002;441:63–68. doi: 10.1007/s00428-001-0560-2. [DOI] [PubMed] [Google Scholar]
- 91.Gotohda N, Itano S, Okada Y, Horiki S, Endo A, Terada N, Isozaki H, Takakura N, Tanaka N. Acute appendicitis caused by amebiasis. J Gastroenterol. 2000;35:861–863. doi: 10.1007/s005350070024. [DOI] [PubMed] [Google Scholar]
- 92.Dorfman S, Cardozo J, Dorfman D, Del Villar A. The role of parasites in acute appendicitis of pediatric patients. Invest Clin. 2003;44:337–340. [PubMed] [Google Scholar]
- 93.Karatepe O, Adas G, Tukenmez M, Battal M, Altiok M, Karahan S. Parasitic infestation as cause of acute appendicitis. G Chir. 2009;30:426–428. [PubMed] [Google Scholar]
- 94.Wani I, Maqbool M, Amin A, Shah F, Keema A, Singh J, Kitagawa M, Nazir M. Appendiceal ascariasis in children. Ann Saudi Med. 2010;30:63–66. doi: 10.4103/0256-4947.59380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Saylam B, Küçük ÖK, Düzgün AP, Özer MV, Coşkun F. Carcinoid tumor of the appendix: report on ten cases. Eur J Trauma Emerg Surg. 2010;19:Epub ahead of print. doi: 10.1007/s00068-010-0066-1. [DOI] [PubMed] [Google Scholar]
- 96.Higgins MJ, Walsh M, Kennedy SM, Hyland JM, McDermott E, O'Higgins NJ. Granulomatous appendicitis revisited: report of a case. Dig Surg. 2001;18:245–248. doi: 10.1159/000050146. [DOI] [PubMed] [Google Scholar]
- 97.Fu TY, Wang JS, Tseng HH. Primary appendiceal lymphoma presenting as perforated acute appendicitis. J Chin Med Assoc. 2004;67:629–632. [PubMed] [Google Scholar]
- 98.Kitamura Y, Ohta T, Terada T. Primary T-cell non-Hodgkin's malignant lymphoma of the appendix. Pathol Int. 2000;50:313–317. doi: 10.1046/j.1440-1827.2000.01037.x. [DOI] [PubMed] [Google Scholar]
- 99.Shiwani MH. Primary malignant lymphoma of the appendix associated with acute appendicitis. J Coll Physicians Surg Pak. 2006;16:79–80. [PubMed] [Google Scholar]
- 100.Pickhardt PJ, Levy AD, Rohrmann CA Jr, Abbondanzo SL, Kende AI. Non-Hodgkin's lymphoma of the appendix: clinical and CT findings with pathologic correlation. AJR Am J Roentgenol. 2002;178:1123–1127. doi: 10.2214/ajr.178.5.1781123. [DOI] [PubMed] [Google Scholar]
- 101.Tsujimura H, Takagi T, Tamaru J, Sakai C. Involvement of the appendix in a relapsed case of primary nasal NK/T-cell lymphoma. Leuk Lymphoma. 2000;37:633–634. doi: 10.3109/10428190009058518. [DOI] [PubMed] [Google Scholar]
- 102.Moradi P, Barakate M, Gill A, Farrow G. Intussusception of the veriform appendix due to endometriosis presenting as acute appendicitis. ANZ J Surg. 2007;77:758–760. doi: 10.1111/j.1445-2197.2007.04232.x. [DOI] [PubMed] [Google Scholar]
- 103.Miettinen M, Sobin LH. Gastrointestinal stromal tumors in the appendix: a clinicopathologic and immunohistochemical study of four cases. Am J Surg Pathol. 2001;25:1433–1437. doi: 10.1097/00000478-200111000-00013. [DOI] [PubMed] [Google Scholar]
- 104.Maes M, Segers K, Cheyns P. Goblet cell carcinoid of the appendix: laparoscopic appendectomy or right hemicolectomy? Acta Chir Belg. 2008;108:447–450. doi: 10.1080/00015458.2008.11680260. [DOI] [PubMed] [Google Scholar]
- 105.Marudanayagam R, Williams GT, Rees BI. Review of the pathological results of 2660 appendicectomy specimens. J Gastroenterol. 2006;41:745–749. doi: 10.1007/s00535-006-1855-5. [DOI] [PubMed] [Google Scholar]
- 106.Calişkan K, Yildirim S, Bal N, Nursal TZ, Akdur AC, Moray G. Mucinous cystadenoma of the appendix: a rare cause of acute abdomen. Ulus Travma Acil Cerrahi Derg. 2008;14:303–307. [PubMed] [Google Scholar]
- 107.Kiyak G, Celik A, Sarikaya SM. Mucocele of the appendix due to mucinous cystadenoma. J Pak Med Assoc. 2009;59:336. [PubMed] [Google Scholar]
- 108.Leanza S, Bekheit M, Coco D, Bellia A, Ferrara F, Sarvà S, Pappalardo A, Piazza L. Carcinoma of the appendix and its natural history in relation to surgical management. A case report. Chir Ital. 2009;61:597–600. [PubMed] [Google Scholar]
- 109.Bhardwaj N, Bains SK, Ortonowski G, Murphy P. A case of Burkitt's lymphoma presenting as suspected acute appendicitis. Afr J Paediatr Surg. 2010;7:214–215. doi: 10.4103/0189-6725.70433. [DOI] [PubMed] [Google Scholar]
- 110.Ghasmei M, Kenari SA. A Primary Diffuse Large B-Cell lymphoma of appendix. IRCMJ. 2010;12:576–578. [Google Scholar]
- 111.Tadele M, Yancovitz S. Diffuse large B-cell lymphoma presenting as acute appendicitis in patients with acquired immunodeficiency syndrome. Infect Dis Clin Pract. 2007;15:411–414. [Google Scholar]
- 112.Khanna M, Buddhavarapu SR. Primary Burkitt’s Lymphoma Of The Appendix Presenting as Acute Abdomen: A Case Report. Radiology Case. 2008;2:9–14. doi: 10.3941/jrcr.v2i5.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Toubai T, Kondo Y, Ogawa T, Imai A, Kobayashi N, Ogasawara M, Kiyama Y, Higa T, Sato K, Miyokawa N, et al. A case of leukemia of the appendix presenting as acute appendicitis. Acta Haematol. 2003;109:199–201. doi: 10.1159/000070971. [DOI] [PubMed] [Google Scholar]
- 114.Hsiao PJ, Kuo SM, Chen JH, Lin HF, Chu PL, Lin SH, Ho CL. Acute myelogenous leukemia and acute leukemic appendicitis: a case report. World J Gastroenterol. 2009;15:5624–5625. doi: 10.3748/wjg.15.5624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Palomino-Portilla EA, Valbuena JR, Quinones-Avila Mdel P, Medeiros LJ. Myeloid sarcoma of appendix mimicking acute appendicitis. Arch Pathol Lab Med. 2005;129:1027–1031. doi: 10.5858/2005-129-1027-MSOAMA. [DOI] [PubMed] [Google Scholar]
- 116.Shivakumar P, Shanmugam RP, Mani CS. Idiopathic granulomatous appendicitis: a rare appendicular pseudo tumor. Trop Gastroenterol. 2010;31:130–131. [PubMed] [Google Scholar]
- 117.Yayla D, Alpman BN, Dolek Y. Granulomatous appendicitis in a 12-year-old boy. J Pediatr Surg. 2010;45:e27–e29. doi: 10.1016/j.jpedsurg.2010.06.017. [DOI] [PubMed] [Google Scholar]
- 118.Gu J, Allan C. Idiopathic granulomatous appendicitis: a report of three consecutive cases. ANZ J Surg. 2010;80:201. doi: 10.1111/j.1445-2197.2010.05237.x. [DOI] [PubMed] [Google Scholar]
- 119.Zissin R, Gayer G, Bernheim J, Kots E, Shapiro-Feinberg M, Hertz M. Granulomatous appendicitis presenting as right lower quadrant pain: CT findings. Abdom Imaging. 2003;28:280–283. doi: 10.1007/s00261-002-0060-0. [DOI] [PubMed] [Google Scholar]
- 120.Ho P, Law WL, Choy C, Chan GS, Chu KW. Granulomatous appendicitis progressing to Crohn's disease with bleeding complication. ANZ J Surg. 2003;73:554–556. doi: 10.1046/j.1445-1433.2003.02663.x. [DOI] [PubMed] [Google Scholar]
- 121.Felekouras E, Kontos M, Kyriakou V, Hatzianagnostou D, Dimaroggona K, Papalampros E, Kordossis T, Bastounis E. Strongyloides stercoralis infection as a cause of acute granulomatous appendicitis in an HIV-positive patient in Athens, Greece. Scand J Infect Dis. 2002;34:856–857. doi: 10.1080/0036554021000026943. [DOI] [PubMed] [Google Scholar]
- 122.Tucker ON, Healy V, Jeffers M, Keane FB. Granulomatous appendicitis. Surgeon. 2003;1:286–289. doi: 10.1016/s1479-666x(03)80047-1. [DOI] [PubMed] [Google Scholar]
- 123.Prieto-Nieto I, Perez-Robledo JP, Hardisson D, Rodriguez-Montes JA, Larrauri-Martinez J, Garcia-Sancho-Martin L. Crohn's disease limited to the appendix. Am J Surg. 2001;182:531–533. doi: 10.1016/s0002-9610(01)00811-x. [DOI] [PubMed] [Google Scholar]
- 124.Mackie SL, Keat A. An unusual complication of appendicitis. Ann Rheum Dis. 2004;63:1526. doi: 10.1136/ard.2003.017426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Madavo C, Hurriez H. Schistosomiasis of the appendix. J R Soc Med. 2006;99:473–474. doi: 10.1258/jrsm.99.9.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Doudier B, Parola P, Dales JP, Linzberger N, Brouqui P, Delmont J. Schistosomiasis as an unusual cause of appendicitis. Clin Microbiol Infect. 2004;10:89–91. doi: 10.1111/j.1469-0691.2004.00805.x. [DOI] [PubMed] [Google Scholar]
- 127.Sartorelli AC, da Silva MG, Rodrigues MA, da Silva RJ. Appendiceal taeniasis presenting like acute appendicitis. Parasitol Res. 2005;97:171–172. doi: 10.1007/s00436-005-1408-5. [DOI] [PubMed] [Google Scholar]
- 128.Lejbkowicz F, Abel AB, Tsilman B, Cohen HI. Taenia infestation in the appendix: a report of two cases. J Med Microbiol. 2002;51:90–91. doi: 10.1099/0022-1317-51-1-90. [DOI] [PubMed] [Google Scholar]
- 129.Gagné F, Fortin P, Dufour V, Delage C. [Tumors of the appendix associating histologic features of carcinoid and adenocarcinoma] Ann Anat Pathol (Paris) 1969;14:393–406. [PubMed] [Google Scholar]


