Abstract
AIM: To evaluate the presence of extracapsular invasion (ECI) in positive nodes as a predictor of disease recurrence disease in colorectal cancer.
METHODS: Two hundred and twenty-eight consecutive patients who underwent colorectal resection were identified for inclusion in this study, of which 46 had positive lymph nodes. Among 46 cases with stage IIIcolorectal cancer, 16 had ECI at positive nodes and 8 had disease recurrence. The clinical and pathological features of these cases were reviewed.
RESULTS: In the univariate analysis, the number of positive lymph nodes and depth of tumor invasion were significantly associated with the presence of ECI at positive nodes. Multivariate analysis demonstrated that only ECI was a predictor of recurrence. The recurrence-free interval differed significantly among patients with ECI at positive nodes.
CONCLUSION: Our results suggest that ECI at metastatic nodes can identify which cases are at high risk of short-term disease recurrence in colorectal cancer.
Keywords: Extracapsular invasion, Lymph node, Metastasis, Colorectal cancer, Risk factor, Adjuvant therapy
INTRODUCTION
The role of systemic adjuvant chemotherapy in colorectal cancer patients with lymph node involvement has been established in a large number of clinical trials[1-3]. Lymph node status is one of the most important prognostic factors for colorectal carcinoma. However, patients with TNM stage III colorectal cancer are a heterogeneous group. Some patients with stage III colorectal cancer have good prognoses, similar to that of patients with stage II disease, whereas others develop disease recurrence. It is of utmost importance to develop markers that can predict which patients are at high risk for disease recurrence.
Previous studies have demonstrated and confirmed that the presence of extracapsular invasion (ECI) at metastatic lymph nodes is significantly related to prognosis in various types of carcinoma including colorectal cancer[4-11]. We have also recently demonstrated that ECI at metastatic nodes in breast and colorectal cancer was strongly associated with further regional nodes metastasis[12]. The purpose of this study was to investigate the correlation between the presence of ECI in positive lymph nodes and disease recurrence in cases of colorectal cancer undergoing curative operation. It will be advantageous to be able to tailor therapy individually, using ECI as an indicator of the risk of recurrence.
MATERIALS AND METHODS
Two hundred and twenty-eight consecutive patients who underwent colorectal resection in the Department of General Surgical Science, Graduate School of Medicine, Gunma University, from January 2007 to December 2009 were identified for inclusion in this study. Patients with recurrence or metastasis at operation, neo-adjuvant chemotherapy, radiation, or incomplete clinical information were excluded. Of the eligible cases, 46 (20.2%) with positive lymph nodes, identified as TNM stage III colorectal, were analyzed in this study. The clinical features of these cases were reviewed according to the presence or not of ECI at positive lymph nodes, and statistical analysis was performed. ECI was defined as extracapsular growth of tumor cells, invasion into perinodal fat or extranodal location of tumor cells[12]. Informed consent was obtained from all patients.
Age, sex, primary tumor size, location, depth of tumor invasion, histological type, lymphovascular invasion at the primary tumor site, number of metastatic lymph nodes, ECI at positive lymph nodes, administration of adjuvant therapy and serum tumor markers (carcinoembryonic antigen) were tested as possible predictors of disease recurrence. Recurrence-free interval was defined as the interval from surgery to the time disease recurrence was diagnosed. The overall median follow-up period was 1.7 years and none of the patients died of surgical complications. Fisher’s exact test, the Chi-squared test, and the Student t-test were used to compare the 2 groups. Multivariate analysis was performed with logistic regression analysis to select covariates (primary tumor size and ECI at positive lymph nodes). The recurrence-free interval was calculated by the Kaplan-Meier method. The log-rank test was used to evaluate differences between recurrence-free intervals. Differences were considered to be significant at P < 0.05.
RESULTS
Table 1 summarizes the characteristics of the patients who underwent colorectal resection with TNM stage III colorectal cancer. The series consisted of 16 cases with ECI at positive nodes and 30 with no ECI at positive nodes. Table 1 also summarizes the results of the univariate analysis conducted to determine the relationship between the clinicopathologic variables and the presence of ECI at positive nodes. The number of positive lymph nodes and the depth of tumor invasion were significantly associated with the presence of ECI at positive nodes.
Table 1.
Patients characteristics and clinicopathological features associated with the presence of extracapsular invasion at lymph node metastases
| ECI | Positiven = 16 | Negativen = 30 | P value | |
| Age (yr) | 65.3 ± 16.1 | 66.5 ± 13.8 | 0.798 | |
| Sex | ||||
| Male | 7 | 21 | 0.082 | |
| Female | 9 | 9 | ||
| Location | ||||
| Colon | 13 | 20 | 0.295 | |
| Rectum | 3 | 10 | ||
| Histological type | ||||
| Tub | 15 | 28 | 0.957 | |
| Muc | 1 | 2 | ||
| pT category | ||||
| T 1,2 | 1 | 10 | 0.040 | |
| T 3,4 | 15 | 20 | ||
| Tumor size (mm) | 47.3 ± 15.1 | 40.4 ± 21.2 | 0.270 | |
| Number of positive LNs | 3.63 ± 2.29 | 1.70 ± 1.27 | 0.001 | |
| Lymphovascular invasion | 16 | 29 | 0.460 | |
| CEA ≥ 3.0 | 3 | 3 | 0.041 | |
| Adjuvant treatment | 13 | 0.720 | 23 | 0.720 |
LN: Lymph node; ECI: Extracapsular invasion; CEA: Carcinoembryonic antigen.
The 46 cases with metastatic lymph nodes were divided into 2 groups based on the presence of disease recurrence. Among 46 cases with stage III colorectal cancer, 8 (17.4%) had disease recurrence. Table 2 summarizes the results of the univariate analysis conducted to determine the relationship between the clinicopathologic variables and disease recurrence. In the univariate analysis ECI at positive nodes and the depth of tumor invasion were the factors significantly associated with disease recurrence. Among those, multivariate analysis demonstrated that only ECI was a predictor of the recurrence (P = 0.016). Time to tumor recurrence by Kaplan-Meier curves was significantly shorter among patients with ECI at positive nodes (Figure 1).
Table 2.
Patient characteristics and clinicopatholo gical features associated with recurrent disease
| Recurrent disease | Positive | Negative | P value | |
| 8 | 38 | |||
| Age (yr) | 68.0 ± 17.2 | 65.7 ± 14.0 | 0.780 | |
| Sex | ||||
| Male | 3 | 25 | 0.278 | |
| Female | 5 | 13 | ||
| Location | ||||
| Colon | 6 | 27 | 0.795 | |
| Rectum | 2 | 11 | ||
| Histological type | ||||
| Tub | 7 | 36 | 0.451 | |
| Muc | 1 | 2 | ||
| PT category | ||||
| T 1, 2 | 0 | 11 | < 0.001 | |
| T 3, 4 | 8 | 27 | ||
| Tumor size (mm) | 44.9 ± 11.6 | 42.5 ± 20.8 | 0.679 | |
| Number of positive LNs | 2.38 ± 1.41 | 2.37 ± 2.02 | 0.906 | |
| ECI | 6 | 10 | 0.009 | |
| Lymphovascular invasion | 8 | 37 | 0.643 | |
| CEA | ≥ 3.0 | 3 | 3 | 0.093 |
| Adjuvant treatment | 5 | 31 | 0.473 |
ECI: Extracapsular invasion; CEA: Carcinoembryonic antigen.
Figure 1.
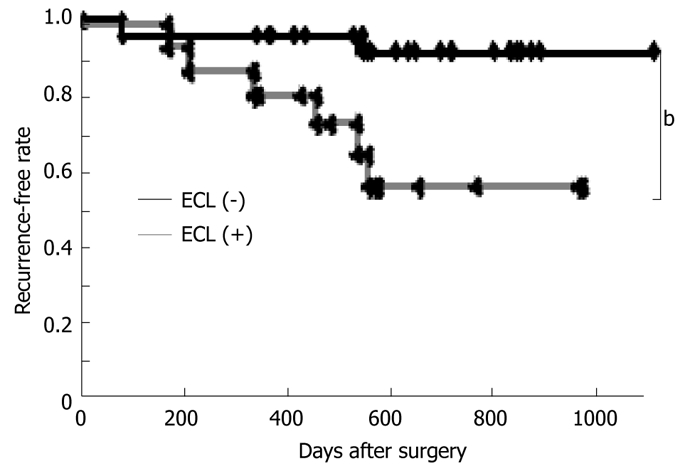
Impact of the presence of extracapsular invasion at positive nodes on postoperative recurrence-free interval. Recurrence-free interval by Kaplan-Meier curves differed significantly among patients with and without extracapsular invasion at positive nodes. bP < 0.01.
DISCUSSION
The key observations made in this study can be summarized as follows: (1) the presence of ECI at positive nodes was significantly associated with the number of positive lymph nodes and depth of tumor invasion; (2) multivariate analysis demonstrated that only ECI was a predictor of recurrence; and (3) the recurrence-free interval by Kaplan-Meier curves was significantly shorter among patients with ECI at positive nodes. These findings suggest that the presence of ECI at positive lymph nodes is a strong predictor for short-term recurrence in cases with colorectal cancer undergoing curative surgery.
The surgical stage remains the most accurate predictor of survival for colorectal cancer[13]. Pathologic prognostic factors of primary tumor invasion and regional node involvement predict the risk of relapse of cases with colorectal cancer undergoing curative operation. In the current study, both the number of positive lymph nodes and the depth of tumor invasion were significantly associated with the presence of ECI at positive nodes. Lymph node metastasis is one of the most important prognostic factors in patients with colorectal cancer, and many studies have indicated that the location and number of metastatic nodes affect prognosis[5,14-16]. Regarding ECI, previous studies have demonstrated and confirmed that the presence of ECI at metastatic lymph nodes is significantly associated with prognoses in various types of carcinoma including colorectal carcinoma[4-11]. The ability of metastatic nodes to recruit degradation factors that permit cancer cells to break through the lymph node capsule is indicative of a very aggressive cancer. We previously demonstrated that the presence of ECI in positive lymph nodes is significantly related to the nodal spread of tumor cells in colorectal and breast cancer patients[12]. These studies imply that ECI is a biologic marker of aggressive cancer and essentially support our findings. Tumor cells are thought to invade the lymphovascular vessels, which enables tumor cells to spread metastatic or recurrent disease.
Adjuvant therapy is systemic treatment administered with the intent of reducing the risk of recurrence. The benefit of adjuvant therapy in patients with lymph node involvement (stage III) has been well established in large prospective randomized trials[1-3]. In Japan, the oxaliplatin plus 5-fluorouracil/leucovorin (LV) (FOLFOX) regimen has not been approved for adjuvant therapy for patients with stage III colorectal cancer at this time. In this Japanese population study, oral chemotherapeutic agents, including capecitabine or UFT (tegafur plus uracil) with oral LV, were used for adjuvant therapy for stage III colorectal cancer. Oral chemotherapeutic agents are advantageous because of their ease of administration. However, in the current series, 13 (81.3%) of the 16 cases with ECI in positive nodes had oral adjuvant therapy but 6 of 16 cases (37.5%) had disease recurrence. These results imply that high-risk patients with ECI at positive nodes should receive stronger adjuvant chemotherapy, including FOLFOX with or without monoclonal antibody.
This study has several potential limitations. The major limitation of our study is that it used retrospective methods of data collection. In addition, the number of cases in our study was relatively small and the follow-up periods were relatively short. However, the clinical implications of this data are very important, and these findings serve to emphasize that ECI at metastatic lymph nodes is an important prognostic factor for stage III colorectal carcinoma and will be advantageous in tailoring therapy to the individual case. In patients treated in phase III adjuvant clinical trials, disease-free survival and overall survival have been highly correlated, both within studies and across trials[17]; however, in a number of patients, improving the quality of life and the length of recurrence-free intervals may be more important statistical parameters than median overall survival. Additional research is needed to explore this putative association between the presence of ECI and the risk of recurrence.
In conclusion, we have demonstrated that ECI at metastatic lymph nodes may predict which cases are at high risk of short-term disease recurrence in colorectal cancer. Thus, it will be possible to tailor therapy individually, using ECI as an indicator of the risk of recurrence. Analyses from large randomized trials or experimental data are warranted to evaluate this relationship between the presence of ECI and disease recurrence.
COMMENTS
Background
The authors have demonstrated that extracapsular invasion (ECI) at metastatic nodes in breast and colorectal cancer was strongly associated with further regional nodes metastasis. The purpose of this study was to evaluate the presence of ECI in positive nodes as a predictor of disease recurrence disease in colorectal cancer.
Research frontiers
Lymph node status is one of the most important prognostic factors for colorectal carcinoma. However, patients with TNM stage III colorectal cancer are a heterogeneous group. It is of utmost importance to develop markers that can predict which patients are at high risk for disease recurrence.
Innovations and breakthroughs
This study suggests that the presence of ECI at positive lymph nodes is a strong predictor for short-term recurrent-free interval in cases with colorectal cancer undergoing curative operation.
Applications
It will be possible to tailor therapy individually, using ECI as an indicator of the risk of recurrence.
Terminology
ECI was defined as extracapsular growth of tumor cells, invasion into perinodal fat or extranodal location of tumor cells.
Peer review
The key observations made in this study suggest that the presence of ECI at positive lymph nodes is a strong predictor of short-term recurrent-free interval in cases with colorectal cancer undergoing curative operation.
Acknowledgments
The authors would like to thank Saitoh Y, Yano T, Ohno M, Matsui Y, Muraoka S and Sekiguchi M for their secretarial assistance
Footnotes
Supported by Grants-in-Aid from the Japanese Ministry of Education, Culture, Sports, Science, and Technology
Peer reviewer: Dr. Vui Heng Chong, Gastroenterology and Hepatology Unit, Department of Medicine, Raja Isteri Pengiran Anak Saleha Hospital, Bandar Seri Begawan BA 1710, Brunei Darussalam
S- Editor Sun H L- Editor Cant MR E- Editor Ma WH
References
- 1.Monga DK, O'Connell MJ. Surgical adjuvant therapy for colorectal cancer: current approaches and future directions. Ann Surg Oncol. 2006;13:1021–1034. doi: 10.1245/ASO.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 2.André T, Boni C, Mounedji-Boudiaf L, Navarro M, Tabernero J, Hickish T, Topham C, Zaninelli M, Clingan P, Bridgewater J, et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med. 2004;350:2343–2351. doi: 10.1056/NEJMoa032709. [DOI] [PubMed] [Google Scholar]
- 3.Kuebler JP, Wieand HS, O'Connell MJ, Smith RE, Colangelo LH, Yothers G, Petrelli NJ, Findlay MP, Seay TE, Atkins JN, et al. Oxaliplatin combined with weekly bolus fluorouracil and leucovorin as surgical adjuvant chemotherapy for stage II and III colon cancer: results from NSABP C-07. J Clin Oncol. 2007;25:2198–2204. doi: 10.1200/JCO.2006.08.2974. [DOI] [PubMed] [Google Scholar]
- 4.Stitzenberg KB, Meyer AA, Stern SL, Cance WG, Calvo BF, Klauber-DeMore N, Kim HJ, Sansbury L, Ollila DW. Extracapsular extension of the sentinel lymph node metastasis: a predictor of nonsentinel node tumor burden. Ann Surg. 2003;237:607–612; discussion 612-613. doi: 10.1097/01.SLA.0000064361.12265.9A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yano H, Saito Y, Kirihara Y, Takashima J. Tumor invasion of lymph node capsules in patients with Dukes C colorectal adenocarcinoma. Dis Colon Rectum. 2006;49:1867–1877. doi: 10.1007/s10350-006-0733-9. [DOI] [PubMed] [Google Scholar]
- 6.Komuta K, Okudaira S, Haraguchi M, Furui J, Kanematsu T. Identification of extracapsular invasion of the metastatic lymph nodes as a useful prognostic sign in patients with resectable colorectal cancer. Dis Colon Rectum. 2001;44:1838–1844. doi: 10.1007/BF02234464. [DOI] [PubMed] [Google Scholar]
- 7.Heide J, Krüll A, Berger J. Extracapsular spread of nodal metastasis as a prognostic factor in rectal cancer. Int J Radiat Oncol Biol Phys. 2004;58:773–778. doi: 10.1016/S0360-3016(03)01616-X. [DOI] [PubMed] [Google Scholar]
- 8.D'Journo XB, Avaro JP, Michelet P, Trousse D, Tasei AM, Dahan L, Doddoli C, Guidicelli R, Fuentes P, Seitz JF, et al. Extracapsular lymph node involvement is a negative prognostic factor after neoadjuvant chemoradiotherapy in locally advanced esophageal cancer. J Thorac Oncol. 2009;4:534–539. doi: 10.1097/jto.0b013e31819c862d. [DOI] [PubMed] [Google Scholar]
- 9.Okamoto T, Tsuburaya A, Kameda Y, Yoshikawa T, Cho H, Tsuchida K, Hasegawa S, Noguchi Y. Prognostic value of extracapsular invasion and fibrotic focus in single lymph node metastasis of gastric cancer. Gastric Cancer. 2008;11:160–167. doi: 10.1007/s10120-008-0473-8. [DOI] [PubMed] [Google Scholar]
- 10.Lagarde SM, ten Kate FJ, de Boer DJ, Busch OR, Obertop H, van Lanschot JJ. Extracapsular lymph node involvement in node-positive patients with adenocarcinoma of the distal esophagus or gastroesophageal junction. Am J Surg Pathol. 2006;30:171–176. doi: 10.1097/01.pas.0000189182.92815.12. [DOI] [PubMed] [Google Scholar]
- 11.Yamashita H, Noguchi S, Murakami N, Toda M, Uchino S, Watanabe S, Kawamoto H. Extracapsular invasion of lymph node metastasis. A good indicator of disease recurrence and poor prognosis in patients with thyroid microcarcinoma. Cancer. 1999;86:842–849. doi: 10.1002/(sici)1097-0142(19990901)86:5<842::aid-cncr21>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 12.Fujii T, Yanagita Y, Fujisawa T, Hirakata T, Iijima M, Kuwano H. Implication of extracapsular invasion of sentinel lymph nodes in breast cancer: prediction of nonsentinel lymph node metastasis. World J Surg. 2010;34:544–548. doi: 10.1007/s00268-009-0389-4. [DOI] [PubMed] [Google Scholar]
- 13.Monga DK, O'Connell MJ. Surgical adjuvant therapy for colorectal cancer: current approaches and future directions. Ann Surg Oncol. 2006;13:1021–1034. doi: 10.1245/ASO.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 14.Tang R, Wang JY, Chen JS, Chang-Chien CR, Tang S, Lin SE, You YT, Hsu KC, Ho YS, Fan HA. Survival impact of lymph node metastasis in TNM stage III carcinoma of the colon and rectum. J Am Coll Surg. 1995;180:705–712. [PubMed] [Google Scholar]
- 15.Adjuvant therapy of colon cancer--results of a prospectively randomized trial. Gastrointestinal Tumor Study Group. N Engl J Med. 1984;310:737–743. doi: 10.1056/NEJM198403223101201. [DOI] [PubMed] [Google Scholar]
- 16.Jass JR, Love SB, Northover JM. A new prognostic classification of rectal cancer. Lancet. 1987;1:1303–1306. doi: 10.1016/s0140-6736(87)90552-6. [DOI] [PubMed] [Google Scholar]
- 17.Sargent DJ, Wieand HS, Haller DG, Gray R, Benedetti JK, Buyse M, Labianca R, Seitz JF, O'Callaghan CJ, Francini G, et al. Disease-free survival versus overall survival as a primary end point for adjuvant colon cancer studies: individual patient data from 20,898 patients on 18 randomized trials. J Clin Oncol. 2005;23:8664–8670. doi: 10.1200/JCO.2005.01.6071. [DOI] [PubMed] [Google Scholar]


