Abstract
Background
Atrial fibrillation (AF) is a common finding in patients with myocardial infarction (MI). AF is not generally perceived by clinicians as a critical event during the acute phase of MI; however, its prognostic influence in MI remains controversial. Furthermore, contradictory data exist concerning death risk according to AF timing. This systematic review and first meta-analysis aim to quantify the mortality risk associated with AF in MI patients and its timing.
Methods and Results
A comprehensive search of several electronic databases (1970–2010, adults, any language) identified MI studies that evaluated mortality related to AF. Evidence was reviewed by 2 blinded reviewers with a formal assessment of the methodological quality of the studies. Adjusted odds ratios (OR's) were pooled across studies using the random-effects model. The I2 statistic was used to assess heterogeneity. In the 43 included studies (278,854 subjects), the mortality OR's and 95% CI associated with AF was: 1.46 (1.35–1.58), I2=76%, 23 studies. This worse prognosis persisted regardless of the timing of AF; OR (95% CI, I2, n studies) of mortality for new AF with no prior history of AF was 1.37 (1.26–1.49), I2=28%, n=9; and for prior AF was 1.28 (1.16–1.40), I2=24%, n=4. The sensitivity analysis of new AF studies adjusting for confounding factors did not show a decrease in death risk.
Conclusions
AF is associated with increased risk of mortality in MI patients. New AF with no history of AF prior to MI remained associated with an increased risk of mortality even after adjusting for several important AF risk factors. These subsequent increases in mortality suggest that AF can no longer be considered as a non severe event during MI.
Keywords: atrial fibrillation, myocardial Infarction, mortality
Introduction
Atrial fibrillation (AF) is a common finding in patients who have myocardial infarction (MI). Both conditions have increased frequency with advancing age, and acute MI is associated with a sharp increase in the occurrence of AF. The incidence of AF among MI patients varies between 2% and 22%.1–7 Compared to severe complications such as ventricular tachycardia or cardiac failure, AF is not generally perceived by clinicians as a critical event during the acute phase of MI; however, in the literature, the prognostic influence of the presence of AF in MI remains controversial. Some studies illustrated an independent adverse effect on mortality,2, 5, 6, 8–12 whereas other studies showed no significant effects.1, 3, 4, 13–15 In 2009, a review of AF in acute MI suggested that AF in patients hospitalized for MI seems to carry adverse prognostic implications for in-hospital and long-term mortality.16 However, no meta-analysis has been published addressing this question.
Furthermore, AF may occur as a complication of the MI or be present (diagnosed or not) at the time of the MI. Some studies demonstrated that new AF at the time of MI is associated with an increased risk of mortality, contrasting with a lack of risk with pre-existing AF10 whereas other studies did not show a different risk of death according to AF timing.9, 17
To address these controversies, we performed a systematic review and a meta-analysis of the data available to date aiming to quantify the mortality risk associated with the presence of AF in MI patients and its timing.
Methods
This meta-analysis is in adherence with the preferred reporting items for systematic reviews and meta-analyses (PRISMA) and the reporting Meta-analyses of Observational Studies in Epidemiology (MOOSE).18, 19
Eligibility Criteria
Eligible studies were randomized controlled clinical trials and observational studies that enrolled patients with acute MI and AF, and evaluated the outcome of mortality.
To be considered for inclusion, studies were also required to (i) perform comparisons with a control group of patients without AF; and (ii) provide sufficient quantitative data on all-cause mortality or cardiovascular death. We excluded studies that evaluated AF after the first week of MI diagnosis and those reporting exclusively AF in the context of surgery or catheter ablation. We also excluded abstracts, editorials, reviews, case reports, and case series.
We classified AF in each study as `New AF', `Prior AF' or `Any AF' according to the authors' classification. New AF was defined by most studies as AF occurring for the first time after the MI with no history of AF prior to MI. Few studies did not report if patients had atrial fibrillation preceding their MI and new AF was defined in these studies as the first occurrence of AF during the infarction period, in the absence of AF on admission ECG records. Prior AF was defined as pre-existing AF to the MI admission. If no distinction about the first occurrence timing of AF was made, AF was classified as Any AF.
We classified mortality to be all-cause or cardiovascular death, if all-cause death was not available.
Data Sources and Search Strategies
A comprehensive search of several electronic databases (from 1970 to February 2010, adults, any language, any population) was conducted. The databases included Ovid Medline In-Process & Other Non-Indexed Citations, Ovid MEDLINE, Ovid EMBASE, Ovid Cochrane Database of Systematic Reviews, Ovid Health Technology Assessment, and Scopus to identify studies that evaluated mortality related to AF in MI patients. The search strategy was designed and conducted by an experienced librarian with input from the study's principal investigator. Controlled vocabulary supplemented with keywords was used to define the concept areas: myocardial infarction, atrial fibrillation/flutter, mortality, as well as to limit to randomized controlled and observational studies. The detailed search strategy is available from the reprint author. In addition, we reviewed the reference sections of eligible studies and available reviews. We also requested potentially eligible studies from content experts.
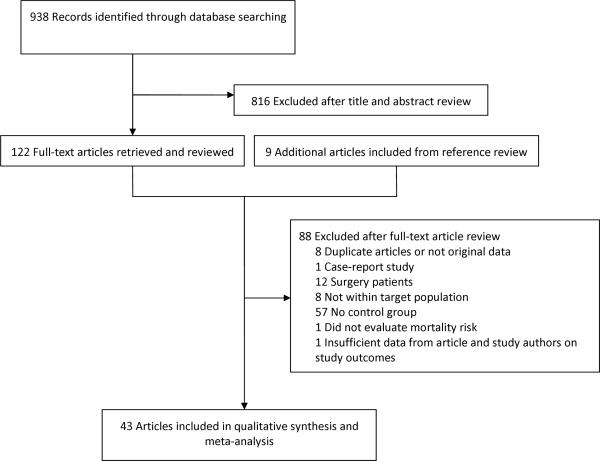
Two reviewers (P.J., A.M.C.) working independently, considered the potential eligibility of each of the abstracts and titles that resulted from executing the search strategy, then reviewed the full text of all potentially eligible studies. The chance adjusted inter-reviewer agreement (kappa statistic) for study eligibility was 0.82 (95% CI, 0.72–0.92). The study selection process is outlined in Figure 1. Disagreements have been harmonized by consensus.
Figure 1.
Literature Search and Study selection
Data Collection
Data extraction included full description of participants enrolled, AF timing and evaluation duration, the confounding factors adjusted for and the outcome measure. Authors were contacted in case of uncertainty about the data. Two reviewers working independently and using a standardized form extracted data from all eligible studies. Data collected included: study characteristics, such as author name, year of publication, study size, patient age, AF description, unadjusted and adjusted estimated risk of mortality and adjustment variables (Appendix 1 and 2 and Table 1). The Newcastle-Ottawa Scale was used to assess the quality of studies.20 A quality score was calculated on the basis of three major components: selection of the study groups (0 to 4 points), quality of the adjustment for confounding (0 to 2 points) and ascertainment of the exposure and outcome (0 to 3 points). A higher score represents better methodological quality.
Table 1.
Characteristics of studies on new atrial fibrillation with no history of atrial fibrillation prior to the myocardial infarction
| Source | Follow-up Duration | Unadjusted OR (95%CI) | Adjusted OR (95%CI) | Key Prognostic Variables |
|||||
|---|---|---|---|---|---|---|---|---|---|
| Age | Diabetes | Hypertension | Prior MI | Heart Failure | Coronary Revascularization | ||||
| Randomized Trials | |||||||||
| Pedersen,111999 | 5 y | NR | 1.4 (1.2–1.7) | X | X | X | X | X | X |
| Lehto,92005 | 3 y | NR | 1.8 (1.4–2.4) | X | X | X | X | ||
| Non Randomized Trials | |||||||||
| Community studies | |||||||||
| Saczynski,122009 | 5 y | NR | 1.3 (1.2–1.4) | X | X | X | X | X | |
| Registries | |||||||||
| Behar,11992 | 6 y | 2.2 (1.8–2.8) | 1.3 (1.1–1.5) | X | X | X | X | ||
| Eldar,31998 | 1y | 3.5 (2.6–4.6) | 1.3 (1.1–1.7) | X | X | X | X | ||
| Mehta,102003 | Hosp | 3.7 (3.1–4.4) | 1.7 (1.3–2.1) | X | X | X | X | X | |
| Lau,322009 | 1 y | 3.3 (2.1–5.3) | 1.4 (0.8–2.2) | X | X | X | X | ||
| Convenience sample | |||||||||
| Klass,301970 | Hosp | 4.7 (2.0–10.9) | NR | ||||||
| Cristal,271976 | Hosp | 4.6 (2.1–10.0) | NR | ||||||
| Liem,481976 | Hosp | 1.8(1.1–3.1) | NR | ||||||
| Liberthson,331976 | Hosp | 0.8 (0.4–1.8) | NR | ||||||
| Kobayashi,471992 | Hosp | 2.6 (0.9–7.5) | NR | ||||||
| Cicek,262003 | 30 day | 7.4 (0.9–70.3) | NR | ||||||
| Asanin,132005 | 7 y | 2.5 (1.8–3.6) | 1.1 (0.7–1.8) | X | X | X | X | X | X |
| Trappolini,152006 | Hosp | 2.4(1.4–4.1) | 1.5(0.8–2.8) | X | X | X | X | X | X |
| Siu,402007 | 3 y | 0.9 (0.4–2.1) | NR | ||||||
| Li,142008 | Hosp | NR | NR | ||||||
| Total | 2.6 (2.1–3.3) | 1.4 (1.3–1.5) | |||||||
OR, Odds Ratio; CI, Confidence Interval; MI, Myocardial Infarcton; NR, Not Reported; Hosp, during hospitalization; y, years.
Statistical Analysis
We chose to use odds ratio (OR) as a measure of effect size because it was one of the most commonly used effect measures in our studies. To avoid unnecessary heterogeneity, we formed homogeneous groups of studies according to the adjustment status of the estimated risk. If several estimates were reported in the same article, we chose the most fully adjusted estimate (ie, multivariate regression was selected over univariate regression) corresponding to the longer follow-up. Unadjusted and adjusted odds ratios (ORs) with 95% confidence interval (CI) of AF impact on mortality were pooled separately across studies using the random-effects model. Statistical heterogeneity across the studies was tested using the Q statistic, and I2 statistic was calculated to quantify inconsistency among studies.21 I2 values of ≤ 25%, 50%, and ≥ 75% represent low, moderate, and high inconsistency, respectively. Because therapies for myocardial infarction have evolved considerably over the time frame of the studies analyzed, we conducted meta-regressions using the effect size as the dependent outcome variable and the year of inclusion in the study as the independent variable. To assess the potential for publication bias, we performed the Begg and Mazumdar rank correlation test.22 A value of p <0.05 (2-sided) was considered statistically significant. Analysis was conducted using Comprehensive Meta-Analysis(®) software (Biostat, Englewood, NJ)
Subgroup and Sensitivity Analyses
Subgroup analyses were conducted by pooling time-specific AF estimates (New AF with no prior history of AF - Prior AF) to evaluate the mortality risk of AF according to its development timing to MI. Then, a sensitivity analysis was conducted by excluding new AF studies that did not adjust for age, diabetes, heart failure and coronary revascularization. Finally, we conducted a sensitivity analysis on new AF studies that adjusted on age, diabetes, hypertension, prior MI, heart failure and coronary revascularization due to the substantial importance of these confounders.
Role of the funding source
The study sponsor had no role in study design, data collection, analysis, or interpretation of data. The sponsor did not participate in the writing of the report or in the decision to submit the paper for publication. All authors had full access to all the data in the study, and all agreed to submit for publication
Results
Study Identification
Nine hundred and thirty-eight potentially relevant studies were identified. After title and abstract screening, 816 studies were excluded and the remaining 122 studies were retrieved for a more detailed evaluation (Figure 1). Nine additional studies were identified through manual review of references. Out of these 131 clinical studies, 88 were excluded as they did not meet eligibility. Finally, 43 studies were included in our review with 8 studies2, 5, 7, 9, 11, 17, 23, 24 derived from randomized trials, 31 cohort studies1, 3, 4, 6, 8, 10, 12, 14, 15, 25–46 and 4 case-control studies.13, 47–49 For the purpose of this study, we dealt with the 8 studies derived from randomized trials as observational studies, the population being analyzed as a whole without taking into account the randomization process.
Appendix 1 summarizes the characteristics of the 43 eligible studies. Sample size ranged from 100 to 106,780 with a median of 967 patients and a mean age, of 65 years. Across the 40 studies that reported participant sex, there were 30% women. The years of MI diagnosis ranged from 1972 to 2007. Exposure was poorly described in the included studies and therefore, it was not always clear whether patients with prior AF were included. In these cases, AF was classified as Any AF. Additionally, new AF included AF on admission electrocardiography in some studies17, 30 while several studies excluded it.4–6, 23, 39 Across the 22 studies that reported the number of participants with any AF, the incidence of AF was 13% (range, 4%–25%). Across the 30 studies that reported the number of participants with new AF, the incidence of AF was 10% (range, 4%–19%). Across the 11 studies that reported the number of participants with prior AF, the incidence of AF was 7% (range, 1%–13%). Evaluation of AF was mostly during the hospital stay or less with only one study9 that evaluated AF during a median of 3 years after the qualifying MI. Follow-up time varied widely across studies. In this meta-analysis, the pooled mortality analysis referred to all-cause mortality except for 1 study38 that reported only cardiovascular mortality. Loss to follow-up was generally low (<5%). Quality score as evaluated by the Newcastle-Ottawa Scale revealed a median score of 7, range (4–8) (Appendix 1). The 20 studies that reported only unadjusted OR's had a median score of 5.
Meta-Analysis
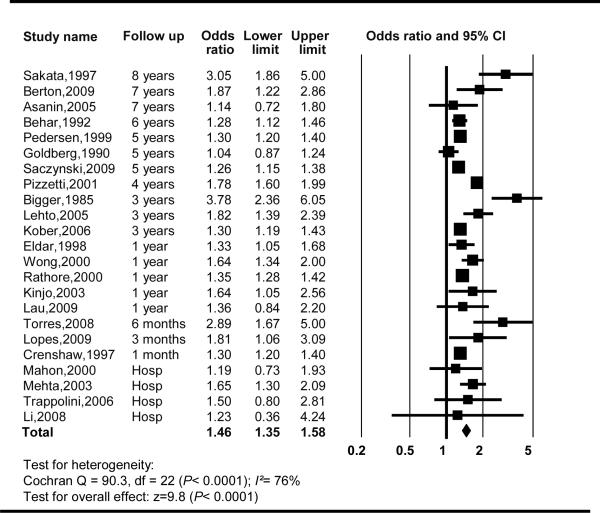
After pooling the 43 eligible studies, there was a significant association between AF and mortality (for both available unadjusted and adjusted OR's. However, the mortality risk estimate was significantly higher for the unadjusted OR's. For this reason, we decided to report only mortality estimates after accounting for the confounding factors. A total of 23 of the 43 studies presented ORs and 95% CIs for mortality following multivariate analysis (Appendix 2). The mortality OR and 95% CI associated with AF was: 1.46 (1.35–1.58), I2=76%) (Figure 2). Although the heterogeneity between the analyzed studies was high, amost all of the studies pointed to a positive association. The meta-regression analysis showed no association between the effect size and the year of inclusion in the studies (p = 0.38) confirming that our findings were consistent over time. There was no statistical evidence of publication bias among the included studies by using Begg's test (P=0.06).
Figure 2.
Mortality and atrial fibrillation in myocardial infarction patients CI, Confidence Interval.
Subgroups and Sensitivity Analyses
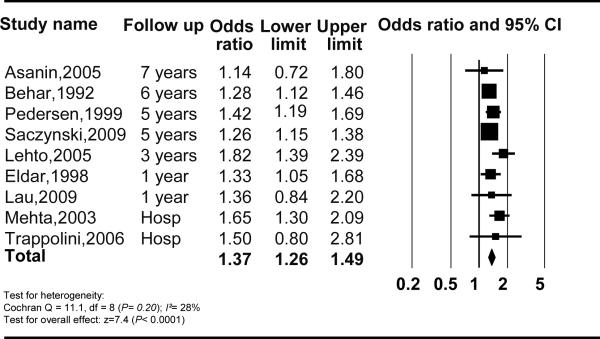
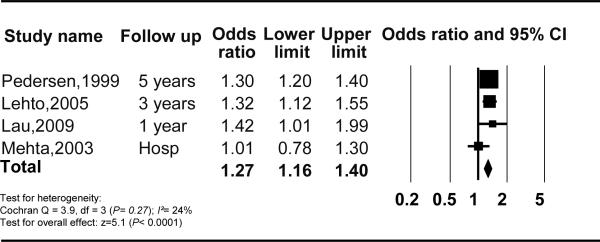
Table 1 summarizes the characteristics of the 17 studies evaluating new AF with no history of AF prior to MI. The significant association between AF and mortality was similar when analysis was performed for new AF and prior AF subgroups; OR (95% CI, I2, n studies) of mortality for new AF with no prior history of AF was 1.37 (1.26–1.49), I2=28%, n=9; and for prior AF was 1.28 (1.16–1.40), I2=24%, n=4) (Figure 3 and 4). There was no statistical evidence of publication bias (P=0.18 and P=1.00 respectively). The heterogeneity between the analyzed studies decreased substantially comparing to the main analysis.
Figure 3.
Mortality and new atrial fibrillation post myocardial infarction CI, Confidence Interval.
Figure 4.
Mortality and atrial fibrillation prior to myocardial infarction CI, Confidence Interval.
We conducted several sensitivity analyses pooling studies according to their follow-up duration; short-term (≤30days), mid-term (>30days-1year) or late-term (> 1 year) mortality. The time frame had relatively little effect on the estimates (data not shown).
Because the selection for confounding factors varied widely between studies evaluating new AF with no prior AF (Table 1), we conducted sensitivity analyses to assess robustness. Analyses of studies that did adjust for age, diabetes, heart failure and coronary revascularization showed a strong association between new AF and mortality: OR's (95% CI, n studies) = 1.49 (1.26–1.76, n=4). Finally, the association between new AF and mortality in studies that adjusted for age, diabetes, hypertension, prior MI, heart failure and coronary revascularization remained almost similar (1.39 (1.19–1.63, n=3).
Discussion
Our findings
This is the first meta-analysis of clinical studies on the prognostic impact of AF in the setting of MI. In this meta-analysis of 43 studies involving 278854 patients, we have demonstrated an increased risk of mortality associated with the presence of AF in the setting of MI. Indeed, AF is associated with at least 40% increase in the risk of mortality compared with control patients in sinus rhythm. Our analysis further demonstrates that this worse prognosis persists regardless of the timing of AF development. Finally, new AF with no history of AF prior to MI remained associated with an increased risk of death even after adjusting for age, diabetes, hypertension, prior MI, heart failure and coronary revascularization status.
The results of previous studies on the impact of AF on survival in patients with MI were conflicting, with some studies showing no significant adverse effect on mortality,1, 3, 4, 13–15 whereas other studies illustrating an independent adverse effect.2, 5, 6, 8–12
It is plausible that studies showing no increased risk were imprecise and the present meta-analysis includes a larger number of events and thus, has increased power. It was also unclear if the presence of prior AF to the MI is associated with an adverse prognosis.6, 10, 11, 32 Our meta-analysis demonstrated that the increased mortality risk seems to be related to AF regardless of its development timing.
There are several potential explanations for the observed association between increased all-cause mortality and AF in MI patients. New AF may lead to adverse outcomes in patients with MI through adverse haemodynamic effects such as loss of atrial contraction, rapid ventricular rates, loss of atrioventricular synchrony and an irregular RR interval leading to a decrease in cardiac output.50 Additionally, the combination of AF and heart failure is particularly ominous in that it appears that the development of either condition has a marked detrimental impact upon the mortality of the other.51–53 Pooling selected studies that adjusted for patient's characteristics, heart failure and acute treatment of MI showed the same association between mortality and new AF. The worse prognosis in patients with MI developing AF seems to be directly correlated with the arrhythmia, in addition to the clinical conditions severity of the patients. However, it is still unclear as to whether AF is a complication of MI or merely demarcates MI severity. Finally, in AF patients with MI requiring percutaneous coronary intervention (PCI) with stent implantation, the optimal association between aspirin, clopidogrel and oral anticoagulant (OAC) remains cumbersome and renders the clinical management of MI in the presence of AF more difficult. Aspirin is given systematically during acute MI and the dual antiplatelet therapy (clopidogrel and aspirin) is the gold standard treatment after acute coronary syndrome and PCI.54 In AF patients, triple therapy and dual therapy using aspirin and OAC are associated with a high frequency of major bleedings55 and the use of clopidogrel and OAC combination is associated with a relatively high incidence of fatal stroke.56 In daily clinical practice, oral anticoagulation is only given to a minority of MI patients with AF, despite the fact that oral anticoagulation is associated with a reduction in 1-year mortality.23, 57 Ongoing and further research is needed to identify ways to prevent the occurrence of AF during MI, and to determine the optimal AF therapeutics for patients with MI to reduce mortality.
Strengths and Limitations
Several limitations should be considered in this study. First, we included some randomized trials that were not designed to capture AF; thus, reporting bias is a possibility. However, the incidence rates of AF were consistent with the rates found in the general population, which suggest that AF was adequately ascertained by appropriate surveillance to capture this event. Second, we could not investigate the effects of persistent AF versus paroxysmal AF on mortality as the studies used for our meta-analysis did not investigate this issue. Third, significant heterogeneity between included studies was noted in the main analysis, as is often the case in meta-analyses of large observational studies.58, 59 Potential sources of heterogeneity include patient demographics, follow up duration, outcome ascertainment, adjustment for confounders and study quality. Moreover, studies included in the review span several decades (published between 1970 and 2009) during which great advances have been made in the treatment of MI. The use of optimal meta-analytic techniques with random-effect models, cannot however account for these differences. However, there was virtually no qualitative heterogeneity and subgroups and sensitivity analyses confirmed robustness by showing similar results to the main analysis. Finally, we did not have patient-level data, the gold standard method to test for interactions at the patient-level covariates.
Our study has several important strengths. We conducted a comprehensive up to date literature search with evidence reviewed by 2 blinded reviewers with adequate inter-reviewer agreement; and formal assessment of the methodological quality of the studies. Also, our pooled estimates are based on multivariate ORs of studies adjusting for several important AF risk factors. Subgroup analyses and sensitivity analyses confirmed the robustness of our main results. Finally, there was no statistical evidence of publication bias.
Conclusion
In conclusion, the presence of AF is associated with an increased risk of mortality in MI patients, irrespective of the timing of AF. These subsequent 40% increases in mortality associated with AF during MI suggest that AF can no longer be considered as a non severe event. New AF with no history of AF prior to MI remained associated with an increased risk of mortality even after adjusting for several important AF risk factors. Closer attention should be paid to patients with AF complicating MI, including diligent monitoring during the acute phase of MI.
Supplementary Material
Acknowledgments
Funding Sources Supported in part by grants from the Public Health Service and the National Institutes of Health (AR30582 and RO1 HL 59205). The post-doc who worked on this research was paid by INSERM, U970, Cardiovascular Epidemiology, Paris-Descartes University-France and the French Emergency Physician Society (SFMU).
Footnotes
Disclosures None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Behar S, Zahavi Z, Goldbourt U, Reicher-Reiss H. Long-term prognosis of patients with paroxysmal atrial fibrillation complicating acute myocardial infarction. SPRINT Study Group. European Heart Journal. 1992;13:45–50. doi: 10.1093/oxfordjournals.eurheartj.a060046. [DOI] [PubMed] [Google Scholar]
- 2.Crenshaw BS, Ward SR, Granger CB, Stebbins AL, Topol EJ, Califf RM. Atrial fibrillation in the setting of acute myocardial infarction: the GUSTO-I experience. Global Utilization of Streptokinase and TPA for Occluded Coronary Arteries. J Am Coll Cardiol. 1997;30:406–413. doi: 10.1016/s0735-1097(97)00194-0. [DOI] [PubMed] [Google Scholar]
- 3.Eldar M, Canetti M, Rotstein Z, Boyko V, Gottlieb S, Kaplinsky E, Behar S. Significance of paroxysmal atrial fibrillation complicating acute myocardial infarction in the thrombolytic era. SPRINT and Thrombolytic Survey Groups. Circulation. 1998;97:965–970. doi: 10.1161/01.cir.97.10.965. [DOI] [PubMed] [Google Scholar]
- 4.Kinjo K, Sato H, Sato H, Ohnishi Y, Hishida E, Nakatani D, Mizuno H, Fukunami M, Koretsune Y, Takeda H, Hori M. Prognostic significance of atrial fibrillation/atrial flutter in patients with acute myocardial infarction treated with percutaneous coronary intervention. Am J Cardiol. 2003;92:1150–1154. doi: 10.1016/j.amjcard.2003.07.021. [DOI] [PubMed] [Google Scholar]
- 5.Pizzetti F, Turazza FM, Franzosi MG, Barlera S, Ledda A, Maggioni AP, Santoro L, Tognoni G. Incidence and prognostic significance of atrial fibrillation in acute myocardial infarction: the GISSI-3 data. Heart (British Cardiac Society) 2001;86:527–532. doi: 10.1136/heart.86.5.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rathore SS, Berger AK, Weinfurt KP, Schulman KA, Oetgen WJ, Gersh BJ, Solomon AJ. Acute myocardial infarction complicated by atrial fibrillation in the elderly: prevalence and outcomes. Circulation. 2000;101:969–974. doi: 10.1161/01.cir.101.9.969. [DOI] [PubMed] [Google Scholar]
- 7.Wong CK, White HD, Wilcox RG, Criger DA, Califf RM, Topol EJ, Ohman EM. New atrial fibrillation after acute myocardial infarction independently predicts death: the GUSTO-III experience. Am Heart J. 2000;140:878–885. doi: 10.1067/mhj.2000.111108. [DOI] [PubMed] [Google Scholar]
- 8.Berton G, Cordiano R, Cucchini F, Cavuto F, Pellegrinet M, Palatini P. Atrial fibrillation during acute myocardial infarction: association with all-cause mortality and sudden death after 7-year of follow-up. Int J Clin Pract. 2009;63:712–721. doi: 10.1111/j.1742-1241.2009.02023.x. [DOI] [PubMed] [Google Scholar]
- 9.Lehto M, Snapinn S, Dickstein K, Swedberg K, Nieminen MS. Prognostic risk of atrial fibrillation in acute myocardial infarction complicated by left ventricular dysfunction: the OPTIMAAL experience. European Heart Journal. 2005;26:350–356. doi: 10.1093/eurheartj/ehi064. [DOI] [PubMed] [Google Scholar]
- 10.Mehta RH, Dabbous OH, Granger CB, Kuznetsova P, Kline-Rogers EM, Anderson FA, Jr., Fox KA, Gore JM, Goldberg RJ, Eagle KA. Comparison of outcomes of patients with acute coronary syndromes with and without atrial fibrillation. Am J Cardiol. 2003;92:1031–1036. doi: 10.1016/j.amjcard.2003.06.001. [DOI] [PubMed] [Google Scholar]
- 11.Pedersen OD, Bagger H, Kober L, Torp-Pedersen C. The occurrence and prognostic significance of atrial fibrillation/-flutter following acute myocardial infarction. TRACE Study group. TRAndolapril Cardiac Evalution. European Heart Journal. 1999;20:748–754. doi: 10.1053/euhj.1998.1352. [DOI] [PubMed] [Google Scholar]
- 12.Saczynski JS, McManus D, Zhou Z, Spencer F, Yarzebski J, Lessard D, Gore JM, Goldberg RJ. Trends in atrial fibrillation complicating acute myocardial infarction. Am J Cardiol. 2009;104:169–174. doi: 10.1016/j.amjcard.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Asanin M, Perunicic J, Mrdovic I, Matic M, Vujisic-Tesic B, Arandjelovic A, Vasiljevic Z, Ostojic M. Prognostic significance of new atrial fibrillation and its relation to heart failure following acute myocardial infarction. Eur J Heart Fail. 2005;7:671–676. doi: 10.1016/j.ejheart.2004.07.018. [DOI] [PubMed] [Google Scholar]
- 14.Li K, Huo Y, Ding YS. Clinical profile and outcomes of atrial fibrillation in elderly patients with acute myocardial infarction. Chin Med J. 2008;121:2388–2391. [PubMed] [Google Scholar]
- 15.Trappolini M, Scorza A, Chillotti FM, Trappolini F, Danese A, De Vito F, Luberti E, Angrisani L, Braucci S. Prognostic significance of atrial fibrillation in thrombolysed and non thrombolysed patients. Minerva Cardioangiol. 2006;54:471–479. [PubMed] [Google Scholar]
- 16.Schmitt J, Duray G, Gersh BJ, Hohnloser SH. Atrial fibrillation in acute myocardial infarction: a systematic review of the incidence, clinical features and prognostic implications. European Heart Journal. 2009;30:1038–1045. doi: 10.1093/eurheartj/ehn579. [DOI] [PubMed] [Google Scholar]
- 17.Kober L, Swedberg K, McMurray JJ, Pfeffer MA, Velazquez EJ, Diaz R, Maggioni AP, Mareev V, Opolski G, Van de Werf F, Zannad F, Ertl G, Solomon SD, Zelenkofske S, Rouleau JL, Leimberger JD, Califf RM. Previously known and newly diagnosed atrial fibrillation: a major risk indicator after a myocardial infarction complicated by heart failure or left ventricular dysfunction. Eur J Heart Fail. 2006;8:591–598. doi: 10.1016/j.ejheart.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 18.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ (Clinical research ed. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 20.Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, Tugwell P. Newcastle-Ottawa Scale. 2006 [Google Scholar]
- 21.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ (Clinical research ed. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. [PubMed] [Google Scholar]
- 23.Lopes RD, Elliott LE, White HD, Hochman JS, Van de Werf F, Ardissino D, Nielsen TT, Weaver WD, Widimsky P, Armstrong PW, Granger CB. Antithrombotic therapy and outcomes of patients with atrial fibrillation following primary percutaneous coronary intervention: results from the APEX-AMI trial. European Heart Journal. 2009;30:2019–2028. doi: 10.1093/eurheartj/ehp213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tangelder MJ, Frison L, Weaver D, Wilcox RG, Bylock A, Emanuelsson H, Held P, Oldgren J. Effect of ximelagatran on ischemic events and death in patients with atrial fibrillation after acute myocardial infarction in the efficacy and safety of the oral direct thrombin inhibitor ximelagatran in patients with recent myocardial damage (ESTEEM) trial. Am Heart J. 2008;155:382–387. doi: 10.1016/j.ahj.2007.08.035. [DOI] [PubMed] [Google Scholar]
- 25.Bigger JT, Jr., Fleiss JL, Rolnitzky LM, Merab JP, Ferrick KJ. Effect of digitalis treatment on survival after acute myocardial infarction. Am J Cardiol. 1985;55:623–630. doi: 10.1016/0002-9149(85)90125-0. [DOI] [PubMed] [Google Scholar]
- 26.Cicek D, Camsari A, Pekdemir H, Kiykim A, Akkus N, Sezer K, Diker E. Predictive value of P-wave signal-averaged electrocardiogram for atrial fibrillation in acute myocardial infarction. Ann Noninvasive Electrocardiol. 2003;8:233–237. doi: 10.1046/j.1542-474X.2003.08311.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cristal N, Peterburg I, Szwarcberg J. Atrial fibrillation developing in the acute phase of myocardial infarction. Prognostic implications. Chest. 1976;70:8–11. doi: 10.1378/chest.70.1.8. [DOI] [PubMed] [Google Scholar]
- 28.Goldberg RJ, Seeley D, Becker RC, Brady P, Chen ZY, Osganian V, Gore JM, Alpert JS, Dalen JE. Impact of atrial fibrillation on the in-hospital and long-term survival of patients with acute myocardial infarction: a community-wide perspective. Am Heart J. 1990;119:996–1001. doi: 10.1016/s0002-8703(05)80227-3. [DOI] [PubMed] [Google Scholar]
- 29.Hunt D, Sloman G, Penington C. Effects of atrial fibrillation on prognosis of acute myocardial infarction. BHJ. 1978;40:303–307. doi: 10.1136/hrt.40.3.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klass M, Haywood LJ. Atrial fibrillation associated with acute myocardial infarction: a study of 34 cases. Am Heart J. 1970;79:752–760. doi: 10.1016/0002-8703(70)90362-5. [DOI] [PubMed] [Google Scholar]
- 31.Koracevic GP, Petrovic S, Damjanovic M, Stanojlovic T. Association of stress hyperglycemia and atrial fibrillation in myocardial infarction. Wiener klinische Wochenschrift. 2008;120:409–413. doi: 10.1007/s00508-008-0983-8. [DOI] [PubMed] [Google Scholar]
- 32.Lau DH, Huynh LT, Chew DP, Astley CM, Soman A, Sanders P. Prognostic impact of types of atrial fibrillation in acute coronary syndromes. Am J Cardiol. 2009;104:1317–1323. doi: 10.1016/j.amjcard.2009.06.055. [DOI] [PubMed] [Google Scholar]
- 33.Liberthson RR, Salisbury KW, Hutter AM, Jr., DeSanctis RW. Atrial tachyarrhythmias in acute myocardial infarction. Am J Med. 1976;60:956–960. doi: 10.1016/0002-9343(76)90566-0. [DOI] [PubMed] [Google Scholar]
- 34.Madias JE, Patel DC, Singh D. Atrial fibrillation in acute myocardial infarction: a prospective study based on data from a consecutive series of patients admitted to the coronary care unit. Clin Cardiol. 1996;19:180–186. doi: 10.1002/clc.4960190309. [DOI] [PubMed] [Google Scholar]
- 35.Mahon NG, Codd MB, McKenna CJ, O'Rorke C, McCann HA, Sugrue DD. Characteristics and outcomes in patients with acute myocardial infarction with ST-segment depression on initial electrocardiogram. Am Heart J. 2000;139:311–319. doi: 10.1067/mhj.2000.101223. [DOI] [PubMed] [Google Scholar]
- 36.Petretta M, Canonico V, Bianchi V, Attisano T, Arrichiello P, Morgano G, Capozzi E, Bonaduce D. Influence of age on the short- and medium-term prognosis in patients with acute myocardial infarct. Giornale Italiano di Cardiologia. 1991;21:395–408. [PubMed] [Google Scholar]
- 37.Rastenyte D, Jancaityte L. Sex differences in one-year mortality after a first-ever myocardial infarction. Medicina (Kaunas, Lithuania) 2005;41:754–759. [PubMed] [Google Scholar]
- 38.Sakata K, Kurihara H, Iwamori K, Maki A, Yoshino H, Yanagisawa A, Ishikawa K. Clinical and prognostic significance of atrial fibrillation in acute myocardial infarction. Am J Cardiol. 1997;80:1522–1527. doi: 10.1016/s0002-9149(97)00746-7. [DOI] [PubMed] [Google Scholar]
- 39.Sankaranarayanan R, James MA, Nuta B, Townsend M, Kesavan S, Burtchaell S, Holloway R, Ewings P. Does atrial fibrillation beget ventricular fibrillation in patients with acute myocardial infarction? Pacing Clin Electrophysiol. 2008;31:1612–1619. doi: 10.1111/j.1540-8159.2008.01234.x. [DOI] [PubMed] [Google Scholar]
- 40.Siu CW, Jim MH, Ho HH, Miu R, Lee SW, Lau CP, Tse HF. Transient atrial fibrillation complicating acute inferior myocardial infarction: implications for future risk of ischemic stroke. Chest. 2007;132:44–49. doi: 10.1378/chest.06-2733. [DOI] [PubMed] [Google Scholar]
- 41.Suarez G, Herrera M, Vera A, Torrado E, Ferriz J, Arboleda JA. Prediction on admission of in-hospital mortality in patients older than 70 years with acute myocardial infarction. Chest. 1995;108:83–88. doi: 10.1378/chest.108.1.83. [DOI] [PubMed] [Google Scholar]
- 42.Sugiura T, Iwasaka T, Takahashi N, Nakamura S, Taniguchi H, Nagahama Y, Matsutani M, Inada M. Atrial fibrillation in inferior wall Q-wave acute myocardial infarction. Am J Cardiol. 1991;67:1135–1136. doi: 10.1016/0002-9149(91)90879-p. [DOI] [PubMed] [Google Scholar]
- 43.Torres M, Rocha S, Marques J, Nabais S, Rebelo A, Pereira MA, Azevedo P, Correia A. Impact of atrial fibrillation in acute coronary syndromes. Rev Port Cardiol. 2008;27:1407–1418. [PubMed] [Google Scholar]
- 44.Cui K, Gu S, Ding YS, Zhang Y, Li Y, Zheng H. Effects of atrial fibrillation/atrial flutter on the short and medium-term prognosis of patients with acute myocardial infarction. J Intervent Radiol. 2008;17:594–596. [Google Scholar]
- 45.Galcera TJ, Melgarejo MA, Garcia AA, Baranco PM, Martinez-Lozano AF, Rodriguez FS. Incidence, clinical characteristics and prognostic significance of supraventricular tachyarrhythmias in acute myocardial infarction. Revista Espanola de Cardiologia. 1999;52:647–655. doi: 10.1016/s0300-8932(99)74984-5. [DOI] [PubMed] [Google Scholar]
- 46.Janion M, Kurzawski J. Myocardial infarction in women complicated by atrial fibrillation. Polski Przeglad Kardiologiczny. 2001;3:41–45. [Google Scholar]
- 47.Kobayashi Y, Katoh T, Takano T, Hayakawa H. Paroxysmal atrial fibrillation and flutter associated with acute myocardial infarction: hemodynamic evaluation in relation to the development of arrhythmias and prognosis. Jpn Circ J. 1992;56:1–11. doi: 10.1253/jcj.56.1. [DOI] [PubMed] [Google Scholar]
- 48.Liem KL, Lie KI, Durrer D, Wellens HJ. Clinical setting and prognostic significance of atrial fibrillation complicating acute myocardial infarction. Eur J Cardiol. 1976;4:59–62. [PubMed] [Google Scholar]
- 49.Nielsen FE, Sorensen HT, Christensen JH, Ravn L, Rasmussen SE. Reduced occurrence of atrial fibrillation in acute myocardial infarction treated with streptokinase. European Heart Journal. 1991;12:1081–1083. doi: 10.1093/oxfordjournals.eurheartj.a059841. [DOI] [PubMed] [Google Scholar]
- 50.Clark DM, Plumb VJ, Epstein AE, Kay GN. Hemodynamic effects of an irregular sequence of ventricular cycle lengths during atrial fibrillation. J Am Coll Cardiol. 1997;30:1039–1045. doi: 10.1016/s0735-1097(97)00254-4. [DOI] [PubMed] [Google Scholar]
- 51.Cha YM, Redfield MM, Shen WK, Gersh BJ. Atrial fibrillation and ventricular dysfunction: a vicious electromechanical cycle. Circulation. 2004;109:2839–2843. doi: 10.1161/01.CIR.0000132470.78896.A8. [DOI] [PubMed] [Google Scholar]
- 52.Ehrlich JR, Nattel S, Hohnloser SH. Atrial fibrillation and congestive heart failure: specific considerations at the intersection of two common and important cardiac disease sets. J Cardiovasc Electr. 2002;13:399–405. doi: 10.1046/j.1540-8167.2002.00399.x. [DOI] [PubMed] [Google Scholar]
- 53.Wang TJ, Larson MG, Levy D, Vasan RS, Leip EP, Wolf PA, D'Agostino RB, Murabito JM, Kannel WB, Benjamin EJ. Temporal relations of atrial fibrillation and congestive heart failure and their joint influence on mortality: the Framingham Heart Study. Circulation. 2003;107:2920–2925. doi: 10.1161/01.CIR.0000072767.89944.6E. [DOI] [PubMed] [Google Scholar]
- 54.Steinhubl SR, Berger PB, Mann JT, 3rd, Fry ET, DeLago A, Wilmer C, Topol EJ. Early and sustained dual oral antiplatelet therapy following percutaneous coronary intervention: a randomized controlled trial. JAMA. 2002;288:2411–2420. doi: 10.1001/jama.288.19.2411. [DOI] [PubMed] [Google Scholar]
- 55.Orford JL, Fasseas P, Melby S, Burger K, Steinhubl SR, Holmes DR, Berger PB. Safety and efficacy of aspirin, clopidogrel, and warfarin after coronary stent placement in patients with an indication for anticoagulation. Am Heart J. 2004;147:463–467. doi: 10.1016/j.ahj.2003.06.004. [DOI] [PubMed] [Google Scholar]
- 56.Ait Mokhtar O, Bonello L, Armero S, Sbragia P, Paganelli F. Early and late outcomes of clopidogrel and coumadin combination for patients on oral anticoagulants undergoing coronary stenting. Cardiovasc Revasc Med. 2010;11:159–162. doi: 10.1016/j.carrev.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 57.Stenestrand U, Lindback J, Wallentin L. Anticoagulation therapy in atrial fibrillation in combination with acute myocardial infarction influences long-term outcome: a prospective cohort study from the Register of Information and Knowledge About Swedish Heart Intensive Care Admissions (RIKS-HIA) Circulation. 2005;112:3225–3231. doi: 10.1161/CIRCULATIONAHA.105.552984. [DOI] [PubMed] [Google Scholar]
- 58.Coory MD. Comment on: Heterogeneity in meta-analysis should be expected and appropriately quantified. Int J Epi. 2008;39:932. doi: 10.1093/ije/dyp157. author reply 933. [DOI] [PubMed] [Google Scholar]
- 59.Higgins JP. Commentary: Heterogeneity in meta-analysis should be expected and appropriately quantified. Int J Epi. 2008;37:1158–1160. doi: 10.1093/ije/dyn204. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.