Abstract
The diversity of HIV-1 and human genetics complicates our ability to determine the impact of treatment during primary HIV-1 infection (PHI) on disease outcome. Here we show in a small group infected with virtually identical HIV-1 strains and treated during PHI that only subjects expressing protective HLA alleles had lower viral loads following treatment discontinuation. These data suggests that genetic factors play a pivotal role in the outcome of HIV-1 infection despite early therapy.
Keywords: acute HIV-1 infection, HLA-B57, HLA-A11, Berlin patient, cluster
Introduction
Although subjects with primary HIV infection (PHI) present with distinct clinical syndromes, only a fraction of patients are diagnosed at this early stage of infection. However, it is during this initial phase that antiviral CD8+ T cell responses first emerge and high-level HIV-1 viremia is controlled by several logs to a set point(1,2). It has been proposed that therapeutic intervention initiated during PHI may alter the immune response of the host to HIV-1 and therefore allow for subsequent immune control of disease progression(3–6).
One of the strongest predictors of HIV-1 disease progression is host genetic factors(10,11), which may also play a key role in influencing control of viral replication following short-term treatment during the acute phase of the infection. For example, subjects expressing the MHC alleles HLA-B57, HLA-B27, HLA-B51 and HLA-A11, which dictate the specificity of the CD8+ T cell response, demonstrate an unusual ability to control viral replication regardless of early therapeutic interventions(10,12). In addition to host factors, viral genetics have been shown to play a substantial role in clinical outcome(13,14). Therefore, differences in both host and viral genetics can contribute substantially to disease progression in the setting of HIV-1 infections.
Unfortunately, however, it remains difficult to interpret the relative impact of an early therapeutic intervention during PHI in the presence of multi-faceted and heterogeneous factors such as host genetics and viral diversity. Here we report on a small epidemiologically-linked group, infected with virtually identical strains of HIV-1, in whom we had the rare opportunity to examine immune control and disease progression following treatment during PHI.
Materials and Methods
Study Subjects
All subjects were enrolled at the HIV-clinic Jessen, Germany [approved by the respective institutional review boards].
Viral Sequencing
Autologous gag and pol was sequenced from plasma RNA using population sequencing, as described previously(16). A neighbour-joining phylogenetic-tree was constructed using ClustalX [reference clade B consensus sequence (LANL)(accession no.xxxx-xxxx)]
Genetic Typing
All subjects were HLA typed using the SSP-Unitray system (Invitrogen). Screening for CCR5, CCR2 and SDF-1 polymorphisms were performed by PCR as described before(17).
Elispot Assays
HIV-1-specific CTL responses were quantified by IFN-γ-Elispot-assay using previously described optimal epitopes(18). A response was considered positive if >55 Spot Forming Cells (SFC)/106 cells, and at least 3 > mean background activity.
Results
Identification of an epidemiologically-linked cluster of HIV-1 infected subjects
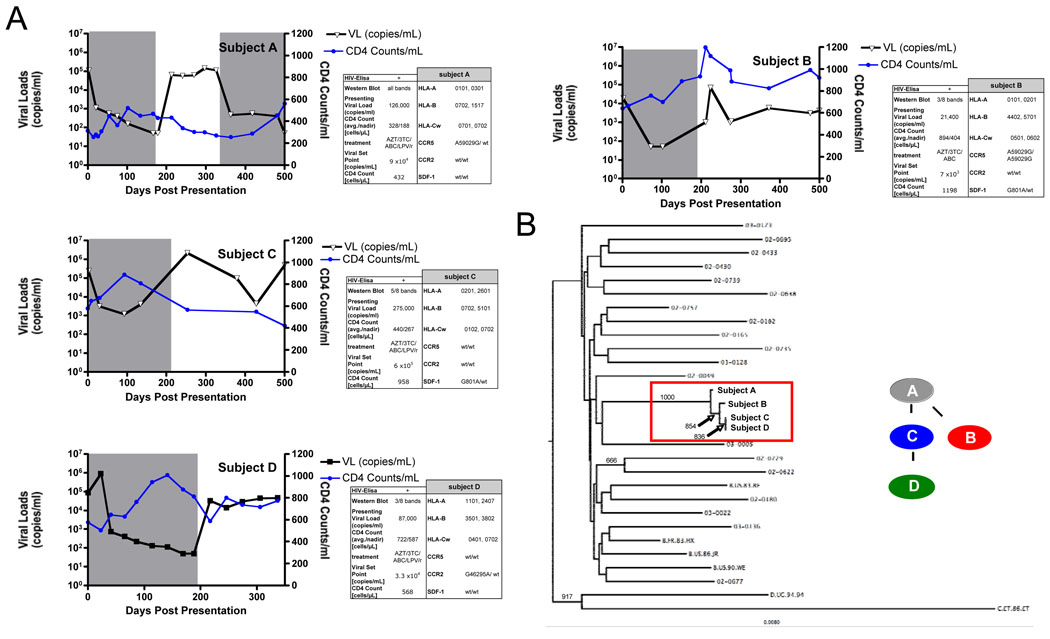
Three subjects with PHI (≤3 WB bands positive) were suspected of having been serially infected within 3 months from a chronically infected index subject following unprotected sex (Fig.1A). The status of the source person was unknown and has been tested HIV- five years ago. To verify the presumed linked transmission events, the gag and pol genes from each subject were amplified and sequenced from plasma RNA. Nearly identical HIV-1 clade B sequences were identified in each subject, with a mean pairwise nucleotide sequence identity between these sequences of 99.6%(±0.2) (Fig.1B, SFig1). These data strongly support the transmission of highly related strains of HIV-1 between these epidemiologically linked subjects over a short period of time.
Figure 1. Epidemiologically-linked transmission of HIV-1.
A) Longitudinal viral loads and CD4+ T cell counts are shown for all four subjects over a period of 900 days after presentation with primary HIV-1 infection. Lines in blue indicate the CD4+ T cell count, while lines in black indicate the viral loads in each subject. Grey shaded areas indicate the time on antiretroviral therapy in each subject. Inset shows the route of transmission of HIV-1 from subject “A” to subject “B” and “C”, while subject “D was subsequently infected by subject ”C”. B) HIV pol sequences from the four transmission subjects and nineteen other HIV chronic infected subjects from the same cohort were compared using a neighbour-joining phylogenetic tree. Sequences from the four subjects (boxed) cluster independently from sequences derived from other subjects. Scale bar indicates the genetic distance along the branches. Bootstrap values >600 are shown.
Subjects infected with nearly identical strains of HIV-1 exhibit different clinical courses
All patients immediately initiated HAART for a period of six months, achieving suppression of viral replication(Fig.1A). Within six months of cessation of therapy subjects “A”, who had already established chronic infection at the time of HAART initiation, and “C” exhibited viral load set points of 9×104 and 6×105 copies/ml, respectively, while subjects “B” and “D” exhibited lower viral load set points of 7×103 and 3.3×104 copies/ml, respectively(Fig.1A). Thus, viral loads in subjects “A” and “C” ranged in the upper quartile, which is predictive of more rapid progression of disease, while subjects “B” and “D” ranged in the second and third quartiles, associated with slower progression of disease according to the MACS studies(19). These data indicate that subjects infected with an identical viral strain, and receiving HAART during the early phase of the infection, can have a substantially different viral setpoint after treatment discontinuation, suggesting that additional factors may be responsible for the distinct clinical courses.
Role of host genetics on control of HIV-1
To examine whether differences in host genetics might account for these differences in viral set points between subjects we examined multiple genetic loci previously identified to contribute to susceptibility to HIV-1 infection and rate of progression to AIDS(11). Here we assessed differences in HLA class I alleles(20), as well as polymorphisms within the chemokine receptors (21). While multiple promotor mutations in CCR5, CCR2, and SDF-1 were observed in this cluster of individuals. Only subject “B” was found to be homozygous for the potential protective A59029G-polymorphism in CCR5(21) (Fig1A). Notably, subjects “B” and “D” also expressed the HLA alleles HLA-B57 and HLA-A11 respectively, which are associated with control of HIV-1 replication and a more favorable disease progression(10). In addition, both subjects immunodominantly targeted specific CTL epitopes (HLA-B57-HW9(Nef), HLA-B57-TW10(Gag), HLA-A11-AK11(Gag)), which have been previously associated with control of viral replication(22,23) (not shown). The immunodominant response B51-TI8(Pol) in subject “C”, with a substantial higher viral load, was in contrast not present as an escape mutation had been transmitted within this epitope. In summary these data suggest that the expression of protective HLA alleles and the immunodominant targeting of key responses by these alleles, had a strong impact on the control of viral replication following cessation of therapy.
Discussion
The identification of tightly linked transmission cases of HIV-1 provides a unique opportunity to understand the different influences of host genetics and early antiretroviral therapy on disease progression. Here we present data documenting the rapid transmission of a strain of HIV-1 clade B within four individuals with different genetic backgrounds, which resulted in distinctly different virologic and immunological outcomes. The high degree of similarity of sequences derived from each subject suggested that little viral diversification had taken place within the first few weeks of infection, and therefore was not substantially contributing to differences in viral control.
After treatment discontinuation only subjects “B” and “D” maintained relative control of viral replication after cessation of HAART, in contrast to subjects “A” and “C”. Both subjects “B” and “D” expressed HLA alleles –especially HLA-B57- associated with control of HIV-1, and they mounted immune responses against immunodominantly targeted CD8 epitopes which have previously been associated with slower disease progression(22,23). This observation is emphasized by revisiting the “Berlin patient”, the first described case of spontaneous control of viral replication following short-term treatment interruption during primary HIV-1 infection(4). Several studies since then have investigated the role of early treatment during acute HIV-1 infection with or without interruptions but have provided contradictory results(6,7,9,24,25). A re-analysis of the host genetic factors of the “Berlin patient”, who to the present date exhibits control of viral replication, has recently revealed expression of the HLA-B57 haplotype (unpublished data). Therefore, it is likely that the expression of HLA-B57 may have been one of the major contributor to the sustained control of HIV-1 replication in this subject, questioning the role of early interventional strategies for the sustained containment of HIV-1 following cessation of therapy. Taken together, these data suggests including host genetic factors into trials investigating the impact of treatment initiation during acute HIV-1 infection in order to accurately discern the relative contribution of early treatment on HIV-1 clinical outcome.
Supplementary Material
Alignment of full length gag sequences from all four study subjects to the clade B consensus sequence, illustrating the high degree of sequence similarity between different subjects. Also shown are sequences from subject “D” before and after superinfection highlighting infection with a distinct second strain of HIV-1.
Acknowledgment
We would like to thank the participating subjects in this study. We also would like to thank Mary Carrington and Yuko Yuki from the NCI Frederick, Andreas Carganico from the HIV-clinic Dupke, Carganico, Baumgarten in Berlin, Zabrina Brumme, Chanson Brumme and Adrianne Gladden from the Partners AIDS Research Center, Boston for their outstanding help to realize this project. This study was funded by NIH grants R01-AI054178 (TMA) and U01-AI052403 (TMA, BDW, MA). H.S. is supported by the Deutscher Akademischer Austauschdiensts (DAAD).
References
- 1.Kassutto S, Rosenberg ES. Primary HIV type 1 infection. Clin Infect Dis. 2004;38:1447–1453. doi: 10.1086/420745. [DOI] [PubMed] [Google Scholar]
- 2.Kahn JO, Walker BD. Acute human immunodeficiency virus type 1 infection. N Engl J Med. 1998;339:33–39. doi: 10.1056/NEJM199807023390107. [DOI] [PubMed] [Google Scholar]
- 3.Lori F, Jessen H, Lieberman J, Finzi D, Rosenberg E, Tinelli C, Walker B, Siliciano RF, Lisziewicz J. Treatment of human immunodeficiency virus infection with hydroxyurea, didanosine, and a protease inhibitor before seroconversion is associated with normalized immune parameters and limited viral reservoir. J Infect Dis. 1999;180:1827–1832. doi: 10.1086/315113. [DOI] [PubMed] [Google Scholar]
- 4.Lisziewicz J, Rosenberg E, Lieberman J, Jessen H, Lopalco L, Siliciano R, Walker B, Lori F. Control of HIV despite the discontinuation of antiretroviral therapy. N Engl J Med. 1999;340:1683–1684. doi: 10.1056/NEJM199905273402114. [DOI] [PubMed] [Google Scholar]
- 5.Lori F, Lewis MG, Xu J, Varga G, Zinn DE, Crabbs C, Wagner W, Greenhouse J, Silvera P, Yalley-Ogunro J, Tinelli C, Lisziewicz J. Control of SIV rebound through structured treatment interruptions during early infection. Science. 2000;290:1591–1593. doi: 10.1126/science.290.5496.1591. [DOI] [PubMed] [Google Scholar]
- 6.Rosenberg ES, Altfeld M, Poon SH, Phillips MN, Wilkes BM, Eldridge RL, Robbins GK, D'Aquila RT, Goulder PJ, Walker BD. Immune control of HIV-1 after early treatment of acute infection. Nature. 2000;407:523–526. doi: 10.1038/35035103. [DOI] [PubMed] [Google Scholar]
- 7.Streeck H, Jessen H, Alter G, Teigen N, Waring MT, Jessen A, Stahmer I, van Lunzen J, Lichterfeld M, Gao X, Allen TM, Carrington M, Walker BD, Rockstroh JK, Altfeld M. Immunological and virological impact of highly active antiretroviral therapy initiated during acute HIV-1 infection. J Infect Dis. 2006;194:734–739. doi: 10.1086/503811. [DOI] [PubMed] [Google Scholar]
- 8.Kinloch-de Loes S, Hoen B, Smith DE, Autran B, Lampe FC, Phillips AN, Goh LE, Andersson J, Tsoukas C, Sonnerborg A, Tambussi G, Girard PM, Bloch M, Battegay M, Carter N, El Habib R, Theofan G, Cooper DA, Perrin L. Impact of therapeutic immunization on HIV-1 viremia after discontinuation of antiretroviral therapy initiated during acute infection. J Infect Dis. 2005;192:607–617. doi: 10.1086/432002. [DOI] [PubMed] [Google Scholar]
- 9.Kaufmann DE, Lichterfeld M, Altfeld M, Addo MM, Johnston MN, Lee PK, Wagner BS, Kalife ET, Strick D, Rosenberg ES, Walker BD. Limited Durability of Viral Control following Treated Acute HIV Infection. Plos Med. 2004;1:e36. doi: 10.1371/journal.pmed.0010036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carrington M, O'Brien SJ. The influence of HLA genotype on AIDS. Annu Rev Med. 2003;54:535–551. doi: 10.1146/annurev.med.54.101601.152346. [DOI] [PubMed] [Google Scholar]
- 11.Berger EA, Murphy PM, Farber JM. Chemokine receptors as HIV-1 coreceptors: roles in viral entry, tropism, and disease. Annu Rev Immunol. 1999;17:657–700. doi: 10.1146/annurev.immunol.17.1.657. [DOI] [PubMed] [Google Scholar]
- 12.Altfeld M, Addo MM, Rosenberg ES, Hecht FM, Lee PK, Vogel M, Yu XG, Draenert R, Johnston MN, Strick D, Allen TM, Feeney ME, Kahn JO, Sekaly RP, Levy JA, Rockstroh JK, Goulder PJ, Walker BD. Influence of HLA-B57 on clinical presentation and viral control during acute HIV-1 infection. AIDS. 2003;17:2581–2591. doi: 10.1097/00002030-200312050-00005. [DOI] [PubMed] [Google Scholar]
- 13.Price DA, Goulder PJ, Klenerman P, Sewell AK, Easterbrook PJ, Troop M, Bangham CR, Phillips RE. Positive selection of HIV-1 cytotoxic T lymphocyte escape variants during primary infection. Proc Natl Acad Sci U S A. 1997;94:1890–1895. doi: 10.1073/pnas.94.5.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feeney ME, Tang Y, Pfafferott K, Roosevelt KA, Draenert R, Trocha A, Yu XG, Verrill C, Allen T, Moore C, Mallal S, Burchett S, McIntosh K, Pelton SI, St John MA, Hazra R, Klenerman P, Altfeld M, Walker BD, Goulder PJ. HIV-1 viral escape in infancy followed by emergence of a variantspecific CTL response. J Immunol. 2005;174:7524–7530. doi: 10.4049/jimmunol.174.12.7524. [DOI] [PubMed] [Google Scholar]
- 15.Allen TM, Altfeld M, Yu XG, O'Sullivan KM, Lichterfeld M, Le Gall S, John M, Mothe BR, Lee PK, Kalife ET, Cohen DE, Freedberg KA, Strick DA, Johnston MN, Sette A, Rosenberg ES, Mallal SA, Goulder PJ, Brander C, Walker BD. Selection, transmission, and reversion of an antigen-processing cytotoxic T-lymphocyte escape mutation in human immunodeficiency virus type 1 infection. J Virol. 2004;78:7069–7078. doi: 10.1128/JVI.78.13.7069-7078.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Altfeld M, Allen TM, Yu XG, Johnston MN, Agrawal D, Korber BT, Montefiori DC, O'Connor DH, Davis BT, Lee PK, Maier EL, Harlow J, Goulder PJ, Brander C, Rosenberg ES, Walker BD. HIV-1 superinfection despite broad CD8+ T-cell responses containing replication of the primary virus. Nature. 2002;420:434–439. doi: 10.1038/nature01200. [DOI] [PubMed] [Google Scholar]
- 17.Kostrikis LG, Huang Y, Moore JP, Wolinsky SM, Zhang L, Guo Y, Deutsch L, Phair J, Neumann AU, Ho DD. A chemokine receptor CCR2 allele delays HIV-1 disease progression and is associated with a CCR5 promoter mutation. Nat Med. 1998;4:350–353. doi: 10.1038/nm0398-350. [DOI] [PubMed] [Google Scholar]
- 18.Altfeld M, Kalife ET, Qi Y, Streeck H, Lichterfeld M, Johnston MN, Burgett N, Swartz ME, Yang A, Alter G, Yu XG, Meier A, Rockstroh JK, Allen TM, Jessen H, Rosenberg ES, Carrington M, Walker BD. HLA Alleles Associated with Delayed Progression to AIDS Contribute Strongly to the Initial CD8(+) T Cell Response against HIV-1. PLoS Med. 2006;3:e403. doi: 10.1371/journal.pmed.0030403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mellors JW, Rinaldo CR, Jr, Gupta P, White RM, Todd JA, Kingsley LA. Prognosis in HIV-1 infection predicted by the quantity of virus in plasma. Science. 1996;272:1167–1170. doi: 10.1126/science.272.5265.1167. [DOI] [PubMed] [Google Scholar]
- 20.Carrington M, Nelson GW, Martin MP, Kissner T, Vlahov D, Goedert JJ, Kaslow R, Buchbinder S, Hoots K, O'Brien SJ. HLA and HIV-1: heterozygote advantage and B*35-Cw*04 disadvantage. Science. 1999;283:1748–1752. doi: 10.1126/science.283.5408.1748. [DOI] [PubMed] [Google Scholar]
- 21.Kaslow RA, Carrington M, Apple R, Park L, Munoz A, Saah AJ, Goedert JJ, Winkler C, O'Brien SJ, Rinaldo C, Detels R, Blattner W, Phair J, Erlich H, Mann DL. Influence of combinations of human major histocompatibility complex genes on the course of HIV-1 infection. Nat Med. 1996;2:405–411. doi: 10.1038/nm0496-405. [DOI] [PubMed] [Google Scholar]
- 22.Frater AJ, Brown H, Oxenius A, Gunthard HF, Hirschel B, Robinson N, Leslie AJ, Payne R, Crawford H, Prendergast A, Brander C, Kiepiela P, Walker BD, Goulder PJ, McLean A, Phillips RE. Effective T-cell responses select human immunodeficiency virus mutants and slow disease progression. J Virol. 2007;81:6742–6751. doi: 10.1128/JVI.00022-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Navis M, Schellens IM, Swieten PV, Borghans JA, Miedema F, Kootstra NA, van Baarle D, Schuitemaker H. A Nonprogressive Clinical Course in HIV-Infected Individuals Expressing Human Leukocyte Antigen B57/5801 Is Associated with Preserved CD8(+) T Lymphocyte Responsiveness to the HW9 Epitope in Nef. J Infect Dis. 2008 doi: 10.1086/528695. [DOI] [PubMed] [Google Scholar]
- 24.Kinloch-De Loes S, Hirschel BJ, Hoen B, Cooper DA, Tindall B, Carr A, Saurat JH, Clumeck N, Lazzarin A, Mathiesen L, et al. A controlled trial of zidovudine in primary human immunodeficiency virus infection. N Engl J Med. 1995;333:408–413. doi: 10.1056/NEJM199508173330702. [DOI] [PubMed] [Google Scholar]
- 25.Hecht FM, Wang L, Collier A, Little S, Markowitz M, Margolick J, Kilby JM, Daar E, Conway B, Holte S. A multicenter observational study of the potential benefits of initiating combination antiretroviral therapy during acute HIV infection. J Infect Dis. 2006;194:725–733. doi: 10.1086/506616. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Alignment of full length gag sequences from all four study subjects to the clade B consensus sequence, illustrating the high degree of sequence similarity between different subjects. Also shown are sequences from subject “D” before and after superinfection highlighting infection with a distinct second strain of HIV-1.