Abstract
Polymeric micelles with cross-linked ionic cores of poly(methacrylic acid) and nonionic shell of poly(ethylene oxide) (cl-micelles) are shown here to readily internalize in epithelial cancer cells but not in normal epithelial cells that form tight junctions (TJ). The internalization of such cl-micelles in the cancer cells proceeded mainly through caveolae-mediated endocytosis. In confluent normal epithelial cells this endocytosis route was absent at the apical side and the cl-micelles sequestered in TJ regions of the cell membrane without entering the cells for at least 24 hours. Disruption of the TJ by calcium deprivation resulted in redistribution of cl-micelles inside the cells. In cancer cells following initial cellular entry the cl-micelles bypassed the early endosomes and reached the lysosomes within 30 minutes. This allowed designing cl-micelles with cytotoxic drug, doxorubicin, linked via pH-sensitive hydrazone bonds, which were cleaved in the acidic environment of lysosomes resulting in accumulation of the drug in the nucleus after 5 hours. Such pH-sensitive cl-micelles displayed selective toxicity to cancer cells but were nontoxic to normal epithelial cells. In conclusion, we describe major difference in interactions of cl-micelles with cancer and normal cells that can lead to development of novel drug delivery system with reduced side effects and higher efficacy in cancer chemotherapy.
INTRODUCTION
Recent years saw rapid emergence of polymeric nanoparticulate materials (NMs) for delivery of drugs, genes and imaging agents [1–5]. Examples of materials under development include liposomes, polymer-drug conjugates, nanogels, polymeric micelles, and the like. The precise delivery of these materials to the sites of the disease, such as tumor cells, is central for successful therapies. For example, passive targeting of nanoparticles, also termed as “Enhanced Permeation and Retention (EPR)” effect, has attracted great attention in delivery of drugs and imaging agents [6, 7]. EPR stems from an intrinsic property of certain tumors to accumulate NMs due to leaky vasculature and poor lymphatic drainage. The mechanisms of subsequent entry of NMs into target cells have also attracted great attention. The size, shape, charge and aggregation state of NMs were most recently revealed as critical determinants for their cellular entry and sub-cellular targeting [8, 9]. Specifically, different NMs employ different endocytosis mechanisms to gain cellular entry. These mechanisms are classified as clathrin dependent (clathrin mediated endocytosis) and clathrin independent pathways (caveolae mediated endocytosis, clathrin-and caveolae-independent pathways and macropinocytosis) [9]. After exploitation of these entry pathways, NMs are processed through complex sorting mechanisms and are driven to specific intracellular compartments. In the field of drug delivery the mechanisms of cellular entry of NMs and their final sub-cellular distribution, could greatly affect the performance of the drugs. Therefore, understanding these mechanisms is of significance.
The purpose of this work was to evaluate cellular entry of core-cross linked polymeric micelles (cl-micelles) of poly(ethylene oxide)-b-poly(methacrylic acid) (PEO-b-PMA) copolymer, that were recently proposed for delivery of anticancer drugs [10]. In an aqueous environment such micelles behave as nanoscale ionic gels, capable of swelling and changing charge in response to environmental changes (pH or ionic strength). Unexpectedly we found here that such micelles in confluent epithelial cells selectively bind with the tight junctions (TJ) and do not enter the cells. However, disruption of the TJ abolishes such localization and promotes entry of the micelles into the cells. In cancer cells with absent or dysfunctional TJ the cellular entry of such micelles is not restricted by the level of cell confluency. The micelles enter the cells selectively through caveolae-mediated endocytosis, bypass early endosomes and reach lysosomes. This suggests that observed control of the delivery of the synthetic NM through formation of TJ in normal epithelial cells, and enhanced entry into cancer cells and can be exploited for design of cancer-specific drug carriers.
MATERIAL AND METHODS
Materials
PEO-b-PMA diblock copolymer (Mw/Mn = 1.45) was purchased from Polymer Source Inc., Canada. The block lengths were 170 and 180 repeating units for PEO and PMA, respectively. The concentration of carboxylate groups in the copolymer samples was determined by potentiometric titration. Dox hydrochloride is a kind gift from Dong-A Pharmaceutical Company, South Korea. Calcium chloride, 1,2-ethylenediamine (ED), cystamine (Cys), 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC), N-hydroxysuccinimide (NHS), triethylamine (TEA), methanol and dimethylformamide (DMF) were obtained from Sigma-Aldrich (St Louis, MO). DOXIL™ was purchased from Ortho Biotech (Horsham, PA) and SP1049C was a kind gift from Supratek Pharma Inc. (Montreal, Canada). Alexa 488-labeled cholera toxin subunit B (Alexa 488-CTB), Alexa 488-labeled transferrin (Alexa 488-Tf), WGA (Wheat Germ agglutinin), DiD (Vybrant® DiD cell-labeling solution), Lysotracker™ (red or green), rab-5 GFP (Organelle lights™ Endosome-GFP), Polystyrene beads (FluoSpheres® carboxylate modified microspheres, blue fluorescent 365/415 and FluoSpheres® amine modified microspheres, red fluorescent 580/605)), fetal bovine serum (FBS) (both dialyzed and heat inactivated), Dulbecco’s Modified Eagle’s Medium (DMEM), were purchased from Invitrogen Inc (Carlsbad, CA). Bovine serum albumin (BSA) and NUNC™ chambered glass coverslips for live cell imaging was purchased from (Fisher Scientific, Waltham, MA). MTT reagent (3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) was purchased from Research Products International (Prospect, IL). All other chemicals were of reagent grade and used without further purification.
Cell Lines
Madin-Darby Canine Kidney epithelial (MDCK) cells, Caco-2 (human epithelial colorectal adenocarcinoma) cells, MCF-7/ADR (Dox resistant human breast adenocarcinoma) cells, 3T3 mouse embryonic fibroblasts (MEFs), KO (knock out) cell line (homozygous for a disruption of the caveolin-1 gene Cav-1 −/−, ATCC CRL-2753) and 3T3 MEFs WT (wild type, Cav-1 +/+) cell line (ATCC CRL-2752) were maintained in DMEM, containing 10% heat inactivated FBS and 0.01% penicillin/streptomycin. Primary mouse micro-vessel lung microvasculature endothelial cells (MVEC) were kind gift from Dr. Joseph Vetro (University of Nebraska Medical Center). MVEC cells were incubated in RPMI-media with 0.1% penicillin/streptomycin in the presence of MEM vitamins and sodium pyruvate. Primary BBMEC were maintained as previously described [11].
Synthesis of Dox-conjugated PEO-b-PMA
NHS (2.34 mg, 0.02 mmol) and EDC (6.0 mg, 0.03 mmol) in CH2Cl2 (1.0 ml) were added to a solution of 100 mg PEO-b-PMA in 20 ml DMF/methanol (1:1 v/v) and stirred for 2 hr at R.T. The Dox (9.1 mg, 0.016 mmol) and TEA (4.5 µl, 0.032 mmol) in methanol were added to this solution and the reaction mixture was stirred continuously for additional 24 hr. Organic solvents were evaporated in vacuum, and resulting mixture was dialyzed against distilled water for 2 days using a dialysis membrane (MW cutoff 3,500 Da). Dox-conjugated PEO-b-PMA was further purified using size exclusion chromatography and lyophilized. The degree of conjugation was 2.7 Dox per copolymer chain as determined by 1H-NMR spectroscopy.
Synthesis of fluoresceinthiocarbamyl ethylenediamine (FITC-ED)
FITC-ED was synthesized using previously reported procedure [12]. Briefly, ED (200 mg, 1.5 mM) was dissolved in the mixture of 50 ml methanol and 0.5 ml TEA. Solution of FITC (117 mg, 0.3 mmol) in 10 ml methanol containing 100 µl TEA was added dropwise to ED solution over a 30 min period followed by stirring for additional 1 hr. The resulting solution was filtered and ED-FITC was recovered by precipitation in 10 ml of methanol. The precipitate was dried in air, and used without further purification.
Synthesis of FITC-labeled PEO-b-PMA
NHS (2 mg, 0.0174 mmol) and EDC (4.8 mg, 0.025 mmol) were dissolved in 1 ml CH2Cl2 and added to a solution of 100 mg PEO-b-PMA (0.78 mmol carboxylic groups) in 2.5 ml DMF. The reaction mixture was stirred overnight at R.T. After that, 4 mg of FITC-ED in 200 µl DMF and 20 µl TEA were added to the mixture and stirred for additional 12 hr. Organic solvents were evaporated in vacuum, and resulting mixture was dialyzed against distilled water for 2 days using a dialysis membrane (MW cutoff 3,500 Da). FITC-labeled PEO-b-PMA was further purified by size exclusion chromatography and lyophilized.
Synthesis of pH-sensitive Dox-conjugated PEO-b-PMA
Dox was conjugated to PEO-b-PMA copolymer through an acid-sensitive hydrazone bond. EDC (6.0 mg, 0.03 mmol) dissolved in CH2Cl2 (1.0 mL), hydrazine hydrate (1.6 mg, 0.03 mmol) were added to a solution of 100 mg PEO-b-PMA (0.78 mmol carboxylic groups) in 20 ml DMF/methanol (1:1 v/v) and stirred for 24 hr at R.T. Organic solvents were evaporated in vacuum, resulting mixture was dialyzed against deionized water for 2 days, and hydrazine-modified PEO-b-PMA was isolated by lyophilization. 10 mg Dox in 2 ml of methanol containing 5 µl TEA were mixed with hydrazine-modified PEO-b-PMA dissolved in 20 ml DMF/Methanol (1:1 v/v) and reacted for 24 hr. Organic solvents were evaporated in vacuum, and the mixture was dialyzed against distilled water for 2 days using a dialysis membrane (MW cutoff 3,500 Da). The pH was adjusted and maintained at 8–9 during the dialysis. The resulting conjugate was further purified using PD-10 columns and lyophilized. The degree of conjugation was 1.3 Dox per copolymer chain as determined by 1H-NMR spectroscopy.
General procedure for the synthesis of cl-micelles
cl-Micelles were prepared by the previously described method [10]. In brief, Dox-conjugated PEO-b-PMA/Ca2+ or FITC-labeled PEO-b-PMA/Ca2+ complexes were prepared by mixing an aqueous solution of corresponding of PEO-b-PMA with a solution of CaCl2 at a molar ratio of [Ca2+]/[COO−]=1.3. EDC was added into solution of PEO-b-PMA/Ca2+ complexes to create an active-ester intermediate with carboxylic groups of PMA segments followed by addition of the solution of ED or Cys as bifunctional cross-linkers. The extent of cross-linking was controlled by the ratio of the amine functional groups to carboxylic acid groups. The reaction mixture was allowed to stir overnight at R.T. After completion of the reaction, ethylenediaminetetraacetic acid (EDTA) (1.5 molar equivalent) was added followed by dialysis, first, against 0.5% aqueous ammonia, and, second, against distilled water to remove metal ions and byproducts of the cross-linking reaction.
Electrokinetic mobility and size measurements
Electrophoretic mobility measurements were performed using a “ZetaPlus" analyzer (Brookhaven Instrument Co.) with a 30 mW solid-state laser operating at a wavelength of 635 nm. ζ-potential of the particles was calculated from the electrophoretic mobility values using Smoluchowski equation [13]. Effective hydrodynamic diameters of the particles were measured by photon correlation spectroscopy (DLS) in a thermostatic cell at a scattering angle of 90° using the same instrument equipped with a Multi Angle Sizing Option (BI-MAS). All measurements were performed at 25°C. Software provided by the manufacturer was used to calculate the size of the particles and polydispersity indices. The diameters mean values were calculated from the measurements performed at least in triplicate.
Atomic force microscopy (AFM)
Samples for AFM imaging were prepared by depositing 5 µl of an aqueous dispersion of cl-micelles (ca. 0.2 mg/ml) onto positively charged 1-(3-aminopropyl) silatrane mica surface (APS-mica) for 10 min. followed by surface washing with deionized water and drying under argon atmosphere. The AFM imaging in air was performed with regular etched silicon probes (TESP) with a spring constant of 42 N/m using a Multimode NanoScope IV system (Veeco, Santa Barbara, CA) operated in a tapping mode. The images were processed and the widths and heights of the particles were measured using Femtoscan software (Advanced Technologies Center, Moscow, Russia).
FACS analysis
MDCK or MCF-7/ADR (5×104 cell/well in 24 well plates) cells were plated and experiments were performed on either confluent or non-confluent cells. Cells were exposed to various concentrations of different NMs that include i) Dox-labeled PEO-b-PMA copolymer, ii) Dox-labeled cl-micelles, iii) FITC-labeled cl-micelles, iv) carboxylate modified polystyrene beads, v) amine modified polystyrene beads. Cells were then washed and trypsinized, centrifuged, re-suspended in PBS (pH7.4, 1% BSA) and followed by FACS analysis. In select experiments, 3T3 MEF Cav KO or WT cells were exposed to i) Dox-labeled cl-micelles, ii) Dox-labeled PEO-b-PMA copolymer, iii) DOXIL™. The cells positive for fluorescence were termed as the % gated cells and the mean fluorescence intensity was analyzed using Becton Dickinson FACStarPlus flow cytometer operating under Lysis II (San Jose, CA) equipped with an argon ion laser. The normalized mean fluorescence was calculated by multiplying the % gated cells to the mean fluorescence. Data were acquired in linear mode and visualized in logarithmic mode. Data from 10,000 events were gated using forward and side scatter parameters to exclude debris and dead cells. All experiments were conducted thrice and measurements were conducted in triplicates. The data presented as means ± SEM.
Confocal analysis on live cells
5×104 MDCK cells/well were grown in chambered glass coverslips for 2–3 days till they reach confluence. Confluent cells were then exposed to different NMs, which include i) Dox-labeled PEO-b-PMA copolymer, ii) cl-micelles (Dox or FITC-labeled, Cys or ED attached cross linker, pH labile or stable bound Dox), iii) carboxylate modified or amine modified polystyrene beads, iv) DOXIL™, and v) SP1049C for 1 hr (or longer periods as indicated in legends) followed by washing with PBS. Cells were then visualized utilizing live cell confocal imaging (Carl Zeiss LSM 510 Meta, Peabody, MA). In select experiments, confluent cells were exposed to Dox labeled cl-micelles for 1 hr and in the final 10 min., WGA (10µg/ml) and DiD (10 µg/ml) were added. Cells were then washed and live cell confocal microscopy was performed. For endocytosis studies, confluent MCF-7/ADR cells were plated in the chambered coverslips and were exposed for 30 min to either FITC or Dox labeled cl-micelles in the presence or absence of 5 g/ml Alexa 488-CTB or 5 µg/ml Alexa 488-Tf. Similarly, non-confluent MDCK and MCF-7/ADR cells were treated with 100 nM of Lysotracker™ for 10 min. Cells were finally washed and kept in complete media for imaging. In select experiments, 3T3MEF Cav WT and KO (1 × 106 cells) were plated in live cell chambered coverslips and exposed to cl-micelles, followed by washing and visualization under the confocal microscope. Confluent MCF-7/ADR cells were exposed to pH-sensitive cl-micelles for 1 hr followed by washing with PBS. Live cell based time lapse studies were performed for 5 hrs and changes in localization were visualized using confocal microscope with pictures taken every 1 hr. cl-micelles (pH stable) were used as a positive control.
Immunocytochemistry on fixed MDCK cells
MDCK cells (confluent or non-confluent) were washed with PBS and fixed with 4% paraformaldehyde. Cells were incubated with rabbit anti-caveolin-1-Cy3 antibody (Sigma Aldrich, St Louis, MO) (1:100) in blocking buffer overnight at 4°C. Cells were washed and examined under confocal microscope.
MTT assay
Cytotoxicity of cl-micelles was assessed in MDCK and MCF-7/ADR cells by MTT assay as described previously [14]. Briefly, cells were seeded in a 96-well microtiter plates and allowed to adhere for 24 hrs or to reach confluency. Cells were treated with free Dox or Dox incorporated in different NMs at various doses (0–100 µg/ml on doxorubicin basis) for 24 h at 37 °C, followed by washing with PBS, and maintaining in DMEM medium (10% FBS) for additional 72 h. 25 µl of MTT indicator dye (5 mg/ml) was added to each well and the cells were incubated for 2 h at 37 °C in the dark. 100 µl of 50% DMF-20% SDS solution was added to each well and kept overnight at 37 °C. Absorption was measured at 570 nm in a microplate reader (SpectraMax M5, Molecular Devices Co., USA) using wells without cells as blanks. The reading taken from the wells with cells cultured with control medium was used as 100% viability value. The cell viability was calculated as Asample/Acontrol × 100%. Based on the results of the test, the IC50 values (the concentration which kills 50% of cells) were calculated by using GraphPad Prism Software (GraphPad Software, San Diego California, USA).
Cell transfection
Cells were transfected using Organelle Lights™ Endosome-GFP kit. This kit contains a Organelle Lights™ reagent which is baculovirus, a efficient delivery system that contains a gene sequence which encodes for Rab5a (targeting sequence, an early endosome marker) fused to a GFP (fluorescent protein) already incorporated into the viral genome and the kit also has an enhancer solution for increased expression of the chimera. Briefly, cells were plated, allowed to attach, and treated with Organelle Lights reagent at R.T. in the dark for 2–4 hrs. The reagent was aspirated and cells were incubated in DMEM medium containing 1X enhancer for 2 hrs followed by washing and addition of complete medium. The transfected cells were treated with Dox labeled cl-micelles for 1 hr, washed and imaged using confocal microscopy
Calcium depletion
MDCK cells were grown in cell culture media containing dialyzed FBS and low content of calcium ions (5 µM Ca2+) for 2 days in 24-well plates. Confluent cells were then exposed to cl-micelles or PEO-b-PMA copolymer and the experiments were performed by FACS or confocal microscopy as discussed above.
Transepithelial electrical resistance (TEER) measurements
MDCK cells were seeded at the density of 5×104 cell per well in HTS Transwell-24 System (Corning Inc., Corning, NY) and TEER was measured by using Epithelial Voltohmmeter (EVOM™, World Precision Instruments, Inc., FL). TEER values (Ω × cm2) were calculated by multiplying the effective membrane area of Transwell-24 System and TEER measurement. TEER values were recorded for five consecutive days after plating the MDCK cells. On each day the apical side of the cells was exposed to cl-micelles for 1 hr. Cells were washed, trypsinized, and resuspended in PBS containing 1% BSA. Uptake of cl-micelles was determined by FACS as described above.
RESULTS
Preparation of cl-micelles
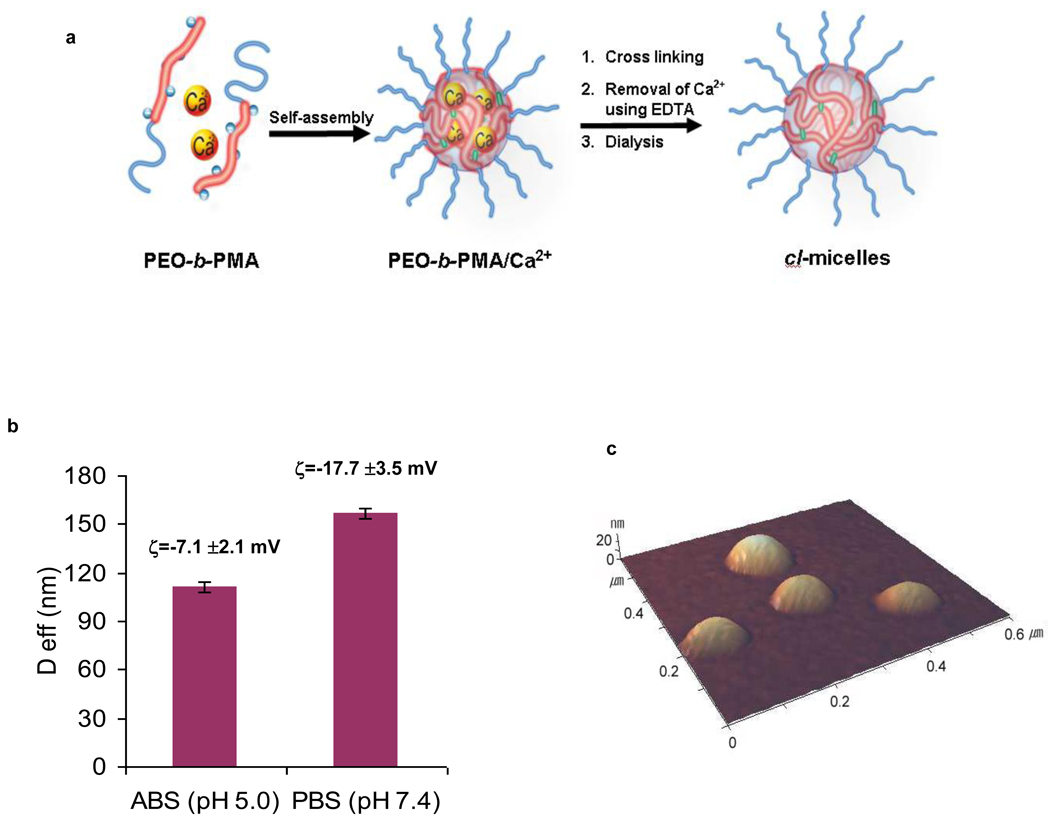
PEO-b-PMA micelles with cross-linked ionic cores were synthesized using a two-step process shown schematically in Fig. 1a. Briefly, the PEO-b-PMA block copolymer was first condensed by CaCl2 resulting in the micelles with PEO shell and PMA/Ca2+ complex core. Second, the core was chemically cross-linked by ethylenediamine and Ca2+ ions were removed by ethylenediaminetetraacetic acid (EDTA). The resulting cl-micelles represent hydrogel-like soft NMs, which have swollen cores of a cross-linked PMA network surrounded by a shell composed of PEO blocks [10]. These micelles swelled considerably as pH increased due to ionization of the PMA chains in the micelles core (Fig. 1b). The swelling was accompanied by an increase in the net negative charge of the particles (Fig. 1c) and was completely reversible. At extracellular pH 7.4 the micelles had a strong net negative charge (zeta potential of about −18 mV) and were ca. 150–160 nm in diameter. At pH 5.0 the zeta-potential increased to about −7 mV and the micelles size decreased to ca. 110 nm. Since the cores were cross-linked the particle sizes did not change even upon 100-fold dilution. Based on the AFM images the cl-micelles had a spherical morphology (Fig. 1c) and were practically uniform (polydispersity index 0.1; such narrow polydispersity was also confirmed by DLS). The carboxylic groups in the micelle cores were used to attach fluorescent moieties such as Doxorubicin (Dox) and fluorescein isothiocyanate (FITC), to track cl-micelles in the cells. This was achieved by, first, labeling the PEO-b-PMA with Dox or FITC and, second, preparing cl-micelles as described above. At relatively low degrees of labeling (1 Dox or FITC per ca. 60 to 90 carboxyl groups) the size, charge or swelling behavior of cl-micelles was not affected by the presence of the labels.
Fig. 1. PEO-b-PMA cl-micelles.
a. Synthesis of the cl-micelles. First, PEO-b-PMA is reacted in aqueous solution with CaCl2 to form the polyion-metal core micelles. Second, the micelle cores are cross-linked by ethylenediamine in the presence of a water-soluble carbodiimide, EDC. Finally, Ca2+ ions are removed by EDTA followed by dialysis. b. Effect of pH on effective diameter (Deff) and ζ-potential of the cl-micelles. c. Typical AFM images of the cl-micelles.
Effect of TJ formation on cl-micelles entry into epithelial cells
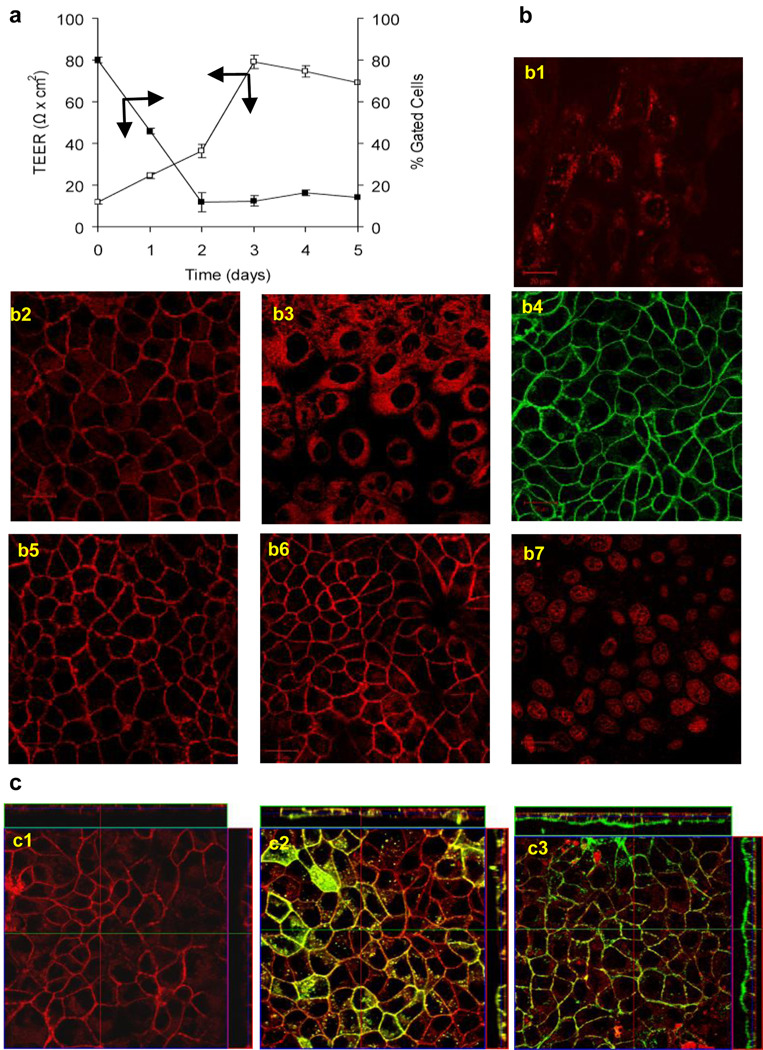
In most experiments cells were exposed to the labeled cl-micelles for 60 min., which allowed accumulating sufficient amounts of the fluorescence to be visualized by different methods in all cell types. Interestingly, there was a drastic difference in cl-micelles internalization in normal epithelial and cancer cell lines. Specifically, in MDCK cells the entry and cellular localization of the cl-micelles was drastically dependent on the cell confluence. The cl-micelles were readily internalized into the sub-confluent MDCK cells but were excluded from internalization and localized at the periphery in the confluent cells (Fig. 2a, 2b1, 2b2). This peripheral staining of the confluent cells by cl-micelles was seen even after 12 hrs exposure (see Supplementary Movie S1). Interestingly, the observed localization pattern for the cl-micelles had a striking resemblance to ZO-1 staining of the TJs in the confluent cells (see Supplementary Fig. S1). Furthermore, this localization pattern of the cl-micelles was abolished upon calcium deprivation (Fig. 2b3), which dysfunctions the TJs. The z-stack suggested that the cl-micelles were localized at the apical sides of the cell-cell contacts (Fig. 2c1). Furthermore, little co-localization of the cl-micelles was observed with the membrane probes, which were either evenly distributed throughout the membrane (DiD) or localized mainly in the basolateral regions of the membranes (WGA) (Fig. 2c2, 2c3).
Fig. 2. Cellular uptake of cl-micelles is regulated by formation of TJ.
a. Uptake of cl-micelles in MDCK cells was measured as a function of time. b. Live cell confocal imaging of MDCK cells exposed to cl-micelles with non-degradable cross-links (b1–b4), pH-sensitive cl-micelles (b5), cl-micelles with a cystamine linker (b6), or Dox alone (b7). The experiments used non confluent cells (b1) or confluent cells without (b2, b4–b7) or with (b3) calcium depleted medium. The cl-micelles were labeled with Dox (b1–b3, b5–b7) or FITC (b4). C. Fluorescence confocal microscopy z stacks made after exposure of confluent cells exposed to Dox-labeled cl-micelles (c1) alone and in the presence DiD (c2) or WGA (c3). (An orthogonal section of the z-stack image is presented). In all experiments, cells were exposed to 200 µg/ml of cl-micelles on copolymer basis or 100 µg/ml of Dox (b7) for 1 hr.
The apparent TJ localization of the cl-micelles appeared to be an intrinsic property of the block copolymer material used for the micelle preparation. First, the same localization pattern was observed independent on which fluorescent group, Dox or FITC, was used to visualize the cl-micelles (Fig. 2b4), or whether this group (Dox) was attached to the micelles via stable amide or pH-labile hydrazone bonds (Fig. 2b5). Second, no difference in localization was observed for micelles containing ethylene diamine or cystamine linker connecting the block copolymer molecules (Fig. 2b6). Third, the same localization was observed for non cross-linked PEO-b-PMA block copolymer (Fig. 3c4). However, the free fluorescent molecule, Dox, was localized in the nucleus (Fig. 2b7).
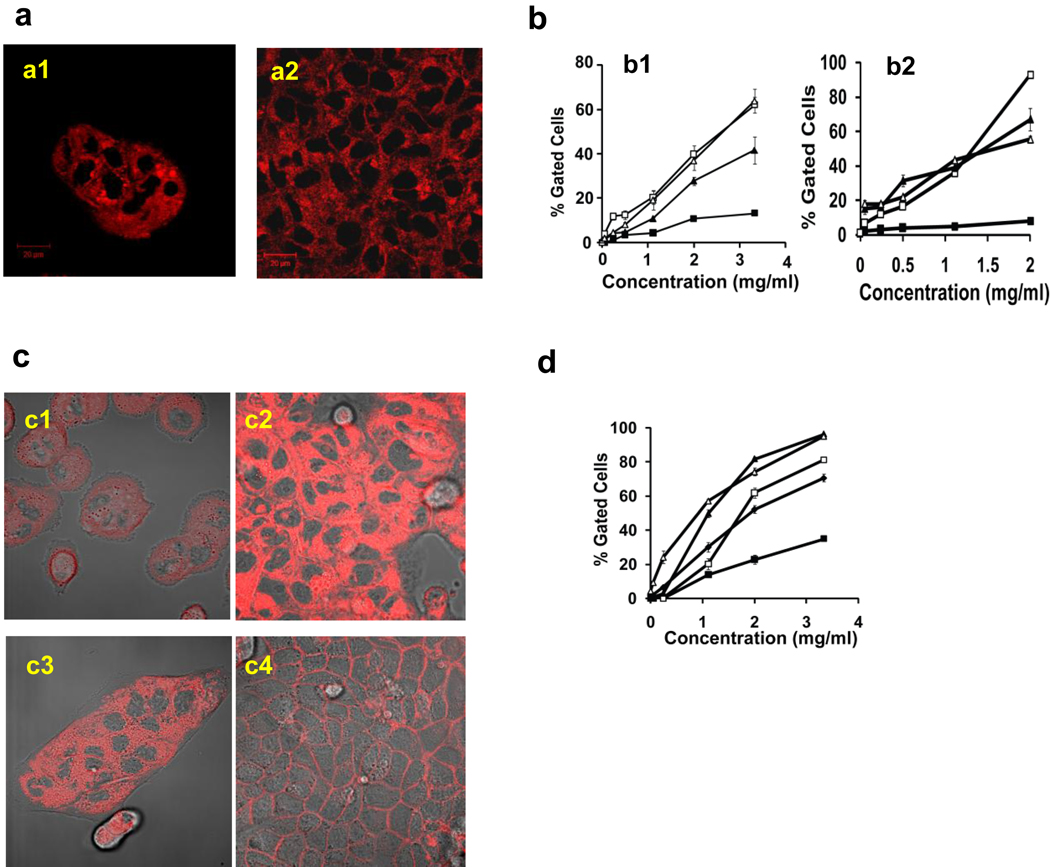
Fig. 3. Cellular uptake of cl-micelles and PEO-b-PMA based copolymer is restricted by formation of tight junctions.
a. Confocal microscope was performed on (a1) Non-confluent or (a2) confluent MCF-7/ADR cells exposed to cl-micelles (200 µg/ml) for 1hr followed by imaging. b. Flow cytometery was performed on cells exposed to DOX-labeled cl-micelle (b1), FITC-labeled cl-micelles (b2) for 1 hr. C. Live cell confocal imaging was performed on MCF-7/ADR (c1, c2) and MDCK cells (c3, c4) exposed to PEO-b-PMA-Dox for 1 hr at 37°C. The localization of copolymer was imaged as function of confluency of these cells: (c1, c3) non confluent and (c2, c4) confluent. d. Flow cytometery was performed after cells were exposed to different concentrations of copolymer was treated for 1 hr. The symbols depict the following: (□) non-confluent or (■) confluent MDCK cells, (◆) confluent MDCK cells deprived of calcium and (△) non-confluent or (▲) confluent MCF-7/ADR cells. Data are mean ± S.E.M. (n = 3).
Quite different behavior was observed in the breast cancer MCF-7/ADR cells, which did not form the TJs [15]. In this case the same localization pattern of cl-micelles was observed independently of the extent of the cell confluence (Fig. 3a). The confocal microscopy suggested that the cl-micelles readily internalized in sub-confluent or confluent MCF-7/ADR cells. Therefore, we quantified the uptake of cl-micelles labeled by Dox or FITC in both cell lines using flow cytometery (Fig. 3b1, 3b2). As already mentioned above, the micelle entry was inhibited in confluent MDCK cells with unaffected TJs. In contrast, substantial uptake was observed in sub-confluent MDCK cells, Ca2+ deprived confluent MDCK cells or MCF-7/ADR cells independently of their confluence. Similar results were obtained with the PEO-b-PMA copolymer alone (without cross-linking) (Fig. 3c, 3d).
Next we examined a very different set of NMs using negatively charged (carboxylate coated) or positively charged (amino coated) polystyrene beads (approximately 200 nm, see Fig. S2a, S2b). Both materials in contrast to cl-micelles or PEO-b-PMA copolymer internalized efficiently (approx. 100% gated cells) in all cell types independently of the cell confluency (Fig. S2c, S2d). Furthermore, the localization pattern of such NMs revealed no apparent TJ staining and suggested that they were entering the confluent MDCK cells (Fig. S2e, S2f). However, the measurements of the normalized mean fluorescence indicated that internalization of these materials was nevertheless decreased in the confluent MDCK cells, while in the cancer cells the internalization was independent on the confluence (Table S1). Generally, this result was consistent with some literature data suggesting the formation of the TJs is accompanied with the decrease in the cell endocytosis [16, 17]. However, in the case of the cl-micelles the inhibition of the internalization in the confluent cells was nearly complete.
Internalization and intracellular routing of cl-micelles
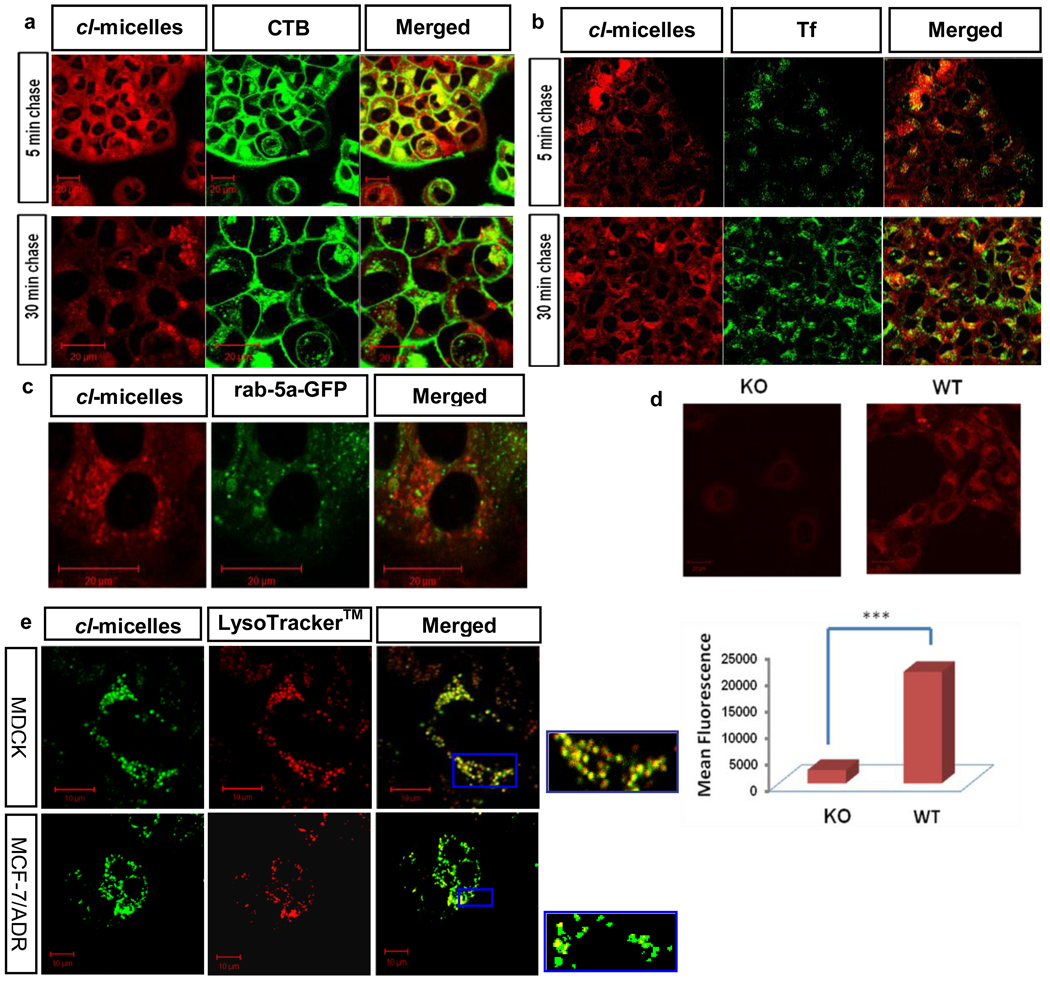
We hypothesized that the unusual behavior of cl-micelles was due to a pathway of their entry into cells, which is affected in confluent MDCK cells but not in the non-confluent or cancer cells. Therefore, we characterized the trafficking of cl-micelles in different cells using cholera toxin B (CTB) or transferrin (Tf) as markers of the caveolae- and clathrin-mediated endocytosis respectively [9]. In the initial experiment, the cl-micelles were pulsed to MCF-7/ADR cells in the presence of CTB for 30 min., followed by a chase of 5 min or 30 min. At 5 min we observed co-localization of the cl-micelles and CTB in vesicular structures, which suggested a caveolae-mediated uptake for the cl-micelles (Fig. 4a). We confirmed that the caveolae indeed was a major pathway for the cl-micelles by comparing the internalization of the cl-micelles in wild type (WT) and caveolae knock out (KO) 3T3 mouse fibroblasts. Interestingly, the cl-micelles internalization in KO cells was negligible compared to the WT cells. This was further reinforced using flow cytometery demonstrating over 20-times difference in the uptake of cl-micelles in these cells (Fig. 4d). Such drastic inhibition of the internalization of cl-micelles in the cells with abolished caveolae pathway suggests that the cl-micelles were highly selective for this pathway. This was reinforced using Tf as a marker for clathrin-mediated endocytosis. In contrast to CTB after 5 min. there was little if any co-localization of Tf with cl-micelles in MCF-7/ADR cells (Fig. 4b). Furthermore, 10 min. post incubation we found little co-localization of cl-micelles with rab 5-GFP, which was transfected in these cells to serve as marker for early endosomes (Fig. 4c, and Movie S2). The co-localization with early endosomes was also not observed at earlier time points, such as 5 min. and 20 min after the onset of incubation (Fig. S3). Altogether, the initial stages of entry of cl-micelles into cancer cells appeared to be restricted to the caveola-emediated endocytosis pathway and do not involve early endosomes.
Fig. 4. The cl-micelles utilize caveolae mediated endocytosis to enter cells bypass the early endosomes and are delivered to the lysosomes.
MCF-7/ADR cells were pulsed for 30 min. with cl-micelles in the presence of (a) Alexa 488 labeled CTB or (b) Alexa 488 labeled Tf and then chased from 5 to 30 min. c. Cells were transfected with Rab-5 GFP and after 16 hrs, exposed to cl-micelles for 30 min. followed by live cell imaging with a 10 min. pulse. d. Confocal microscopy and flow cytometery analysis on KO and WT mouse 3T3 fibroblasts exposed to Dox-labeled cl-micelles for 1hr. (data are mean ± SEM, *** p<0.001). e. Cells (MDCK or MCF-7/ADR) were exposed to FITC-labeled cl-micelles for 1hr. and to LysoTracker™ red (red) for 10 min. at 37°C followed by live cell imaging. Inset shows localization of polymer within the vesicular compartment of the lysosomes. In all experiments cells were exposed to 200 µg/ml of cl-micelles.
However, at the later stages of 30 min. the co-localization of the cl-micelles and CTB in MCF-7/ADR cells was significantly decreased (Fig. 4a). To the contrary, the co-localization of cl-micelles with Tf was increased (Fig. 4b). At this time point Tf is known to reach the lysosomes, while CTB is routed to Golgi and ER [18]. Therefore, we suggested that the cl-micelles following the initial caveolae-mediated entry into cells after 30 min. were routed to the lysosomes. This was directly confirmed by co-localization of cl-micelles with LysoTracker™ in both cancer and non-confluent MDCK cells (Fig 4e, and Movie S3).
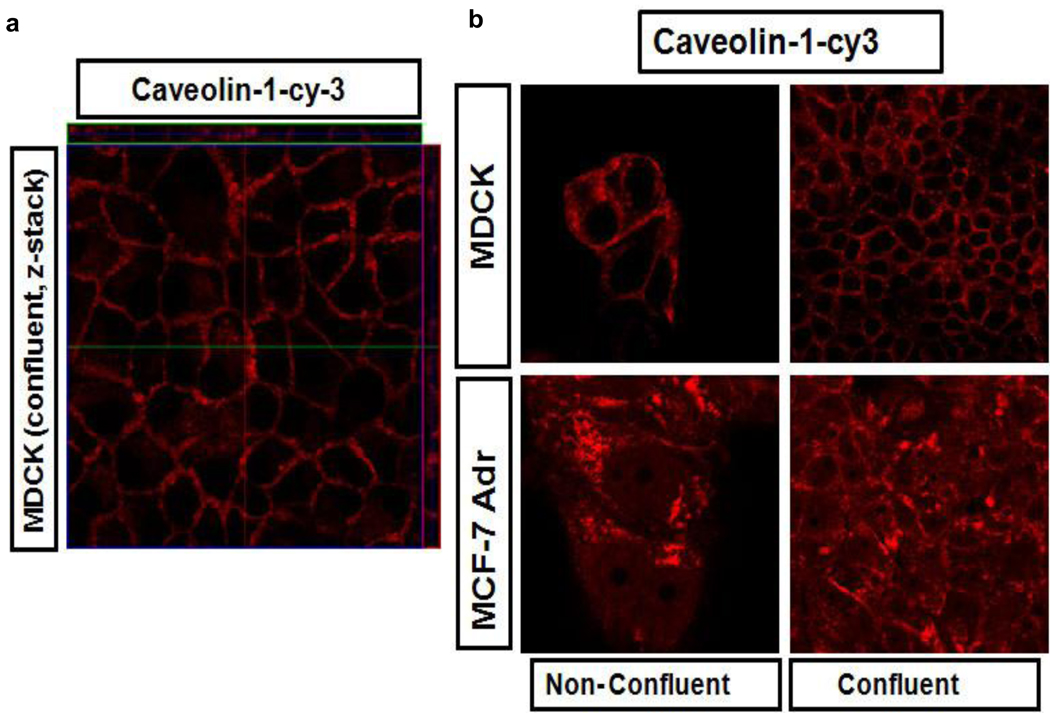
Interestingly, we noticed that in the confluent MDCK cells the uptake of CTB was decreased although not completely abolished. Indeed, using the antibodies to caveolin-1 we demonstrated that in the confluent MDCK cells the intracellular caveolin-1 staining was diminished and located at the lateral regions of the plasma membrane (Fig. 5a). This appears to be exactly the same localization pattern as was observed for the cl-micelles (Fig. 2). In contrast, in non-confluent MDCK cells and in MCF-7/ADR cells caveolin-1 was present in the intracellular compartments as well as membranes (Fig. 5b).
Fig. 5. Caveolin-1 localization in non-confluent and confluent MDCK and MCF7/ADR cells.
a. The z-stack image of confluent MDCK cells. b. Comparison of localization of caveolin-1 in non-confluent and confluent in MDCK and MCF-7/ADR cells. Immunocytochemistry was performed using caveolin-1-cy3 antibody.
Therefore, we believe that the cl-micelles cannot enter the confluent MDCK cells because these cells have altered caveolae-mediated pathway, which is necessary for these micelles entry. The selectivity of the cl-micelles to the caveolae-mediated pathway stands out compared to other materials. For example, CTB is known to exploit caveolae and non-caveolae pathways, which may explain some internalization of this marker in confluent MDCK cells discussed above. Moreover, we also saw decreased but still present internalization of CTB in caveolae-deficient KO fibroblasts. Likewise, we found that both types of polystyrene beads used in this study easily entered the KO fibroblasts. Thus even though the entry of such nanoparticles was somewhat decreased in confluent MDCK cells, their internalization and localization was different than those of the cl-micelles. To the contrary the free PEO-b-PMA block copolymer revealed exactly the same behavior as the cross-linked micelles in KO fibroblast and co-localized with CTB at the initial stages (5 min) of the entry in MCF-7/ADR (Fig. S4).
Finally, we examined a different epithelial cell line, Caco-2, which forms TJ. In these confluent cells the cl-micelles exhibited a similar pattern of peripheral localization as in confluent MDCK cells (Fig. S5a). There, however, existed few vesicular structures with cl-micelles within the intracellular compartments, which could be due to enhanced endocytic activity of Caco-2 cells since they were derived from a tumor cell line. The non-multidrug resistant breast carcinoma MCF-7 cells, which are capable of formation of TJ [19], demonstrated both the peripheral localization and decreased intracellular vesicular structures (Fig. S5b). At the same time in the ovarian carcinoma cells A2780 with impaired TJ [15], the cl-micelles were readily internalized and did not show the TJ localization pattern (Fig. S5c). Interestingly, the cl-micelles also internalized in the primary bovine brain microvessel endothelial cells, BBMEC, and mouse lung microvasculature endothelial cells (Fig. S5d, S5e). This suggests that the relationship between formation of TJ and inhibition of cellular entry of cl-micelles might be a distinctive property of epithelial cells.
Lysosomes-specific delivery of Dox with cl-micelles
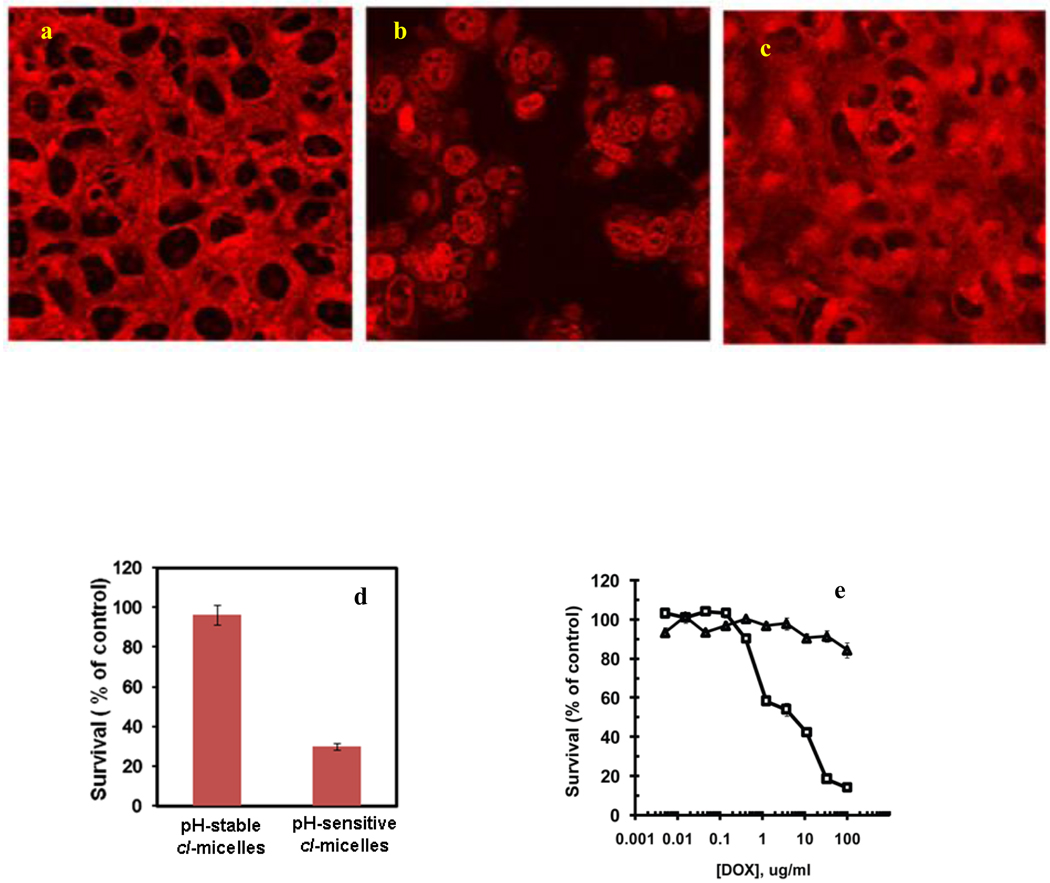
Since our analysis concluded that in the cancer cells following the caveolae-mediated uptake the cl-micelles are transported to the lysosomes, we decided to exploit this property for the organelle-specific delivery of the cytotoxic drug. To achieve this goal we conjugated Dox to the micelle core via a pH-sensitive hydrazone linker, which is stable at extracellular pH 7.4 but degrades upon acidification. Since the loading degree may affect the physicochemical properties and internalization behavior of the cl-micelles, in this experiment the Dox loading was kept the same as in the internalization studies. After 1 hr incubation of such pH-sensitive micelles with MCF7/ADR cells the Dox fluorescence was mainly constrained to the vesicular structures (Fig. 6a). However, after 5 hrs this localization pattern was changed and Dox entered the nucleus (Fig. 6b). In contrast, in cl-micelles with stably conjugated Dox no nuclear fluorescence was observed for at least 5 hrs (Fig. 6c). Importantly, the pH-sensitive micelles exhibited significant toxicity in MCF-7/ADR cells, while the pH-stable micelles were non-toxic (Fig. 6d).
Fig. 6. The pH-sensitive cl-micelles release Dox which reach the nucleus within 5 hrs and leads to significant toxicity in MCF-7/ADR cells but toxicity in case of MDCK cells is dependent on confluence.
a–c. MCF-7/ADR cells were exposed for 1 hr. to cl-micelles containing Dox conjugated via pH-sensitive hydrazone link (a, b) or non-sensitive link (c). Then cells were washed and visualized immediately (a), or after additional 4 hrs. incubation in the fresh media (b, c). d. MCF-7/ADR were exposed to cl-micelles with pH-sensitive or stably conjugated Dox (100 µg/ml) and cytotoxicity studies were performed. e. The confluent (□) or non-confluent (△) MDCK cells were exposed to the cl-micelles and cell viability was assessed using MTT assay.
Furthermore, since the pH-sensitive cl-micelles showed impaired entry in confluent MDCK cells (Fig. 2b5), we evaluated whether their cytotoxicity also depended on the cell confluence. Indeed, such micelles were cytotoxic to the non-confluent cells and totally non-toxic to the confluent cells (Fig. 6e). We interpreted this by the inability of cl-micelles to internalize and reach the acidic compartments of the confluent cells, which is necessary to release the drug. Even after 24 hrs of incubation with confluent MDCK cells Dox linked to the cl-micelles via hydrazone bonds was not released and remained at the cell periphery (Fig. S6a). In contrast the free Dox was cytotoxic to both non-confluent and confluent cells, although its IC50 was considerably higher in confluent cells (Table 1). Furthermore, we compared the pH-sensitive cl-micelles with two different NMs - a liposomal Dox formulation, DOXIL™ and a Pluronic-based micellar Dox formulation, SP1049C, which are clinically approved (DOXIL™) or in clinical development (SP1049C). Surprisingly, in non-confluent and confluent MDCK cells DOXIL™ exhibited similar cytotoxicity pattern as the cl-micelles, while SP1049C behaved more like the free Dox (Table 1, Fig. S6b, S6c).
Table 1.
IC50 values (µg/ml) of Dox-loaded polymeric nanoformulations in different cell types (the cytotoxicity curves are provided in Fig. S5b and S5c).
| Sample | MDCKa | MDCKb | MCF-7/ADR |
|---|---|---|---|
| Free DOX | 0.049 ± 0.33 | 6.93 ± 1.14 | 15.4 ± 1.16 |
| pH-sensitive DOX cl-micelles | 2.76 ± 0.31 | NT | 60.4 ± 1.79 |
| SP1049C | 0.046 ± 0.011 | 1.55 ± 0.13 | 12.6 ± 1.20 |
| DOXIL™ | 13.6 ± 1.44 | NT | ca. 200 |
non confluent;
confluent, NT stands for non-toxic up to 100 µg/ml.
Interestingly, the cellular entry of DOXIL™ nanoparticles (effective diameter 85.8 nm and zeta potential −2.6 mV in PBS, pH 7.4) was caveolae-mediated (co-localization with CTB) and it was routed to the lysosomes in cancer cells similarly to cl-micelles (co-localization with LysoTracker™ Green) (Fig. S7a, S7b). Furthermore, DOXIL™ exhibited the same localization pattern as the cl-micelles in the confluent MDCK cells (Fig. S7c) and was unable to enter caveolae-deficient KO cells (Fig. S7e). In contrast, Dox delivered with SP1049C in the confluent MDCK cells was able to enter cells and reach the nucleus like the free drug (Fig. S7d).
DISCUSSION
Polymeric nanocarriers have gained significant interest for chemotherapy of cancer, which remains one of the most deadly diseases with nearly 12 million new cases reported worldwide each year [2, 20]. Nearly 90% of all cancers including breast, lung, prostrate and colon cancer originate from the epithelial cells [15]. The epithelial cells function as a boundary between the external environment and the tissue. They form TJ on the apical side, which hold cells together, prevent passage of integral membrane proteins from apical to basolateral side and restrict transfer of molecules and ions between cells. These functions contribute to the apical-basolateral polarity of the epithelial cells [15, 21]. Normal epithelial cells are sealed by TJ, which serve as a molecular sieve that excludes molecules and ions of a radius exceeding 15 Å (ca. 3.5 kDa) [22]. Most macromolecules and NMs are above this size limit. Therefore, they cannot transport into normal epithelium through paracellular route and have to utilize transcellular routes of internalization. The one notable exception is charged dendrimers and chitosan nanoparticles, which were reported to open TJs and facilitate trans- and para-cellular transport [23–25]. However, neither the copolymer nor the cl-micelles appear to perturb TJs as shown by ZO-1 staining (Fig. S8). This suggests that such NMs are more likely to engage transcellular routes. Such routes include (i) clathrin-mediated endocytosis, (ii) caveolae-mediated endocytosis, (iii) clathrin- and caveolae-independent endocytosis, and (iv) micropinocytosis [16, 26, 27]. Notably, loss of TJs has been widely reported as one of the contributing factors for transition of normal epithelia to a cancerous phenotype [28]. It has been proposed that overactive endocytic rate, derailed endocytic trafficking and dissolution of cell-cell contacts potentiate metastasis and serve as a hallmark of cancer [28]. Hence, the transport of macromolecules and NMs can also drastically change in cancer.
Remarkably, we demonstrate here for the that there is one type of NMs, cl-micelles that enter cells selectively through caveolae-mediated endocytosis and exhibit drastic difference in transport in TJ forming and non-forming epithelial cells. The cl-micelles belong to a broader class of polymeric micelles, which have core-shell architecture. For example, polymeric micelles formed by amphiphilic block copolymers have hydrophobic core capable of incorporating water-insoluble drugs and hydrophilic shell surrounding the core and preventing aggregation of the micelles [1]. Several such polymeric micelle formulations for cancer chemotherapy are in Phase I and Phase II clinical trials and one of them, SP1049C, already reached the Phase III stage [29, 30]. The cl-micelles are a distinct class of NMs formed by doubly hydrophilic block copolymers containing ionic and nonionic water-soluble blocks [10, 31]. They are soft, hydrogel-like structures that have cross-linked PMA polyanion cores and nonionic PEO shell. Surprisingly, such cl-micelles display peripheral localization and little entry in TJ-forming epithelial cells but unrestricted entry in the sub-confluent cells or cancer epithelial cells that do not form TJ. A characteristic property of such cl-micelles is the negative charge of the PMA chains of the copolymer. However, the particle charge alone cannot account for their unusual behavior, since both positively- and negatively charged polystyrene-based nanoparticles were able to internalize and had different localization in the TJ-forming cells. Nevertheless, another NM, DOXIL™, based on negatively charged PEGylated (i.e. covered by PEO) liposomes loaded with Dox displayed a similar behavior as the cl-micelles. Thus, we conclude that TJ forming epithelial cells can restrict the entry of selected NMs, such as negatively charged PEO-covered cl-micelles and DOXIL™ liposomes. In contrast, the sub-confluent cells and cancer cells with impaired TJ readily internalize such materials.
We present data suggesting that striking differences in localization and intracellular trafficking of cl-micelles in epithelial cells with and without TJ are due to the differences in endocytosis pathways displayed in these cells. Surprisingly, the initial stages of endocytosis of cl-micelles were strongly restricted to the caveolae-mediated pathway. Caveolae are flask shaped cholesterol-rich micro-domains that are employed by viruses to internalize [18]. In the TJ forming MDCK cells the caveolae are absent at the apical side of the plasma membrane and localize either in the lateral regions (Fig. 5) or the basolateral membrane [32]. In fact, the peripheral localization of caveolae marker, caveolin-1, in confluent MDCK cells was very similar to that of the cl-micelles. In contrast, in sub-confluent MDCK cells and MCF-7/ADR cells caveolin-1 appeared to be partially internalized. Interestingly, it was shown that over-expression of a member of the apoptosis-regulating protein family, Bcl-2, in MDCK and MCF-7 cells, results in disruption of junctional complexes concurrent with intracellular redistribution of their components [19]. Furthermore, in confluent MDCK cells internalization of CTB was considerably decreased, while in MCF-7 cells the CTB readily internalized and, at the early time point, co-localized with the cl-micelles. Thus, the lack of internalization of cl-micelles in TJ forming epithelial cells appears to be tied to the alterations of caveolae at the apical side of such cells. Disruption of TJ is accompanied by loss of polarity, concomitant redistribution of caveolae from the basolateral to the apical side and increase in the caveolae mediated endocytosis. We believe that the latter is one reason for the increased cellular entry of cl-micelles in sub-confluent normal epithelial cells or cancer cells that lack TJ. Interestingly, the relationship between TJ and caveolae has been discussed in literature already. For example, it was demonstrated that disruption of TJ in mouse brain endothelial cells by proinflammatory cytokine CCL2 involves caveolae-dependent internalization of transmembrane TJ proteins [33]. However, we present evidence that disruption of TJ in epithelial cells results in enhanced caveolae-mediated transport of a man-made NM. This conclusion is reinforced using DOXIL™, another NM characterized by caveolae-mediated cellular transport. As mentioned both materials are modified with PEO chain, however, PEGylation alone cannot explain the observed behavior since other materials, such Pluronic micelles, were shown to enter cells through different routes [9]. Notably, contrary to epithelial cells, endothelial cells express caveolae at both apical and basolateral sides even after TJ-formation [34]. This may explain why the entry of cl-micelles in endothelial cells was not restricted to the same extent as in the epithelial cells.
We further demonstrate that in epithelial cancer cells following the initial entry the cl-micelles bypass the early endosomes and are transported to the lysosomes. This result appears to contradict the general belief that the materials that are internalized via caveolae route usually avoid the lysosomal compartments [35]. However, some studies already suggested that caveolae-mediated route may communicate at later stages of entry with the classical clathrin route, which in most cases delivers its cargo to lysosomes [36]. Even more importantly, it has been recently proposed that during cancer progression the endocytic trafficking becomes deregulated and caveolae directs its cargo towards lysosomes. In particular, the TJ proteins, which use caveolae for internalization and recycling in normal epithelia, in cancerous cells are routed to lysosomes for degradation [28]. This initiates the loss of cell-cell contacts and increases metastatic potential of cancer. Therefore, the delivery of the cl-micelles to the lysosomes may be characteristic for cancers derived from epithelial cells.
Based on this it was of interest to evaluate whether such property can be useful and translated to the design of anti-cancer nanomedicines. For this purpose we synthesized pH-sensitive cl-micelles that can release the drug in acidic environment of lysosomes. Consistent with this design Dox was initially delivered with cl-micelles to the lysosomes but then after 5 hrs was released and accumulated in the nucleus of the MCF-7/ADR cells. This resulted in significant toxicity of such pH-sensitive cl-micelles with respect to the cancer cells. However, the growth of confluent MDCK cells was practically unaffected upon treatment with such micelles, because these cells lack the mechanism for the micelles internalization and delivery to the lysosomes. Interestingly, a similar cytotoxicity pattern was observed with DOXIL™ liposomes, which are also known to stably incorporate Dox in extracellular media but enhance drug release in lysosomes environment [37]. In contrast, free Dox as well as SP1049C, which rapidly releases Dox outside of the cells, were highly toxic to confluent MDCK cells. Therefore, we have shown that pH-sensitive cl-micelles can deliver and release Dox in cancer but not in normal kidney cells.
CONCLUSIONS
This study describes cl-micelles that display a selectivity of cellular entry via caveolae-mediated endocytosis. We also show that the entry of such cl-micelles is inhibited in TJ-forming epithelial cells but permitted in cancer cells that do not form TJ. This difference is due to lack of caveolae-mediated endocytosis at the apical side of TJ-forming epithelial cells. In cancer cells, following initial stages of cellular entry cl-micelles avoid early endosomes but are ultimately routed to lysosomes. A possibility of creating cl-micelles containing a drug linked to the micelles via pH sensitive bond, which is stable at extracellular pH but cleavable in lysosomes, is further demonstrated. Such pH-sensitive drug loaded cl-micelles can release drug in lysosomes compartments and exhibit selective toxicity to cancer cells but are not toxic to normal epithelial cells that form TJ. Overall this work demonstrates that cellular trafficking of selected NMs can be very different in cancer and normal cells and reinforces the need of investigation of peculiarities of such interactions for design of safe and efficient nanomedicines.
Supplementary Material
Acknowledgement
This study was supported National Institutes of Health R01 R01CA089225 to AVK, R01CA116590 to TKB and UNMC Bukey Fellowship to GS. We will like to thank Dr. Keith Johnson for a fruitful discussion; Daria Alakhova for assistance in drawing cl-micelles, Yi Zhao and Sheila Higginbotham for assistance to maintain MCF-7/ADR and BBMEC cells, respectively. We would also like to thank the Confocal Microscopy and Flow Cytometery Core Facilities at UNMC.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author’s contributions: JOK and GS contributed equally to this work; AVK and TKB designed research; all authors contributed to data analysis, discussions and writing the paper.
REFERENCES
- 1.Kabanov AV, Alakhov VY. Pluronic block copolymers in drug delivery: from micellar nanocontainers to biological response modifiers. Crit Rev Ther Drug Carrier Syst. 2002;19(1):1–72. doi: 10.1615/critrevtherdrugcarriersyst.v19.i1.10. [DOI] [PubMed] [Google Scholar]
- 2.Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R, Langer R. Nanocarriers as an emerging platform for cancer therapy. Nat Nanotechnol. 2007;2(12):751–760. doi: 10.1038/nnano.2007.387. [DOI] [PubMed] [Google Scholar]
- 3.Duncan R. The dawning era of polymer therapeutics. Nat Rev Drug Discov. 2003;2(5):347–360. doi: 10.1038/nrd1088. [DOI] [PubMed] [Google Scholar]
- 4.Torchilin VP. Recent advances with liposomes as pharmaceutical carriers. Nat Rev Drug Discov. 2005;4(2):145–160. doi: 10.1038/nrd1632. [DOI] [PubMed] [Google Scholar]
- 5.Otsuka H, Nagasaki Y, Kataoka K. PEGylated nanoparticles for biological and pharmaceutical applications. Adv Drug Deliv Rev. 2003;55(3):403–419. doi: 10.1016/s0169-409x(02)00226-0. [DOI] [PubMed] [Google Scholar]
- 6.Maeda H. The enhanced permeability and retention (EPR) effect in tumor vasculature: the key role of tumor-selective macromolecular drug targeting. Adv Enzyme Regul. 2001;41:189–207. doi: 10.1016/s0065-2571(00)00013-3. [DOI] [PubMed] [Google Scholar]
- 7.Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumor tropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986;46(12):6387–6392. [PubMed] [Google Scholar]
- 8.Gratton SE, Ropp PA, Pohlhaus PD, Luft JC, Madden VJ, Napier ME, et al. The effect of particle design on cellular internalization pathways. Proc Natl Acad Sci U S A. 2008;105(33):11613–11618. doi: 10.1073/pnas.0801763105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sahay G, Batrakova EV, Kabanov AV. Different internalization pathways of polymeric micelles and unimers and their effects on vesicular transport. Bioconjug Chem. 2008;19(10):2023–2029. doi: 10.1021/bc8002315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bronich TK, Keifer PA, Shlyakhtenko LS, Kabanov AV. Polymer micelle with cross-linked ionic core. J Am Chem Soc. 2005;127(23):8236–8237. doi: 10.1021/ja043042m. [DOI] [PubMed] [Google Scholar]
- 11.Batrakova EV, Li S, Miller DW, Kabanov AV. Pluronic P85 increases permeability of a broad spectrum of drugs in polarized BBMEC and Caco-2 cell monolayers. Pharm Res. 1999;16(9):1366–1372. doi: 10.1023/a:1018990706838. [DOI] [PubMed] [Google Scholar]
- 12.Pourfarzaneh M, White GW, Landon J, Smith DS. Cortisol directly determined in serum by fluoroimmunoassay with magnetizable solid phase. Clin Chem. 1980;26(6):730–733. [PubMed] [Google Scholar]
- 13.Woodle MC, Collins LR, Sponsler E, Kossovsky N, Papahadjopoulos D, Martin FJ. Sterically stabilized liposomes. Reduction in electrophoretic mobility but not electrostatic surface potential. Biophys J. 1992;61(4):902–910. doi: 10.1016/S0006-3495(92)81897-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferrari M, Fornasiero MC, Isetta AM. MTT colorimetric assay for testing macrophage cytotoxic activity in vitro. J Immunol Methods. 1990;131(2):165–172. doi: 10.1016/0022-1759(90)90187-z. [DOI] [PubMed] [Google Scholar]
- 15.Gonzalez-Mariscal L, Lechuga S, Garay E. Role of tight junctions in cell proliferation and cancer. Prog Histochem Cytochem. 2007;42(1):1–57. doi: 10.1016/j.proghi.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 16.Harush-Frenkel O, Altschuler Y, Benita S. Nanoparticle-cell interactions: drug delivery implications. Crit Rev Ther Drug Carrier Syst. 2008;25(6):485–544. doi: 10.1615/critrevtherdrugcarriersyst.v25.i6.10. [DOI] [PubMed] [Google Scholar]
- 17.Foerg C, Ziegler U, Fernandez-Carneado J, Giralt E, Merkle HP. Differentiation restricted endocytosis of cell penetrating peptides in MDCK cells corresponds with activities of Rho-GTPases. Pharm Res. 2007;24(4):628–642. doi: 10.1007/s11095-006-9212-1. [DOI] [PubMed] [Google Scholar]
- 18.Carver LA, Schnitzer JE. Caveolae: mining little caves for new cancer targets. Nat Rev Cancer. 2003;3(8):571–581. doi: 10.1038/nrc1146. [DOI] [PubMed] [Google Scholar]
- 19.Li L, Backer J, Wong AS, Schwanke EL, Stewart BG, Pasdar M. Bcl-2 expression decreases cadherin-mediated cell-cell adhesion. J Cell Sci. 2003;116(18):3687–3700. doi: 10.1242/jcs.00644. [DOI] [PubMed] [Google Scholar]
- 20.Duncan R. Polymer conjugates as anticancer nanomedicines. Nat Rev Cancer. 2006;6(9):688–701. doi: 10.1038/nrc1958. [DOI] [PubMed] [Google Scholar]
- 21.Tsukita S, Furuse M, Itoh M. Multifunctional strands in tight junctions. Nat Rev Mol Cell Biol. 2001;2(4):285–293. doi: 10.1038/35067088. [DOI] [PubMed] [Google Scholar]
- 22.Salama NN, Eddington ND, Fasano A. Tight junction modulation and its relationship to drug delivery. Adv Drug Deliv Rev. 2006;58(1):15–28. doi: 10.1016/j.addr.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 23.Sweet DM, Kolhatkar RB, Ray A, Swaan P, Ghandehari H. Transepithelial transport of PEGylated anionic poly(amidoamine) dendrimers: implications for oral drug delivery. J Control Release. 2009;138(1):78–85. doi: 10.1016/j.jconrel.2009.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kitchens KM, Kolhatkar RB, Swaan PW, Eddington ND, Ghandehari H. Transport of poly(amidoamine) dendrimers across Caco-2 cell monolayers: Influence of size, charge and fluorescent labeling. Pharm Res. 2006;23(12):2818–2826. doi: 10.1007/s11095-006-9122-2. [DOI] [PubMed] [Google Scholar]
- 25.Smith J, Wood E, Dornish M. Effect of chitosan on epithelial cell tight junctions. Pharm Res. 2004;21(1):43–49. doi: 10.1023/b:pham.0000012150.60180.e3. [DOI] [PubMed] [Google Scholar]
- 26.Conner SD, Schmid SL. Regulated portals of entry into the cell. Nature. 2003;422(6927):37–44. doi: 10.1038/nature01451. [DOI] [PubMed] [Google Scholar]
- 27.Giepmans BN, van Ijzendoorn SC. Epithelial cell-cell junctions and plasma membrane domains. Biochim Biophys Acta. 2009;1788(4):820–831. doi: 10.1016/j.bbamem.2008.07.015. [DOI] [PubMed] [Google Scholar]
- 28.Mosesson Y, Mills GB, Yarden Y. Derailed endocytosis: an emerging feature of cancer. Nat Rev Cancer. 2008;8(11):835–850. doi: 10.1038/nrc2521. [DOI] [PubMed] [Google Scholar]
- 29.Armstrong A, Brewer J, Newman C, Alakhov V, Pietrzynski G, Campbell S, et al. SP1049C as first-line therapy in advanced (inoperable or metastatic) adenocarcinoma of the oesophagus: A phase II window study. J Clin Oncology ASCO Annual Meeting Proceedings Part I. 2006;24:4080. [Google Scholar]
- 30.Danson S, Ferry D, Alakhov V, Margison J, Kerr D, Jowle D, et al. Phase I dose escalation and pharmacokinetic study of pluronic polymer-bound doxorubicin (SP1049C) in patients with advanced cancer. Br J Cancer. 2004;90:2085–2091. doi: 10.1038/sj.bjc.6601856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bontha S, Kabanov AV, Bronich TK. Polymer micelles with cross-linked ionic cores for delivery of anticancer drugs. J Control Release. 2006;114(2):163–174. doi: 10.1016/j.jconrel.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 32.Vogel U, Sandvig K, van Deurs B. Expression of caveolin-1 and polarized formation of invaginated caveolae in Caco-2 and MDCK II cells. J Cell Sci. 1998;111(Pt 6):825–832. doi: 10.1242/jcs.111.6.825. [DOI] [PubMed] [Google Scholar]
- 33.Stamatovic SM, Keep RF, Wang MM, Jankovic I, Andjelkovic AV. Caveolae-mediated internalization of occludin and claudin-5 during CCL2-induced tight junction remodeling in brain endothelial cells. J Biol Chem. 2009;284(28):19053–19066. doi: 10.1074/jbc.M109.000521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Frank P, Pavlides S, Lisanti M. Caveolae and transcytosis in endothelial cells: role in atherosclerosis. Cell Tissue Res. 2009;335(1):41–47. doi: 10.1007/s00441-008-0659-8. [DOI] [PubMed] [Google Scholar]
- 35.Rejman J, Bragonzi A, Conese M. Role of clathrin- and caveolae-mediated endocytosis in gene transfer mediated by lipo- and polyplexes. Mol Ther. 2005;12(3):468–474. doi: 10.1016/j.ymthe.2005.03.038. [DOI] [PubMed] [Google Scholar]
- 36.Kiss AL, Botos E. Endocytosis via caveolae: alternative pathway with distinct cellular compartments to avoid lysosomal degradation. J Cell Mol Med. 2009;13(7):1228–1237. doi: 10.1111/j.1582-4934.2009.00754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gregoriadis G. Engineering liposomes for drug delivery: progress and problems. Trends Biotechnol. 1995;13(12):527–537. doi: 10.1016/S0167-7799(00)89017-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.