Abstract
The small and large subunits of the ribosome are held together by a series of bridges, involving RNA–RNA, RNA–protein and protein–protein interactions. Some 12 bridges have been described for the Escherichia coli 70S ribosome. In this work, we have targeted for mutagenesis, some of the 16S rRNA residues involved in the formation of intersubunit bridges B3, B5, B6, B7b and B8. In addition to effects on subunit association, the mutant ribosomes also affect the fidelity of translation; bridges B5, B6 and B8 increase decoding errors during elongation, while disruption of bridges B3 and B7b alters the stringency of start codon selection. Moreover, mutations in the bridge B5, B6 and B8 regions of 16S rRNA also correct the growth and decoding defects associated with alterations in ribosomal protein S12. These results link bridges B5, B6 and B8 with the decoding process and are consistent with the recently described location of translation factor EF-Tu on the ribosome and the proposed involvement of h14 in activating Guanosine-5′-triphosphate (GTP) hydrolysis by aminoacyl-tRNA•EF-Tu•GTP. These observations are consistent with a model in which bridges B5, B6 and B8 contribute to the fidelity of translation by modulating GTP hydrolysis by aminoacyl-tRNA•EF-Tu•GTP ternary complexes during the elongation phase of protein synthesis.
INTRODUCTION
A universal feature of translation systems is the presence of two unequally-sized ribosomal subunits. The large and small subunits are held together by a series of non-covalent bridges that form upon subunit association, during the initiation phase of protein synthesis and are disrupted in the recycling step. In the Escherichia coli 70S ribosome, some 12 different bridges connect the two subunits and six of these are conserved in eukaryal, cytoplasmic and mitochondrial ribosomes (1–3). Structural analyses of various 70S ribosomal complexes suggest that in addition to holding the two subunits together, the intersubunit bridges may also serve to modulate ribosomal activities; bridges have been proposed to serve as communication conduits between large and small subunits, to regulate translocation and the interaction of the ribosome with release and recycling factors (4–6). Moreover, at least some intersubunit contacts are disrupted during the translocation step, which is accompanied by a ratchet-like movement of the two subunits (1).
The function of the ribosomal bridges has also been studied using biochemical and genetic approaches. Modification/interference experiments have identified 6 nt in 16S rRNA that are essential for subunit association (7). Five of these bases are components of bridges while the sixth is base paired to a bridge nucleotide. A catalog of the RNA and protein components of the intersubunit bridges was derived from a 5.5Å map of the 70S ribosome (6) and since then, base substitution mutations in several of the rRNA bases involved in bridge formation have been constructed and analyzed (5,8–10). In this work, we have constructed substitutions in 16S rRNA bases involved in formation of bridges B3, B5, B6, B7b and B8 and expressed these mutant ribosomes in a strain of E. coli (Δ7 prrn) expressing only plasmid-encoded rRNA. As was observed with the 23S rRNA bridge mutants, several of the 16S rRNA bridge mutants showed little effect on growth or subunit association in vivo (9). Analyses of the decoding properties of the mutant ribosomes indicate that bridges B3 and B7b influence the fidelity of initiation codon selection while bridges B5, B6 and B8 contribute to the tRNA selection process during elongation.
Among the mutations that were constructed were several two-base substitutions at C345–G346 in helix 14 (h14) of 16S rRNA. This helix contacts residues in the large subunit ribosomal proteins L14 and L19 to form bridge B8. Previous genetic analyses by Maisnier-Patin et al. (11) showed that mutations in another bridge B8 component, protein L19, ameliorated the phenotypes of certain error-restrictive mutations in ribosomal protein S12. In the work described here, we show that mutations in the 16S rRNA nucleotides of bridges B5, B6 and B8 also suppress some of the decoding and growth defects caused by mutations in ribosomal protein S12. The effects of h14, h44 and L19 mutations on decoding likely derive from effects on ribosome–EF-Tu interactions, since cryo-EM analyses and X-ray crystallography of ribosomal complexes has shown that conserved elements of EF-Tu contact h14 upon binding of aminoacyl-tRNA•EF-Tu•GTP to the ribosome (12–14). Together, these data lead us to propose that a network of interactions, involving ribosomal proteins L14 and L19, together with helices h14 and h44 in 16S rRNA contribute to the fidelity of the decoding process, through effects on the function of aminoacyl-tRNA•EF-Tu•GTP on the ribosome during elongation.
MATERIALS AND METHODS
Bacterial strains and plasmids
Strain pop2136 which carries the temperature-sensitive λcI repressor was used as host strain for cloning experiments. The Δ7 prrn strain MC338 [ΔrrnA ΔrrnB ΔrrnCΔrrnD ΔrrnE ΔrrnG ΔrrnH ΔlacZ ΔrecA/pTRNA67, pCsacB7 (15)] was derived from SQ351, a kind gift of Drs Selwyn Quan and Catherine Squires, Tufts University, Boston. Streptomycin resistant derivatives of MC338 were isolated using the underlay technique (16). Briefly, cells were plated on antibiotic-free LB agar and incubated for 3 h at 37°C, after which time, a 200 µl aliquot of a 25 mg/ml solution of streptomycin was introduced underneath the agar disc. The streptomycin was allowed to diffuse into the agar and the pates were reincubated for 3–6 days. Resistant colonies were recovered and the rpsL genes were sequenced. Several of the slow-growing isolates were found to contain the K42N substitution in protein S12 and one such allele was designated rpsL2225. The pCsacB7 plasmid in MC338 rpsL2225 was replaced with the rrnB plasmid, prrnS12 (17) to give strain MC343. The Δ7 prrn strains, MC333, MC334 and MC335, carrying streptomycin-dependent mutations in rpsL have been described previously (17). A further streptomycin-dependent rpsL mutation that replaces glycine at position 91 in S12 with an aspartate residue (G91D) was isolated in subsequent selections with MC338, to give strain MC345.
The 16S mutant rRNAs were expressed from plasmid pKK3535, which expresses the rrnB operon from the native, constitutive P1P2 promoters.
All of the lacZ reporter plasmids were constructed in plasmid pLG339 (18). This kanamycin and tetracycline resistant plasmid carries a pSC101 origin and is compatible with the rrnB plasmid, pKK3535 and the pACYC184-derived plasmid used to express tRNAs in the Δ7 prrn strains. The lacZ genes from the previously-described pSG series of lacZ frameshift, nonsense and initiation codon reporter constructs (19) were amplified by PCR using primers upstream of the Ptac promoter and downstream of the lacZ termination codon, respectively. The amplified fragments were cloned into the BamHI or EcoRV sites of pLG339 and the resulting constructs were verified by nucleotide sequencing.
Construction of 16S rRNA bridge mutants
Site-directed mutagenesis of 16S rRNA was carried out using a PCR-based extension-overlap protocol (20). Generation of Δ7 prrn strains expressing mutant rRNA exclusively involves replacing the resident wild-type rrn plasmid with plasmids encoding mutant rRNA. In MC338, plasmid pCsacB7 carries the wild-type rrnC operon as well as a kanamycin resistance marker and the sacB gene, conferring sensitivity to sucrose. The ampicillin resistant, wild-type rrnB plasmid pKK3535 and its mutant derivatives were introduced into MC338 by transformation. Ampicillin resistant transformants were purified, grown in liquid medium overnight and plated on sucrose- and ampicillin-containing plates. The sucrose resistant colonies were then tested for loss of kanamycin resistance. The rpsL2225-containing strain MC343 expresses wild-type rRNA from plasmid prrnS12 and is streptomycin sensitive since it also carries the wild-type rpsL gene on prrnS12 and streptomycin resistance is recessive. Upon transformation of MC343 with pKK3535-type plasmids, streptomycin resistance can be generated through loss of prrnS12 and this was confirmed by testing for kanamycin sensitivity.
Growth rate determinations, sucrose gradient analysis of ribosomes and β-galactosidase assays
Doubling times of strains growing in liquid LB medium at 37°C were carried out as described (17). The growth of 16S bridge mutants on solid medium was observed by streaking cells on LB agar plates containing ampicillin and incubated for 24 h and 72 h at 30°C and 37°C, respectively.
Sucrose gradient analysis of ribosomes was carried out as described previously (21). Cells were lysed in buffers containing either 5 mM or 10 mM MgCl2, loaded onto gradients containing the same concentration of MgCl2 and centrifuged at 17 000 rpm for 18 h in a Beckman SW28 rotor at 4°C. Ribosomal subunits, 70S ribosomes and polysomes were fractionated using an ISCO gradient fractionator connected to a UV detector, by displacement with 70% Glycerol.
The β-galactosidase activities of the rRNA mutants were assayed as described previously and activities were expressed in Miller units (19,22).
Structural analysis
Distances between bridge atoms were measured with UCSF Chimera (23). Electron density maps were calculated with Phenix (24) to ensure that the measured atomic positions were consistent with the experimental crystallographic data. Briefly, PDB entries 2J00, 2J01, 2J02 and 2J03 were submitted to a few rounds of rigid body refinement against the publicly available structure factors. The generated maps were visualized with UCSF Chimera (23).
RESULTS
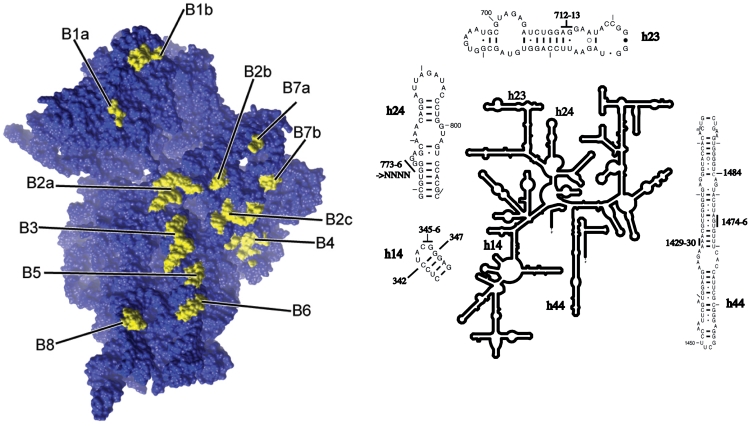
In this work, we targeted for mutagenesis the 16S rRNA bridge nucleotides C1484 (bridge B3), U1474, G1475, A1476 (bridge B5), A1429, A1430 (bridge B6) A712, G713, G773, G774, G775, G776 (bridge B7b) and C345, G346, G347 (bridge B8; Figure 1). At the initiation of this study, these nucleotides had not previously been analyzed by mutagenesis.
Figure 1.
Locations of bridges on the 30S ribosomal subunit and of the 16S rRNA bridge residues mutagenized in this study. The intersubunit bridges on the Thermus thermophilus 30S subunit (PDB entry # 2J00) are depicted on the left panel. The right hand panel shows the E. coli 16S rRNA secondary structure, with insets indicating the mutations described in the text.
Effects of bridge B3, B5, B6, B7b and B8 mutations on viability and subunit association
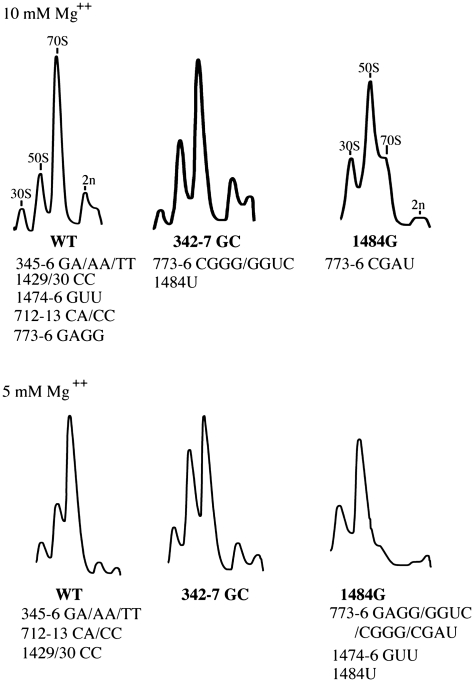
Helix 44 is a prominent structural feature on the subunit interface of the 30S subunit and makes multiple contacts with the 50S subunit at bridges B2a, B3, B5 and B6 (Figure 1). Bridge B5 comprises several distinct interactions between the U1420/G1475 region of h44 and protein L14, as well as with helices 62 and 64 of the 50S subunit. In bridge B6, the A1429/A1430 region of h44 contacts the G1702–C1704 region of 23S rRNA. All three single base substitutions were constructed at positions U1474, G1475, A1476 (bridge B5) and at A1429 and A1430 (bridge B6). When expressed in the Δ7 prrn strain MC338, none of the single base mutations had any effect on growth rate or subunit association, as assayed by analyses of cell lysates on sucrose gradients (data not shown). Consequently, two more radical, multi-base mutations were constructed in bridges B5 and B6, U1474G/G1475U/A1486U and A1429C/A1430C, respectively that were predicted to disrupt pairing in h44. (Hereafter, these mutations are referred to as 1474-6 GUU and 1429-30 CC). Despite this, the 1429-30 CC bridge B6 mutation had no detectable effect on subunit association in vivo, as judged by the relative proportions of free subunits and 70S ribosomes in sucrose gradients containing either 5 mM or 10 mM Mg++ (Figure 2). In addition, the 1429-30 CC mutation had no effect on cell growth and these results demonstrate that disruption of this region of h44 is well-tolerated in vivo without detectable effects on 70S ribosome formation. The 1474-6 GUU mutation in bridge B5 had no effect on growth in liquid or solid medium at 37°C, but had a substantial effect on growth on solid medium at 30°C. The 3-base mutation had little or no effect on subunit association in gradients containing 10 mM Mg++. However, when the Mg++ concentration in the lysis buffers and sucrose gradients was lowered to 5 mM, this mutation had a severe effect on the formation of 70S ribosomes (Figure 2). These results suggest that 30S–50S subunit contacts are altered in this B6 mutant, but that the in vivo concentrations of Mg++ and polyamines are sufficient to mask these effects on subunit association.
Figure 2.
Sucrose gradient analyses of wild-type and mutant ribosomes. Gradient profiles on the top and bottom rows are from experiments carried out in buffers containing 10 mM and 5 mM Mg++, respectively. Mutants were grouped according to the relative abundance of free 50S and 30S subunits versus 70S ribosomes. The identities of the representative gradients are indicated in bold and the identities of other members of each gradient group are indicated beneath.
In bridge B3, the 1483–1486 region of h44 contacts h71 of 23S rRNA. In contrast to the bridge B5 and B6 mutations, all three single base mutations at C1484 in bridge B3 had substantial effects on growth and subunit association. When expressed from the native P1P2 promoters of rrnB in MC338, the C1484A mutation had a dominant lethal phenotype and no transformants were recovered when MC338 was transformed with pKK3535-derived plasmids carrying this mutation. The C1484U and C1484G mutants were viable, but had substantially increased doubling times (108 and 140 min, respectively). The C1484U mutation had only modest effects on subunit association when ribosomes were extracted and analyzed at 10 mM Mg++. However, at lower Mg++ concentrations, 70S ribosomes were barely detectable (Figure 2). The C1484G mutant 30S subunits associated poorly with 50S subunits at both Mg++ concentrations. These results highlight the importance of bridge B3 for subunit association. It is also noteworthy that among a collection of 23S rRNA bridge mutations previously constructed in our laboratory, some of the single base substitutions affecting bridge B3 had the greatest effects on subunit association in vivo (9).
In order to explore the basis for the lethality of the C1484A mutation, plasmid pKK1484A was mutagenized either by passage through a mutator strain or via error-prone PCR and viable mutants were recovered in MC338. A majority of the viable transformants had lost the C1484A mutation and three isolates had undergone an A→G change at 1484, replacing a lethal mutation with a highly deleterious, but nonetheless viable mutation. Three further mutants that retained the C1484A mutation also carried a T1414C mutation in 16S rRNA while a single isolate carried the original C1484A mutation and a G1416T base change. Reconstruction experiments confirmed that these second site mutations in h44 alone were responsible for the suppression of C1484A’s lethal phenotype. The results of both mutageneses indicate that the lethality caused by the C1484A mutation can be rescued by additional base changes at adjacent positions in helix 44 (Figure 3). The T1414C mutation has the result of replacing a U–G wobble pair with a G–C base pair, while the G1416T mutation replaces a G–A mismatch (caused by the C1484A base change) with a U–A base pair. Together, these results suggest that the helical stability of this region of h44 is critical for ribosomal function.
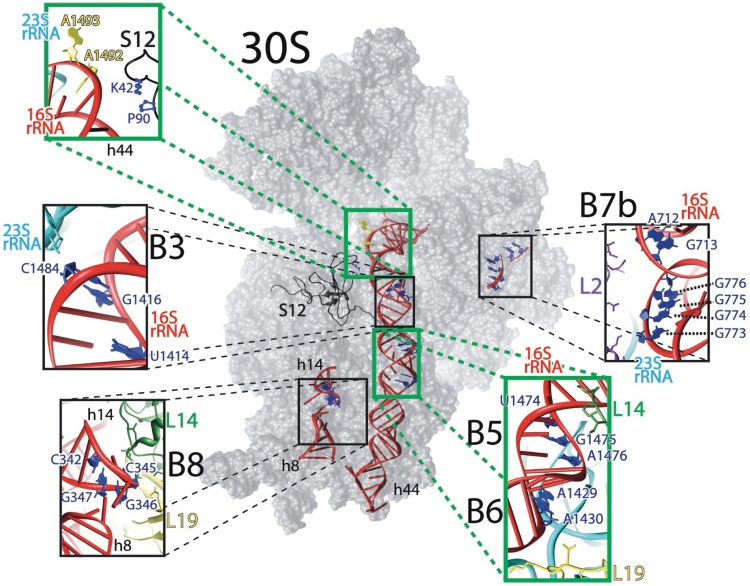
Figure 3.
The subunit interface of the 30S ribosomal subunit. The E. coli 30S ribosomal subunit (PDB entry # 2I2P) with insets depicting detailed views of bridges B3, B5, B6, B7b and B8. The figure was rendered using UCSF Chimera (23).
In bridge B7b, nucleotides A712 and G713 in h23 and G773, G774, G775 and G776 in h24 interact with specific residues of ribosomal protein L2 in the 50S subunit. None of the three single base mutations at A712 or G713C mutation had any effect on cell growth or subunit association when expressed in the Δ7 prrn strain MC338 (data not shown). Two two-base mutations, A712C/G713A and A712C/G713C were constructed and both mutations were also without apparent effect on growth or subunit association (Table 1 and Figure 2).
Table 1.
Growth characteristics of 16S rRNA bridge mutations in wild type and mutant S12 strains
| rRNA mutation | Bridge | Doubling time | Growth on solid medium | Doubling time | |
|---|---|---|---|---|---|
| 37°C | 30°C | 37°C | 37°C | ||
| WT S12 | WT S12 | WT S12 | K42N S12 | ||
| Wild-type | 46 ± 2 | ++++ | ++++ | 106 ± 9 | |
| 342/47 GC | B8 | 48 ± 1 | ++++ | ++++ | 145 ± 14 |
| 345-6 TT | B8 | 51 ± 1 | ++ | +++ | 47 ± 2 |
| 345-6 GA | B8 | 52 ± 3 | ++ | ++++ | 50 ± 1 |
| 345-6 AA | B8 | 48 ± 1 | ++ | +++ | 54 ± 2 |
| 712/13 CA | B7b | 45 ± 1 | ++++ | ++++ | 105 ± 16 |
| 712/13 CC | B7b | 44 ± 2 | ++++ | ++++ | 113 ± 14 |
| 773-6 GGUC | B7b | 43 ± 3 | +++ | ++++ | 119 ± 12 |
| 773-6 CGGG | B7b | 75 ± 3 | + | ++ | 124 ± 32 |
| 773-6 CGAU | B7b | 142 ± 9 | − | + | ND |
| 773-6 GAGG | B7b | 51 ± 2 | ++++ | ++++ | 121 ± 5 |
| 1429-30 CC | B6 | 47 ± 2 | ++++ | ++++ | 62 ± 2 |
| 1474-6 GUU | B5 | 47 ± 1 | + | ++++ | 96 ± 5 |
| 1484U | B3 | 108 ± 4 | ++ | +++ | 164 ± 16 |
| 1484G | B3 | 140 ± 13 | + | + | 154 ± 23 |
Doubling times were determined from growth of cultures in LB broth. Growth on solid LB medium was determined after 24 or 72 h of incubation at 37°C or 30°C, respectively. ++++, +++, ++, + and −, reflect normal, slightly inhibited, moderate, slow and no growth, respectively; ND, mutant not analyzed due to inviability in this strain.
For mutagenesis of the 773–776 region of h24, the four consecutive G residues at these positions were randomized and 11 different base combinations were recovered and tested for viability and effects on subunit association. The sequences of the multi-base mutations were as follows: 5′GGUC3′, 5′GAGU3′, 5′CGGG3′, 5′AGGG3′, 5′CGAU3′, 5′GAGG3′, 5′GGUU3′, 5′GGUA3′, 5′GGAG3′, 5′AGUG3′, 5′GGCC3′ (the wild-type sequence is 5′GGGG3′ and the mutant bases are represented in bold). All eleven mutations were viable in MC338 and examination of the sucrose gradients showed that all mutants (5′CGGG3′, 5′AGGG3′, 5′CGAU3′, 5′AGUG3′) with alterations at G773 had substantially increased levels of free subunits and decreased amounts of 70S ribosomes. In contrast, mutants carrying a G at position 773 but with alterations at G774, G775 or G776 had little effects on subunit association (data not shown). From these results, it appeared that G773 was important for bridge B7b function in subunit association. From this collection of eleven variants, four mutants (5′GGUC3′, 5′CGGG3′, 5′CGAU3′ and 5′GAGG3′) were studied further. Analyses of cell lysates on sucrose gradients containing only 5 mM Mg++ showed that all four mutants had substantial effects on subunit association (Figure 2). However, at 10 mM, much less drastic effects were observed. At the higher Mg++ concentration, the 5′GAGG3′ mutant had no effect on the levels of subunits while the 5′GGUC3′ and 5′CGGG3′ mutants had moderate effects on 70S ribosome formation. The 5′CGAU3′ mutant 50S subunits associated poorly at both Mg++ concentrations (Figure 2). Comparison of the effects of the 5′CGGG3′ and 5′CGAU3′ mutants indicates that while the identity of the base at 773 is critical, the bases at 775 and 776 also contribute to bridge function.
In bridge B8, residues 345–347 and 338–339 in h14 make contacts with proteins L14 and L19. Since G347 is paired with C342, we constructed mutants that either randomized the loop residues C345 and G346, or replaced the C342–G347 base pair with a G342–C347 pair. From randomization of nt 345–346, AA, GA and TT combinations were recovered. All three two-base mutations were viable in the Δ7 prrn strain MC338, in the absence of any wild-type rRNA and had at most, only modest effects on doubling times in liquid medium at 37°C (Table 1). However, as was observed with several other bridge mutants, these h14 mutants grew substantially slower at 30°C. Analyses of cell lysates on sucrose gradients containing 5 mM or 10 mM Mg++ showed that none of the three mutants was affected in subunit association (Figure 2). The h14 mutant that replaced the C342–G347 base pair with a G342–C347 pair (342–347 GC) was also viable in MC338 and had no detectable effects on growth at 30°C or 37°C (Table 1). However, sucrose gradient analyses showed that ribosomes carrying the C342–G347 base pair flip (342–347 GC) had modestly increased levels of free subunits at 10 mM Mg++ and displayed a substantial subunit association defect at 5 mM Mg++ (Figure 2), consistent with the involvement of this helix in subunit joining.
Effects of bridge mutations on decoding
Mutations at several of the 23S rRNA nucleotides involved in intersubunit bridges affect reading frame maintenance and decoding of stop codons (5,9,15). The effects of the 16S rRNA bridge mutations on recognition of initiation and termination triplets and maintenance of reading frame were analyzed using a series of lacZ reporter constructs (Table 2).
Table 2.
Effects of 16S rRNA bridge mutations on stop codon readthrough, −1 frameshifting and initiation from CUG codons
| rRNA mutation | Bridge |
lacZ plasmids |
|||
|---|---|---|---|---|---|
| pLG3/4 UGA | pLG12-6 (UAG) | pLG12DP (–1 fs) | pLG413 (CUG) | ||
| Wild-type | − | 148 ± 8 | 8 ± 1 | 56 ± 2 | 58 ± 3 |
| 342/47 GC | B8 | 238 ± 4 | 8 ± 1 | 76 ± 2 | 69 ± 2 |
| 345-6 TT | B8 | 290 ± 27 | 25 ± 1 | 45 ± 2 | 44 ± 4 |
| 345-6 GA | B8 | 218 ± 19 | 12 ± 1 | 52 ± 3 | 52 ± 6 |
| 345-6 AA | B8 | 304 ± 15 | 12 ± 1 | 54 ± 6 | 56 ± 2 |
| 712/13 CA | B7b | 188 ± 14 | 8 ± 1 | 69 ± 2 | 63 ± 4 |
| 712/13 CC | B7b | 179 ± 15 | 8 ± 1 | 71 ± 2 | 72 ± 5 |
| 773-6 GGUC | B7b | 218 ± 27 | 9 ± 1 | 69 ± 2 | 67 ± 2 |
| 773-6 CGGG | B7b | 169 ± 2 | 8 ± 1 | 71 ± 2 | 95 ± 3 |
| 773-6 CGAU | B7b | 170 ± 10 | 8 ± 1 | 73 ± 4 | 114 ± 3 |
| 773-6 GAGG | B7b | 214 ± 6 | 7 ± 1 | 71 ± 2 | 72 ± 2 |
| 1484U | B3 | 167 ± 15 | 9 ± 1 | 77 ± 3 | 65 ± 4 |
| 1484G | B3 | 192 ± 14 | 11 ± 1 | 80 ± 10 | 114 ± 2 |
| 1474-6 GUU | B5 | 470 ± 19 | 12 ± 1 | 76 ± 2 | 167 ± 20 |
| 1429-30 CC | B6 | 316 ± 31 | 10 ± 1 | 40 ± 4 | 50 ± 1 |
All rRNAs were expressed in the Δ7 prrn strain MC338, from pKK3535-derived plasmids. β-Galactosidase activities are expressed in Miller units (22). Each value is the result of 3–5 independent experiments and the assay conditions are described in the text. Plasmids pLG3/4 UGA and pLG12-6 carry UGA and UAG stop codons, respectively, while plasmid pLG12DP carries a −1 frameshift mutation, in the 5′ coding region. In pLG413, the AUG initiation codon has been replaced with a CUG triplet.
All four bridge B8 mutations in h14 increased readthrough of UGA stop codons (1.5–2-fold increases) while only the CG345-6TT mutant increased readthrough of UAG codons. The same h14 mutants had no effects on frameshifting or initiation from non-AUG codons. The h44 mutants 1474-6 GUU (bridge B5) and C1484G (bridge B3) increased initiation from a CUG codon while the 1474-6 GUU and 1429-30 CC (bridge B6) mutants increased readthrough of UGA stop codons. The h23 mutations affecting bridge B7b did not affect the levels of β-galactosidase in any of the constructs used. However, two of the h24 mutants in bridge B7b (773-6 5′CGGG3′ and 5′CGAU3′) increased initiation from CUG, but had no effects on decoding of stop codons or maintenance of reading frame. These data suggest that bridges B3, B5 and B7b contribute to the fidelity of the initiation process while bridges B5, B6 and B8 alter discrimination between sense and termination codons.
Interaction of ribosomal protein S12 with bridges B5, B6 and B8
Ribosomal protein S12 plays a major role in decoding and in the ribosome’s response to the error-inducing antibiotic, streptomycin. Many of the mutations in the rpsL gene, encoding protein S12, that confer resistance to streptomycin decrease, or restrict miscoding errors. Classical genetic studies showed that the effects of S12 mutations on decoding could be reversed by certain S4 and S5 mutations, which on their own, increased the frequency of decoding errors. More recently, Maisnier-Patin et al. (11,25,26) have isolated a range of different suppressor mutations that relieve the growth defects associated with an error-restrictive K42N substitution in protein S12. In addition to the expected S4 and S5 mutations, they also recovered mutations in the large subunit protein, L19. When genetically separated from the rpsL mutation, ribosomes carrying the mutant L19 protein supported increased misreading levels, thus linking L19 with decoding activities. Protein L19 forms part of bridges B8 and B6 (Figure 3) and one interpretation of the Maisnier-Patin et al. result is that the conformation of the intersubunit bridges affects S12 functions. To address the potential linkage between bridge functions and S12, we have asked if any of the mutations in the rRNA residues of bridges B3, B5, B6, B7b or B8 affect the phenotypes of error-restrictive S12 mutants.
The Δ7 prrn strain MC343, carrying the K42N mutation in protein S12 and expressing only wild-type rRNA from a plasmid, is extremely slow growing (doubling time of 106 min). Growth of MC343 was not improved when the wild-type rrn plasmid in MC343 was replaced with rrn plasmids carrying mutations affecting bridges B3 or B7b. However, replacement of the wild-type rrn plasmid with plasmids carrying mutations affecting bridges B5, B6 and B8 led to a remarkable improvement in the growth of MC343 (Table 1). In the most dramatic examples, MC343 strains expressing rRNAs with mutations in the bridge B8 residues 345–346 had doubling times (47–54 min) close to that of MC338 (doubling time of 46 min) expressing wild-type rRNA and the wild-type S12 protein. The two-base mutation affecting bridge B6 (1429-30 CC) also improved growth of MC343 (doubling time of 62 min), while less dramatic effects were seen with the multi-base bridge B5 mutation (doubling time of 96 min). Notably, the h14 342/347 GC mutant did not lead to any recovery of the doubling time of MC343.
β-Galactosidase assays using the pLG3/4 UGA construct, showed that a strain carrying wild-type rRNA and the K42N mutant S12 protein had greatly reduced readthrough of UGA stop codons (5 U of activity), compared with strains expressing fully wild-type ribosomes (148 U). This very low level of UGA readthrough was increased in strains carrying both the K42N mutant S12 as well as base changes at nt 345–346 in 16S rRNA (17–37 Miller units) or at nt 1429–1430 or 1474–1476 in h44 (8 and 9 Miller units, respectively). None of the other rRNA mutations affected UGA readthrough in the S12 K42N mutant strain (data not shown). While the recovery of UGA readthrough levels is only partial in the S12/rRNA double mutants, nonetheless the same mutant rRNAs ameliorate both the growth defects and the low UGA readthrough levels of the K42N S12 mutant. This suggests that altered decoding underlies the slow growth of the mutant S12 strain and its recovery by rRNA bridge mutations.
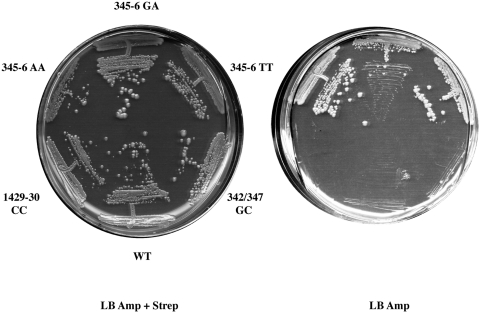
In addition to the amino acid substitutions in S12 that confer resistance to streptomycin, other substitutions have been described that render growth dependent on the presence of streptomycin in the medium. Such streptomycin-dependent (SmD) rpsL alleles show the greatest effects on decoding and require streptomycin to counteract the extreme effects of the altered S12 on tRNA selection. In a further genetic test to probe the interaction of protein S12 with the intersubunit bridges, we asked if any of the 16S rRNA mutations affecting bridge residues could suppress the streptomycin dependence associated with substitutions at positions P90 and G91 in S12. Four different streptomycin-dependent mutants were used and these carried either single (P90R, G91D) or double (P90R + R85S; P90L + R85S) amino acid substitutions. Mutations at position 85 in S12 do not affect streptomycin dependence, but are encountered as ‘ancillary’ mutations in several streptomycin-dependent mutants (27). Each SmD strain was transformed with wild-type or mutant rrn plasmids, plated on media containing streptomycin plus ampicillin and then tested for streptomycin independence on media containing ampicillin only. Only strains carrying the P90R substitution showed any response to the mutant rrn plasmids; all three h14 mutants carrying base changes at positions 345–346 in16S rRNA (bridge B8) allowed growth of these strain in the absence of streptomycin at 30°C (Figure 4). No growth on streptomycin-free media was observed in these transformants at 37°C and none of the other mutant rrn plasmids allowed growth in the absence of streptomycin, at either temperature.
Figure 4.
Suppression of streptomycin dependence by rRNA mutations. Derivatives of the streptomycin-dependent strain MC333, carrying the P90R substitution in S12 and expressing wild-type or the indicated mutant 16S rRNAs were streaked on plates containing ampicillin plus streptomycin (left) or ampicillin only (right) and incubated at 30°C for 96 h.
From both sets of experiments combining mutant rRNA and protein S12, it is clear that the h14 mutations at positions 345–346 (bridge B8) can ameliorate the defects associated with the K42N, as well as the P90R mutations in S12, while the h44 (bridges B5 and B6) base changes only affect the K42N substitution. The failure of the h44 mutants to relieve streptomycin dependence may be due to the more stringent requirements for suppression of streptomycin dependence, compared with improvement of the growth properties of strains carrying the K42N change in S12. The experiments reported here are consistent with previous reports that show genetic interactions between proteins L19 and S12. Together, these data establish a link between decoding and bridge functions and suggest that the step(s) in decoding that are affected by S12 can be modulated by bridges B5, B6 and B8.
DISCUSSION
The intersubunit bridges are conserved features of the ribosome and in the work presented here, we show that in addition to contributing to subunit association, mutations at several of the regions of 16S rRNA involved in bridge formation have unanticipated effects on decoding, both at the initiation and elongation phases of protein synthesis. These results suggest that rearrangements of the intersubunit connections occur throughout the translation cycle.
One expectation of altering the intersubunit bridges through mutations is that the association of mutant ribosomal subunits should be affected. However, while some of the bridge mutations constructed here decrease subunit association, 70S ribosome levels were relatively unaffected by the mutations affecting bridges B5, B6 and B8, and at positions 712–713 in h23 (bridge B7b), at least in buffers containing 10 mM Mg++. Metal ions appear to contribute to the stability of the interactions at bridges B5, B6 and B8 (28). However, only in the case of bridge B5 mutant ribosomes (1474-6 GUU) does subunit association appear to be sensitive to Mg++ concentration. The lack of discernable effects of some bridge mutations on subunit association likely derives from the redundancy of bridging interactions between the subunits; multiple interactions comprise each bridge and twelve different bridges hold the two subunits together.
In contrast to the mutations in bridges B5, B6 and B8, base changes at position 1484 in bridge B3 and nt 773–776 in bridge B7b have substantial effects on subunit association. In bridge B3, two consecutive sheared G–A pairs in h44 of 16S rRNA, A1418–G1482 and G1417–A1483, interact with two G–C pairs in h71 of 23S rRNA via A-minor interactions (29). In addition, the RNA backbone of both helices comes in close juxtaposition exactly at position 1484 (Figure 3). The mutagenesis results showing that the structural intactness of h44 around 1484 is crucial for maintaining ribosomal function strongly suggests that the packing of helices 44 and 71 in this region is affected by mutations at C1484. In bridge B7b, nt 712–713 and 773–776 of 16S rRNA contact residues in protein L2 on the 50S subunit. Mutations at nt 712–713 had little effect on any of the parameters examined in this study. In contrast, base changes at nt 773–776, which disrupt the stability of h24 in 16S rRNA, had substantial effects on subunit association and the selection of the correct initiation codon. Of the four consecutive G residues at positions 773–776 that were randomized in our mutagenesis experiments, the identity of G773 was identified as being the most critical for ribosomal function. G773 is also the closest of these residues to L2 (Figure 3), suggesting that the disruption of rRNA–L2 interaction hampers the formation of 70S ribosomes.
Several of the bridge mutations studied here affect the fidelity of decoding, either by affecting the choice of initiation codon (bridges B3, B5 and B7b), or by increasing the readthrough of stop codons (bridges B5, B6 and B8). Both local sequence context, a specialized, initiator tRNA and initiation factors contribute to the specificity of initiation. In addition, mutations in a number of the 16S residues that constitute the ribosomal P site, to which the initiator tRNA binds, have been isolated that also affect the specificity of initiation (19,30). Recent kinetic experiments suggest that conformational rearrangements of the 30S initiation complex are required for association with 50S subunits (31). The conformational rearrangements of the 30S initiation complex to a form that can be bound by the 50S subunit requires the action of initiation factors and are predicted to involve rearrangement of some ribosomal bridges. Our results indicate that bridges B3, B5 and B7b are involved in these conformational rearrangements. The initiation factor IF3 plays a major role in the fidelity of initiation (32,33) and mutations in the factor itself as well as the 16S rRNA nucleotides that interact with IF3 affect the choice of initiation codon (21,30). Among the 16S rRNA mutations that affect IF3 function are A790, G791 and A792, at the tip of the h24 hairpin (21,30,34). Conceivably, the 773–776 mutations analyzed here and which are located at the other end of h24 (Figures 1 and 3) may affect the structure and orientation of this hairpin within the 30S subunit and the binding of IF3, thus accounting for the observed defects in the choice of initiation codon. The 1484 (bridge B3) and 1474-6 (bridge B5) regions of h44 are distant from the ribosomal P site and the known sites of IF3 interaction and it is not obvious how mutations in these regions influence the initiation process (Table 2). Potentially, these mutations may affect the conformational rearrangements of the 30S initiation complex required for association with 50S subunits and/or the ordered formation of bridging interactions during subunit joining (31,35).
Selection of cognate tRNAs during decoding involves an initial selection of ternary complexes and a second inspection, or proofreading step, that occurs following GTP hydrolysis, which is greatly accelerated by cognate, but not by near-cognate ternary complexes. Mutations in ribosomal, or ternary complex components that increase GTP hydrolysis by near-cognate ternary complexes allow these complexes to evade discrimination during the initial selection phase and thus have the potential to increase the frequency of decoding errors (36). Mutations in bridges B5, B6 and B8 increase stop codon readthrough. In principle, increased readthrough of stop codons can occur though perturbation of the decoding process during elongation, or by altering ribosome-release factor interactions at termination. The same bridge mutations analyzed here that increase stop codon readthrough, also ameliorate the effects of error-restrictive S12 mutants. Since S12 mutations affect tRNA selection during elongation without affecting peptide release (37), this suggests that these rRNA mutations affect decoding processes during elongation.
Bridges B5, B6 and B8 are clearly remote from the decoding site; however, recent cryo-EM analyses of ribosome–aminoacyl-tRNA•EF-Tu•GTP complexes stalled with kirromycin suggest a possible mechanism by which these bridge mutations might affect decoding fidelity. In two different studies, an interaction between a conserved region of EF-Tu (the switch 1 region) and the h14 region of the 30S subunit has been observed (13,38). Villa et al. (38) have proposed that the interaction of the switch 1 region of EF-Tu with h14 results in conformational changes in EF-Tu that allow the critical His-84 residue to activate a water molecule for nucleophilic attack on GTP. Recently, McClory et al. (39) have reported the isolation of a series of 16S rRNA mutations that promote missense decoding. Among the mutations recovered were base changes at G346, G347, G348 and A349 in h14 and A1430 in h44, some of which are also described in this work. Their analyses showed that mutations in h14 stimulated GTP hydrolysis by near-cognate ternary complexes in vitro; leading them to suggest that bridge B8 normally acts to counter the movement of the 30S subunit that allows it to contact EF-Tu and activate GTP hydrolysis. Our results are consistent with such a model and in addition suggest that the adjacent bridges B5 and B6 also modulate conformational changes in the 30S subunit in response to ternary complex binding. The large subunit proteins L14 and L19 are components of bridge B8 (Figure 3) and, by affecting the conformation of bridge B8, could conceivably transmit the structural alteration in bridge B5 and B6 mutant ribosomes to the GTPase center of EF-Tu.
A mechanism involving inappropriate activation of EF-Tu-dependent GTP hydrolysis likely also underlies the reversal of the growth and decoding defects of protein S12 mutants by alterations in the protein (11,26) or rRNA (this work) components of bridges B5, B6 and B8. Previous analyses of other ribosomal mutations that suppress S12 defects have shown that many of these mutant ribosomes support high levels of miscoding and that the combination of error-prone and error restrictive (S12) ribosomal mutations generates a quasi wild-type error level. Ribosomes carrying the error-restrictive K42N substitution in S12, or a truncated S4 protein that increases decoding errors have recently been analyzed biochemically (37). These in vitro studies show that the S12 mutation affects the proofreading step and that the altered S4 protein overcomes this defect by increasing the stability of near-cognate ternary complexes during initial selection and enhancing their rates of subsequent GTP hydrolysis. Here we show that the phenotypes of the K42N mutant S12, the same S12 mutant studied by Zaher and Green (37), can be suppressed by bridge B5, B6 and B8 alterations. According to our interpretations discussed above and the model proposed by McClory et al. (39), ribosomes carrying bridge mutations trigger GTP hydrolysis inappropriately in response to the binding of near-cognate ternary complexes. In so doing, they increase the flow of near-cognate tRNAs that enter the proofreading phase, where mutant S12 exerts its effects, and thus, increase the chances of incorporation of near-cognate amino acids.
Based on their biochemical analyses of mutant ribosomes, Zaher and Green (37) have proposed that the disruption of the S4–S5 interface and the formation of contacts between protein S12 and 16S rRNA occur sequentially, at the initial recognition and proofreading steps, respectively. The EF-Tu-bridge B8 contacts observed in cryo-EM experiments and analyzed genetically in this study, represent yet another stage in the ribosome-ternary complex interaction pathway, which can be altered by mutations that have profound effects on the accuracy of tRNA selection.
FUNDING
National Science Foundation (MCB0745025 to M.O.C.); Science Foundation Ireland to John Atkins, University College Cork, Ireland (to A.V.S.). Funding for open access charge: National Science Foundation grant plus funds from the School of Biological Sciences, University of Missouri-Kansas City.
Conflict of interest statement. None declared.
ACKNOWLEDGEMENTS
We are indebted to Drs Catherine Squires and Selwyn Quan for supplying the Δ7 prrn strain and to Drs Steven Gregory and Jennifer Carr for their comments on the manuscript.
REFERENCES
- 1.Gao H, Sengupta J, Valle M, Korostelev A, Eswar N, Stagg SM, Van Roey P, Agrawal RK, Harvey SC, Sali A, et al. Study of the structural dynamics of the E coli 70S ribosome using real-space refinement. Cell. 2003;113:789–801. doi: 10.1016/s0092-8674(03)00427-6. [DOI] [PubMed] [Google Scholar]
- 2.Spahn CM, Beckmann R, Eswar N, Penczek PA, Sali A, Blobel G, Frank J. Structure of the 80S ribosome from Saccharomyces cerevisiae–tRNA-ribosome and subunit-subunit interactions. Cell. 2001;107:373–386. doi: 10.1016/s0092-8674(01)00539-6. [DOI] [PubMed] [Google Scholar]
- 3.Sharma MR, Koc EC, Datta PP, Booth TM, Spremulli LL, Agrawal RK. Structure of the mammalian mitochondrial ribosome reveals an expanded functional role for its component proteins. Cell. 2003;115:97–108. doi: 10.1016/s0092-8674(03)00762-1. [DOI] [PubMed] [Google Scholar]
- 4.Ali IK, Lancaster L, Feinberg J, Joseph S, Noller HF. Deletion of a conserved, central ribosomal intersubunit RNA bridge. Mol. Cell. 2006;23:865–874. doi: 10.1016/j.molcel.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 5.Komoda T, Sato NS, Phelps SS, Namba N, Joseph S, Suzuki T. The A-site finger in 23 S rRNA acts as a functional attenuator for translocation. J. Biol. Chem. 2006;281:32303–32309. doi: 10.1074/jbc.M607058200. [DOI] [PubMed] [Google Scholar]
- 6.Yusupov MM, Yusupova GZ, Baucom A, Lieberman K, Earnest TN, Cate JH, Noller HF. Crystal structure of the ribosome at 5.5 A resolution. Science. 2001;292:883–896. doi: 10.1126/science.1060089. [DOI] [PubMed] [Google Scholar]
- 7.Pulk A, Maivali U, Remme J. Identification of nucleotides in E. coli 16S rRNA essential for ribosome subunit association. RNA. 2006;12:790–796. doi: 10.1261/rna.2275906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sergiev PV, Kiparisov SV, Burakovsky DE, Lesnyak DV, Leonov AA, Bogdanov AA, Dontsova OA. The conserved A-site finger of the 23S rRNA: just one of the intersubunit bridges or a part of the allosteric communication pathway? J. Mol. Biol. 2005;353:116–123. doi: 10.1016/j.jmb.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 9.Liiv A, O’Connor M. Mutations in the intersubunit bridge regions of 23 S rRNA. J. Biol. Chem. 2006;281:29850–29862. doi: 10.1074/jbc.M603013200. [DOI] [PubMed] [Google Scholar]
- 10.Rackham O, Wang K, Chin JW. Functional epitopes at the ribosome subunit interface. Nat. Chem. Biol. 2006;2:254–258. doi: 10.1038/nchembio783. [DOI] [PubMed] [Google Scholar]
- 11.Maisnier-Patin S, Paulander W, Pennhag A, Andersson DI. Compensatory evolution reveals functional interactions between ribosomal proteins S12, L14 and L19. J. Mol. Biol. 2007;366:207–215. doi: 10.1016/j.jmb.2006.11.047. [DOI] [PubMed] [Google Scholar]
- 12.Li W, Agirrezabala X, Lei J, Bouakaz L, Brunelle JL, Ortiz-Meoz RF, Green R, Sanyal S, Ehrenberg M, Frank J. Recognition of aminoacyl-tRNA: a common molecular mechanism revealed by cryo-EM. EMBO J. 2008;27:3322–3331. doi: 10.1038/emboj.2008.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schuette JC, Murphy FVt, Kelley AC, Weir JR, Giesebrecht J, Connell SR, Loerke J, Mielke T, Zhang W, Penczek PA, et al. GTPase activation of elongation factor EF-Tu by the ribosome during decoding. EMBO J. 2009;28:755–765. doi: 10.1038/emboj.2009.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schmeing TM, Voorhees RM, Kelley AC, Gao YG, Murphy FVt, Weir JR, Ramakrishnan V. The crystal structure of the ribosome bound to EF-Tu and aminoacyl-tRNA. Science. 2009;326:688–694. doi: 10.1126/science.1179700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O’Connor M. Helix 69 in 23S rRNA modulates decoding by wild type and suppressor tRNAs. Mol. Genet. Genomics. 2009;282:371–380. doi: 10.1007/s00438-009-0470-6. [DOI] [PubMed] [Google Scholar]
- 16.Bjare U, Gorini L. Drug dependence reversed by a ribosomal ambiguity mutation, ram, in Escherichia coli. J. Mol. Biol. 1971;57:423–435. doi: 10.1016/0022-2836(71)90101-x. [DOI] [PubMed] [Google Scholar]
- 17.Vila-Sanjurjo A, Lu Y, Aragonez JL, Starkweather RE, Sasikumar M, O’Connor M. Modulation of 16S rRNA function by ribosomal protein S12. Biochim. Biophys. Acta. 2007;1769:462–471. doi: 10.1016/j.bbaexp.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 18.Stoker NG, Fairweather NF, Spratt BG. Versatile low-copy-number plasmid vectors for cloning in Escherichia coli. Gene. 1982;18:335–341. doi: 10.1016/0378-1119(82)90172-x. [DOI] [PubMed] [Google Scholar]
- 19.O’Connor M, Thomas CL, Zimmermann RA, Dahlberg AE. Decoding fidelity at the ribosomal A and P sites: influence of mutations in three different regions of the decoding domain in 16S rRNA. Nucleic Acids Res. 1997;25:1185–1193. doi: 10.1093/nar/25.6.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ito W, Ishiguro H, Kurosawa Y. A general method for introducing a series of mutations into cloned DNA using the polymerase chain reaction. Gene. 1991;102:67–70. doi: 10.1016/0378-1119(91)90539-n. [DOI] [PubMed] [Google Scholar]
- 21.Tapprich WE, Goss DJ, Dahlberg AE. Mutation at position 791 in Escherichia coli 16S ribosomal RNA affects processes involved in the initiation of protein synthesis. Proc. Natl Acad. Sci. USA. 1989;86:4927–4931. doi: 10.1073/pnas.86.13.4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miller JH. A Short Course in Bacterial Genetics. New York: Cold Spring Harbor Laboratory Press; 1991. [Google Scholar]
- 23.Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE. UCSF Chimera–a visualization system for exploratory research and analysis. J. Comput. Chem. 2004;25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- 24.Adams PD, Afonine PV, Bunkoczi G, Chen VB, Davis IW, Echols N, Headd JJ, Hung LW, Kapral GJ, Grosse-Kunstleve RW, et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 2010;66:213–221. doi: 10.1107/S0907444909052925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bjorkman J, Samuelsson P, Andersson DI, Hughes D. Novel ribosomal mutations affecting translational accuracy, antibiotic resistance and virulence of Salmonella typhimurium. Mol. Microbiol. 1999;31:53–58. doi: 10.1046/j.1365-2958.1999.01142.x. [DOI] [PubMed] [Google Scholar]
- 26.Maisnier-Patin S, Berg OG, Liljas L, Andersson DI. Compensatory adaptation to the deleterious effect of antibiotic resistance in Salmonella typhimurium. Mol. Microbiol. 2002;46:355–366. doi: 10.1046/j.1365-2958.2002.03173.x. [DOI] [PubMed] [Google Scholar]
- 27.Timms AR, Bridges BA. Double, independent mutational events in the rpsL gene of Escherichia coli: an example of hypermutability? Mol. Microbiol. 1993;9:335–342. doi: 10.1111/j.1365-2958.1993.tb01694.x. [DOI] [PubMed] [Google Scholar]
- 28.Selmer M, Dunham CM, Murphy FVt, Weixlbaumer A, Petry S, Kelley AC, Weir JR, Ramakrishnan V. Structure of the 70S ribosome complexed with mRNA and tRNA. Science. 2006;313:1935–1942. doi: 10.1126/science.1131127. [DOI] [PubMed] [Google Scholar]
- 29.Schuwirth BS, Borovinskaya MA, Hau CW, Zhang W, Vila-Sanjurjo A, Holton JM, Cate JH. Structures of the bacterial ribosome at 3.5 A resolution. Science. 2005;310:827–834. doi: 10.1126/science.1117230. [DOI] [PubMed] [Google Scholar]
- 30.Qin D, Abdi NM, Fredrick K. Characterization of 16S rRNA mutations that decrease the fidelity of translation initiation. RNA. 2007;13:2348–2355. doi: 10.1261/rna.715307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Milon P, Konevega AL, Gualerzi CO, Rodnina MV. Kinetic checkpoint at a late step in translation initiation. Mol. Cell. 2008;30:712–720. doi: 10.1016/j.molcel.2008.04.014. [DOI] [PubMed] [Google Scholar]
- 32.O’Connor M, Gregory ST, Rajbhandary UL, Dahlberg AE. Altered discrimination of start codons and initiator tRNAs by mutant initiation factor 3. RNA. 2001;7:969–978. doi: 10.1017/s1355838201010184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sacerdot C, de Cock E, Engst K, Graffe M, Dardel F, Springer M. Mutations that alter initiation codon discrimination by Escherichia coli initiation factor IF3. J. Mol. Biol. 1999;288:803–810. doi: 10.1006/jmbi.1999.2737. [DOI] [PubMed] [Google Scholar]
- 34.Santer M, Bennett-Guerrero E, Byahatti S, Czarnecki S, O’Connell D, Meyer M, Khoury J, Cheng X, Schwartz I, McLaughlin J. Base changes at position 792 of Escherichia coli 16S rRNA affect assembly of 70S ribosomes. Proc. Natl Acad. Sci. USA. 1990;87:3700–3704. doi: 10.1073/pnas.87.10.3700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hennelly SP, Antoun A, Ehrenberg M, Gualerzi CO, Knight W, Lodmell JS, Hill WE. A time-resolved investigation of ribosomal subunit association. J. Mol. Biol. 2005;346:1243–1258. doi: 10.1016/j.jmb.2004.12.054. [DOI] [PubMed] [Google Scholar]
- 36.Dinman JD, O’Connor M. Mutants that affect recoding. In: Atkins JF, Gesteland RF, editors. Recoding: Expansion of Decoding Rules Enriches Gene Expression. 2010. Springer, New York, Dordrecht, Heidelberg, London, pp. 321–344. [Google Scholar]
- 37.Zaher HS, Green R. Hyperaccurate and error-prone ribosomes exploit distinct mechanisms during tRNA selection. Mol. Cell. 2010;39:110–120. doi: 10.1016/j.molcel.2010.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Villa E, Sengupta J, Trabuco LG, LeBarron J, Baxter WT, Shaikh TR, Grassucci RA, Nissen P, Ehrenberg M, Schulten K, et al. Ribosome-induced changes in elongation factor Tu conformation control GTP hydrolysis. Proc. Natl Acad. Sci. USA. 2009;106:1063–1068. doi: 10.1073/pnas.0811370106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McClory SP, Leisring JM, Qin D, Fredrick K. Missense suppressor mutations in 16S rRNA reveal the importance of helices h8 and h14 in aminoacyl-tRNA selection. RNA. 2010;16:1925–1934. doi: 10.1261/rna.2228510. [DOI] [PMC free article] [PubMed] [Google Scholar]