Abstract
Background
Benign prostatic hyperplasia (BPH), a common condition among older men, confers its morbidity through potentially bothersome lower urinary tract symptoms. Treatments for BPH include drugs such as alpha adrenergic receptor blockers and 5-alpha reductase inhibitors, minimally invasive therapies that use heat to damage or destroy prostate tissue, and surgery including transurethral resection of the prostate. Complementary and alternative medicines are gaining popularity in the U.S. Two phytotherapies commonly used for BPH are extracts of the fruit of Serenoa repens, the Saw palmetto dwarf palm that grows in the Southeastern U.S., and extracts of the bark of Pygeum africanum, the African plum tree.
Purpose
The objective of the Complementary and Alternative Medicines for Urological Symptoms (CAMUS) clinical trial is to determine if phytotherapy is superior to placebo in the treatment of BPH.
Methods
CAMUS was originally designed as a 3300-participant, four-arm trial of Serenoa repens, Pygeum africanum, an alpha adrenergic blocking drug, and placebo with time to clinical progression of BPH, a measure of long-term efficacy, as the primary endpoint. Before enrollment started, a randomized, double-blind, placebo-controlled, single institution clinical trial showed that Serenoa repens at the usual dose did not demonstrate any benefit over placebo with respect to symptom relief at one year. Consequently, the focus of CAMUS shifted from evaluating long-term efficacy to determining if any short-term (6-18 month) symptom relief could be achieved with increasing doses of Serenoa repens, the phytotherapy most commonly used in the U.S. for BPH.
Results
Results are anticipated in 2011.
Conclusions
Trial design occurs in an environment of continually evolving information. In this case, emerging results from another trial suggested that a study of long-term efficacy was premature, and that an effective dose and preparation of Serenoa repens had to be established before proceeding to a long-term clinical trial.
Keywords: botanical therapy, benign prostatic hyperplasia, clinical trial, trial re-design
Background
Benign prostatic hyperplasia (BPH), a common condition among older men in the United States and other developed countries, confers its morbidity through potentially bothersome lower urinary tract symptoms (LUTS) such as a weak stream, frequency and urgency. Prevalence of BPH increases with age, and affects 17% of men age 50-59, 27% of men age 60-69, and 35% of men age 70-79 [1]. The aging of the U.S. population [2] is expected to result in a concomitant increase in the number of men with LUTS associated with BPH. The broad range of treatments for BPH includes two classes of medications, alpha adrenergic blocking agents and 5-alpha reductase inhibitors, minimally invasive therapies that use heat to damage or destroy prostate tissue, and surgical therapies, including transurethral resection of the prostate (TURP) [3].
As BPH is a progressive disease, a number of clinical trials have evaluated medical therapy with time to clinical progression of BPH as the primary endpoint [4]. The Medical Treatment of Prostatic Symptoms (MTOPS) trial compared the ability of doxazosin, an alpha adrenergic blocking agent, and finasteride, a 5-alpha reductase inhibitor, alone or in combination, against placebo to delay BPH progression [5, 6]. Both drugs were equally effective at reducing the rate of BPH progression, while combination therapy was significantly more effective than either agent alone [5]. In MTOPS, clinical progression of BPH was defined as experiencing any one of the following conditions: acute urinary retention, renal insufficiency, recurrent urinary tract infections, incontinence, or an increase of at least 4 points in the American Urological Association (AUA) symptom score.
Complementary and alternative medicines are gaining popularity in the U.S. and are used by about one-fifth of adults [6, 7]. Two of the most studied phytotherapies for BPH are extracts of the fruit of Serenoa repens, the Saw palmetto dwarf palm that grows in the Southeastern U.S.; and, to a much lesser extent, extracts of the bark of Pygeum africanum, the African plum tree [8, 9]. The proposed mechanisms of action for Saw palmetto include 5-alpha reductase inhibition, intraprostatic androgen receptor blockage, and adrenergic receptor antagonism as well as an anti-inflammatory effect [10]. In vitro studies have shown that Pygeum extracts have anti-inflammatory and immunomodulatory properties, effects on bladder contractility, modulation of androgen production, and direct effects on the function of prostate epithelium [9, 11]. Although a recent Cochrane meta-analyses of Serenoa repens concluded that it was no better than placebo in the treatment of urinary symptoms related to BPH [12], Cochrane meta-analyses of both phytotherapies at the time that the multicenter clinical trial of phytotherapy for BPH was conceived, found modest favorable effects on LUTS and uroflow rates with few side effects [13, 14]. Although existing trials of these agents had methodological limitations and their mechanisms of action remain undefined, they are widely used. If they are effective at reducing LUTS, BPH patients might find them preferable to medical therapy based on the appeal of “natural therapy”, their minimal side effects, and their cost.
Purpose
In January 2002, the National Institutes of Health (NIH) issued a request for applications (RFA) entitled “Alternative Therapies for Benign Prostate Symptoms – Clinical Trials Consortium.” The purpose of the consortium was to develop and conduct a multi-center, randomized, placebo-controlled clinical trial to determine the effect of Serenoa repens and Pygeum africanum on clinical progression of BPH. It was anticipated that the study would require 7 years at a cost of $21 million (http://grants.nih.gov/grant/guide/rfa_files/RFA-DK-02-029/html).
Three NIH agencies participated in the RFA: National Institute of Diabetes and Digestive Diseases (NIDDK), National Center for Complementary and Alternative Medicines (NCCAM) and the Office of Dietary Supplements (ODS). Eleven clinical centers, 10 in the U.S. and one in Canada, and a data coordinating center (DCC) were funded under a cooperative agreement mechanism (U01). In anticipation of this RFA, NCCAM released a request for proposal (RFP) for suppliers of phytotherapies to be used in the trial. Two European based companies were selected to provide Serenoa repens and Pygeum africanum, and their matching placeboes for the study.
Methods
Protocol development was initiated in October 2002 (Table 1) using the MTOPS design as a starting point for the Complementary and Alternative Medicines for Urological Symptoms (CAMUS) trial. Both phytotherapies were to be given at commonly used dosages: 100 mg daily for Pygeum africanum and 320 mg daily for Serenoa repens.
Table 1.
Eligibility Criteria
Eligibility Criteria
|
Exclusion Criteria
|
The two botanical products differed in color and odor. Developing placeboes that would mask the odor required each purveyor to test preparations with various additives. One of the firms also conducted a test to determine participants’ perception of whether or not they were given the product or placebo. Stability tests were required for the placeboes and botanical products. To avoid enhancing the odor of an agent by having multiple gelcaps in a bottle, the products were to be distributed in blister packs. Because of the difference in color and odor of the two agents, participants were to take two gelcaps, one for each phytotherapy (product or placebo) daily to mask the study.
As phytotherapies were not expected to benefit those with the most bothersome symptoms, the upper limit of the American Urological Association Symptom Index (AUASI) score for eligibility was lowered from 30 in MTOPS to 24 (Table 1). The age criterion was lowered to 45 years of age from 50 years of age in MTOPS to increase the generalizability and practicality of the trial results. Since most MTOPS participants who crossed over to invasive or medical therapy did so due to clinical progression of BPH, the primary endpoint in CAMUS was time to clinical progression of BPH or crossover to invasive or open-label therapy for BPH.
More controversial was whether or not to include an active comparator from one of the two classes of drugs used in the MTOPS study to aid in the interpretation of potentially negative results. If phytotherapies and the active comparator did not demonstrate efficacy, then the finding might be attributed to differences between the MTOPS and CAMUS study populations. There was a concern, however, that study participants interested in botanical therapies might be deterred from participation by the inclusion of a prescription medical therapy.
The Prostate Cancer Prevention Trial (PCPT) finding that finasteride was effective in reducing the incidence of prostate cancer, but that prostate cancer cases that occurred on finasteride were more aggressive [15] eliminated 5-alpha reductase inhibitors from consideration as the active comparator, and an alpha adrenergic blocking agent was chosen. The addition of the active comparator arm required a substantial increase in the sample size and a supplier for the alpha blocker.
CAMUS was originally a four-arm study of Pygeum africanum, Serenoa repens, an alpha adrenergic blocking agent, and placebo designed to determine if either phytotherapy or the active comparator was superior to placebo in prolonging the time to clinical progression of BPH or crossover to invasive or open label therapy for BPH. The main intent was to evaluate each phytotherapy agent against placebo. There were no plans to compare the two phytotherapy agents, especially since doing so would require enlarging the sample size to adjust for an additional treatment comparison. The study was to enroll 3300 patients evenly distributed across the four treatment arms. Initiation of participant enrollment was delayed while NIH unsuccessfully sought a donor of the alpha adrenergic blocking agent. Ultimately, NIH obtained additional funds to purchase the alpha adrenergic blocking agent and its matching placebo.
Dissemination of Results from a Related Study
Shortly after funding was obtained for the active comparator and before CAMUS began enrollment, the results of the NIH-sponsored STEP (Saw palmetto Treatment for Enlarged Prostates) study, an investigator-initiated randomized, double-blind, placebo-controlled single institution clinical trial of a saw palmetto extract for reducing BPH-related symptoms were reported to the CAMUS investigators (Figure 1). Two hundred twenty-five men ≥ 50 years of age with baseline AUASI scores ≥ 8 were randomized to Saw palmetto 160 mg twice a day or a matching placebo. Although well-tolerated, Serenoa repens did not demonstrate any benefit over placebo with respect to change in AUASI at one year, the primary outcome measure [16]. It was not clear if these results, which contradicted results of some previous trials, reflected chance or called into question the efficacy of at least this particular preparation of Serenoa repens, the same preparation selected for use in CAMUS, at the standard dose. Because saw palmetto is more widely used in the U.S. than Pygeum africanum, the funding agencies felt that any phytotherapy trial in BPH had to include saw palmetto so dropping the saw palmetto arm and proceeding with a 3-arm study was not an option.
Figure 1.
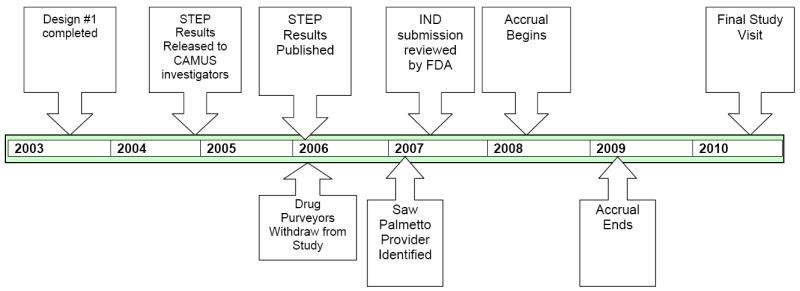
CAMUS Timeline
The negative findings from two NCCAM-funded clinical trials of two complementary and alternative medicines had an impact on the CAMUS study. In a study of St. John’s wort for depression, it was speculated that its lack of efficacy might have resulted from inadequate dosing [17]. The finding that three preparations of Echinacea were ineffective against the common cold [18] was accompanied by an editorial that suggested that NCCAM was selecting “implausible agents” for study based on their popularity and recommended that the agency conduct clinical trials in treatments where there is knowledge-based evidence that there is a reasonable chance of efficacy [19]. The funding agencies strongly encouraged the investigators to change the focus of the CAMUS study from evaluating long-term efficacy in preventing progression of LUTS to determining if there was a dose of either botanical product that could provide short-term (6-18 months) reduction of LUTS.
Dose-ranging Study Designs
With the study now focused on evaluating multiple doses of the two botanical products, two types of study designs were discussed: 1) an intrapatient dose escalation study in which study participants on the phytotherapy agents would receive escalating doses at 6-month intervals: saw palmetto at 320 mg, 640 mg, 960 mg daily or Pygeum africanum at 100 mg, 200 mg, and 300 mg, and 2) a fixed dose design in which participants were assigned to placebo or a dose level of one of the phytotherapies to be taken over 12 months. The intrapatient dose escalation design would address the question of whether either phytotherapy, at any dose, would provide short-term efficacy.
The investigators opted for the fixed dose design, because it would directly compare each dose level against placebo. There was considerable discussion about the number of dose levels to study for each product. It was unclear whether there was a need to re-evaluate the 320 mg daily dose that had been used in STEP, but including it would provide an opportunity to determine if the STEP results could be replicated. The purveyors expressed some concern about the higher doses since studies had not previously been conducted at these dose levels. To maintain masking would have required all participants to ingest 6 gelcaps daily. Concerns about participant adherence led to the decision to conduct separate studies for the two agents, each with four arms, placebo and 3 doses of a botanical product. Clinical centers would be randomly assigned to participate in the saw palmetto or Pygeum africanum trial.
Each trial was designed to detect a 3-point difference (with standard deviation of 6) between each dose level and placebo with respect to the change in AUA symptom score from baseline to 12 months, the same outcome measure used in STEP. To preserve an overall 0.05 two-sided significance level between the two studies, each with 4 arms, placebo and 3 phytotherapy dose levels, would have required 125 patients per arm for a total of 1000 patients. NIH informed the investigators that this sample size was not feasible with the remaining funds available for the study, and that the investigators should aim for a study with 600 patients.
Since the STEP results did not detect a 3 point difference in the change in the AUA symptom score between saw palmetto and placebo, it was suggested that the study be designed to detect a smaller difference of 2 points. Using the intrapatient dose escalation design with 3 dose levels and dose escalation at 6 month intervals, study duration for each participant would be 18 months. With this design, each study would have two arms and would require 332 patients to detect a 2 point difference (with standard deviation of 6) between the botanical product and placebo with respect to the change in AUA symptom score from baseline. Design characteristics of the intrapatient dose escalation study design varied from the fixed dose design in that the one-sided significance level of 0.05 was used in each study which allowed a lower difference between treatment arms with respect to the change in AUA symptom index to be detected with a smaller sample size.
Loss of Purveyors for Botanical Products
When the STEP results were published [16], the accompanying editorial speculated that the lack of efficacy associated with Serenoa repens might be attributed to the specific preparation used [20], the same preparation planned for CAMUS. Shortly following the publication, the purveyor for saw palmetto withdrew support for the study. The purveyor for Pygeum africanum was acquired by another firm and it, too, withdrew from the study.
A significant delay ensued as NIH decided to proceed with a study solely focused on saw palmetto and issued a new RFP for a supplier of saw palmetto. The saw palmetto product selected for use in CAMUS is an ethanolic extract of saw palmetto berries and differs from the saw palmetto product tested in the STEP trial, which was produced with a CO2 extraction process.
Final CAMUS Design
In the final design, CAMUS is a prospective, randomized, double-blind, multicenter, placebo-controlled clinical trial to determine if escalating doses of saw palmetto (320 mg, 640 mg, 960 mg daily) reduce LUTS over an 18-month period in comparison to placebo and whether there is sufficient short-term efficacy and tolerability to merit testing for long-term efficacy in a trial focused on the prevention of BPH progression. Its primary objective is to determine if escalating doses of Serenoa repens reduces the AUA symptom score compared to placebo over 72 weeks of treatment and is well tolerated. Secondary objectives are to determine if Serenoa repens has a beneficial effect on subjective global assessment; assess its impact on the BPH Impact Index, the Quality of Life item score from the IPSS, the nocturia item score from the IPSS, peak uroflow, post-void residual volume, prostate specific antigen (PSA) level, erectile and ejaculatory function, ICSmale Incontinence scale, Jenkins Sleep Dysfunction scale, and NIH Chronic Prostatitis Symptom Index and to assess its impact on complete blood counts, coagulation studies and basic blood chemistries. Because the saw palmetto arm uses doses higher than those commonly used, participants have their doses slowly increased at 24-week intervals and are evaluated for safety 4 weeks after each dosage level change. (Table 3) The frequency of hematology, serum chemistry and EKG testing was increased to occur every 12 weeks in response to the recommendation by the Food and Drug Administration’s review of the Investigational New Drug (IND) application.
Eligible participants (Table 2) are randomized equally to one of two treatment arms within two AUASI-defined strata (8-15, 16-24): extract of Serenoa repens 320 mg once daily for 24 weeks (one gelcap); followed by 640 mg daily for 24 weeks (two gelcaps) followed by 960 mg daily for 24 weeks (three gelcaps) or placebo. To maintain masking, all participants take one gelcap daily for the first 24 weeks, two gelcaps daily during the second 24 weeks and three gelcaps daily during the next 24 weeks. Protocol treatment is discontinued if the participant develops unacceptable toxicity, or meets one of the protocol-defined reasons for treatment discontinuation: noncompliance, withdrawal of informed consent, investigator discretion, diagnosis of prostate or bladder cancer, crossover to invasive or open-label therapy for BPH, or death. The study was approved by the Data Safety and Monitoring Board, and is conducted under an IND held by the NIH.
Table 2.
Schedule of Procedures
| Screening | Week from Randomization | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Procedure | SV1.0 | SV2.0 | 0 | 4 | 12 | 24 | 28 | 36 | 48 | 52 | 60 | 72 |
| Informed Consent | ● | |||||||||||
| Eligibility and randomization | ● | |||||||||||
| Medical History | ● | |||||||||||
| Medical Follow-up | ● | ● | ● | ● | ● | ● | ● | ● | ● | |||
| Assessment of all medications | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ||
| Vital Signs | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | |
| Physical and digital rectal exam | ● | ● | ||||||||||
| Prostate serum antigen (PSA) | ● | ● | ● | ● | ||||||||
| Uroflow measurement | ● | ● | ● | ● | ● | ● | ● | ● | ||||
| Hematology, Serum chemistries, EKG | ● | ● | ● | ● | ● | ● | ● | |||||
| Urinalysis | ● | ● | ||||||||||
| Serum for banking | ● | ● | ||||||||||
| Study drug administration and compliance | ● | ● | ● | ● | ● | ● | ● | |||||
| Discontinuation of study drug | ● | |||||||||||
| Questionnaires | ||||||||||||
| Jenkins Sleep Dysfunction Scale | ● | ● | ● | ● | ||||||||
| Erectile Function | ● | ● | ● | ● | ||||||||
| Ejaculatory Function | ● | ● | ● | ● | ||||||||
| Bladder Function | ● | ● | ● | ● | ● | ● | ● | |||||
| AUASI Score | ● | ● | ● | ● | ● | ● | ● | ● | ||||
Results
Enrollment began in July 2008 and ended in April 2009. Patient study participation should be completed by the end of 2010. Study results should be available in 2011.
Discussion
Why did the STEP findings cause the CAMUS study to be completed re-designed? Timing, the challenges of assessing botanical products, and the changing level of acceptable risk for the various stakeholders all played a role in that decision.
The initial major alteration in the design of the study was the inclusion of an active comparator, a drug therapy with proven efficacy against BPH. Since funds had not been allotted for an active comparator, the initial approach was to request a donation from an alpha blocker manufacturer. The attempt to identify a donor was unsuccessful due to the lack of perceived benefit to the prospective donors. Since the efficacy of alpha blockers in the treatment of BPH had been established, its manufacturers were unlikely to benefit from the CAMUS study. The risk was that the CAMUS study would not confirm the alpha blocker’s efficacy or would demonstrate comparability with the phytotherapy agents, both unappealing prospects. Once it became clear that the active comparator would not be donated, additional time was expended in securing funds for purchasing the alpha blocker. Without the delay, it was anticipated that CAMUS would have enrolled 75% of its participants by the time STEP was completed.
Studies of phytotherapy are complicated by variations in formulations and extraction processes [21] and difficulties in masking appearance and odor. Limited information on their mechanisms of action makes it difficult to determine the appropriate dose to be studied. BPH is defined, in large part, by self-reported symptoms precluding the use of animal studies to provide preliminary assessments of dose-related efficacy. The rationale for the large-scale trial of long-term efficacy of the botanical agents with respect to BPH was to conduct a study without the methodological limitations of previously reported clinical trials The STEP trial had considerable credibility because it was considered to be methodologically sound. Although the results were negative, it was not initially clear that saw palmetto’s lack of short-term efficacy meant that it would not be effective in the long-term. In MTOPS, finasteride was no better than placebo with respect to the short-term AUA symptom score change, but had a longer time to BPH progression [5]. The STEP results could have been viewed as confirmation of 5-alpha reducatase inhibition as saw palmetto’s mechanism of action.
For the NIH agencies involved, the CAMUS study represented a major investment. Although equipoise is required for the initiation of a clinical trial designed to assess superiority of one or more agents in comparison to placebo, it was hoped that the investment would result in a payoff by establishing efficacy of one or more of the experimental agents. The negative findings from other clinical trials of complementary and alternative medicine decreased the funding agencies’s tolerance for risk. For the drug purveyors who responded to the initial request for donations of botanical products and matching placeboes for the large-scale trial, the CAMUS study represented an opportunity to demonstrate the efficacy of their product for BPH at minimal cost since NIH funded the costs of developing and conducting the trial. The prolongation of the protocol development process meant that the priority for the CAMUS study within the internal organizations at the purveyors diminished with time.
Implicit in the decision to change the focus of the study to evaluation of botanical therapies at dose levels higher than previously reported is the underlying assumption that there is a dose-response effect. Although a dose-response relationship has not been established for the botanical therapies, a dose-response effect on suppression of dihydrotestosterone (DHT) has been shown with dutasteride, a 5-alpha reductase inhibitor [22]. For the drug purveyors, the fixed dose design was more appealing. If higher doses induced greater reduction in LUTS, there was the potential for increasing demand their product.
A positive result in the intrapatient dose escalation design would mean that the botanical product was effective in reducing LUTS without defining the effective dose. Since there was already evidence for those findings, there was little motivation for the purveyors to remain committed to the study.
At the time the two initial purveyors withdrew and a new saw palmetto provider was sought for the intrapatient dose escalation design, the trial design was not reconsidered. This may, in part, have been due to the requirement that the study protocol accompany the new RFP for a provider to define the scope of work. Investigator fatigue with the lengthy process was also a factor. The new provider of saw palmetto agreed to provide product for the intrapatient dose escalation study.
The CAMUS experience confirms other reports that actions taken in response to new scientific information are influenced by multiple factors [23, 24]. All sponsors of the project have goals that must be considered and external forces can have an impact. With CAMUS, the risks of proceeding with the initial trial to evaluate long-term efficacy of botanical therapies were deemed too high. The result is a smaller, less ambitious clinical trial.
Acknowledgments
Supported by cooperative agreements from the National Institute of Diabetes and Digestive and Kidney Diseases U01DK063825, U01DK063795, U01DK063833, U01DK063883, U01DK063862, U01DK063831, U01DK063840, U01DK063788, U01DK063866, U01DK063778, U01DK063797, and U01DK063835, the Office of Dietary Supplements and National Center for Complementary and Alternative Medicine at the National Institutes of Health. Serenoa repens and placebo were donated by Madaus/EUROMED
Acknowledgements – CAMUS Study Group
Steering Committee Chair
-
Harvard Medical School
Michael J. Barry, MD
Data Coordinating Center
-
University of Alabama at Birmingham
O. Dale Williams, PhD (Director)
Sreeletha Meleth, PhD (Associate Director)
Lisa R. Allen, MPA
Clinical Sites
-
New York University
Andrew McCollough, MD (PI)
Brianne Goodwin, BSN, RN (Study Coordinator)
Dara Herman, BSN, RN (Study Coordinator)
Artrit Butuci (Research Data Associate)
-
Northern California Kaiser Permanante
Andrew L. Avins, MD, MPH
Harley Goldberg, DO (Co-I)
Luisa Hamilton (Study Coordinator)
-
Northwestern University Fineberg School of Medicine
Kevin T. McVary, MD (PI)
Maria I. Velez (Study Coordinator)
Sharon Lynn Stafford (Program Assistant)
Nancy Schoenecker, RN, CCRC (Clinical Research Coordinator)
-
Queens University
J. Curtis Nickel, MD (PI)
Alvaro Morales (Co-I)
D. Robert Siemens, MD (Co-I)
Joe Downey, MSc, CCRP (Study Coordinator)
Janet Clark-Pereira, CCRP (Study Coordinator)
-
University of Colorado at Denver Health Sciences Center
E. David Crawford, MD (PI)
Shandra S. Wilson, MD (Co-I)
James A. Lugg, MD (Co-I)
Al Barqawi, MB, FRCS (Co-I)
Patricia DeVore, BS (Clinical Research Coordinator)
Cliff Jones (Study Coordinator)
-
University of Iowa
Karl J. Kreder, MD (PI)
Victoria Sharp, MD (Co-I)
Diane Meyerholz, RN, BSN (Study Coordinator)
Mary Eno, RN (Study Coordinator)
-
University of Maryland
Michael J. Nasland, MD (PI)
Andrew Kramer, MD (Co-I)
Ganine Markowitz-Chrystal, MS, CCRC (Study Coordinator)
Myra Collins (Study Coordinator)
-
University of Texas, Southwestern Medical Center
Claus G. Roehrborn, MD (PI)
Brad Hornberger, PA-C) (Co-I)
Allison Beaver, RN (Study Coordinator)
Beth Petty, RN (Study Coordinator)
Suzie Carter (Data Manager)
-
Washington University School of Medicine
Gerald L. Andriole, MD (PI)
Linda Black, RN (Study Coordinator)
Karen Whitmore (Supervisor Patient Services)
-
Weil Medical College of Cornell University
Steven A Kaplan, MD (PI)
Alexis E. Te, MD (Co-I)
Maritza Rodriguez, RNP (Study Coordinator)
-
Yale University School of Medicine
Harris E. Foster, Jr., MD (PI)
Karen Stavris, RN MSN, CCRC (Study Coordinator)
Biostatistics Consultant
-
University of Arkansas for Medical Sciences
Jeannette Y. Lee, PhD
Data Safety Monitoring Board
-
University of Minnesota VA Medical Center
Timothy J. Wilt, MD, MPH (Chair)
-
University of Illinois at Chicago
Harry H.S. Fong, Ph.D.
-
University of Chicago
Glenn S. Gerber, MD
-
University of Virginia
Mikel Gray, RN, PhD, CUNP, FAAN
-
HeteroGeneity LLC
Freddie Ann Hoffman, MD
-
University of North Carolina
Gary Koch, PhD
-
University of California at Los Angeles
Mark Litwin, MD, MPH
-
US Environmental Protection Agency
Warren E. Lux, MD
-
Harvard Medical School
Michael P. O’Leary, MD, mPH
-
Alliance for Prevention of Prostate Cancer
Col (Ret.) James E. Williams, Jr.
-
Hines VA Hospital CSPCC
Domenic Reda, PhD
Sponsor, Supplier of Serenoa repens
-
EUROMED USA
Joseph J. Veilleux, MD
Sponsors, National Institutes of Health
-
National Institute of Diabetes, Digestive & Kidney Diseases
John W. Kusek, PhD
Leroy M. Nyberg, MD, PhD
-
National Center for Complementary and Alternative Medicine
Laura K. Moen, PhD
Qi-Ying Liu, PhD
-
Office of Dietary Supplements
Joseph Betz, PhD
Paul M. Coates, MD
References
- 1.Jacobsen SJ, et al. New diagnostic and treatment guidelines for benign prostatic hyperplasia. Potential impact in the United States. Arch Intern Med. 1995;155(5):477–81. [PubMed] [Google Scholar]
- 2.Litman HJ, McKinlay JB. The future magnitude of urological symptoms in the USA: projections using the Boston Area Community Health survey. BJU Int. 2007;100(4):820–5. doi: 10.1111/j.1464-410X.2007.07018.x. [DOI] [PubMed] [Google Scholar]
- 3.Committee APG. AUA guideline on management of benign prostatic hyperplasia. Chapter 1. Diagnosis and treatment recommendations. J Urol. 2003:530–47. doi: 10.1097/01.ju.0000078083.38675.79. [DOI] [PubMed] [Google Scholar]
- 4.Roehrborn CG, et al. The benign prostatic hyperplasia registry and patient survey: study design, methods and patient baseline characteristics. BJU Int. 2007;100(4):813–9. doi: 10.1111/j.1464-410X.2007.07061.x. [DOI] [PubMed] [Google Scholar]
- 5.McConnell JD, et al. The long-term effect of doxazosin, finasteride, and combination therapy on the clinical progression of benign prostatic hyperplasia. N Engl J Med. 2003;349(25):2387–98. doi: 10.1056/NEJMoa030656. [DOI] [PubMed] [Google Scholar]
- 6.Bautista OM, et al. Study design of the Medical Therapy of Prostatic Symptoms (MTOPS) trial. Control Clin Trials. 2003;24(2):224–43. doi: 10.1016/s0197-2456(02)00263-5. [DOI] [PubMed] [Google Scholar]
- 7.Gardiner P, et al. Factors associated with herbal therapy use by adults in the United States. Altern Ther Health Med. 2007;13(2):22–9. [PubMed] [Google Scholar]
- 8.Fourcade RO, Theret N, Taieb C. Profile and management of patients treated for the first time for lower urinary tract symptoms/benign prostatic hyperplasia in four European countries. BJU Int. 2008;101(9):1111–8. doi: 10.1111/j.1464-410X.2008.07498.x. [DOI] [PubMed] [Google Scholar]
- 9.Dedhia RC, McVary KT. Phytotherapy for lower urinary tract symptoms secondary to benign prostatic hyperplasia. J Urol. 2008;179(6):2119–25. doi: 10.1016/j.juro.2008.01.094. [DOI] [PubMed] [Google Scholar]
- 10.Gerber GS. Saw palmetto for the treatment of men with lower urinary tract symptoms. J Urol. 2000;163(5):1408–12. [PubMed] [Google Scholar]
- 11.Ishani A, et al. Pygeum africanum for the treatment of patients with benign prostatic hyperplasia: a systematic review and quantitative meta-analysis. Am J Med. 2000;109(8):654–64. doi: 10.1016/s0002-9343(00)00604-5. [DOI] [PubMed] [Google Scholar]
- 12.Tacklind J, et al. Serenoa repens for benign prostatic hyperplasia. Cochrane Database Syst Rev. 2009;(2):CD001423. doi: 10.1002/14651858.CD001423.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilt T, Ishani A, Mac Donald R. Serenoa repens for benign prostatic hyperplasia. Cochrane Database Syst Rev. 2002;(3):CD001423. doi: 10.1002/14651858.CD001423. [DOI] [PubMed] [Google Scholar]
- 14.Wilt T, et al. Pygeum africanum for benign prostatic hyperplasia. Cochrane Database Syst Rev. 2002;(1):CD001044. doi: 10.1002/14651858.CD001044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thompson IM, et al. The influence of finasteride on the development of prostate cancer. N Engl J Med. 2003;349(3):215–24. doi: 10.1056/NEJMoa030660. [DOI] [PubMed] [Google Scholar]
- 16.Bent S, et al. Saw palmetto for benign prostatic hyperplasia. N Engl J Med. 2006;354(6):557–66. doi: 10.1056/NEJMoa053085. [DOI] [PubMed] [Google Scholar]
- 17.Group, H.D.T.S. Effect of Hypericum perforatum (St John’s wort) in major depressive disorder: a randomized controlled trial. JAMA. 2002;287(14):1807–14. doi: 10.1001/jama.287.14.1807. [DOI] [PubMed] [Google Scholar]
- 18.Turner RB, et al. An evaluation of Echinacea angustifolia in experimental rhinovirus infections. N Engl J Med. 2005;353(4):341–8. doi: 10.1056/NEJMoa044441. [DOI] [PubMed] [Google Scholar]
- 19.Sampson W. Studying herbal remedies. N Engl J Med. 2005;353(4):337–9. doi: 10.1056/NEJMp058130. [DOI] [PubMed] [Google Scholar]
- 20.DiPaola RS, Morton RA. Proven and unproven therapy for benign prostatic hyperplasia. N Engl J Med. 2006;354(6):632–4. doi: 10.1056/NEJMe058301. [DOI] [PubMed] [Google Scholar]
- 21.Habib FK, Wyllie MG. Not all brands are created equal: a comparison of selected components of different brands of Serenoa repens extract. Prostate Cancer Prostatic Dis. 2004;7(3):195–200. doi: 10.1038/sj.pcan.4500746. [DOI] [PubMed] [Google Scholar]
- 22.Clark RV, et al. Marked suppression of dihydrotestosterone in men with benign prostatic hyperplasia by dutasteride, a dual 5alpha-reductase inhibitor. J Clin Endocrinol Metab. 2004;89(5):2179–84. doi: 10.1210/jc.2003-030330. [DOI] [PubMed] [Google Scholar]
- 23.Breitner JC, Martin BK, Meinert CL. The suspension of treatments in ADAPT: concerns beyond the cardiovascular safety of celecoxib or naproxen. PLoS Clin Trials. 2006;1(8):e41. doi: 10.1371/journal.pctr.0010041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nissen SE. ADAPT: the wrong way to stop a clinical trial. PLoS Clin Trials. 2006;1(7):e35. doi: 10.1371/journal.pctr.0010035. [DOI] [PMC free article] [PubMed] [Google Scholar]


