The dynamic regulation of blood flow in the peripheral circulation comprises multiple mechanisms that work in concert to provide adequate control of blood supply to the various vascular beds that feed organ systems. Blood vessels actively respond to different stimuli to adapt vessel diameter according to the continuously changing metabolic requirements of such organ systems. Among these vasoactive stimuli, shear stress as well as endocrine and paracrine factors play a substantial role in the maintenance of vascular homeostasis and the balance between vasoconstriction and vasodilation by acting on the different cell types that form the vascular wall, such as vascular endothelial and smooth muscle cells (SMCs) (Patterson et al., 2002).
Intracellular calcium concentration ([Ca2+]i) is critical for vascular smooth muscle function, as increased [Ca2+]i triggers SMC contraction. In response to vasoactive ligands that bind to Gq/phospholipase C–coupled receptors, inositol 1,4,5-trisphosphate (IP3) is produced. IP3 binds to its receptor (IP3R) in the membrane of the SMC SR, which facilitates Ca2+ release from the SR, raising [Ca2+]i and inducing cell contraction. SMCs can also respond to membrane depolarization via voltage-dependent Ca2+ channels (VDCCs) that mediate Ca2+ entry from the extracellular milieu independently of SR Ca2+ release (Xi et al., 2008; Zhao et al., 2008), triggering vasoconstriction. Moreover, vascular SMCs express plasma membrane large-conductance, Ca2+-activated K+ (BKCa) channels, which are gated by Ca2+ with relatively low (micromolar) affinity. As a consequence of a large increase in [Ca2+]i after IP3-mediated depletion of internal stores, a BKCa-dependent hyperpolarizing K+ current will induce inactivation of VDCCs, thereby preventing vasoconstriction (Patterson et al., 2002).
The interplay between the different cellular mechanisms that increase [Ca2+]i is important for the control of vascular tone. In this context, the report we review here (Zhao et al., 2010) sheds an interesting light onto how a negative feedback mechanism involving type 1 IP3 receptors (IP3R1) and BKCa channels is established in cerebral artery SMCs. The findings of Zhao et al. (2010) bring to our attention the possibility that IP3 may further regulate vascular tone by increasing the apparent Ca2+ sensitivity of the BKCa channel through a mechanism involving an interaction between the channel and IP3R1, independently of SR Ca2+ release. Using electrophysiological approaches on freshly isolated cerebral artery SMCs, the authors found that IP3 increased the open probability (PO) of BKCa channels in both cell-attached and excised inside-out membrane patches in a dose-dependent manner. Neither fast Ca2+ buffering with BAPTA nor elevated pipette Ca2+ concentration (2 mM) altered the ability of IP3 to increase PO of BKCa channels, suggesting that the observed phenomenon was independent of both intracellular calcium release and plasma membrane calcium influx. Moreover, when the authors examined the Ca2+ sensitivity of BKCa channels in inside-out patches at a physiological membrane potential of −40 mV, they found that 10 µM IP3 induced a decrease of the mean apparent dissociation constant (Kd) for Ca2+ of the channel from ∼20 to ∼12 µM.
Other reports have suggested that IP3 may regulate vasoconstriction by inducing a direct coupling between IP3R1 and canonical transient receptor potential (TRPC) type 3 (TRPC3) channels (Xi et al., 2008; Adebiyi et al., 2010), raising the question of whether IP3 regulates BKCa activity by means of IP3R1. Indeed, Zhao et al. (2010) found that heparin, an IP3R blocker as well as a monoclonal antibody against the cytosolic C terminus of IP3R1, prevented the IP3-mediated increase in BKCa channel PO. Moreover, experiments performed on cerebral artery SMCs isolated from IP3R1 knockout mice showed that IP3 is unable to increase BKCa channel PO in the absence of the IP3R. This requirement for the IP3R suggests that the receptor could interact directly with the BKCa channel, a hypothesis that was tested by coimmunoprecipitation and immunofluorescence resonance energy transfer (immuno-FRET) studies. Coimmunoprecipitation experiments revealed that both α and β1 subunits of the BKCa channel are precipitated by an anti-IP3R1 antibody, suggesting that the three polypeptides coexist in a higher-order macromolecular complex, whereas immuno-FRET showed that BKCa and IP3R1 localized in close spatial proximity. The data suggest that IP3 can modulate vascular tone by regulating BKCa channel activity independently of the canonical SR Ca2+ release pathway, and highlight that this phenomenon requires an interaction between the BKCa channel and IP3R1.
The paper by Zhao et al. (2010) advances our knowledge of the regulation of vascular function by revealing a novel molecular signaling mechanism for IP3, a ubiquitous second messenger that mediates the actions of many Gq/phospholipase C–coupled vasoconstrictor agonists such as endothelin-1 and angiotensin II (Patterson et al., 2002; Adebiyi et al., 2010). The same group has contributed to the understanding of this non-canonical IP3 signaling by providing evidence that IP3Rs can directly couple to plasma membrane ion channels such as TRPC3, thereby modifying their activity (Xi et al., 2008; Adebiyi et al., 2010). In arterial myocytes, both endothelin-1 and IP3 activate a nonselective cation current (ICat) that depends on the functional coupling of TRPC3 and IP3R1, an interaction that proved to be isoform selective and independent of SR Ca2+ release. Although TRPC3-mediated ICat would cause membrane depolarization and activation of VDCCs, thereby inducing vasoconstriction, the novel finding that BKCa channels are also activated by IP3 in an IP3R1-dependent manner raises an interesting question regarding the balance between depolarizing and hyperpolarizing currents in arterial myocytes, which could play a significant role in the control of basal vascular tone and of other active vascular processes such as the myogenic response or endothelium-mediated SMC hyperpolarization. In fact, BKCa channels have been implicated in the control of myogenic tone as a hemodynamic autoregulatory mechanism (Hill et al., 2010), and the fact that IP3 is able to simultaneously regulate TRPC3 and BKCa channels provides insight into the role that paracrine factors may play in the modulation of the myogenic response and the local regulation of blood flow. The role of TRPC channels in the control of vasoconstriction has been addressed before, and it has been suggested that the main isoform involved in the myogenic response (i.e., vasoconstriction in response to increased transmural pressure) is TRPC6, whereas TRPC3 would be implicated in the response to vasoconstrictor agents that elevate IP3 (Welsh et al., 2002; Adebiyi et al., 2010). Beyond local regulation, it will be interesting to probe the importance of this specific coupling between IP3R1 and BKCa channels in the control of blood flow distribution and its role in other vascular beds.
Despite the compelling evidence discussed above, some questions still remain. For instance, all IP3 stimulations in inside-out patch clamp experiments were performed at concentrations of 10 µM IP3 or higher, with the support of an IP3 dose–response curve that yielded maximal stimulations at 10 µM and an apparent Kd for IP3 of ∼4.1 ± 1.3 µM. Although the authors’ discussion about arterial myocyte global [IP3]i is enlightening, we agree that more accurate measurements of basal and agonist-stimulated arterial myocyte [IP3]i are required to obtain a clearer picture of the physiologically relevant IP3 levels that will induce IP3R1-mediated activation of BKCa channels. To this effect, electrophysiological experiments performed at ∼4 µM IP3 (the apparent Kd indicated by the data) may further clarify the relevance of submaximal IP3 levels that may be encountered in a physiological context. It was reported previously that Bt-IP3, a cell-permeable IP3 analogue, could induce TRPC3-mediated depolarization in endothelium-denuded, SR Ca2+-depleted arteries pressurized at 20 mmHg at concentrations as low as 1 µM (Xi et al., 2008), whereas the data provided by Zhao et al. (2010) show no increase in the mean PO of BKCa channels at such IP3 levels. This discrepancy may be a result of the experimental approach, but we cannot rule out the possibility that the very nature of the interaction between IP3R1 and plasma membrane ion channels may be different. IP3Rs have multiple IP3-binding sites with varying affinities (Foskett et al., 2007), and receptor-activating conformational changes induced by IP3 binding at different sites could favor interactions with different proteins.
We think that elucidating whether the IP3-induced shift in Ca2+ sensitivity of the BKCa channel (from ∼20 to ∼12 µM) is relevant for its biophysical properties, as well as for its physiological role, would be an important step in the clarification of the general picture this paper conveys. However, it remains unsolved whether this effect is in fact a result of a direct molecular interaction between IP3R1 and the BKCa channel. We think that the authors do not provide a conclusive demonstration for the occurrence of a direct interaction, despite that evidence in favor of the requirement of IP3R1 for IP3-mediated activation of BKCa channels is presented. Heparin, a general IP3R blocker, and an antibody against IP3R1 both abolish IP3-induced BKCa activation in inside-out membrane patches. The effect of these IP3R1-blocking strategies would require an intact and functional receptor (i.e., able to bind IP3 and activate BKCa) excised with the patch. In other work by this group, it was shown that a synthetic peptide corresponding to the N-terminal sequence of the IP3R containing the TRPC3-binding region activated a Gd3+-sensitive, nonselective cation current (ICat). Also, membrane-permeant (TAT-conjugated) synthetic peptides were used to probe the coupling between IP3R1 and TRPC3. Data revealed the presence of a calmodulin- and IP3R-binding domain in TRPC3 channels that supports the occurrence of the proposed interaction (Adebiyi et al., 2010). To our knowledge, the presence of calmodulin- and IP3R-binding domains has not been described in BKCa channels. The use of these experimental approaches could prove helpful in the validation of the authors’ hypothesis by establishing the actual domains that may be mediating the interaction between IP3R1 and BKCa channels.
IP3R1 was also shown to localize in close proximity to BKCa channels by immuno-FRET. As opposed to the standard FRET approach, which makes use of genetically encoded fluorophores directly coupled to the proteins of interest, immuno-FRET is based on the energy transfer between fluorophores coupled to the secondary antibodies of a standard indirect immunofluorescence. In both methodologies, resonance energy transfer occurs between donor and acceptor molecules located within a specific distance (the Förster radius, R0) characteristic of each FRET pair, and it is unlikely to occur when the distance between fluorophores is >100 Å (Pietraszewska-Bogiel and Gadella, 2011). Positive immuno-FRET signals are a good indicator of the interaction between IP3R1 and BKCa if the distance between donor and acceptor represents the same separation between these proteins, but this is hard to determine because the three-dimensional structure of the immunocomplex is unknown. Moreover, antibodies are large proteins with a molecular weight of ∼150 kD. Analysis of the crystal structure of various immunoglobulins has shown that the distance between antigen-binding sites can vary between 118 and 185 Å (Saphire et al., 2002). Because R0 for the FRET pair used by the authors (Cy2/Cy3) is ∼50–60 Å (Zhao et al., 2010), it is possible that the actual distance between IP3R1 and BKCa may be >100 Å, suggesting that energy transfer could be a consequence of the interaction between components of the immunocomplex, regardless of whether or not IP3R1 and BKCa interact and/or localize nearby.
The hypothesis that IP3R1 and BKCa channels are found in close proximity is also supported by coimmunoprecipitation experiments showing that an IP3R1 antibody is able to pull down both subunits of the BKCa channel. Although it is not conclusive evidence of direct interaction, coimmunoprecipitation data clearly suggests the existence of a macromolecular complex that comprises both entities. Despite this attractive inference, Zhao et al. (2010) do not provide a control reverse immunoprecipitation to strengthen their conclusions. Also, probing the presence of molecular chaperones that may stabilize these interactions could provide insight on the trafficking and signaling properties of the IP3R1–BKCa complex, as well as providing explanation for the intracellular normalized FRET data the authors obtained. However, the fact that the regulatory β1 subunit of the channel was coimmunoprecipitated is particularly important; its role as a crucial regulator of channel activity and Ca2+ sensitivity (Brenner et al., 2000; Patterson et al., 2002) could provide insight on the mechanisms of BKCa modulation by IP3 and IP3R1. Experiments aimed at discriminating which BKCa subunit mediates the interaction between the channel and the IP3R or other components of the immunoprecipitated complex will help clarify this issue.
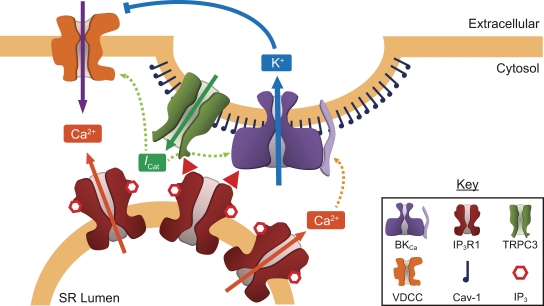
It has been shown previously that TRPC3 and TRPC6 channels can associate with BKCa channels in differentiated cells of a podocyte cell line (Kim et al., 2009), whereas recent work shows that, in arterial SMCs, caveolin-1, a lipid raft scaffolding protein, assembles IP3R1 and TRPC3 channels to signaling complexes (Adebiyi et al., 2011). Moreover, it has been shown that BKCa channels are localized to lipid rafts in glioma cells, an interaction that places BKCa channels in close proximity to IP3Rs (Weaver et al., 2007). The involvement of lipid rafts and caveolin-1 in the architecture of these macromolecular signaling complexes brings a bridging element between the different components that constitute them, and paves the road to further inquiry regarding the physiological role of such complexes and the potential interaction with other elements known to localize to plasma membrane microdomains. However, other potential interactions may take place between plasma membrane ion channels and SR membrane components. The STIM/Orai family of proteins has been shown to mediate store-operated Ca2+ entry in many different tissues including vascular smooth muscle, and they have been proposed to interact with members of the TRPC channel family to regulate Ca2+ entry at cellular domains where the plasma membrane is in close proximity to the SR membrane (Wang et al., 2008). Therefore, BKCa channels, IP3, and IP3Rs may also be subject to interaction with these ion channel families in the control of SMC Ca2+ signals, cell contractility, and vascular tone. Fig. 1 summarizes the potential interactions discussed above.
Figure 1.
IP3-induced interactions between IP3Rs and ion channels in arterial SMCs. Induction of Ca2+ release from the SR via IP3R (solid orange arrows) is the best-known mechanism of action of IP3. Non-canonical IP3 signaling is independent of SR Ca2+ release and involves an IP3-induced interaction of IP3R1 with plasma membrane channels such as TRPC3 and BKCa (red arrowheads), possibly localized in membrane caveolae. TRPC3 activation by IP3R1 induces a nonselective cation current (ICat; solid green arrow) that depolarizes the cell membrane, activating VDCC and BKCa channels (dashed green arrows). BKCa channels are also activated by Ca2+ (dashed orange arrows). Whereas VDCCs mediate Ca2+ influx through the plasma membrane (purple arrow) leading to contraction, K+ efflux through BKCa channels (blue arrow) leads to hyperpolarization of the cell membrane, which closes VDCCs, leading to SMC relaxation.
The evidence regarding the regulation of vascular homeostasis and, in particular, arterial smooth muscle physiology by non-canonical IP3 signaling suggests that macromolecular signaling complexes that physically and functionally link the plasma and SR membranes may be an important common theme in terms of the fine modulation of signals that establish vascular SMC excitability and vascular tone, thereby controlling active processes such as the myogenic response. At the same time, they hint at the specific possibility that IP3-mediated regulation of BKCa channels may not only involve the IP3R but also other molecules present in these signaling microdomains. The recent determination of the crystal structure of the cytoplasmic Ca2+-sensing domain of the BKCa channels (Wu et al., 2010; Yuan et al., 2010) will certainly provide further insight to this matter by means of structure-driven mutational analysis to elucidate the specific regions of BKCa channels that interact with IP3R1. Careful dissection of their components and integration of their independent and interdependent activities in a physiologically sound model of vascular function will be the next step in the advancement of this field.
Please participate in a discussion of this Journal Club article on the JGP Facebook page (www.facebook.com/JGenPhysiol).
Acknowledgments
The authors would like to thank Drs. Jorge E. Contreras, Walter N. Durán and Martha C. Nowycky, Mrs. Ammy M. Santiago, Mr. Rafael Contreras, and Mr. Jorge Cantero for their helpful comments and suggestions.
The authors are supported by National Institutes of Health grants 5R01 HL070634 and 5R01 HL088479.
Jorge E. Contreras served as faculty advisor.
Lawrence G. Palmer served as editor.
Footnotes
Abbreviations used in this paper:
- BKCa
- large-conductance, Ca2+-activated K+
- [Ca2+]i
- intracellular calcium concentration
- immuno-FRET
- immunofluorescence resonance energy transfer
- IP3
- inositol 1,4,5-trisphosphate
- IP3R
- IP3 receptor
- SMC
- smooth muscle cell
- TRPC
- canonical transient receptor potential
- VDCC
- voltage-dependent Ca2+ channel
References
- Adebiyi A., Zhao G., Narayanan D., Thomas-Gatewood C.M., Bannister J.P., Jaggar J.H. 2010. Isoform-selective physical coupling of TRPC3 channels to IP3 receptors in smooth muscle cells regulates arterial contractility. Circ. Res. 106:1603–1612 10.1161/CIRCRESAHA.110.216804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adebiyi A., Narayanan D., Jaggar J.H. 2011. Caveolin-1 assembles type 1 inositol 1,4,5-trisphosphate receptors and canonical transient receptor potential 3 channels into a functional signaling complex in arterial smooth muscle cells. J. Biol. Chem. 286:4341–4348 10.1074/jbc.M110.179747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner R., Peréz G.J., Bonev A.D., Eckman D.M., Kosek J.C., Wiler S.W., Patterson A.J., Nelson M.T., Aldrich R.W. 2000. Vasoregulation by the β1 subunit of the calcium-activated potassium channel. Nature. 407:870–876 10.1038/35038011 [DOI] [PubMed] [Google Scholar]
- Foskett J.K., White C., Cheung K.-H., Mak D.-O.D. 2007. Inositol trisphosphate receptor Ca2+ release channels. Physiol. Rev. 87:593–658 10.1152/physrev.00035.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill M.A., Yang Y., Ella S.R., Davis M.J., Braun A.P. 2010. Large conductance, Ca2+-activated K+ channels (BKCa) and arteriolar myogenic signaling. FEBS Lett. 584:2033–2042 10.1016/j.febslet.2010.02.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E.Y., Alvarez-Baron C.P., Dryer S.E. 2009. Canonical transient receptor potential channel (TRPC)3 and TRPC6 associate with large-conductance Ca2+-activated K+ (BKCa) channels: role in BKCa trafficking to the surface of cultured podocytes. Mol. Pharmacol. 75:466–477 10.1124/mol.108.051912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson A.J., Henrie-Olson J., Brenner R. 2002. Vasoregulation at the molecular level: a role for the β1 subunit of the calcium-activated potassium (BK) channel. Trends Cardiovasc. Med. 12:78–82 10.1016/S1050-1738(01)00146-3 [DOI] [PubMed] [Google Scholar]
- Pietraszewska-Bogiel A., Gadella T.W.J. 2011. FRET microscopy: from principle to routine technique in cell biology. J. Microsc. 241:111–118 10.1111/j.1365-2818.2010.03437.x [DOI] [PubMed] [Google Scholar]
- Saphire E.O., Stanfield R.L., Crispin M.D., Parren P.W., Rudd P.M., Dwek R.A., Burton D.R., Wilson I.A. 2002. Contrasting IgG structures reveal extreme asymmetry and flexibility. J. Mol. Biol. 319:9–18 10.1016/S0022-2836(02)00244-9 [DOI] [PubMed] [Google Scholar]
- Wang Y., Deng X., Hewavitharana T., Soboloff J., Gill D.L. 2008. Stim, ORAI and TRPC channels in the control of calcium entry signals in smooth muscle. Clin. Exp. Pharmacol. Physiol. 35:1127–1133 10.1111/j.1440-1681.2008.05018.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver A.K., Olsen M.L., McFerrin M.B., Sontheimer H. 2007. BK channels are linked to inositol 1,4,5-triphosphate receptors via lipid rafts: a novel mechanism for coupling [Ca(2+)](i) to ion channel activation. J. Biol. Chem. 282:31558–31568 10.1074/jbc.M702866200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh D.G., Morielli A.D., Nelson M.T., Brayden J.E. 2002. Transient receptor potential channels regulate myogenic tone of resistance arteries. Circ. Res. 90:248–250 10.1161/hh0302.105662 [DOI] [PubMed] [Google Scholar]
- Wu Y., Yang Y., Ye S., Jiang Y. 2010. Structure of the gating ring from the human large-conductance Ca(2+)-gated K(+) channel. Nature. 466:393–397 10.1038/nature09252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi Q., Adebiyi A., Zhao G., Chapman K.E., Waters C.M., Hassid A., Jaggar J.H. 2008. IP3 constricts cerebral arteries via IP3 receptor-mediated TRPC3 channel activation and independently of sarcoplasmic reticulum Ca2+ release. Circ. Res. 102:1118–1126 10.1161/CIRCRESAHA.108.173948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan P., Leonetti M.D., Pico A.R., Hsiung Y., MacKinnon R. 2010. Structure of the human BK channel Ca2+-activation apparatus at 3.0 A resolution. Science. 329:182–186 10.1126/science.1190414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao G., Adebiyi A., Blaskova E., Xi Q., Jaggar J.H. 2008. Type 1 inositol 1,4,5-trisphosphate receptors mediate UTP-induced cation currents, Ca2+ signals, and vasoconstriction in cerebral arteries. Am. J. Physiol. Cell Physiol. 295:C1376–C1384 10.1152/ajpcell.00362.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao G., Neeb Z.P., Leo M.D., Pachuau J., Adebiyi A., Ouyang K., Chen J., Jaggar J.H. 2010. Type 1 IP3 receptors activate BKCa channels via local molecular coupling in arterial smooth muscle cells. J. Gen. Physiol. 136:283–291 10.1085/jgp.201010453 [DOI] [PMC free article] [PubMed] [Google Scholar]