Voltage-gated potassium (Kv) channels play an essential role in regulating membrane excitability. Among ion channels, Kv channels are exceptional in their genetic diversity and in that their gene products can coassemble with one another and with auxiliary subunits to generate a broad spectrum of channel structures and functions (Vacher et al., 2008). As a consequence, individual Kv channel subtypes activate at distinct membrane potentials, impacting their contribution to intrinsic excitability. In addition, dynamic and reversible posttranslational modifications (PTMs) confer additional diversity to Kv channel function. Kv2.1 is a Kv channel that is widely expressed in neurons, myocytes, and pancreatic β cells (Vacher et al., 2008). In many of these cell types, Kv2.1 contributes the majority of the delayed rectifier current (e.g., Murakoshi and Trimmer, 1999; Guan et al., 2007; Jacobson et al., 2007). During periods of increased activity, the voltage-dependent activation of Kv2.1 is shifted to more negative membrane potentials, resulting in homeostatic suppression of excitability (Du et al., 2000; Misonou et al., 2004, 2005, 2006; Mohapatra et al., 2009). Many of these changes in Kv2.1 gating are mediated through extensive phosphorylation of the cytosolic carboxyl (C) terminus (Murakoshi et al., 1997; Park et al., 2006), and mass spectrometric analyses have identified almost two dozen sites chemically modified in vivo with phosphate (Phospho) on Kv2.1 subunits in the mammalian brain (Park et al., 2006; Cerda et al., 2011). The increased levels of intracellular calcium arising from neuronal activity lead to activation of the protein phosphatase calcineurin and Kv2.1 dephosphorylation (Misonou et al., 2004, 2005). Conversely, acute activity blockade induces rapid hyperphosphorylation of Kv2.1 (Misonou et al., 2006). As such, Kv2.1 is exceptional in the wide range of functionalities that can be generated from a single-channel type through reversible multisite phosphorylation.
In this issue, Plant et al. provide compelling evidence for a distinct additional mode of posttranslational regulation of Kv2.1 gating. Their work demonstrates that endogenous Kv2.1 in neurons, and recombinant Kv2.1 in heterologous cells, is directly modified by a small ubiquitin-like modifier protein (SUMO). There are three isoforms in humans (SUMO 1–3) that range from ≈75–100 amino acids in length and 9–12 kD in molecular mass. Sumoylation is the covalent linkage of the SUMO tag to the epsilon amino group on side chains of lysine residues of target proteins (Gareau and Lima, 2010). Sumoylation is similar to ubiquitination in that both involve an E1-activating enzyme, an E2-conjugating enzyme, and an E3 ligase, and is reversible through the activity of desumoylating proteases such as members of the SENP and Ulp families (Hay, 2005; Liu and Shuai, 2008; Gareau and Lima, 2010). Sumoylation regulates numerous cellular processes, including protein translocation between the cytoplasm and nucleus, transcription, apoptosis, stress responses, and cell cycle (Hay, 2005). A recent study showed that overexpression of SUMO diminished the Kv2.1-based delayed rectifier current density in pancreatic β cells and insulinoma cells, leading to changes in their membrane excitability (Dai et al., 2009). Plant et al. (2011) now show that Kv2.1 is directly tagged by SUMO, that sumoylation regulates the gating of Kv2.1 in neurons and in heterologous cells, and that the SUMO-induced changes in Kv2.1 gating impact neuronal firing. Using mass spectrometric analyses, the authors show that bacterially expressed Kv2.1 is directly modified by the ligation of SUMO to one residue, K470. Moreover, mutation of K470 eliminates all of the effects of sumoylation on Kv2.1 gating. The authors provide evidence that not only is sumoylation of two of the four subunits in a Kv2.1 channel tetramer sufficient to mediate the maximal effects of sumoylation on Kv2.1 gating, but also that Kv2.1 channels are sumoylated on only one or two subunits, and not more. These findings open a new window into the regulation of ion channel gating by a PTM best described for its functions in regulating transcription within the nucleus (Gareau and Lima, 2010). Here, we discuss similarities in the impact of adding SUMO and Phospho tags to Kv2.1, the potential for cross talk between these PTMs, mechanisms whereby these structurally distinct tags could exert such similar effects on channel gating, and how the reversible modification of Kv2.1 by SUMO and Phospho may be dynamically regulated within cells to modulate excitability.
SUMO and Phospho: structurally disparate tags have a similar effect
In spite of rather drastic molecular differences between them (weighing in at ≈10 kD, the heavyweight SUMO protein tags a single Kv2.1 lysine, whereas the ≈0.1-kD flyweight inorganic Phospho group tags approximately two dozen Kv2.1 serine and threonine residues), the effects of changing the sumoylation state of Kv2.1 are strikingly similar to those seen with reversible multisite phosphorylation. The bulk (21/23) of the identified Kv2.1 Phospho sites are found on the cytosolic C terminus (Cerda et al., 2011), and many have been shown to affect voltage-dependent gating (Park et al., 2006). The K470 SUMO site defined by Plant et al. (2011) is also on the C terminus, located near two sites (S453 and S480) dynamically tagged with Phospho. Plant et al. (2011) show that treating neurons or heterologous cells expressing Kv2.1 with SENP1, a SUMO-specific protease that causes desumoylation of target proteins, leads to an approximately −20-mV shift in the half-maximal activation voltage, whereas the addition of SUMO to cells to enhance Kv2.1 sumoylation yields +15- to +20-mV shifts. Desumoylation- and sumoylation-induced shifts are in the same direction and magnitude as Kv2.1 dephosphorylation and phosphorylation, respectively. The effects of saturating levels of SENP1 or SUMO presumably push the Kv2.1 population of control cells into a state where all of the channels are either desumoylated or sumoylated. This suggests that some but not all of the Kv2.1 channels in control cells are tagged with endogenous SUMO at steady state. This also resembles Kv2.1 Phospho tagging in that bidirectional activity-dependent changes in the Phospho state of neuronal Kv2.1 have also been observed, with enhanced activity (triggering dephosphorylation) or acute activity blockade (triggering enhanced phosphorylation) driving these changes. The novel discoveries of Plant et al. (2011) raise several interesting questions for future study. Do the SUMO and Phospho pathways operate independently or as a team? Does the population of Kv2.1 channels remain in their intermediate state of phosphorylation, or is this altered with changes in the sumoylation state? The Phospho state of the Kv2.1 channels was determined neither in the cells subjected to treatments that Plant et al. (2011) show yield large SUMO-dependent differences in gating, nor in Kv2.1 channels harboring the mutation at K470 that eliminates sumoylation. Likewise, there are no available data as to the SUMO state of Kv2.1 in cells subjected to the various treatments that lead to changes in the Phospho state of Kv2.1, or in Kv2.1 channels carrying gain or loss of function mutations in Phospho sites. Such information is crucial to an understanding of the interplay between SUMO and Phospho regulation of Kv2.1.
Cross talk between SUMO and Phospho tags has been observed in the numerous examples of proteins that exhibit Phospho-dependent sumoylation (Hietakangas et al., 2006). This is mediated by a conserved phosphorylation-dependent sumoylation motif (PDSM) that generates a “Phospho-SUMOyl switch” (Yang and Grégoire, 2006). The canonical PDSM consists of a SUMO tetrapeptide consensus motif ψKxE, where ψ represents any aliphatic amino acid, K is the sumoylated lysine, x can be any amino acid, and E is a glutamic acid. The PDSM sequence is ψKxExxSP, where S is the phosphorylated serine residue, and P is an adjacent proline. Phosphorylation of the serine would presumably be mediated by proline-directed protein kinases such as p38MAPK, cyclin-dependent kinases, and GSK3, among others. Phospho tagging of the PDSM is thought to generate a negative charge that binds to a basic domain on the Ubc9 SUMO-conjugating enzyme to stabilize enzyme–substrate interaction (Hietakangas et al., 2006; Yang and Grégoire, 2006). Interestingly, about half of the identified Kv2.1 Phospho sites are adjacent to proline (Cerda et al., 2011). Although the sumoylation site at K470 identified by Plant et al. (2011) does not conform to the canonical PDSM motif, there is a proline-associated Phospho site at S480 (469AKKDKVQDNHLSP481). The exact spatial relationship between K470 and S480 (or any of the other proline-associated sites on Kv2.1) in the intact Kv2.1 channel is not known, as no detailed structural information is available for Kv2.1. Much is known about the protein phosphatases that modulate the Phospho state of Kv2.1, and of particular interest to a discussion of cross talk between Kv2.1 SUMO and Phospho tags is that the Phospho-SUMOyl switch in the transcription factor MEF2 is regulated via dephosphorylation by calcineurin (Yang and Grégoire, 2006), the same protein phosphatase that mediates the activity-dependent dephosphorylation of Kv2.1. Whether SUMO tagging of specific lysine residues (such as K470 on Kv2.1) could entice or deter protein kinases and phosphatases to add or remove Phospho tags from neighboring sites (i.e., a SUMOyl-Phospho switch) has not been determined. However, it is interesting to note that consensus sites for many protein kinases (e.g., PKA, PKC, CaMKII, etc.) can contain a lysine residue, which if SUMOylated would presumably negatively impact the efficiency of Phospho tagging at these sites. Future studies will be required to assess interplay between the SUMO and Phospho tags on Kv2.1, using combinations of treatments and mutations known to affect the level of these tags in concert to determine the extent and nature of any cross talk.
How does tagging the C terminus of Kv2.1 with SUMO and Phospho impact gating?
Kv2.1 has a cytoplasmic C terminus (amino acids [a.a.] 411–853) unique to Kv2.1. How SUMO tagging of K470 and multisite Phospho tagging throughout the extended C-terminal tail exert such potent effects on Kv2.1 gating is not known. Although SUMO-dependent regulation of two other ion channels has been demonstrated previously, the effects and underlying mechanisms are distinct from sumoylation-dependent regulation of Kv2.1. On the background leak potassium channel K2P1 (TWIK1), the action of the SUMO tag is consistent with a steric block of the channel pore (Rajan et al., 2005), and in Kv1.5, sumoylation of the N-terminal T1–S1 region alters steady-state inactivation without affecting activation (Benson et al., 2007). It is interesting to note that the SUMO-tagged residue K470 identified by Plant et al. (2011) is located in the membrane proximal region (a.a. 444–477) of the Kv2.1 C terminus that interacts with a specific segment (a.a. 55–71) within the T1 domain in the Kv2.1 N terminus (Ju et al., 2003; Mohapatra et al., 2008). Interaction of the Kv2.1 N and C termini with one another has long been recognized to impact voltage-dependent gating (e.g., VanDongen et al., 1990). More recent studies using fluorescence resonance energy transfer microscopy and patch clamp techniques showed that N-/C-terminal interaction is primarily between as opposed to within subunits of a tetramer, and that the C terminus moves relative to the N terminus in response to membrane depolarization (Kobrinsky et al., 2006). Loss of N/C interaction through deletion of the C-terminal interaction segment (a.a. 55–71) within the T1 domain mimics and occludes the effects of C-terminal phosphorylation on activation gating (Mohapatra et al., 2008), suggesting that N/C interaction is crucial to Phospho-dependent gating regulation. It will be of interest to determine whether disrupting N/C interaction impacts SUMO-dependent regulation of Kv2.1 gating as described by Plant et al. (2011).
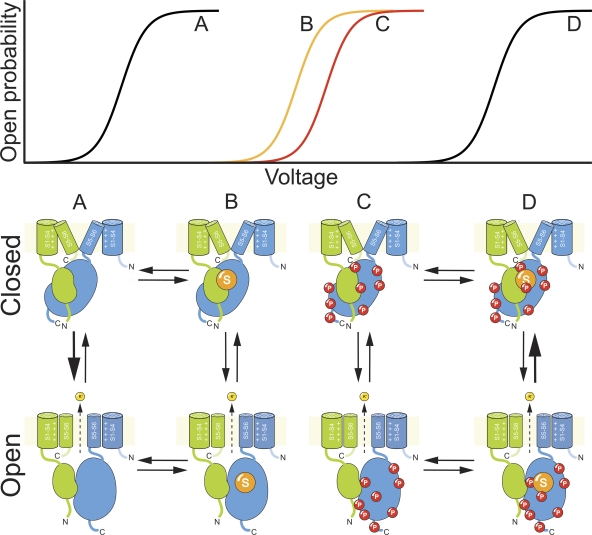
Single-particle image reconstruction of the Kv2.1 structure at 25-Å resolution suggests that the T1 domains of Kv2.1 form a core cylindrical cytoplasmic structure, with the large cytosolic C termini draped around the exterior of the internal T1 bundle (Adair et al., 2008), similar to that proposed previously (Ju et al., 2003). Collectively, these studies suggest a possible structural platform for dynamic rearrangements within the Kv2.1 C terminus during activation gating that affect N/C interaction (Fig. 1). The sumoylated residue in Kv2.1, K470, is located within the segment of the Kv2.1 C terminus (a.a. 444–477) that interacts with the N terminus. One can imagine a scenario whereby altering the SUMO state alters N/C interaction, with subsequent effects on the conformational changes associated with voltage-dependent activation (Fig. 1). Given the positive shifts in the half-maximal activation voltage, SUMO tagging of K470 appears to stabilize closed Kv2.1 channels. Perhaps the channels are held closed by SUMO tagging increasing the affinity of an N/C interaction that promotes channel closure, leading to a requirement for larger membrane depolarizations to provide the energy needed to drive channel opening. Conversely, desumoylation could reduce the affinity of N/C interaction, thus stabilizing open channels. Previous findings are consistent with phosphorylation acting in a similar manner, the distinction being that Phospho tagging of a large number of C-terminal sites is needed to obtain a summed impact equivalent to adding a SUMO tag at K470 (Fig. 1). A prediction, not yet experimentally determined, of a situation where SUMO and Phospho independently stabilize the same closed state is that modification of the C terminus with both SUMO and Phospho tags would lead to further stabilization of the closed state and the most right-shifted voltage-dependent activation curve (Fig. 1).
Figure 1.
A possible model for the effects of SUMO and Phospho tags on Kv2.1 gating. For simplicity, the cartoon depicts only two subunits of the Kv2.1 tetramer and shows intersubunit N/C interaction between only the N terminus of the green subunit and the C terminus of the blue subunit. Panels show Kv2.1 in both closed and open states: (A) lacking C-terminal modification; (B) with a SUMO tag only; (C) with Phospho tags only; and (D) with both SUMO and Phospho tags. The presence or absence of SUMO and Phospho tags affects the affinity of N/C interaction, which then impacts the closed-to-open–state transitions in the Kv2.1 channel that involve movement of N and C termini away from one another. These effects of SUMO and Phospho tags are reflected in the shifts in the voltage-dependent activation curves depicted in the top panel.
An alternative model for how sumoylation could alter gating arises from consideration of the effect it could have on the movement of the transmembrane gating machinery, specifically the voltage sensor module formed by the S1–S4 transmembrane segments. Both the SUMO (net −3 to −5 charge, depending on SUMO isotype) and the Phospho (net −2 charge) tags have an overall acidic nature at physiological pH. The proximity of K470 to the S6 transmembrane domain (ending at a.a. 410) in the primary structure of Kv2.1 suggests that the acidic SUMO tag could be near enough to the membrane to exert an immobilizing electrostatic effect on the positively charged S4 segment within the voltage sensor. The activation curve of the delayed rectifier Kv current in squid giant axons is also right-shifted after phosphorylation (Perozo and Bezanilla, 1990). A detailed analysis suggested that phosphorylation increases the density of negative surface charges in the vicinity of the voltage sensor, leading to electrostatic interactions that presumably yield a partial immobilization of the transmembrane charge movements associated with gating and a positive shift in activation potential (Perozo and Bezanilla, 1990), similar to that seen upon SUMO and Phospho tagging of Kv2.1. Future studies using detailed measurements of the kinetics and voltage dependence of gating currents, and a determination of the charge movement associated with activation gating, in SUMO- and Phospho-tagged Kv2.1 channels relative to their unmodified counterpart may help to resolve these mechanistic questions.
Physiological regulation of Kv2.1 and cellular plasticity
Kv2.1 is dynamically regulated by the addition or removal of Phospho tags in response to changes in activity. A compelling question stemming from the studies of Plant et al. (2011) is whether dynamic changes in SUMO tagging are used to regulate gating of Kv2.1 channels in native cells. Although Plant et al. (2011) show that Kv2.1 gating and neuronal excitability can be modulated experimentally by exposure to SUMO and SENP1, or by mutation of K470, they did not provide evidence that dynamic changes in the sumoylation state are used in physiological (or pathophysiological) regulation of Kv2.1 channel activity or cell excitability.
SUMO tags are added to target proteins via a multistep ligation reaction and removed by SENP or ULP proteases (Gareau and Lima, 2010). An emerging literature suggests that various signaling pathways can acutely regulate the activity of these enzymes via PTMs (Liu and Shuai, 2008). As one example, reactive oxygen species are inhibitory, whereas hypoxia is stimulatory, for the reaction leading to Ubc9-SUMO thioester formation, the penultimate step in SUMO ligation to target proteins. This suggests that these pathophysiological conditions could affect cell excitability via regulation of the Kv2.1 sumoylation state.
An interesting distinction from Phospho tags shown by Plant et al. (2011) is that sumoylation of Kv2.1 acts more like a classical molecular switch, with one possible SUMO site for every two subunits in a channel tetramer. Plant et al. (2011) provide compelling data that the SUMO code yields three states: no subunits tagged, one subunit tagged (intermediate SUMO effect), and two subunits tagged (maximal SUMO effect). In contrast, the approximately two dozen Phospho tags present on each Kv2.1 subunit generate a complex Phospho code that could generate a huge array of distinct functional states. Moreover, this large and diverse collection of distinct Phospho sites allows for complex patterns of regulation via the wide array of protein kinases and phosphatases, whose individual activities can be dynamically in play at any given moment. The quasi “digital versus analog” nature of SUMO and Phospho tags, respectively, suggests that SUMO tags are modulated as a high impact digital switch, perhaps under conditions of stress (reactive oxygen species, hypoxia), similar to the role of sumoylation in regulating transcription, whereas Phsecbospho tags are used for incremental changes in Kv2.1 function that are involved in fine-tuning neuronal excitability.
Acknowledgments
The work from our laboratory on Phospho tagging of Kv2.1 was supported by a grant from the National Institutes of Health (grant R01-NS42225).
References
- Adair B., Nunn R., Lewis S., Dukes I., Philipson L., Yeager M. 2008. Single particle image reconstruction of the human recombinant Kv2.1 channel. Biophys. J. 94:2106–2114 10.1529/biophysj.107.118562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson M.D., Li Q.J., Kieckhafer K., Dudek D., Whorton M.R., Sunahara R.K., Iñiguez-Lluhí J.A., Martens J.R. 2007. SUMO modification regulates inactivation of the voltage-gated potassium channel Kv1.5. Proc. Natl. Acad. Sci. USA. 104:1805–1810 10.1073/pnas.0606702104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerda O., Baek J.H., Trimmer J.S. 2011. Mining recent brain proteomic databases for ion channel phosphosite nuggets. J. Gen. Physiol. 137:3–16 10.1085/jgp.201010555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai X.Q., Kolic J., Marchi P., Sipione S., Macdonald P.E. 2009. SUMOylation regulates Kv2.1 and modulates pancreatic beta-cell excitability. J. Cell Sci. 122:775–779 10.1242/jcs.036632 [DOI] [PubMed] [Google Scholar]
- Du J., Haak L.L., Phillips-Tansey E., Russell J.T., McBain C.J. 2000. Frequency-dependent regulation of rat hippocampal somato-dendritic excitability by the K+ channel subunit Kv2.1. J. Physiol. 522:19–31 10.1111/j.1469-7793.2000.t01-2-00019.xm [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gareau J.R., Lima C.D. 2010. The SUMO pathway: emerging mechanisms that shape specificity, conjugation and recognition. Nat. Rev. Mol. Cell Biol. 11:861–871 10.1038/nrm3011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan D., Tkatch T., Surmeier D.J., Armstrong W.E., Foehring R.C. 2007. Kv2 subunits underlie slowly inactivating potassium current in rat neocortical pyramidal neurons. J. Physiol. 581:941–960 10.1113/jphysiol.2007.128454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay R.T. 2005. SUMO: a history of modification. Mol. Cell. 18:1–12 10.1016/j.molcel.2005.03.012 [DOI] [PubMed] [Google Scholar]
- Hietakangas V., Anckar J., Blomster H.A., Fujimoto M., Palvimo J.J., Nakai A., Sistonen L. 2006. PDSM, a motif for phosphorylation-dependent SUMO modification. Proc. Natl. Acad. Sci. USA. 103:45–50 10.1073/pnas.0503698102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson D.A., Kuznetsov A., Lopez J.P., Kash S., Ammälä C.E., Philipson L.H. 2007. Kv2.1 ablation alters glucose-induced islet electrical activity, enhancing insulin secretion. Cell Metab. 6:229–235 10.1016/j.cmet.2007.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju M., Stevens L., Leadbitter E., Wray D. 2003. The roles of N- and C-terminal determinants in the activation of the Kv2.1 potassium channel. J. Biol. Chem. 278:12769–12778 10.1074/jbc.M212973200 [DOI] [PubMed] [Google Scholar]
- Kobrinsky E., Stevens L., Kazmi Y., Wray D., Soldatov N.M. 2006. Molecular rearrangements of the Kv2.1 potassium channel termini associated with voltage gating. J. Biol. Chem. 281:19233–19240 10.1074/jbc.M601231200 [DOI] [PubMed] [Google Scholar]
- Liu B., Shuai K. 2008. Regulation of the sumoylation system in gene expression. Curr. Opin. Cell Biol. 20:288–293 10.1016/j.ceb.2008.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misonou H., Mohapatra D.P., Park E.W., Leung V., Zhen D., Misonou K., Anderson A.E., Trimmer J.S. 2004. Regulation of ion channel localization and phosphorylation by neuronal activity. Nat. Neurosci. 7:711–718 10.1038/nn1260 [DOI] [PubMed] [Google Scholar]
- Misonou H., Mohapatra D.P., Menegola M., Trimmer J.S. 2005. Calcium- and metabolic state-dependent modulation of the voltage-dependent Kv2.1 channel regulates neuronal excitability in response to ischemia. J. Neurosci. 25:11184–11193 10.1523/JNEUROSCI.3370-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misonou H., Menegola M., Mohapatra D.P., Guy L.K., Park K.S., Trimmer J.S. 2006. Bidirectional activity-dependent regulation of neuronal ion channel phosphorylation. J. Neurosci. 26:13505–13514 10.1523/JNEUROSCI.3970-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohapatra D.P., Siino D.F., Trimmer J.S. 2008. Interdomain cytoplasmic interactions govern the intracellular trafficking, gating, and modulation of the Kv2.1 channel. J. Neurosci. 28:4982–4994 10.1523/JNEUROSCI.0186-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohapatra D.P., Misonou H., Pan S.J., Held J.E., Surmeier D.J., Trimmer J.S. 2009. Regulation of intrinsic excitability in hippocampal neurons by activity-dependent modulation of the KV2.1 potassium channel. Channels (Austin). 3:46–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakoshi H., Trimmer J.S. 1999. Identification of the Kv2.1 K+ channel as a major component of the delayed rectifier K+ current in rat hippocampal neurons. J. Neurosci. 19:1728–1735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakoshi H., Shi G., Scannevin R.H., Trimmer J.S. 1997. Phosphorylation of the Kv2.1 K+ channel alters voltage-dependent activation. Mol. Pharmacol. 52:821–828 [DOI] [PubMed] [Google Scholar]
- Park K.S., Mohapatra D.P., Misonou H., Trimmer J.S. 2006. Graded regulation of the Kv2.1 potassium channel by variable phosphorylation. Science. 313:976–979 10.1126/science.1124254 [DOI] [PubMed] [Google Scholar]
- Perozo E., Bezanilla F. 1990. Phosphorylation affects voltage gating of the delayed rectifier K+ channel by electrostatic interactions. Neuron. 5:685–690 10.1016/0896-6273(90)90222-2 [DOI] [PubMed] [Google Scholar]
- Plant L.D., Dowdell E.J., Dementieva I.S., Marks J.D., Goldstein S.A.N. 2011. SUMO modification of cell surface Kv2.1 potassium channels regulates the activity of rat hippocampal neurons. J. Gen. Physiol. 137:441–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajan S., Plant L.D., Rabin M.L., Butler M.H., Goldstein S.A. 2005. Sumoylation silences the plasma membrane leak K+ channel K2P1. Cell. 121:37–47 10.1016/j.cell.2005.01.019 [DOI] [PubMed] [Google Scholar]
- Vacher H., Mohapatra D.P., Trimmer J.S. 2008. Localization and targeting of voltage-dependent ion channels in mammalian central neurons. Physiol. Rev. 88:1407–1447 10.1152/physrev.00002.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanDongen A.M., Frech G.C., Drewe J.A., Joho R.H., Brown A.M. 1990. Alteration and restoration of K+ channel function by deletions at the N- and C-termini. Neuron. 5:433–443 10.1016/0896-6273(90)90082-Q [DOI] [PubMed] [Google Scholar]
- Yang X.J., Grégoire S. 2006. A recurrent phospho-sumoyl switch in transcriptional repression and beyond. Mol. Cell. 23:779–786 10.1016/j.molcel.2006.08.009 [DOI] [PubMed] [Google Scholar]