Abstract
Recent data from knockouts, human disease, and transport studies suggest that solute carrier (SLC) and ATP binding cassette (ABC) multispecific “drug” transporters maintain effective organ and body fluid concentrations of key nutrients, signaling molecules, and antioxidants. These processes involve transcellular movement of solutes across epithelial barriers and fluid compartments (e.g., blood, cerebrospinal fluid, urine, bile) via “matching” or homologous sets of SLC (e.g., SLC21, SLC22, SLC47) and ABC transporters. As described in the “Remote Sensing and Signaling Hypothesis” (Biochem Biophys Res Commun 323:429–436, 2004; Biochem Biophys Res Commun 351:872–876, 2006; J Biol Chem 282:23841–23853, 2007; Nat Clin Pract Nephrol 3:443–448, 2007; Mol Pharmacol 76:481–490, 2009), highly regulated transporter networks with overlapping substrate preferences are involved in sensing and signaling to maintain homeostasis in response to environmental changes (e.g., substrate imbalance and injury). They function in parallel with (and interact with) the endocrine and autonomic systems. Uric acid (urate), carnitine, prostaglandins, conjugated sex steroids, cGMP, odorants, and enterobiome metabolites are discussed here as examples. Xenobiotics hitchhike on endogenous carrier systems, sometimes leading to toxicity and side effects. By regulation of the expression and/or function of various remote organ multispecific transporters after injury, the overall transport capacity of the remote organ to handle endogenous toxins, metabolites, and signaling molecules may change, aiding in recovery. Moreover, these transporters may play a role in communication between organisms. The specific cellular components involved in sensing and altering transporter abundance or functionality depend upon the metabolite in question and probably involve different types of sensors as well as epigenetic regulation.
Introduction
The ∼400 solute carriers (SLCs) (He et al., 2009) and ∼50 ATP binding cassette (ABC) transporters (Vasiliou et al., 2009) represent two superfamilies of ancient genes in humans. In general, ABC transporters are membrane-anchored proteins that use the energy from ATP hydrolysis to mediate the export of cytoplasmic solutes to the extracellular spaces. In contrast, many SLC transporters are importers moving solutes from the extracellular milieu into the cell, either by passive diffusion along its concentration gradient, by cotransport, or counter-transport against its concentration gradient by co-opting the concentration gradient of another solute. That gradient is ultimately derived from ATP hydrolysis-driven transporters such as the sodium-potassium-ATPase pump.
Evidence from structural, anatomical, genomic, genetic, and functional studies over the last two decades indicates that together, SLC and ABC solute carriers coordinately mediate the transepithelial movement of a wide variety of substrates, including nutrients, toxins, signaling molecules, neurotransmitters, and xenobiotic compounds.
Mutations or abnormal epigenetic modulation of the expression of some of these carrier genes have been demonstrated not only to result in disease conditions, many of which are rare “metabolic” syndromes, but also to confer drug resistance in cancer treatment, thereby suggesting a regulatory role of these transporters in maintaining homeostatic balance between the multiple levels of the cells, organs, organisms, and their respective external environments. A number of the substrates are well known signaling molecules such as cyclic nucleotides, prostaglandins, odorants, and conjugated sex steroids. Others, such as uric acid (urate), are key regulators of redox states within cells and tissues. Still others are rate-limiting intermediaries in bioenergetics and other “chemical” pathways, whereas some transported substrates are key cofactors in essential enzymatic reactions.
From a systems biology perspective, the central questions in the field of transporter research revolve around 1) discovering exactly how these transporters function in vivo, in light of a growing appreciation of their potential for “sensing” fluctuations in the chemical/biochemical composition of their local environment (e.g., blood, cerebrospinal fluid, amniotic fluid, urine); and 2) defining how these functions translate to the multiple levels of systemic communication between the cells and organs in which these transporters are present and possibly between organisms. A “remote sensing and signaling hypothesis” has been proposed to explain how transporters work together to sense and control fluctuations in the levels of substrates found in the organism (Monte et al., 2004; Kaler et al., 2006, 2007; Nigam et al., 2007; Ahn and Nigam, 2009). This hypothesis was initially based mainly on analyses of a group of organic anion transporters (Oat) of the Slc22 family, although the argument is broadly applicable to other SLC (e.g. SLC21, SLC47) and ABC family members conventionally viewed as “drug transporters.” The physiological substrates of Oats include many endogenous metabolites, toxins such as mercurials, and many commonly prescribed medications such as nonsteroidal anti-inflammatory drugs, antivirals, antihypertensives, statins and diuretics. This, together with the growing understanding of the complex tissue-specific expression and regulation of isoforms in epithelial and endothelial cells lining body fluid compartments, suggests that in higher organisms, these transporters work together in remote communication between tissues and organisms.
The Oat transporters of the SLC22 family have some distinctive genomic, structural, and functional features that are consistent with a key role in a broader remote-sensing system. The Oats are often clustered together on a chromosome, probably as a result of tandem duplication of a common ancestral gene during evolution (Eraly et al., 2003b, 2004; Wu et al., 2009). The Oat genes encode transporters each with 12 distinctive transmembrane domains and a significant extracellular as well as an intracellular domain harboring multiple post-translational modification sites that can also be commonly observed in seven-transmembrane G-protein-coupled receptors (GPCRs) (Srimaroeng et al., 2008). The classic Oats, Oat1 (originally identified as NKT) and Oat3, possess the capability to handle a broad spectrum of substrates, much like their counterpart of ABC family transporters of Mrp and Mdrs (Ahn and Nigam, 2009); however, many of the Oats are expressed in a tissue-specific fashion, often with their greatest expression in the kidney and the choroid plexus. Oat isoforms can recognize and handle similar substrates; this is particularly true for those encoded by genes located in the same cluster.
A growing body of data suggest a transporter-mediated mechanism of communication between cells and organs in the same individual, communication between individuals, and even between species (Ahn and Nigam, 2009). Expression patterns during development suggest potential roles in organogenesis. This Minireview summarizes recent advancements in the field and highlights the emerging remote sensing and signaling perspective for these ABC and SLC transporters in the context of several substrates whose handling is well understood and important in clinical contexts. We examine recent advances in the settings of urate, carnitine, and enterobiome metabolite handling, as well as injury models and recent data on their potential role in remote communication between individuals and species. The evidence suggests that these carrier proteins can not only mediate the movement of substrates across an epithelial barrier but can somehow also “sense” related SLC and ABC transporters in barrier organs in a manner somewhat analogous to that observed in the hormonal feedback mechanism to maintain biochemical balance in the organism's internal environment. It seems that the transporter networks act in parallel and collectively with the other regulatory systems of endocrine and autonomous nervous systems to coordinately maintain homeostasis. Indirect connections to signaling via G-protein-coupled receptors are also emerging. The following examples are discussed to emphasize the variegated role of transporters in the context of a multilevel coordinated and regulated system that may play a role in remote sensing and signaling enabling communication between cells, organs, and organisms.
Urate Handling
Uric acid is the primary nitrogenous end product of protein and purine metabolism in many animals, including humans. In mammals other than humans and higher primates, the enzyme uricase oxidizes uric acid to the water-soluble compound allantoin, which is excreted in urine to maintain the relatively low uric acid levels found in plasma. In humans and higher primates, however, functional uricase enzyme is not present, and the primary route of uric acid elimination is by active secretion into the urine by the proximal tubule cells of the kidney (Keebaugh and Thomas, 2010). Compared with most other mammals, humans have relatively higher uric acid levels in their blood that range between 3.6 and 8.3 mg/dL. Although the advantage of this elevated level of uric acid has been debated, it is commonly believed to benefit humans by functioning as an antioxidant. In addition, the loss of functional uricase in higher primates and humans is paralleled evolutionarily by the loss of the ability to synthesize ascorbic acid, which is also a potent antioxidant. Thus, uric acid may have replaced ascorbic acid as an endogenous antioxidant agent; more than half of the antioxidant capacity of blood plasma could potentially come from uric acid (Foti and Amorati, 2009).
Uric acid may also be neuroprotective. It has been suggested that elevated levels of blood urate reduce the risk of developing Parkinson's disease and thus seem to correlate with slower disease progression (Elbaz and Moisan, 2008). Nevertheless, high uric acid concentrations can be toxic, and although high plasma uric acid may be protective in some circumstances by acting as a trap for free radicals, hyperuricemia is actually a risk factor for several metabolic syndromes (Heinig and Johnson, 2006; Puig and Martínez, 2008; Sachs et al., 2009). At physiological pH, the solubility threshold of uric acid is 6.7 mg/dl, which is in the midrange of concentrations found in humans; thus, many people with uric acid levels in the “high normal” range may live with supersaturated plasma uric acid. Such individuals seem predisposed to the formation of uric acid crystals and some common metabolic syndromes such as gout and nephrolithiasis. Hyperuricemia is also associated with Lesch-Nyhan disease, an X-linked recessive condition affecting male children beginning in the first year of life (Nyhan, 1968).
Because humans live with a nearly saturated or supersaturated level of plasma uric acid, regulation of its concentration is critical. This is suggested by the number of genes, particularly transporters, involved in its handling, as revealed by GWAS studies of hyperuricemia (Table 1) (Li et al., 2007; Dehghan et al., 2008; Döring et al., 2008; Vitart et al., 2008; Kolz et al., 2009). For example, SLC22A12, predominantly expressed in the apical membrane of the proximal tubule cells in the kidney and closely related to Oat1 and Oat3, mediates the initial uric acid reabsorption from the ultrafiltrate, a critical step in maintaining blood uric acid levels. Initially identified in mice as Rst, its human ortholog is known as URAT1. Mutations (or single nucleotide polymorphisms) in this transporter gene have been found to predispose the individuals to hypouricemia, a condition particularly observed in the Japanese population (Komatsuda et al., 2006).
TABLE 1.
Tissue and developmental preferential expressions of genes associated with hyperuricemia
| Gene | Adult Tissue Expression | Developmental Stages | Tumor |
|---|---|---|---|
| SLC2A9 | Kidney, ovary, bone | Adult, fetus | Ovarian/uterine tumor and chondrosarcoma |
| SLC16A9 | Parathyroid gland kidney | Embryonic body, fetus, adult | Non-neoplasia, head-and-neck tumor |
| SLC17A1 | Kidney, liver | Fetus, adult | Kidney tumor, normal |
| SLC17A3 | Kidney, brain | Fetus | Normal |
| SLC22A11 | Placenta, kidney | Juvenile, adult, fetus | Normal tissues, liver tumor |
| SLC22A12 | Kidney | Adult | Normal |
| ABCG2 | Placenta, pharynx, brain, kidney | Juvenile, neonate, adult, blastocyst | Bladder carcinoma, germ cell tumor, non-neoplasia |
| GCKR | Liver | Fetus, adult | Normal |
| LRRC16A | Lung, intestine, brain, kidney | Adult, fetus | Normal tissues, colorectal tumor |
| PDZK1 | Kidney, liver, brain | Adult, fetus | Normal |
Urate can be handled by more than one transporter. Another uric acid transporter SLC22A11 found predominantly in the placenta and in kidney has also been associated with perturbed uric acid levels (Hagos et al., 2007). Both SLC22A11 and SLC22A12 transport urate across the cell membrane. SLC22A11 is a “sister” gene of SLC22A12, situated in cis conformation less than 20 kilobases from SLC22A12 on human chromosome 11. It is believed that this was generated through tandem duplication of an ancestral gene during evolution, suggesting a potential similar functionality of the genes. Paired or sister genes are a common feature in the SLC22 family (Eraly et al., 2003b, 2004; Wu et al., 2009). For example, SLC22A6/OAT1 and SLC22A8/OAT3 are also paired on human chromosome 11, less than 1 Mb from the SLC22A11 and SLC22A12 pair (Eraly et al., 2003b, 2004; Wu et al., 2009). Although they have not been associated in GWAS studies with hyperuricemia so far, both SLC22A6/OAT1 and SLC22A8/OAT3 are able to handle urate. Urate handling was disturbed in Slc22a6/Oat1, Slc22a8/Oat3, and Slc22a12 (Rst in mouse) knockout mice (Eraly et al., 2008). These urate transporters, which are expressed in different cells and in different tissues, provide a basis for the complex handling of urate and are possibly connected to “sensing” urate in the context of altered oxidative states.
Along these lines, it is interesting to note that the expression of SLC22A6/OAT1 and SLC22A8/OAT3 genes is coordinately regulated. In mice, deletion of either one of the pair results in reduced renal expression of the other transporter although this could be due to the gene-targeting approaches used (Sweet et al., 2002; Eraly et al., 2006). Thus, although the mechanism for this coordinated regulation remains to be explained, it is possible that a feedback epigenetic regulation exists, given that both Oat1 and Oat3 are broad-spectrum transporters and many substrates such as urate can be handled by either or both Oat1 and Oat3. In addition, Slc22a6 and Slc22a8 are the next two closest homologs of Slc22a12 in mouse genome (Wu et al., 2009).
In the proximal tubule of the kidney, unlike the SLC22A11/SLC22A12 paired genes, which are apically localized mediating the absorption from glomerular ultrafiltrate, the SLC22A6/SLC22A8 paired genes are basolaterally localized and mediate the initial uptake of organic anions (including urate) from the blood. For excretion from the apical side of the renal epithelial cell, ABCG2 is also involved in urate excretion and has been implicated to be associated with hyperuricemia (Table 1). Together, these genes may mediate the vectorial movement of active excretion of urate and other solutes from blood to urine (Wright et al., 2010).
Urate handling illustrates how several SLC and ABC genes work together to handle urate in humans via their functioning as apical and basolateral proximal tubule cell urate transporters that are regulated and responsible for disease when mutations in transporter genes occur. Genetic, genomic, biochemical, and functional characteristics of the paired SLC22A6/OAT1 and SLC22A8/OAT3 genes thus raises the interesting possibility that these transporters are connected via a “sensing” of shared substrates, which, in turn, could potentially modulate gene expression. It is possible that this “sensing” connection acts locally to provide some level of redundancy in the handling of shared substrates, a phenomenon that has been observed in the genetic models of Oat1 deletion and Oat3 deletion and in knockouts of the Octs, another set of paired/clustered organic cation transporter genes in the Slc22 family (Jonker et al., 2003). It is also possible that this connection could act to signal remotely between organs. For example, the SLCs and ABCs, which are expressed in barrier epithelia at the interface of fluid compartments in a wide variety of organs, including the brain, eye, ear, liver, and kidney, are charged with handling a number of circulating endogenous metabolites and/or toxins. These endogenous substrates generated in one organ could be secreted into the circulation via a set of solute carriers. In turn, the endogenous substrates could be transported and eliminated from the body via urine in the kidney by another set of SLC/ABC transporters. According to this view, the SLCs and ABCs in the kidney maintain the organism's (whole body blood circulation) substrate homeostasis, whereas the transporters in the target (remote) organs maintain the substrate homeostasis locally. This coordinated substrate handling can also be achieved by more than one transporter (either an SLC-SLC combination or an ABC-ABC or SLC-ABC combination) with shared substrate-specificity. For example, in the case of urate handling, urate generated in the fetus is probably handled by SLC22A11 (preferentially expressed in placenta) for the movement of urate into the blood of the mother, which is then handled by SLC22A12 in the maternal kidney to maintain urate homeostasis.
Examination of the expression of urate handling genes further supports this notion of a tissue-specific urate handling and sensing mechanism. Table 1 is partly derived from hyperuricemia GWAS and National Center for Biotechnology Information human expressed sequence tag databases. Higher expressed sequence tag counts can be found for most of the carrier genes in discrete organs. There are several genes coding for human membrane proteins related to urate handling: SLC2A9, SLC16A9, SLC17A1, SLC17A3, SLC22A11, SLC22A12 (URAT1), ABCG2, and PDZK1 (Wright et al., 2010). A common feature of these transporters/membrane proteins is that the kidney, a major site of expression, is the primary organ charged with the excretion of urate. Known physiological and presumably epigenetic regulation in the kidney results in a narrow range of blood urate concentrations. In addition to the kidney, each of these transporters may also have other preferred organs of expression; SLC2A9 is also expressed in the ovary and bone; SLC16A9 is expressed in the parathyroid gland; SLC17A1 is in the liver; SLC17A3 is in the brain; and SLC22A11 is in the placenta. These distinctive expression patterns suggest the intriguing but unproven possibility that urate levels in a particular organ and in the kidney are coordinately regulated via a remote communication of urate concentration, possibly by sensing the cell or tissue oxidation state, which is potentially tied to the regulation of the expression of the respective transporters. Presumably, the result of urate concentration sensing and subsequent regulation of transporter expression ultimately maintains urate homeostasis and the appropriate oxidative states in organs and in the circulation. One expects that, as with other SLC and ABC multispecific transporters, stress or injury to one organ may alter urate handling and urate transporter expression (in another organ), but this remains to be systematically tested.
Nevertheless, it is clear that active uric acid handling uses a number of apical and basolateral SLC and ABC transporters, including multispecific “drug” transporters, in an organ-specific manner and reflects the dualistic nature of uric acid in physiology and pathology. Uric acid handlers/transporters in different organs not only help to maintain the balance of plasma uric acid, but they might also play a key role in preventing uric acid crystal formation within various organs. For example, the hyperuricemia-associated transporter SLC2A9, implicated by GWAS, is not only expressed in the kidney (on both the apical and basolateral aspects of proximal tubule cells and thus may be able to mediate the vectorial absorption of some urate in the nephron) but is also expressed in a number of other tissues (Table 1) (Cheeseman, 2009). As an antioxidant, a higher level of urate presumably benefits the organism. Because it is at or near the saturation state for crystal formation, however, it can be detrimental to the organism. Thus, sensing and handling of uric acid in each individual organ and maintaining the overall urate homeostasis become critical. In cells, tissues, and body fluids, presumably all of this regulates the oxidative state upon which so many essential metabolic reactions and signaling events depend.
Some published work has raised the possibility that multiple SLC22 genes sense urate levels. Although urate handling by solute carriers has been established genetically, the extent of epigenetic regulation is unclear. Simiao is one of the more frequently prescribed traditional Chinese medicines used to treat the symptoms of gouty arthritis (Shi et al., 2008). The effective ingredient of the pill is not known, but the pill has been shown to be a successful uricosuric in experimental mice that affects the expression of renal organic ion transporters (Hu et al., 2010). Analysis of renal expression of SLC genes in Simiao-treated mice revealed decreased expression of Slc22a12/Rst and Scl2a9. Both have been implicated in mediating the reabsorption of urate from ultrafiltrates and consequently increasing the urinary urate excretion. In addition to the well established organic anion urate-handling pathway, administration of the Simiao pill also led to increased expression of other renal solute carriers of the Scl22 family. These results suggest the following: 1) the expression of urate transporters can be modulated by uricosuric agents; and 2) there may be cross-talk between classic renal organic anion and urate-handling pathways.
The complex regulation of uric acid is only beginning to be understood. Available genetic, functional, and expression-profiling data suggest urate handling is regulated at levels of the cell, tissue, organ, and organism. Coordinated urate handling at these levels through substrate/urate sensing, signaling, and transporter regulation works to maintain homeostasis (Fig. 1). Optimal urate handling at these multiple levels may maximize its metabolic capacity and anti–free-radical functionality by creating an optimal redox environment for key enzymatic reactions and signal transduction events while minimizing the serious side effect of cell toxicity.
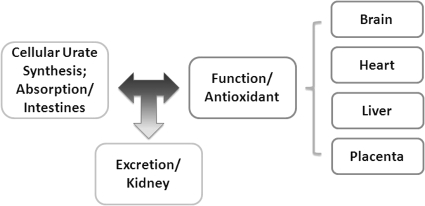
Fig. 1.
Diagram depicting the remote sensing and signaling mechanism among organs mediating urate handling. Urate is either absorbed in the intestine or synthesized in the liver. Its absorption, distribution, and excretion are mediated by a battery of solute carriers in individual organs to achieve balance and homeostasis in the cell, organ, and at the system level.
Carnitine Handling
Carnitine [3-hydroxy-4-(trimethylazaniumyl) butanoate], a natural product synthesized from lysine and methionine in liver and kidney, plays a critical role in the generation of metabolic energy because of its ability to facilitate the movement of fatty acids into mitochondria. Carnitine is also available from the diet after being absorbed in the intestine and distributed by active transport into the cells of muscle, heart, and others. High concentrations of carnitine are present in the heart and other organs, and these processes of preferential distribution are mediated by carnitine transporters of the SLC22 family, particularly SLC22A5/OCTN2, and probably OCTs (OCT3) as well as Flipt1 and Flipt2 (also known as CT1/SLC22A15 and CT2/SLC22A16, carnitine transporter 2).
SLC22A5 is strongly expressed in skeletal muscle, intestine, liver, kidney, heart, placenta, and other organs. SLC22A5 encodes a gene product with 12 transmembrane domains, which mediates cellular absorption of small-molecule organic cations and serves as a high-affinity sodium-dependent carnitine transporter. Genetic mutations in the coding and noncoding regions of SLC22A5 have been implicated in systemic primary carnitine deficiency (Nezu et al., 1999). Mice with genetic deletion of the SLC22A5 gene (Shekhawat et al., 2007) develop enlarged fatty livers, hypertrophic cardiomyopathy, steatosis with cellular accumulation of fatty acid in other organs, and intestinal villous atrophy, similar to that observed in human cases with SLC22A5 mutations. This suggests that carnitine transporter-dependent cellular oxidation of fatty acids in mitochondria is essential.
Similar to urate, both SLC and ABC transporters regulate carnitine levels. Absorption of carnitine from dietary sources occurs in the small intestine. Initially, uptake of carnitine from the lumen of the intestine across the apical membrane into the enterocyte is mediated in a sodium-dependent manner (Kato et al., 2006). Mrp3 and Abca1 mediate subsequent movement across the basolateral membrane of the enterocyte and into the circulation (Klaassen and Aleksunes, 2010). These are ABC family cellular exporters with broad substrate specificities, and here, the apical absorption may be the rate-limiting step. Enteric absorption of carnitine from diet may be saturatable at 2 g daily, suggesting a feedback mechanism that maintains balance of absorption and excretion (Rebouche and Seim, 1998).
In the heart, SLC22A5 is expressed in cardiomyocytes (Iwata et al., 2008). Slc22a5 mutant mice develop progressive cardiomyopathy, similar to what is observed in patients with primary carnitine deficiency resulting from mutations in SLC22A5. Even mice heterozygous for Slc22a5 deficiency demonstrated a cardiovascular phenotype under stress (Takahashi et al., 2007), supporting the notion that carnitine uptake in cardiomyocytes is important in maintaining cardiac function.
In the kidney, the SLC22A5 gene product is located on the apical membrane of proximal tubule cells. As for other organic cation substrates, SLC22A5 mediates the absorption of carnitine from renal ultrafiltrate into the proximal tubule epithelial cells. Similar to uric acid absorption, renal carnitine handling is a critical step in maintaining systemic carnitine homeostasis. The systemic balance of l-carnitine is primarily maintained by renal excretion, and disturbances in its handling would presumably cause other remote organ dysfunctions in heart, muscle, and placenta. Carnitine handling represents another potential example of remote sensing and signaling in which homeostasis in remote target organs is regulated coordinately.
Another organ with strong expression of Slc22a5 is the placenta, which is found in the apical surface of syncytiotrophoblasts (Grube et al., 2005). The absorption of carnitine by the placenta is sodium-dependent and can be inhibited by Slc22a5 inhibitors, including some antiseizure drugs taken by pregnant women (Grube et al., 2005). In Slc22a5-deficient mice, the levels of carnitine in the fetus and placenta are reduced, whereas the enzymes involved in metabolic oxidation and energy generation are increased (perhaps in an attempt to compensate for the loss of carnitine). This suggests that Slc22a5 plays a major role in the delivery of carnitine to the fetus and that disruption of the fetal carnitine supply, either by genetic modification/mutations in transporters involved in its handling or inhibition of its absorption [which may occur in mothers using antiseizure drugs during pregnancy (Grube et al., 2005)] increases the risk of fetal developmental defects.
It is noteworthy that l-carnitine is an essential nutrient for newborn infants because the mechanism of its biosynthesis is not fully developed until later in postnatal development. The primary source of carnitine for a newborn is from the mother's milk (Sandor et al., 1982). As discussed earlier, the intestine has an avid carnitine absorption system of solute carriers of SLC and ABC families. Likewise, carnitine is handled by a network of transporters in the mammary gland featuring SLC22A5, SLC22A4, and SLC22A3 (Kwok et al., 2006). The carnitine produced in the mammary gland is excreted into the milk and then is absorbed in the baby's intestine. Mother's milk is the only source of carnitine for the newborn infant and is required for the β-oxidation of fatty acids. This process of carnitine transfer from mother to infant represents a coordinated mechanism of solute handling via SLC and ABC transporters between individuals.
The production and handling of carnitine can be regulated systemically between organs. Under normal physiological conditions, the primary site of carnitine production is in the liver. During lactation, the production of carnitine in the mammary gland increases, apparently at the expense of production in the mother's liver, which is also accompanied by reduced hepatic enzymatic and transcriptional activity, as well as solute carrier activities associated with carnitine synthesis and handling (Gutgesell et al., 2009). This complex physiological regulation of carnitine synthesis and handling is necessary to provide the infant with carnitine for β-oxidation and energy production to use fatty acids. In this instance of carnitine production, transfer from mother to infant, and carnitine utilization in the infant, all via solute carrier networks, mediates the enzymatic activities between and within cells of the mother and the infant (Fig. 2).
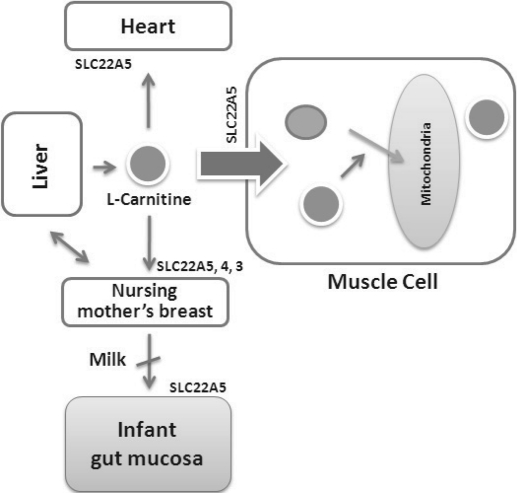
Fig. 2.
l-Carnitine and its carrier SLC22A5 mediated sensing and signaling among organs and between mother and infant. Liver is the primary site of l-carnitine biosynthesis. Circulating l-carnitine is distributed/transported through its carriers, primarily SLC22A5, depicted here, as well as Oct3, Flipt1 and Flipt2 (CT1/SLC22A15 and CT2/SLC22A16) and others to the target organs. The synthesis and distribution of l-carnitine is regulated physiologically. For example, during lactation, l-carnitine is preferentially distributed to the nursing mother's breast through increased expression of its carriers at the expense of the liver, because mother's milk is the only source of carnitine for the infant and is essential for the survival/growth of the baby.
Enterobiome Metabolite Handling between Organs
The intestine is a large organ averaging 26 feet in length in adult humans. The primary functions of this organ are for food processing, nutrient absorption, and waste disposal. These functions are largely processed by a complex microflora ecosystem. The end products of this process include small-molecule nutrients and toxins, which can be taken up by the epithelial solute carrier system (Cheng and Klaassen, 2009). Other organs can then use absorbed small-molecule nutrients in the circulation for metabolism, whereas the toxins can be further metabolized, particularly in liver and kidney, for disposition. Integral to these processes of solute movement crossing epithelial barriers are the organic solute carriers.
Similar to the other sensing, signaling, and handling systems for urate and carnitine described in previous sections, there is significant overlap of the expression of the transporters in intestine and other organs. For enterocyte uptake, Octs and Octns are important for organic cations and Oatps (SLC21), for organic anions. Among the exporters, Mdrs, Mrps, and ABCG2 are expressed in intestine. Expression of these transporters can also be found in liver and kidney (Klaassen and Aleksunes, 2010). From a whole-body perspective, this increases the possibility that the same carriers in the other organs, such as liver and kidney, can handle the uptake of small-molecule nutrients and toxins in the intestine. On the other hand, enterobiome substrates can be handled with a “mix and match” of transporters in liver or kidney, given that most of these transporters have broad-spectrum and overlapping substrate specificities (Kaler et al., 2007; Truong et al., 2008; Ahn et al., 2009). Furthermore, an enterobiotic or an enteric toxin picked up in the intestine by a set of carriers (e.g., an organic anion absorbed by Oatp1a5) can be metabolized in the liver, and then excreted in the kidney by the Oats (Wikoff et al., 2009). Antibiotics would apparently dramatically change the levels of these substrates in varying body compartments.
The intestine is also the first site for metabolism of ingested nutrients and drugs. Mdr1/Abcb1/p-glycoprotein, the best studied drug-resistant carriers, has been linked to this process. Abcb1 is located on the villi of the enterocytes and exports substrates back to the lumen. Strong expression of the metabolizing enzyme Cyp3a4 is also found in the intestine, suggesting that Cyp3a4 and Abcb1 may work together (Bruyere et al., 2010). Indeed, there is a significant overlap of substrate specificities between Cyp3a4 and Abcb1, suggesting that potentially both are involved in handling of the same substrates or their metabolites. In addition, the expressions of both genes can be coordinately regulated in the intestine, further supporting the notion that solute carriers and metabolizing enzymes work together in substrate handling (Benet et al., 1999). For example, the expressions of both Cyp3a and Abcb1 can be up-regulated in the intestine by dexamethasone, consequently affecting the bioavailability of their common substrate indinavir (Lin et al., 1999).
Abcb1 and Cyp3a are also found in the liver. This is consistent with the notion that substrates excreted from one organ can be recognized and processed in another remote organ by the same (or a related) set of genes. Indeed, more than half of the ingested cyclosporine, an immunosuppressive agent, is metabolized in the enterocytes of the intestine, and some of the biotransformation also occurs in the liver hepatocytes (Serkova et al., 2004).
An exogenous substrate or toxin may be initially absorbed into the enterocyte by an Slc transporter such as an Oatp (SLC21) or Octn (SLC22) because the substrate may conform to the specificities of the importer. As a defensive mechanism, a first level of metabolism of oxidation may occur in the cytoplasm with cytochrome P450 enzymes, whereas the apical exporters of Mdrs pump it back to the lumen of the intestine. The remainder of the substrate escapes to the blood circulation. It is again picked up by the hepatocyte through the Oatps, in which the same set of cytochrome P450 and solute carriers modifies the substrate in detoxification reactions (Fig. 3). This relay system is another example of organ-organ communication with linkage of enzymatic activities together with transporter-mediated activities that are regulated by hormones and other mediators of signaling.
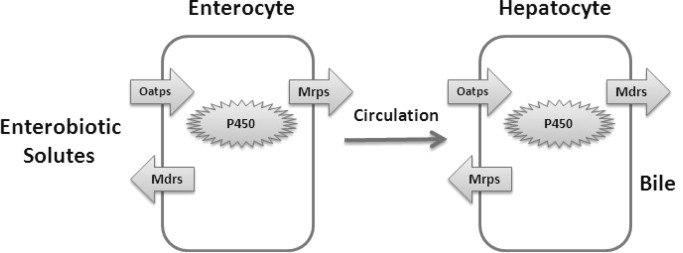
Fig. 3.
Diagram depicting iterative enzymatic- and transporter-mediated organic anion solute handling in intestine and in liver.
Implications of Toxin Sensing and Handling in Tissue Injury
Acute kidney injury is characterized by rapid progressive reduction in renal function with decreased glomerular filtration rate. Indoxyl sulfate (IS) is one of many potential uremic toxins accumulated in the blood during renal failure. Indoxyl sulfate is an endogenous substrate of Slc22a6/Oat1 and a substrate for Slc22A8/Oat3. Together, SLC22A6 and SLC22A8 uptake mediate the initial step of organic anion secretion in the kidney (Nigam et al., 2007). Reduction in the expression of these transporter genes has been reported in the initial stages of ischemic acute kidney injury, followed by increased expression in parallel with recovery of renal function during reperfusion in rodent models (Di Giusto et al., 2008).
Disturbance of homeostasis can also induce coordinated changes of transporter gene expression in remote organs, perhaps through epigenetic signaling mechanisms. In a hepatic ischemia-reperfusion model, injury to the liver reduced the hepatic expression of Abcc2/Mrp2, whereas its expression was up-regulated in the kidney (Tanaka et al., 2008). This interorgan regulation suggests a potential compensatory mechanism that increased transporter capacity in the kidney that may handle the excretory or metabolic capacity lost in hepatic ischemia settings. Although the signaling pathway mediating this adaptation is not clearly defined, this cross-organ solute carrier regulation is presumably mediated epigenetically, potentially by pathways involving growth factors and/or cytokines known to be expressed in injured and remote organs (Nigam and Lieberthal, 2000). Although these SLC and ABC transporters are multispecific and have overlapping substrate specificity, they clearly have distinct overall substrate preferences (Kaler, 2007). By altering the expression or function of these SLC and ABC transporters in a remote organ after toxic injury, the overall substrate preferences of the remote organ may be affected, thereby altering elimination of endogenous toxins and changing the handling of key metabolites and signaling molecules.
Circulating uremic toxins, such as indoxyl sulfate, 3-carboxy-4-methyl-5-propyl-2-furanpropionate, indole acetate, and hippurate, in renal failure settings can actually accumulate in the proximal tubule cell and aggravate renal dysfunction. In addition to their ability to directly compete with endogenous substrates, they can also potentially affect nonrenal drug handling, particularly in the liver by alteration in the expression of organic ion transporters of the SLC22, SLC21, and the ABC family of carriers (Dreisbach, 2009). Oats, Octs, Oatps, and Mrp1/ABCC1 have been implicated. Changes in their expression probably affect the bioavailability of endogenous substrates and toxins and xenobiotics for phase II metabolism in liver and in kidney cells (Sun et al., 2006). In hepatocytes treated with uremic toxins of IS or 3-carboxy-4-methyl-5-propyl-2-furanpropionate, the expression of Oatp transporters is reduced (Sun et al., 2006). The decreased expressions of these transporters is also observed in models of chronic renal failure in rats (Di Giusto et al., 2008). In addition, decreased Mrp transport can also be observed in the intestines of these rats, suggesting that the absorption of xenobiotics and enterobiotic substrates may also be adversely affected in chronic renal failure. Direct competition by uremic solutes for drug binding on SLC and ABC transporters is also a possibility. This may also be the case for endogenous metabolites and signaling molecules regulated by the remote sensing and signaling system and thus may partly explain metabolic disturbances associated with kidney disease.
As with urate and carnitine, the larger solute carrier network may be quite complex. Precursors of IS are derived from dietary amino acid tryptophan, which is then metabolized into indole in the intestine by bacterial tryptophanase and then moved across the intestinal barrier into the blood. Circulating indole is then absorbed into hepatocyte and metabolized to IS, which is handled by Oats and Oatps and (as described above) affects SLC and ABC transporter expression. It is clear that one of the functions of this “transporter” network is to handle/distribute dietary or enterobiotic solutes across multiple barriers in the body, which may serve as nutrient for the cell or be metabolized. Because of their importance to cellular functions and toxicity, this solute-handling process is likely to be monitored and regulated, and sensing and signaling by solute carriers is likely to be integral to this process (Fig. 4).
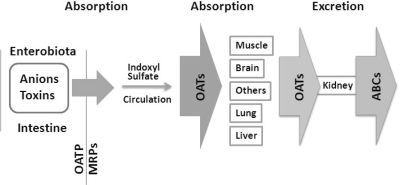
Fig. 4.
Flow diagram depicting distribution and handling of enterobiome metabolites by solute carriers.
Interaction of SLC and ABC Transporters with Other Signaling Pathways
Second-Messenger Molecules.
In the sensing of injury, the solute transporters in remote organs are somehow communicating with each other, and their expression and activities are regulated in response to remote injury. The mechanism of this communication is far from clear but may involve growth factors, hormones, and small molecules. These SLC and ABC transporters can also potentially serve as regulators of other signaling pathways. cGMP is a second messenger mediating cellular responses to a variety of surrounding stimuli. It is also a high-affinity substrate for human OAT2 (SLC22A7). Pharmacogenetic analysis of the SLC22A7 and pharmacokinetic analysis of its gene products suggest that it plays a role in mediating the balance of intra/extracellular cGMP and consequently regulates this second-messenger signaling pathway (Cropp et al., 2008). In addition, cGMP can serve as an intracellular substrate modulating the expression/activities of other organic ion transporters of the SLC22 family (Schlatter et al., 2002). This cross-talk between different SLC transporters through a transported signaling molecule remains to be explored.
On the other hand, second-messenger systems and hormones such as angiotensin, bradykinin, and progesterone can regulate the transporter activities as well. The intracellular domains of the solute carriers harbor sites for enzymatic modifications mediated by classic signaling pathways. For example, phosphorylation of SLC22A6 and SLC22A8 promotes transporter activity by mobilizing the OATs from intracellular sites (Srimaroeng et al., 2008; Barros et al., 2009; Duan and You, 2009, 2010).
Prostaglandins.
Handling of prostaglandins is another example of how a signaling pathway and so-called drug transporter may be intertwined. Prostaglandins are a group of fatty-acid derivatives containing 20 carbon atoms with a 5-carbon ring. As signaling molecules, prostaglandins exert their diverse physiological regulations through the binding of cell-membrane anchored seven-transmembrane G-protein coupled receptors located on various cell types. For example, prostaglandin E2 can bind EP1 receptor to elicit smooth muscle contraction, bind to EP2 to mediate vasodilatation, and bind to EP3 to mediate uterus contraction during pregnancy. Thus, prostaglandins are strong physiological regulators functioning as classic GPCR receptor ligands, and their levels must be strictly regulated.
Prostaglandins are produced in most organs and tissues. They are considered to be local hormones acting in a paracrine or autocrine fashion. Releasing of prostaglandins from the cell was once believed to occur through passive diffusion because of their high lipophilicities. It is now clear that transporters, particularly MRP4/ABCC4, mediate the release of many prostaglandins. Other ABC transporters may also play a role in the release of cellular prostaglandins. On the other hand, there are multiple prostaglandin importers of SLC families involved in the cellular uptake of certain prostaglandins. The first prostaglandin importer identified was prostaglandin transporter PGT/SLCO2A1 (Kanai et al., 1995). PGT is an organic anion transporter of the SLCO family. Several more have since been identified, including the Oats of the SLC22 family. Their tissue distribution seems consistent with the fact that prostaglandins, which act via paracrine or autocrine regulation, are able to be removed locally to keep the systemic circulating levels low. The recent identification of a renal-specific prostaglandin transporter, OAT-PG in the SLC22 family, suggests how the uptake and elimination may be coordinately handled (Shiraya et al., 2010). Unlike other PG transporters, OAT-PG has narrower substrate specificities with a preference for PGE2, PGF2a, and PGD2. Upon the uptake of these substrates in the proximal tubule cells, the PGs can be rapidly inactivated by cytoplasmic 15-hydroxyprostaglandin dehydrogenase to generate 15-keto-PGE2. This represents a case of local PG handling by combining ligand signaling through a GPCR receptor, uptake by SLC transporters, and inactivation by metabolism.
What would happen if the PG is allowed to accumulate in the circulation? In humans, the circulating PGE2 is very low in the low nanomolar range during resting states (Sánchez-Moreno et al., 2004). Upon cytokine stimulation or fever, the level of circulating PGE2 can increase up to 4-fold. This elevated level of PGE2 is sufficient to trigger PG transporters. Some of these have a Km in the low nanomolar range. In fact, cytokines increase the level of PGE2 in the blood and in cerebrospinal fluid, which is probably due to the entry of PGE2 into the cerebral ventricle from the peripheral circulation (Davidson et al., 2001). It is noteworthy that multiple OATs, including Oat3, are highly expressed in the choroid plexus, and Oat3 knockouts have decreased choroid plexus handling of organic anions. Higher levels of PGE2 in the central nervous system, particularly in the hypothalamus, could conceivably alter thermoregulation (Sauvant et al., 2006). Thus, SLC and ABC transporters both transport classic signaling molecules such as cGMP and prostaglandins (and indeed have evolved specific transporters for them) and also are regulated by these signaling molecules at both the level of the transporter function and epigenetically (Sauvant et al., 2006; Miller, 2010). In addition, the molecules mediating remote communication remain to be deciphered but could include growth factors, hormone, metabolites, and signaling molecules. The sensor may be a protein (conceivably even certain transporters or orphan transporters themselves) or cellular machinery associated with the transporters (e.g., PDZ domain-contained proteins or vesicular components). The machinery of sensing must then somehow alter transporter regulation at the protein or transcriptional epigenetic level.
Perspective on Remote Signaling via SLC and ABC Transporters
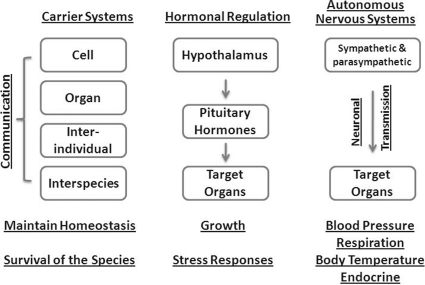
How should we view the sensing and signaling by SLC and ABC transporters from the perspective of systemic physiological metabolites and regulatory molecules such as urate, carnitine, cGMP, and prostaglandins? Taking neuroendocrine regulation as an example, hormones released from pituitary gland under the influence of hypothalamic stimuli enter the blood stream, reach the target organ, interact with respective receptors, and then exert an effect through a second-messenger system of the target cell. Somewhat analogously, the transporter system (composed of SLCs and/or ABCs) will obtain a substrate, generated or absorbed, in an organ (such as urate in the intestines), distribute it to its target organ, and carry out its function with cells (regulating redox state). The level of circulating urate is regulated through sensing and excretion/reabsorption control in the renal proximal tubules. This sensing and regulation seems to be relatively autonomous and may not involve regulation by the central nervous system. Similar arguments can be made for carnitine, cGMP, and prostaglandins. This process resembles that of the autonomous nervous systems in regulation of lung respiration rate, heart rate, blood pressure, and endocrine secretion, all of the fundamental processes to maintain homeostasis (Fig. 5). At present, the molecular and cellular machinery involved in sensing remains largely unidentified. It may be vary with what is being sensed (e.g., redox stress, toxins, endogenous organic amines generated by the enterobiome, or organ injury.
Fig. 5.
The solute carrier-mediated remote sensing and signaling mechanism of solute handling maintains balance and homeostasis at multiple levels. This process resembles other regulatory mechanisms of autonomous nervous systems and endocrine systems.
Finally, a number of studies have demonstrated spatiotemporal patterns of SLC and ABC multispecific transporters consistent with a potential role in organ development or differentiation. For example, a number of studies of SLC22 genes have revealed transient or early developmental expression in certain organs or shown high expression in the embryo compared with the adult (Pavlova et al., 2000; Eraly et al., 2003a; Sweet et al., 2006; Wu et al., 2009). Although the role of these transporters in development needs to be explored in more detail, one possibility is that they transport key small molecules, such as cyclic nucleotides, prostaglandins, or conjugated steroids, involved in morphogenesis or differentiation.
Intra- and Interspecies Sensing and Handling of Signaling Molecules
Olfactory Receptor Expression in Non-Nasal Epithelial Tissues.
Olfactory receptor (OR) genes constitute possibly the largest single family in human genome, with expression mainly in the nasal epithelial cell mediating the sense of smell. Transcriptomic profiling has revealed the presence of “olfactory” receptors in non-nasal tissues. For example, a recent microarray analysis of human OR described 58 OR in the liver, 108 in heart, 83 in testis, and 93 in the lung. The expression patterns of some of these genes are found to be conserved in chimpanzee, consistent with the possibility that these OR genes may be functional in these tissues (De la Cruz et al., 2009). The non-nasal expression of OR has been referred to as ectopic expression (Feldmesser et al., 2006; Zhang et al., 2007). However, more evidence is emerging, suggesting that these ORs may mediate the signaling responses of ligands in the organs in which they are expressed. The question therefore arises as to whether the odorant signaling ligand/molecules can reach internal organs, and if so, how?
An organic anion transporter of the Slc22 family, Slc22a20/Oat6, has restricted expression in nasal epithelial and in testis (Monte et al., 2004). Detailed analysis of its substrate preferences suggests that Oat6 can mediate the uptake of small odorant organic anion signaling molecules, as well as estrone sulfate (Kaler et al., 2006) and dehydroepiandrosterone sulfate. It has been suggested that these volatile signaling molecules derived from urine could be absorbed by organic anion transporters (Oat6 and/or Oat3) located on nasal epithelial cells and then can be presented to neurons to elicit neuronal responses. An alternative route would transfer these molecules to the circulatory system for presentation to the remote internal organs in which the ORs have also been shown to be expressed, for example, testis (Kaler et al., 2006). At the remote organ, the signaling of the same odorant/solute could be mediated by the same OR interacting with the “olfactory” molecule in the nasal epithelium. However, the levels of steroid-derivatives need to be regulated. It seems that the same Oat (Oat6) in Sertoli cells may provide a critical link mediating testicular steroidogenesis (Schnabolk et al., 2010). This example illustrates a potential mechanism of remote sensing and handling of signaling molecules important for the propagation and survival of the species.
Can the metabolites secreted in the urine/feces from an animal (rodent) be picked up by the organic ion transporters of a predator animal (cat) or a potential mate? This notion is supported by the fact that the molecular signatures of molecules that convey social cues in mouse urine share a number of intrinsic characteristics of Oat6 and Oat1 substrates, including low molecular weight, moderate hydrophobicity, and a negative electric charge. Moreover, a recent study found that some of the radiolabeled olfactory ligands/substrates from a urine sample were present in the brain (Hsu et al., 2008; Meeks et al., 2010) and, perhaps, the limbic system of another animal exposed to the urine sample. Thus, although the mechanism of this transport is not understood, these findings do provide circumstantial support for the hypothesis proposed above.
Analysis of the substrate specificities of Slc22a20/Oat6 is interesting in that, like its homologs of Oat3 or Oat1, Oat6 has a broad substrate specificity (Kaler et al., 2006). However, its expression is rather tissue-specific, primarily found in the nasal epithelia. It is noteworthy that Oat6 has more selectivity for volatile organic anions including those known to function as odorants (Kaler et al., 2006, 2007; Ahn et al., 2009). Emerging structural analyses of the transporters and pharmacophore modeling of substrates suggest that there are multiple small-molecule binding sites/pathways mediating the transmembrane processes of different groups of organic ions depending on the size, charges, and polarities (Kaler et al., 2006, 2007; Ahn et al., 2009). Nevertheless, how (or if) these carriers mediate the initial steps of interindividual (or interspecies) sensing and signaling remains to be elucidated (Fig. 6).
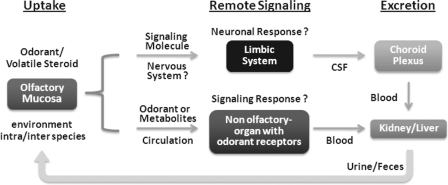
Fig. 6.
Speculative diagram of roles of odorant transporters such as Oats (Oat6) in proposed remote communication. The odorants/volatile steroid derivatives from the environment (including intra- and interspecies sensing) can be, after eliciting an odorant response (via odorant receptors) in the olfactory mucosa, recycled/delivered to internal organs to trigger remote responses. We speculate that these responses might be manifested as neuronal signaling, perhaps in the limbic system, or a signaling response in a remote organ (testis, adrenal gland, liver, kidney, etc.) mediated by nonolfactory odorant receptors. These signaling molecules can conceivably then be excreted by kidney, large intestine, or sweat gland, where they can potentially be involved in the subsequent round of remote sensing. The handling of these molecules is dependent on the combination of SLC and ABC transporters and their localization and expression.
Summary
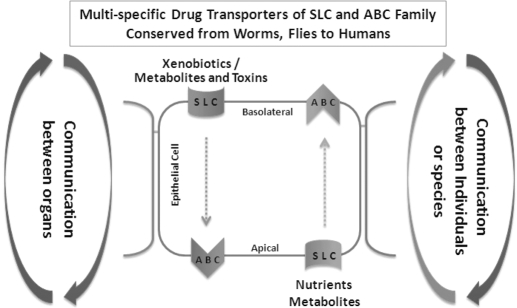
As one of the largest gene superfamilies in the human genome, the SLC family, along with the ABC transporter family, coordinately maintains the solute balance between multiple compartments separated by an epithelium in individual organs and homeostasis systematically (Fig. 7). The transporter genes are evolutionarily conserved; their homologs can be traced back to worms and flies. The evidence increasingly suggests that the primary function of the carriers is to handle endogenous metabolites and toxins, to distribute and maintain effective concentration of nutrients and antioxidants in the organ, and to maintain overall system homeostasis. These processes/pathways are centered on transcellular movement of solutes crossing a single layer of epithelial cells by “matching” pairs of SLC importers and ABC exporters. Homeostasis is accomplished through coordinated transporter networks either through the sensing and signaling by the same carriers in different cells or organs or by handling, sensing, and signaling of the same substrates by a set of carriers with shared substrate preferences. Thus, these transporter proteins are crucial in maintaining physiologically normal metabolic profiles in various compartments (at the cellular level and between organs) as the organism continues to interact with the environment (nutrients, toxins, and other organisms). Moreover, these transporters are sensitive to changes in metabolic profiles as a result of changes in the external environment as evinced in the case of toxicant-induced injuries to organs. At the ecological level, the role of the transporters is crucial for the communication between various organisms because most living systems primarily communicate through metabolic byproducts that act as substrates (e.g., pheromones). These networks, which are regulated by hormones and growth factors, are sensitive to environmental changes that include substrate imbalance, injury, and xenobiotics, which can “hijack” and affect the existing endogenous carrier systems. The remote sensing and signaling system of SLC and ABC multispecific “drug” transporters may function in parallel with the endocrine and autonomic nervous system. Important questions remain. What is the nature of the sensing mechanism? How is the information transmitted to local and remote transporters? Does this regulation occur at the transporter protein level, epigenetic level, or both?
Fig. 7.
Diagram depicting solute carrier-mediated communication within cell, between organs, between individuals of the same species, and between individuals of different species.
Acknowledgments
We thank Kevin Bush, Megan Bettilyon, and Satish Eraly for help and comments.
This work was supported by the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases [Grant DK079784]; the National Institutes of Health National Institute of Allergy and Infectious Diseases [Grant AI057695]; the National Institutes of Health National Institute of Neurological Disorders and Stroke [Grant NS062156]; the National Institutes of Health National Institute of General Medical Sciences [Grant GM88824]; and by a postdoctoral fellowship from the Society of Toxicology and Colgate Palmolive (to A.V.D.).
Article, publication date, and citation information can be found at http://molpharm.aspetjournals.org.
doi:10.1124/mol.110.070607.
- SLC
- solute carrier
- ABC
- ATP-binding cassette
- Oat
- organic anion transporter
- GWAS
- genome-wide association study
- IS
- indoxyl sulfate
- PG
- prostaglandin
- OR
- olfactory receptor
- PGE2
- prostaglandin E2
- GPCR
- G-protein-coupled receptor.
Authorship Contributions
Wrote or contributed to the writing of the manuscript: Wu, Dnyanmote, and Nigam.
References
- Ahn SY, Eraly SA, Tsigelny I, Nigam SK. (2009) Interaction of organic cations with organic anion transporters. J Biol Chem 284:31422–31430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn SY, Nigam SK. (2009) Toward a systems level understanding of organic anion and other multispecific drug transporters: a remote sensing and signaling hypothesis. Mol Pharmacol 76:481–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barros SA, Srimaroeng C, Perry JL, Walden R, Dembla-Rajpal N, Sweet DH, Pritchard JB. (2009) Activation of protein kinase Czeta increases OAT1 (SLC22A6)- and OAT3 (SLC22A8)-mediated transport. J Biol Chem 284:2672–2679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benet LZ, Izumi T, Zhang Y, Silverman JA, Wacher VJ. (1999) Intestinal MDR transport proteins and P-450 enzymes as barriers to oral drug delivery. J Control Release 62:25–31 [DOI] [PubMed] [Google Scholar]
- Bruyere A, Decleves X, Bouzom F, Ball K, Marques C, Treton X, Pocard M, Valleur P, Bouhnik Y, Panis Y, et al. (2010) Effect of variations in the amounts of P-glycoprotein (ABCB1), BCRP (ABCG2) and CYP3A4 along the human small intestine on PBPK models for predicting intestinal first-pass. Mol Pharm 7:1596–1607 [DOI] [PubMed] [Google Scholar]
- Cheeseman C. (2009) Solute carrier family 2, member 9 and uric acid homeostasis. Curr Opin Nephrol Hypertens 18:428–432 [DOI] [PubMed] [Google Scholar]
- Cheng X, Klaassen CD. (2009) Tissue distribution, ontogeny, and hormonal regulation of xenobiotic transporters in mouse kidneys. Drug Metab Dispos 37:2178–2185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cropp CD, Komori T, Shima JE, Urban TJ, Yee SW, More SS, Giacomini KM. (2008) Organic anion transporter 2 (SLC22A7) is a facilitative transporter of cGMP. Mol Pharmacol 73:1151–1158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson J, Abul HT, Milton AS, Rotondo D. (2001) Cytokines and cytokine inducers stimulate prostaglandin E2 entry into the brain. Pflugers Arch 442:526–533 [DOI] [PubMed] [Google Scholar]
- De la Cruz O, Blekhman R, Zhang X, Nicolae D, Firestein S, Gilad Y. (2009) A signature of evolutionary constraint on a subset of ectopically expressed olfactory receptor genes. Mol Biol Evol 26:491–494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehghan A, Köttgen A, Yang Q, Hwang SJ, Kao WL, Rivadeneira F, Boerwinkle E, Levy D, Hofman A, Astor BC, et al. (2008) Association of three genetic loci with uric acid concentration and risk of gout: a genome-wide association study. Lancet 372:1953–1961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Giusto G, Anzai N, Endou H, Torres AM. (2008) Elimination of organic anions in response to an early stage of renal ischemia-reperfusion in the rat: role of basolateral plasma membrane transporters and cortical renal blood flow. Pharmacology 81:127–136 [DOI] [PubMed] [Google Scholar]
- Döring A, Gieger C, Mehta D, Gohlke H, Prokisch H, Coassin S, Fischer G, Henke K, Klopp N, Kronenberg F, et al. (2008) SLC2A9 influences uric acid concentrations with pronounced sex-specific effects. Nat Genet 40:430–436 [DOI] [PubMed] [Google Scholar]
- Dreisbach AW. (2009) The influence of chronic renal failure on drug metabolism and transport. Clin Pharmacol Ther 86:553–556 [DOI] [PubMed] [Google Scholar]
- Duan P, You G. (2009) Novobiocin is a potent inhibitor for human organic anion transporters. Drug Metab Dispos 37:1203–1210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan P, You G. (2010) Short-term regulation of organic anion transporters. Pharmacol Ther 125:55–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbaz A, Moisan F. (2008) Update in the epidemiology of Parkinson's disease. Curr Opin Neurol 21:454–460 [DOI] [PubMed] [Google Scholar]
- Eraly SA, Blantz RC, Bhatnagar V, Nigam SK. (2003a) Novel aspects of renal organic anion transporters. Curr Opin Nephrol Hypertens 12:551–558 [DOI] [PubMed] [Google Scholar]
- Eraly SA, Hamilton BA, Nigam SK. (2003b) Organic anion and cation transporters occur in pairs of similar and similarly expressed genes. Biochem Biophys Res Commun 300:333–342 [DOI] [PubMed] [Google Scholar]
- Eraly SA, Monte JC, Nigam SK. (2004) Novel slc22 transporter homologs in fly, worm, and human clarify the phylogeny of organic anion and cation transporters. Physiol Genomics 18:12–24 [DOI] [PubMed] [Google Scholar]
- Eraly SA, Vallon V, Rieg T, Gangoiti JA, Wikoff WR, Siuzdak G, Barshop BA, Nigam SK. (2008) Multiple organic anion transporters contribute to net renal excretion of uric acid. Physiol Genomics 33:180–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eraly SA, Vallon V, Vaughn DA, Gangoiti JA, Richter K, Nagle M, Monte JC, Rieg T, Truong DM, Long JM, et al. (2006) Decreased renal organic anion secretion and plasma accumulation of endogenous organic anions in OAT1 knock-out mice. J Biol Chem 281:5072–5083 [DOI] [PubMed] [Google Scholar]
- Feldmesser E, Olender T, Khen M, Yanai I, Ophir R, Lancet D. (2006) Widespread ectopic expression of olfactory receptor genes. BMC Genomics 7:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foti MC, Amorati R. (2009) Non-phenolic radical-trapping antioxidants. J Pharm Pharmacol 61:1435–1448 [DOI] [PubMed] [Google Scholar]
- Grube M, Meyer Zu Schwabedissen H, Draber K, Präger D, Möritz KU, Linnemann K, Fusch C, Jedlitschky G, Kroemer HK. (2005) Expression, localization, and function of the carnitine transporter octn2 (slc22a5) in human placenta. Drug Metab Dispos 33:31–37 [DOI] [PubMed] [Google Scholar]
- Gutgesell A, Ringseis R, Brandsch C, Stangl GI, Hirche F, Eder K. (2009) Peroxisome proliferator-activated receptor alpha and enzymes of carnitine biosynthesis in the liver are down-regulated during lactation in rats. Metabolism 58:226–232 [DOI] [PubMed] [Google Scholar]
- Hagos Y, Stein D, Ugele B, Burckhardt G, Bahn A. (2007) Human renal organic anion transporter 4 operates as an asymmetric urate transporter. J Am Soc Nephrol 18:430–439 [DOI] [PubMed] [Google Scholar]
- He L, Vasiliou K, Nebert DW. (2009) Analysis and update of the human solute carrier (SLC) gene superfamily. Hum Genomics 3:195–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinig M, Johnson RJ. (2006) Role of uric acid in hypertension, renal disease, and metabolic syndrome. Cleve Clin J Med 73:1059–1064 [DOI] [PubMed] [Google Scholar]
- Hsu FF, Nodari F, Kao LF, Fu X, Holekamp TF, Turk J, Holy TE. (2008) Structural characterization of sulfated steroids that activate mouse pheromone-sensing neurons. Biochemistry 47:14009–14019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu QH, Jiao RQ, Wang X, Lv YZ, Kong LD. (2010) Simiao pill ameliorates urate underexcretion and renal dysfunction in hyperuricemic mice. J Ethnopharmacol 128:685–692 [DOI] [PubMed] [Google Scholar]
- Iwata D, Kato Y, Wakayama T, Sai Y, Kubo Y, Iseki S, Tsuji A. (2008) Involvement of carnitine/organic cation transporter OCTN2 (SLC22A5) in distribution of its substrate carnitine to the heart. Drug Metab Pharmacokinet 23:207–215 [DOI] [PubMed] [Google Scholar]
- Jonker JW, Wagenaar E, Van Eijl S, Schinkel AH. (2003) Deficiency in the organic cation transporters 1 and 2 (Oct1/Oct2 [Slc22a1/Slc22a2]) in mice abolishes renal secretion of organic cations. Mol Cell Biol 23:7902–7908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaler G, Truong DM, Khandelwal A, Nagle M, Eraly SA, Swaan PW, Nigam SK. (2007) Structural variation governs substrate specificity for organic anion transporter (OAT) homologs. Potential remote sensing by OAT family members. J Biol Chem 282:23841–23853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaler G, Truong DM, Sweeney DE, Logan DW, Nagle M, Wu W, Eraly SA, Nigam SK. (2006) Olfactory mucosa-expressed organic anion transporter, Oat6, manifests high affinity interactions with odorant organic anions. Biochem Biophys Res Commun 351:872–876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanai N, Lu R, Satriano JA, Bao Y, Wolkoff AW, Schuster VL. (1995) Identification and characterization of a prostaglandin transporter. Science 268:866–869 [DOI] [PubMed] [Google Scholar]
- Kato Y, Sugiura M, Sugiura T, Wakayama T, Kubo Y, Kobayashi D, Sai Y, Tamai I, Iseki S, Tsuji A. (2006) Organic cation/carnitine transporter OCTN2 (Slc22a5) is responsible for carnitine transport across apical membranes of small intestinal epithelial cells in mouse. Mol Pharmacol 70:829–837 [DOI] [PubMed] [Google Scholar]
- Keebaugh AC, Thomas JW. (2010) The evolutionary fate of the genes encoding the purine catabolic enzymes in hominoids, birds, and reptiles. Mol Biol Evol 27:1359–1369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaassen CD, Aleksunes LM. (2010) Xenobiotic, bile acid, and cholesterol transporters: function and regulation. Pharmacol Rev 62:1–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolz M, Johnson T, Sanna S, Teumer A, Vitart V, Perola M, Mangino M, Albrecht E, Wallace C, Farrall M, et al. (2009) Meta-analysis of 28,141 individuals identifies common variants within five new loci that influence uric acid concentrations. PLoS Genet 5:e1000504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsuda A, Iwamoto K, Wakui H, Sawada K, Yamaguchi A. (2006) Analysis of mutations in the urate transporter 1 (URAT1) gene of Japanese patients with hypouricemia in northern Japan and review of the literature. Ren Fail 28:223–227 [DOI] [PubMed] [Google Scholar]
- Kwok B, Yamauchi A, Rajesan R, Chan L, Dhillon U, Gao W, Xu H, Wang B, Takahashi S, Semple J, et al. (2006) Carnitine/xenobiotics transporters in the human mammary gland epithelia, MCF12A. Am J Physiol Regul Integr Comp Physiol 290:R793–R802 [DOI] [PubMed] [Google Scholar]
- Li S, Sanna S, Maschio A, Busonero F, Usala G, Mulas A, Lai S, Dei M, Orrù M, Albai G, et al. (2007) The GLUT9 gene is associated with serum uric acid levels in Sardinia and Chianti cohorts. PLoS Genet 3:e194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JH, Chiba M, Chen IW, Nishime JA, deLuna FA, Yamazaki M, Lin YJ. (1999) Effect of dexamethasone on the intestinal first-pass metabolism of indinavir in rats: evidence of cytochrome P-450 3A [correction of P-450 A] and p-glycoprotein induction. Drug Metab Dispos 27:1187–1193 [PubMed] [Google Scholar]
- Meeks JP, Arnson HA, Holy TE. (2010) Representation and transformation of sensory information in the mouse accessory olfactory system. Nat Neurosci 13:723–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DS. (2010) Regulation of P-glycoprotein and other ABC drug transporters at the blood-brain barrier. Trends Pharmacol Sci 31:246–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monte JC, Nagle MA, Eraly SA, Nigam SK. (2004) Identification of a novel murine organic anion transporter family member, OAT6, expressed in olfactory mucosa. Biochem Biophys Res Commun 323:429–436 [DOI] [PubMed] [Google Scholar]
- Nezu J, Tamai I, Oku A, Ohashi R, Yabuuchi H, Hashimoto N, Nikaido H, Sai Y, Koizumi A, Shoji Y, et al. (1999) Primary systemic carnitine deficiency is caused by mutations in a gene encoding sodium ion-dependent carnitine transporter. Nat Genet 21:91–94 [DOI] [PubMed] [Google Scholar]
- Nigam S, Lieberthal W. (2000) Acute renal failure. III. The role of growth factors in the process of renal regeneration and repair. Am J Physiol Renal Physiol 279:F3–F11 [DOI] [PubMed] [Google Scholar]
- Nigam SK, Bush KT, Bhatnagar V. (2007) Drug and toxicant handling by the OAT organic anion transporters in the kidney and other tissues. Nat Clin Pract Nephrol 3:443–448 [DOI] [PubMed] [Google Scholar]
- Nyhan WL. (1968) Lesch-Nyhan syndrome. Summary of clinical features. Fed Proc 27:1034–1041 [PubMed] [Google Scholar]
- Pavlova A, Sakurai H, Leclercq B, Beier DR, Yu AS, Nigam SK. (2000) Developmentally regulated expression of organic ion transporters NKT (OAT1), OCT1, NLT (OAT2), and Roct. Am J Physiol Renal Physiol 278:F635–F643 [DOI] [PubMed] [Google Scholar]
- Puig JG, Martínez MA. (2008) Hyperuricemia, gout and the metabolic syndrome. Curr Opin Rheumatol 20:187–191 [DOI] [PubMed] [Google Scholar]
- Rebouche CJ, Seim H. (1998) Carnitine metabolism and its regulation in microorganisms and mammals. Annu Rev Nutr 18:39–61 [DOI] [PubMed] [Google Scholar]
- Sachs L, Batra KL, Zimmermann B. (2009) Medical implications of hyperuricemia. Med Health R I 92:353–355 [PubMed] [Google Scholar]
- Sánchez-Moreno C, Dashe JF, Scott T, Thaler D, Folstein MF, Martin A. (2004) Decreased levels of plasma vitamin C and increased concentrations of inflammatory and oxidative stress markers after stroke. Stroke 35:163–168 [DOI] [PubMed] [Google Scholar]
- Sandor A, Pecsuvac K, Kerner J, Alkonyi I. (1982) On carnitine content of the human breast milk. Pediatr Res 16:89–91 [DOI] [PubMed] [Google Scholar]
- Sauvant C, Holzinger H, Gekle M. (2006) Prostaglandin E2 inhibits its own renal transport by down-regulation of organic anion transporters rOAT1 and rOAT3. J Am Soc Nephrol 17:46–53 [DOI] [PubMed] [Google Scholar]
- Schlatter E, Mönnich V, Cetinkaya I, Mehrens T, Ciarimboli G, Hirsch JR, Popp C, Koepsell H. (2002) The organic cation transporters rOCT1 and hOCT2 are inhibited by cGMP. J Membr Biol 189:237–244 [DOI] [PubMed] [Google Scholar]
- Schnabolk GW, Gupta B, Mulgaonkar A, Kulkarni M, Sweet DH. (2010) Organic anion transporter 6 (Slc22a20) specificity and Sertoli cell-specific expression provide new insight on potential endogenous roles. J Pharmacol Exp Ther 334:927–935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serkova NJ, Christians U, Benet LZ. (2004) Biochemical mechanisms of cyclosporine neurotoxicity. Mol Interv 4:97–107 [DOI] [PubMed] [Google Scholar]
- Shekhawat PS, Srinivas SR, Matern D, Bennett MJ, Boriack R, George V, Xu H, Prasad PD, Roon P, Ganapathy V. (2007) Spontaneous development of intestinal and colonic atrophy and inflammation in the carnitine-deficient jvs (OCTN2(−/−)) mice. Mol Genet Metab 92:315–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi XD, Li GC, Qian ZX, Jin ZQ, Song Y. (2008) Randomized and controlled clinical study of modified prescriptions of Simiao Pill in the treatment of acute gouty arthritis. Chin J Integr Med 14:17–22 [DOI] [PubMed] [Google Scholar]
- Shiraya K, Hirata T, Hatano R, Nagamori S, Wiriyasermkul P, Jutabha P, Matsubara M, Muto S, Tanaka H, Asano S, et al. (2010) A novel transporter of SLC22 family specifically transports prostaglandins and co-localizes with 15-hydroxyprostaglandin dehydrogenase in renal proximal tubules. J Biol Chem 285:22141–22151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srimaroeng C, Perry JL, Pritchard JB. (2008) Physiology, structure, and regulation of the cloned organic anion transporters. Xenobiotica 38:889–935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H, Frassetto L, Benet LZ. (2006) Effects of renal failure on drug transport and metabolism. Pharmacol Ther 109:1–11 [DOI] [PubMed] [Google Scholar]
- Sweet DH, Eraly SA, Vaughn DA, Bush KT, Nigam SK. (2006) Organic anion and cation transporter expression and function during embryonic kidney development and in organ culture models. Kidney Int 69:837–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweet DH, Miller DS, Pritchard JB, Fujiwara Y, Beier DR, Nigam SK. (2002) Impaired organic anion transport in kidney and choroid plexus of organic anion transporter 3 (Oat3 (Slc22a8)) knockout mice. J Biol Chem 277:26934–26943 [DOI] [PubMed] [Google Scholar]
- Takahashi R, Asai T, Murakami H, Murakami R, Tsuzuki M, Numaguchi Y, Matsui H, Murohara T, Okumura K. (2007) Pressure overload-induced cardiomyopathy in heterozygous carrier mice of carnitine transporter gene mutation. Hypertension 50:497–502 [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Chen C, Maher JM, Klaassen CD. (2008) Ischemia-reperfusion of rat livers decreases liver and increases kidney multidrug resistance associated protein 2 (Mrp2). Toxicol Sci 101:171–178 [DOI] [PubMed] [Google Scholar]
- Truong DM, Kaler G, Khandelwal A, Swaan PW, Nigam SK. (2008) Multi-level analysis of organic anion transporters 1, 3, and 6 reveals major differences in structural determinants of antiviral discrimination. J Biol Chem 283:8654–8663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasiliou V, Vasiliou K, Nebert DW. (2009) Human ATP-binding cassette (ABC) transporter family. Hum Genomics 3:281–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitart V, Rudan I, Hayward C, Gray NK, Floyd J, Palmer CN, Knott SA, Kolcic I, Polasek O, Graessler J, et al. (2008) SLC2A9 is a newly identified urate transporter influencing serum urate concentration, urate excretion and gout. Nat Genet 40:437–442 [DOI] [PubMed] [Google Scholar]
- Wikoff WR, Anfora AT, Liu J, Schultz PG, Lesley SA, Peters EC, Siuzdak G. (2009) Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc Natl Acad Sci USA 106:3698–3703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright AF, Rudan I, Hastie ND, Campbell H. (2010) A ‘complexity’ of urate transporters. Kidney Int 78:446–452 [DOI] [PubMed] [Google Scholar]
- Wu W, Baker ME, Eraly SA, Bush KT, Nigam SK. (2009) Analysis of a large cluster of SLC22 transporter genes, including novel USTs, reveals species-specific amplification of subsets of family members. Physiol Genomics 38:116–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, De la Cruz O, Pinto JM, Nicolae D, Firestein S, Gilad Y. (2007) Characterizing the expression of the human olfactory receptor gene family using a novel DNA microarray. Genome Biol 8:R86. [DOI] [PMC free article] [PubMed] [Google Scholar]