Abstract
Systemic administration of local anesthetics has beneficial perioperative properties and an anesthetic-sparing and antiarrhythmic effect, although the detailed mechanisms of these actions remain unclear. In the present study, we investigated the effects of a local anesthetic, lidocaine, on hyperpolarization-activated and cyclic nucleotide-gated (HCN) channels that contribute to the pacemaker currents in rhythmically oscillating cells of the heart and brain. Voltage-clamp recordings were used to examine the properties of cloned HCN subunit currents expressed in Xenopus laevis oocytes and human embryonic kidney (HEK) 293 cells under control condition and lidocaine administration. Lidocaine inhibited HCN1, HCN2, HCN1-HCN2, and HCN4 channel currents at 100 μM in both oocytes and/or HEK 293 cells; it caused a decrease in both tonic and maximal current (∼30–50% inhibition) and slowed current activation kinetics for all subunits. In addition, lidocaine evoked a hyperpolarizing shift in half-activation voltage (ΔV1/2 of ∼−10 to −14 mV), but only for HCN1 and HCN1-HCN2 channels. By fitting concentration-response data to logistic functions, we estimated half-maximal (EC50) concentrations of lidocaine of ∼30 to 40 μM for the shift in V1/2 observed with HCN1 and HCN1-HCN2; for inhibition of current amplitude, calculated EC50 values were ∼50 to 70 μM for HCN1, HCN2, and HCN1-HCN2 channels. A lidocaine metabolite, monoethylglycinexylidide (100 μM), had similar inhibitory actions on HCN channels. These results indicate that lidocaine potently inhibits HCN channel subunits in dose-dependent manner over a concentration range relevant for systemic application. The ability of local anesthetics to modulate Ih in central neurons may contribute to central nervous system depression, whereas effects on If in cardiac pacemaker cells may contribute to the antiarrhythmic and/or cardiovascular toxic action.
Introduction
Local anesthetics have been widely used in surgical anesthesia and for short- and long-term pain management since they were first discovered by Koller in 1884 (Koller, 1928). Although typically used for regional anesthesia, where they are relatively safe, toxic systemic reactions have been a problem resulting from administration of an excessive dose (Brown et al., 1995). In addition, local anesthetics have also been used purposefully in systemic application, in which a low and moderate intravenous dose can produce beneficial actions (Kingery, 1997; Koppert et al., 2004). For example, it is well known that systemic administration of local anesthetics has antiarrhythmic (Pinter and Dorian, 2001), anesthetic-sparing, and perioperative analgesic effects (Smith et al., 2004). Lidocaine is the most important class 1B antiarrhythmic drug; it is used intravenously for the treatment of ventricular arrhythmias (Trujillo and Nolan, 2000; Pinter and Dorian, 2001). Lidocaine reduces minimal alveolar concentration of volatile anesthetics for the suppression of responses to painful stimuli in animals by 20 to 40% (DiFazio et al., 1976; Himes et al., 1977; Smith et al., 2004) and decreases the requirement of intravenous anesthetic propofol (Senturk et al., 2002). The local anesthetic lidocaine can produce ∼0.4 minimal alveolar concentration (low-dose systemic application) (DiFazio et al., 1976) and decrease the bispectral index to 0 (higher-dose systemic application) (Gaughen and Durieux, 2006). Perioperative intravenous lidocaine prevents postoperative and neuropathic pain and decreases postoperative morphine consumption (Kingery, 1997; Koppert et al., 2004). Overall, these actions are generally considered to reflect cardiovascular and central neural systemic depressive actions of local anesthetics (Gaughen and Durieux, 2006).
Because blockade of voltage-gated sodium channels by local anesthetics represents the main mechanism for inhibition of action potential propagation, inhibition of sodium channels by local anesthetics was believed to play an important role in producing local anesthetics systemic actions (Ragsdale et al., 1994). However, increasing evidence reveals that it is unlikely that blocking of sodium channel can account for the full spectrum of systemic actions of local anesthetics. For example, tetrodotoxin, a potent sodium-channel blocker and local anesthetic (Narahashi, 1972), is of special interest in this context because it is believed to induce no sedation by systemic application (Marcil et al., 2006), unlike lidocaine. Thus, the mechanisms mediating various systemic actions of local anesthetics remain unknown, and molecular substrates other than sodium channels that contribute to those systemic actions should be sought.
In this study, we provide evidence that local anesthetic lidocaine potently inhibits the HCN channels that underlie the hyperpolarization-activated Na+/K+ current that has been designated If or Ih in cardiac myocytes and many central neurons (Biel et al., 1999). We find that lidocaine inhibits all HCN channels tested, including homomeric HCN1, HCN2, and HCN4 as well as heteromeric HCN1-HCN2 by decreasing tonic and peak current and slowing voltage-dependent channel activation; in addition, a hyperpolarizing shift in voltage dependence was observed for channels containing HCN1 subunits. We suggest that the ability of local anesthetics to modulate Ih in central neurons may contribute to the central nervous system depression seen with systemic administration, whereas their effects on If in cardiac pacemaker cells may contribute to antiarrhythmic and cardiovascular toxic action.
Materials and Methods
Voltage-Clamp Recording of HCN Channels Expressed in Xenopus laevis Oocytes.
We obtained mHCN1, mHCN2, and mHCN4 from Drs. B. Santoro and S. A. Siegelbaum (Columbia University, New York, NY) in pGHE or pcDNA3 expression vector and subcloned them into pcDNA3-HE3 for recording in X. laevis oocytes and HEK 293 cells. The concatemeric HCN1-HCN2 construct was made by using overlap extension polymerase chain reaction to produce a PshAI-NheI fragment that spliced the final leucine of HCN1 directly in frame with the initiating methionine of HCN2, as described previously (Chen et al., 2005b). To prepare RNA, in vitro transcription was performed with NheI-linearized DNA (HCN1), SphI-linearized DNA (HCN2), XbaI-linearized DNA (HCN1-HCN2), or XbaI-linearized DNA (HCN4) using T7 RNA polymerase (Promega Biotech Co., Ltd., Beijing, China). X. laevis oocytes (Maosheng Biologic Science and Technology Development Co., Ltd, Shanghai, China) were injected with 46 nl of RNA (50–200 ng/μl) using a Nanoliter 2000 microinjector (WPI, Sarasota, FL). After injection, oocytes were incubated at 17°C for 1 to 3 days in ND-96 solution, containing 96 mM NaCl, 2 mM KCl, 1.8 mM CaCl2, 1 mM MgCl2, and 5 mM HEPES, pH 7.5, that was supplemented with 50 mg/l gentamicin sulfate. Whole-cell currents were recorded from oocytes in solution containing 107 mM KCl, 5 mM NaCl, 10 mM HEPES, 1 mM MgCl2, and 1 mM EGTA, pH 7.3, with the two-microelectrode voltage-clamp technique using a Warner OC-725B amplifier (Warner Instruments, Hamden, CT). An Ag-AgCl ground wire was connected to the bath solution by 2% agar salt bridge (in 3 M KCl) placed downstream of the oocyte. Recordings were obtained at room temperature (22–24°C). Voltage-recording and current-injecting electrodes were filled with 3 M KCl (1–3 MΩ).
Heterologous Expression of HCN Channel Constructs in HEK 293 Cells.
HEK 293 cells were cultured using standard procedures and transiently transfected with HCN channel constructs, together with a green fluorescent protein plasmid (pEGFP; Clontech, Mountain View, CA), using Lipofectamine 2000 reagent (Invitrogen, Carlsbad, CA). Recordings were obtained 1 to 2 days after transfection. Whole-cell recordings were obtained at room temperature using 3 to 5 MΩ patch pipettes and an Axopatch 200B amplifier in a HEPES-buffered bath solution composed of 118 mM NaCl, 25 mM KCl, 2 mM MgCl2, 2 mM CaCl2, 10 mM HEPES, and 10 mM glucose, pH 7.3 that was perfused continuously (∼2 ml/min). Internal solution contained 120 mM KCH4SO3, 4 mM NaCl, 1 mM MgCl2, 0.5 mM CaCl2, 10 mM HEPES, 10 mM EGTA, 3 mM Mg-ATP, and 0.3 mM GTP-Tris, pH 7.2 (Himes et al., 1977). Stock solutions of lidocaine hydrochloride (Sigma-Aldrich, St. Louis, MO) and the lidocaine metabolite monoethylglycinexylidide (MEGX; Ryan Scientific, Inc., Mt. Pleasant, SC) were prepared in water and dimethyl sulfoxide (100 mM) and brought to the indicated concentrations in HEPES-buffered bath solution, at pH 7.3.
Data Acquisition and Analysis.
Data were acquired using pCLAMP software (Molecular Devices, Sunnyvale, CA) and a Digidata 1322A or a Digidata 1200 digitizer (Molecular Devices). For voltage-clamp recording, time-dependent hyperpolarization-activated currents (Ih, HCN) were evoked with incrementing (Δ −10 mV) hyperpolarizing pulses (3–4 s) from a holding potential of −40 mV, followed immediately by a step to fixed potential (−90 mV) to obtain tail currents. Amplitude of voltage-dependent HCN currents were derived at each potential as the difference between “instantaneous” current, measured immediately after the capacitive transient before time-dependent HCN activation, and the steady-state current at the end of hyperpolarizing voltage steps; maximal available voltage-dependent current was determined at −120 mV (or −110 mV for some HCN1 channels). Input conductance at the holding potential was calculated from the slope of instantaneous I-V curves, with tonic (constitutive) Ih defined as the Cs+-sensitive component of instantaneous current. Test pulses from −40 to −120 mV are a typical protocol to evaluate HCN channel function. The extreme hyperpolarization allows maximal voltage-dependent channel activation that is required to characterize maximal current amplitude and to normalize tail currents for analysis of voltage dependence of channel gating. Tail currents were normalized, plotted as a function of the preceding hyperpolarization step voltage and fitted with Boltzmann curves for derivation of half-activation voltage (V1/2) by using a least-squares analysis and the “Solver” add-in of Excel (Microsoft Corp., Redmond, WA). Time constants (τ) were determined by fitting currents evoked during hyperpolarizing steps to a biexponential function (Clampfit; Molecular Devices).
Concentration-response relationships for lidocaine effects on V1/2 and maximal current amplitude were fitted in Prism 3.0 according to the equation E/Emax = {1 + ([lidocaine]/EC50)nH}−1, where fitted parameters were the concentration of half-maximal effect (EC50), Hill coefficient (nH), and either the maximal shift of V1/2 or maximal inhibition of current amplitude by lidocaine (Emax). Results are presented as mean ± S.E.M. Statistical tests included two-way analysis of variance or Student's t test, as indicated. Differences in mean values were considered significant if P < 0.05.
Results
HCN1 and HCN2 Channel Subunits Are Differentially Modulated by Lidocaine.
The HCN family of ion channels represents the molecular substrate for Ih in neurons and If in cardiomyocytes. HCN1 and HCN2 are abundant subunits expressed in brain and heart (Santoro et al., 2000; Biel et al., 2009). These two HCN subunits produce homomeric channels that differ markedly in activation properties; HCN2 currents activate more slowly and at more hyperpolarized potentials than HCN1 currents, and they are more sensitive to cAMP (Biel et al., 1999).
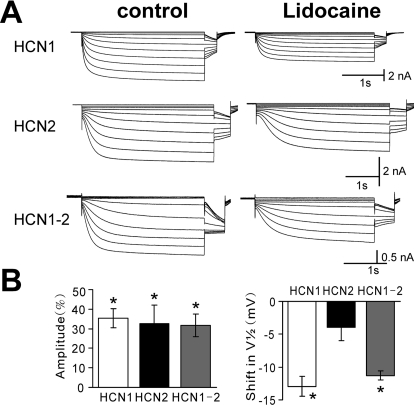
We expressed HCN1 and HCN2 homomeric channels in both X. laevis oocytes and HEK 293 cells and found that they also differed in their modulation by local anesthetic lidocaine (Figs. 1 and 2). Lidocaine effects on HCN channels were fast (4.5 ± 0.3 min in HEK 293 cells, n = 23) and reversible (data not shown). In oocytes expressing HCN1 subunits, lidocaine (100 μM) caused a hyperpolarizing shift in voltage dependence of activation (ΔV1/2 of −12.9 ± 1.5 mV) and a decrease in maximal current amplitude of 35.5 ± 4.9% (Fig. 1, A and B, top). Lidocaine also decreased HCN2 current amplitude (32.5 ± 9.5%) but did not substantially change the voltage range of HCN2 activation (ΔV1/2 of −3.9 ± 2.0 mV; Fig. 1, A and B, middle). Note that the initial V1/2 of HCN1 (−79.3 ± 9.1 mV, n = 27) was substantially more depolarized than that of HCN2 (−100.7 ± 1.3 mV, n = 25), as expected for these cloned channels (Biel et al., 1999). It is now clear that HCN subunits can form heteromeric channels (Biel et al., 2009). To test the effects of lidocaine on heteromeric HCN channels, we expressed a linked HCN1-HCN2 construct in oocytes; this HCN1-HCN2 heteromeric channel produced hyperpolarization-activated currents with kinetic and voltage-dependent properties intermediate to those of the constituent HCN1 and HCN2 subunits (initial V1/2 −89.9 ± 1.9 mV, n = 16), as reported previously (Chen et al., 2005a). Effects of lidocaine on linked HCN1-HCN2 heteromeric channel currents were most like those on mHCN1, inducing a hyperpolarizing shift in V1/2 (ΔV1/2 of −11.3 ± 0.7 mV) and a decrease in maximal current amplitude (31.7 ± 5.8%; Fig. 1, A and B, bottom).
Fig. 1.
Local anesthetic lidocaine differentially inhibits HCN channel currents expressed in X. laevis oocytes. A, sample currents from X. laevis oocytes expressing mHCN1, mHCN2, and mHCN1-mHCN2 channel constructs evoked by hyperpolarizing voltage steps from −40 to −120 mV before and during exposure to lidocaine (100 μM); conditioning voltage steps were of different duration for the three constructs (3, 4, and 3 s) followed by a step to −90 mV for tail current analysis. B, summary data showing averaged (± S.E.M.) current inhibition (percentage from control; left) and shift in half-activation potential (V1/2; right) evoked by lidocaine for each of the indicated HCN channel constructs. *, P < 0.05 by analysis of variance for lidocaine versus control (n = 6, 5, and 8 for mHCN1, mHCN2, and mHCN1-mHCN2, respectively).
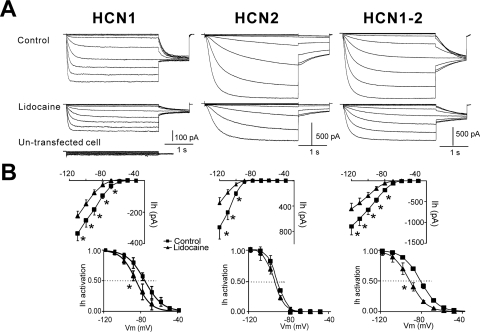
Fig. 2.
Local anesthetic inhibits HCN channel currents in HEK 293 cells. A, sample currents from HEK 293 cells expressing mHCN1, mHCN2, or a linked heteromeric mHCN1-mHCN2 construct evoked by hyperpolarizing voltage steps (Δ −10 mV) from −40 to −120 mV before and during exposure to lidocaine (100 μM); voltage steps were followed by a step to −90 mV for tail current analysis. A sample current trace from untransfected HEK 293 cell is also shown at the bottom. B, activation curves were determined from tail currents (bottom) and steady-state I-V curves from currents at the end of the voltage steps (top) under control conditions (■), during exposure to lidocaine (▴) for mHCN1, mHCN2, or a linked mHCN1-HCN2 constructs. *, P < 0.05 versus control (n = 5, 5, and 6 for mHCN1, mHCN2, and mHCN1-HCN2, respectively).
We repeated these studies in a mammalian heterologous expression system in which we also found differential modulation of HCN1 and HCN2 channel currents by lidocaine (Fig. 2). Similar to uninjected oocytes (data not shown), untransfected HEK 293 cells expressed undetectable HCN currents (Fig. 2A), which indicated that all measured HCN currents were attributable to the transfected HCN channel construct. In HEK 293 cells expressing HCN1 subunits, lidocaine (100 μM) caused a hyperpolarizing shift in voltage dependence of activation (ΔV1/2 of −11.8 ± 0.3 mV) and a decrease in maximal current amplitude of 30.9 ± 5.2% (Fig. 2, A and B, left). Again, lidocaine decreased maximal HCN2 current amplitude (46.6 ± 4.2%) without substantially changing the voltage range of HCN2 activation (ΔV1/2 of −3.2 ± 1.5 mV; Fig. 2, A and B, middle). For the linked HCN1-HCN2 subunit currents, lidocaine induced a shift in V1/2 (ΔV1/2 of −10.1 ± 2.0 mV) and a decrease in maximal current amplitude (38.7 ± 3.2%; Fig. 2, A and B, right).
Note that although we evaluated inhibition by lidocaine of current amplitude at −120 mV, a potential at which HCN channels are maximally activated, this should not be misconstrued to suggest that lidocaine actions occur only at these extreme potentials. Rather, as depicted in the I-V and activation curves, the effects of lidocaine on voltage-activated HCN channel currents are manifest in a smooth and continuous fashion over the entire voltage range examined, including at physiologically relevant membrane potentials (Figs. 1A and 2A). Moreover, as shown below, lidocaine also inhibits a tonic HCN current component that is active even at depolarized potentials.
Lidocaine Inhibits HCN4 Channel Currents Expressed in HEK 293 Cells.
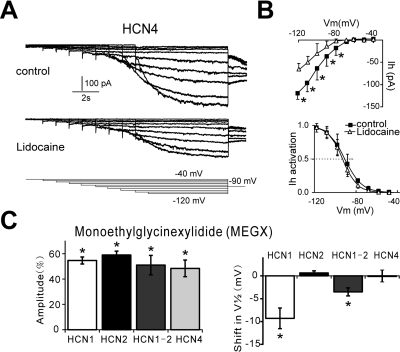
We also examined effects of lidocaine on HCN4 channel currents, which, unlike HCN1 and HCN2, are predominantly expressed in heart with only limited expression in some brain areas. In HEK 293 cells expressing the slowly activating HCN4 subunits, lidocaine (100 μM) caused the inhibition of maximal current amplitude of 30.4 ± 4.2% (Fig. 3, A and B), with little effects in voltage dependence of activation (ΔV1/2 of −2.6 ± 0.9 mV) (Fig. 3, A and B). Thus, the modulation of HCN4 by lidocaine was similar to that of HCN2 (i.e., decrease in current amplitude with essentially no effect on V1/2).
Fig. 3.
Lidocaine inhibits HCN4 channel currents. A, sample currents from HEK 293 cells expressing mHCN4 channel construct evoked by hyperpolarizing voltage steps from −40 to −120 mV before and during exposure to lidocaine (100 μM); conditioning voltage steps (6–14 s) were followed by a step to −90 mV for tail current analysis. B, steady-state I-V curves from currents at the end of the voltage steps (top), and activation curves were determined from tail currents (bottom) under control conditions (■) and during exposure to lidocaine (▵). C. Summary data showing averaged (± S.E.M.) current inhibition (percentage from control; left) and shift in half-activation potential (V1/2; right) evoked by lidocaine metabolite MEGX for each of the indicated HCN channel constructs. *, P < 0.05 versus control.
Lidocaine Metabolite MEGX Inhibits HCN Channel Currents in HEK 293 Cells.
It has been reported that a lidocaine metabolite, MEGX, can produce systemic actions similar to those of lidocaine (Halkin et al., 1975). Therefore, we examined the effects of MEGX on HCN channel currents. In HEK 293 cells expressing HCN1 subunits, MEGX (100 μM) caused a hyperpolarizing shift in voltage dependence of activation (ΔV1/2 of −9.3 ± 2.3 mV) and a decrease in maximal current amplitude of 54.7 ± 2.8% (Fig. 3C). With HCN2 channels, MEGX decreased maximal current amplitude (59.3 ± 3.5%) without substantially changing the voltage range of HCN2 activation (ΔV1/2 of 0.6 ± 0.5 mV; Fig. 3C), as we noted also for lidocaine. For HCN1-HCN2 heteromeric channel currents, MEGX induced a shift in V1/2 (ΔV1/2 of −3.5 ± 0.9 mV) and a decrease in maximal current amplitude (51.0 ± 7.7%, Fig. 3C). Finally, MEGX caused the inhibition of maximal amplitude of HCN4 current (48.4 ± 6.6%; Fig. 3C, left) with little effect on voltage dependence of activation (ΔV1/2 of −0.1 ± 2.3 mV; Fig. 3C, right). Thus, the modulation of HCN channels by MEGX was similar to that by lidocaine.
Lidocaine Inhibits Tonic Currents of All HCN Channels.
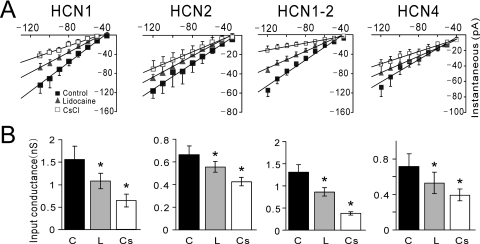
A tonic component of current, in addition to the voltage- and time-dependent component, has been observed in recordings from cloned HCN channels (Proenza et al., 2002; Macri and Accili, 2004). This current component represents constitutive activation of HCN channels at holding potentials depolarized to the threshold for voltage-dependent activation and, at least for HCN2 channels, can represent ∼10% of the total available current (Chen et al., 2005a). We therefore tested whether lidocaine inhibits tonic currents from HCN channels and whether it does so in a subunit-specific manner. In HEK 293 cells expressing HCN1, HCN2, HCN1-HCN2, and HCN4 channels, we measured input conductance as the slope of I-V relationships from instantaneous currents (i.e., measured immediately after the capacitive transient and before development of time-dependent currents) evoked by hyperpolarizing voltage steps from a holding potential of −40 mV. As evident in Fig. 4, instantaneous currents from cells expressing all four HCN constructs were inhibited by 3 mM CsCl, which completely blocks HCN channel currents (Biel et al., 2009); this Cs+-sensitive instantaneous current component reflects tonic HCN current. It is noteworthy that lidocaine inhibited tonically active currents from all four HCN channels. When expressed relative to the Cs+-sensitive, tonic HCN current, lidocaine (100 μM) inhibited 45.7% of tonic HCN1 current, 53.3% of tonic HCN1-HCN2 current, 54.3% of tonic HCN2 current, and 40.6% of tonic HCN4 current. These data confirm earlier observations of constitutive activity of HCN channel currents at depolarized membrane potentials (Proenza et al., 2002; Macri and Accili, 2004), and they demonstrate that lidocaine inhibits tonic currents from HCN1, HCN1-HCN2, HCN2, and HCN4 channels.
Fig. 4.
Lidocaine inhibits tonic HCN channel currents. A, instantaneous I-V relationships were obtained from a holding potential of −40 mV in HEK 293 cells expressing mHCN1, mHCN2, mHCN1-HCN2, and mHCN4 under control conditions (■), in the presence of 100 μM lidocaine (▴) and 3 mM CsCl (□). Solid lines represent linear fits through averaged data (± S.E.M.; n = 5, 5, 6, and 5 for HCN1, HCN2, HCN1-HCN2, and HCN4, respectively), representing the input conductance at −40 mV. B, input conductance was determined from slopes of instantaneous I-V curves in individual cells expressing the HCN channel constructs and averaged for each condition as indicated (C, control; L, lidocaine; Cs, CsCl). Lidocaine decreased input conductance in all HCN-expressing cells (*, P < 0.05 versus control).
Lidocaine Causes a Slowing of HCN Current Activation.
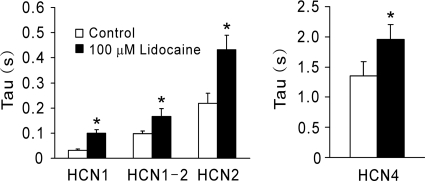
In addition to inhibitory actions of lidocaine on current amplitude or V1/2 in HCN channels shown above, lidocaine also modulated HCN channel current kinetics. As shown in Figs. 1, 2, and 5 and consistent with previous reports, HCN channel currents differ in their activation properties, with HCN1 currents activating the fastest and HCN4 currents activating the slowest (Biel et al., 1999). Lidocaine caused a slowing of current activation (fast τ at −120 mV) for all HCN channels examined (HCN1, from 31.8 ± 4.8 to 99.3 ± 13.9 ms; HCN2, 217.8 ± 40.8 to 432.4 ± 57.9 ms; HCN1-HCN2, 98.3 ± 10.9 to 166.6 ± 31.8 ms; and HCN4, 1357.0 ± 227.9 to 1961.0 ± 240.3 ms).
Fig. 5.
Lidocaine causes a slowing of HCN subunit currents. Activation data were obtained from biexponential fits at −120 mV, and the time constant (τ) describing the fastest (and largest) current component were determined under control and during lidocaine application for mHCN1, mHCN1-mHCN2, mHCN2, and mHCN4 subunit currents under control conditions and in the presence of 100 μM lidocaine. Lidocaine caused a slowing of all HCN subunits current activation examined. *, P < 0.05 versus control (n = 5, 5, 6, and 5 for HCN1, HCN2, HCN1-HCN2, and HCN4, respectively).
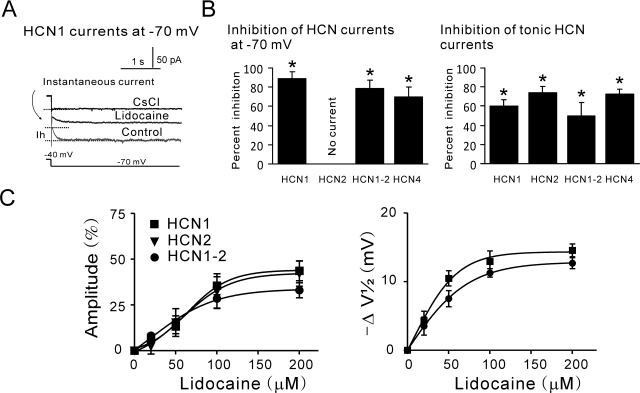
Lidocaine Inhibits HCN Channels at Physiological Membrane Potentials.
To this point, our data indicate that lidocaine inhibits current amplitude on HCN1, HCN2, HCN1-HCN2, and HCN4 channels, with a subunit-specific negative shift of voltage gating only in HCN1 subunit-containing channels (HCN1 and HCN1-HCN2). Lidocaine also inhibits tonic currents and slows current activation of all four channels. At intermediate, physiologically relevant potentials (e.g., −70 mV), these effects together determine the degree of HCN inhibition by lidocaine. As shown in Fig. 6A, for a cell expressing HCN1, a voltage step from −40 to −70 mV induces an instantaneous current followed by the voltage- and time-dependent component. The voltage- and time-dependent HCN1 current was strongly inhibited by lidocaine (by 89.0 ± 6.8%; Fig. 6, A and B), reflecting both the negative shift of voltage gating and the decrease in maximum available current induced by lidocaine. In addition, lidocaine and Cs+ significantly decreased instantaneous HCN1 current; lidocaine inhibited 60.3 ± 6.2% of the Cs+-sensitive instantaneous (i.e., tonic) HCN1 current (Fig. 6, A and B). For HCN2 channels, which have a more negative voltage range of activation, the tonic component accounts for most of the current at −70 mV and was significantly inhibited by lidocaine (74.4 ± 6.3%; Fig. 6B). For HCN1-HCN2 and HCN4 currents, lidocaine inhibits both voltage-dependent and tonic current components, as shown in Fig. 6B.
Fig. 6.
Lidocaine modulates HCN channel currents at −70 mV and at clinically relevant concentrations. A, sample HCN1 current at −70 mV under control conditions and during administration of lidocaine (100 μM) and CsCl (3 mM), an HCN channel blocker. HCN1 currents include two components: a voltage- and time-dependent component that was almost strongly inhibited by lidocaine and totally blocked by Cs+, and a Cs+-sensitive instantaneous current component that was partly reduced by lidocaine. B, summary data showing effects of lidocaine on voltage- and time-dependent (left) and tonic (right) HCN currents measured at −70 mV in cells expressing HCN1, HCN2, HCN1-HCN2, and HCN4 channel constructs. Calculation of the percentage of inhibition of tonic current is relative to the Cs+-sensitive instantaneous current component (i.e., the HCN current). Lidocaine inhibited instantaneous and voltage-dependent currents for HCN1, HCN1-HCN2, and HCN4 channels; for HCN2, the instantaneous current is predominant at −70 mV and was also reduced by lidocaine. *, P < 0.05 versus control (n = 5, 5, 6, and 5 for HCN1, HCN2, HCN1-HCN2, and HCN4, respectively). C, averaged values for shift in amplitude inhibition (left) and V1/2 (right) of mHCN1 (squares), mHCN2 (triangles), and heteromeric mHCN1-mHCN2 (circles) currents expressed in oocytes at different concentrations of lidocaine. The effects of lidocaine (0, 20, 50, 100, and 200 μM) on current amplitude and V1/2 were averaged (± S.E.M.) and fitted with logistic equations.
Lidocaine Modulates HCN1 and HCN2 Channel Currents at Clinically Relevant Concentrations.
We tested the effects of lidocaine on HCN1, HCN2, and HCN1-HCN2 heteromeric channels expressed in oocytes over a range of concentrations that encompasses those achieved clinically (20–200 μM). Lidocaine suppressed the amplitude of heteromeric HCN channel currents (Fig. 6C, left) and induced a hyperpolarizing shift in the voltage dependence of activation (Fig. 6C, right) in a dose-dependent manner. By fitting amplitude inhibition data to logistic functions, we estimated half-maximal (EC50) concentrations of lidocaine of, respectively, 67.6 ± 9.6, 66.8 ± 15.3, and 51.6 ± 9.5 μM for HCN1, HCN2, and HCN1-HCN2, with corresponding maximal values of 43.8 ± 3.5, 41.7 ± 5.2, and 32.4 ± 3.0% inhibition. For the shift in V1/2, calculated EC50 values were 34.0 and 41.3 μM for HCN1 and HCN1-HCN2, with corresponding maximal values of −13.9 and −12.2 mV, respectively. For both actions, the effects of lidocaine were essentially maximal at 100 μM. These results indicate that lidocaine inhibits HCN channels in a dose-dependent manner over a relevant concentration range for various systemic actions.
Discussion
In the present study, we demonstrated that the local anesthetic lidocaine inhibits constitutive and voltage-dependent mouse HCN channel currents expressed in both oocytes and HEK 293 cells systems. For channels containing HCN1 subunits (either homomeric HCN1 or heteromeric HCN1-HCN2), lidocaine caused a negative shift in V1/2, decreased tonic and maximal currents, and slowed activation kinetics. We also found that lidocaine modulated cloned HCN2 and HCN4 homomeric channel currents via a decrease in tonic and maximal current amplitude, but with no change in voltage dependence of activation. Our observations regarding the effects of lidocaine on mouse HCN4 currents are generally consistent with an earlier report of rabbit HCN4 current modulation by lidocaine (Tamura et al., 2009). HCN channel inhibition was observed at concentrations that can be readily achieved during systemic administration of lidocaine (Edvardsson and Olsson, 1987; Estes et al., 1989; Heavner, 2002), whether purposeful or accidental. It is worth noting that effects of lidocaine on HCN channels were observed at physiologically relevant membrane potentials for neurons and cardiac cells; lidocaine inhibited the tonic current component at depolarized potentials and the voltage- and time-dependent component over the entire voltage range of activation. Therefore, these data suggest that inhibition of neuronal Ih or cardiac If currents could contribute to various beneficial and/or untoward systemic actions of local anesthetics, which remain poorly understood.
There have been no previous studies detailing the effects of lidocaine on the multiple HCN channels that could contribute to its systemic actions (e.g., antiarrhythmic, anesthetic-sparing, or perioperative analgesic effects). The HCN family of channels underlie neuronal Ih and cardiac If currents (Biel et al., 1999). The four HCN channel transcripts and proteins are widely and variously distributed throughout the mammalian central nervous system (Santoro et al., 2000) and in cardiac sinoatrial node and Purkinje cells (Moosmang et al., 2001), in which they display distinct but often overlapping patterns of expression: HCN1 and HCN2 have the broadest neuronal distribution, whereas HCN3 and HCN4 expression is more restricted (Santoro et al., 2000). Each subunit makes functional homomeric channels with distinctly different voltage-dependence, kinetics, and/or cyclic nucleotide sensitivity. Our results show that lidocaine inhibits HCN1, HCN2, HCN4, and the HCN1-HCN2 heteromeric channel currents, although the form of modulation is different: lidocaine caused both a hyperpolarizing shift in V1/2 and a decrease in current amplitude for HCN1 and HCN1-HCN2 but only a decrease in current amplitude for HCN2 and HCN4. It is noteworthy that we found previously that inhalational anesthetics also differentially modulate voltage dependence and maximal amplitude of HCN1 and HCN2 channels (Chen et al., 2005b), an effect that could be attributed to difference in intrinsic allosteric inhibition of HCN channel gating that is conferred by distinct C-terminal domains (Wainger et al., 2001; Chen et al., 2005b).
The current study extends previous work on HCN4 (Tamura et al., 2009) and demonstrates that HCN channel inhibition by lidocaine includes HCN1 and HCN2, the two other HCN subunits expressed in cardiomyocytes. These results could thus be relevant for both classic antiarrhythmic actions and cardiotoxic effects of systemic lidocaine (Trujillo and Nolan, 2000; Pinter and Dorian, 2001). For example, it is well known that If plays an important pacemaker role in cardiac cells (DiFrancesco, 1981; Irisawa et al., 1993), and indeed, lidocaine was reported to reduce an inward current activated by hyperpolarization in the rabbit sinoatrial node (Satoh and Hashimoto, 1984). In isolated sinoatrial node cells HCN1, HCN2, and HCN4 channels are known to contribute to cardiac pacemaking activity (Moosmang et al., 2001), whereas in ventricular myocytes, HCN2 and HCN4 subunits are prominently expressed. These If-expressing cardiac cells that are located in regions other than the sinoatrial node (e.g., in atrioventricular tissues) can beat spontaneously and trigger abnormal automaticity (Cerbai et al., 1997; Hoppe and Beuckelmann, 1998a). Moreover, HCN channel expression is reportedly enhanced in extranodal areas under pathophysiological conditions, and it is possible that the corresponding increase in If may contribute to arrhythmogenesis (Hoppe et al., 1998b). Therefore, inhibition by lidocaine of pacemaker current in these extranodal areas may contribute to its well known antiarrhythmic actions. On the other hand, strong block of HCN currents at high doses could induce cardiac toxicity (e.g., by decreasing sinoatrial nodal function and lowering heart rate to dangerous levels).
Also of importance to this study, it has been shown that Ih is a prominent current near resting membrane potential in thalamocortical neurons and cortical pyramidal cells (McCormick and Pape, 1990; Spain et al., 1991). In cortical and hippocampal pyramidal cells, HCN1 and HCN2 expression seems predominant (Santoro et al., 2000), whereas in thalamocortical cells, HCN2 subunits account for the majority of current (Ludwig et al., 2003). We have shown previously that general anesthetics such as propofol and ketamine produce anesthesia partly through inhibition of Ih in thalamocortical circuit neurons (Chen et al., 2009). We therefore suggest that inhibitory actions of local anesthetics on Ih in thalamocortical cells and cortical or hippocampal pyramidal neurons may contribute to their central anesthetic-sparing actions. In addition, because inhibition of HCN channels in sensory neurons can reduce pain sensation (Chaplan et al., 2003), it is possible that anesthetic sparing (DiFazio et al., 1976; Himes et al., 1977; Senturk et al., 2002) or analgesic actions in postoperative or neuropathic contexts (Kingery, 1997; Smith et al., 2004) could be due to effects of systemic local anesthetics on HCN1 or HCN2 subunits expressed in nociceptors (Chaplan et al., 2003).
We did not include the HCN3 subunit in these studies. There is limited expression of HCN3 in central nervous system and little to no expression of HCN3 in the cardiac conduction system or the myocardium (Biel et al., 2009). However, HCN3 transcripts have been detected in heart muscle (Biel et al., 2009), and it remains possible that lidocaine may have some effect on HCN3, which could also contribute to its systemic actions.
Any suggestion of a role for Ih inhibition in the clinical actions of lidocaine presupposes modulation of the channels over a concentration range that is achieved clinically. In this respect, circulating concentrations of lidocaine that produce antiarrhythmic actions are reported to be from 7 to 40 μM (Edvardsson and Olsson, 1987; Estes et al., 1989). However, the plasma concentration for significant systemic actions such as cardiovascular depressive actions can be as high as 120 μg/ml (417 μM) (Heavner, 2002). We showed in the current study that inhibitory effects of lidocaine on HCN channels occur within this concentration range, with IC50 values from 20 to 70 μM. An earlier report suggested an IC50 of ∼274 μM for lidocaine inhibition of homomeric HCN4 current at −70 mV (Tamura et al., 2009). However, that analysis was based on a three-point curve that did not establish a saturating concentration and did not consider the effects on activation kinetics or on maximal or tonic current (Tamura et al., 2009). In this respect, inhibition of tonic current and slowing of current kinetics may be particularly important for the slowly activating HCN4 channel (fast τ >1 s) in rapidly firing heart tissue (∼ 4–5 beats/s in mouse). Moreover, exemplar data in that earlier report (Tamura et al., 2009) shows clear effects on maximal voltage-dependent HCN4 channel currents of only 30 μM lidocaine [see step to −140 mV in Fig. 4, top, in Tamura et al., (2009)], well within expected concentrations for antiarrhythmic actions of lidocaine.
We also found that MEGX, a lidocaine metabolite, inhibited all HCN channel subunits tested in a manner that was qualitatively identical with lidocaine-mediated inhibition. In the liver, lidocaine is nearly completely metabolized by CYP3A4 to MEGX (Wang et al., 2000), a pharmacologically active metabolite that is almost as potent as lidocaine in terms of systemic toxicity (Halkin et al., 1975). Thus, our current results suggest that both lidocaine and its principal metabolite MEGX could contribute to HCN-channel mediated systemic actions of lidocaine.
In conclusion, these data suggest that Ih in neurons and If in cardiomyocytes are likely targets for systemic actions of local anesthetics. The ability of local anesthetics to modulate Ih in sensory and central neurons may contribute to anesthetic-sparing, analgesic, and central nervous system depressive actions, whereas their effects on If in cardiac pacemaker cells may contribute to the antiarrhythmic and cardiovascular toxic actions.
This work was supported by the National Natural Science Foundation of China [Grant 30772076]; the 2009 National Alliance for Research on Schizophrenia and Depression Young Investigator Award Program; and the National Institutes of Health National Institute of General Medical Sciences [Grant GM66181].
Article, publication date, and citation information can be found at http://molpharm.aspetjournals.org.
doi:10.1124/mol.110.070227.
- HCN
- hyperpolarization-activated and cyclic nucleotide-gated
- MEGX
- monoethylglycinexylidide
- HEK
- human embryonic kidney
- I-V
- current-voltage.
Authorship Contributions
Participated in research design: Xia, Liu, Bayliss, and Chen.
Conducted experiments: Meng and Chen.
Contributed new reagents or analytic tools: Bayliss and Chen.
Performed data analysis: Meng and Chen.
Wrote or contributed to the writing of the manuscript: Bayliss and Chen.
References
- Biel M, Ludwig A, Zong X, Hofmann F. (1999) Hyperpolarization-activated cation channels: a multi-gene family. Rev Physiol Biochem Pharmacol 136:165–181 [DOI] [PubMed] [Google Scholar]
- Biel M, Wahl-Schott C, Michalakis S, Zong X. (2009) Hyperpolarization-activated cation channels: from genes to function. Physiol Rev 89:847–885 [DOI] [PubMed] [Google Scholar]
- Brown DL, Ransom DM, Hall JA, Leicht CH, Schroeder DR, Offord KP. (1995) Regional anesthesia and local anesthetic-induced systemic toxicity: seizure frequency and accompanying cardiovascular changes. Anesth Analg 81:321–328 [DOI] [PubMed] [Google Scholar]
- Cerbai E, Pino R, Porciatti F, Sani G, Toscano M, Maccherini M, Giunti G, Mugelli A. (1997) Characterization of the hyperpolarization-activated current, I(f), in ventricular myocytes from human failing heart. Circulation 95:568–571 [DOI] [PubMed] [Google Scholar]
- Chaplan SR, Guo HQ, Lee DH, Luo L, Liu C, Kuei C, Velumian AA, Butler MP, Brown SM, Dubin AE. (2003) Neuronal hyperpolarization-activated pacemaker channels drive neuropathic pain. J Neurosci 23:1169–1178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Shu S, Bayliss DA. (2005a) Suppression of ih contributes to propofol-induced inhibition of mouse cortical pyramidal neurons. J Neurophysiol 94:3872–3883 [DOI] [PubMed] [Google Scholar]
- Chen X, Shu S, Bayliss DA. (2009) HCN1 channel subunits are a molecular substrate for hypnotic actions of ketamine. J Neurosci 29:600–609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Sirois JE, Lei Q, Talley EM, Lynch C, 3rd, Bayliss DA. (2005b) HCN subunit-specific and cAMP-modulated effects of anesthetics on neuronal pacemaker currents. J Neurosci 25:5803–5814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFazio CA, Neiderlehner JR, Burney RG. (1976) The anesthetic potency of lidocaine in the rat. Anesth Analg 55:818–821 [DOI] [PubMed] [Google Scholar]
- DiFrancesco D. (1981) A study of the ionic nature of the pace-maker current in calf Purkinje fibres. J Physiol 314:377–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edvardsson N, Olsson SB. (1987) Clinical value of plasma concentrations of antiarrhythmic drugs. Eur Heart J 8 (Suppl L):83–89 [DOI] [PubMed] [Google Scholar]
- Estes NA, 3rd, Manolis AS, Greenblatt DJ, Garan H, Ruskin JN. (1989) Therapeutic serum lidocaine and metabolite concentrations in patients undergoing electrophysiologic study after discontinuation of intravenous lidocaine infusion. Am Heart J 117:1060–1064 [DOI] [PubMed] [Google Scholar]
- Gaughen CM, Durieux M. (2006) The effect of too much intravenous lidocaine on bispectral index. Anesth Analg 103:1464–1465 [DOI] [PubMed] [Google Scholar]
- Halkin H, Meffin P, Melmon KL, Rowland M. (1975) Influence of congestive heart failure on blood vessels of lidocaine and its active monodeethylated metabolite. Clin Pharmacol Ther 17:669–676 [DOI] [PubMed] [Google Scholar]
- Heavner JE. (2002) Cardiac toxicity of local anesthetics in the intact isolated heart model: a review. Reg Anesth Pain Med 27:545–555 [DOI] [PubMed] [Google Scholar]
- Himes RS, Jr, DiFazio CA, Burney RG. (1977) Effects of lidocaine on the anesthetic requirements for nitrous oxide and halothane. Anesthesiology 47:437–440 [DOI] [PubMed] [Google Scholar]
- Hoppe UC, Beuckelmann DJ. (1998a) Characterization of the hyperpolarization-activated inward current in isolated human atrial myocytes. Cardiovasc Res 38:788–801 [DOI] [PubMed] [Google Scholar]
- Hoppe UC, Jansen E, Südkamp M, Beuckelmann DJ. (1998b) Hyperpolarization-activated inward current in ventricular myocytes from normal and failing human hearts. Circulation 97:55–65 [DOI] [PubMed] [Google Scholar]
- Irisawa H, Brown HF, Giles W. (1993) Cardiac pacemaking in the sinoatrial node. Physiol Rev 73:197–227 [DOI] [PubMed] [Google Scholar]
- Kingery WS. (1997) A critical review of controlled clinical trials for peripheral neuropathic pain and complex regional pain syndromes. Pain 73:123–139 [DOI] [PubMed] [Google Scholar]
- Koller C. (1928) Historical notes on the beginning of local anesthesia. JAMA 90:1742–1743 [Google Scholar]
- Koppert W, Weigand M, Neumann F, Sittl R, Schuettler J, Schmelz M, Hering W. (2004) Perioperative intravenous lidocaine has preventive effects on postoperative pain and morphine consumption after major abdominal surgery. Anesth Analg 98:1050–1055 [DOI] [PubMed] [Google Scholar]
- Ludwig A, Budde T, Stieber J, Moosmang S, Wahl C, Holthoff K, Langebartels A, Wotjak C, Munsch T, Zong X, et al. (2003) Absence epilepsy and sinus dysrhythmia in mice lacking the pacemaker channel HCN2. EMBO J 22:216–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macri V, Accili EA. (2004) Structural elements of instantaneous and slow gating in hyperpolarization-activated cyclic nucleotide-gated channels. J Biol Chem 279:16832–16846 [DOI] [PubMed] [Google Scholar]
- Marcil J, Walczak JS, Guindon J, Ngoc AH, Lu S, Beaulieu P. (2006) Antinociceptive effects of tetrodotoxin (TTX) in rodents. Br J Anaesth 96:761–768 [DOI] [PubMed] [Google Scholar]
- McCormick DA, Pape HC. (1990) Properties of a hyperpolarization-activated cation current and its role in rhythmic oscillation in thalamic relay neurones. J Physiol 431:291–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moosmang S, Stieber J, Zong X, Biel M, Hofmann F, Ludwig A. (2001) Cellular expression and functional characterization of four hyperpolarization-activated pacemaker channels in cardiac and neuronal tissues. Eur J Biochem 268:1646–1652 [DOI] [PubMed] [Google Scholar]
- Narahashi T. (1972) Mechanism of action of tetrodotoxin and saxitoxin on excitable membranes. Fed Proc 31:1124–1132 [PubMed] [Google Scholar]
- Pinter A, Dorian P. (2001) Intravenous antiarrhythmic agents. Curr Opin Cardiol 16:17–22 [DOI] [PubMed] [Google Scholar]
- Proenza C, Angoli D, Agranovich E, Macri V, Accili EA. (2002) Pacemaker channels produce an instantaneous current. J Biol Chem 277:5101–5109 [DOI] [PubMed] [Google Scholar]
- Ragsdale DS, McPhee JC, Scheuer T, Catterall WA. (1994) Molecular determinants of state-dependent block of Na+ channels by local anesthetics. Science 265:1724–1728 [DOI] [PubMed] [Google Scholar]
- Santoro B, Chen S, Luthi A, Pavlidis P, Shumyatsky GP, Tibbs GR, Siegelbaum SA. (2000) Molecular and functional heterogeneity of hyperpolarization-activated pacemaker channels in the mouse CNS. J Neurosci 20:5264–5275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh H, Hashimoto K. (1984) Effect of lidocaine on membrane currents in rabbit sino-atrial node cells. Arch Int Pharmacodyn Ther 270:241–254 [PubMed] [Google Scholar]
- Senturk M, Pembeci K, Menda F, Ozkan T, Gucyetmez B, Tugrul M, Camci E, Akpir K. (2002) Effects of intramuscular administration of lidocaine or bupivacaine on induction and maintenance doses of propofol evaluated by bispectral index. Br J Anaesth 89:849–852 [DOI] [PubMed] [Google Scholar]
- Smith LJ, Bentley E, Shih A, Miller PE. (2004) Systemic lidocaine infusion as an analgesic for intraocular surgery in dogs: a pilot study. Vet Anaesth Analg 31: 53–63 [DOI] [PubMed] [Google Scholar]
- Spain WJ, Schwindt PC, Crill WE. (1991) Post-inhibitory excitation and inhibition in layer V pyramidal neurones from cat sensorimotor cortex. J Physiol 434:609–626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura A, Ogura T, Uemura H, Reien Y, Kishimoto T, Nagai T, Komuro I, Miyazaki M, Nakaya H. (2009) Effects of antiarrhythmic drugs on the hyperpolarization-activated cyclic nucleotide-gated channel current. J Pharmacol Sci 110:150–159 [DOI] [PubMed] [Google Scholar]
- Trujillo TC, Nolan PE. (2000) Antiarrhythmic agents: drug interactions of clinical significance. Drug Saf 23:509–532 [DOI] [PubMed] [Google Scholar]
- Wainger BJ, DeGennaro M, Santoro B, Siegelbaum SA, Tibbs GR. (2001) Molecular mechanism of cAMP modulation of HCN pacemaker channels. Nature 411:805–810 [DOI] [PubMed] [Google Scholar]
- Wang JS, Backman JT, Taavitsainen P, Neuvonen PJ, Kivistö KT. (2000) Involvement of CYP1A2 and CYP3A4 in lidocaine N-deethylation and 3-hydroxylation in humans. Drug Metab Dispos 28:959–965 [PubMed] [Google Scholar]