Abstract
Focal cortical dysplasia (FCD) are associated with neurological disorders and cognitive impairments in humans. Molecular layer ectopia, clusters of misplaced cells in layer I of the neocortex, have been identified in patients with developmental dyslexia and psychomotor retardation. Mouse models of this developmental disorder display behavioral impairments and increased seizure susceptibility. Although there is a correlation between cortical malformations and neurological dysfunction, little is known about the morphological and physiological properties of cells within cortical malformations. In the present study we used electrophysiological and immunocytochemical analyses to examine the distribution of neuronal and non-neuronal cell types within and surrounding layer I neocortical ectopia in NXSMD/EiJ mice. We show that cells within ectopia have membrane properties of both pyramidal and a variety of nonpyramidal cell types, including fast-spiking cells. Immunocytochemical analysis for different interneuronal subtypes demonstrates that ectopia contain nonpyramidal cells immunoreactive for calbindin-D28K (CALB), parvalbumin (PARV), and calretinin (CR). Ectopia also contain astrocytes, positive for glial fibrillary acidic protein (GFAP) and oligodendrocyte precursor cells positive for NG2 proteoglycan (NG2). Lastly, we provide electrophysiological and morphological evidence to demonstrate that cells within ectopia receive input from cells within layers I, upper and deeper II/III, and V and provide outputs to cells within deep layer II/III and layer V, but not layers I and upper II/III. These results indicate that ectopia contain cells of different lineages with diverse morphological and physiological properties, and appear to cause disruptions in local cortical circuitry.
Keywords: FCD, heterotopias, circuitry, electrophysiology, interneuron, hyperexcitabiltiy
1. Introduction
Focal cortical dysplasia are associated with neurological disorders and cognitive impairments in humans (Arnold et al., 1991; Battaglia et al., 1997; Blümcke et al. 2010; Calcagnotto et al., 2005; Chang et al. 2005; Guerrini et al., 2010; Humphreys et al., 1990; Kaufmann and Galaburda, 1989; Kuzniecky et al., 1989; Kuzniecky and Barkovich, 1996; Palmini et al., 1991b; Palmini et al., 1991c; Thom et al., 2004). Heterotopias, focal disruptions during cortical development leading to clusters of misplaced cells in grey matter and/or white matter, are associated with reading disability (Chang et al. 2007; Galaburda and Kemper, 1979; Humphreys et al., 1990; Kaufmann and Galaburda, 1989; McCann etal. 2008) and epilepsy (Aghakhani et al., 2005; Blümcke et al. 2010; Guerrini et al., 2010; Kobayashi et al., 2006; Krsek et al. 2009; Kuzniecky et al., 1989; Kuzniecky and Barkovich, 1996; Sisodiya 2004; Thom et al., 2004). Although there is a clear association between FCDs and neurological dysfunction, in order to determine a causal relationship we must first characterize the cells within the dysplastic tissue and their consequences on cortical function.
Heterotopia have been identified in patients with developmental dyslexia and psychomotor retardation (Caviness et al., 1978; Humphreys et al., 1990; Kaufmann and Galaburda, 1989). Ectopic formations located in layer I of the neocortex, virtually identical to those described in humans, have been characterized in three strains of mice: NZB/BlNJ, BXSB/MpJ, and NXSMD/EiJ (Sherman et al., 1985; Sherman et al., 1987; Sherman et al., 1990). Molecular layer ectopia in these mice contain 50 or more cells, are located in either the somatosensory (NXSMD/EiJ, and NZB/BlNJ) or frontal/motor (BXSB/MpJ) cortices, and occur in 40–85% of mice (Boehm et al., 1996; Denenberg et al., 1991; Gabel and LoTurco, 2001; Sherman et al., 1990). Behaviorally, mice with ectopia are associated with spatial and non-spatial working memory deficits (Balogh et al., 1998; Boehm et al., 1996; Denenberg et al., 1991; Schrott et al., 1993; Spencer et al., 1986), as well as auditory processing deficits (Clark et al., 2000; Frenkel et al., 2000).
It has been shown that cells within molecular layer ectopia have diverse morphological and physiological properties. Morphologically, ectopia in NZB/BlNJ and NXSMD/EiJ mice contain both atypically oriented pyramidal, aspiny and sparsely spiny non-pyramidal cells (Gabel and LoTurco, 2001). Physiologically, cells within layer I ectopia display regular spiking, burst-discharge accommodating (bAc) and burst-discharge non-accommodating (bNac) action potential properties (Gabel and LoTurco, 2001). Despite the previous characterization of neurons within ectopia, a more detailed analysis is needed. For example, cells within ectopia contain GABA-positive non-pyramidal cells (Gabel and LoTurco, 2001), but the classic fast-spiking interneuron subtype (Galarreta and Hestrin, 1999; Kawaguchi, 2001; McCormick et al., 1985); for review see (Connors and Gutnick, 1990) has not yet been identified within ectopia.
Ectopia in humans contain both neurons and glia (Caviness et al., 1978; Humphreys et al., 1990; Kaufmann and Galaburda, 1989), but histological studies have not yet determined if ectopia in animal models contain similar cell types. Furthermore, examination of the distribution of different cell types within and surrounding the malformation may provide some insight as to possible mechanisms of cortical dysfunction. For example, increased excitability in animal models cortical maldevelopment, such as 4-layered microgyria and tish animal models, has been correlated with a decrease in parvalbumin immunoreactivity within the epileptogenic region surrounding the malformation (Rosen et al., 1998), concomitant with a reduction in spontaneous inhibitory post synaptic current amplitude and in GABAergic terminals surrounding pyramidal cells was reported in the tish cortex and normotopic cortex (Trotter et al. 2006). We previously reported that slices containing ectopia display an increased level of excitability (Gabel and LoTurco, 2002), but the expression of parvalbumin, and other calcium binding proteins present in nonpyramidal cells, has not yet been examined within or surrounding molecular layer ectopia.
In the present study we used electrophysiological and immunocytochemical analyses to examine the distribution of neuronal and non-neuronal cell types within and surrounding layer I neocortical ectopia in NXSMD/EiJ mice. We show that cells within ectopia have membrane properties of both pyramidal and a variety of non-pyramidal cell types, including fast-spiking cells. Immunocytochemical analysis for different interneuronal subtypes demonstrates that ectopia contain nonpyramidal cells immunoreactive for calbindin-D28K (CALB), parvalbumin (PARV), and calretinin (CR). Ectopia also contain astrocytes, positive for glial fibrillary acidic protein (GFAP) and oligodendrocyte precursor cells positive for NG2 proteoglycan (NG2). Lastly, we provide electrophysiological and morphological evidence to demonstrate that cells within ectopia receive input from cells within layers I, upper and deeper II/III, and V and provide outputs to cells within deep layer II/III and layer V, but not layers I and upper II/III. These results indicate that layer I ectopia contain cells of different lineages with diverse morphological and physiological properties. Furthermore molecular layer ectopia appear to cause disruptions in local cortical circuitry.
2. Results
Ectopia
Molecular layer ectopia, ectopic collections of cells located in layer I of the neocortex, were identified in 300 µm free-floating sections under oblique illumination using a dissecting microscope. In this study, at least one molecular layer ectopia was identified in, 61.5 % (40/65) of all NXSMD/EiJ mice; 66.7% (32/48) of male and 47.1% (8/17) of female NXSMD/EiJ mice. In the majority of mice only one ectopia was present per animal, with the highest rate of occurrence within the somatosensory cortex (82.5%, 33/40), although ectopia were occasionally identified within the frontal cortex (17.5%, 7/40). Multiple ectopia were present in 10% (4/40) of the brains examined, with only 1 of the 4 brains containing two ectopia within the same hemisphere of a 300 µm section. Multiple ectopia predominately occurred in male NXSMD/EiJ mice (75%, 3/4) and all were identified within the somatosensory cortex.
Cells Within Ectopia Display Diverse Membrane Properties
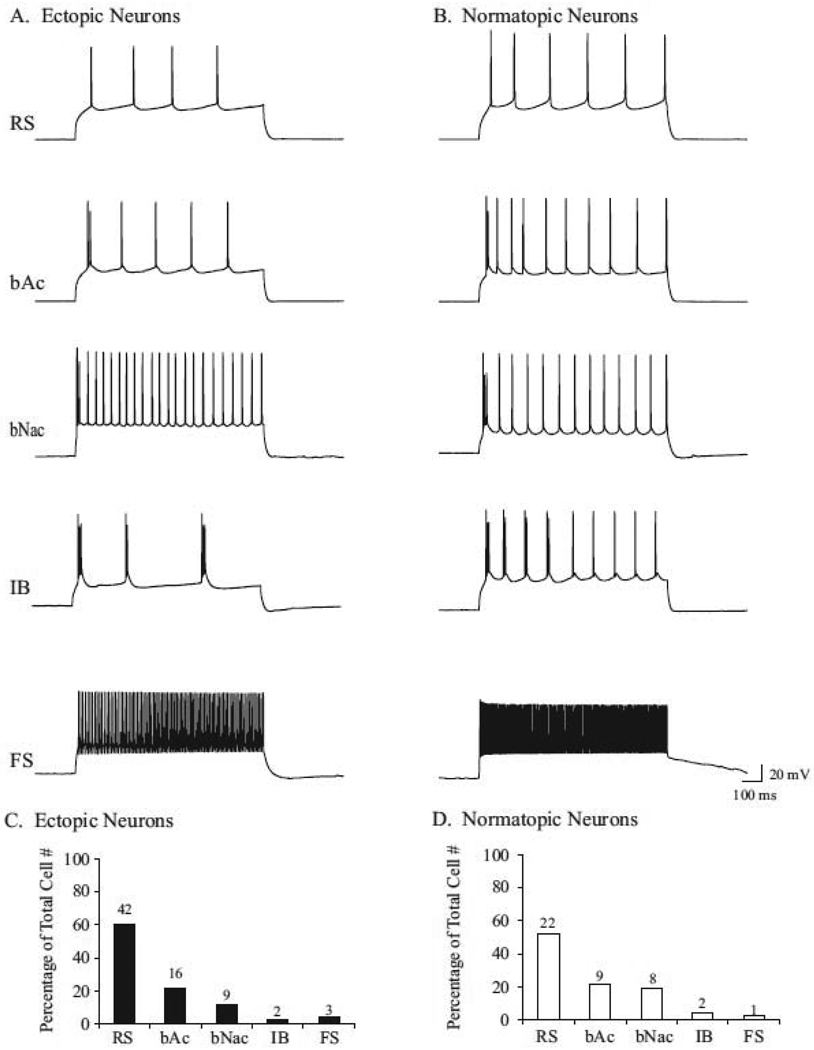
Whole cell recordings were made from 121 neurons in slices containing ectopia in order to examine the membrane properties of cells within and surrounding ectopia. Following depolarizing current injections (900 ms in duration), cells within and surrounding ectopia exhibit a variety of different firing patterns including regular spiking (RS), burst-discharge accommodating (bAc), burst-discharge non-accommodating (bNac), intrinsically bursting (IB), and fast-spiking (FS) action potential patterns. Out of 76 recordings of cells within ectopia a variety of action potential properties were identified with varying degrees of frequency. The majority of cells were RS (60.6%, 46/76), followed by bAc (21.1%, 16/76), bNac (11.8%, 9/76), IB (2.6%, 2/76), and FS cells (3.9%, 3/76; Fig. 1 A & C). Similarly, cells within layers I–V of the normotopic cortex exhibited a variety of action potential properties. The majority of cells were RS (52.4%, 22/42), followed by bAc (21.4% ,9/42), bNac (19%, 8/42), IB (4.8%, 2/42), and FS cells (2.4%, 1/42; Fig. 1 B & D). Together these results suggest that ectopia contain neurons with diverse membrane properties; similar to firing patterns exhibited by neurons within the surrounding normotopic cortex.
Figure 1.
Neurons within and surrounding ectopia exhibit diverse firing patterns. Neurons within ectopia (A) and the normotopic cortex (B) exhibit regular spiking (RS), burst-discharge accommodating (bAc), burst-discharge non-accommodating (bNac), intrinsically bursting (IB), and fast spiking (FS) action potential patterns. (C & D) Percentage of each type of firing pattern identified out of the total number of cells recorded within (C), or surrounding (D) ectopia.
Ectopia contain a variety of neuronal and glial cell types
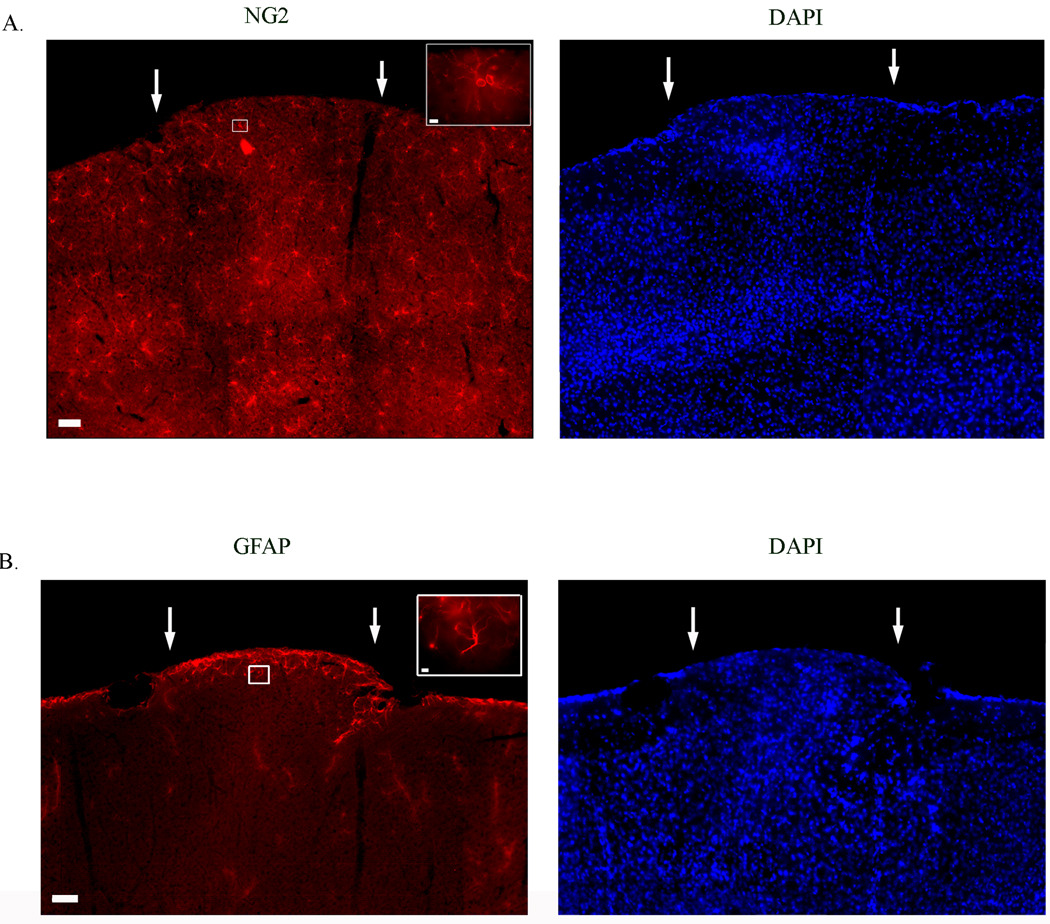
To determine the cellular constituents of layer I neocortical ectopia we performed immunohistochemical analysis of astrocytes (GFAP), oligodendrocyte precursor cells (NG2), pyramidal neurons and a variety of interneuron subtypes by examining the immunoreactive of cells for different calcium binding proteins (i.e. CALB, CR, PARV). Ectopia contain both NG2- (Fig. 2A) and GFAP- (Fig. 2B) positive cells, indicating that ectopia contain both astrocytes and oligodendrocyte precursor cells. NG2+ cells are uniformly distributed in all cortical layers of slices containing ectopia, and appear to be distributed without regard to the boundaries of the ectopia (Fig. 2A). GFAP positive cells are located throughout the cortex, with the greatest concentration of GFAP+ cells localized to the pial surface and the white matter (not shown) of the surrounding cortex. Within ectopia there is a higher concentration of GFAP+ cells located at the pial surface and at the superficial borders of ectopia, but there are no GFAP+ present below the superficial extent of the ectopia (Fig. 2B). These data suggest that ectopia contain differentially distributed astrocytes and oligodendrocyte precursor cells.
Figure 2.
Expression patterns on non-neuronal cells in slices containing layer I neocortical ectopia. (A & B) DAPI, a nuclear marker, expression patterns reveals the cluster of misplaced cells in layer I of the cortex, ectopia (arrows). (A) Expression pattern of oligodendrocyte precursor cells positive for NG2 proteoglycan. (B) Examples of astrocyte expression using an antibody against GFAP. Scale bars A & B = 50 µm; Inset = 5 µm.
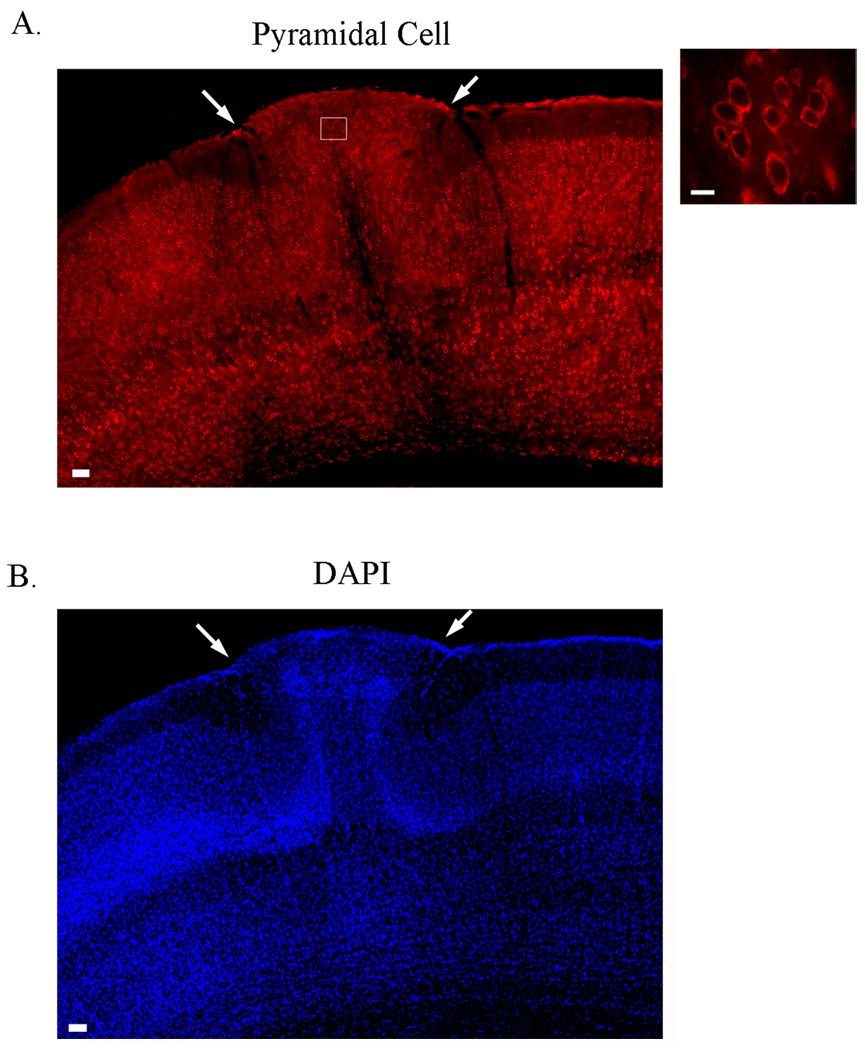
Postmortem analysis of cells immunopositive for the pyramidal cell marker within ectopia (n = 96) and within normotopic cortex (n = 119 within layer II/III ; n= 111 within layers V and VI) demonstrate a range of soma sizes within the ectopia (4.791 – 17.172 microns); comparable to cells in layer II/III (4.545– 13.657 microns) and infragranular layers (5.917 – 16.735 microns), with a median value of 8.6745 microns, 9.122 microns and 10.633 microns, respectively (Fig. 3). Both large and small pyramidal neurons located within ectopia can be atypically oriented, with some pyramidal cells being completely inverted. These results suggest that abnormally oriented large and small pyramidal cells are distributed throughout ectopia.
Figure 3.
Ectopia contain pyramidal cells. (A) Expression pattern of cells immunoreactive for pyramidal cells within ectopia. Both large and small abnormally oriented pyramidal shaped cells are distributed throughout ectopia. (B) DAPI expression patterns shows the cluster of misplaced cells in layer I of the cortex, ectopia (arrows). Scale bars A = 50 µm; Inset = 10 µm.
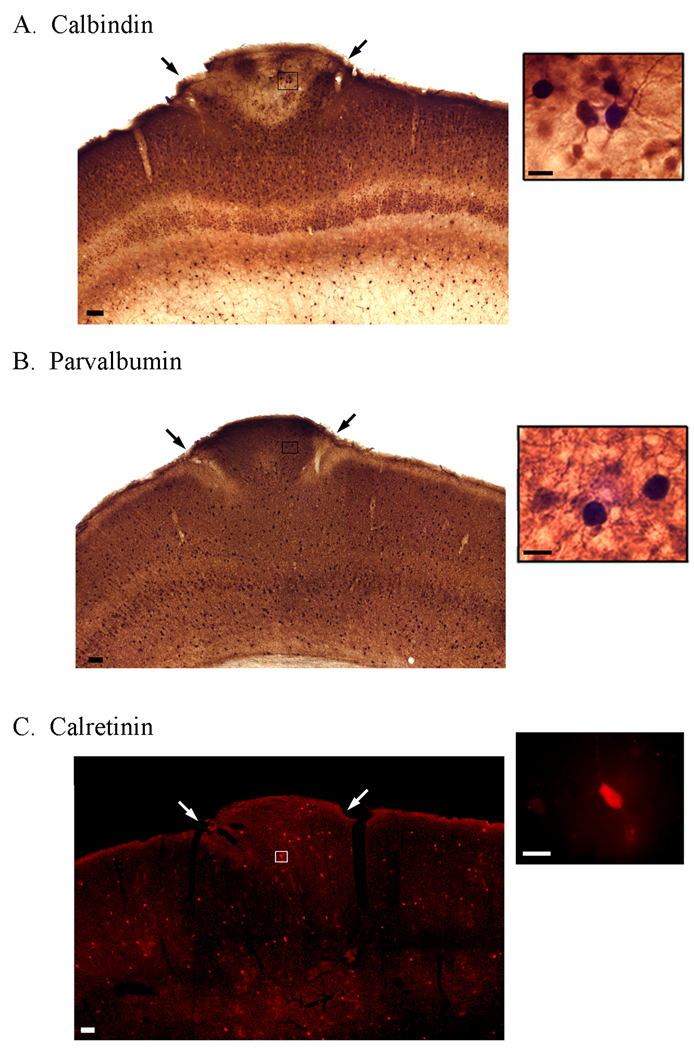
Nonpyramidal cells were identified within ectopia using antibodies that recognize calbindin (CALB), parvalbumin (PARV) and calretinin (CR). Immunohistochemical analysis demonstrated that cells within ectopia are immunopositive for CALB, PARV, and CR subtypes (Fig. 4). Within the normotopic cortex surrounding ectopia supragranular layer pyramidal cells, and infragranular layer nonpyramidal cells are CALB+. Within ectopia CALB+ pyramidal cells appear to be localized to the base of ectopia, whereas non-pyramidal CALB+ cells are localized near the top of the malformation (Fig. 4A). PARV+ cells are located in all cortical layers below layer I within the normotopic cortex, and are distributed throughout ectopia (Fig. 4B). CR+ cells are sparsely distributed throughout all cortical layers within the normotopic cortex, and throughout ectopia (Fig. 4C). These results demonstrate that cells within ectopia contain CALB, PARV, and CR positive nonpyramidal cells.
Figure 4.
Ectopia contain a variety of interneuron subtypes. Expression patterns of neurons immunoreactive for CALB (A), PARV (B), and CR (C) within slices containing ectopia (arrows). Scale bars A–C = 50 µm; Insets = 10 µm.
Local cortical circuitry in slices containing ectopia
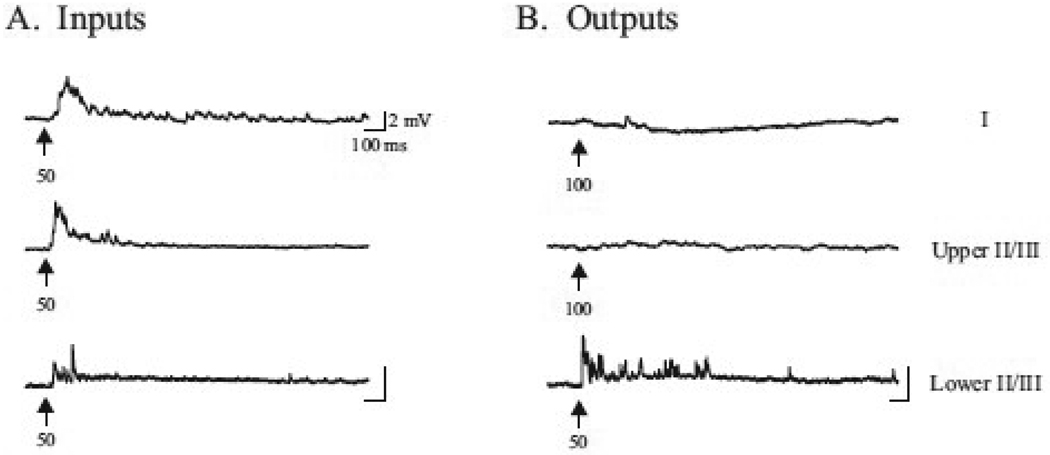
Whole cell recordings were made either within ectopia (inputs) or within the normotopic cortex surrounding ectopia (outputs) while simultaneously applying 1 mM glutamate for brief periods of time (5–300 ms) either within ectopia (outputs), within the normotopic cortex surrounding ectopia (inputs). On average, a positive response was elicited with glutamate applications that lasted 71.5 ± 12.1 ms in duration, with a mode of 40 ms. Positive responses within ectopia were recorded when local application of glutamate was applied for 50 ms within layers I and upper and deeper II/III (fig. 5A). Positive responses were also evident when glutamate was applied to layer V (data not shown). Fig 5A provides examples of recordings made within layers I–III, when the glutamate pipette was placed within ectopia. Positive responses were only elicited in cells located in deep layer II/III (Fig. 5A) and layer V (data not shown), following application of 1 mM glutamate, but never in layer I or upper II/III adjacent to ectopia. Figure 5B demonstrates the percentage of positive and negative responses elicited when examining inputs and outputs between cells within ectopia and cells within layers I–V in the surrounding normotopic cortex.
Figure 5.
Intracortical synaptic inputs and outputs within neocortical ectopia. Cells within ectopia receive input from cells within layers I, II/III and V in the normotopic cortex, but outputs are only made to cells in deep layer II/III and V. (A) Whole cell recordings were made from neurons within ectopia at holding potentials of −80mV, while locally applying 1 mM glutamate for brief periods of time (arrow, time in ms) either in layers I, upper and deeper II/III, or layer V (data not shown). Fast rising post-synaptic responses are evident due to glutamate application in layers I, II/III, and V (data not shown). (B) Whole cell recordings were made from neurons in layers I, upper and deeper II/III, or layer V (data not shown) of the normotopic cortex at holding potentials of −80mV, while locally applying 1 mM glutamate for brief periods of time (arrow, time in ms) within ectopia. Fast-rising post-synaptic responses are only evident in deep layer II/III, and layer V (data not shown) with application of glutamate in ectopia.
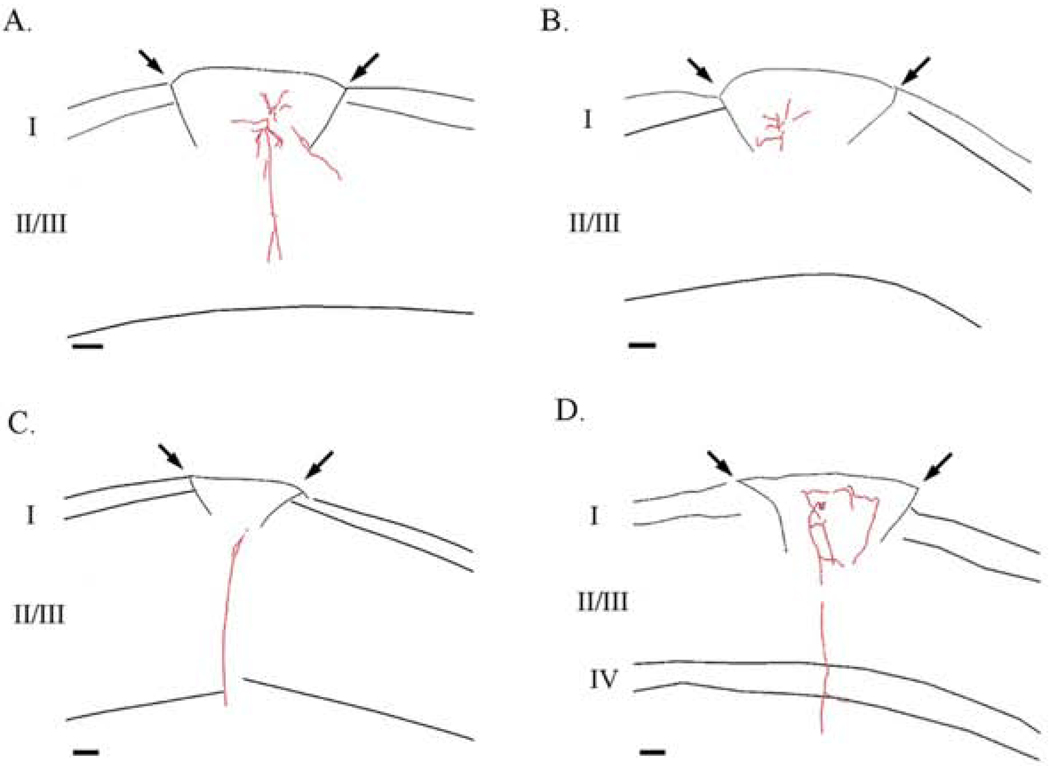
Consistent with electrophysiological data, camera lucida reconstructions of axons of cells within ectopia demonstrates that cells within ectopia send axons vertically through the base of the malformation (Fig. 6 A, C, and D), but do not appear to send axons horizontally to layers I and upper II/III (Fig. 6 A, B, and D). Furthermore, axons of cells within ectopia appear to turn when they reach the borders of the ectopia (Fig. 6 B and D). Together these data suggest that cells within ectopia can receive input from cells in all cortical layers, but may only make reciprocal connections with cells in deep layer II/III and layer V below the malformation.
Figure 6.
Properties of axons within ectopia. (A–D) Camera lucida reconstructions of the axons of neurons within ectopia. The axon of ectopic neurons can project vertically through the base of ectopia (A, C, & D), but axons of ectopic neurons turn at the superficial borders of the ectopia (B & D).
3. Discussion
Constituent cells within ectopia
In previous reports we demonstrated that neurons within ectopia can be identified as either atypically oriented spiny pyramidal cells, or aspiny and sparsely spiny nonpyramidal cells which display RS and irregular spiking (bAc and bNac) action potential properties (Gabel and LoTurco, 2001). Here we show that cells within ectopia exhibit RS and irregular spiking firing patterns, as well as FS and IB firing properties. It is important to note, that although cells were randomly selected for recording, the high percentage of RS cells reflect the accessibility of these cells for recording, rather than indicating that there are quantitatively more RS cells within an ectopia. Within the normotopic cortex of rodents pyramidal cells display RS and IB firing patterns, and non-pyramidal cells exhibit FS and irregular spiking action potential properties (Landry et al., 1984; McCormick et al., 1985; Agmon et al., 1989; Chagnac-Amitai et al., 1989; Kawaguchi et al. 1993, Kawaguchi and Kubota, 1993; Cauli et al., 1997; Gupta et al., 2000; for review see Connors and Gutnick, 1990). Consistent with physiological characteristics of cells within ectopia, and reports of heterotopia in patients with epilepsy (Thom et al. 2004), ectopic cells have morphologies of pyramidal and a variety of non pyramidal cells based on their immunoreactivity with antibodies against pyramidal cells and their differential expression of CALB, PARV and CR. Within the normotopic cortex of rodents firing properties and expression of calcium binding proteins can be correlated to define subpopulations of nonpyramidal cells. For example, FS nonpyramidal cells are primarily immunoreactive for PARV (Cauli et al., 1997; Kawaguchi and Kubota, 1993) irregular bursting cells are predominately CR positive (Cauli et al., 1997) and CALB positive cells are both regular spiking pyramidal cells of layers II/III (Cauli et al., 1997; for review see DeFelipe, 1997), and regular spiking non-pyramidal cells of layers II/III and V (Cauli et al., 1997).
The distribution of both pyramidal and CALB positive cells suggests that ectopia may contain cells from both supragranular and infragranular layers. For example, ectopia contain pyramidal cells with soma sizes ranging from 4.791 – 17.172 µm in diameter. The sizes of pyramidal cells within ectopia are similar to small pyramidal cells within layer II/III and large pyramidal cells within layer V of the normotopic cortex (for review see Cajal, 1995). Furthermore, CALB immunoreactivity is present in pyramidal cells within layer II/III, as well as in non-pyramidal neurons in infragranular layers (Cauli et al., 1997; for review see DeFelipe, 1997), and both CALB positive pyramidal and nonpyramidal cells are located within ectopia. In order confirm that ectopia contain cells from all cortical layers birthdating studies, using an S phase marker at different stages during neurogenesis, need to be performed. Perhaps these studies will not only confirm these results, but also demonstrate an overall organization of cells within ectopia. Together histological and electrophysiological findings suggest that cells within ectopia have similar morphological and physiological characteristics to cells located within supragranular and infragranular layers of the normotopic cortex.
Disruptions in intracortical circuitry within slices containing ectopia
Previously, using electrophysiological and histochemical analysis of cells within and surrounding ectopia, we demonstrated that cells within ectopia receive input from the normotopic cortex surrounding the malformation (Gabel and LoTurco, 2001). Studies using anterograde and retrograde tracers have shown the borders of ectopia restrict projections from ectopia (Jenner et al., 2000). Here we examined the direct connections between cells within ectopia and cells within layers I, upper and deeper II/III, and V by applying 1 mM glutamate to sites within (outputs) and surrounding (inputs) ectopia. Using this method we showed that neurons within ectopia are reciprocally connected with cells in deep layer II/III and layer V, but connections between cells within ectopia and upper layer II/III and layer V are restricted to inputs into ectopia. Consistent with electrophysiological data, camera lucida reconstructions of cells within ectopia demonstrate that axons are restricted by the superficial borders of ectopia, but are able to project out near the base of the malformation. It is important to note that only a small percentage of neurons (~8% of all recorded cells) were reconstructed using camera lucida, therefore further examination of neurons within and surrounding the malformation may reveal a more complex local circuitry between normotopic and ectopic neurons.
Within the somatosensory cortex of rodents neurons within layers I–III project to layer V, make horizontally connections with other neurons within the layer, and receive input from layers V and VI (Akers and Killackey, 1978; Cauller et al., 1998; Chapin et al., 1987; Fink and Heimer, 1967; Fonseca et al., 1988; Lapenko and Podladchikova, 1983). Layer V pyramidal neurons project to layer VI, as well as horizontally to other layer V neurons (Lapenko and Podladchikova, 1983). In other models of cortical malformations, the specificity of cortical connections is maintained even after disruptions in cortical lamination occur (Caviness, 1976; Caviness and Frost, 1983; Simmons et al., 1982; Yorke and Caviness, 1975). More specifically, heterotopia composed of neocortical neurons located in CA1 of the hippocampus still maintain neocortical connections despite their location in the hippocampus (Chevassus-au-Louis et al., 1998; Tschuluun et al. 2005). Consistent with these results, misplaced pyramidal and nonpyramidal cells in layer I of the neocortex receive input from neurons within layers I, II/III, and V, however they appear to only send projections to deep layer II/III and layer V neurons with axon projecting through the base of the malformation. Ultrastructural analysis of ectopia showed that pericyte septa (thin invaginations of an intact pia) are located at the superficial border of ectopia (i.e. the dorsal aspect of the ectopia) and seem to provide a boundary between ectopia and the normotopic cortex (personal communication with Dr. AmyJo Stavnezer, The College of Wooster, Wooster, OH). It is possible that axons within ectopia are unable to project across this border resulting in the restricted output of ectopic neurons through the ventral surface of the malformation.
Relationship to other animal models with cortical dysplasias
Cortical dysplasia, such as subcortical band heterotopias, nodular heterotopias, and poly-microgyria, are associated with epilepsy in humans (Andrade 2009; Leventer et al. 2008; Meencke and Janz 1984; Palmini, 1991b; Palmini, 1991c; Fusco, 1992; Meencke and Veith 1992; Chugani, 1993,). Similarly, animal models of cortical malformations display increased seizure susceptibility in vivo and in vitro (Chen et al., 2000; Jacobs et al., 1999; Lee et al., 1997; Luhmann and Raabe, 1996; Sarkisian et al., 1999). Previously we showed that mice with spontaneously developing ectopia also exhibit increased seizure susceptibility in vivo and in vitro (Gabel and LoTurco, 2002), but mechanisms underlying increased excitability in slices containing ectopia remain unclear. In other animal models of cortical dysplasia imbalances between excitation and inhibition and disruptions in cortical circuitry have been suggested to underlie increased excitability.
A reduction in the number of PARV positive interneurons (Zhou & Roper 2010; Roper et al., 1999; Rosen et al., 1998; Sarkisian et al., 2001), a down regulation of GABA receptors, and an increase in NMDA and AMPA receptors (Babb et al., 2000; DeFazio and Hablitz, 2000; Luhmann et al., 1998; Redecker et al., 2000) have been shown to occur in models of cortical dysplasia and humans with FCD (Andrade 2009; Spreafico et al. 1998). In contrast, preliminary examination of the density of PARV+ cells in slices containing ectopia did not show a significant change compared to neurons in the opposite hemisphere (data not shown). Previously reported electrophysiological data also did not suggest an increase, or decrease in NMDA, AMPA, or GABAA receptor–mediated events within ectopia, compared to normotopic cortical cells (Gabel and LoTurco, 2001). However, synaptic properties of neurons surrounding ectopia have not yet been performed.
Disruptions in cortical and subcortical circuitry within the dysplastic cortex have also been suggested to cause increased excitability in animal models of cortical malformations. For example, callosal and thalamic afferents made to missing lamina within the dysplastic cortex of microgyric rats were re-routed to the paramicrogyrial cortex surrounding the malformation (Rosen et al., 1989; Rosen et al., 2000). This disruption was suggested to lead to an increase in strength of excitatory connections within the paramicrogyrial zone thus causing increased excitability within this region (Jacobs et al., 1999). Similarly, examination of the cortical circuitry in animal models of heterotopia indicated that ectopia are aberrantly connected with the surrounding neocortex overlying the heterotopia (Ackman et al. 2009) as well as thalamus (Jenner et al., 2000); receive input from subcortical structures (Jenner et al., 2000), and extend axons along axon fascicles underlying ectopia (Gabel and LoTurco, 2001; Jenner et al., 2000) which terminate in cortical areas ipsilateral to ectopia (Jenner et al., 2000). Here we show that ectopia in layer I of the neocortex receive inputs from neurons in layer I, II/III and V, but outputs from ectopia to neurons in layers I and upper II/III appear to be inhibited. Together, the data suggests that molecular layer ectopia contain a variety of cell types indicative of cells from multiple layers, resulting in disruptions in intracortical and subcortical circuitry, which may lead to increased excitability in vivo and in vitro in mice with ectopia.
4. Experimental Procedure
Preparation of Brain Slices for Whole-cell Recordings
Sixty-five adult male and female NXSMD/EiJ mice (68 to 272 days postnatal) were deeply anesthetized with halothane and decapitated. Following decapitation, the brains were rapidly removed from the skull and immersed in an ice-cold, oxygenated sucrose solution containing (concentrations in mM): Sucrose 124.0, KCl 5.0, MgCl2 2.0, NaH2PO4 (2• H2O) 1.23, NaHCO3 23.8, CaCl2 2.0; pH = 7.4. The brains were blocked and 300 µm thick coronal serial sections were cut using a Vibroslice (Campden Instruments, London, England). Slices were transferred to a petri dish containing ice-cold, oxygenated artificial cerebral spinal fluid (aCSF) and examined with oblique illumination under a dissecting microscope to identify slices containing ectopia (Fig. 1A & B). aCSF contained (concentrations in mM): NaCl 124.0, KCl 5.0, MgCl2 2.0, NaH2PO4 (2• H2O) 1.23, NaHCO3 23.8, CaCl2 2.0; pH = 7.2, osmolarity = 300 ± 5 mOsm/liter. Slices selected for recording were affixed to 18 mm circular microscope cover slips (Fisher Scientific, Pittsburgh, PA) with plasma/thrombin clots. Slices were maintained at room temperature (20 22° C) in aCSF for ≥1 h in an oxygenated holding chamber before recording began. Prior to whole-cell recording, the 18 mm circular microscope cover slips containing slices were mounted onto the stage of a Nikon Eclipse E-600 FN microscope. Recordings were performed at room temperature (RT, 20 – 22° C).
Whole-cell Recordings
Whole cell recordings were made from cells within and surrounding neocortical ectopia in the somatosensory cortex. Slices were visualized, and neurons were targeted using infrared differential interference contrast (IR-DIC) microscopy. Electrodes were pulled from capillary tubing (Garner Glass N51A, Garner Glass Co., Claremont, CA) using a Narishige multi-step electrode puller (Model PP-830) and had resistances of ~ 10 MΩ. The intracellular solution contained (concentrations in mM): Potassium Gluconate 120.0, HEPES 10.0, KCl 1.0, MgCl2 1.0; the pH was adjusted to 7.4 ± 0.05 with HCl 5.0 N and osmolarity was adjusted to 275 ± 5 mOsm/liter. Current-clamp recordings were made using an Axon Instruments 200B amplifier (Axon Instruments Inc., Foster City, CA), and low pass filtered at 1 kHz. Currents were digitally sampled at 10 kHz and monitored with pCLAMP 8.0 software (Axon Instruments Inc., Foster City, CA) running on a PC pentium computer.
Identification of inputs and outputs
In order to examine the local circuitry between cells within ectopia and the surrounding cortex, we used a picospritzer (Picospritzer II, General Valve Corporation) to locally apply 1 mM glutamate dissolved in aCSF for brief periods of time (5–200 ms) to either the inside of ectopia (outputs) or within layers I, upper and deeper II/III, or V surrounding (inputs) the malformation. Glutamate electrodes, made from glass micropipettes (~5 MΩ), were pulled from capillary tubing (Garner Glass) using a Narishige multi-step electrode puller (Model PP-830). Whole cell recordings were made within layers I, upper and deeper II/III, or V surrounding (outputs), or inside (inputs) ectopia. Upper and lower II/III were identified based on pyramidal cell soma size using IR-DIC microscopy. Following a 300 ms baseline recording a brief pulse (5–300 ms) of 1 mM glutamate was locally applied either within (outputs) or surrounding (inputs) ectopia and the response of the cell to the application of glutamate was monitored for durations of 1–2 s. A positive response was defined as either a fast-rising, complex post-synaptic potential, or an increase in the frequency of post-synaptic potentials that occurred due to the application of glutamate. A negative response was defined as no change in the spontaneous post-synaptic activity following the application of glutamate. Glutamate was applied for increasing durations until a positive response was elicited or until glutamate was applied for a maximum duration of 300 ms. Post-synaptic potentials (PSPs) were identified using Clampfit 8 software (Axon Instruments).
Histological procedures
In all recordings Biocytin (Sigma-Aldrich, St. Louis, MO), ~1–2%, was included in the internal pipette solution. Following electrophysiological recording, slices were fixed in 4% paraformaldehyde for at least 24 hours at 4°C. The tissue was rinsed in phosphate buffered saline (PBS) and sections were treated in 0.5% H2O2 in PBS for 20–30 minutes at room temperature (RT) to eliminate endogenous peroxidase activity. Sections were then permeabilized and blocked with 0.2% Triton X-100 and 1% Normal Goat Serum (NGS) in PBS overnight at 4°C. Tissue was washed in PBS, and then incubated with VECTISTAIN elite ABC reagent (Vector Laboratories, Burlingame, CA) overnight at 4°C. The tissue was washed in PBS and then reacted with diaminobenzidine tetrahydrochloride substrate (DAB) (Vector Laboratories) for 10–15 min at RT. Sections were rinsed in PBS and cleared with 100% glycerol for viewing with a Nikon Eclipse E-400 microscope. In some cases camera lucida reconstructions of axons were performed using a Nikon Eclipse microscope and Neuralucida and Neuralexplorer software (MicroBrightfield Inc., Colchester, VT).
For immunocytochemical analysis, adult NXSMD/EiJ male and female mice were transcardially perfused with PBS followed by 4% paraformaldehyde. Brains were post-fixed overnight at 4°C, blocked in 1.5 – 1.9 % agar, and sectioned in the coronal plane on a vibratome, and free floating sections (50 µm) were collected. Standard immunocytochemical procedures were used. Sections were incubated in the following primary antibodies: mouse anti-GFAP (1:500) (Sigma-Aldrich), rabbit anti-NG2 (1:2000) (generous gift from A. Nishiyama), mouse anti-calretinin (1:50) (Chemicon, Temecula,CA) mouse anti-calbindin D28K (1:1000) (Sigma-Aldrich), mouse anti-parvalbumin (1:1000, Chemicon), or mouse anti-pyramidal cell (1:1000, catalog #345, Swant Swiss Antibodies, Bellinoza, Switzerland) in 0.1% Triton X-100, 2.5% NGS, and PBS at 4°C for 40 hours. Sections were rinsed several times with 2.5% NGS in PBS and then incubated in Alexa Fluor 594 goat-anti-mouse or goat-anti-rabbit (1:200) (Molecular Probes, Eugene, OR), or biotinylated goat-anti-mouse IgG (1:200) (Vector Laboratories) secondary antibody for 2 hours at RT. Nuclei in some sections were labeled with 4,6-diamino-2-phenylindole (DAPI) (1:50000). Sections for florescence were collected onto gelatin-coated slides, and coverslipped with Prolong Antifade mounting media (Molecular Probes). Sections incubated in biotinylated secondary antibodies were incubated in avidin and biotinylated horseradish peroxidase mixture for 1 hour at RT. The tissue was rinsed in PBS and then reacted with DAB. These sections were collected onto gelatin-coated slides, dried for several hours and coverslipped with Cytoseal. Images were collected on a Nikon Eclipse E400 Epi-fluorescence microscope (Tokyo, Japan) equipped with a digital Spot Camera (Diagnostic Instruments, USA).
Characterization of pyramidal cell soma size was conducted following immunostaining procedures using the pyramidal cell marker. Overlapping images of the ectopia and normotopic cortex were taken using a Nikon Eclipse E400 Epi-fluorescence microscope (Tokyo, Japan) equipped with a digital Spot Camera (Diagnostic Instruments, USA), reconstructed using Photoshop software. Soma width within the upper layers (layer II/III extent) and deep layers (layers V and VI) of the cortex, as well as within the malformation, were analyzed using ImageJ freeware (rsbweb.nih.gov/ij/). Soma width was measured in pyramidal cells located within as well as medial and lateral to the molecular layer ectopia.
Table 1.
Intracortical synaptic inputs and outputs within slices containing ectopia
| Cortical Layer | Inputs | Outputs | |
|---|---|---|---|
| I | 33% (3) | 0% (3) | |
| II/III | Upper II/III | 0% (6) | |
| 67% (6) | |||
| Lower II/III | 71% (7) | ||
| IV | 100% (5) | ||
| V | 50% (6) | 50% (10) | |
Note* Data represent percent of positive inputs and outputs between cells within ectopia and cells within layers I, upper and deeper II/III, and V. The number of cases per condition is indicated in parentheses (n).
Acknowledgements
I would like to thank Joe LoTurco, Ph.D., Physiology & Neurobiology Department, University of Connecticut, Storrs, CT for guidance and support provided during the completion of this work. This work was supported by NIH grant HD020806.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ackman JB, Aniksztejn L, Crepel V, Becq H, Pellegrino C, Cardoso C, Ben-Ari Y, Represa A. Abnormal network activity in a targeted genetic model of human double cortex. J Neurosci. 2009;29:313–327. doi: 10.1523/JNEUROSCI.4093-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aghakhani Y, Kinay D, Gotman J, Soualmi L, Andermann F, Olivier A, Dubeau F. The role of periventricular nodular heterotopia in epileptogenesis. Brain. 2005;128:641–651. doi: 10.1093/brain/awh388. [DOI] [PubMed] [Google Scholar]
- Agmon A, Connors B. Repetitive burst-firing neurons in the deep layers of mouse somatosensory cortex. Neurosci Lett. 1989;99:137–141. doi: 10.1016/0304-3940(89)90278-4. [DOI] [PubMed] [Google Scholar]
- Akers R, Killackey H. Organization of corticocortical connections in the parietal cortex of the rat. J Comp Neurol. 1978;181:513–537. doi: 10.1002/cne.901810305. [DOI] [PubMed] [Google Scholar]
- Andrade DM. Genetic basis in epilepsies caused by malformations of cortical development and in those with structurally normal brain. Hum Genet. 2009;126:173–193. doi: 10.1007/s00439-009-0702-1. [DOI] [PubMed] [Google Scholar]
- Arnold S, Hyman B, Van Hoesen G, Damasio A. Some cytoarchitectural abnormalities of the entorhinal cortex in schizophrenia. Arch Gen Psychiatry. 1991;48:625–632. doi: 10.1001/archpsyc.1991.01810310043008. [DOI] [PubMed] [Google Scholar]
- Babb T, Ying Z, Mikuni N, Nishiyama K, Drazba J, Bingaman W, Wyllie E, Wylie C, Yacubova K. Brain plasticity and cellular mechanisms of epileptogenesis in human and experimental cortical dysplasia. Epilepsia. 2000;41 Suppl 6:S76–S81. doi: 10.1111/j.1528-1157.2000.tb01561.x. [DOI] [PubMed] [Google Scholar]
- Balogh S, Sherman G, Hyde L, Denenberg V. Effects of neocortical ectopias upon the acquisition and retention of a non-spatial reference memory task in BXSB mice. Brain Res Dev Brain Res. 1998;111:291–293. doi: 10.1016/s0165-3806(98)00138-2. [DOI] [PubMed] [Google Scholar]
- Battaglia G, Granata T, Farina L, D'Incerti L, Franceschetti S, Avanzini G. Periventricular nodular heterotopia: epileptogenic findings. Epilepsia. 1997;38:1173–1182. doi: 10.1111/j.1528-1157.1997.tb01213.x. [DOI] [PubMed] [Google Scholar]
- Battaglia G, Franceschetti S, Chiapparini L, Freri E, Bassanini S, Giavazzi A, Finardi A, Taroni F, Granata T. Electroencephalographic recordings of focal seizures in patients affected by periventricular nodular heterotopia: role of the heterotopic nodules in the genesis of epileptic discharges. J Child Neurol. 2005;20:369–377. doi: 10.1177/08830738050200041701. [DOI] [PubMed] [Google Scholar]
- Blümcke I, pieper T, Pauli E, Hildebrandt M, Kundernatsch M, Winkler P, Karlmeier A, Holthausen H. A distinct variant of focal cortical dysplasia type I characterized by magnetic resonance imaging and neuropathological examination in children with severe epilepsies. Epileptic Disord. 2010;12:172–180. doi: 10.1684/epd.2010.0321. [DOI] [PubMed] [Google Scholar]
- Boehm G, Sherman G, Hoplight Bn, Hyde L, Waters N, Bradway D, Galaburda A, Denenberg V. Learning and memory in the autoimmune BXSB mouse: effects of neocortical ectopias and environmental enrichment. Brain Res. 1996;726:11–22. [PubMed] [Google Scholar]
- Cajal SRY. Histology of the Nervous System of Man and Vertebrates, Vol. I & II, Translated (from the French) by Neely and Larry Swanson. New York, NY: Oxford University Press, Oxford; 1995. [Google Scholar]
- Calcagnotto M, Paredes M, Tihan T, Barbaro N, Baraban S. Dysfunction of synaptic inhibition in epilepsy associated with focal cortical dysplasia. J Neurosci. 2005;25:9649–9657. doi: 10.1523/JNEUROSCI.2687-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauli B, Audinat E, Lambolez B, Angulo M, Ropert N, Tsuzuki K, Hestrin S, Rossier J. Molecular and physiological diversity of cortical nonpyramidal cells. J Neurosci. 1997;17:3894–3906. doi: 10.1523/JNEUROSCI.17-10-03894.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauller L, Clancy B, Connors B. Backward cortical projections to primary somatosensory cortex in rats extend long horizontal axons in layer I. J Comp Neurol. 1998;390:297–310. [PubMed] [Google Scholar]
- Caviness VJ. Patterns of cell and fiber distribution in the neocortex of the reeler mutant mouse. J Comp Neurol. 1976;170:435–447. doi: 10.1002/cne.901700404. [DOI] [PubMed] [Google Scholar]
- Caviness VJ, Evrard P, Lyon G. Radial neuronal assemblies, ectopia and necrosis of developing cortex: a case analysis. Acta Neuropathol. 1978;41:67–72. doi: 10.1007/BF00689559. [DOI] [PubMed] [Google Scholar]
- Caviness VJ, Frost D. Thalamocortical projections in the reeler mutant mouse. J Comp Neurol. 1983;219:182–202. doi: 10.1002/cne.902190205. [DOI] [PubMed] [Google Scholar]
- Chagnac-Amitai Y, Connors B. Synchronized excitation and inhibition driven by intrinsically bursting neurons in neocortex. J Neurophysiol. 1989;62:1149–1162. doi: 10.1152/jn.1989.62.5.1149. [DOI] [PubMed] [Google Scholar]
- Chang BS, Ly J, Appignani B, Bodell A, Apse KA, Ravenscroft RS, Sheen VL, Doherty MJ, Hackney DB, O Connor M, Galaburda AM, Walsh CA. Reading impairment in the neuronal migration disorder of periventricular nodular heterotopia. Neurology. 2005;64:799–803. doi: 10.1212/01.WNL.0000152874.57180.AF. [DOI] [PubMed] [Google Scholar]
- Chang BS, Katzir T, Liu T, Corriveau K, Barzillai M, Apse KA, Bodell A, Hackney D, Alsop D, Wong ST, Walsh CA. A structural basis for reading fluency: white matter defects in a genetic brain malformation. Neurology. 2007;69:2146. doi: 10.1212/01.wnl.0000286365.41070.54. [DOI] [PubMed] [Google Scholar]
- Chapin J, Sadeq M, Guise J. Corticocortical connections within the primary somatosensory cortex of the rat. J Comp Neurol. 1987;263:326–346. doi: 10.1002/cne.902630303. [DOI] [PubMed] [Google Scholar]
- Chen ZF, Schottler F, Bertram E, Gall CM, Anzivino MJ, Lee KS. Distribution and inititation of seizure activity in a rat brain with subcortical band heterotopias. Epilepsia. 2000;41:493. doi: 10.1111/j.1528-1157.2000.tb00201.x. [DOI] [PubMed] [Google Scholar]
- Chevassus-au-Louis N, Ben-Ari Y, Vergnes M. Decreased seizure threshold and more rapid rate of kindling in rats with cortical malformation induced by prenatal treatment with methylazoxymethanol. Brain Res. 1998;812:252–255. doi: 10.1016/s0006-8993(98)00932-9. [DOI] [PubMed] [Google Scholar]
- Chugani HT, Shewmon DA, Shields WD, Sankar R, Comair Y, vinters HV, Peacock WJ. Surgery from intractable infantile spasms: neuroimaging perspectives. Epilepsia. 1993;34:764–771. doi: 10.1111/j.1528-1157.1993.tb00459.x. [DOI] [PubMed] [Google Scholar]
- Clark M, Sherman G, Bimonte H, Fitch R. Perceptual auditory gap detection deficits in male BXSB mice with cerebrocortical ectopias. Neuroreport. 2000;11:693–696. doi: 10.1097/00001756-200003200-00008. [DOI] [PubMed] [Google Scholar]
- Connors B, Gutnick M. Intrinsic firing patterns of diverse neocortical neurons. Trends Neurosci. 1990;13:99–104. doi: 10.1016/0166-2236(90)90185-d. [DOI] [PubMed] [Google Scholar]
- DeFazio R, Hablitz J. Alterations in NMDA receptors in a rat model of cortical dysplasia. J Neurophysiol. 2000;83:315–321. doi: 10.1152/jn.2000.83.1.315. [DOI] [PubMed] [Google Scholar]
- DeFelipe J. Types of neurons, synaptic connections and chemical characteristics of cells immunoreactive for calbindin-D28K, parvalbumin and calretinin in the neocortex. J Chem Neuroanat. 1997;14:1–19. doi: 10.1016/s0891-0618(97)10013-8. [DOI] [PubMed] [Google Scholar]
- Denenberg V, Mobraaten L, Sherman G, Morrison L, Schrott L, Waters N, Rosen G, Behan P, Galaburda A. Effects of the autoimmune uterine/maternal environment upon cortical ectopias, behavior and autoimmunity. Brain Res. 1991a;563:114–122. doi: 10.1016/0006-8993(91)91522-3. [DOI] [PubMed] [Google Scholar]
- Denenberg V, Sherman G, Schrott L, Rosen G, Galaburda A. Spatial learning, discrimination learning, paw preference and neocortical ectopias in two autoimmune strains of mice. Brain Res. 1991b;562:98–104. doi: 10.1016/0006-8993(91)91192-4. [DOI] [PubMed] [Google Scholar]
- Fink R, Heimer L. Two methods for selective silver impregnation of degenerating axons and their synaptic endings in the central nervous system. Brain Res. 1967;4:369–374. doi: 10.1016/0006-8993(67)90166-7. [DOI] [PubMed] [Google Scholar]
- Fonseca M, DeFelipe J, Fairén A. Local connections in transplanted and normal cerebral cortex of rats. Exp Brain Res. 1988;69:387–398. doi: 10.1007/BF00247584. [DOI] [PubMed] [Google Scholar]
- Frenkel M, Sherman G, Bashan K, Galaburda A, LoTurco J. Neocortical ectopias are associated with attenuated neurophysiological responses to rapidly changing auditory stimuli. Neuroreport. 2000;11:575–579. doi: 10.1097/00001756-200002280-00029. [DOI] [PubMed] [Google Scholar]
- Fusco L, bertini E, Vigevano F. Epilepsia partialis continua and neuronal migration anomalies. Brain Development. 1992;14:323–328. doi: 10.1016/s0387-7604(12)80152-5. [DOI] [PubMed] [Google Scholar]
- Gabel L, LoTurco J. Electrophysiological and morphological characterization of neurons within neocortical ectopias. J Neurophysiol. 2001;85:495–505. doi: 10.1152/jn.2001.85.2.495. [DOI] [PubMed] [Google Scholar]
- Gabel L, LoTurco J. Layer I ectopias and increased excitability in murine neocortex. J Neurophysiol. 2002;87:2471–2479. doi: 10.1152/jn.2002.87.5.2471. [DOI] [PubMed] [Google Scholar]
- Gabel L, Gibson C, Gruen J, Loturco J. Progress towards a cellular neurobiology of reading disability. Neurobiol Dis. 2009 doi: 10.1016/j.nbd.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galaburda A, Kemper T. Cytoarchitectonic abnormalities in developmental dyslexia: a case study. Ann Neurol. 1979;6:94–100. doi: 10.1002/ana.410060203. [DOI] [PubMed] [Google Scholar]
- Galaburda A, Sherman G, Rosen G, Aboitiz F, Geschwind N. Developmental dyslexia: four consecutive patients with cortical anomalies. Ann Neurol. 1985;18:222–233. doi: 10.1002/ana.410180210. [DOI] [PubMed] [Google Scholar]
- Galaburda A. The pathogenesis of childhood dyslexia. Res Publ Assoc Res Nerv Ment Dis. 1988;66:127–137. [PubMed] [Google Scholar]
- Galarreta M, Hestrin S. A network of fast-spiking cells in the neocortex connected by electrical synapses. Nature. 1999;402:72–75. doi: 10.1038/47029. [DOI] [PubMed] [Google Scholar]
- Guerrini R, Barba C. Malformations of cortical development and aberrant cortical networks: epileptogenesis and functional organization. J Clin Neurophysiol. 2010;27:372–379. doi: 10.1097/WNP.0b013e3181fe0585. [DOI] [PubMed] [Google Scholar]
- Gupta A, Wang Y, Markram H. Organizing principles for a diversity of GABAergic interneurons and synapses in the neocortex. Science. 2000;287:273–278. doi: 10.1126/science.287.5451.273. [DOI] [PubMed] [Google Scholar]
- Humphreys P, Kaufmann W, Galaburda A. Developmental dyslexia in women: neuropathological findings in three patients. Ann Neurol. 1990;28:727–738. doi: 10.1002/ana.410280602. [DOI] [PubMed] [Google Scholar]
- Jacobs K, Hwang B, Prince D. Focal epileptogenesis in a rat model of polymicrogyria. J Neurophysiol. 1999;81:159–173. doi: 10.1152/jn.1999.81.1.159. [DOI] [PubMed] [Google Scholar]
- Jenner A, Galaburda A, Sherman G. Connectivity of ectopic neurons in the molecular layer of the somatosensory cortex in autoimmune mice. Cereb Cortex. 2000;10:1005–1013. doi: 10.1093/cercor/10.10.1005. [DOI] [PubMed] [Google Scholar]
- Kaufmann W, Galaburda A. Cerebrocortical microdysgenesis in neurologically normal subjects: a histopathologic study. Neurology. 1989;39:238–244. doi: 10.1212/wnl.39.2.238. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y. Groupings of nonpyramidal and pyramidal cells with specific physiological and morphological characteristics in rat frontal cortex. J Neurophysiol. 1993;69:416–431. doi: 10.1152/jn.1993.69.2.416. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y, Kubota Y. Correlation of physiological subgroupings of nonpyramidal cells with parvalbumin- and calbindinD28k-immunoreactive neurons in layer V of rat frontal cortex. J Neurophysiol. 1993;70:387–396. doi: 10.1152/jn.1993.70.1.387. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y. Distinct firing patterns of neuronal subtypes in cortical synchronized activities. J Neurosci. 2001;21:7261–7272. doi: 10.1523/JNEUROSCI.21-18-07261.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi E, Bagshaw A, Grova C, Gotman J, Dubeau F. Grey matter heterotopia: what EEG-fMRI can tell us about epileptogenicity of neuronal migration disorders. Brain. 2006;129:366–374. doi: 10.1093/brain/awh710. [DOI] [PubMed] [Google Scholar]
- Krsek P, Peiper T, Karlmeier A, Hildebrandt M, Kolodziejczyk D, Winkler P, Pauli E, Blumcke I, Holthausen H. Different presurgical characteristics and seizure outcomes in children with focal cortical dysplasia type I or II. Epilepsia. 2009;50:125–137. doi: 10.1111/j.1528-1167.2008.01682.x. [DOI] [PubMed] [Google Scholar]
- Kuzniecky R, Andermann F, Tampieri D, Melanson D, Olivier A, Leppik I. Bilateral central macrogyria: epilepsy, pseudobulbar palsy, and mental retardation--a recognizable neuronal migration disorder. Ann Neurol. 1989;25:547–554. doi: 10.1002/ana.410250604. [DOI] [PubMed] [Google Scholar]
- Kuzniecky R, Barkovich A. Pathogenesis and pathology of focal malformations of cortical development and epilepsy. J Clin Neurophysiol. 1996;13:468–480. doi: 10.1097/00004691-199611000-00002. [DOI] [PubMed] [Google Scholar]
- Landry P, Wilson CJ, Kitai ST. Morphological and electrophysiological characteristics of pyramidal tract neurons in the rat. Exp Brain Res. 1984;84:1. doi: 10.1007/BF00231144. [DOI] [PubMed] [Google Scholar]
- Lapenko T, Podladchikova O. Study of intracortical connections between groups of neurons in the somatosensory region of the cortex in the rat using the technic of retrograde axonal transport of horseradish peroxidase. Neirofiziologiia. 1983;15:22–26. [PubMed] [Google Scholar]
- Lee KS, Schottler F, Collins JL, Lanzino G, Couture D, Rao A, Hiramatsu K, Goto Y, hong S, Caner H, Yamamoto H, chen Z, Bertram E, Berr S, Omary R, Scrable H, Jackson T, Goble J, Eisenman L. A genetic animal model of human neocortical heterotopias associated with seizures. J Neurosci. 1997;17:6236–6242. doi: 10.1523/JNEUROSCI.17-16-06236.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventer RJ, Guerrini R, Dobyns WB. Malformations of cortical development and epilepsy. Dialogues Clin Neurosci. 2008;10:47–62. doi: 10.31887/DCNS.2008.10.1/rjleventer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luhmann H, Karpuk N, Qü M, Zilles K. Characterization of neuronal migration disorders in neocortical structures. II. Intracellular in vitro recordings. J Neurophysiol. 1998;80:92–102. doi: 10.1152/jn.1998.80.1.92. [DOI] [PubMed] [Google Scholar]
- Luhmann H, Raabe K. Characterization of neuronal migration disorders in neocortical structures: I. expression of epileptiform activity in an animal model. Epilp Res. 1996;26:67–74. doi: 10.1016/s0920-1211(96)00041-1. [DOI] [PubMed] [Google Scholar]
- McCann MV, Pongonis SJ, Golomb MR, Edwards-Brown M, Christensen CK, Sokol DK. Like father, like son: periventricular nodular heterotopias and nonverbal learning disorder. J child Neurol. 2008;23:950–953. doi: 10.1177/0883073808315415. [DOI] [PubMed] [Google Scholar]
- McCormick D, Connors B, Lighthall J, Prince D. Comparative electrophysiology of pyramidal and sparsely spiny stellate neurons of the neocortex. J Neurophysiol. 1985;54:782–806. doi: 10.1152/jn.1985.54.4.782. [DOI] [PubMed] [Google Scholar]
- Meencke H, Veith G. Migration disturbances in epilepsy. Epilep Res [Suppl] 1992;9:31–40. [PubMed] [Google Scholar]
- Meencke HJ, Janz D. Neuropathological findings in primary generalized epilepsy: a study of eight cases. Epilepsia. 1984;25:8–21. doi: 10.1111/j.1528-1157.1984.tb04149.x. [DOI] [PubMed] [Google Scholar]
- Palmini A, Andermann F, Olivier A, Tampieri D, Robitaille Y. Focal neuronal migration disorders and intractable partial epilepsy: results of surgical treatment. Ann Neurol. 1991a;30:750–757. doi: 10.1002/ana.410300603. [DOI] [PubMed] [Google Scholar]
- Palmini A, Andermann F, Olivier A, Tampieri D, Robitaille Y, Andermann E, Wright G. Diffuse cortical dysplasia, or the double cortex syndrome: the clinical and epileptic spectrum in 10 patients. Neurology. 1991b;41:1656–1662. doi: 10.1212/wnl.41.10.1656. [DOI] [PubMed] [Google Scholar]
- Palmini A, Andermann F, Olivier A, Tampieri D, Robitaille Y, Andermann E, Wright G. Focal neuronal migration disorders and intractable partial epilepsy: a study of 30 patients. Ann Neurol. 1991c;30:741–749. doi: 10.1002/ana.410300602. [DOI] [PubMed] [Google Scholar]
- Peiffer A, Dunleavy C, Frenkel M, Gabel L, LoTurco J, Rosen G, Fitch R. Impaired detection of variable duration embedded tones in ectopic NZB/BINJ mice. Neuroreport. 2001;12:2875–2879. doi: 10.1097/00001756-200109170-00024. [DOI] [PubMed] [Google Scholar]
- Redecker C, Luhmann HJ, Hagemann G, Fritschy JM, Witte OW. Differential downregulation of GABA-A receptor subunits in widespread brain regions in the freeze-lesion model of focal cortical malformations. J Neurosci. 2000;20:5045–5053. doi: 10.1523/JNEUROSCI.20-13-05045.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roper S, Eisenschenk S, King M. Reduced density of parvalbumin- and calbindin D28-immunoreactive neurons in experimental cortical dysplasia. Epilepsy Res. 1999;37:63–71. doi: 10.1016/s0920-1211(99)00035-2. [DOI] [PubMed] [Google Scholar]
- Rosen G, Galaburda A, Sherman G. Cerebrocortical microdysgenesis with anomalous callosal connections: a case study in the rat. Int J Neurosci. 1989;47:237–247. doi: 10.3109/00207458908987438. [DOI] [PubMed] [Google Scholar]
- Rosen G, Jacobs K, Prince D. Effects of neonatal freeze lesions on expression of parvalbumin in rat neocortex. Cereb Cortex. 1998;8:753–761. doi: 10.1093/cercor/8.8.753. [DOI] [PubMed] [Google Scholar]
- Rosen G, Burstein D, Galaburda A. Changes in efferent and afferent connectivity in rats with induced cerebrocortical microgyria. J Comp Neurol. 2000;418:423–440. [PubMed] [Google Scholar]
- Sarkisian M, Frenkel M, Li W, Oborski J, LoTurco J. Altered interneuron development in the cerebral cortex of the flathead mutant. Cereb Cortex. 2001;11:734–743. doi: 10.1093/cercor/11.8.734. [DOI] [PubMed] [Google Scholar]
- Sarkisian MR, Rattan S, D Mello SR. A new genetic model of epilepsy in early postnatal development. Epilepsia. 1999;40:394–400. doi: 10.1111/j.1528-1157.1999.tb00732.x. [DOI] [PubMed] [Google Scholar]
- Schrott L, Waters N, Boehm G, Sherman G, Morrison L, Rosen G, Behan P, Galaburda A, Denenberg V. Behavior, cortical ectopias, and autoimmunity in BXSB-Yaa and BXSB-Yaa+ mice. Brain Behav Immun. 1993;7:205–223. doi: 10.1006/brbi.1993.1022. [DOI] [PubMed] [Google Scholar]
- Sherman G, Galaburda A, Geschwind N. Cortical anomalies in brains of New Zealand mice: a neuropathologic model of dyslexia? Proc Natl Acad Sci U S A. 1985;82:8072–8074. doi: 10.1073/pnas.82.23.8072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman G, Galaburda A, Behan P, Rosen G. Neuroanatomical anomalies in autoimmune mice. Acta Neuropathol. 1987;74:239–242. doi: 10.1007/BF00688187. [DOI] [PubMed] [Google Scholar]
- Sherman G, Morrison L, Rosen G, Behan P, Galaburda A. Brain abnormalities in immune defective mice. Brain Res. 1990;532:25–33. doi: 10.1016/0006-8993(90)91737-2. [DOI] [PubMed] [Google Scholar]
- Simmons P, Lemmon V, Pearlman A. Afferent and efferent connections of the striate and extrastriate visual cortex of the normal and reeler mouse. J Comp Neurol. 1982;211:295–308. doi: 10.1002/cne.902110308. [DOI] [PubMed] [Google Scholar]
- Sisodiya SM. Malformations of cortical development: burdens and insights from important causes of human epilepsy. Lancet Neurol. 2004;3:29–38. doi: 10.1016/s1474-4422(03)00620-3. [DOI] [PubMed] [Google Scholar]
- Spencer DJ, Humphries K, Mathis D, Lal H. Behavioral impairments related to cognitive dysfunction in the autoimmune New Zealand black mouse. Behav Neurosci. 1986;100:353–358. doi: 10.1037//0735-7044.100.3.353. [DOI] [PubMed] [Google Scholar]
- Spreafico R, Battaglia G, Arcelli P, Andermann F, Dubeau F, Palmini A, Olivier A, Villemure JG, Tampieri D, Avanzini G, Avoli M. Cortical dysplasia: an immunocytochemical study of three patients. Neurology. 1998;50:27–36. doi: 10.1212/wnl.50.1.27. [DOI] [PubMed] [Google Scholar]
- Thom M, Martinian L, Parnavelas J, Sisodiya S. Distribution of cortical interneurons in grey matter heterotopia in patients with epilepsy. Epilepsia. 2004;45:916–923. doi: 10.1111/j.0013-9580.2004.46603.x. [DOI] [PubMed] [Google Scholar]
- Trotter S, Kapur J, Anzivino M, Lee K. GABAergic synaptic inhibition is reduced before seizure onset in a genetic model of cortical malformation. J Neurosci. 2006;26:10756–10767. doi: 10.1523/JNEUROSCI.2323-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschuluun N, Wenzel JH, katleba K, Schwartzkroin PA. Initiation and spread of epileptiform discharges in the methylazoxymethanol acetate rat model of cortical dysplasia: functional and structural connectivity between CA1 heterotopia and hippocampus/neocortex. Neuroscience. 2005;133:327–342. doi: 10.1016/j.neuroscience.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Yorke CJ, Caviness VJ. Interhemispheric neocortical connections of the corpus callosum in the normal mouse: a study based on anterograde and retrograde methods. J Comp Neurol. 1975;164:233–245. doi: 10.1002/cne.901640206. [DOI] [PubMed] [Google Scholar]
- Zhou FW, Roper SN. Densities of glutamatergic and GABA ergic presynaptic terminals are altered in experimental cortical dysplasia. Epilepsia. 2010;51:1468–1476. doi: 10.1111/j.1528-1167.2010.02583.x. [DOI] [PubMed] [Google Scholar]