Abstract
Lavage of the ductal systems of the breast provides fluid (ductal lavage fluid, DLF) containing hormones and products of hormone actions that may represent more accurately the composition of the breast than samples collected from blood or urine. The present study was undertaken to assess the presence of potential cancer biomarkers, their variation among individuals at high risk for breast cancer, and differences associated with menopause and tamoxifen treatment. Seventy seven tamoxifen-eligible subjects with a 5-year breast cancer risk estimate (Gail > 1.6%; N = 53) or recently diagnosed breast cancer (N = 24) were offered tamoxifen therapy; those not accepting tamoxifen were under observation only. After 6 months, all subjects underwent ductal lavage (DL) in an unaffected breast. Estradiol (E2), estrone sulfate, androstenedione, dehydroepiandrosterone (DHEA), DHEA sulfate, progesterone, cathepsin D, and epidermal growth factor (EGF) were measured in ductal lavage fluid (DLF) by immunoassays. Data were expressed as the mass of analyte per milligram of protein in DLF and normalized by natural log transformation. With the exception of DHEA, none of the analytes measured were significantly lower in postmenopausal women than in premenopausal women. The mean loge concentration difference in estradiol was 10.9%. Tamoxifen treatment for 6 months did not result in a significantly greater concentration of E2 or in any of the other analytes in DLF of pre- or postmenopausal women. The between-duct variance of the concentration of free steroids within the same breast averaged 51% less than that between subjects, and was similar to that of non-diffusible proteins. The maintenance of estradiol concentrations in the breast after menopause demonstrates the importance of local biosynthesis. The fact that DLF E2 does not reflect the high serum concentrations of E2 during tamoxifen treatment indicates that breast concentrations of estradiol may be under feedback control. Unlike studies of low risk populations, progesterone concentrations were not significantly less in postmenopausal than in premenopausal women. The similarity in variance of free steroids and protein analytes between ducts of a breast indicates little transfer of steroids between lobules.
Keywords: Breast, Cancer, Risk, Lavage, Hormones
Introduction
There is a clear connection between estrogen exposure and risk of breast cancer [1]. Recent studies show that women in the top quartile of estradiol levels may have a relative risk of breast cancer of more than two [2–4]. Such data support the principle of the relationship between estradiol and breast cancer but the association would have to be much higher to be a useful biomarker for risk of breast cancer in individuals. The reason that serum estrogen concentrations do not have a higher association with breast cancer may, in part, be related to differences between concentrations of steroid hormones in serum and breast tissues. Studies of E2 and other hormones in nipple aspirate fluid (NAF) clearly indicate a very poor correlation with serum levels [5–8] as do correlations between tissue and serum concentrations [9–12]. Recently, Lonning et al. [13] reported a correlation between plasma and normal breast tissue concentrations of estradiol, but only in breasts that had an estrogen receptor-α positive tumor. The general lack of correlation with the normal breast is not surprising because of the significant local biosynthesis of estrogens in breast tissue [5, 14–16].
Sampling of breast fluid either by collection of NAF or by ductal lavage is a means of obtaining information about the milieu of the breast directly. Ductal lavage has the advantage that epithelial cells that are shed from the ducts may be obtained for assessment of atypical forms from the breasts of healthy high-risk women [17]. We have previously reported initial results from a Phase 2 prevention study designed to assess the utility of ductal lavage for the measurement of cellular markers of breast cancer risk and tamoxifen response in a population at increased risk of breast cancer [18]. Tamoxifen-eligible women either accepted or declined tamoxifen therapy after appropriate counseling, and all subjects were asked to return for a 6-month follow-up procedure which included ductal lavage. We now report results on the analysis of endocrine parameters (hormones and hormone-related proteins) in the ductal lavage supernatant fluid in this study. Ductal lavage fluid (DLF) provides a sample similar to NAF, but with more thorough sampling of the entire ductal tree [19]. In addition, samples from individual ducts can be examined separately. In NAF collection, when vacuum is applied to the nipple and there is more than one duct producing fluid, the NAF is collected as a single sample from the surface of the nipple, so individual ducts cannot be assessed separately. However, with ductal lavage we are able to compare the hormonal composition from different ducts within a breast.
We sought to assess the relationship among androgens, estrogens, estrogen response proteins, and epidermal growth factor (EGF) in ductal lavage fluid and how these relate to menopausal state, tamoxifen treatment, and measurements of the same hormonal products in NAF. Because tamoxifen treatment is associated with a two- to threefold increase in serum estradiol [20–22], it was important to determine whether a similar increase occurs within the breast. Such an increase in estradiol may compete with tamoxifen or its metabolites for the estrogen receptor, reducing the effectiveness of the antiestrogen.
Methods
Subjects
Subjects were recruited from the Bluhm Family Program for Breast Cancer Early Detection and Prevention, at the Lynn Sage Breast Center of Northwestern Memorial Hospital. Data on known breast cancer risk factors were used to estimate the 5-year breast cancer risk using a statistical model [23]. Eligible women were all at high risk for breast cancer; no breasts with cancer or that had evidence of cancer were lavaged in this study. Subjects included (a) 53 unaffected healthy women with a 5-year risk estimate of >1.6 and (b) 24 women who had completed local therapy for unilateral estrogen receptor positive ductal carcinoma in situ or who had invasive cancer of <1 cm in size and who did not require chemotherapy. In group “b” only the unaffected breast was lavaged. No subjects had taken tamoxifen or dietary supplements such as soy products for prevention. Both premenopausal and postmenopausal women were included in the study.
Ductal Lavage Procedure
Subjects applied an anesthetic cream EMLA (2.5% lidocaine and 2.5% prilocaine; AstraZeneca, Wilmington, DE) 2 h before the lavage procedure. Nitroglycerin cream (glyceryl trinitrate, E. Fougera & Co., Melville, NY) was applied to the nipple of each breast to be studied 20 min before the procedure along with a warm compress. The breast was massaged, and the Cytyc aspirator (Cytyc Corp., Boxborough, MA) was used to elicit nipple aspirate fluid. Lavage of fluid-yielding ducts and visualized non-fluid-yielding ducts was performed through a microcatheter (Cytyc), using Plasmalyte, an isotonic solution containing 140 mM Na+, 5.0 mM K+, 98 mM Cl−, 3.0 mM Mg++, 27 mM acetate, and 23 mM gluconate (Baxter Healthcare Corp., Deerfield, IL). The lavage effluent was collected in Cytolyte (Cytyc). The sample from each duct was collected, diluted to 20 ml with Cytolyte, and centrifuged at 1,500 g for 10 min. The supernatant fluid (DLF) was decanted carefully and processed as described below.
DL was performed under local anesthesia in the office setting. The details of the procedure have been described previously [18]. Women were informed of the cytologic findings and allowed to choose tamoxifen therapy at a dose of 20 mg daily or observation. All subjects were asked to return for DL 6 months after the first procedure or 6 months after initiation of tamoxifen. The latter samples were assayed for hormones and related substances. The protocol was approved by the Institutional Review Board of Northwestern University and written informed consent was obtained from all subjects.
Analysis of DLF
Briefly, sample volumes were reduced in a centrifugal evaporator to remove methanol from the Cytolyte. They were then lyophilized to dryness, and made up to 4.0 ml with water. The lyophilization flask was rinsed with water and then with ethyl acetate–hexane (3:2), and the aqueous solution was extracted a second time with additional organic solvent to recover all unconjugated steroids. Recovery of protein and 3H-E2 from the lyophilization flask was 83.8 ± 3.8 and 82.6 ± 2.4%, respectively. Procedures for analysis of the individual analytes were as described in detail previously [6]. Estrone sulfate, DHEA sulfate, cathepsin D, and EGF were measured in the aqueous fraction. The ethyl acetate–hexane fraction was evaporated and the residue was partitioned into phenolic and non-phenolic fractions (0.4 N aq NaOH and isooctane, respectively). Estradiol was measured in the phenolic fraction and androstenedione, progesterone, and dehydroepiandrosterone (DHEA) were measured in the non-phenolic fraction. Estradiol, progesterone, DHEA sulfate, and estrone sulfate were measured by RIAs. Cathepsin D, EGF, androstenedione, and DHEA were measured by ELISAs. A quality-control preparation was prepared from a pool of DLF that was collected from repeat samples not used for this study but from the same subject group. The quality-control preparation was measured in each assay. Buffer blanks were carried through the procedure as well, and the value of the blank was subtracted from each sample value. All samples were assayed in duplicate. Because the volume of fluid within the breast is diluted by a large volume of buffer during the lavage procedure, it is necessary to use a reference with which to express the hormone values. For this reason we chose to express the amount of analytes per milligram of protein extracted with the analytes in the lavage solution. The protein concentration was measured by the BioRad Coomassie Blue assay procedure. The intra- and interassay coefficients of variation for the assays were <15% and <20%, respectively. All immunoassays were from commercial sources and had limits of detection which were established by the manufacturers.
Statistical Procedures
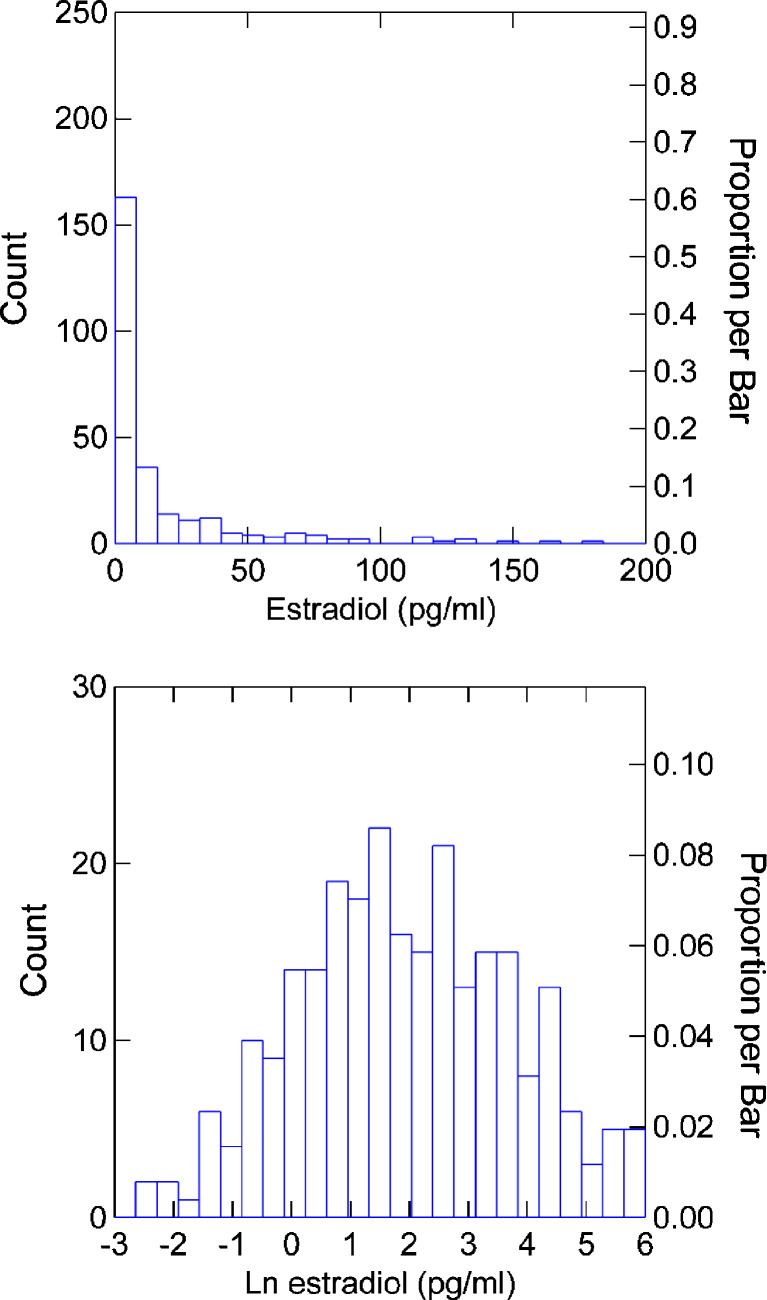
All data were loge-transformed. This provided adequate normalization for parametric analyses. The distribution of untransformed and transformed estradiol data are shown in Fig. 1.
Fig. 1.
Effect of natural log transformation on the distribution of estradiol values
Means and standard deviations of the analytes were calculated, as were geometric means of the values for interpretability. For comparisons of two groups of independent observations, F tests and two-sample Student’s t tests were used to compare variance estimates and means for groups, respectively. Spearman correlation coefficients were also calculated to describe the correspondence of DLF and NAF measurements. F tests were used to compare variance estimates within- and between-subjects. Within-subject estimates were calculated using the residual mean squared error from an ANOVA model on the complete data set controlling for subject.
Subjects receiving tamoxifen were compared separately for pre- and postmenopausal women to the appropriate “observation” group. A total of 20 subjects not receiving tamoxifen had both NAF and DLF collected from the same duct; paired t tests were used to compare means.
Results
An adequate lavage sample was obtained from 86.3% of women who volunteered for ductal lavage. The sources of attrition of study subjects has been described previously [24]. There were a total of 77 subjects who had adequate protein in the lavage sample for analyses; 45 were premenopausal: 30 controls and 15 who received tamoxifen (age range 30–55, median 46 years; BMI range 17.5 to 44.0, median 26.2 kg/m2). There were 32 postmenopausal women: 15 controls and 17 who received tamoxifen (age range 43–67, median 55 years; BMI range 18.2 to 40.9, median 26.2 kg/m2). Among women who chose tamoxifen, the median BMI was 27.1 kg/m2, not significantly different from those who chose observation. The mean Gail risk estimate was also not different between those who chose tamoxifen and those who did not, and there was no difference in diagnosis or biomarkers in ductal epithelial cells including Ki67, Cox2, or estrogen receptor-α between the two groups [24].
The protein per breast in DLF of premenopausal women was 0.98 ± 35.9 (SD) mg. There were no significant differences in the amount of protein in lavage samples between the pre- and postmenopausal women or between subjects receiving tamoxifen and controls. The mean number of ducts lavaged was 1.10 (range, 0–4) per breast in premenopausal women, 1.57 (range 0–10) in postmenopausal women, and 1.33 (range 0–10) in patients on tamoxifen. These do not represent the number available for lavage; only one or two ducts were chosen from a breast for the study.
Differences between hormones and hormone-related products in DLF of pre- and postmenopausal women are shown in Table 1. Differences in hormone levels in breast fluid between mid-follicular and mid-luteal phases were not found to be significant in a previous study [5] so stage of the menstrual cycle was not considered for this analysis. The analyte with the greatest concentration in DLF is DHEA sulfate, 3 × 105-fold greater than that of estradiol. Nevertheless, 11 subjects had no detectable DHEA sulfate. Negligible values were found among other analytes also: estradiol, 8 (three premenopausal, five postmenopausal); estrone sulfate, 10; androstenedione, 8; DHEA, 13; progesterone, 6 (one premenopausal, five postmenopausal); EGF, 5; cathepsin D, 11. In addition, 17 and 21 samples in the cathepsin D and progesterone assays were lost and results are not available. Smaller numbers of other analytes were lost due to being out of range or outliers. The small amount of analytes extracted from DLF usually does not permit a second assay of the analyte. Despite the major decline in serum estradiol concentrations after menopause, the mean loge concentration of estradiol in DLF was only 10.9% less in postmenopausal than in premenopausal women (Table 1). A product of estrogen action, cathepsin D, was also not significantly lower in the postmenopausal women. Potential estrogen precursors estrone sulfate and the androgens were all lower in postmenopausal women but only DHEA was significantly lower.
Table 1.
Analyte concentrations in DL fluid of pre- and postmenopausal women not taking tamoxifen
| Analyte | Premenopausal | Postmenopausal | p value | ||||
|---|---|---|---|---|---|---|---|
| N | Mean loge (SD) | Geometric mean | N | Mean loge (SD) | Geometric mean | ||
| min,max | min,max | ||||||
| Estradiol pg/mg | 22 | 2.20 (1.65) | 9.03 | 10 | 1.96 (2.12) | 7.10 | 0.91 |
| 1.20,5.37 | 1.27,4.45 | ||||||
| Estrone sulfate ng/mg | 25 | 2.96 (1.40) | 19.3 | 12 | 2.24 (1.95) | 9.39 | 0.27 |
| −1.04,5.34 | −1.90,5.13 | ||||||
| Progesterone ng/mg | 21 | −1.10 (1.84) | 0.33 | 11 | −1.69 (1.76) | 0.18 | 0.38 |
| −4.34,2.25 | −4.42,1.37 | ||||||
| Androstenedione, ng/mg | 23 | −2.54 (1.44) | 0.079 | 12 | −2.84 (1.37) | 0.058 | 0.55 |
| −5.81,−0.34 | −5.12,−0.71 | ||||||
| DHEA ng/mg | 21 | −0.60 (1.47) | 0.55 | 14 | −1.95 (1.67) | 0.142 | 0.02 |
| −3.02,2.21 | −5.52,−0.04 | ||||||
| DHEA sulfate μg/mg | 22 | 1.15 (1.46) | 3.16 | 14 | 0.97 (1.71) | 2.64 | 0.75 |
| −2.36,3.81 | −3.61,3.18 | ||||||
| Cathepsin D ng/mg | 17 | 2.28 (2.37) | 9.78 | 7 | 3.53 (1.22) | 34.1 | 0.10 |
| −3.51,4.94 | 1.86,5.03 | ||||||
| EGF ng/mg | 27 | 1.30 (1.13) | 3.67 | 16 | 1.25 (2.04) | 3.49 | 0.93 |
| −1.94,2.86 | −5.81,3.24 | ||||||
p values are from two-sample t tests
Concentrations of analytes in DLF of patients who had received tamoxifen for a period of 6 months are shown in Table 2. Loge mean values are compared with the corresponding values in control subjects. Overall, the mean loge estradiol was greater by 33% in premenopausal women and 6.6% in postmenopausal women but neither difference was significant. Neither cathepsin D nor the androgens were significantly greater in subjects receiving tamoxifen.
Table 2.
Analyte concentrations in DL fluid in subjects with and without tamoxifen
| Analyte | Control | Tamoxifen | p value | ||||
|---|---|---|---|---|---|---|---|
| N | Mean loge (SD) | Geometric mean | N | Mean loge (SD) | Geometric mean | ||
| Premenopausal | |||||||
| Estradiol pg/mg | 22 | 2.20 (1.65) | 9.05 | 12 | 2.93 (1.86) | 18.75 | 0.27 |
| Estrone sulfate ng/mg | 25 | 2.96 (1.40) | 19.25 | 14 | 2.43 (1.51) | 11.3 | 0.29 |
| Progesterone ng/mg | 21 | −1.10 (1.84) | 0.33 | 9 | −1.14 (1.49) | 0.32 | 0.94 |
| Androstenedione, ng/mg | 23 | −2.54 (1.44) | 0.079 | 13 | −2.54 (2.10) | 0.079 | 1.00 |
| DHEA ng/mg | 21 | −0.60 (1.47) | 0.55 | 9 | 0.18 (2.27) | 1.19 | 0.36 |
| DHEA sulfate μg/mg | 22 | 1.15 (1.46) | 3.15 | 13 | 0.97 (1.49) | 2.64 | 0.73 |
| Cathepsin D ng/mg | 17 | 2.28 (2.37) | 9.76 | 13 | 3.02 (1.57) | 20.46 | 0.31 |
| EGF ng/mg | 27 | 1.30 (1.13) | 3.69 | 13 | 1.18 (1.16) | 3.26 | 0.75 |
| Postmenopausal | |||||||
| Estradiol pg/mg | 10 | 2.12 (1.96) | 8.32 | 13 | 2.26 (2.34) | 9.58 | 0.88 |
| Estrone sulfate ng/mg | 12 | 2.24 (1.95) | 9.36 | 13 | 1.77 (1.73) | 5.89 | 0.54 |
| Progesterone ng/mg | 11 | −1.69 (1.76) | 0.19 | 8 | −2.76 (2.33) | 0.063 | 0.29 |
| Androstenedione, ng/mg | 12 | −2.84 (1.37) | 0.058 | 13 | −3.21 (1.82) | 0.041 | 0.57 |
| DHEA ng/mg | 14 | −1.95 (1.67) | 0.14 | 13 | −0.93 (2.25) | 0.39 | 0.20 |
| DHEA sulfate μg/mg | 14 | 0.97 (1.71) | 2.63 | 11 | 1.03 (1.30) | 2.79 | 0.92 |
| Cathepsin D ng/mg | 7 | 3.53 (1.22) | 34.19 | 9 | 3.68 (1.02) | 39.82 | 0.79 |
| EGF ng/mg | 16 | 1.25 (2.04) | 3.50 | 14 | 0.86 (1.05) | 2.35 | 0.50 |
Variability of measurement was assessed across subjects and among ducts within a breast (Table 3). In subjects in whom more than one duct was analyzed, the value for a single randomly selected duct was used to estimate between-subject variability. A comparison of mean square errors (MSE) from ANOVA revealed that variability among ducts within a breast for estradiol was 44% less than variability between subjects. Variability of other analytes was similarly less within breasts. When variability was compared separately in pre- and postmenopausal women, the pattern was not different. For greater statistical power data from all subjects were combined in Table 3.
Table 3.
F tests comparing log variance estimates within and between subjects not taking tamoxifen (MSE mean square error from the analysis of variance, df degrees of freedom)
| Within-subject MSE (df) | Between-subject MSE (df) | F statistic p value | |
|---|---|---|---|
| Estradiol pg/mg | 1.66 (49) | 2.96 (31) | 0.08 |
| Estrone sulfate ng/mg | 1.06 (57) | 2.59 (36) | 0.00 |
| Progesterone ng/mg | 1.61 (45) | 3.28 (31) | 0.01 |
| Androstenedione ng/mg | 0.97 (52) | 1.99 (34) | 0.01 |
| DHEA ng/mg | 1.23 (51) | 2.79 (34) | 0.00 |
| DHEA sulfate μg/mg | 1.76 (50) | 2.37 (35) | 0.17 |
| EGF ng/mg | 1.55 (64) | 2.28 (42) | 0.04 |
In a group of 20 subjects NAF and DLF were both collected from the same ducts (one per subject). A comparison of the concentrations is shown in Table 4. Only the protein analytes cathepsin D and EGF were significantly higher in DLF. Estradiol and the other free steroids as well as the steroid sulfates were not different between NAF and DLF by paired t tests.
Table 4.
Comparison of analytes in NAF and DLF from the same duct
| Analyte | N | NAF | DLF | SDD | p valuea | ||
|---|---|---|---|---|---|---|---|
| Mean log | Geometric mean | Mean log | Geometric mean | t | |||
| Estradiol pg/mg | 19 | 1.73 | 5.63 | 1.17 | 3.23 | 2.55 | 0.36 |
| Estrone sulfate ng/mg | 13 | 2.35 | 10.51 | 2.79 | 16.25 | 1.27 | 0.24 |
| Androstenedione ng/mg | 11 | −1.94 | 0.14 | −1.59 | 0.20 | 3.32 | 0.74 |
| DHEA ng/mg | 20 | −1.435 | 4.20 | −1.41 | 4.08 | 1.57 | 0.94 |
| DHEA sulfate μg/mg | 16 | 1.55 | 4.71 | 1.86 | 6.42 | 1.46 | 0.63 |
| Progesterone ng/mg | 10 | −1.76 | 0.17 | −2.13 | 0.12 | 2.06 | 0.60 |
| Cathepsin D ng/mg | 19 | 3.00 | 20.0 | 4.43 | 84.0 | 1.23 | 0.00 |
| EGF ng/mg | 20 | 1.01 | 2.74 | 1.82 | 6.16 | 1.14 | 0.00 |
SDD standard deviation of differences
ap values are from paired t tests
Discussion
In the present study, we performed measurements of hormones and hormone-related proteins in the supernatants of the DL samples, which presumably provides a sampling of the deeper reaches of an individual ductal tree than samples of NAF [19]. This may provide a more accurate estimate of the hormonal milieu of the entire breast than is obtained from samples of NAF.
We observed that E2 concentrations in breast fluid were not significantly lower in postmenopausal than in premenopausal women. This is consistent with previous studies of NAF [7], as well as with studies of tissue concentrations of estrogens [9–12]. The E2 concentrations in breast fluid are different under some circumstances as evidenced by the threefold lower concentrations in women taking oral contraceptives and the almost sevenfold greater values in women taking hormone replacement that was observed in a previous study from this laboratory [7]. The potential E2 precursors, androstenedione, DHEA, and estrone sulfate all had lower loge mean values in postmenopausal women but only DHEA concentrations were significantly lower. Even so, there would appear to be an abundance of precursor steroids for E2 biosynthesis in the breasts of postmenopausal women. Androstenedione concentrations were eight- to ninefold greater than the concentrations of estradiol. Further support for meaningful local biosynthesis of estradiol in the postmenopausal breast comes from the lack of significant difference between cathepsin D in pre- and postmenopausal women. One of the factors regulating the biosynthesis of cathepsin D is estrogen. If estrogenic activity in the breast declines after menopause, a decrease in cathepsin D should occur [25, 26]. These data may explain, in part, why there is no sharp drop in incidence of breast cancer associated with the decline in serum estradiol at menopause [27].
Additionally, progesterone concentrations in the DLF of postmenopausal women, while lower, were not significantly lower than in premenopausal women. This is different from the highly significantly lower concentrations reported earlier on young women who had a low risk of breast cancer [7]. The possibility that cytokines such as IL-4 are increased with risk of breast cancer exists and may be the cause of greater progesterone concentrations in DLF of this group. Based on in vitro studies, progesterone synthesis in the breast is not expected to be significant in the absence of stimulation by IL-4 [28]; this and other cytokines deserve investigation in relation to progesterone concentrations in future studies of women at risk for breast cancer.
Several previous studies have shown that plasma E2 is increased during tamoxifen treatment of premenopausal women by 100% to 300% [20, 21] and to some degree in postmenopausal women as well [22]. This increase in circulating estradiol in response to tamoxifen is not exerted through stimulation of LH or FSH secretion but apparently by a direct action on the ovary [20, 21]. In DLF, the mean loge values of E2 were greater by only 33% in premenopausal women, far less than the anticipated difference in serum, indicating that the high serum measurements of estradiol seen with tamoxifen therapy are not reflected in breast tissue. The lack of higher concentrations of E2 in the breast could be caused by feedback suppression of local biosynthesis of E2 by the elevated serum concentrations of E2 or its metabolites. Thus, biosynthesis of E2 in the breast may be stimulated only when there is insufficient E2 from the serum, i.e., when feedback inhibition is relieved. Such feedback inhibition has not been studied in the breast but it is known that metabolites of estradiol such as 2-methoxyestrone are potent inhibitors of 17β-hydroxysteroid dehydrogenase [29, 30], an enzyme essential for the biosynthesis of estradiol.
Because each duct represents a separate lobule of the breast [31], differences among ducts may be considered differences among lobules. The variances (MSEs) between subjects in the several analytes were generally quite similar and the reduction in variation within a breast, represented by ducts, was also similar among the analytes. The variance among unconjugated steroids (diffusible substances) averaged 50.6% less within breasts, and that for proteins and steroid sulfates (non-diffusible substances) was 46.4% less. The similarity in variance between the two types of substances is an indication that little diffusion of steroids occurs between lobules of the breast. Elevated production of estradiol in one lobule is unlikely to have a significant effect on its concentration in other lobules.
Since this is the first analysis of soluble substances in ductal lavage samples, a comparison of data from NAF and DLF from the same ducts was made. All substances with the exception of cathepsin D and EGF were not different when analyzed in the two fluids from the same duct. The mean loge concentrations of cathepsin D and EGF in DLF were 32% and 44% higher than the respective concentrations in NAF. This is evidence for differences in the concentrations of these proteins in the nipple vs. the lower ductal branches of the lobules when compared to the total protein in the sample. The significance of this observation is not known at this time.
In summary, these analyses of ductal lavage fluid provide us with a picture of the endocrine environment of the breast which is somewhat different from that provided in our previous studies of nipple aspirate fluid. From the comparison of NAF and DLF from the same ducts it is apparent that DLF provides quantitatively more of EGF and cathepsin D. Whether qualitative differences may exist is not determined by the present study. Nevertheless, concentrations of most analytes in NAF and DLF from the same duct were generally similar and, therefore, there is no apparent advantage in performing the more labor intensive and invasive procedure of ductal lavage for most purposes. The exceptions may be for studies in which differences between ductal systems are of interest and for subjects in whom NAF collection is not possible. The studies differ in the subject populations; the former work [6, 7] was carried out in younger women with a low risk of breast cancer; the present study was conducted in high-risk women, so other direct comparisons between the studies are not possible. However, methodological differences between the studies employing NAF and DLF are minimal. The study confirms previous evidence that estradiol levels are maintained in the breast after menopause in levels that are similar to those of premenopausal women. The lack of greater estradiol concentrations in the breast during tamoxifen treatment suggests that feedback mechanisms exist to maintain levels of estradiol in the breast. The maintenance of progesterone levels in postmenopausal women at high risk of breast cancer is an observation that should be investigated further. The studies emphasize the importance of E2 and progesterone as breast cancer risk markers. Further, they support the concept that the local breast environment of both pre- and postmenopausal women is a rich source of markers of risk, and may provide measures of the efficacy of preventive interventions which affect the concentrations of the steroid hormones.
Acknowledgments
Supported by grants from the NIH, NCI: P50 CA89018, and R21 CA105056
Conflict of interest
The authors declare that they have no conflict of interest.
Contributor Information
Robert Treat Chatterton, Jr., Email: chat@northwestern.edu
Seema A. Khan, Email: skhan@nmh.org
References
- 1.Russo IH, Russo J. Role of hormones in mammary cancer initiation and progression. J Mammary Gland Biol Neoplasia. 1998;3(1):49–61. doi: 10.1023/A:1018770218022. [DOI] [PubMed] [Google Scholar]
- 2.Eliassen AH, Missmer SA, Tworoger SS, Spiegelman D, Barbieri RL, Dowsett M, Hankinson SE. Endogenous steroid hormone concentrations and risk of breast cancer among premenopausal women. J Natl Cancer Inst. 2006;98(19):1406–1415. doi: 10.1093/jnci/djj376. [DOI] [PubMed] [Google Scholar]
- 3.Hankinson SE, Eliassen AH. Circulating sex steroids and breast cancer risk in premenopausal women. Horm Cancer. 2010;1:2–10. doi: 10.1007/s12672-009-0003-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Missmer SA, Eliassen AH, Barbieri RL, Hankinson SE. Endogenous estrogen, androgen, and progesterone concentrations and breast cancer risk among postmenopausal women. J Natl Cancer Inst. 2004;96(24):1856–1865. doi: 10.1093/jnci/djh336. [DOI] [PubMed] [Google Scholar]
- 5.Chatterton RT, Jr, Geiger AS, Gann PH, Khan SA. Formation of estrone and estradiol from estrone sulfate by normal breast parenchymal tissue. J Steroid Biochem Mol Biol. 2003;86:159–166. doi: 10.1016/S0960-0760(03)00266-8. [DOI] [PubMed] [Google Scholar]
- 6.Chatterton RT, Jr, Geiger AS, Khan SA, Helenowski IB, Jovanovic BD, Gann PH. Variation in estradiol, estrogen precursors, and estrogen-related products in nipple aspirate fluid from normal premenopausal women. Cancer Epidemiol Biomark Prev. 2004;13(6):928–935. [PubMed] [Google Scholar]
- 7.Chatterton RT, Jr, Geiger AS, Mateo ET, Helenowski IB, Gann PH. Comparison of hormone levels in nipple aspirate fluid of pre- and postmenopausal women: effect of oral contraceptives and hormone replacement. J Clin Endocrinol Metab. 2004;90:1686–1691. doi: 10.1210/jc.2004-1861. [DOI] [PubMed] [Google Scholar]
- 8.Gann PH, Geiger AS, Helenowski IB, Vonesh EF, Chatterton RT. Estrogen and progesterone levels in nipple aspirate fluid of healthy premenopausal women: relationship to steroid precursors and response proteins. Cancer Epidemiol Biomark Prev. 2006;15(1):39–44. doi: 10.1158/1055-9965.EPI-05-0470. [DOI] [PubMed] [Google Scholar]
- 9.Geisler J. Breast cancer tissue estrogens and their manipulation with aromatase inhibitors and inactivators. J Steroid Biochem Mol Biol. 2003;86(3–5):245–253. doi: 10.1016/S0960-0760(03)00364-9. [DOI] [PubMed] [Google Scholar]
- 10.Blankenstein MA, Szymczak J, Daroszewski J, Milewicz A, Thijssen JH. Estrogens in plasma and fatty tissue from breast cancer patients and women undergoing surgery for non-oncological reasons. Gynecol Endocrinol. 1992;6(1):13–17. doi: 10.3109/09513599209081001. [DOI] [PubMed] [Google Scholar]
- 11.Thijssen JH, van Landeghem AA, Poortman J. Uptake and concentration of steroid hormones in mammary tissues. Ann NY Acad Sci. 1986;464:106–116. doi: 10.1111/j.1749-6632.1986.tb15998.x. [DOI] [PubMed] [Google Scholar]
- 12.Thijssen JH, Blankenstein MA, Miller WR, Milewicz A. Estrogens in tissues: uptake from the peripheral circulation or local production. Steroids. 1987;50(1–3):297–306. doi: 10.1016/0039-128X(83)90079-X. [DOI] [PubMed] [Google Scholar]
- 13.Lonning PE, Helle H, Duong NK, Ekse D, Aas T, Geisler J. Tissue estradiol is selectively elevated in receptor positive breast cancers while tumour estrone is reduced independent of receptor status. J Steroid Biochem Mol Biol. 2009;117(1–3):31–41. doi: 10.1016/j.jsbmb.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 14.Blankenstein MA, Maitimu-Smeele I, Donker GH, Daroszewski J, Milewicz A, Thijssen JH. On the significance of in situ production of oestrogens in human breast cancer tissue. J Steroid Biochem Mol Biol. 1992;41(3–8):891–896. doi: 10.1016/0960-0760(92)90443-M. [DOI] [PubMed] [Google Scholar]
- 15.Chetrite GS, Cortes-Prieto J, Philippe JC, Wright F, Pasqualini JR. Comparison of estrogen concentrations, estrone sulfatase and aromatase activities in normal, and in cancerous, human breast tissues. J Steroid Biochem Mol Biol. 2000;72(1–2):23–27. doi: 10.1016/S0960-0760(00)00040-6. [DOI] [PubMed] [Google Scholar]
- 16.Pasqualini JR, Cortes-Prieto J, Chetrite G, Talbi M, Ruiz A. Concentrations of estrone, estradiol and their sulfates, and evaluation of sulfatase and aromatase activities in patients with breast fibroadenoma. Int J Cancer. 1997;70(6):639–643. doi: 10.1002/(SICI)1097-0215(19970317)70:6<639::AID-IJC2>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 17.Dooley WC, Ljung B-M, Veronesi U, Cassaniga M, Elledge RM, O'Shaughnessy JA, Kuerer HM, Hung DT, Khan SA, Phillips RF, Ganz PA, Euhus DM, Esserman LJ, Haffty BG, King BL, Kelley MC, Anderson MM, Schmit PJ, Clark RR, Kass FC, Anderson BO, Troyan SL, Arias RD, Quiring JN, Love SM, Page DL, King EB. Ductal lavage for detection of cellular atypia in women at high risk for breast cancer. J Natl Cancer Inst. 2001;93:1624–1632. doi: 10.1093/jnci/93.21.1624. [DOI] [PubMed] [Google Scholar]
- 18.Bhandare D, Nayar R, Bryk M, Hou N, Cohn R, Golewale N, Parker NP, Chatterton RT, Rademaker A, Khan SA. Endocrine biomarkers in ductal lavage samples from women at high risk for breast cancer. Cancer Epidemiol Biomark Prev. 2005;14(11):2620–2627. doi: 10.1158/1055-9965.EPI-05-0302. [DOI] [PubMed] [Google Scholar]
- 19.Love SM. Re: ductal lavage findings in women with known breast cancer undergoing mastectomy. J Natl Cancer Inst. 2005;97(11):857–858. doi: 10.1093/jnci/dji145. [DOI] [PubMed] [Google Scholar]
- 20.Jordan VC, Fritz NF, Langan-Fahey S, Thompson M, Tormey DC. Alteration of endocrine parameters in premenopausal women with breast cancer during long-term adjuvant therapy with tamoxifen as the single agent. J Natl Cancer Inst. 1991;83:1488–1491. doi: 10.1093/jnci/83.20.1488. [DOI] [PubMed] [Google Scholar]
- 21.Groom GV, Griffiths K. Effect of the anti-oestrogen tamoxifen on plasma levels of luteinizing hormone, follicle-stimulating hormone, prolactin, oestradiol and progesterone in normal pre-menopausal women. J Endocrinol. 1976;70(3):421–428. doi: 10.1677/joe.0.0700421. [DOI] [PubMed] [Google Scholar]
- 22.Kostoglou-Athanassiou I, Ntalles K, Gogas J, Markopoulos C, Evizou-Terzaki V, Athanassiou P, Georgiou E, Proukakis C. Sex hormones in postmenopausal women with breast cancer on tamoxifen. Horm Res. 1997;47(3):116–120. doi: 10.1159/000185445. [DOI] [PubMed] [Google Scholar]
- 23.Gail MH. The estimation and use of absolute risk for weighing the risks and benefits of selective estrogen receptor modulators for preventing breast cancer. Ann NY Acad Sci. 2001;949:286–291. doi: 10.1111/j.1749-6632.2001.tb04034.x. [DOI] [PubMed] [Google Scholar]
- 24.Khan SA, Lankes HA, Patil DB, Bryk M, Hou N, Ivancic D, Nayar R, Masood S, Rademaker A. Ductal lavage is an inefficient method of biomarker measurement in high-risk women. Cancer Prev Res. 2009;2(3):265–273. doi: 10.1158/1940-6207.CAPR-08-0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rochefort H. Cathepsin D in breast cancer. Breast Cancer Res Treat. 1990;16(1):3–13. doi: 10.1007/BF01806570. [DOI] [PubMed] [Google Scholar]
- 26.Couissi D, Dubois V, Remacle C, Schonne E, Trouet A. Western immunoblotting and enzymatic activity analysis of cathepsin D in human breast cancer cell lines of different invasive potential. Regulation by 17beta-estradiol, tamoxifen and ICI 182, 780. Clin Exp Metastasis. 1997;15(4):349–360. doi: 10.1023/A:1018489819092. [DOI] [PubMed] [Google Scholar]
- 27.Costantino JP, Gail MH, Pee D, Anderson S, Redmond CK, Benichou J, Wieand HS. Validation studies for models projecting the risk of invasive and total breast cancer incidence. J Natl Cancer Inst. 1999;91(18):1541–1548. doi: 10.1093/jnci/91.18.1541. [DOI] [PubMed] [Google Scholar]
- 28.Gingras S, Cote S, Simard J. Multiple signaling pathways mediate interleukin-4-induced 3beta-hydroxysteroid dehydrogenase/delta5-delta4 isomerase type 1 gene expression in human breast cancer cells. Mol Endocrinol. 2000;14(2):229–240. doi: 10.1210/me.14.2.229. [DOI] [PubMed] [Google Scholar]
- 29.Purohit A, Singh A, Reed MJ. Regulation of steroid sulphatase and oestradiol 17 beta-hydroxysteroid dehydrogenase in breast cancer. Biochem Soc Trans. 1999;27(2):323–327. doi: 10.1042/bst0270323. [DOI] [PubMed] [Google Scholar]
- 30.Purohit A, Tutill HJ, Day JM, Chander SK, Lawrence HR, Allan GM, Fischer DS, Vicker N, Newman SP, Potter BV, Reed MJ. The regulation and inhibition of 17beta-hydroxysteroid dehydrogenase in breast cancer. Mol Cell Endocrinol. 2006;248(1–2):199–203. doi: 10.1016/j.mce.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 31.Going JJ. Ductal-lobar organisation of human breast tissue, its relevance in disease and a research objective: vector mapping of parenchyma in complete breasts (the Astley Cooper project) Breast Cancer Res. 2006;8(4):107–107. doi: 10.1186/bcr1527. [DOI] [PMC free article] [PubMed] [Google Scholar]