Abstract
Purpose
Intensity-modulated radiation therapy (IMRT) and laparoscopic or robotic minimally invasive radical prostatectomy (MIRP) are costlier alternatives to three-dimensional conformal radiation therapy (3D-CRT) and open radical prostatectomy for treating prostate cancer. We assessed temporal trends in their utilization and their impact on national health care spending.
Methods
Using Surveillance, Epidemiology, and End Results–Medicare linked data, we determined treatment patterns for 45,636 men age ≥ 65 years who received definitive surgery or radiation for localized prostate cancer diagnosed from 2002 to 2005. Costs attributable to prostate cancer care were the difference in Medicare payments in the year after versus the year before diagnosis.
Results
Patients received surgery (26%), external RT (38%), or brachytherapy with or without RT (36%). Among surgical patients, MIRP utilization increased substantially (1.5% among 2002 diagnoses v 28.7% among 2005 diagnoses, P < .001). For RT, IMRT utilization increased substantially (28.7% v 81.7%; P < .001) and for men receiving brachytherapy, supplemental IMRT increased significantly (8.5% v 31.1%; P < .001). The mean incremental cost of IMRT versus 3D-CRT was $10,986 (in 2008 dollars); of brachytherapy plus IMRT versus brachytherapy plus 3D-CRT was $10,789; of MIRP versus open RP was $293. Extrapolating these figures to the total US population results in excess spending of $282 million for IMRT, $59 million for brachytherapy plus IMRT, and $4 million for MIRP, compared to less costly alternatives for men diagnosed in 2005.
Conclusion
Costlier prostate cancer therapies were rapidly and widely adopted, resulting in additional national spending of more than $350 million among men diagnosed in 2005 and suggesting the need for comparative effectiveness research to weigh their costs against their benefits.
INTRODUCTION
With approximately 180,000 new diagnoses per year,1 prostate cancer has been cited as a litmus test for health care spending and reform due to its rising costs of care.2 Over the past decade, newer and more expensive alternatives have been introduced for the treatment of prostate cancer. For men who choose surgery, minimally invasive radical prostatectomy (MIRP), which includes either laparoscopic or robotic-assisted surgery, is a costlier alternative to the traditional open RP due to the greater cost of disposables, equipment, and increased operating room time during a lengthy learning curve.3 For men who choose radiation, intensity-modulated radiation therapy (IMRT) is a more expensive alternative to traditional three-dimensional conformal radiation therapy (3D-CRT) due to more intense physics planning and quality assurance time, as well as treatment delivery time and software and hardware costs.4
Despite interest from patients and providers in these newer technologies, and belief by advocates that they could improve outcomes, there was only limited comparative effectiveness data when they were introduced, and to date there have been no randomized trials testing their clinical efficacy compared to traditional, less expensive counterparts. The purpose of this study is to characterize the adoption of these more expensive therapies among Medicare beneficiaries and to estimate the excess health care spending attributable to the increased utilization of these newer modalities.
METHODS
Data Source
Our study was approved by the Brigham and Women's institutional review board and a data-use agreement was in place with the Centers for Medicare and Medicaid Services; patient data were de-identified and the requirement for consent was waived. We used Surveillance, Epidemiology, and End Results (SEER) –Medicare data for analyses, composed of a linkage of population based cancer registry data from 16 SEER areas covering approximately 26% of the US population with Medicare administrative data. The Medicare program provides benefits to 97% of Americans age 65 years or older.5
Defining the Study Cohort and Exclusion Criteria
We identified 103,363 men age 65 years or older in the SEER registry with pathologically confirmed prostate cancer from 2002 to 2005, who had no history of other malignancies. We excluded men enrolled in a health maintenance organization or not enrolled in both Medicare Part A and Part B throughout the duration of the study because claims are not reliably submitted for such men. We also excluded men who were missing a date of diagnosis or had metastatic disease. This reduced the cohort to 71,674 men, of which 58,571 men underwent some form of treatment with follow-up through December 31, 2007. The focus of our study was men who underwent surgery or radiation, so we excluded 11,093 men who received primary androgen deprivation therapy and 1,205 who received cryotherapy. We also excluded 619 men who all received proton therapy at a single center because their trends results would not be generalizable. The final study cohort was 45,636 patients.
Determination of Surgery and Radiation Therapies
Treatment type was identified from Medicare inpatient, outpatient, and carrier component files (formerly physician/provider B files) based on the presence of Current Procedural Terminology, Fourth Edition (CPT-4) codes listed in Appendix Table A1 (online only). Brachytherapy and external RT were considered as part of a combination therapy if they were given within 6 months of each other.
Determination of Treatment Cost
To determine the cost of therapy, we summed the total amount paid by Medicare for inpatient, outpatient, and physician services within 12 months of prostate cancer diagnosis.6 To ensure that we adequately captured the cost of treatment, we included in our cost analysis only men who began treatment within 6 months of the prostate cancer diagnosis. Using each subject as his own control, we subtracted health expenditures accrued in the 12 months before prostate cancer diagnosis, which we considered baseline annual health care costs, from 12-month expenditures after prostate cancer diagnosis.7 This difference captures the cost of treatment and other services such as preoperative evaluation, imaging, laboratory tests, and treatment of complications within 1 year. The mean cost of each therapy was then tabulated and stratified by the year of diagnosis. All costs were adjusted to 2008 dollars using the 2007 Annual Report of the Boards of Trustees of the Federal Hospital Insurance and Federal Supplementary Medical Insurance Trust Fund Table 5.B.1 HI and SMI Average Per Beneficiary Costs (HI = Part A; SMI = Part B).
Determination of the Excess Direct Medical Spending on More Expensive Therapies at the National Level
To estimate the total amount spent nationwide on more expensive prostate cancer therapies for men of any age, we identified the total number of patients in the US diagnosed with nonmetastatic prostate cancer in 2005 from the SEER limited-use registry treated with surgery, external beam radiation, or brachytherapy plus external beam radiation.8 We divided these figures by 0.26 to extrapolate national estimates of the number of people receiving each treatment since the SEER registry captures 26% of the US population. We multiplied the number in each treatment category (eg, surgery), by the proportion expected to receive the more expensive therapy to determine the expected number of people receiving the expensive therapy nationwide. The observed rates of utilization found in our cohort were adjusted for demographic differences between the cohort and the US population to develop expected utilization rates applicable to the US population. The number of people receiving each expensive therapy was then multiplied by the mean cost of each therapy to estimate national spending.9
Statistical Analyses
Temporal trends in use of the more expensive therapy were examined using the Mantel-Haenszel test for trend. The χ2 test was used to determine the factors associated with the receipt of the more expensive therapy. A P value of lower than .05 was considered statistically significant. We developed directly standardized rates of utilization that would be expected in the general population by weighing each patient in our cohort by the ratio of patients in general population to SEER-Medicare for the strata of demographic characteristics to which each patient belongs.10 All analyses were performed using SAS version 9.1.3 (SAS Institute Inc, Cary, NC).
RESULTS
Utilization Trends
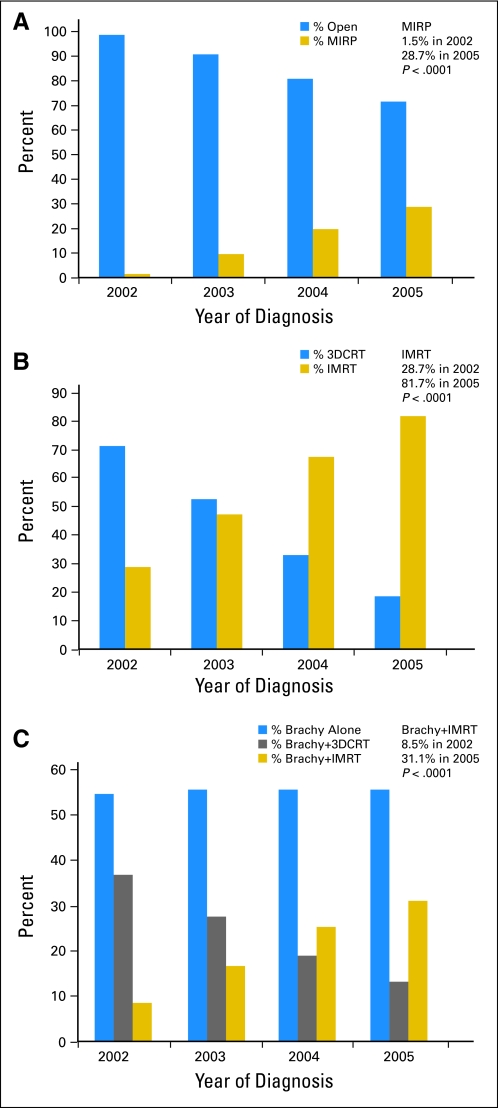
The characteristics of the study cohort are listed in Table 1, stratified by treatment modality. Of the cohort, 11,894 (26%) received surgery, 17,274 (38%) received external radiation, and 16,468 (36%) received brachytherapy with or without external radiation as their primary therapy (year-by-year analysis in Appendix Table A2, online only). Figures 1A-C demonstrate rapidly increased utilization of the more expensive therapies over the study period. Among men undergoing surgery, MIRP was used by 1.5% of those diagnosed in 2002 versus 28.7% of those diagnosed in 2005 (P < .001), while IMRT was used by 28.7% in 2002 versus 81.7% in 2005 (P < .001) of those undergoing external radiation, and supplemental IMRT was used for 8.5% in 2002 versus 31.1% in 2005 (P < .001) among those receiving brachytherapy. Among just the subgroup of brachytherapy patients receiving supplemental external radiation, supplemental IMRT was used by 18.7% versus 70.2% (P < .001). Correspondingly, the use of each of the less expensive therapies (open RP, 3D conformal RT, and brachytherapy plus 3D conformal RT) decreased.
Table 1.
Baseline Patient Characteristics Stratified by Primary Curative Modality Chosen
| Variable | Brachytherapy |
External RT |
Surgery |
P | |||
|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | ||
| Race | |||||||
| White | 13,247 | 80.44 | 13,326 | 77.14 | 9,498 | 79.86 | < .001 |
| Black | 1,470 | 8.93 | 1,716 | 9.93 | 910 | 7.65 | |
| Hispanic | 842 | 5.11 | 1,058 | 6.12 | 904 | 7.60 | |
| Asian | 592 | 3.59 | 795 | 4.60 | 441 | 3.71 | |
| Other/unknown | 317 | 1.92 | 379 | 2.19 | 141 | 1.19 | |
| Age at diagnosis, years | |||||||
| 65-69 | 5,591 | 33.95 | 3,969 | 22.98 | 7,435 | 62.51 | < .0001 |
| 70-74 | 5,915 | 35.92 | 5,793 | 33.54 | 3,589 | 30.17 | |
| 75-79 | 4,962 | 30.13 | 7,512 | 43.49 | 870 | 7.31 | |
| High school education in patient's census region, % | |||||||
| < 75/unknown | 3,453 | 20.97 | 3,906 | 22.61 | 2,377 | 19.98 | < .0001 |
| 75-84 | 3,546 | 21.53 | 4,064 | 23.53 | 2,368 | 19.91 | |
| 85-89 | 3,118 | 18.93 | 3,255 | 18.84 | 2,213 | 18.61 | |
| 90+ | 6,351 | 38.57 | 6,049 | 35.02 | 4,936 | 41.50 | |
| Median income, $ | |||||||
| < 35,000/unknown | 5,244 | 31.85 | 6,686 | 38.70 | 3,590 | 30.18 | < .0001 |
| 35,000-44,000 | 3,905 | 23.71 | 4,017 | 23.25 | 2,812 | 23.64 | |
| 45,000-59,000 | 3,921 | 23.81 | 3,634 | 21.04 | 2,736 | 23.00 | |
| ≥ 60,000 | 3,398 | 20.63 | 2,937 | 17.00 | 2,756 | 23.17 | |
| Region* | |||||||
| Northeast | 4,936 | 29.97 | 4,362 | 25.25 | 1,414 | 11.89 | < .0001 |
| South | 3,365 | 20.43 | 2,733 | 15.82 | 1,975 | 16.61 | |
| Midwest | 1,751 | 10.63 | 3,202 | 18.54 | 1,634 | 13.74 | |
| West | 6,416 | 38.96 | 6,977 | 40.39 | 6,871 | 57.77 | |
| SEER registry | |||||||
| San Francisco | 605 | 3.67 | 592 | 3.43 | 488 | 4.10 | < .0001 |
| Michigan | 1,137 | 6.90 | 2,029 | 11.75 | 916 | 7.70 | |
| New Mexico/Georgia/Hawaii | 1,526 | 9.27 | 1,145 | 6.63 | 770 | 6.47 | |
| Iowa | 614 | 3.73 | 1,173 | 6.79 | 718 | 6.04 | |
| Seattle | 1,092 | 6.63 | 745 | 4.31 | 909 | 7.64 | |
| Utah | 959 | 5.82 | 209 | 1.21 | 693 | 5.83 | |
| Connecticut | 978 | 5.94 | 1,552 | 8.98 | 448 | 3.77 | |
| San Jose | 433 | 2.63 | 375 | 2.17 | 246 | 2.07 | |
| Los Angele | 672 | 4.08 | 1,283 | 7.43 | 1,275 | 10.72 | |
| Greater California | 2,199 | 13.35 | 2,943 | 17.04 | 2,742 | 23.05 | |
| Kentucky | 1,178 | 7.15 | 1,261 | 7.30 | 684 | 5.75 | |
| Louisiana | 1,117 | 6.78 | 1,157 | 6.70 | 1,039 | 8.74 | |
| New Jersey | 3,958 | 24.03 | 2,810 | 16.27 | 966 | 8.12 | |
| Population density | |||||||
| Metropolitan | 15,192 | 92.25 | 15,619 | 90.42 | 10,896 | 91.61 | < .0001 |
| Nonmetropolitan | 1,276 | 7.75 | 1,655 | 9.58 | 998 | 8.39 | |
| Marital status | |||||||
| Not married | 3,024 | 18.36 | 3,579 | 20.72 | 1,792 | 15.07 | < .0001 |
| Married | 12,106 | 73.51 | 11,959 | 69.23 | 9,509 | 79.95 | |
| Unknown | 1,338 | 8.12 | 1,736 | 10.05 | 593 | 4.99 | |
| Grade | |||||||
| Well | 224 | 1.36 | 224 | 1.30 | 158 | 1.33 | < .001 |
| Moderate | 11,067 | 67.20 | 9,210 | 53.32 | 6,451 | 54.24 | |
| Poorly/undifferentiated | 4,849 | 29.44 | 7,530 | 43.59 | 5,211 | 43.81 | |
| Unknown | 328 | 1.99 | 310 | 1.79 | 74 | 0.62 | |
| Clinical stage | |||||||
| T1 | 7,880 | 47.85 | 7,246 | 41.95 | 5,149 | 43.29 | < .001 |
| T2 | 8,049 | 48.88 | 8,905 | 51.55 | 6,365 | 53.51 | |
| T3 | 267 | 1.62 | 603 | 3.49 | 174 | 1.46 | |
| T4 | 16 | 0.10 | 137 | 0.79 | 21 | 0.18 | |
| Unknown | 256 | 1.55 | 383 | 2.22 | 185 | 1.56 | |
| Charlson score | |||||||
| 0 | 11,860 | 72.02 | 11,516 | 66.67 | 9,412 | 79.13 | < .001 |
| 1 | 3,230 | 19.61 | 3,765 | 21.80 | 1,760 | 14.80 | |
| 2+ | 1,153 | 7.00 | 1,763 | 10.21 | 448 | 3.77 | |
| Unknown | 225 | 1.37 | 230 | 1.33 | 274 | 2.30 | |
| Total | 16,468 | 36 | 17,274 | 38 | 11,894 | 26 | |
NOTE. Education had 24 unknown, income had 26 unknown. For men diagnosed in 2002, well differentiated refers to a Gleason score of 2-4, moderately differentiated is Gleason 5-7, and poorly differentiated is Gleason 8-10, but for men diagnosed from January 1, 2003 onward, poorly differentiated was designated as Gleason 7. Region categorization: northeast: Connecticut and New Jersey; south, Atlanta, rural Georgia, Kentucky, and Louisiana; west: San Francisco, Hawaii, New Mexico, Seattle, Utah, San Jose, Los Angeles, and greater California; and midwest: Detroit and Iowa. Comorbidity is the Klabunde modification of the Charlson Index.21
Abbreviation: RT, radiation therapy.
Fig 1.
(A) Increasing use of minimally invasive radical prostatectomy (MIRP) among patients receiving surgery. (B) Increasing use of intensity-modulated radiation therapy (IMRT) among patients receiving external radiation. (C) Increasing use of supplemental IMRT among patients receiving brachytherapy (Brachy). 3D-CRT, three-dimensional conformal radiation therapy.
Predictors of Utilization
Table 2 presents a multivariable logistic regression of the factors associated with receiving more expensive therapy. Univariable analysis is listed in Appendix Table A3 (online only). The factors consistently associated with receiving the more expensive therapy regardless of whether they chose surgery or radiation were living in an area with median income ≥ $60,000, living in a metropolitan rather than rural area, having T1c disease, and being of Asian descent (all P < .05). The pattern of association with other demographic variables was less consistent. In our cohort of patients older than 65 years, the patients older than 75 years made up only 7% of those receiving MIRP, but were 33% of those receiving brachytherapy plus IMRT and 44% of those receiving IMRT. However, age was not a consistent significant predictor of utilization of more expensive therapies.
Table 2.
Multivariable Logistic Analysis of Factors Associated With More Expensive Therapy
| Variable | MIRP v Open RP |
IMRT v 3DCRT |
Brachy/IMRT v Brachy/3DCRT |
||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95%CI | P | OR | 95%CI | P | OR | 95%CI | P | |
| Outcome | MIRP | IMRT | Brachy/IMRT | ||||||
| Age at diagnosis, years | |||||||||
| 65-69 | 1.09 | 0.88 to 1.36 | .4204 | 1.18 | 1.09 to 1.28 | < .001 | 0.96 | 0.84 to 1.08 | .4813 |
| 70-74 | 1.1 | 0.87 to 1.38 | .4312 | 1.05 | 0.98 to 1.13 | .1522 | 1.03 | 0.91 to 1.16 | .6409 |
| 75+ | 1.00 | ref | 1.00 | ref | 1.00 | ref | |||
| Comorbidity | |||||||||
| 0 | 1.1 | 0.82 to 1.48 | .5253 | 1.14 | 1.03 to 1.26 | .0135 | 0.97 | 0.81 to 1.17 | .7458 |
| 1 | 0.96 | 0.7 to 1.33 | .8258 | 1.01 | 0.9 to 1.13 | .876 | 0.99 | 0.81 to 1.21 | .9107 |
| 2+ | 1.00 | ref | 1.00 | ref | 1.00 | ref | |||
| Race | |||||||||
| White/Non-Hispanic | 1.00 | ref | 1.00 | ref | 1.00 | ref | |||
| Black/Non-Hispanic | 0.91 | 0.71 to 1.15 | .4284 | 1.18 | 1.06 to 1.33 | .0034 | 1.17 | 0.99 to 1.38 | .0608 |
| Hispanic | 0.74 | 0.57 to 0.98 | .0342 | 1.16 | 1 to 1.35 | .0461 | 1.33 | 1.06 to 1.66 | .0121 |
| Asian/Non-Hispanic | 1.51 | 1.18 to 1.93 | .0011 | 1.49 | 1.27 to 1.76 | < .001 | 1.43 | 1.11 to 1.86 | .0062 |
| Other/unknown | 1.03 | 0.65 to 1.66 | .8868 | 1.21 | 0.97 to 1.51 | .0894 | 1.27 | 0.84 to 1.93 | .2561 |
| High school education in patient's census region, % | |||||||||
| < 75 | 1.00 | ref | 1.00 | ref | 1.00 | ref | |||
| 75-84.99 | 0.99 | 0.8 to 1.22 | .9448 | 1.24 | 1.12 to 1.38 | < .001 | 1.06 | 0.9 to 1.25 | .4966 |
| 85-89.99 | 0.79 | 0.62 to 0.99 | .0402 | 1.3 | 1.16 to 1.46 | < .001 | 1.25 | 1.04 to 1.51 | .0176 |
| 90+ | 0.74 | 0.58 to 0.93 | .0111 | 1.52 | 1.35 to 1.73 | < .001 | 1.15 | 0.95 to 1.4 | .1619 |
| Median income, $ | |||||||||
| < 35,000 | 1.00 | ref | 1.00 | ref | 1.00 | ref | |||
| 35,000-44,999 | 1.49 | 1.24 to 1.79 | < .001 | 1.02 | 0.93 to 1.12 | .6857 | 0.99 | 0.85 to 1.15 | .8532 |
| 45,000-59,999 | 1.91 | 1.57 to 2.33 | < .001 | 1.13 | 1.02 to 1.26 | .0228 | 0.99 | 0.83 to 1.17 | .8912 |
| ≥ 60,000 | 3.1 | 2.49 to 3.85 | < .001 | 1.47 | 1.29 to 1.67 | < .001 | 1.31 | 1.07 to 1.59 | .0075 |
| Region | |||||||||
| West | 1.00 | ref | 1.00 | ref | 1.00 | ref | |||
| Northeast | 0.95 | 0.8 to 1.12 | .5351 | 1.03 | 0.95 to 1.12 | .4834 | 2.17 | 1.91 to 2.47 | < .001 |
| South | 0.73 | 0.61 to 0.88 | .0009 | 0.74 | 0.67 to 0.82 | < .001 | 1.65 | 1.43 to 1.91 | < .001 |
| Midwest | 1.39 | 1.19 to 1.63 | < .001 | 0.64 | 0.58 to 0.7 | < .001 | 0.57 | 0.47 to 0.7 | < .001 |
| Marital status | |||||||||
| Unmarried | 1.00 | ref | 1.00 | ref | 1.00 | ref | |||
| Married | 0.99 | 0.84 to 1.16 | .8818 | 1.04 | 0.96 to 1.12 | .3355 | 1 | 0.88 to 1.13 | .9599 |
| Unknown | 2.37 | 1.86 to 3.04 | < .001 | 1.17 | 1.03 to 1.32 | .0132 | 1.92 | 1.54 to 2.4 | < .001 |
| Population density | |||||||||
| Metropolitan | 1.00 | ref | 1.00 | ref | 1.00 | ref | |||
| Nonmetropolitan county | 0.75 | 0.58 to 0.97 | .0307 | 0.76 | 0.67 to 0.85 | < .001 | 0.52 | 0.41 to 0.66 | < .001 |
| Grade/differentiation | |||||||||
| Well | 1.00 | ref | 1.00 | ref | 1.00 | ref | |||
| Moderately | 1.09 | 0.62 to 1.93 | .7538 | 1.13 | 0.86 to 1.49 | .3752 | 0.86 | 0.5 to 1.46 | .5726 |
| Poorly | 1.58 | 0.9 to 2.78 | .1149 | 1.73 | 1.32 to 2.28 | < .001 | 1.1 | 0.65 to 1.88 | .7175 |
| Unknown/missing | 1.26 | 0.51 to 3.13 | .6222 | 0.96 | 0.67 to 1.38 | .8371 | 0.73 | 0.38 to 1.38 | .3313 |
| Clinical stage | |||||||||
| T1 | 1.00 | ref | 1.00 | ref | 1.00 | ref | |||
| T2 | 0.61 | 0.54 to 0.68 | < .001 | 0.71 | 0.66 to 0.76 | < .001 | 0.63 | 0.57 to 0.7 | < .001 |
| T3 | 0.53 | 0.33 to 0.86 | .0104 | 0.67 | 0.57 to 0.8 | < .001 | 0.71 | 0.53 to 0.94 | .0169 |
| T4 | 0.36 | 0.08 to 1.62 | .1853 | 0.45 | 0.32 to 0.65 | < .001 | 0.71 | 0.23 to 2.23 | .5637 |
| Unknown/missing | 0.29 | 0.15 to 0.56 | .0002 | 0.72 | 0.58 to 0.9 | .0038 | 0.8 | 0.51 to 1.25 | .3183 |
NOTE. Boldface indicates statistical significance.
Abbreviations: MIRP, minimally invasive radical prostatectomy; Open RP, open radical prostatectomy; IMRT, intensity-modulated radiation therapy; 3DCRT, three-dimensional conformal radiation therapy; Brachy, brachytherapy; ref, referent.
Cost of Therapy
Table 3 displays the mean cost of each primary therapy in 2008 dollars stratified by their year of diagnosis. Costs for each treatment declined significantly from 2002 to 2005 (all P ≤ .001). For example, in constant 2008 dollars, IMRT costs fell by 15% from $37,125 to $31,574, brachytherapy plus IMRT costs fell by 16% from $43,723 to $36,795, and MIRP costs fell by 23% from $21,325 (in 2003 since the 2002 estimates are based on small numbers) to $16,469. Nevertheless, newer, more expensive treatments remained costlier than their less expensive alternatives over the study period. Specifically, among men diagnosed in 2005, the mean cost difference between IMRT and 3D-CRT was $10,986. Similarly, the cost difference between brachytherapy plus IMRT and brachytherapy plus 3D-CRT was $10,789, while the cost difference between MIRP and open RP was only $293. In Appendix Table A4 (online only), costs were alternatively estimated by matching controls from the Medicare 5% noncancer sample as outlined by Brown et al.6
Table 3.
Mean Cost of Each Primary Therapy Among Medicare Enrollees, Stratified by Year of Diagnosis
| Year | $ |
||||||
|---|---|---|---|---|---|---|---|
| 3DCRT | IMRT | Brachy | Brachy+ 3DCRT | Brachy+ IMRT | Open RP | MIRP | |
| 2002 | 22,384 | 37,125 | 21,117 | 28,770 | 43,723 | 18,070 | 29,988 |
| 2003 | 23,542 | 37,418 | 19,476 | 27,320 | 43,364 | 17,423 | 21,325 |
| 2004 | 22,023 | 33,237 | 18,308 | 26,756 | 39,453 | 16,930 | 17,645 |
| 2005 | 20,588 | 31,574 | 17,076 | 26,006 | 36,795 | 16,469 | 16,762 |
| P trend | < .001 | < .001 | < .001 | < .001 | < .001 | < .001 | .001 |
Abbreviations: 3DCRT, three-dimensional conformal radiation therapy; IMRT, intensity-modulated radiation therapy; Brachy, brachytherapy; Open RP, open radical prostatectomy; MIRP, minimally invasive radical prostatectomy.
Estimate of Excess Direct Medical Spending on Costlier Therapies at the National Level
Compared to the less costly alternative, the nationwide excess direct spending (Table 4) for the rapid adoption of more expensive therapies was $282 million for IMRT, $59 million for brachytherapy plus IMRT, and $4 million for MIRP for men diagnosed in 2005 (assuming that all treatments were reimbursed at Medicare rates).
Table 4.
Estimates of Additional Direct Costs As a Result of Newer Technologies
| Year | MIRP v Open RP |
||||||
|---|---|---|---|---|---|---|---|
| Utilization of MIRP From Our Cohort | Weighted Estimated Utilization of MIRP in US | Total No. in SEER Who Underwent Surgery | Estimated Total No. in the US Who Underwent Surgery | Estimated No. of MIRP in the US | Mean Cost Difference Between MIRP and Open RP ($) | Total Cost Savings If All MIRP in US Changed to Open RP ($) | |
| 2002 | 1.49 | 1.14 | 15,368 | 59,108 | 674 | 11,918 | 8,030,720 |
| 2003 | 9.48 | 7.78 | 14,760 | 56,769 | 4,417 | 3,902 | 17,233,683 |
| 2004 | 19.59 | 18.17 | 15,360 | 59,077 | 10,734 | 715 | 7,675,018 |
| 2005 | 28.66 | 25.17 | 13,866 | 53,331 | 13,423 | 293 | 3,933,060 |
| Year | IMRT v 3D-CRT |
||||||
|---|---|---|---|---|---|---|---|
| Utilization of IMRT From Our Cohort | Weighted Estimated Utilization of IMRT in US | Total No. in SEER Who Underwent RT | Estimated Total No. in the US Who Underwent RT | Estimated No. of IMRT in the US | Mean Cost Difference Between IMRT and 3DCRT ($) | Total Saving Cost If All IMRT in US Changed to 3DCRT ($) | |
| 2002 | 28.65 | 23.35 | 10,656 | 40,985 | 9,570 | 14,741 | 141,071,333 |
| 2003 | 47.20 | 39.62 | 10,148 | 39,031 | 15,464 | 13,876 | 214,579,605 |
| 2004 | 67.31 | 58.80 | 10,006 | 38,485 | 22,629 | 11,214 | 253,763,625 |
| 2005 | 81.66 | 74.18 | 8990 | 34,577 | 25,649 | 10,986 | 281,782,316 |
| Year | Brachy/IMRT v Brachy/3D-CRT |
||||||
|---|---|---|---|---|---|---|---|
| Utilization of Brachy/IMRT From Our Cohort | Weighted Estimated Utilization of Brachy/IMRT in US | Total No. in SEER Who Underwent Brachy + RT | Estimated Total No. in the US Who Underwent Brachy + RT | Estimated No. of Brachy/IMRT in the US | Mean Cost Difference Between Brachy/IMRT and Brachy/EBRT ($) | Total Cost Savings If All Brachy/IMRT in US Changed to Brachy/EBRT ($) | |
| 2002 | 18.66 | 15.51 | 2,914 | 11,208 | 1,738 | 14,953 | 25,993,709 |
| 2003 | 37.54 | 36.49 | 2,136 | 8,215 | 2,998 | 16,044 | 48,094,353 |
| 2004 | 57.26 | 53.72 | 1,931 | 7,427 | 3,990 | 12,697 | 50,658,293 |
| 2005 | 70.19 | 71.27 | 2,000 | 7,692 | 5,482 | 10,789 | 59,146,252 |
Abbreviations: MIRP, minimally invasive radical prostatectomy; Open RP, open radical prostatectomy; SEER, Surveillance, Epidemiology, and End Results database; IMRT, intensity-modulated radiation therapy; Brachy, brachytherapy; 3DCRT, three-dimensional conformal radiation therapy.
DISCUSSION
Our study has several important findings. First, we found a rapid and substantial increase in the utilization of MIRP, IMRT, and brachytherapy plus IMRT, which are more expensive alternatives to traditional open RP, 3D-CRT, and brachytherapy plus 3D-CRT, respectively. Men who received the more expensive therapies tended to reside in wealthier areas, and in metropolitan as opposed to rural areas, possibly due to the greater availability of newer technologies in these locations or greater marketing efforts directed toward their inhabitants. Of note, Asian race was consistently associated with 1.5-fold odds of receiving a more expensive therapy compared with white race, but the underlying reasons for this could not be determined from this study. Men undergoing the more expensive therapies also tended to have lower stage disease, which may reflect increased screening in more affluent populations, or perhaps a provider bias of offering these therapies to patients who will likely be cured of their prostate cancer and thereby have more time to benefit from any perceived reduction in long-term toxicity.
There are no randomized trials assessing whether newer treatments such as MIRP or IMRT have any clinical benefit over their less-expensive counterparts; the only available data currently come from retrospective studies. For instance, an observational, population-based study comparing outcomes after MIRP versus open RP found that MIRP appeared to be associated with a shorter length of stay (2 v 3 days), fewer transfusions (2.7% v 20.8%), fewer postoperative respiratory complications (4.3% v 6.6%), and fewer anastomotic strictures (5.8% v 14.0%). However, MIRP was also associated with an increased risk of genitourinary complications (4.7% v 2.1%) and diagnoses of incontinence (15.9 per v 12.2 per 100 person-years) and erectile dysfunction (26.8 v 19.2 per 100 person-years).11 For external radiation, retrospective studies seem to consistently suggest that IMRT is associated with a significant reduction in long-term rectal bleeding compared to 3D-CRT. Zelefsky et al demonstrated that men treated to 81 Gy with IMRT versus conformal radiation experienced a significantly lower risk of ≥ grade 2 rectal bleeding, (2% v 14%, respectively), and other retrospective series have had similar findings.12–14
However, even if there is some underlying clinical benefit to these newer more expensive therapies, it is still important to ask whether the marginal benefit of these therapies is large enough to justify their higher cost.
We found that the rapid shift to more expensive therapies versus less costly counterparts resulted in a national cost burden of more than $350 million among patients diagnosed in 2005. Specifically, Medicare expenditures for IMRT were nearly $11,000 greater per case compared to 3D-CRT and were also nearly $11,000 greater per case for brachytherapy plus IMRT compared to brachytherapy plus 3D-CRT. While the Medicare expenditures for MIRP appeared to be only $236 more per case than for open radical prostatectomy, this surgical amount only approximates the difference in Medicare reimbursed surgeon fees between MIRP and open RP, and does not nearly reflect the full extent of the underlying cost difference between the surgical procedures. For instance, the most widespread form of MIRP presently is robotic-assisted prostatectomy, which requires at least a $1.4 million upfront investment to purchase the robot and then a $140,000 annual maintenance for the robot.3 Importantly, while private health plans may reimburse a facility fee, Medicare does not reimburse for the use of the robot. Therefore, this fixed component of the costs cannot be accounted for by a Medicare claims–based analysis, which makes the cost difference between open RP and MIRP seem artificially small. Moreover, our Medicare-based cost estimates likely underestimate the true expense of the rapid shift to newer, more costly technologies, as Medicare typically reimburses a lower amount compared to private health plans.
Just as the newer technologies have been widely adopted without rigorous efficacy trials, they have also been adopted without robust cost-effectiveness analysis. To our knowledge, there are no data on the cost-effectiveness of MIRP. As for the cost-effectiveness of IMRT, a study by Konski et al suggested that based on its likely reduction in rectal toxicity, IMRTs incremental cost per quality-adjusted life year was $40,101, which meets the typical requirement that treatments have an incremental cost/quality-adjusted life year lower than $50,000 to be considered cost-effective.15 However, that article was not published until 2006, and this study suggests that by then, 81% of external radiation patients were already receiving IMRT, making it likely that even if IMRT were found to not be cost effective, it would have been nearly impossible to reverse the nationwide trend in its use.
This research has implications for predicting the patterns of use of other newer and more expensive technologies in health care, as these trends are likely not unique to prostate cancer. It suggests that when a newer expensive technology becomes available and is reimbursed by health plans, it is likely to be rapidly adopted even before there is adequate data on its clinical benefits and cost effectiveness. This study may also inform the debate about the use of proton therapy for prostate cancer. Proton therapy carries a significantly higher price tag than IMRT, with some estimates showing it is about twice as expensive.16 There are also significant marketing efforts promoting protons for prostate cancer and growing patient interest in receiving it. While protons are likely less toxic for certain pediatric and CNS tumors,17,18 it remains unknown whether protons for prostate cancer are superior to IMRT in terms of cancer control or toxicity, and there is great uncertainty about whether proton therapy for prostate cancer could be cost-effective.16,19 Nevertheless, if protons become more widely available, the trends seen in the rapid uptake of IMRT for prostate cancer may well be repeated with proton therapy.
Proponents of allowing the widespread adoption of higher-cost therapies before they are proven may point out that as a technology becomes more widely used, its costs will decrease over time. This is in fact reflected in Table 3, which shows the mean cost of IMRT falling by 20% from 2002 to 2005, and of MIRP falling by 12% over the same time period. These drops in the inflation-adjusted cost of each prostate cancer therapy are corroborated by other reports.7 As the prices of these newer technologies falls, the likelihood that they will become cost effective can theoretically increase. However, it should be noted that the costs of the less-expensive therapies were also falling over that same time period. If the cost of the less expensive therapy is also falling, then the more expensive therapy may remain equally cost-ineffective despite its lower absolute price tag.
This study has certain limitations. First, we may have overestimated the excess costs of the new therapies because we could only look at direct Medicare costs, and could not factor in the potential indirect cost benefits, such as MIRP potentially leading to fewer missed working days for patients. In addition, our 12-month cost methodology cannot capture potential long-term savings from toxicity reduction, such as IMRT potentially reducing the need for late interventions for rectal bleeding. We also could not account for any potential long-term savings that could be due to higher cure rates and lower need for salvage therapies. Also, as more surgeons performing MIRP overcome their learning curves, the cost differentials between MIRP and open RP may fall. Conversely, we may have underestimated the excess costs because to be consistent with other cost studies we only accounted for direct Medicare payments and excluded payments made by beneficiaries and supplemental insurance. Accounting for these additional payments would have increased our estimated excess expenditures by approximately 30%. Finally, as mentioned above, the cost estimates were entirely based on patients enrolled in Medicare, and applying the mean Medicare costs to younger patients who may have private insurance that reimburses at higher rates likely leads to an underestimate of the true nationwide expenditures on the more expensive therapies.
Despite limited comparative effectiveness research, newer and costlier prostate cancer therapies were rapidly and widely adopted, resulting in an excess national spending of more than $350 million among men diagnosed in 2005. This pattern of rapid adoption may provide some empirical evidence for why health care costs account for 17% of the US gross domestic product,20 and suggests the need for increased comparative effectiveness research to accurately weigh costs and benefits.
Appendix
Table A1.
Codes Used to Determine Treatment
| Modality | Code |
|---|---|
| 3DCRT | CPT-4 codes 77402 77403 77404 77406 77407 77408 77409 77411 77412 77413 77414 77416 77373 77421 77435 and ICD-9 procedure code 9224 |
| Brachytherapy | ICD-9 codes 9227 4604 4610 or CPT-4 codes 55859 55860 55862 55865 76873 76968 77326 77327 77328 77761 77762 77763 77776 77777 77778 77781 -77784 77790 77799 C1164 C1174 C1325 C1350 C1700-C1712 C1715-C1720 C1728 C1790-C1806 G0256 G0261 Q3001 77785- 77787 55875 C2638 C2639 C2640 C2641 |
| IMRT | CPT-4 77418 |
| MIRP | CPT-4 55866 |
| Open RP | CPT-4 codes 55840 55842 and 55845 |
Abbreviations: 3DCRT, three-dimensional conformal radiation therapy; CPT-4, Current Procedural Terminology, fourth edition; ICD-9, International Classification of Diseases, ninth revision; IMRT, intensity-modulated radiation therapy; MIRP, minimally invasive radical prostatectomy; Open RP, open radical prostatectomy.
Table A2.
Percent Utilization of Each Major Type of Treatment in Our Cohort, Stratified by Age
| Parameter | % |
|||
|---|---|---|---|---|
| Surgery | External RT | Brachy + RT | Brachy | |
| Total cohort | ||||
| 2002 | 24 | 39 | 17 | 20 |
| 2003 | 26 | 38 | 16 | 20 |
| 2004 | 27 | 37 | 16 | 20 |
| 2005 | 27 | 37 | 16 | 20 |
| Total | 26 | 38 | 16 | 20 |
| Age 65-74 | ||||
| 2002 | 32 | 31 | 17 | 20 |
| 2003 | 34 | 31 | 16 | 19 |
| 2004 | 35 | 30 | 15 | 20 |
| 2005 | 35 | 29 | 15 | 20 |
| Total | 34 | 30 | 16 | 20 |
| Age 75+ | ||||
| 2002 | 6 | 57 | 17 | 20 |
| 2003 | 6 | 56 | 17 | 21 |
| 2004 | 7 | 56 | 16 | 20 |
| 2005 | 7 | 56 | 17 | 20 |
| Total | 7 | 56 | 17 | 20 |
Abbreviations: RT, radiation therapy; Brachy, brachytherapy.
Table A3.
Factors Associated With Use of the More Expensive Therapy (univariable analysis)
| Category | 3DCRT |
IMRT |
P | Open RP |
MIRP |
P | Brachy/3DCRT |
Brachy/IMRT |
P | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | ||||
| Race | |||||||||||||||
| White | 6,110 | 77.91 | 7,216 | 76.51 | < .001 | 8,089 | 79.62 | 1,409 | 81.21 | < .001 | 3,218 | 78.66 | 2,448 | 75.18 | .007 |
| Black | 824 | 10.51 | 892 | 9.46 | 810 | 7.97 | 100 | 5.76 | 433 | 10.58 | 395 | 12.13 | |||
| Hispanic | 486 | 6.20 | 572 | 6.06 | 807 | 7.94 | 97 | 5.59 | 228 | 5.57 | 224 | 6.88 | |||
| Asian | 266 | 3.39 | 529 | 5.61 | 340 | 3.35 | 101 | 5.82 | 159 | 3.89 | 133 | 4.08 | |||
| Other/unknown | 156 | 1.99 | 223 | 2.36 | 113 | 1.11 | 28 | 1.61 | 53 | 1.30 | 56 | 1.72 | |||
| Age at diagnosis, years | |||||||||||||||
| 65-69 | 1,733 | 22.10 | 2,236 | 23.71 | .032 | 6,341 | 62.42 | 1,094 | 63.05 | .382 | 1,364 | 33.34 | 1,050 | 32.25 | .201 |
| 70-74 | 2,637 | 33.63 | 3,156 | 33.46 | 3,061 | 30.13 | 528 | 30.43 | 1,460 | 35.69 | 1,228 | 37.71 | |||
| 75+ | 3,472 | 44.27 | 4,040 | 42.83 | 757 | 7.45 | 113 | 6.51 | 1,267 | 30.97 | 978 | 30.04 | |||
| % with a high school education in patient's census region | |||||||||||||||
| < 75/unknown | 2,057 | 26.23 | 1,849 | 19.60 | < .001 | 2,129 | 20.96 | 248 | 14.29 | < .001 | 931 | 22.76 | 746 | 22.91 | .174 |
| 75-84 | 1,988 | 25.35 | 2,076 | 22.01 | 2,046 | 20.14 | 322 | 18.56 | 902 | 22.05 | 651 | 19.99 | |||
| 85-89 | 1,524 | 19.43 | 1,731 | 18.35 | 1,918 | 18.88 | 295 | 17.00 | 758 | 18.53 | 613 | 18.83 | |||
| 90+ | 2,273 | 28.98 | 3,776 | 40.03 | 4,066 | 40.02 | 870 | 50.14 | 1,500 | 36.67 | 1,246 | 38.27 | |||
| Median income in census region, $ | |||||||||||||||
| < 35,000/unknown | 3,494 | 44.56 | 3,192 | 33.84 | < .001 | 3,262 | 32.11 | 328 | 18.90 | < .001 | 1,362 | 33.29 | 936 | 28.75 | < .001 |
| 35,000-44,000 | 1,893 | 24.14 | 2,124 | 22.52 | 2,450 | 24.12 | 362 | 20.86 | 984 | 24.05 | 743 | 22.82 | |||
| 45,000-59,000 | 1,488 | 18.97 | 2,146 | 22.75 | 2,305 | 22.69 | 431 | 24.84 | 974 | 23.81 | 769 | 23.62 | |||
| ≥ 60,000 | 967 | 12.33 | 1,970 | 20.89 | 2,142 | 21.08 | 614 | 35.39 | 771 | 18.85 | 808 | 24.82 | |||
| Region | |||||||||||||||
| Northeast | 1,718 | 21.91 | 2,644 | 28.03 | < .001 | 1,185 | 11.66 | 229 | 13.20 | < .001 | 1,082 | 26.45 | 1,446 | 44.41 | < .001 |
| South | 1,480 | 18.87 | 1,253 | 13.28 | 1,793 | 17.65 | 182 | 10.49 | 854 | 20.88 | 723 | 22.21 | |||
| Midwest | 1,757 | 22.40 | 1,445 | 15.32 | 1,332 | 13.11 | 302 | 17.41 | 617 | 15.08 | 188 | 5.77 | |||
| West | 2,887 | 36.81 | 4,090 | 43.36 | 5,849 | 57.57 | 1,022 | 58.90 | 1,538 | 37.59 | 899 | 27.61 | |||
| SEER registry | |||||||||||||||
| San Francisco | 299 | 3.81 | 293 | 3.11 | < .001 | 387 | 3.81 | 101 | 5.82 | < .001 | 181 | 4.42 | 115 | 3.53 | < .001 |
| Michigan | 994 | 12.68 | 1,035 | 10.97 | 653 | 6.43 | 263 | 15.16 | 411 | 10.05 | 143 | 4.39 | |||
| New Mexico/Georgia/Hawaii | 431 | 5.50 | 714 | 7.57 | 711 | 7.00 | 59 | 3.40 | 462 | 11.29 | 470 | 14.43 | |||
| Iowa | 763 | 9.73 | 410 | 4.35 | 679 | 6.68 | 39 | 2.25 | 206 | 5.04 | 45 | 1.38 | |||
| Seattle | 434 | 5.53 | 311 | 3.30 | 824 | 8.11 | 85 | 4.90 | 267 | 6.53 | 111 | 3.41 | |||
| Utah | 128 | 1.63 | 81 | 0.86 | 634 | 6.24 | 59 | 3.40 | 397 | 9.70 | 17 | 0.52 | |||
| Connecticut | 711 | 9.07 | 841 | 8.92 | 382 | 3.76 | 66 | 3.80 | 211 | 5.16 | 138 | 4.24 | |||
| San Jose | 174 | 2.22 | 201 | 2.13 | 207 | 2.04 | 39 | 2.25 | 111 | 2.71 | 62 | 1.90 | |||
| Los Angeles | 331 | 4.22 | 952 | 10.09 | 1,049 | 10.33 | 226 | 13.03 | 137 | 3.35 | 181 | 5.56 | |||
| Greater California | 1,237 | 15.77 | 1,706 | 18.09 | 2,281 | 22.45 | 461 | 26.57 | 333 | 8.14 | 311 | 9.55 | |||
| Kentucky | 905 | 11.54 | 356 | 3.77 | 579 | 5.70 | 105 | 6.05 | 312 | 7.63 | 96 | 2.95 | |||
| Louisiana | 428 | 5.46 | 729 | 7.73 | 970 | 9.55 | 69 | 3.98 | 192 | 4.69 | 259 | 7.95 | |||
| New Jersey | 1,007 | 12.84 | 1,803 | 19.12 | 803 | 7.90 | 163 | 9.39 | 871 | 21.29 | 1,308 | 40.17 | |||
| Population density | |||||||||||||||
| Metropolitan | 6,846 | 87.30 | 8,773 | 93.01 | < .001 | 9,241 | 90.96 | 1,655 | 95.39 | < .001 | 3,726 | 91.08 | 3,139 | 96.41 | < .001 |
| Non-metropolitan | 996 | 12.70 | 659 | 6.99 | 918 | 9.04 | 80 | 4.61 | 365 | 8.92 | 117 | 3.59 | |||
| Marital status | |||||||||||||||
| Not married | 1,684 | 21.47 | 1,895 | 20.09 | .055 | 1,563 | 15.39 | 229 | 13.20 | < .001 | 776 | 18.97 | 611 | 18.77 | < .001 |
| Married | 5,395 | 68.80 | 6,564 | 69.59 | 8,163 | 80.35 | 1,346 | 77.58 | 3,103 | 75.85 | 2,355 | 72.33 | |||
| Unknown | 763 | 9.73 | 973 | 10.32 | 433 | 4.26 | 160 | 9.22 | 212 | 5.18 | 290 | 8.91 | |||
| Grade | |||||||||||||||
| Well/unknown | 287 | 3.66 | 247 | 2.62 | < .001 | 208 | 2.05 | 24 | 1.38 | < .001 | 113 | 2.76 | 81 | 2.59 | .002 |
| Moderate | 4,502 | 57.41 | 4,708 | 49.92 | 5,631 | 55.43 | 820 | 47.26 | 2,156 | 52.70 | 1,578 | 48.46 | |||
| Poorly/undifferentiated | 3,053 | 38.93 | 4,477 | 47.47 | 4,320 | 42.52 | 891 | 51.35 | 1,822 | 44.54 | 1,597 | 49.05 | |||
| Clinical stage* | |||||||||||||||
| T1/unknown | 3,132 | 39.94 | 4,497 | 47.68 | < .001 | 4,389 | 43.20 | 945 | 54.47 | < .001 | 1,553 | 37.96 | 1,679 | 51.56 | < .001 |
| T2 | 4,349 | 55.46 | 4,556 | 48.30 | 5,597 | 55.09 | 768 | 44.27 | 2,387 | 58.35 | 1,479 | 45.42 | |||
| T3/T4 | 361 | 4.60 | 379 | 4.01 | 173 | 1.71 | 22 | 1.27 | 151 | 3.70 | 98 | 3.01 | |||
| Charlson comorbidity score | |||||||||||||||
| 0 | 5,069 | 64.64 | 6,447 | 68.35 | < .001 | 7,992 | 78.67 | 1,420 | 81.84 | .022 | 2,897 | 70.81 | 2,262 | 69.47 | .435 |
| 1 | 1,800 | 22.95 | 1,965 | 20.83 | 1,535 | 15.11 | 225 | 12.97 | 848 | 20.73 | 702 | 21.56 | |||
| 2+ | 847 | 10.80 | 916 | 9.71 | 391 | 3.85 | 57 | 3.29 | 295 | 7.21 | 251 | 7.71 | |||
| Unknown | 126 | 1.61 | 104 | 1.10 | 241 | 2.37 | 33 | 1.90 | 51 | 1.25 | 41 | 1.26 | |||
NOTE. Education had 17 unknown, income had 18 unknown, stage had 659 unknown, grade had 516 unknown. For men diagnosed in 2002, well-differentiated referred to Gleason 2 to 4, moderately differentiated was Gleason 5 to 7, and poorly/undifferentiated was Gleason 8 to 10, but for patients diagnosed from January 1, 2003 onward, Gleason 7 was designated poorly/undifferentiated.
Abbreviations: 3DCRT, three-dimensional conformal radiation therapy; IMRT, intensity-modulated radiation therapy; Open RP, open radical prostatectomy; MIRP, minimally invasive radical prostatectomy; Brachy, brachytherapy.
Table A4.
Mean Cost of Each Primary Therapy Among Medicare Enrollees, Stratified by Year of Diagnosis: Using the 5% Non-Cancer Sample As Controls
| Years | $ |
||||||
|---|---|---|---|---|---|---|---|
| 3DCRT | IMRT | Brachy | Brachy+ 3DCRT | Brachy+ IMRT | Open RP | MIRP | |
| 2002 | 17,996 | 32,917 | 17,977 | 24,814 | 34,526 | 12,962 | 26,032 |
| 2003 | 19,794 | 33,977 | 15,800 | 23,680 | 36,585 | 13,138 | 16,326 |
| 2004 | 18,283 | 29,823 | 14,312 | 24,050 | 30,887 | 13,187 | 12,768 |
| 2005 | 17,279 | 27,476 | 12,300 | 20,377 | 28,379 | 13,198 | 13,490 |
Abbreviations: 3DCRT, three-dimensional conformal radiation therapy; IMRT, intensity-modulated radiation therapy; Brachy, brachytherapy; Open RP, open radical prostatectomy; MIRP, minimally invasive radical prostatectomy.
Footnotes
See accompanying editorial on page 1503
Supported by Department of Defense Physician Training Award W81XWH-08-1-0283 (J.C.H.) , a Joint Center for Radiation Therapy foundation grant (P.L.N.), and a Robert and Kathy Salipante Minimally Invasive Urologic Oncology Fellowship (W.W.C.).
This study used the linked Surveillance, Epidemiology, and End Results (SEER) –Medicare database. The interpretation and reporting of these data are the sole responsibility of the authors. The authors acknowledge the efforts of the Applied Research Program, National Cancer Institute; the Office of Research, Development and Information, Center for Medicare and Medicaid Services; Information Management Services, Inc.; and the SEER Program tumor registries in the creation of the SEER-Medicare Database. The sponsor was not involved with the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript.
Presented in part at the Genitourinary Cancers Symposium, San Francisco, CA, March 5-7, 2010.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Paul L. Nguyen, Xiangmei Gu, Stuart R. Lipsitz, Toni K. Choueiri, Wesley W. Choi, Yin Lei, Jim C. Hu
Financial support: Toni K. Choueiri, Jim C. Hu
Provision of study materials or patients: Jim C. Hu
Collection and assembly of data: Paul L. Nguyen, Xiangmei Gu
Data analysis and interpretation: Paul L. Nguyen, Xiangmei Gu, Stuart R. Lipsitz, Toni K. Choueiri, Wesley W. Choi, Karen E. Hoffman, Jim C. Hu
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Leonhardt D. In Health Reform, a Cancer Offers an Acid Test. The New York Times. 2009 [Google Scholar]
- 3.Bolenz C, Gupta A, Hotze T, et al. Cost comparison of robotic, laparoscopic, and open radical prostatectomy for prostate cancer. Eur Urol. 57:453–458. doi: 10.1016/j.eururo.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 4.Cahlon O, Hunt M, Zelefsky MJ. Intensity-modulated radiation therapy: Supportive data for prostate cancer. Semin Radiat Oncol. 2008;18:48–57. doi: 10.1016/j.semradonc.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 5.Warren JL, Klabunde CN, Schrag D, et al. Overview of the SEER-Medicare data: Content, research applications, and generalizability to the United States elderly population. Med Care. 2002;40:IV-3-18. doi: 10.1097/01.MLR.0000020942.47004.03. [DOI] [PubMed] [Google Scholar]
- 6.Brown ML, Riley GF, Schussler N, et al. Estimating health care costs related to cancer treatment from SEER-Medicare data. Med Care. 2002;40:IV-104-17. doi: 10.1097/00005650-200208001-00014. [DOI] [PubMed] [Google Scholar]
- 7.Zeliadt SB, Etzioni R, Ramsey SD, et al. Trends in treatment costs for localized prostate cancer: The healthy screenee effect. Med Care. 2007;45:154–159. doi: 10.1097/01.mlr.0000241044.09778.3f. [DOI] [PubMed] [Google Scholar]
- 8.SEER. Surveillance Epidemiology and End Results Program ( www.seer.cancer.gov) SEER*Stat Database: Incidence - SEER 17 Regs Limited-Use, Nov 2008 Sub (1969-2006 varying) - Linked To County Attributes - Total U.S., 1969-2006 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch, released April 2009, based on the November 2008 submission.
- 9.Warren JL, Yabroff KR, Meekins A, et al. Evaluation of trends in the cost of initial cancer treatment. J Natl Cancer Inst. 2008;100:888–897. doi: 10.1093/jnci/djn175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fleiss J, Levin B, Paik MC. New York, NY: John Wiley & Sons; 2003. Statistical Methods for Rates and Proportions (ed 3) [Google Scholar]
- 11.Hu JC, Gu X, Lipsitz SR, et al. Comparative effectiveness of minimally invasive vs open radical prostatectomy. JAMA. 2009;302:1557–1564. doi: 10.1001/jama.2009.1451. [DOI] [PubMed] [Google Scholar]
- 12.Jani AB, Su A, Correa D, et al. Comparison of late gastrointestinal and genitourinary toxicity of prostate cancer patients undergoing intensity-modulated versus conventional radiotherapy using localized fields. Prostate Cancer Prostatic Dis. 2007;10:82–86. doi: 10.1038/sj.pcan.4500910. [DOI] [PubMed] [Google Scholar]
- 13.Zelefsky MJ, Fuks Z, Hunt M, et al. High dose radiation delivered by intensity modulated conformal radiotherapy improves the outcome of localized prostate cancer. J Urol. 2001;166:876–881. [PubMed] [Google Scholar]
- 14.Zelefsky MJ, Chan H, Hunt M, et al. Long-term outcome of high dose intensity modulated radiation therapy for patients with clinically localized prostate cancer. J Urol. 2006;176:1415–1419. doi: 10.1016/j.juro.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 15.Konski A, Watkins-Bruner D, Feigenberg S, et al. Using decision analysis to determine the cost-effectiveness of intensity-modulated radiation therapy in the treatment of intermediate risk prostate cancer. Int J Radiat Oncol Biol Phys. 2006;66:408–415. doi: 10.1016/j.ijrobp.2006.04.049. [DOI] [PubMed] [Google Scholar]
- 16.Konski A, Speier W, Hanlon A, et al. Is proton beam therapy cost effective in the treatment of adenocarcinoma of the prostate? J Clin Oncol. 2007;25:3603–3608. doi: 10.1200/JCO.2006.09.0811. [DOI] [PubMed] [Google Scholar]
- 17.Krejcarek SC, Grant PE, Henson JW, et al. Physiologic and radiographic evidence of the distal edge of the proton beam in craniospinal irradiation. Int J Radiat Oncol Biol Phys. 2007;68:646–649. doi: 10.1016/j.ijrobp.2007.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gunderson LL, Tepper JE. Philadelphia, PA: Elsevier Churchill Livingstone; 2007. Clinical Radiation Oncology (ed 2) [Google Scholar]
- 19.Nguyen PL, Trofimov A, Zietman AL. Proton-beam vs intensity-modulated radiation therapy. Which is best for treating prostate cancer? Oncology (Williston Park) 2008;22:748–754. discussion 754:757, 2008. [PubMed] [Google Scholar]
- 20.Truffer CJ, Keehan S, Smith S, et al. Health spending projections through 2019: The recessions's impact continues. Health Aff (Millwood) 2010;29:522–529. doi: 10.1377/hlthaff.2009.1074. [DOI] [PubMed] [Google Scholar]
- 21.Klabunde CN, Potosky AL, Legler JM, et al. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000;53:1258–1267. doi: 10.1016/s0895-4356(00)00256-0. [DOI] [PubMed] [Google Scholar]