Abstract
Purpose
To further clarify the relationship between total cholesterol and cancer, which remains unclear.
Methods
We prospectively examined the association between total cholesterol and site-specific and all-cancer incidence among 1,189,719 Korean adults enrolled in the National Health Insurance Corporation who underwent a standardized biennial medical examination in 1992 to 1995 and were observed for 14 years until cancer diagnosis or death.
Results
Over follow-up, 53,944 men and 24,475 women were diagnosed with a primary cancer. Compared with levels less than 160 mg/dL, high total cholesterol (≥ 240 mg/dL) was positively associated with prostate cancer (hazard ratio [HR], 1.24; 95% CI, 1.07 to 1.44; P trend = .001) and colon cancer (HR, 1.12; 95% CI, 1.00 to 1.25; P trend = .05) in men and breast cancer in women (HR, 1.17; 95% CI, 1.03 to 1.33; P trend = .03). Higher total cholesterol was associated with a lower incidence of liver cancer (men: HR, 0.42; 95% CI, 0.38 to 0.45; P trend < .001; women: HR, 0.32; 95% CI, 0.27 to 0.39; P trend < .001), stomach cancer (men: HR, 0.87; 95% CI, 0.82 to 0.93; P trend ≤ .001; women: HR, 0.86; 95% CI, 0.77 to 0.97; P trend = .06), and, in men, lung cancer (HR, 0.89; 95% CI, 0.82 to 0.96; P trend < .001). Results for liver cancer were slightly attenuated after additional adjustment for liver enzyme levels and hepatitis B surface antigen status (men: HR, 0.60; P trend < .001; women: HR, 0.46; P trend = .003) and exclusion of the first 10 years of follow-up (men: HR, 0.59; P trend < .001; women: HR, 0.44; P trend < .001). Total cholesterol was inversely associated with all-cancer incidence in both men (HR, 0.84; 95% CI, 0.81 to 0.86; P trend < .001) and women (HR, 0.91; 95% CI, 0.87 to 0.95; P trend < .001), but these associations were attenuated after excluding incident liver cancers (men: HR, 0.95; P trend < .001; women: HR, 0.98; P trend = .32).
Conclusion
In this large prospective study, we found that total cholesterol was associated with the risk of several different cancers, although these relationships differed markedly by cancer site.
INTRODUCTION
High total cholesterol is a well-established risk factor for coronary heart disease and stroke.1,2 However, the relationship between total cholesterol and the risk of cancer remains uncertain. Cholesterol plays an important role in cellular structure and function and as a precursor to several biochemical pathways, including the synthesis of vitamin D and steroid hormones, thought to be involved in the etiology of certain cancers.2–5 In some prospective studies, low total cholesterol levels were associated with an increased risk of cancer,6–11 spurring concern about the potential health risks of cholesterol-lowering drug use.12 These inverse associations were generally restricted to the first few years of follow-up,6,8–11 possibly as a result of a decline in cholesterol levels among participants with prediagnostic cancer at baseline.13 Furthermore, prospective studies on the relationship between total cholesterol and site-specific cancer risk have been limited and somewhat contradictory. Some of the more consistent findings include inverse associations with the risk of stomach10,14,15 and premenopausal breast cancer16,17 and positive associations with risk of high-grade, organ-confined or advanced prostate cancer.10,18–20 Few prospective studies have had the power to examine total cholesterol in relation to less commonly occurring cancers.
We examined the association between total cholesterol and risk of all and site-specific cancer incidence in a prospective study of Korean men and women with comprehensive data on health indicators and health behavior. With approximately 1.2 million participants and 79,000 incident cancers, to our knowledge, this is the largest study of the relationship between total cholesterol and cancer incidence to date.
METHODS
Study Population
Between 1992 and 1995, 1,329,525 Korean men and women aged 30 to 95 years participated in one biennial National Health Insurance Corporation (NHIC) medical evaluation. Of the participants, 784,870 (59.0%) were enrolled in 1992; 367,903 (27.7%) were enrolled in 1993; 98,417 (7.4%) were enrolled in 1994; and 78,335 (5.9%) were enrolled in 1995.
We excluded participants who died before January 1, 1993 (n = 904); who reported having cardiovascular disease, cancer, liver disease, or a respiratory disease at or before the initial visit (n = 91,712); or who had missing information on regular exercise, blood pressure, fasting blood glucose, body mass index (BMI), or alcohol, or extremely low levels of BMI (< 16 kg/m2), weight (≤ 30 kg), or height (≤ 1.30 m; n = 44,557). We further excluded participants with missing or implausibly high or low total cholesterol levels (< 80 or ≥ 350 mg/dL; n = 2,633). After applying these exclusions, the final study population consisted of 1,189,719 participants (756,604 men and 433,115 women).
Because the study used routinely collected data, consent was not specifically obtained. The institutional review boards of Yonsei University and the Johns Hopkins Bloomberg School of Public Health approved the study.
Data Collection
Enrollees in the Korean NHIC undergo standardized biennial examinations at local hospitals. During visits from 1992 through 1995, information on reproductive history, medical history, and lifestyle factors, including smoking, alcohol consumption, and physical activity, was obtained through self-administered questionnaires. Weight and height measurements were recorded with participants wearing light clothing. Participants were seated for blood pressure measurements. Fasting serum glucose and total cholesterol were obtained during these visits for routine clinical purposes. Quality control procedures were in accordance with the Korean Association of Laboratory Quality Control.
Follow-Up and Outcome Classification
Follow-up of each participant began on the date in which fasting blood samples were collected (1992 to 1995) and ended on the date of cancer diagnosis, death, loss to follow-up, or administrative censoring (December 31, 2006). Because reporting to the national cancer registry in Korea was only approximately 90% complete during follow-up,21 claims data for the NHIC generated from hospital admission files were used to identify additional first admission events for cancer. In Korea, professionally trained and certified medical chart recorders abstracted charts and assigned discharge diagnoses and codes in a standardized fashion. Since 2003, this system has also become the main data source for the Korean Central Cancer Registry.22 In this study, participants with a primary cancer were identified by either a positive report from the national cancer registry or by a hospital admission for a cancer diagnosis.
Statistical Analysis
Total cholesterol was categorized using cut points of less than 160, 160 to 179, 180 to 199, 200 to 239, and ≥ 240 mg/dL, in line with a recent prospective study of total cholesterol and cancer risk in Japanese men and women.10 We considered total cholesterol levels between 200 and 240 mg/dL as borderline high and levels ≥ 240 mg/dL as high.
Cox proportional hazards models with attained age as the underlying time metric, with late entry defined as the age in which participants enrolled onto the cohort, were used to calculate hazard ratios (HRs) and 95% CIs for all and site-specific cancer incidence according to total cholesterol level, modeled both categorically and continuously (per 20 mg/dL). Trend tests for all and site-specific cancers were conducted using the Wald test statistic for total cholesterol modeled as a continuous variable. All models were stratified by sex and adjusted for cigarette smoking (never-smoker, former smoker, currently smoking < 20 cigarettes per day, or currently smoking ≥ 20 cigarettes per day), alcohol consumption (0, < 25, 25 to 49, 50 to 99, or ≥ 100 g/d), BMI (calculated as weight in kilograms divided by height in meters squared), physical activity (exercises v does not exercise), hypertension (systolic blood pressure ≥ 140 mmHg or diastolic blood pressure > 90 mmHg), and fasting serum glucose (continuous). Because information on family history of cancer and reproductive variables was only available for a subset of participants and adjustment for these variables did not materially change the results, we did not include these covariates in the final models. All models were tested for and met the proportional hazards assumption.
In sensitivity analyses, liver cancer models were further adjusted for hepatitis B surface antigen status and fasting ALT and AST levels, although restricting the analysis to participants with this information meant that the number of incident liver cancers was substantially reduced (by 2,881 in men and 1,457 in women). We examined the results after excluding the first 5 years of follow-up to investigate the potential influence of undiagnosed cancer on total cholesterol levels. Breast cancer models were stratified by attained age (before v after age 50) to investigate potential differences by menopausal status.
All analyses were conducted using SAS version 9.1 (SAS Institute, Cary, NC). All statistical tests were two sided, and P < .05 was considered statistically significant.
RESULTS
The mean age at cohort entry was 44.9 years for men and 49.3 years for women. Mean total cholesterol levels were 190.9 mg/dL in men and 194.1 mg/dL in women. Compared with participants with low total cholesterol levels (< 160 mg/dL) at baseline, participants with high levels (≥ 240 mg/dL) were typically older; had a higher BMI and higher fasting serum glucose; and were more likely to have high blood pressure, to drink alcohol, and to exercise. Compared with men, women were less likely to be current or former smokers, to consume any alcohol, and to exercise (Table 1).
Table 1.
Sex-Specific Distributions of Baseline Characteristics by Total Cholesterol in the Korean Cancer Prevention Study (1992 to 2006)
| Total Cholesterol |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| < 160 mg/dL |
160-179 mg/dL |
180-199 mg/dL |
200-239 mg/dL |
≥ 240 mg/dL |
||||||
| Characteristic | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD |
| Men | ||||||||||
| No. | 143,906 | 156,876 | 171,241 | 209,137 | 75,444 | |||||
| Age, years | 44 | 12 | 44 | 11 | 45 | 11 | 46 | 11 | 47 | 10 |
| Body mass index, kg/m2 | 22.5 | 2.5 | 22.9 | 2.5 | 23.2 | 2.5 | 23.6 | 2.5 | 24.1 | 2.5 |
| Systolic blood pressure, mmHg | 122.3 | 15.4 | 123.1 | 15.3 | 124.2 | 15.6 | 125.7 | 16.2 | 128.1 | 17.0 |
| Fasting serum glucose, mg/dL | 89.9 | 21.7 | 90.6 | 21.7 | 91.9 | 22.8 | 94.2 | 25.5 | 99.0 | 32.9 |
| Smoking status, % | ||||||||||
| Never | 21.3 | 21.1 | 21.0 | 20.3 | 19.4 | |||||
| Former | 19.0 | 19.5 | 20.0 | 20.7 | 21.1 | |||||
| Current | 59.7 | 59.4 | 59.1 | 59.1 | 59.5 | |||||
| High blood pressure, %* | 16.8 | 18.3 | 20.3 | 23.7 | 28.7 | |||||
| Any alcohol use, % | 76.2 | 76.7 | 77.0 | 77.3 | 77.3 | |||||
| Physically active, % | 27.4 | 28.0 | 28.8 | 29.4 | 29.7 | |||||
| Women | ||||||||||
| No. | 77,808 | 85,800 | 92,675 | 123,567 | 53,265 | |||||
| Age, years | 44 | 12 | 47 | 12 | 49 | 12 | 52 | 12 | 55 | 11 |
| Body mass index, kg/m2 | 22.2 | 2.9 | 22.7 | 3.0 | 23.2 | 3.1 | 23.7 | 3.1 | 24.3 | 3.1 |
| Systolic blood pressure, mmHg | 116.6 | 16.6 | 118.8 | 17.7 | 121.1 | 18.7 | 123.9 | 19.8 | 127.5 | 21.1 |
| Fasting serum glucose, mg/dL | 86.2 | 17.5 | 87.6 | 18.7 | 89.4 | 20.6 | 92.0 | 24.8 | 97.0 | 32.8 |
| Smoking status, % | ||||||||||
| Never | 96.1 | 95.1 | 94.3 | 93.8 | 90.9 | |||||
| Former | 1.3 | 1.7 | 1.9 | 2.5 | 3.1 | |||||
| Current | 2.6 | 3.3 | 3.8 | 4.8 | 6.0 | |||||
| High blood pressure, %* | 11.7 | 15.1 | 19.1 | 24.2 | 31.0 | |||||
| Any alcohol use, % | 13.8 | 14.4 | 14.4 | 14.5 | 14.6 | |||||
| Physically active, % | 14.4 | 15.7 | 16.8 | 17.7 | 18.1 | |||||
Abbreviation: SD, standard deviation.
Systolic blood pressure ≥ 140 mmHg or diastolic blood pressure > 90 mmHg.
Of 756,604 men and 433,115 women included in the study, 53,945 men and 24,475 women were diagnosed with cancer during a mean follow-up time of 12.7 years. Stomach (n = 18,012), lung (n = 10,866), and liver (n = 10,161) cancers were the most commonly diagnosed malignancies in this population (Appendix Table A1, online only, lists age-specific incidence rates).
Relationship Between Cholesterol and Site-Specific Cancer Incidence
Compared with low total cholesterol (< 160 mg/dL), high total cholesterol (≥ 240 mg/dL) was associated with a reduced risk of liver cancer in men (HR, 0.42; 95% CI, 0.38 to 0.45; P trend < .001) and women (HR, 0.32; 95% CI, 0.27 to 0.39; P trend < .001; Tables 2 and Table 3). Additional adjustment for liver enzymes (ALT and AST) and hepatitis B surface antigen status slightly attenuated these associations (men: HR, 0.60; 95% CI, 0.54 to 0.67; P trend < .001; women: HR, 0.46; 95% CI, 0.24 to 0.87; P trend = .003). A significant inverse dose-response association between total cholesterol and risk of stomach cancer was observed in men (HR, 0.87; 95% CI, 0.82 to 0.93; P trend < .001), whereas a borderline significant trend was observed for stomach cancer in women (HR, 0.86; 95% CI, 0.77 to 0.97; P trend = .06). Total cholesterol was also significantly inversely associated with lung cancer in men (HR, 0.89; 95% CI, 0.82 to 0.96; P trend < .001) but not in women. Although a significant inverse trend association was observed for esophageal cancer in men (P trend = .01), the risk in the highest compared with lowest cholesterol group was not significantly reduced (HR, 0.92; 95% CI, 0.76 to 1.11), and no association was observed in women.
Table 2.
Total Cholesterol in Relation to Cancer Incidence in Korean Men in the Korean Cancer Prevention Study (1992 to 2006)
| Total Cholesterol |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| < 160 mg/dL |
160-179 mg/dL |
180-199 mg/dL |
200-239 mg/dL |
≥ 240 mg/dL |
|||||||||||
| Cancer Site | No. | HR† | No. | HR† | 95% CI | No. | HR† | 95% CI | No. | HR† | 95% CI | No. | HR† | 95% CI | P Trend* |
| Esophagus | 299 | 1.0 | 284 | 0.85 | 0.73 to 1.00 | 318 | 0.84 | 0.71 to 0.98 | 340 | 0.69 | 0.59 to 0.80 | 173 | 0.92 | 0.76 to 1.11 | .01 |
| Stomach | 2,578 | 1.0 | 2,770 | 0.93 | 0.88 to 0.98 | 3,111 | 0.90 | 0.85 to 0.94 | 4,054 | 0.89 | 0.85 to 0.94 | 1,536 | 0.87 | 0.82 to 0.93 | < .001 |
| Colon | 687 | 1.0 | 864 | 1.06 | 0.96 to 1.17 | 1,023 | 1.06 | 0.96 to 1.17 | 1,418 | 1.10 | 1.00 to 1.20 | 569 | 1.12 | 1.00 to 1.25 | .05 |
| Rectum | 627 | 1.0 | 775 | 1.05 | 0.95 to 1.17 | 900 | 1.04 | 0.94 to 1.15 | 1,169 | 1.01 | 0.92 to 1.12 | 478 | 1.06 | 0.94 to 1.19 | .75 |
| Liver | 2,193 | 1.0 | 1,790 | 0.69 | 0.65 to 0.74 | 1,879 | 0.62 | 0.58 to 0.66 | 1,944 | 0.48 | 0.45 to 0.51 | 670 | 0.42 | 0.38 to 0.45 | < .001 |
| Pancreas | 331 | 1.0 | 342 | 0.88 | 0.76 to 1.03 | 385 | 0.84 | 0.73 to 0.97 | 528 | 0.86 | 0.75 to 0.99 | 213 | 0.88 | 0.74 to 1.05 | .17 |
| Lung | 1,683 | 1.0 | 1,740 | 0.92 | 0.86 to 0.98 | 1,907 | 0.88 | 0.82 to 0.94 | 2,444 | 0.87 | 0.81 to 0.92 | 956 | 0.89 | 0.82 to 0.96 | < .001 |
| Prostate | 366 | 1.0 | 456 | 1.06 | 0.93 to 1.22 | 562 | 1.10 | 0.97 to 1.26 | 777 | 1.14 | 1.01 to 1.29 | 329 | 1.24 | 1.07 to 1.44 | .001 |
| Kidney | 211 | 1.0 | 221 | 0.87 | 0.72 to 1.05 | 309 | 1.03 | 0.86 to 1.22 | 427 | 1.05 | 0.89 to 1.24 | 170 | 1.04 | 0.85 to 1.28 | .29 |
| Gallbladder | 176 | 1.0 | 183 | 0.89 | 0.73 to 1.10 | 201 | 0.84 | 0.68 to 1.03 | 268 | 0.84 | 0.69 to 1.02 | 115 | 0.91 | 0.71 to 1.15 | .27 |
| Bladder | 315 | 1.0 | 378 | 1.03 | 0.89 to 1.20 | 449 | 1.05 | 0.91 to 1.21 | 671 | 1.18 | 1.03 to 1.35 | 245 | 1.10 | 0.93 to 1.30 | .10 |
| All cancer | 10,249 | 1.0 | 10,641 | 0.89 | 0.87 to 0.92 | 11,946 | 0.86 | 0.84 to 0.88 | 15,166 | 0.83 | 0.81 to 0.85 | 5,943 | 0.84 | 0.81 to 0.86 | < .001 |
| All cancer minus liver cancer | 8,056 | 1.0 | 8,851 | 0.94 | 0.92 to 0.97 | 10,067 | 0.92 | 0.90 to 0.95 | 13,222 | 0.92 | 0.89 to 0.94 | 5,273 | 0.95 | 0.91 to 0.98 | < .001 |
Abbreviation: HR, hazard ratio.
Calculated by including total cholesterol as a continuous variable in the multivariable models.
Models used attained age as the underlying time metric and were adjusted for cigarette smoking, alcohol drinking, body mass index, fasting serum glucose, hypertension, and physical activity.
Table 3.
Total Cholesterol in Relation to Cancer Incidence in Korean Women in the Korean Cancer Prevention Study (1992 to 2006)
| Total Cholesterol |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| < 160 mg/dL |
160-179 mg/dL |
180-199 mg/dL |
200-239 mg/dL |
≥ 240 mg/dL |
|||||||||||
| Cancer Site | No. | HR† | No. | HR† | 95% CI | No. | HR† | 95% CI | No. | HR† | 95% CI | No. | HR† | 95% CI | P Trend* |
| Esophagus | 11 | 1.0 | 12 | 0.82 | 0.36 to 1.87 | 22 | 1.24 | 0.60 to 2.57 | 25 | 0.91 | 0.44 to 1.87 | 14 | 1.06 | 0.47 to 2.37 | .91 |
| Stomach | 575 | 1.0 | 656 | 0.89 | 0.80 to 1.00 | 897 | 1.01 | 0.91 to 1.12 | 1,265 | 0.93 | 0.84 to 1.03 | 570 | 0.86 | 0.77 to 0.97 | .06 |
| Colon | 240 | 1.0 | 312 | 1.00 | 0.84 to 1.18 | 384 | 1.00 | 0.85 to 1.18 | 629 | 1.07 | 0.92 to 1.24 | 328 | 1.14 | 0.96 to 1.35 | .06 |
| Rectum | 203 | 1.0 | 288 | 1.09 | 0.91 to 1.30 | 335 | 1.03 | 0.87 to 1.23 | 497 | 0.99 | 0.84 to 1.17 | 278 | 1.13 | 0.94 to 1.36 | .60 |
| Liver | 399 | 1.0 | 342 | 0.63 | 0.54 to 0.72 | 348 | 0.50 | 0.44 to 0.58 | 410 | 0.37 | 0.32 to 0.42 | 186 | 0.32 | 0.27 to 0.39 | < .001 |
| Pancreas | 100 | 1.0 | 121 | 0.87 | 0.67 to 1.14 | 164 | 0.93 | 0.72 to 1.19 | 247 | 0.85 | 0.67 to 1.08 | 144 | 0.96 | 0.74 to 1.24 | .83 |
| Lung | 293 | 1.0 | 354 | 0.91 | 0.78 to 1.06 | 439 | 0.92 | 0.79 to 1.07 | 711 | 0.95 | 0.83 to 1.09 | 339 | 0.91 | 0.78 to 1.07 | .62 |
| Kidney | 37 | 1.0 | 64 | 1.37 | 0.91 to 2.05 | 66 | 1.18 | 0.79 to 1.77 | 114 | 1.35 | 0.93 to 1.97 | 52 | 1.28 | 0.83 to 1.98 | .48 |
| Gallbladder | 68 | 1.0 | 86 | 0.93 | 0.68 to 1.28 | 130 | 1.12 | 0.83 to 1.50 | 180 | 0.96 | 0.72 to 1.27 | 86 | 0.89 | 0.64 to 1.23 | .26 |
| Bladder | 31 | 1.0 | 45 | 1.10 | 0.70 to 1.74 | 72 | 1.44 | 0.94 to 2.19 | 91 | 1.15 | 0.76 to 1.75 | 57 | 1.45 | 0.92 to 2.27 | .22 |
| Breast | 701 | 1.0 | 802 | 1.08 | 0.98 to 1.20 | 854 | 1.12 | 1.01 to 1.24 | 1,035 | 1.11 | 1.01 to 1.23 | 413 | 1.17 | 1.03 to 1.33 | .03 |
| Cervix | 406 | 1.0 | 443 | 0.99 | 0.87 to 1.13 | 521 | 1.08 | 0.95 to 1.24 | 616 | 0.97 | 0.85 to 1.10 | 268 | 0.99 | 0.84 to 1.16 | .67 |
| All cancer | 3,952 | 1.0 | 4,530 | 0.95 | 0.91 to 0.99 | 5,349 | 0.97 | 0.93 to 1.01 | 7,284 | 0.91 | 0.88 to 0.95 | 3,360 | 0.91 | 0.87 to 0.95 | < .001 |
| All cancer minus liver cancer | 3,553 | 1.0 | 4,188 | 0.98 | 0.94 to 1.03 | 5,001 | 1.02 | 0.98 to 1.07 | 6,874 | 0.98 | 0.94 to 1.02 | 3,174 | 0.98 | 0.94 to 1.03 | .32 |
Abbreviation: HR, hazard ratio.
Calculated by including total cholesterol as a continuous variable in the multivariable models.
Models used attained age as the underlying time metric and were adjusted for cigarette smoking, alcohol drinking, body mass index, fasting serum glucose, hypertension, and physical activity.
High (≥ 240 mg/dL) versus low (< 160 mg/dL) total cholesterol was positively associated with risk of prostate cancer in men (HR, 1.24; 95% CI, 1.07 to 1.44; P trend = .001; Table 2) and breast cancer in women (HR, 1.17; 95% CI, 1.03 to 1.33; P trend = .03; Table 3). Although we lacked data on menopausal status for all of the women, a sensitivity analysis of breast cancer incidence stratified by follow-up time showed a similar association between total cholesterol (≥ 240 v < 160 mg/dL) and breast cancer before age 50 years (HR, 1.17; 95% CI, 0.94 to 1.46) versus after age 50 years (HR, 1.14; 95% CI, 0.96 to 1.35; P interaction = .07). We observed a borderline significant positive dose-response association for colon cancer in men (HR, 1.12; 95% CI, 1.00 to 1.25; P trend = .05; Table 2).
After excluding the first 5 years of follow-up, most of the results, including the association for prostate cancer (2,168 men; HR, 1.23; 95% CI, 1.05 to 1.45; P trend = .002) and colon cancer (3,836 men; HR, 1.12; 95% CI, 0.99 to 1.27; P trend = .05) in men, were not substantially different. There were some slight differences, however. For instance, the association for breast cancer risk was slightly stronger (2,748 women; HR, 1.21; 95% CI, 1.04 to 1.41; P trend = .003), and the association for colon cancer in women was stronger and statistically significant (1,478 women; HR, 1.28; 95% CI, 1.06 to 1.56; P trend = .004). The inverse associations for liver cancer risk remained even after exclusion of the first 10 years of follow-up (men: n = 2,658; HR, 0.59; 95% CI, 0.51 to 0.68; P trend < .001; women: n = 407; HR, 0.44; 95% CI, 0.31 to 0.64; P trend < .001).
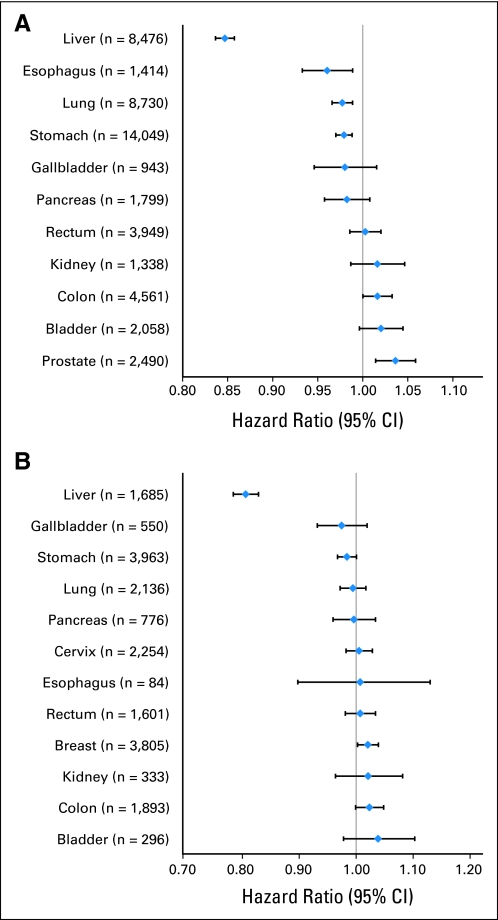
The site-specific HRs per 20 mg/dL difference in total cholesterol for men and women are shown in Figs 1A and 1B, respectively. The relationship of total cholesterol with site-specific cancer incidence varied greatly, with approximately half of the HRs falling below unity (ie, cancers of the liver, lung, stomach, gallbladder, pancreas, and, in men, esophagus) and half falling above unity (ie, cancers of the rectum, kidney, colon, bladder, prostate, breast, cervix, and, in women, esophagus). However, with the exception of liver cancer, these risks were fairly modest, with HRs between 0.95 and 1.05 per 20 mg/dL.
Fig 1.
Hazard ratios and 95% CIs for total cholesterol (per 20 mg/dL) in relation to site-specific cancer incidence in (A) Korean men and (B) Korean women in the Korean Cancer Prevention Study (1992 to 2006). Models used attained age as the underlying time metric and were adjusted for cigarette smoking, alcohol drinking, body mass index, fasting serum glucose, hypertension, and physical activity.
Relationship Between Cholesterol and All-Cancer Incidence
After adjusting for smoking, alcohol consumption, BMI, fasting glucose, hypertension, and physical activity, all-cancer incidence decreased with increasing total cholesterol in both men (≥ 240 v < 160 mg/dL; HR, 0.84; 95% CI, 0.81 to 0.86; P trend < .001; Table 2) and women (HR, 0.91; 95% CI, 0.87 to 0.95; P trend < .001; Table 3). Excluding the first 5 years did not change the association with all-cancer incidence considerably (men: n = 40,056; HR, 0.87; 95% CI, 0.84 to 0.91; P trend < .001; women: n = 17,146; HR, 0.94; 95% CI, 0.89 to 1.00; P trend = .007). In women, there was no association for total cholesterol with all-cancer incidence after excluding liver cancers from the analysis; in men, the association was attenuated but remained statistically significant (HR, 0.95; 95% CI, 0.91 to 0.98; P trend < .001; Tables 2 and 3).
To assess the internal validity of our study, we examined the association of total cholesterol (≥ 240 v < 160 mg/dL) with the risk of ischemic heart disease. The HR was 1.90 in men (95% CI, 1.81 to 1.99; P trend < .001) and 1.38 in women (95% CI, 1.28 to 1.48; P trend < .001).
DISCUSSION
Data from this large prospective study in Korea suggest that total cholesterol levels have differing associations with cancer, depending on cancer site. Total cholesterol levels were positively associated with breast cancer in women and prostate and colon cancer in men. Conversely, total cholesterol was inversely associated with liver and stomach cancer in both men and women and lung cancer in men. Although we observed an inverse dose-response association for total cholesterol with all-cancer incidence, these results seemed to be driven mainly by the strong inverse association with liver cancer. The potential influence of prediagnostic cancer on total cholesterol levels may have been a source of bias in the current study,13 because the exclusion of incident cancer cases occurring in early follow-up strengthened the positive associations of colon and breast cancer in women and slightly attenuated the association with liver cancer.
Undiagnosed liver disease could inhibit cholesterol metabolism,23,24 possibly exaggerating the negative association with liver cancer incidence. However, further adjustment for serum ALT and AST levels, two markers of liver inflammation and liver cell damage, and hepatitis B surface antigen status slightly attenuated the results, suggesting that undiagnosed liver cancer may only partially explain this association. Two other prospective studies have examined the association between total cholesterol and liver cancer incidence.10,11 Our results are consistent with the findings from a smaller cohort of 33,000 Japanese men and women, in which an inverse association with liver cancer was observed in both men and women, even after adjustment for risk factors such as hepatitis C infection, alcohol intake, and smoking; exclusion of the first 3 years of follow-up; and restriction of the analysis to patients with nonadvanced cancer.10 In contrast, an inverse trend association between total cholesterol and liver cancer risk observed in a cohort of Finnish male smokers was attenuated after excluding the first 9 years of follow-up.11 The low cholesterol comparison group (< 203.9 mg/dL)11 included relatively high cholesterol levels compared with that of both the Japanese study (< 160 mg/dL)10 and our study; therefore, a possible increased risk of liver cancer at lower total cholesterol levels may not have been observable in the Finnish cohort.
Nine prospective studies have examined the relationship between total cholesterol and prostate cancer incidence.6,7,10,11,18–20,25,26 Apart from the current study, only one other prospective study showed a positive association between total cholesterol and total prostate cancer incidence.10 Given the small number of incident prostate cancers, most prospective studies were unlikely to have sufficient power to detect modest relative risks (ie, < 1.5). However, some prospective studies have suggested that high total cholesterol may increase the risk of high-grade or advanced, rather than total, prostate cancer,18–20 and cholesterol-lowering drugs have been associated with a reduced risk of advanced prostate cancers in observational studies.27 Although we lacked information on cancer stage and grade, we might expect a higher proportion of advanced prostate cancers during follow-up relative to most Western populations because prostate-specific antigen screening was relatively uncommon in South Korea during this time.
We also observed modest positive associations for total cholesterol with risk of colon cancer in men and breast cancer in women. Evidence from prospective studies generally does not support an association between cholesterol and risk of colorectal cancer7,10,11,25,28 or breast cancer,10,25,29–33 except possibly an inverse association with premenopausal breast cancer.16,17 Although the exact mechanisms whereby high total cholesterol levels could lead to an increased risk of cancer are unclear, cholesterol is involved in numerous biochemical pathways that are potentially relevant in cancer initiation and/or progression, including vitamin D and steroid hormone synthesis.3–5 In addition, compared with their normal counterparts, cancer cells have higher levels of cholesterol-rich lipid rafts in the plasma membrane, which may be important in the transduction of several signaling pathways, including those relevant to cancer cell survival, such as Akt.34
Considering that our data were originally collected for routine clinical purposes, it is plausible that confounding as a result of certain unmeasured factors was a source of bias in this study. Our results were not substantially changed after adjusting for several cancer risk factors, including BMI, alcohol consumption, cigarette smoking, physical activity, and fasting serum glucose.35 Although we cannot rule out potential confounding by diet in this study, previous studies found no difference in the associations between total cholesterol and cancer after further adjustment for total vegetable,10 total fat,7,15 saturated and polyunsaturated fat,11 fiber,7,15 and vitamin15 intakes. We also cannot rule out potential confounding by socioeconomic status because data on socioeconomic indicators, such as education, income, or place of residence, were not ascertained. Total cholesterol may also be related to other cancer risk factors that were not measured in this study, such as hepatitis C virus infection, although the prevalence of anti–hepatitis C virus is only approximately 1% among adults in South Korea.36 Another limitation of this study is the lack of information on participants' use of cholesterol-lowering drugs, which may influence the development of cancer through a variety of mechanisms independent of cholesterol lowering, including the inhibition of tumor growth, induction of apoptosis, inhibition of angiogenesis, increases in mitotic abnormalities, and immunosuppression.37 However, evidence from randomized trials, although limited, does not suggest a strong link between cholesterol-lowering drug use and cancer incidence.37,38 We estimate that the prevalence of cholesterol-lowering drug use would have been low (between 1% and 2%) in this Korean population. We also lacked data on lipoprotein subfractions and cancer subtypes (ie, estrogen receptor/progesterone receptor-positive v -negative breast cancer, advanced v nonadvanced prostate cancer, and proximal v distal colon cancer). Finally, total cholesterol was measured from baseline blood draws only; thus, intraindividual variation in cholesterol over time may have attenuated our results.
Because the study included only government employees, teachers, and their dependents, the study population was generally older and included a higher proportion of men and middle-class individuals compared with the general population of South Korea. Although the incidence rates of cancer may differ for these reasons, we have little reason to suspect that the associations would differ between our study and the general population.
Our study does have several important strengths. The large size of our study and the wide range of total cholesterol values provided sufficient power to detect relatively modest effects, in contrast with many previous studies. Comprehensive information on potential confounding factors, such as alcohol consumption, cigarette smoking, BMI, physical activity, fasting serum glucose, blood pressure, hepatitis B surface antigen status, ALT, and AST, was collected. With more than 14 years of follow-up, we were able to assess the potential bias caused by preclinical disease by excluding a considerable amount of early follow-up time (up to 10 years).
This large prospective study of Korean adults provides novel evidence that high cholesterol, or factors correlated with it, may be positively associated with the risk of several malignancies, including cancers of the prostate, breast, and colon. Although the relative risks were modest, these effects, if causal, may translate into a reasonable number of incident cancers in populations where high cholesterol levels are more prevalent, such as the United States.39 The strong inverse association between cholesterol and liver cancer incidence was consistent with previous studies and seemed to be partly, but not fully, explained by undiagnosed liver disease.
Acknowledgment
We thank the staff of the Korean National Health Insurance Corporation.
Appendix
Table A1.
Age-Specific Cancer Incidence Rates per 100,000 Person-Years in Korean Men and Women in the Korean Cancer Prevention Study (1992 to 2006)
| Attained Age (cancer incidence rate per 100,000 person-years) |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cancer Site | 30.0-34.9 Years | 35.0-39.9 Years | 40.0-44.9 Years | 45.0-49.9 Years | 50.0-54.9 Years | 55.0-59.9 Years | 60.0-64.9 Years | 65.0-69.9 Years | 70.0-74.9 Years | 75.0-79.9 Years | 80.0-84.9 Years | ≥ 85.0 Years |
| Men | ||||||||||||
| Esophagus | 0 | 0.5 | 1.2 | 2.4 | 8.3 | 16.3 | 32.9 | 37.7 | 53.0 | 83.5 | 67.1 | 18.1 |
| Stomach | 14.6 | 15.5 | 37.7 | 55.5 | 106.7 | 180.7 | 281.2 | 369.3 | 460.0 | 480.9 | 437.3 | 292.2 |
| Colon | 3.2 | 4.2 | 9.6 | 16.7 | 30.3 | 58.3 | 69.7 | 107.7 | 169.0 | 167.5 | 157.0 | 145.3 |
| Rectum | 3.2 | 2.8 | 7.5 | 20.0 | 25.7 | 49.0 | 71.6 | 101.5 | 120.0 | 140.7 | 112.4 | 164.1 |
| Liver | 3.2 | 12.2 | 26.9 | 50.2 | 91.9 | 129.5 | 163.9 | 176.3 | 194.7 | 206.1 | 246.4 | 127.1 |
| Pancreas | 0 | 1.9 | 1.5 | 5.4 | 9.4 | 23.3 | 35.7 | 56.5 | 65.2 | 74.5 | 111.8 | 72.5 |
| Lung | 0 | 4.7 | 12.6 | 16.1 | 39.7 | 94.4 | 162.0 | 264.1 | 391.9 | 516.3 | 539.9 | 475.1 |
| Prostate | 0 | 0 | 0.3 | 1.8 | 5.6 | 18.6 | 49.8 | 91.6 | 147.3 | 176.9 | 120.0 | 145.8 |
| Kidney | 3.2 | 2.4 | 5.7 | 7.9 | 13.6 | 15.1 | 24.4 | 32.1 | 50.4 | 20.9 | 29.8 | 0 |
| Gallbladder | 0 | 1.4 | 1.8 | 4.7 | 8.3 | 9.3 | 13.6 | 16.0 | 31.2 | 47.7 | 37.3 | 36.2 |
| Bladder | 1.6 | 1.9 | 5.1 | 5.6 | 13.6 | 20.6 | 37.6 | 58.0 | 77.6 | 89.7 | 119.8 | 164.1 |
| All cancer | 38.8 | 65.0 | 135.0 | 221.2 | 402.1 | 696.6 | 1033.3 | 1458.8 | 1893.2 | 2250.2 | 2192.5 | 1753.2 |
| Women | ||||||||||||
| Esophagus | 0 | 0 | 0.8 | 0.7 | 0 | 0.7 | 2.2 | 2.7 | 1.3 | 0 | 14.1 | 0 |
| Stomach | 3.8 | 12.3 | 27.9 | 36.8 | 46.6 | 68.5 | 97.1 | 120.7 | 152.6 | 216.6 | 147.1 | 152.2 |
| Colon | 0 | 2.7 | 6.4 | 11.2 | 23.3 | 28.0 | 39.5 | 66.5 | 87.4 | 101.0 | 75.6 | 57.0 |
| Rectum | 0 | 2.7 | 4.0 | 9.8 | 21.4 | 20.2 | 44.5 | 56.6 | 70.2 | 62.0 | 66.1 | 47.5 |
| Liver | 0 | 4.1 | 5.6 | 11.8 | 23.3 | 35.8 | 47.3 | 58.3 | 68.7 | 71.0 | 89.6 | 85.4 |
| Pancreas | 0 | 1.4 | 2.4 | 2.6 | 3.7 | 12.4 | 16.5 | 26.9 | 31.7 | 36.6 | 94.3 | 37.9 |
| Lung | 7.6 | 4.1 | 6.4 | 8.5 | 11.0 | 36.5 | 43.7 | 53.9 | 105.8 | 144.6 | 208.2 | 104.5 |
| Kidney | 3.8 | 0 | 3.2 | 4.6 | 4.9 | 5.9 | 5.7 | 11.7 | 14.5 | 6.9 | 18.9 | 0 |
| Gallbladder | 0 | 0 | 2.4 | 1.3 | 6.1 | 6.5 | 15.1 | 20.6 | 21.1 | 29.8 | 33.0 | 37.9 |
| Bladder | 0 | 0 | 0 | 2.6 | 3.1 | 3.9 | 5.7 | 8.1 | 13.2 | 22.9 | 23.6 | 19.0 |
| Breast | 18.9 | 40.9 | 104.8 | 120.0 | 79.4 | 92.2 | 55.4 | 46.8 | 38.4 | 16.0 | 9.4 | 0 |
| Cervix | 15.2 | 25.9 | 37.5 | 50.0 | 41.1 | 36.6 | 51.8 | 29.7 | 33.1 | 39.0 | 18.9 | 9.5 |
| All cancer | 79.6 | 128.6 | 263.7 | 344.5 | 366.4 | 477.5 | 533.1 | 620.8 | 772.9 | 919.9 | 929.2 | 689.9 |
Footnotes
Supported by Grant No. 10526 from Korean Seoul City Research and Grant No. 0920330 from the National Research and Development Program for Cancer Control, Ministry for Health, Welfare and Family Affairs, Republic of Korea. This study was also supported in part by the Intramural Research Program of the National Cancer Institute, National Institutes of Health.
Presented in part at the 43rd Annual Meeting of the Society for Epidemiologic Research, June 23-26, 2010, Seattle, WA.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Cari M. Kitahara, Amy Berrington de González, Sun Ha Jee
Financial support: Sun Ha Jee
Collection and assembly of data: Sun Ha Jee
Data analysis and interpretation: Cari M. Kitahara, Amy Berrington de González, Neal D. Freedman, Rachel Huxley, Yejin Mok, Sun Ha Jee
Manuscript writing: Cari M. Kitahara, Amy Berrington de González, Neal D. Freedman, Rachel Huxley, Yejin Mok, Sun Ha Jee, Jonathan M. Samet
Final approval of manuscript: Cari M. Kitahara, Amy Berrington de González, Neal D. Freedman, Rachel Huxley, Yejin Mok, Sun Ha Jee, Jonathan M. Samet
References
- 1.Heart Protection Study Collaborative Group MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: A randomized placebo-controlled trial. Lancet. 2002;360:7–22. [Google Scholar]
- 2.Huxley R, Lewington S, Clarke R. Cholesterol, coronary heart disease and stroke: A review of published evidence from observational studies and randomized controlled trials. Semin Vasc Med. 2002;2:315–323. doi: 10.1055/s-2002-35402. [DOI] [PubMed] [Google Scholar]
- 3.Toner CD, Davis CD, Milner JA. The vitamin D and cancer conundrum: Aiming at a moving target. J Am Diet Assoc. 2010;110:1492–1500. doi: 10.1016/j.jada.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 4.Eliassen AH, Hankinson SE. Endogenous hormone levels and risk of breast, endometrial and ovarian cancers: Prospective studies. Adv Exp Med Biol. 2008;630:148–165. [PubMed] [Google Scholar]
- 5.Crawford ED. Understanding the epidemiology, natural history, and key pathways involved in prostate cancer. Urology. 2009;73(suppl 5):S4–S10. doi: 10.1016/j.urology.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 6.Knekt P, Reunanen A, Aromaa A, et al. Serum cholesterol and risk of cancer in a cohort of 39,000 men and women. J Clin Epidemiol. 1988;41:519–530. doi: 10.1016/0895-4356(88)90056-x. [DOI] [PubMed] [Google Scholar]
- 7.Schatzkin A, Hoover RN, Taylor PR, et al. Site-specific analysis of total serum cholesterol and incident cancer in the National Health and Nutrition Examination Survey I Epidemiologic Follow-up Study. Cancer Res. 1988;48:452–458. [PubMed] [Google Scholar]
- 8.Törnberg SA, Holm LE, Carstensen JM, et al. Cancer incidence and cancer mortality in relation to serum cholesterol. J Natl Cancer Inst. 1989;81:1917–1921. doi: 10.1093/jnci/81.24.1917. [DOI] [PubMed] [Google Scholar]
- 9.Strasak AM, Pfeiffer RM, Brant LJ, et al. Time-dependent association of total serum cholesterol and cancer incidence in a cohort of 172,210 men and women: A prospective 19-year follow-up study. Ann Oncol. 2009;20:1113–1120. doi: 10.1093/annonc/mdn736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iso H, Ikeda A, Inoue M, et al. Serum cholesterol levels in relation to the incidence of cancer: The JPHC study cohorts. Int J Cancer. 2009;125:2679–2686. doi: 10.1002/ijc.24668. [DOI] [PubMed] [Google Scholar]
- 11.Ahn J, Lim U, Weinstein SJ, et al. Prediagnostic total and high-density lipoprotein cholesterol and risk of cancer. Cancer Epidemiol Biomarkers Prev. 2009;18:2814–2821. doi: 10.1158/1055-9965.EPI-08-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kritchevsky SB, Kritchevsky D. Serum cholesterol and cancer risk: An epidemiologic perspective. Annu Rev Nutr. 1992;12:391–416. doi: 10.1146/annurev.nu.12.070192.002135. [DOI] [PubMed] [Google Scholar]
- 13.Kritchevsky SB, Wilcosky TC, Morris DL, et al. Changes in plasma lipid and lipoprotein cholesterol and weight prior to the diagnosis of cancer. Cancer Res. 1991;51:3198–3203. [PubMed] [Google Scholar]
- 14.Törnberg SA, Carstensen JM, Holm LE. Risk of stomach cancer in association with serum cholesterol and beta-lipoprotein. Acta Oncol. 1988;27:39–42. doi: 10.3109/02841868809090316. [DOI] [PubMed] [Google Scholar]
- 15.Asano K, Kubo M, Yonemoto K, et al. Impact of serum total cholesterol on the incidence of gastric cancer in a population-based prospective study: The Hisayama study. Int J Cancer. 2008;122:909–914. doi: 10.1002/ijc.23191. [DOI] [PubMed] [Google Scholar]
- 16.Törnberg SA, Holm LE, Carstensen JM. Breast cancer risk in relation to serum cholesterol, serum beta-lipoprotein, height, weight, and blood pressure. Acta Oncol. 1988;27:31–37. doi: 10.3109/02841868809090315. [DOI] [PubMed] [Google Scholar]
- 17.Vatten LJ, Foss OP. Total serum cholesterol and triglycerides and risk of breast cancer: A prospective study of 24,329 Norwegian women. Cancer Res. 1990;50:2341–2346. [PubMed] [Google Scholar]
- 18.Platz EA, Clinton SK, Giovannucci E. Association between plasma cholesterol and prostate cancer in the PSA era. Int J Cancer. 2008;123:1693–1698. doi: 10.1002/ijc.23715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mondul AM, Clipp SL, Helzlsouer KJ, et al. Association between plasma total cholesterol concentration and incident prostate cancer in the CLUE II cohort. Cancer Causes Control. 2010;21:61–68. doi: 10.1007/s10552-009-9434-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Platz EA, Till C, Goodman PJ, et al. Men with low serum cholesterol have a lower risk of high-grade prostate cancer in the placebo arm of the Prostate Cancer Prevention Trial. Cancer Epidemiol Biomarkers Prev. 2009;18:2807–2813. doi: 10.1158/1055-9965.EPI-09-0472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park SK, Sakoda LC, Kang D, et al. Rising prostate cancer rates in South Korea. Prostate. 2006;66:1285–1291. doi: 10.1002/pros.20419. [DOI] [PubMed] [Google Scholar]
- 22.Berrington de Gonzalez A, Yun JE, Lee SY, et al. Pancreatic cancer and factors associated with the insulin resistance syndrome in the Korean cancer prevention study. Cancer Epidemiol Biomarkers Prev. 2008;17:359–364. doi: 10.1158/1055-9965.EPI-07-0507. [DOI] [PubMed] [Google Scholar]
- 23.Chen Z, Keech A, Collins R, et al. Prolonged infection with hepatitis B virus and association between low blood cholesterol concentration and liver cancer. BMJ. 1993;306:890–894. doi: 10.1136/bmj.306.6882.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cicognani C, Malavolti M, Morselli-Labate AM, et al. Serum lipid and lipoprotein patterns in patients with liver cirrhosis and chronic active hepatitis. Arch Intern Med. 1997;157:792–796. [PubMed] [Google Scholar]
- 25.Hiatt RA, Fireman BH. Serum cholesterol and the incidence of cancer in a large cohort. J Chronic Dis. 1986;39:861–870. doi: 10.1016/0021-9681(86)90034-2. [DOI] [PubMed] [Google Scholar]
- 26.Chyou PH, Nomura AM, Stemmermann GN, et al. Prospective study of serum cholesterol and site-specific cancers. J Clin Epidemiol. 1992;45:287–292. doi: 10.1016/0895-4356(92)90089-6. [DOI] [PubMed] [Google Scholar]
- 27.Platz EA. Does statin use affect the risk of developing prostate cancer? Nat Clin Pract Urol. 2009;6:70–71. doi: 10.1038/ncpuro1295. [DOI] [PubMed] [Google Scholar]
- 28.Törnberg SA, Holm LE, Carstensen JM, et al. Risks of cancer of the colon and rectum in relation to serum cholesterol and beta-lipoprotein. N Engl J Med. 1986;315:1629–1633. doi: 10.1056/NEJM198612253152601. [DOI] [PubMed] [Google Scholar]
- 29.Høyer AP, Engholm G. Serum lipids and breast cancer risk: A cohort study of 5,207 Danish women. Cancer Causes Control. 1992;3:403–408. doi: 10.1007/BF00051352. [DOI] [PubMed] [Google Scholar]
- 30.Eliassen AH, Colditz GA, Rosner B, et al. Serum lipids, lipid-lowering drugs, and the risk of breast cancer. Arch Intern Med. 2005;165:2264–2271. doi: 10.1001/archinte.165.19.2264. [DOI] [PubMed] [Google Scholar]
- 31.Gaard M, Tretli S, Urdal P. Risk of breast cancer in relation to blood lipids: A prospective study of 31,209 Norwegian women. Cancer Causes Control. 1994;5:501–509. doi: 10.1007/BF01831377. [DOI] [PubMed] [Google Scholar]
- 32.Kaye JA, Meier CR, Walker AM, et al. Statin use, hyperlipidaemia, and the risk of breast cancer. Br J Cancer. 2002;86:1436–1439. doi: 10.1038/sj.bjc.6600267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ha M, Sung J, Song YM. Serum total cholesterol and the risk of breast cancer in postmenopausal Korean women. Cancer Causes Control. 2009;20:1055–1060. doi: 10.1007/s10552-009-9301-7. [DOI] [PubMed] [Google Scholar]
- 34.Wu H, Jiang H, Lu D, et al. Effect of simvastatin on glioma cell proliferation, migration, and apoptosis. Neurosurgery. 2009;65:1087–1096. doi: 10.1227/01.NEU.0000360130.52812.1D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.World Cancer Research Fund: Food, Nutrition, Physical Activity, and the Prevention of Cancer: A Global Perspective. Washington, DC: World Cancer Research Fund and American Institute for Cancer Research; 2007. [Google Scholar]
- 36.Shin HR. Epidemiology of hepatitis C virus in Korea. Intervirology. 2006;49:18–22. doi: 10.1159/000087258. [DOI] [PubMed] [Google Scholar]
- 37.Gonyeau MJ, Yuen DW. A clinical review of statins and cancer: Helpful or harmful? Pharmacotherapy. 2010;30:177–194. doi: 10.1592/phco.30.2.177. [DOI] [PubMed] [Google Scholar]
- 38.Heart Protection Study Collaborative Group. The effects of cholesterol lowering with simvastatin on cause-specific mortality and on cancer incidence in 20,536 high-risk people: A randomized placebo-controlled trial [ISRCTN48489393] BMC Med. 2005;3:6. [Google Scholar]
- 39.Carroll MD, Lacher DA, Sorlie PD, et al. Trends in serum lipids and lipoproteins of adults, 1960-2002. JAMA. 2005;294:1773–1781. doi: 10.1001/jama.294.14.1773. [DOI] [PubMed] [Google Scholar]