Abstract
The authors describe the case of a middle-aged women who presented with an acute myocardial infarction due to thrombotic occlusion of angiographically normal coronary arteries. Coronary thrombosis was caused by a hypercoagulable state related to a haemolytic crisis of paroxysmal nocturnal haemoglobinuria and the patient was treated conservatively with antithrombotic agents. The clinical course was complicated by both severe bleeding and thrombotic complications and the patient eventually died of a massive intracerebral haemorrhage. The rapid occurrence of complications inhibited a timely administration of a specific treatment for complement-mediated haemolysis (eculizumab).
Background
To highlight paroxysmal nocturnal haemoglobinuria (PNH) as a possible cause of arterial and venous thrombosis and to point out novel treatment options in PNH.
Case presentation
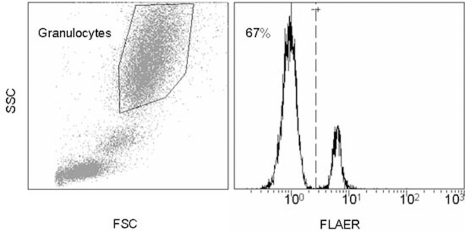
A 67-year-old woman with arterial hypertension, hypercholesterolaemia and a positive family history for myocardial infarction developed an acute chest pain radiating to her left arm. On admission at the hospital, ECG confirmed inferior ST segment elevation myocardial infarction. A primary percutaneous intervention revealed a thrombotic distal occlusion of the right posterolateral branch and a non-occlusive thrombus in the proximal part of the left anterior descending artery (figure 1). Given the distal location of the right posterolateral branch occlusion no intervention was performed and anticoagulation with heparin, aspirin and clopidogrel was initiated. At this point, potential causes of myocardial infarction with angiographically normal coronary arteries were considered.1 Echocardiographically, there was no evidence for a right-to-left shunt. The angiographic picture did not suggest vasospasms and the laboratory values showed no evidence for an inflammatory process. However, the patient’s medical history was remarkable for aplastic anaemia 9 years earlier, which had been successfully treated with two cycles of antithymocyte globuline and cyclosporine. Ever since, the patient was in a complete remission. However, 3 years prior to the current hospitalisation, flow cytometry demonstrated a small PNH clone, with 5% of granulocytes being fluorescein-labelled proaerolysin variant (FLAER)-negative. Anticoagulation was not initiated at that time because the PNH clone was small and because there were signs of haemolysis. Moreover, there was a medical history of no prior thromboembolic events. Unfortunately, the patient was lost to follow-up and flow cytometry could not be repeated. During the current hospitalisation, the patient presented with a Coombs-negative haemolytic anaemia (haemoglobin 93 g/l, reticulocyte count 160×109/l, lactate dehydrogenase 2741 U/l, bilirubine 22 mmol/l, unmeasurably low haptoglobin) and a normal platelet count. The PNH clone had increased to 67% suggesting a diagnosis of haemolysing PNH (figure 2) a condition which is strongly associated with both venous and arterial thromboembolic events.
Figure 1.
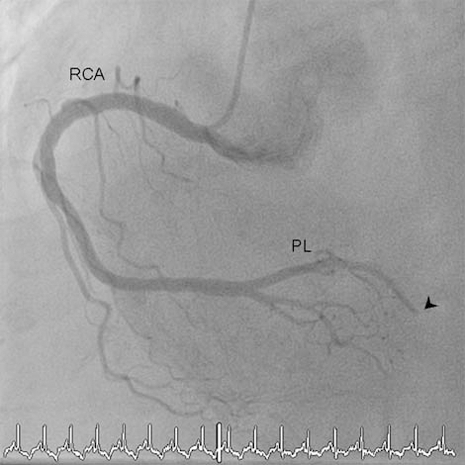
Coronary angiography. LAO/cranial view of the right coronary artery (RCA) showing distal thrombotic occlusion (arrowhead) of the posterolateral branch (PL).
Figure 2.
PNH flow cytometry. Analysis from peripheral blood using a fluorescein-labelled proaerolysin variant (FLAER) that binds selectively to the GPI anchor allowing quantification of GPI expression on the cell surface. FSC and SSC denotes forward and sideward scatter. Primary gating on granulocytes, 67% of all granulocytes are FLAER-negative (PNH cells).
Outcome and follow-up
The patient’s further clinical course was complicated by a retroperitoneal haematoma and an aneurysm at the catheter insertion site. Anticoagulation was temporarily interrupted and the haematoma surgically evacuated. Two weeks later, the patient had to be readmitted with a non-ST-elevation myocardial infarction despite combination therapy with aspirin and a vitamin K antagonist with a subtherapeutic international normalised ratio (INR) level of 1.8 on admission. Because the patient remained haemodynamically stable and chest pain could be controlled conservatively we did not perform an emergency coronary angiography but intensified the antithrombotic treatment by adding unfractionated heparin. The following morning, the patient awoke with severe headaches. Laboratory values at that time revealed a platelet count of 121 000×109/l (150–450 000), an INR value of 2.6, a fibrinogen level of 4.4g/l (1.5–3) and a thrombin clotting time above the therapeutic range. The CT scan showed a massive intracerebral bleeding and an immediate neurosurgical intervention was performed. However, the patient’s neurological condition deteriorated rapidly and she died only few days later.
Discussion
PNH is a rare acquired disorder of haematopoietic stem cells primarily characterised by haemolytic anaemia and recurrent thromboembolic events.2 Pathogenetically, a mutation in the PIG-A gene leads to the loss of glycosylphosphatidylinositol (GPI) anchor proteins and the GPI-associated membrane proteins such as complement regulating proteins CD55 and CD59.3–5 This results in complement-mediated intravascular haemolysis. Recurrent thromboembolic complications are the hallmark of the disease and the leading cause of death among these patients. Although both venous and arterial thrombosis may occur, myocardial infarction due to thrombotic occlusions of coronary arteries is extremely rare accounting for less than 2% of all thromboembolic events.6 Like Ulysses being constraint to sail the strait between the two sea monsters Scylla and Charybdis, our patient turned out to be at the same time at high risk of major bleeding and re-thrombosis. Risk of major bleeding is considerably increased in patients treated with triple anticoagulation, whereas re-thrombosis is common in haemolysing PNH.7 8 But in contrast to the mythical hero, it seems that today’s PNH patients might profit from a solution to their dilemma. Few years ago, the introduction of a monoclonal antibody targeting complement protein C5 has revolutionised the treatment of PNH.9 10 Blocking of C5 inhibits the formation of the membrane-attack complex and thereby completely abrogates intravascular haemolysis, subsequently normalising the risk of thrombosis.6 8 11 Unfortunately, in 2009 eculizumab was still not approved for treatment in Switzerland and administrative hurdles retarded a timely administration. Hence, we do not know how eculizumab would have worked in our patient and it is not clear if it would have changed the course given the rapid occurrence of complications. However, we think that administration of eculizumab soon after the first coronary thrombosis could eventually have prevented myocardial reinfarction and reduced the need for a more aggressive antithrombotic treatment.
Learning points.
-
▶
In patients with arterial or venous thrombosis and Coombs-negative haemolytic anaemia the differential diagnosis must include PNH
-
▶
Eculizumab has been proven to effectively treat haemolysis and prevent thrombotic complications in PNH
-
▶
Treatment with eculizumab should be evaluated in all PNH patients with thrombotic events as soon as possible
-
▶
Eculizumab is an expensive drug. The cost per standard maintenance dose of eculizumab is £250 000 per year
-
▶
The risk of bleeding increases with the number of antithrombotic drugs used.
Footnotes
Competing interests GS received honoraria from Alexion Pharmaceuticals and is a member of the scientific board of Alexion Pharmaceuticals.
Patient consent Obtained.
References
- 1.Ammann P, Marschall S, Kraus M, et al. Characteristics and prognosis of myocardial infarction in patients with normal coronary arteries. Chest 2000;117:333–8 [DOI] [PubMed] [Google Scholar]
- 2.Hillmen P, Lewis SM, Bessler M, et al. Natural history of paroxysmal nocturnal hemoglobinuria. N Engl J Med 1995;333:1253–8 [DOI] [PubMed] [Google Scholar]
- 3.Miyata T, Takeda J, Iida Y, et al. The cloning of PIG-A, a component in the early step of GPI-anchor biosynthesis. Science 1993;259:1318–20 [DOI] [PubMed] [Google Scholar]
- 4.Takeda J, Miyata T, Kawagoe K, et al. Deficiency of the GPI anchor caused by a somatic mutation of the PIG-A gene in paroxysmal nocturnal hemoglobinuria. Cell 1993;73:703–11 [DOI] [PubMed] [Google Scholar]
- 5.Miyata T, Yamada N, Iida Y, et al. Abnormalities of PIG-A transcripts in granulocytes from patients with paroxysmal nocturnal hemoglobinuria. N Engl J Med 1994;330:249–55 [DOI] [PubMed] [Google Scholar]
- 6.Hillmen P, Muus P, Dührsen U, et al. Effect of the complement inhibitor eculizumab on thromboembolism in patients with paroxysmal nocturnal hemoglobinuria. Blood 2007;110:4123–8 [DOI] [PubMed] [Google Scholar]
- 7.Sørensen R, Hansen ML, Abildstrom SZ, et al. Risk of bleeding in patients with acute myocardial infarction treated with different combinations of aspirin, clopidogrel, and vitamin K antagonists in Denmark: a retrospective analysis of nationwide registry data. Lancet 2009;374:1967–74 [DOI] [PubMed] [Google Scholar]
- 8.Hillmen P, Young NS, Schubert J, et al. The complement inhibitor eculizumab in paroxysmal nocturnal hemoglobinuria. N Engl J Med 2006;355:1233–43 [DOI] [PubMed] [Google Scholar]
- 9.Brodsky RA. How I treat paroxysmal nocturnal hemoglobinuria. Blood 2009;113:6522–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parker C. Eculizumab for paroxysmal nocturnal haemoglobinuria. Lancet 2009;373:759–67 [DOI] [PubMed] [Google Scholar]
- 11.Brodsky RA, Young NS, Antonioli E, et al. Multicenter phase 3 study of the complement inhibitor eculizumab for the treatment of patients with paroxysmal nocturnal hemoglobinuria. Blood 2008;111:1840–7 [DOI] [PubMed] [Google Scholar]