Abstract
The authors report the case of a 25-year-old patient with cystic fibrosis (CF) who developed pandemic influenza A/H1N1 during a visit to the USA in August 2010. The patient has severe CF lung disease and takes maintenance oral corticosteroids. The influenza virus was positive for the H275Y oseltamivir-resistance mutation despite the patient never having received oseltamivir. The patient has remained sputum-positive for over 4 months despite inhaled zanamivir therapy. This is the first reported case of transmission of oseltamivir-resistant H1N1 influenza to a patient with CF. The frequency of prolonged sputum carriage of pandemic influenza and transmission of oseltamivir-resistant strains are unknown on a population level. However, if our observations are replicated in other CF patients, they are potentially of considerable importance to clinical and infection-control practices in this patient group.
Background
Influenza A/H1N1 virus is known to develop the H275Y oseltamivir-resistance mutation, in a proportion of cases, in response to treatment with oseltamivir.1 We report the first case of primary acquisition of H275Y-positive pandemic influenza in a patient with cystic fibrosis (CF). The patient had never previously received oseltamivir, which implies that she acquired a resistant virus. Influenza virus continues to be detected in the patient’s sputum over 4 months, later suggesting ongoing viral replication and a risk of onward transmission of infection.
Although most reported cases of swine-origin influenza A/H1N1 in CF patients led to relatively mild illnesses,2–5 CF patients are known to be at risk of severe influenza A infection.6 The possibility of strains resistant to antiviral therapy is an important consideration for therapy. Awareness of person-to-person transmission of oseltamivir-resistant pandemic influenza and the potential for prolonged sputum carriage are of substantial importance to both clinical and infection-control practices in the CF patient group.
Case presentation
A 25-year-old woman with CF was admitted in September 2010 with breathlessness and a productive cough. Her CF genotype was ΔF508 homozygous and her latest spirometry revealed an FEV1 of 34% predicted. She had chronic infection with Achromobacter spanius and Mycobacterium abscessus. Her CF was complicated by allergic bronchopulmonary aspergillosis (ABPA), CF-related diabetes mellitus, osteopaenia and exocrine pancreatic insufficiency. She was taking prednisolone 10 mg daily and itraconazole 200 mg twice daily for ABPA. M abscessus was treated with long-term ciprofloxacin, rifampicin, azithromycin and nebulised amikacin. The patient had not been vaccinated against pandemic influenza A/H1N1. Neither the patient nor any other close contact had been treated with oseltamivir previously.
The patient’s acute symptoms began 2 weeks prior to presentation during a visit to Las Vegas, Nevada. Her travelling companion had symptoms of a mild upper respiratory tract infection at the same time. On her return from the USA, she developed worsening breathlessness, increased sputum load and a fever of 38.4°C. She was admitted to hospital and treated with intravenous meropenem, amikacin and tigecycline.
Investigations
On admission to hospital, the total white cell count was 12.4×109/l with a mild lymphopenia of 1.45×109/l. C reactive protein was elevated to 99 mg/l. Sputum cultures grew Aspergillus fumigatus but no bacterial pathogens. The chest radiograph revealed severe bilateral changes of CF consistent with the findings of a previous high-resolution CT scan of the chest (figure 1).
Figure 1.
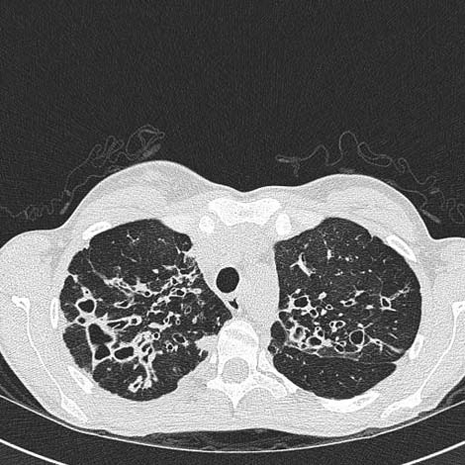
High resolution CT scan demonstrating evidence of bilateral upper lobe bronchiectasis.
On admission, sputum was sent for virological testing using real-time reverse-transcription PCR (RT-PCR) assays for the detection of respiratory viruses including influenza A.7 The sputum sample taken during admission was positive for pandemic influenza A/H1N1. Nose and throat swabs were negative for influenza. Further testing subsequently revealed the influenza virus identified in the sputum to be positive for the H275Y oseltamivir-resistance mutation.
Treatment
The initial sputum virology result confirming influenza A/H1N1 infection was received on day 5 of the admission by which time her fever had settled and her symptoms were improving. Oseltamivir treatment was therefore not given. During a second admission 1 month later, the patient received a 10-day course of inhaled zanamivir, by which time the oseltamivir-resistance result was available. Inhaled zanamivir was tolerated well with no evidence of bronchospasm but, unfortunately, the patient’s sputum remained positive for influenza A/H1N1.
Outcome and follow-up
The patient’s sputum remains positive for influenza A/H1N1 over 4 months after its first identification. She has shown a progressive deterioration in her lung function and symptoms of chronic endobronchial infection. She has required admission for intravenous antibiotics and treatment of a pulmonary exacerbation of CF on three further occasions since the initial episode.
Discussion
The 2009 pandemic influenza A/H1N1 virus was first identified in April 2009 in North America.8 A case death rate of less than 0.5% has been quoted but the pandemic was still responsible for over 18 000 deaths worldwide by July 2010.1 The impact of pandemic influenza A/H1N1 on patients with CF has not been characterised fully.
Risk factors for adverse outcomes with pandemic A/H1N1 influenza include chronic lung disease and immunosuppression, such as with oral corticosteroid use.9 Pandemic influenza has also been shown to have the highest attack rates among young adults and children, potentially placing the CF population at increased risk.1 9 An early single-centre, observational study of 11 Australian adults with CF reported a mild illness in association with pandemic influenza.2 All 11 patients made a good recovery with oral oseltamivir. Small case series from the UK and Europe concurred with this finding,3–5 although an Italian multicentre study of 68 patients with influenza A/H1N1 infection reported three fatalities.4 Many CF patients remain at high risk for adverse outcomes from influenza through concomitant lung disease, oral corticosteroid therapy and poor nutritional status.
This is the first case of primary acquisition of H275Y-positive pandemic influenza A/H1N1 in a patient with CF. A case of influenza A/H1N1 infection has been described in an 8-year-old child with CF in which the H275Y mutation developed only after treatment with oseltamivir.10 Inhaled zanamivir was administered safely in that case, as with our patient, but with a successful clinical and virological response. In the non-CF population, person-to-person transmission of oseltamivir-resistant pandemic influenza has been reported in a cluster of seven previously healthy individuals in Vietnam, during a long bus journey.11 Aside from this, other reported cases of resistance have developed during oseltamivir therapy. Intravenous zanamivir has been used with success in patients with haematological malignancy and severe oseltamivir-resistant pandemic influenza.12 13
The case in this report showed considerably prolonged sputum-positivity for pandemic influenza using PCR techniques. In comparison, 426 non-CF patients with pandemic A/H1N1 influenza in China had a median duration of PCR positivity of just 6 days (range 1–17).14 Risk factors for persistence beyond 5 days in this study were age less than 14 years (OR 1.94), male gender (OR 1.69) and a delay of more than 48 h from onset of symptoms to start of oseltamivir therapy (OR 4.46). No data on use of corticosteroids were provided. Beyond this, case reports have documented prolonged viral shedding of upto 28 days in patients with severe influenza A/H1N1 infection.15
In our patient, it is not clear whether the persistence of influenza infection is due to CF itself, immunosuppression from oral corticosteroids or the presence of concurrent respiratory pathogens. The explanation is likely to be multifactorial and further investigation to unravel each of these issues is required.
Learning points.
-
▶
Person-to-person transmission of oseltamivir-resistant pandemic influenza A/H1N1 involving patients with CF may occur.
-
▶
Pandemic influenza A/H1N1 may persist for many months in patients with CF who are receiving oral corticosteroids.
-
▶
Prolonged periods of barrier nursing and other infection-control measures may be needed in CF patients with pandemic influenza.
-
▶
Inhaled zanamivir can be tolerated in patients with severe CF lung disease, although without virological response in this case.
Footnotes
Competing interests None.
Patient consent Obtained.
References
- 1.World Health Organization Pandemic (H1N1) 2009 – Update 111. Geneva: World Health Organization, 2010 [Google Scholar]
- 2.France MW, Tai S, Masel PJ, et al. The month of July: an early experience with pandemic influenza A (H1N1) in adults with cystic fibrosis. BMC Pulm Med 2010;10:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nash EF, Whitmill R, Barker B, et al. Clinical outcomes of pandemic (H1N1) 2009 influenza (swine flu) in adults with cystic fibrosis. Thorax 2011;66:259. [DOI] [PubMed] [Google Scholar]
- 4.Colombo C, Battezzati PM, Lucidi V, et al. Influenza A/H1N1 in patients with cystic fibrosis in Italy: a multicentre cohort study. Thorax 2011;66:260–1 [DOI] [PubMed] [Google Scholar]
- 5.Whitaker P, Etherington C, Denton M, et al. A/H1N1 flu pandemic. A/H1N1 and other viruses affecting cystic fibrosis. BMJ 2009;339:b3958. [DOI] [PubMed] [Google Scholar]
- 6.Conway SP, Simmonds EJ, Littlewood JM. Acute severe deterioration in cystic fibrosis associated with influenza A virus infection. Thorax 1992;47:112–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ellis J, Iturriza M, Allen R, et al. Evaluation of four real-time PCR assays for detection of influenza A(H1N1)v viruses. Euro Surveill 2009;14:9–11 [DOI] [PubMed] [Google Scholar]
- 8.Dawood FS, Jain S, Finelli L, et al. ; Novel Swine-Origin Influenza A (H1N1) Virus Investigation Team Emergence of a novel swine-origin influenza A (H1N1) virus in humans. N Eng J Med 2009;360:2605–15 [DOI] [PubMed] [Google Scholar]
- 9.Bautista E, Chotpitayasunondh T, Gao Z, et al. ; Writing Committee of the WHO Consultation on Clinical Aspects of Pandemic (H1N1) 2009 Influenza Clinical Aspects of Pandemic 2009 Influenza A (H1N1) Virus Infection. N Eng J Med 2010;362:1708–19 [DOI] [PubMed] [Google Scholar]
- 10.Esposito S, Molteni CG, Colombo C, et al. Oseltamivir-induced resistant pandemic A/H1N1 influenza virus in a child with cystic fibrosis and Pseudomonas aeruginosa infection. J Clin Virol 2010;48:62–5 [DOI] [PubMed] [Google Scholar]
- 11.Le QM, Wertheim HF, Tran ND, et al. A community cluster of oseltamivir-resistant cases of 2009 H1N1 influenza. N Engl J Med 2010;362:86–7 [DOI] [PubMed] [Google Scholar]
- 12.Gaur AH, Bagga B, Barman S, et al. Intravenous zanamivir for oseltamivir-resistant 2009 H1N1 influenza. N Engl J Med 2010;362:88–9 [DOI] [PubMed] [Google Scholar]
- 13.Kidd IM, Down J, Nastouli E, et al. H1N1 pneumonitis treated with intravenous zanamivir. Lancet 2009;374:1036. [DOI] [PubMed] [Google Scholar]
- 14.Cao B, Li XW, Mao Y, et al. Clinical features of the initial cases of 2009 pandemic influenza A (H1N1) virus infection in China. N Engl J Med 2009;361:2507–17 [DOI] [PubMed] [Google Scholar]
- 15.Fleury H, Burrel S, Balick Weber C, et al. Prolonged shedding of influenza A(H1N1)v virus: two case reports from France 2009. Euro Surveill 2009;14:705–6 [PubMed] [Google Scholar]


