Abstract
Background
Alcohol and other substance use disorders (AUD/SUD) are common among youth and often continue into adulthood; therefore, the neurocognitive effects of substance use are of great concern. Because neuromaturation continues into young adulthood, youth with AUD/SUD may be at risk for lasting cognitive decrements. This study prospectively examines neuropsychological functioning over 10 years as a function of AUD/SUD history and outcomes.
Methods
The 51 participants consisted of 18 youth with persisting AUD/SUD, 19 youth with remitted AUD/SUD, and 14 community youth with no AUD/SUD history followed over 10 years (ages 16 to 27 on average) with neuropsychological testing and substance use interviews on 8 occasions. Neuropsychological performance from baseline to 10-year follow-up was compared between the three groups.
Results
Despite scoring higher than controls at intake, both AUD/SUD groups showed a relative decline in visuospatial construction at 10-year follow-up (p=.001). Regressions showed that alcohol use (β=−.33, p < .01) and drug withdrawal symptoms (β=−.31, p<.05) over follow-up were predictive of year 10 visuospatial function. Alcohol use also predicted verbal learning and memory (β=−.28, p<.05), while stimulant use predicted visual learning and memory function (β=−.33, p=.01). More recent substance use was associated with poorer executive function (β=.28, p<.05).
Discussion
These findings confirm prior studies suggesting that heavy, chronic alcohol and other substance use persisting from adolescence to young adulthood may produce cognitive disadvantages, primarily in visuospatial and memory abilities. Youth who chronically consume heavy quantities of alcohol and/or experience drug withdrawal symptoms may be particularly at risk for cognitive deterioration by young adulthood.
Keywords: adolescence, young adulthood, alcohol, substance use disorders, withdrawal, neurocognition, memory, visuospatial function, executive function
Introduction
Alcohol and other substance use disorders (AUD/SUD) are common among youth and often continue into adulthood. In 2008, 72% of high school seniors reported having at least tried alcohol, and 25% reported heavy drinking (five or more drinks in a row) in the past two weeks (Johnston, O'Malley, Bachman, & Schulenberg, 2009). Use tends to increase in young adulthood, with 40% of college students reporting recent heavy drinking (Johnston et al., 2009). Because neuromaturation continues through adolescence and young adulthood (Toga, Thompson, & Sowell, 2006), the neurocognitive effects of alcohol and other drug use are of great concern, and youth with AUD/SUD may be at particular risk for cognitive dysfunction.
In adult populations, chronic heavy alcohol use leads to neurocognitive deficits. Specifically, broad cognitive impairment is common among adult alcoholics during the acute detoxification period (Fein, Bachman, Fisher, & Davenport, 1990), including visuospatial, memory, and executive deficits (Reed, Grant, & Rourke, 1992; Sher, Martin, Wood, & Rutledge, 1997; Sullivan, Rosenbloom, & Pfefferbaum, 2000) as well as gait and balance abnormalities (Sullivan et al., 2000). After several weeks of abstinence, attention and concentration, reaction time, and memory tend to improve (Bates, Voelbel, & Buckman, 2005; Fein et al., 1990). Executive function, processing speed, and non-verbal skills appear more resistant to recovery, while the improvement of verbal abilities is less clear (Bates et al., 2005; Fein et al., 1990). After years of abstinence, adults appear to broadly recover neurocognitive abilities (Reed et al., 1992), although spatial processing may remain affected (Fein, Torres, Price, & Di Sclafani, 2006). The degree of neurocognitive recovery in adults depends on age, premorbid cognitive ability, duration of abstinence, and amount of drinking in the intervening time (Bates et al., 2004; Fein et al., 1990; Rourke & Grant, 1999).
Neuropsychological function among adolescents with heavy alcohol use has not been as thoroughly examined. However, adolescents with alcohol use disorders (AUD) have shown decrements in visuospatial and attention performance (Tapert, Granholm, Leedy, & Brown, 2002); retention of verbal and non-verbal material (Brown, Tapert, Granholm, & Delis, 2000); language and executive function (Moss, Kirisci, Gordon, & Tarter, 1994); and intellectual function, academic achievement, speed of processing, and sustained attention (Tapert & Brown, 2000; Tarter, Mezzich, Hsieh, & Parks, 1995). However, because intellectual abilities differed between groups in some cases (Moss et al., 1994; Tarter et al., 1995), the causes of the neuropsychological abnormalities are less clear.
In addition to cognitive deficits, adolescents with AUD have shown altered patterns of brain activation in response to a spatial working memory task during functional magnetic resonance imaging (fMRI), despite similar performance as controls (Caldwell et al., 2005; Tapert et al., 2004). In particular, the degree of abnormal activation was greater in adolescents who had more extensive histories of withdrawal/hangover symptoms and alcohol consumption. The results suggest possible reorganization of neuronal circuits that are detectable during the early stages of AUD and may suggest that certain brain areas compensate for decreased activation in other areas (Tapert et al., 2004). Although the groups performed similarly on neuropsychological tests, it is unclear whether continued alcohol use would result in behavioral differences (Caldwell et al., 2005).
Certain brain areas may be especially vulnerable to the effects of heavy alcohol consumption. Animal studies have shown decreased neurogenesis in the adolescent forebrain and hippocampal areas subsequent to ethanol exposure (Crews, Mdzinarishvili, Kim, He, & Nixon, 2006). In humans, the volume of the prefrontal cortex appears reduced in adolescent heavy drinkers (De Bellis et al., 2005; Medina et al., 2008), as does the hippocampus (De Bellis et al., 2000; Medina, Schweinsburg, Cohen-Zion, Nagel, & Tapert, 2007; Nagel, Schweinsburg, Phan, & Tapert, 2005). Alcohol-related prefrontal and hippocampal dysmorphology may help explain the cognitive and memory disadvantages among AUD adolescents.
Most of the studies above are cross-sectional, and considerably less is known about the neurocognitive effects of continued heavy alcohol use by youth as they transition into adulthood. To examine the long-term effects of AUD and SUDs, our group is conducting a longitudinal study of adolescents who received treatment for alcohol and other substance use problems. Previous examinations of the data showed that continued substance use following treatment was associated with poorer visuospatial and attention functioning 4 years post-treatment (Tapert & Brown, 1999), and lower visuospatial, attention, learning, and retention function 8 years post-treatment (Tapert et al., 2002). Withdrawal symptoms (especially from alcohol) were associated with poorer visuospatial functioning (Brown et al., 2000; Tapert & Brown, 1999; Tapert et al., 2002), as well as verbal and non-verbal retrieval (Brown et al., 2000).
The current study prospectively examines neuropsychological functioning of youth after 10 years as a function of cumulative alcohol and drug involvement. In addition to examining differences between healthy young adults and those with persisting AUD/SUD, we were interested in whether individuals with remitted AUD/SUD would show long-term improvements in neurocognitive performance. Based on previous findings, we hypothesized that, relative to controls, young adults with persisting AUD/SUD would show decrements in visuospatial skills, attention, executive function, learning, and retention, while those with remitted AUD/SUD would show similar performance to controls by the 10-year follow-up. We also hypothesized that greater cumulative use of alcohol and other drugs as well as substance withdrawal symptoms over follow-up would be linked to poorer neurocognition at the 10-year follow-up, particularly in visuospatial functioning and retention.
Methods
Participants
Participants were part of a longitudinal research project following adolescents (N = 187; ages 13–18 years) with and without alcohol and other substance use disorders who received neuropsychological examinations at baseline and semi-annually thereafter up to 10 years (Brown, Myers, Mott, & Vik, 1994; Brown, Vik, & Creamer, 1989; Tapert et al., 2002). Clinical participants were consecutive admissions to inpatient adolescent substance abuse treatment centers who met diagnostic criteria for alcohol abuse or dependence at project intake. The majority of youth also met criteria for at least one other SUD. The comparison sample was solicited through advertisements in the same communities from which clinical participants originated. Community adolescents were selected to be similar to clinical participants on age (±1 year), gender, socioeconomic status, ethnic background, years of education (±1 year), and prevalence of family history of AUD and SUD. Community adolescents were excluded if they had a history of AUD, SUD, or alcohol or drug use problems at project intake.
Recruitment procedures excluded youths who (1) did not have a resource person (typically a parent) to corroborate biographical, health, and substance involvement information; (2) lived >50 miles from the research facility; (3) had an Axis I psychiatric disorder other than conduct disorder predating the onset of regular substance use; (4) did not speak English fluently; (5) had a history of head trauma with loss of consciousness >2 minutes; (6) had any medical or physical condition that could compromise neuropsychological (NP) performance (e.g., epilepsy, migraine); or (7) had prenatal exposure to alcohol (mother drank 3 or more drinks on an occasion or >3 times per month during pregnancy). Informed assent and consent, approved by University of California, San Diego Institutional Review Board and clinical agencies, was independently obtained from each participant and parent.
Intake assessments were conducted in the treatment facility approximately 3 weeks after admission for clinical participants, and at the research site for community youths. Follow-up neuropsychological assessments were conducted 2, 4, 6, 8, and 10 years after intake evaluations. Substance involvement information was obtained on over 90% of the cohort through in-person or phone interviews at each follow-up time-point (Anderson, Ramo, Cummins, & Brown, under review). At year 10, individuals who did not complete the study did not differ from included cases on the basis of demographics (age, ethnicity, handedness, education) or substance use patterns at intake. However, individuals who did not complete the 10 year substance use assessment were slightly more likely to be male and have lower parental SES at intake relative to participants who completed the 10 year assessment.
NP testing rates were not as high, due to participant relocations beyond the 50 mile limit. Of the 187 participants who received a NP assessment at intake, 79 participants (42.2%; 51 clinical and 28 community youth) had a 10-year follow-up NP assessment. Reasons for non-completion of the NP at year 10 included (1) participant relocation (n=68); (2) not able to be contacted (n=31); (3) study dropout (n=5); (4) death (n=3); and (5) incarceration (n=1). No statistically significant differences were found between youths who did and did not receive a NP assessment at 10-year follow-up on (1) intake demographics (age, gender, ethnicity, handedness, education, parental SES), substance involvement, or NP performance; or (2) 10-year demographics or substance involvement, although participants who did not complete a NP assessment at year 10 were slightly more likely to use stimulants more frequently at year 10 (intake-only NP: mean = 1.8±6.1 days per month; 10 year NP: mean = 1.2±5.6). In addition, ten community controls developed substance-related problems by the 10-year follow-up point and were excluded from the current study because (1) of the small size of this group, and (2) they did not logically fit into the other groups.
For the current evaluation, participants were excluded for recent substance use at the 10-year assessment (<48 hours; n=18) to avoid the effects of intoxication or hangover. This resulted in a sample with at least 2 days since last alcohol or other substance use (range = 2 to >1000). Participants in this sample reported the last substance used as (1) alcohol (n=35), (2) marijuana (n=4), (3) stimulants (n=2), (4) hallucinogens (n=1), or (5) opiates (n=1), and 8 participants denied recent use of any substances (i.e., within 1000 days of assessment). Please refer to Table 2 for more detailed recent and cumulative substance use patterns.
Table 2.
Substance use characteristics of youths followed over 10 years.
| Controls | Persisting AUD/SUD | Remitted AUD/SUD | |
|---|---|---|---|
| Substance use at intake | |||
| # Alcohol dependence sxs ever***a | 0.1 (0.3) | 4.7 (4.2) | 5.8 (4.5) |
| # Drug dependence sxs ever***a | 0.3 (1.1) | 17.9 (6.8) | 19.1 (8.7) |
| Substance use in past 2 years | |||
| Days since last substance use | 162.0 (356) | 50.8 (109) | 371.5 (468) |
| Alcohol, drinks per month, last 3 months | 5.1 (5.5) | 53.2 (84.5) | 9.0 (17.2) |
| Alcohol dependence sxs, past 2 yrs***b | 0.0 (0.0) | 1.7 (1.5) | 0.0 (0.0) |
| Alcohol withdrawal sxs, past 2 yrs | 0.0 (0.0) | 0.7 (2.4) | 0.0 (0.0) |
| Drug withdrawal sxs, past 2 yrs***b | 0.0 (0.0) | 4.3 (7.4) | 0.0 (0.0) |
| Drug dependence sxs, past 2 yrs***b | 0.0 (0.0) | 2.3 (1.9) | 0.0 (0.0) |
| Average use over follow-up period: Intake to year 10 | |||
| Drug dependence sxs***a,d | 0.1 (0.3) | 5.8 (5.0) | 1.6 (2.2) |
| Drug withdrawal sxs***a,d | 0.0 (0.0) | 3.6 (3.3) | 1.5 (2.3) |
| Alcohol dependence sxs***c,d | 0.2 (0.3) | 2.0 (1.7) | 0.7 (0.9) |
| Alcohol withdrawal sxs**a | 0.0 (0.2) | 0.5 (0.6) | 0.3 (0.5) |
| Alcohol, avg. drinks per month***c,d | 5.4 (5.1) | 48.4 (37.8) | 9.8 (16.9) |
| Marijuana, avg. use days per month***c,d | 0.1 (0.2) | 7.1 (7.0) | 0.8 (1.5) |
| Stimulants, avg. use days per month**a | 0.1 (0.2) | 1.8 (2.9) | 0.5 (0.8) |
Note. Values represent means (and standard deviations). Refer to methods section for description of composite variables summarizing substance use over the 10-year follow-up. Sxs = symptoms.
*p<.05
p<.01
p<.001 between three groups.
Mann Whitney post-hoc tests:
Persisting and Remitted AUD/SUD > Controls
Persisting AUD/SUD > Remitted AUD/SUD and Controls
Persisting AUD/SUD > Controls
Persisting AUD/SUD > Remitted AUD/SUD.
Within this sample (N = 51), three groups of individuals were studied:
-
(1)
community controls with no history of AUD/SUD at intake through follow-up (controls; n = 14);
-
(2)
participants who met AUD/SUD criteria at intake, and had (a) alcohol or drug dependence symptoms or problems in the last two years or (b) past 3-month drug or heavy alcohol use (5 or more drinks on one occasion) at year 10 (persisting AUD/SUD; n = 18); and
-
(3)
participants who met AUD/SUD criteria at intake but reported (a) no alcohol or drug dependence symptoms or problems in the past two years and (b) no recent drug or heavy alcohol use at year 10 (remitted AUD/SUD; n = 19).
Measures
Structured clinical interview
A 90-minute confidential structured clinical interview (Brown et al., 1994; Brown et al., 1989) administered to youth and parents assessed demographic information, as well as social, academic, and occupational functioning, family history of AUD/SUD, maternal drinking during pregnancy, physical and emotional health problems, and socioeconomic status (SES; higher scores reflect lower SES) (Hollingshead, 1965).
Customary Drinking and Drug Use Record (CDDR)
The CDDR (Anderson, Ramo, Schulte, Cummins, & Brown, 2007; Brown et al., 1998) gathered self-report information on lifetime and current alcohol and drug involvement. The follow-up version obtained information on the use of alcohol (beer, wine, liquor) and drugs (marijuana, amphetamines, barbiturates, hallucinogens, cocaine, inhalants, opiates, and prescription medications or other drugs not previously specified), life problems related to alcohol and drug use, DSM–III–R and DSM–IV alcohol and drug dependence criteria (American Psychiatric Association, 1987, 1994), and alcohol and drug withdrawal symptomatology. The CDDR incorporates the Cahalan drinking classification procedure (Cahalan, 1970), Drug Indulgence Index (Lu, 1974), and Alcohol Dependence Scale (Horn, Skinner, Wanberg, & Foster, 1984) items. At each follow-up assessment, alcohol and drug information was collected for the past 3 months and since the previous assessment (e.g., 2 years). Good internal consistency, test–retest reliability, and interrater reliability have been demonstrated in adolescent and young adult samples using these time periods (Brown et al., 1998; Stewart & Brown, 1995).
Neuropsychological test battery
The NP battery at project intake included: Wechsler Intelligence Scale for Children–Revised (WISC–R) (Wechsler, 1974) Vocabulary, Similarities, Block Design, Arithmetic, and Digit Span (digits forward, digits backward) subtests; Controlled Oral Word Association Test (COWAT) (Benton, Hamsher, Varney, & Spreen, 1983); Wechsler Memory Scale (WMS) Visual Reproduction subtest (Wechsler, 1945); California Verbal Learning Test (CVLT) (Delis, Kramer, Kaplan, & Ober, 1987); and Trail Making Test (Reitan & Wolfson, 1993). The year 10 battery included: Wechsler Adult Intelligence Scale–Revised (WAIS–R) (Wechsler, 1981) Vocabulary, Similarities, Block Design, Arithmetic, and Digit Span subtests; COWAT; CVLT-II (Delis, Kramer, Kaplan, & Ober, 2000); and Trail Making Test. In addition, WMS Visual Reproduction was replaced with the Rey-Osterrieth Complex Figures test (ROCF) (Osterrieth, 1944) copy and 30-min delay, employing the Taylor system for scoring accuracy (Lezak, 1995). To reduce the influence of practice effects, alternate forms of tests were administered when possible. Testing was approximately 2 hours at each time point. Finally, each NP measure was normed using T-scores based on the control group's raw scores at intake and year 10. Therefore, the mean of the control group at both time points is T = 50.
Procedures
Clinical and community youths were administered the NP test battery and structured interview by a trained psychometrist at project intake and at each follow-up. Adolescents recruited from treatment programs received the initial assessment during inpatient stay after a 3-week detoxification period. The other assessments were conducted at the research facility or the participant's residence. Youths and parents were each interviewed privately by different interviewers. At each follow-up, 10% of youths were randomly selected for urine drug toxicology screening to confirm self-reported substance use. Discrepancies in self-report and toxicology results (<1% of cases) were scored for use on toxicology-identified substances.
Data Analyses
Neuropsychological data reduction
To reduce the number of comparisons and Type I error risk, composite scores were generated to summarize the cognitive domains tested. We used a hybrid method that relied on established cognitive domains (Lezak, Howieson, & Loring, 2004) and reliability analyses (Delis, Jacobson, Bondi, Hamilton, & Salmon, 2003). After performing necessary log10 transformations to meet the assumptions of parametric analysis (intake and year 10 Trails A and B time-to-completion), standard scores were created for each individual cognitive measure for the entire sample (N = 51) at both intake and year 10. For each measure, higher standard scores represented superior performance. Next, tests were grouped to form distinct cognitive domains derived from a theoretical background, and standard scores were averaged within each domain. To ensure internal consistency of the individual measures in each composite score, we calculated standardized Cronbach's α coefficients. We reexamined composites with Cronbach's α coefficients less than .60 until good reliability was obtained. In two cases, the neuropsychological measure was best represented by standing alone (i.e., Trails A: time-to-completion and WISC-R or WAIS-R Block Design).
Six summary scores representing performance at intake and year 10 were derived: (1) Language (WISC-R or WAIS-R Vocabulary and Similarities subtests); (2) Psychomotor Speed (Trails A: time-to-completion); (3) Visuospatial Construction (WISC-R or WAIS-R Block Design); (4) Visuospatial Learning & Memory (WMS Visual Reproduction Immediate and Delayed Recall [intake]; ROCF copy and delayed recall [year 10]), (5) Verbal Learning & Memory (CVLT or CVLT-II: total of trials 1–5, short-delay free recall, long-delay free recall, percent savings [(total words correctly recalled on long-delay free recall / total words recalled on trial 5) × 100]); and (6) Executive Function (Trails B: time-to-completion, COWAT, WISC-R or WAIS-R Arithmetic and Digit Span subtests [total number of digits recalled in forward and backward sequence]). Cronbach's α for each composite score ranged from .72 to .83 with two exceptions: Language at intake (α = .66) and Executive Function at year 10 (α = .65).
Substance use, dependence, and withdrawal scores
To examine the effects of specific substances on neurocognition, indices were created to represent cumulative consumption of the three most commonly used substances in this sample (Tapert et al., 2002). Alcohol consumption was calculated as the average number of standard ethanol consumption units per month (over the preceding 3 months) at each follow-up time point over the 10-year period. Marijuana use was quantified as the average days per month of marijuana use at each follow-up time point. Stimulant use was calculated as the average days per month on which amphetamine or cocaine use occurred at each follow-up. Alcohol withdrawal was computed as the average number of different types of alcohol withdrawal symptoms endorsed for the preceding two years at 4-, 6-, 8-, and 10-year follow-ups, plus the number of different alcohol withdrawal symptoms endorsed for the preceding 3 months at the 6-, 12-, and 24-month follow-up time points. Drug withdrawal and alcohol and drug dependence symptoms were estimated in the same manner. The substance use assessment portion of the follow-up includes over 90% of the sample at each time point over the 10 year period, and all 51 participants had substance use data from at least four follow-up time points to facilitate computing these summary scores. Thus, we can be confident of the composite substance use scores derived herein. Distributions of variables were examined, and all substance use, dependence, and withdrawal scores were log10 transformed to meet the assumptions for parametric analysis (Tabachnick & Fidell, 2007).
Demographic comparison
One-way ANOVAs and Fisher's Exact tests compared groups on demographic variables, including age, gender, ethnicity, handedness, SES, education, and the number of neuropsychological testing sessions completed. The non-parametric Kruskall-Wallis procedure examined group differences in alcohol and other substance use. As groups differed on age at intake (1.3 years) and years of education at year 10 (2.5 years), these were entered as covariates for subsequent group comparisons. SES also differed between groups, but since year 10 participant SES (Hollingshead score) and education were highly correlated (Spearman's rho = −.66, p < .001), education was selected as the most appropriate covariate for this age group. Gender, education at year 10, and participant SES were not linked to year 10 NP scores. Analyses used SPSS 14.0 (Chicago, IL).
Longitudinal analyses
Repeated measures ANCOVAs compared groups on each of the six NP composite scores at intake and year 10 time points (α = .05) controlling for age at intake and years of education at year 10. Hierarchical regression analyses were conducted to ascertain which composite substance involvement variables most strongly predicted performance at year 10. For the first set of regressions, Block 1 entered intake age, intake NP composite score, and year 10 education; Block 2 entered (step-wise) the cumulative alcohol, marijuana, and stimulant scores. In the second set of regressions, Block 1 entered intake age, intake NP composite score, and year 10 education; Block 2 entered (step-wise) cumulative alcohol withdrawal symptoms, cumulative drug withdrawal symptoms, and days since last substance use at year 10. Dependent variables were the six 10-year NP composite scores.
Results
Demography and Substance Use
The three groups were similar in gender, ethnicity, and handedness distributions, and parent SES at baseline (see Table 1). Remitted AUD/SUD youth were about a year older than controls at intake, but this age difference was non-significant at 10-year follow-up. On average, the remitted and persisting AUD/SUD groups had lower levels of education and SES at year 10 than controls (note that higher Hollingshead SES scores reflect lower SES). Because the effects of repeated testing can confound the interpretation of NP performance (Matarazzo & Herman, 1984), the number of times the NP battery was administered to each participant was totaled. Importantly, groups were statistically equivalent with regard to the number of times tested. A repeated measures ANOVA showed that groups were similar in their estimated IQ levels at intake and year 10 [WISC-R and WAIS-R Vocabulary scores: group: F(2,48) = 1.19, NS, ηp2 = .05; group × time: F(2,48) = 2.03, NS, ηp2 = .08]. Substance use was comparable among persisting AUD/SUD and remitted AUD/SUD participants at intake, and generally higher among persisting AUD/SUD throughout follow-up (see Table 2).
Table 1.
Demographic characteristics of youths followed over 10 years.
| Controls n = 14 | Persisting AUD/SUD n = 18 | Remitted AUD/SUD n = 19 | |
|---|---|---|---|
| Age at intake*a | 15.2 (1.6) | 15.8 (1.2) | 16.5 (0.8) |
| Age at year 10 testing | 25.4 (1.7) | 26.7 (2.2) | 26.8 (1.1) |
| Gender (males : females) | 6 : 8 | 14 : 4 | 9 : 10 |
| Caucasian (%) | 64% | 67% | 79% |
| Right-handed (%) | 86% | 89% | 84% |
| Parent SES† at intake | 31.8 (13.4) | 31.7 (15.1) | 25.8 (9.3) |
| Participant SES† at year 10**b | 32.4 (13.9) | 47.8 (14.2) | 44.3 (12) |
| Years of education at year 10***b | 15.1 (2.1) | 12.6 (1.9) | 12.7 (1.9) |
| # of NP testings by year 10 | 7.43 (1.3) | 6.94 (1.1) | 7.16 (1.3) |
Note. Values represent means (and standard deviations) unless otherwise noted.
SES determined by Hollingshead scores (higher scores reflect lower SES).
p<.05
p<.01
p<.001 between three groups.
Post-hoc tests:
Remitted AUD/SUD > Controls
Controls > Persisting and Remitted AUD/SUD.
Neuropsychological Functioning
Repeated measures analysis
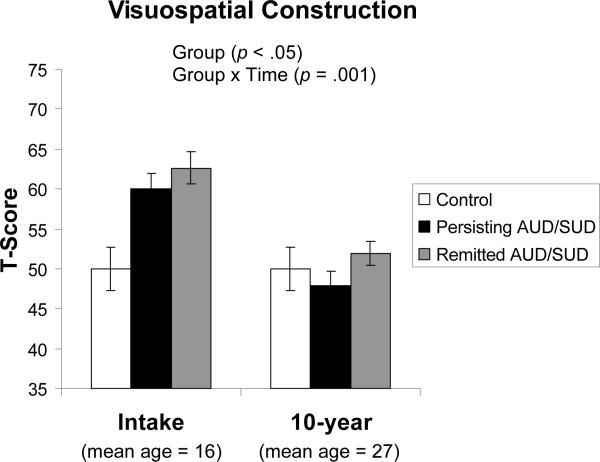
After controlling for age at intake and education at year 10, a main effect of group [F(2,46) = 3.67, p < .05, ηp2 = .14] was found for Visuospatial Construction (i.e., Block Design), with controls performing worse than the remitted and persisting AUD/SUD groups at intake. A group × time interaction for Visuospatial Construction [F(2,46) = 7.73, p = .001, ηp2 = .25] indicated that, despite performing better than controls at baseline, both persisting and remitted AUD/SUD groups showed greater declines in visuospatial construction abilities from intake to 10-year follow-up (see Figure 1). A marginally significant group × time effect was also detected for the Language composite score [F(2,46) = 2.78, p = .073, ηp2 = .11]. Again, both abuse groups performed better than controls at intake but showed greater declines than controls over the 10-year follow-up period.
Figure 1.
Persisting and remitted AUD/SUD groups show reduced Block Design T-Scores from intake to 10-year follow-up, relative to control participants.
Note. Each NP measure was normed using T-scores based on the control group's raw scores at intake and year 10. Therefore, the mean of the control group at both time points is T = 50. Error bars depict standard error of the mean.
Substance use and withdrawal regressions
Hierarchical regressions ascertained whether alcohol and other substance use over the 10-year follow-up period predicted NP performance at year 10. Greater cumulative alcohol use significantly predicted poorer year 10 Visuospatial Construction performance [F(4,46) = 12.09, p < .001; R2Δ = 10.3%, β = −.33, p < .01] and Verbal Learning & Memory [F(4,44) = 8.27, p < .001; R2Δ = 7.4%, β = −.28, p < .05] above and beyond the effects accounted for by age, intake Visuospatial Construction or Verbal Learning & Memory performance, and year 10 education, while cumulative marijuana and stimulant use were not predictive in either case. However, greater stimulant use over the 10-year period significantly predicted poorer Visual Learning & Memory performance at year 10 [F(4,46) = 6.64, p < .001; R2Δ = 10.1%, β = −.33, p = .01] above and beyond the effects accounted for by age, intake Visual Learning & Memory functioning, and education at year 10 (see Table 3).
Table 3.
Substance use and withdrawal over 10-year period and recent substance use predict year 10 neuropsychological performance of young adults.
| Standardized βs for Year 10 substance use |
||||||
|---|---|---|---|---|---|---|
| Dependent variable | Alcohol Use | Marijuana Use | Stimulant Use | Alcohol Withdrawal | Drug Withdrawal | Days since last substance use |
| Language | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
| Psychomotor Speed | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
| Visuospatial Construction | −.33** | n.s. | n.s. | n.s. | −.31** | n.s. |
| Visual Learning & Memory | n.s. | n.s. | −.33** | n.s. | n.s. | n.s. |
| Verbal Learning & Memory | −.28* | n.s. | n.s. | n.s. | n.s. | n.s. |
| Executive Function | n.s. | n.s. | n.s. | n.s. | n.s. | .28** |
Note. All regressions controlled for intake age, intake neuropsychological performance, and years of education at year 10.
p < .05
p ≤ .01
A second set of regressions examined whether alcohol and other drug withdrawal symptoms over the 10-year follow-up period or recency of substance use at year 10 predicted NP performance at year 10. After controlling for age and Visuospatial Construction performance at intake and year 10 education, having more drug withdrawal symptoms over the follow-up period was predictive of poorer year 10 Visuospatial Construction performance [F(4,46) = 10.85, p < .001; R2Δ = 7.6%, β = −.31, p < .05], while alcohol withdrawal over follow-up and days since last substance use at year 10 were not predictive of year 10 performance. Additionally, more recent use of alcohol or other drugs predicted poorer Executive Function at year 10 [F(4,42) = 13.54, p < .001; R2Δ = 7%, β = .28, p < .05] above and beyond the effects accounted for by age, intake Executive Function, and education at year 10.
Exploratory Analyses
We examined potential gender differences between groups for each of the six NP domains using repeated measure ANCOVAs, controlling for age at intake and education at year 10. No significant gender or group × gender effects were detected.
Discussion
We studied the long-term NP consequences of protracted AUD/SUD over a 10-year period from mid-adolescence to young adulthood. Previous studies have reported visuospatial, memory, and attention deficits among individuals with continued heavy drinking or drug use (Brown et al., 2000; Tapert & Brown, 1999; Tapert et al., 2002). The primary aim of the current study was to examine whether youth with remitted AUD/SUD would show improvements in NP functioning relative to youth with persisting AUD/SUD. Community controls with no history of AUD/SUD were also examined. The three groups performed similarly in visuospatial learning and memory, verbal learning and memory, executive function, and psychomotor speed at project intake and at a 10-year follow-up assessment. However, young adults with a history of AUD/SUD, whether persisting or remitted, showed a relative decline in visuospatial construction performance from intake to the 10-year follow-up relative to controls without a history of substance-related problems, even after controlling for age and education. A similar pattern was found for language abilities, although this finding was only marginally significant. These findings suggest that an individual's lifetime history of substance-related problems may negatively influence his/her cognitive function long after he/she has discontinued heavy or disordered use of alcohol or other drugs.
When examining multiple substance use indicators simultaneously, average alcohol use and average drug withdrawal symptoms over the 10-year follow-up appeared most predictive of visuospatial construction skills in young adulthood. Alcohol use during the follow-up period also predicted verbal learning and memory, while stimulant use significantly predicted visual learning and memory performance. In all cases, more use or withdrawal symptoms over the follow-up period was associated with poorer NP outcomes. Furthermore, having used alcohol or other drugs more recently at the 10-year assessment predicted poorer executive function. Overall, this pattern of findings suggests that youth with remitted AUD/SUD may not perform substantially better than youth who continue to have alcohol or drug-related problems. Rather, the cumulative effects of protracted substance use and withdrawal during mid to late adolescence and into young adulthood appear related to long-term cognitive functioning in the mid-twenties, particularly visuospatial construction and visual and verbal memory. Additionally, recent substance use may influence executive function in young adulthood.
The association of poorer visuospatial and memory performance with protracted substance use is in concordance with previous reports from this longitudinal study (Tapert & Brown, 1999; Tapert et al., 2002), as well as other investigators (Fein et al., 1990; Fein et al., 2006; Reed et al., 1992; Sher et al., 1997; Sullivan et al., 2000). Consistent with our previous reports on NP functioning at 4 and 8 years after substance use treatment during adolescence (Tapert & Brown, 1999; Tapert et al., 2002), more drug withdrawal symptoms over the follow-up period were associated with poorer visuospatial function. However, in the current study, alcohol withdrawal symptoms were not related to visuospatial performance, and we did not find decrements in attention (Brown et al., 2000; Tapert & Brown, 1999; Tapert et al., 2002) perhaps due to limited power. This could suggest that adolescent substance-use-related visuospatial and memory problems may be particularly enduring, or vulnerable to the impact of chronic use.
Possible reasons for discrepancies with previous reports include different statistical approaches and sample characteristics. For example, due to upgrades in the neuropsychological battery over the follow-up period, the current study included measures that were similar at intake and 10 year follow-up, while earlier reports included a wider range of NP tests. Further, measures included in each of the composite scores varied between studies. The study inclusion/exclusion criteria at project intake were the same for all participants, but previous reports may have excluded participants from analyses for different reasons. For example, while the current study excluded participants for recent substance use prior to NP testing, previous reports used even more stringent substance exposure exclusions due to larger sample sizes (Tapert & Brown, 1999; Tapert et al., 2002). Further, a unique aspect of the current study was the examination of individuals with remitted AUD/SUD relative to those with persisting AUD/SUD and healthy controls. Previous reports from this sample examined only two groups or defined the groups slightly differently. In the current study, we did not detect deficits in intellectual function or processing speed (Tarter et al., 1995). Possible reasons for this discrepancy include use of different recruitment sources, distributions of male and female participants, neuropsychological batteries, inclusion/exclusion criteria, length of abstinence, and approaches to data analysis.
Although our sample size is modest, the current findings suggest that heavy, chronic alcohol and other substance use during late adolescence and early adulthood may produce long-term cognitive disadvantages, primarily in visuospatial and memory abilities. Further, by including a group of remitted users, this investigation demonstrated that having a lifetime history of heavy alcohol or other substance use during adolescence and young adulthood appears to be more important than whether or not individuals continued to have substance-related problems into their mid-twenties. Youth who consume heavy quantities of alcohol and/or consistently experience drug withdrawal symptoms over several years may especially be at risk. Chronic stimulant use may also influence visual memory functioning, and more recent substance use may have a specific impact on executive functions, which in the current study focused primarily on verbal working memory. Alternatively, some youth may have had a disadvantageous developmental course predetermined by factors other than substance use, which adversely influenced both cognitive performance and substance use outcomes.
Alcohol-related cognitive deficits may have important negative consequences for adolescents and young adults. For example, in the early phases of abstinence, recall deficits may disadvantage youth in academic environments (Brown et al., 2000). Cognitive ability also appears to moderate the relationship between coping skills and substance use after treatment (Tapert, Brown, Myers, & Granholm, 1999). That is, among adolescents with poor cognitive abilities, those with fewer coping skills had worse substance use outcomes one year after treatment, while having a larger repertoire of coping skills appeared to protect these individuals from subsequent drug and alcohol use. For those with average or better intellectual abilities, other factors such as temperament, family, community and work environments may account for future substance exposure (Brown et al., 2008). Thus, treatment for substance use problems may be improved with the integration of coping skills training, as well as cognitive habilitation (Moss et al., 1994). The cognitive decline evident in this and prior longitudinal studies of AUD/SUD youth is subtle and seldom obvious to the individual substance abuser or those in the academic environments. Consequently, youth in treatment may benefit from personal feedback regarding their neurocognitive functioning and potential consequences of continued use (Ramo, Myers, & Brown, 2007). It is likely that substance-related cognitive deficits have other consequences for adolescents and young adults, but more research is needed to examine such characteristics.
Some limitations with the current study should be considered. First, our sample size is relatively small due to the stringent inclusion/exclusion criteria and participant relocations over a 10-year study period. However, participants who received a NP assessment at year 10 were generally similar to those who did not on baseline demographic, substance use, and NP variables, and on 10-year demographic and substance use characteristics. While it is possible that heavier substance users were lost during follow-up, our data suggests that the majority of the original participants at least completed the substance use assessment at year 10. Second, practice effects could influence results given the repeated testing over the 10-year follow-up period, although groups did not differ in the number of NP assessments received during the study. Third, while participants were excluded for using substances before testing to avoid effects of acute intoxication, this did not rule out potential effects of protracted hangover or withdrawal symptoms on NP functioning. Nevertheless, this abstinence duration generalizes readily to substance users with frequent use patterns. Fourth, this clinical sample consists of individuals who have a lifetime history of AUD and most are polysubstance users; consequently, the effects of any single substance cannot be isolated. Furthermore, the community sample was matched to the clinical sample on demographics and family history of AUD/SUD, and it could be that the family history and SES factors modify what would be expected in a true representative community sample. Therefore, the current findings suggest that heavy, chronic alcohol use in the context of other substance involvement may be associated with visuospatial and memory decrements relative to individuals of similar demographics.
Finally, for this study we summarized substance use patterns by creating composite scores that reflected average use of substances, dependence symptoms, and withdrawal symptoms over the 10-year period. While this approach enabled us to retain more participants in this longitudinal study, important information about the long-term effects of alcohol and drugs on cognition may be gained by examining the fluctuating patterns of substance use in more detail. Given the low base rate of alcohol withdrawal symptoms and the high prevalence and intensity of use, consideration of alternative means to assess alcohol withdrawal symptoms may be merited. Future studies might examine the neurocognitive outcomes of various substance use trajectories particularly during critical periods of brain maturation (Anderson et al., under review) as well as factors that either contribute to cognitive decline or are neuroprotective during the transition to young adulthood. The clinical and occupational consequences, as well as the neural correlates (e.g., structural and functional neuroimaging studies), of these substance-related cognitive deficits may have important ramifications.
Acknowledgements
This research was supported by the following National Institutes of Health grants and fellowships: R37 AA07033 (PI: Brown), R01 DA021182 (PI: Tapert), T32 AA013525 (fellow: Hanson; PI: Riley), and F32 DA020206 (PI: Medina). Portions of this study were presented at the Research Society on Alcoholism Annual Meeting, Chicago, IL, 2007. The authors would like to acknowledge the contributions of David Muchin, Kevin Cummins, and Mark Nakamura in making this research possible.
References
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 3rd ed., rev ed. American Psychiatric Association; Washington, DC: 1987. [Google Scholar]
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 4th ed. American Psychiatric Association; Washington, DC: 1994. [Google Scholar]
- Anderson KG, Ramo DE, Cummins K, Brown SA. Alcohol and drug involvement after adolescent treatment and functioning during emerging adulthood. Journal of Consulting and Clinical Psychology. doi: 10.1016/j.drugalcdep.2009.10.005. under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson KG, Ramo DE, Schulte MT, Cummins K, Brown SA. Substance use treatment outcomes for youth: Integrating personal and environmental predictors. Drug and Alcohol Dependence. 2007;88(1):42–48. doi: 10.1016/j.drugalcdep.2006.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates ME, Barry D, Labouvie EW, Fals-Stewart W, Voelbel G, Buckman JF. Risk factors and neuropsychological recovery in clients with alcohol use disorders who where exposed to different treatments. Journal of Consulting and Clinical Psychology. 2004;72(6):1073–1080. doi: 10.1037/0022-006X.72.6.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates ME, Voelbel GT, Buckman JF. Short term neuropsychological recovery in clients with substance use disorders. Alcoholism: Clinical and Experimental Research. 2005;29(3):367–377. doi: 10.1097/01.alc.0000156131.88125.2a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton AL, Hamsher K, Varney NR, Spreen O. Contributions to neuropsychological assessment: A clinical manual. Oxford University Press; New York: 1983. [Google Scholar]
- Brown SA, McGue M, Maggs J, Schulenberg J, Hingson R, Swartzwelder S, et al. A developmental perspective on alcohol and youths 16 to 20 years of age. Pediatrics. 2008;121(Suppl. 4):S290–310. doi: 10.1542/peds.2007-2243D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SA, Myers MG, Lippke L, Tapert SF, Stewart DG, Vik PW. Psychometric evaluation of the Customary Drinking and Drug Use Record (CDDR): A measure of adolescent alcohol and drug involvement. Journal of Studies on Alcohol. 1998;59(4):427–438. doi: 10.15288/jsa.1998.59.427. [DOI] [PubMed] [Google Scholar]
- Brown SA, Myers MG, Mott MA, Vik PW. Correlates of success following treatment for adolescent substance abuse. Applied & Preventive Psychology. 1994;3:61–73. [Google Scholar]
- Brown SA, Tapert SF, Granholm E, Delis DC. Neurocognitive functioning of adolescents: Effects of protracted alcohol use. Alcoholism: Clinical and Experimental Research. 2000;24:164–171. [PubMed] [Google Scholar]
- Brown SA, Vik PW, Creamer VA. Characteristics of relapse following adolescent substance abuse treatment. Addictive Behaviors. 1989;14:291–300. doi: 10.1016/0306-4603(89)90060-9. [DOI] [PubMed] [Google Scholar]
- Cahalan D. Problem drinkers. Jossey-Bass; San Francisco: 1970. [Google Scholar]
- Caldwell LC, Schweinsburg AD, Nagel BJ, Barlett VC, Brown SA, Tapert SF. Gender and adolescent alcohol use disorders on BOLD (blood oxygen level dependent) response to spatial working memory. Alcohol & Alcoholism. 2005;40:194–200. doi: 10.1093/alcalc/agh134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Mdzinarishvili A, Kim D, He J, Nixon K. Neurogenesis in adolescent brain is potently inhibited by ethanol. Neuroscience. 2006;137:437–445. doi: 10.1016/j.neuroscience.2005.08.090. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Clark DB, Beers SR, Soloff PH, Boring AM, Hall J, et al. Hippocampal volume in adolescent-onset alcohol use disorders. American Journal of Psychiatry. 2000;157(5):737–744. doi: 10.1176/appi.ajp.157.5.737. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Narasimhan A, Thatcher DL, Keshavan MS, Soloff P, Clark DB. Prefrontal cortex, thalamus and cerebellar volumes in adolescents and young adults with adolescent onset alcohol use disorders and co-morbid mental disorders. Alcoholism: Clinical and Experimental Research. 2005;29(9):1590–1600. doi: 10.1097/01.alc.0000179368.87886.76. [DOI] [PubMed] [Google Scholar]
- Delis DC, Jacobson M, Bondi MW, Hamilton JM, Salmon DP. The myth of testing construct validity using factor analysis or correlations with normal or mixed clinical populations: Lessons from memory assessment. Journal of the International Neuropsychological Society. 2003;9:936–946. doi: 10.1017/S1355617703960139. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Ober BA. Manual for the California Verbal Learning Test. 2nd edition (CVLT-II) Psychological Corp.; San Antonio, TX: 2000. [Google Scholar]
- Delis DC, Kramer JK, Kaplan E, Ober BA. California Verbal Learning Test Manual. Research Edition Psychological Corporation; San Antonio: 1987. [Google Scholar]
- Fein G, Bachman L, Fisher S, Davenport L. Cognitive impairments in abstinent alcoholics. Addiction Medicine [Special Issue] 1990;152:531–537. [PMC free article] [PubMed] [Google Scholar]
- Fein G, Torres J, Price LJ, Di Sclafani V. Cognitive performance in long-term abstinent alcoholic individuals. Alcoholism: Clinical and Experimental Research. 2006;30(9):1538–1544. doi: 10.1111/j.1530-0277.2006.00185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingshead AB. Two-factor index of social position. Yale University Press; New Haven, CT: 1965. [Google Scholar]
- Horn JI, Skinner HA, Wanberg K, Foster FM. Alcohol Dependence Scale (ADS) Addiction Research Foundation; Toronto: 1984. [Google Scholar]
- Johnston LD, O'Malley PM, Bachman JG, Schulenberg JE. Monitoring the Future national survey results on drug use, 1975–2008. Volume I: Secondary school students. National Institute on Drug Abuse; Bethesda, MD: 2009. NIH Publication No. 09-7402. [Google Scholar]
- Lu KH. The indexing and analysis of drug indulgence. International Journal of the Addictions. 1974;9:785–804. doi: 10.3109/10826087409022175. [DOI] [PubMed] [Google Scholar]
- Matarazzo JD, Herman DO. Base rate data for the WAIS-R: Test-retest stability and VIQ-PIQ differences. Journal of Clinical Neuropsychology. 1984;6:351–366. doi: 10.1080/01688638408401227. [DOI] [PubMed] [Google Scholar]
- Medina KL, McQueeny T, Nagel BJ, Hanson KL, Schweinsburg AD, Tapert SF. Prefrontal cortex volumes in adolescents with alcohol use disorders: Unique gender effects. Alcoholism: Clinical and Experimental Research. 2008;32:386–394. doi: 10.1111/j.1530-0277.2007.00602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina KL, Schweinsburg AD, Cohen-Zion M, Nagel BJ, Tapert SF. Effects of alcohol and combined marijuana and alcohol use during adolescence on hippocampal volume and asymmetry. Neurotoxicology & Teratology. 2007;29:141–152. doi: 10.1016/j.ntt.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss HB, Kirisci L, Gordon HW, Tarter RE. A neuropsychologic profile of adolescent alcoholics. Alcoholism: Clinical and Experimental Research. 1994;18:159–163. doi: 10.1111/j.1530-0277.1994.tb00897.x. [DOI] [PubMed] [Google Scholar]
- Nagel BJ, Schweinsburg AD, Phan V, Tapert SF. Reduced hippocampal volume among adolescents with alcohol use disorders without psychiatric comorbidity. Psychiatry Research. 2005;139(3):181–190. doi: 10.1016/j.pscychresns.2005.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterrieth PA. Le test de copie d'une figure complexe. Archives of Psychology. 1944;30:206–356. [Google Scholar]
- Ramo DE, Myers MG, Brown SA. Relapse Prevention for Adolescent Substance Abuse: Overview and Case Examples. In: Witkiewitz K, Marlatt GA, editors. Therapist's Guide to Evidence-Based Relapse Prevention. Elsevier; New York: 2007. pp. 293–311. [Google Scholar]
- Reed RJ, Grant I, Rourke SB. Long-term abstinent alcoholics have normal memory. Alcoholism: Clinical & Experimental Research. 1992;16:677–683. doi: 10.1111/j.1530-0277.1992.tb00660.x. [DOI] [PubMed] [Google Scholar]
- Reitan RM, Wolfson D. The Halstead-Reitan Neuropsychological Test Battery: Theory and clinical interpretation. Neuropsychology Press; Tucson, AZ: 1993. [Google Scholar]
- Rourke SB, Grant I. The interactive effects of age and length of abstinence on the recovery of neuropsychological functioning in chronic male alcoholics: A 2-year follow-up study. Journal of the International Neuropsychological Society. 1999;5(3):234–246. doi: 10.1017/s1355617799533067. [DOI] [PubMed] [Google Scholar]
- Sher KJ, Martin ED, Wood PK, Rutledge PC. Alcohol use disorders and neuropsychological functioning in first-year undergraduates. Experimental and Clinical Psychopharmacology. 1997;5(3):304–315. doi: 10.1037//1064-1297.5.3.304. [DOI] [PubMed] [Google Scholar]
- Stewart DG, Brown SA. Withdrawal and dependency symptoms among adolescent alcohol and drug abusers. Addiction. 1995;90:627–635. doi: 10.1046/j.1360-0443.1995.9056274.x. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Rosenbloom MJ, Pfefferbaum A. Pattern of motor and cognitive deficits in detoxified alcoholic men. Alcoholism: Clinical and Experimental Research. 2000;24(5):611–621. [PubMed] [Google Scholar]
- Tabachnick BG, Fidell LS. Using multivariate statistics. 5th ed. Pearson; Boston: 2007. [Google Scholar]
- Tapert SF, Brown SA. Neuropsychological correlates of adolescent substance abuse: Four year outcomes. Journal of the International Neuropsychological Society. 1999;5(6):481–493. doi: 10.1017/s1355617799566010. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Brown SA. Substance dependence, family history of alcohol dependence and neuropsychological functioning in adolescence. Addiction. 2000;95(7):1043–1053. doi: 10.1046/j.1360-0443.2000.95710436.x. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Brown SA, Myers MG, Granholm E. The role of neurocognitive abilities in coping with adolescent relapse to alcohol and drug use. Journal of Studies on Alcohol. 1999;60(4):500–508. doi: 10.15288/jsa.1999.60.500. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Granholm E, Leedy NG, Brown SA. Substance use and withdrawal: Neuropsychological functioning over 8 years in youth. Journal of the International Neuropsychological Society. 2002;8(7):873–883. doi: 10.1017/s1355617702870011. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Schweinsburg AD, Barlett VC, Brown SA, Frank LR, Brown GG, et al. Blood oxygen level dependent response and spatial working memory in adolescents with alcohol use disorders. Alcoholism: Clinical and Experimental Research. 2004;28(10):1577–1586. doi: 10.1097/01.alc.0000141812.81234.a6. [DOI] [PubMed] [Google Scholar]
- Tarter RE, Mezzich AC, Hsieh Y-C, Parks SM. Cognitive capacity in female adolescent substance abusers. Drug & Alcohol Dependence. 1995;39:15–21. doi: 10.1016/0376-8716(95)01129-m. [DOI] [PubMed] [Google Scholar]
- Toga AW, Thompson PM, Sowell ER. Mapping brain maturation. TRENDS in Neurosciences. 2006;29(3):148–159. doi: 10.1016/j.tins.2006.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler memory scale. Psychological Corporation; New York: 1945. [Google Scholar]
- Wechsler D. Manual for the Wechsler Intelligence Scale for Children-Revised. The Psychological Corporation; San Antonio, TX: 1974. [Google Scholar]
- Wechsler D. Manual for the Wechsler Adult Intelligence Scale -Revised. Psychological Corporation; San Antonio, TX: 1981. [Google Scholar]