Abstract
Background
Most studies of vascular disease in transplanted organs have used combinations involving disparities determined by genes of the major histocompatibility complex (MHC). This report describes examples of coronary vascular disease occurring in transplanted mouse hearts involving isolated, non-H2-determined incompatibilities.
Methods
Mice, incompatible in respect of HY, H4, or H60, were selected. For H60, the incompatibility depended on breeding congenic pairs or the introduction of H60 by transgenic methods because the latter method results in more widespread expression. Transplant survival was determined, and the appearance and prevalence of coronary artery vasculopathy (CAV) was established by appropriate histologic methods.
Results
Advanced changes of CAV were found at 56 days in transplants involving incompatibilities confined to HY or H4. In both combinations, skin grafts were also rejected. H60 incompatibility does not result in skin graft rejection and only a minority of heart transplants shows evidence of CAV. If heart transplants are preceded by skin grafts bearing both H60 and HY incompatibilities to promote “help” in generating immunity, H60 incompatible hearts develop advanced CAV. Heart transplants in all non-MHC categories ostensibly survive in excellent condition throughout this period despite their CAV.
Conclusions
CAV can develop as a consequence of non-MHC incompatibilities alone and even when antigens are sparsely expressed on cardiac tissue. Presensitization leads to much more severe vascular disease. Human leukocyte antigen compatible kidney transplants may also develop vascular disease and patients manifesting reactivity to MHC antigens should also be more prone to develop vascular disease because of undetectable non-MHC incompatibilities.
Keywords: Coronary artery disease, Non-H2-determined incompatibilities, Heart transplant
The practical importance and biological interest of the chronic proliferative vascular changes that take place in arterial vessels of histoincompatible organs transplanted with primary vascular union are appreciated. The most studied situation in which such vascular changes occur is that in which donors and recipients differ by multiple, major histocompatibility complex (MHC)-determined histocompatibility factors. To permit chronic changes to emerge, the immune responses of recipients are commonly muted by various forms of immunosuppression. From such experiments, there is considerable evidence that these impressive vascular lesions are heavily dependent on immune responses to the histoincompatibilities concerned.
Efforts have been made to isolate different elements of the immune response of recipients to define more precisely how these lesions come about. For example, it has been found that if transplanted hearts are met by a specific immune response that is confined only to humoral factors in isolation or only cell-mediated immunity typical coronary artery vascular (CAV) disease in transplanted mouse hearts can be produced. It is also possible that innate immunity makes a contribution (1).
The experiments reported here were designed to evaluate the possible contribution of selected incompatibilities determined by genes outside the H2 complex to the formation of CAV. In particular, we have explored the importance of defined minor histocompatibility antigens created between congenic pairs by appropriate breeding or by transgenic techniques. Minor histocompatibility antigens consist of self-peptides generated by proteolytic processing of normal cellular proteins that act as barriers to tissue transplantation (2). Those selected here for evaluation were encoded by the male-specific HY minor histocompatibility genes (Smcy, Uby, and Dby) (3), first detected by Eichwald and Silmser (4) for their ability to bring about the rejection of skin grafts from male to otherwise isologous female mice, and the autosomally encoded H4 and H60 minor histocompatibility antigens (5, 6). Like HY, the originally defined H4 “antigen” is the result of allelically variant processed peptides encoded by three closely linked minor histocompatibility genes (H46, H47, and Emp3) (6–8). The H60 minor histocompatibility antigen is a simpler genetic configuration in that it is a peptide encoded by the H60a gene (9), but it is notable in that of all minor histocompatibility antigens, the H60 peptide (LTFNYRNL) can stimulate the most immunodominant CD8 T-cell response (10–12). Moreover, the native H60 glycoprotein is a stimulatory ligand for the NKG2D receptor expressed by natural killer cells(13)and may also act as a costimulatory ligand for CD8 T cells (14). Here, we investigate whether incompatibilities limited to HY, H4, and H60 antigens can cause CAV and heart rejection. The results from our studies indicate that CAV disease can proceed in heart transplants from donors disparate at these minor histocompatibility antigens despite their functional survival.
RESULTS
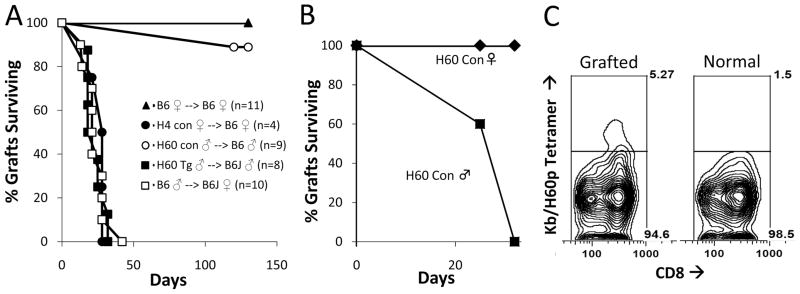
Table 1 summarizes the prevalence of CAV in the presence of each of the three non-H2-determined incompatibilities studied. The behavior of skin grafts in the same combinations is indicated in Figure 1. It is apparent that the HY-determined antigens were strong and consistent generators of responses sufficient to produce CAV over the selected period of observation (Table 1, Grp A). Nevertheless, continuing functional integrity of transplanted hearts was excellent as determined by their maintenance of both normal contractions throughout the period of observation and a normal pink color at the time of sacrifice of their recipients at 54 to 60 days. Because hearts transplanted in this heterotopic style do not support the circulation, a full assessment of their functional capacity was not possible. Examination of the coronary arteries that developed lesions during this 8-week period of observation showed marked intimal thickening that sometimes extended into distal intramyocardial arteries. The intima developed varying degrees of inflammation and fibrosis and this commonly extended to the adventia (Fig. 2A). In contrast, the myocardium showed no evidence of myocardial cell injury or destruction. Finally, and as expected, HY-disparate skin grafts transplanted onto a separate cohort of mice were fully rejected within 35 days (Fig. 1A).
TABLE 1.
Effect on coronary arteries of hearts transplanted to recipients with defined non-H2 incompatibilities
| Group | Heart donor | Recipient | Initial skin graft donor | Day killed (range) | Score (range)a | No. animals | No. animals positive | Pb |
|---|---|---|---|---|---|---|---|---|
| A | B6 male | B6 female | 54–60 | 2–3 | 11 | 11/11 | ||
| B | H4 congenic femalec | B6 female | 56–57 | 3 | 5 | 5/5 | ||
| C | H60 congenic female | B6 female | 56–58 | 2–3 | 11 | 3/11 | >0.022 | |
| D | H60 congenic female | B6 female | H60 congenic male | 56–60 | 2–4 | 16 | 12/16 | |
| E | H60 transgenic male | B6 male | 56 | 3 | 2 | 2/2 | ||
| F | H60 transgenic male | B6.RAG1−/− male | 56 | 3 | 5 | 0/5 | ||
| G | H60 congenic female | B6 female | H60 transgenic female | 61 | 3 | 3 | 1/3 | |
| H | B6 female | B6 female | 55–57 | 3 | 5 | 0/5 |
Functional status of individual transplanted hearts on scale of 0–4 (see Materials and Methods section).
Two-tail P value using Fisher exact test.
H4 congenic = B6.129-H4b.
FIGURE 1.
Skin graft survival of minor histocompatibility antigen disparate skin grafts. Survival times are expressed as means and the number of animals is indicated in parentheses. (A) Tail skins of B6 background mice with the designated congenic segments or transgenes were grafted onto the tails of 6- to 8-week-old B6 recipient mice (Tg, transgenic; Con, congenic). (B) H60 congenic skin grafts are only rejected when the graft is also disparate at HY. In the case of the double skin graft experiments, grafts from H60 congenic ♀; mice and H60 congenic ♂; mice were placed on B6 ♀; mice (n=5). H60 congenic ♀; grafts showed no evidence for rejection whereas H60 congenic ♂; grafts placed on the same mice were fully rejected by 32 days. (C) Flow cytometry demonstrating increased frequency of recipient CD8 T cells binding to H60/H2Kb tetramers from an animal that rejected an H60 skin graft as in (B) (see Materials and Methods section).
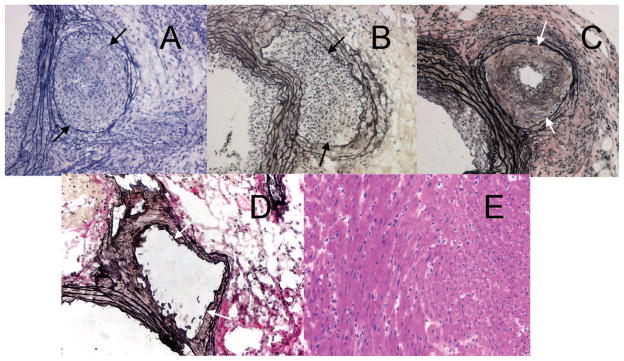
FIGURE 2.
Photomicrographs of minor histocompatibility mismatched heart allografts (A–C) showing advanced coronary artery vascular disease (CAV). (A) Male B6 heart to female B6 recipient. (B) H4 congenic female heart to B6 female recipient. (C and E) H60 congenic female heart into a female B6 presensitized with a H60 transgenic male skin graft. Despite marked CAV shown in (C), the myocardium was spared signs of cellular rejection (E). (D) B6 to B6 isograft shows no evidence of CAV. All sections taken at 8 weeks posttransplant. Sections A, B, C, and D are taken through the proximal coronaries with the aorta to the left. The allografts in each strain combination show coronary artery infiltration by mononuclear cells and intimal fibrosis with severe luminal stenosis. The internal elastica is indicated with arrows. The tissue between the arrows and the lumen is the neointima. Perivascular mononuclear infiltrates are also present in A, B, and C. A, B, C, D, Weigert’s elastic stain; E, H&E; original magnification ×200.
Hearts from H4-congenic female donors transplanted to B6 female recipients behaved in a manner similar to HY incompatible hearts in that they ostensibly survived well as assessed by external inspection but underwent marked endovascular changes (Table 1, Grp B). Although we did not perform formal time course studies of the onset of these vascular lesions, we suspect from previous observations of this process in other settings that these lesions probably formed gradually over at least a 3- to 4-week period and that many weeks later transplants become increasingly ischemic and fibrotic. Histologic examination of the H4-bearing donor hearts showed marked intimal thickening of the proximal coronary arteries (Fig. 2B) that sometimes extended distally. These lesions were not distinguishable from those in the HY antigen mismatched combination. There was again no evidence of injury or destruction of myocardial cells. However, H4-congenic tail skin grafts performed in a separate cohort were rejected within 35 days (Fig. 1A).
The H60 antigen resulted in a different pattern. Of 11 congenic H60 female hearts transplanted to female B6 recipients, three manifested evidence of CAV development, whereas the remaining eight remained entirely normal throughout the period of observation (Table 1, Grp C). The CAV lesions observed were localized to the proximal coronaries. The myocardium seemed normal, without evidence of parenchymal cell damage. T cells were detected and enumerated in the myocardium at low levels (55±53 cells/mm2). Despite the absence of skin allograft rejection in the same strain combination (Fig. 1A), the H60 antigen induced sporadic CAV but not in the pronounced manner observed with HY and H4 disparate hearts.
As only a minority of hearts from H60 congenic donors was found to develop CAV, we sought to determine whether prior sensitization under conditions that should provide help from CD4 T cells would affect this outcome. For this experiment, wild-type B6 female recipients received skin grafts from male H60 congenic donors. Three weeks later, when the skin grafts were progressing to active rejection, these mice received hearts from H60 congenic female donors. Of the 16 recipients, 12 developed CAV (Table 1, Grp D). The incidence of CAV after prior activation of the immune response was thus significantly increased (P<0.022). Histologically, CAV lesions were similar to those in HY and H4 incompatible transplants (Fig. 2C). However, the myocardium showed no evidence of rejection (Fig. 2E) and had a minimal T-cell infiltrate (22±9 cells/mm2). These results showed that prior immunization to H60 dramatically increased CAV, although not impairing early myocardial function.
With a limited number of available mice, we then explored whether the transgenic augmentation of H60 could affect CAV development. Hearts from donor mice carrying an H60 transgene driven by chicken β-actin/cytomegalovirus regulatory sequences developed florid and typical CAV lesions in the two wild-type male recipients of such hearts (Table 1, Grp E). These hearts remained grossly normal, but on histologic examination, a prominent diffuse infiltrate of T cells (averaging 186 cells/mm2) without obvious myocyte injury was present. These results suggested that transgenically reinforced H60 expression was sufficient to cause substantial CAV and myocardial T-cell infiltration, both of which were dependent on an adaptive immune response because no lesions were found in lymphocyte deficient B6.RAG1−/− recipients (Table 1, Grp F).
We also determined the fate of heart transplants compared with that of skin grafts. Tail skin grafts from congenic H60 positive male donors transplanted to sex-matched B6 recipients survived for at least 130 days (Fig. 1A). Furthermore, congenic H60 disparate skin grafts placed adjacent to skin grafts that were disparate from the host at H60+HY showed no evidence of rejection at least up to 32 days after transplantation despite the fact that the H60+HY skin grafts were rejected acutely (Fig. 1B) and that there was an appreciable CD8 T-cell response to H60 as indicated by an appreciable H60p/H2Kb tetramer response (average 5.9%±1.45% compared with 1.5% in nongrafted B6 mice; Fig. 1C). These results indicated that the response to the H60 disparity even when augmented by a cosensitizing graft was unable to evoke appreciable skin graft rejection.
Finally, as H60 transgenic heart transplants developed frank CAV and skin grafts were acutely rejected, we considered the possibility that presensitization with female H60 transgenic skin grafts might provoke CAV development in female H60 congenic hearts transplanted subsequently into female B6 recipients (Table 1, Grp G). Of the three recipients analyzed, only one showed evidence for CAV suggesting that presensitization in this manner did not appreciably affect the outcome.
DISCUSSION
This study has revealed that the vascular disease typical of chronic heart rejection can arise from genetically limited minor histocompatibility antigen disparities, although heart function is maintained for at least a considerable period. Yang et al. examined incompatibility at the H13 minor histocompatibility antigen, which is a barrier to skin graft survival. They also found vascular changes consistent with CAV (15). Unlike the results reported here, there was concurrent full heart rejection. In the case of the incompatibility determined by HY, the antigenic difference between males and females is sufficient to provoke skin graft rejection and gradual formation of CAV. The same proved to be the case for H4. Both these antigens are expressed on the cells of different tissues. For example, it has been shown that HY is present on parathyroid glands in the mouse (16). This antigenic difference was also used by He et al. (17) in an elegant study in which vascular changes were observed in transplanted hearts, and the tempo of graft destruction of skin grafts was related to both the number of recipient T lymphocytes directed to the donor transplant and the size of the donor graft.
Analysis of heart allografts differing only at H60 illustrates a more novel pattern for this immunodominant histocompatibility antigen. Unlike the comparatively broadly expressed minor histocompatibility antigens described above, the expression of H60 is generally considered to be more limited. Our findings of a failure of H60 to promote the rejection of skin allografts would support this limited expression pattern. However, there is clear functional evidence that H60 is potently expressed by cells of the hemopoietic lineage (11, 18). Moreover, H60 transcripts have been detected in extracts of noninflamed whole tissues including cardiac, skeletal muscles, and skin (19), and generally speaking, H60 is believed to be upregulated in tissues, including the heart, under inflammatory/stress conditions (20–22). Previous studies in which donor hearts differed from the recipient at multiple minor histocompatibility antigens show the invasion of H60-specific CD8 T cells in heart allografts (23). However, its expression in the coronary vasculature and cardiac muscle at sufficient levels both to evoke a response and to act as the target for adaptive immune CD8 T cells is unknown. We hoped to gather evidence regarding H60 expression by direct observation of selective antibody binding to H60 in tissue sections using immunohistochemical techniques. Unfortunately, despite reports to the affirmative (21, 24, 25), the commercially available antibodies to H60 (goat anti-mouse H60[C-20], Santa Cruz Biotechnology, Inc.; monoclonal anti-mouse H60, R&D Systems, Inc.) failed to react in several trials even with transgenic samples. However, hearts transplanted from mice congenic for H60 (and thus differing only at H60 expressed by its endogenous control elements) developed clear evidence of CAV, although only in a minority (3 of 11) of B6 recipients. CAV was exacerbated by presensitization, a finding that is consistent with reports showing that the CD8 T-cell response developed to H60 is weak without a source of help from CD4 T cells (26). The exacerbated CAV that developed after preimmunization in this manner can therefore be explained by clonally expanded and activated CD8 T cells specific for the H60 peptide that are more able to directly or indirectly injure the coronary vasculature.
The level of expression of histocompatibility antigens can vary considerably under the influence of ambient factors. Indeed, the systemic administration in vivo of interferon-γ can greatly increase the level of expression of both class I and II histocompatibility antigens in the mouse (27). The signals that control the expression of H60 are less predictable. Type I and II interferons suppress H60 on tumor cells (28), whereas stress stimuli, such as DNA damage and apoptotic responses, upregulate NKG2D ligands (20). CAV was clearly induced in transplanted hearts from H60 transgenic donors, although the number of animals analyzed was necessarily small. The finding of an increased level of T-cell infiltration in the transgenic versus the H60 congenic animals suggests a broadened tissue expression of the transgenic H60 antigen. Prior systemic stimulation of responsiveness to H60 by skin grafts from congenic male donors also significantly increased the incidence of CAV in congenic female hearts placed thereafter. These results support the possibility that the H60 alloantigen is expressed in the coronary vessels at levels usually sufficient to serve as an effective target in a sensitized recipient, but that are insufficient to promote sensitization regularly in a naïve recipient. One feature of the chronic rejection process seen regularly in transplanted mouse hearts is its apparent selectivity for the coronary vessels of such transplants. The vascular linings of transplanted organs clearly present the first contact with recipient blood, and it could be that this factor is an adequate explanation for the selectivity. The stress response caused by vascular injury as the result of direct cytolysis or localized cytokine release by H60-specific CD8 T cells could thus augment and sustain the typical chronic proliferative changes in the walls of coronary vessels, while sparing the myocardium. Alternatively, given the fact that leukocytes express congenic H60 in a immunogenic manner, this CD8 T-cell ligand is expected to be well represented on passenger leukocytes carried in the donor heart. That their presence might have indirectly influenced the development of endovascular changes should not, perhaps, be dismissed, although how such cells, mainly scattered through the parenchyma of the donor organ with only a few in the vascular compartment, may influence events in the linings of vessels is obscure.
The clinical relevance of the present findings lies in the fact that the lesions of CAV can result from minor antigens alone and even when such antigens are expressed only at low levels. This may explain some instances of late allograft loss in human leukocyte antigen-identical recipients (29, 30), a setting in which we have observed chronic allograft vasculopathy (Colvinetal., unpublished data, 2006). This outcome should be more likely, for example, when recipients of human leukocyte antigen-identical organs have previously manifested sensitization as suggested by reactivity to a representative panel of cells.
Thus, a suggested consequence of the present findings is that potential organ recipients manifesting high levels of immunity to MHC-determined antigens may face an increased likelihood of developing CAV even though they receive organs fully compatible in respect to the MHC.
MATERIALS AND METHODS
Mice
C57BL/6J female or male mice (B6) 5 to 7 weeks old, or C57BL/6J-RAG1−/− mice of the same age, were used as recipients according to the needs of the individual experiment. Skin and heart donors consisted of male B6, female B6.129-H4b/DCR, male and female H60 congenic (B6.C-H60c/DCR), or male and female H60 transgenic {B6.Tg(H60a)114}/DCR mice. All congenic stocks were the result of 10 or more backcross generations onto a B6/J background. B6 mice expressing a complete H60 cDNA transgene {B6.Tg(H60a)114/DCR} were produced by conventional transgenic techniques. The transgenic construct comprised a complete H60a cDNA cloned into a vector containing the chicken β-actin enhancer and a cytomegalovirus promoter, which is known to result in a broad tissue expression pattern. All mice were obtained from The Jackson Laboratory, Bar Harbor, ME, and were cared for in a pathogen-free environment according to methods approved by the American Association for the Accreditation of Laboratory Animal Care.
Transplantation Technique
Full-thickness skin grafts were performed according to the technique originally described by Billingham and Medawar (31). In some experiments, tail-to-tail skin grafts were used and rejection scoring was performed as described previously (32). Hearts were transplanted in heterotopic style to the abdominal cavity with primary vascular union between the donor and recipient vessels according to our previously described microsurgical technique (33). Cold ischemia times of less than 25 min were maintained throughout. Technically successful transplants resulted in approximately 95% of trials. The survival status of transplanted hearts was appraised by palpation of the cardiac impulse, which was recorded on a basis of one to four plus. Isotransplants function persistently with scores of at least three plus.
Immunohistology and Flow Cytometry
Cardiac tissue blocks were embedded and frozen in optimal cutting temperature compound (Sakura Finetek USA, Inc., Torrance, CA), and stored at −20°C. Sections including coronary arteries were cut at 4 to 6 μm and stained using Weigert’s method for elastic fibers. CAV was evaluated in the proximal coronary arteries according to our previously described system with coronaries positive for CAV ranging from stage I (10% occlusion) to stage III (>50% occlusion) (34, 35). Frozen sections were stained with peroxidase-conjugated antibody using standard techniques. Immunoperoxidase-stained sections were then developed, postfixed in 4% formaldehyde, counterstained, and mounted as described previously (34). Infiltrating T cells were appraised by counting CD3+ cells using polyclonal rabbit anti-human CD3 (Dako North America, Inc., Carpinteria, CA) in whole slide scanned images of myocardium (ScanScope CS; Aperio, Vista, CA) at 400×. Results are given as CD3+ cells/mm2. This was accomplished with samples representing the myocardium of transplanted hearts in situations in which a comparison of the severity of such infiltrates in different settings was to be made. Flow cytometric analysis to monitor CD8 T cells that are reactive to the H60 minor histocompatibility antigen was performed using H60p/H2Kb tetramers as described previously (11).
Acknowledgments
The authors thank Dr. Eun Young Choi (University of Seoul) for generously providing H60p/H2Kb tetramers.
This work was supported by grants from the National Institutes of Health, HL071932 (J.C.M.) and AI28802 (D.C.R.), and by ROTRF research grants 313867044 (R.B.C.) and 980940345 (J.C.M.).
Footnotes
The authors declare no conflict of interest.
P.S.R., R.B.C., and D.C.R. participated in research design, writing the article, and data analysis; C.M.C. participated in research design, writing the article, performing the research, and data analysis; J.C.M. participated in research design; T.H. participated in performing the research; L.D.C. participated in data analysis; and T.J.S. participated in performing research and data analysis.
References
- 1.Uehara S, Chase CM, Kitchens WH, et al. NK cells can trigger allograft vasculopathy: The role of hybrid resistance in solid organ allografts. J Immunol. 2005;175:3424. doi: 10.4049/jimmunol.175.5.3424. [DOI] [PubMed] [Google Scholar]
- 2.Rötzschke O, Falk K, Wallny HJ, et al. Characterization of naturally occurring minor histocompatibility peptides including H-4 and H-Y. Science. 1990;249:283. doi: 10.1126/science.1695760. [DOI] [PubMed] [Google Scholar]
- 3.Simpson E, Scott D, James E, et al. Minor H antigens: Genes and peptides. Transpl Immunol. 2002;10:115. doi: 10.1016/s0966-3274(02)00057-6. [DOI] [PubMed] [Google Scholar]
- 4.Eichwald EJ, Silmser CR. Skin. Transplant Bull. 1955;2:148. [PubMed] [Google Scholar]
- 5.Snell GD, Stevens LC. Histocompatibility genes of mice. III. H-1 and H-4, two histocompatibility loci in the first linkage group. Immunology. 1961;4:366. [PMC free article] [PubMed] [Google Scholar]
- 6.Mendoza LM, Villaflor G, Eden P, et al. Distinguishing self from non-self: Immunogenicity of the murine H47 locus is determined by a single amino acid substitution in an unusual peptide. J Immunol. 2001;166:4438. doi: 10.4049/jimmunol.166.7.4438. [DOI] [PubMed] [Google Scholar]
- 7.Sahara H, Shastri N. Second class minors: Molecular identification of the autosomal H46 histocompatibility locus as a peptide presented by major histocompatibility complex class II molecules. J Exp Med. 2003;197:375. doi: 10.1084/jem.20021961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roopenian D, Choi EY, Brown A. The immunogenomics of minor histocompatibility antigens. Immunol Rev. 2002;190:86. doi: 10.1034/j.1600-065x.2002.19007.x. [DOI] [PubMed] [Google Scholar]
- 9.Luedtke B, Pooler LM, Choi EY, et al. A single nucleotide polymorphism in the Emp3 gene defines the H4 minor histocompatibility antigen. Immunogenetics. 2003;55:284. doi: 10.1007/s00251-003-0581-x. [DOI] [PubMed] [Google Scholar]
- 10.Choi EY, Christianson GJ, Yoshimura Y, et al. Immunodominance of H60 is caused by an abnormally high precursor T cell pool directed against its unique minor histocompatibility antigen peptide. Immunity. 2002;17:593. doi: 10.1016/s1074-7613(02)00428-4. [DOI] [PubMed] [Google Scholar]
- 11.Choi EY, Yoshimura Y, Christianson GJ, et al. Quantitative analysis of the immune response to mouse non-MHC transplantation antigens in vivo: The H60 histocompatibility antigen dominates over all others. J Immunol. 2001;166:4370. doi: 10.4049/jimmunol.166.7.4370. [DOI] [PubMed] [Google Scholar]
- 12.Malarkannan S, Shih PP, Eden PA, et al. The molecular and functional characterization of a dominant minor H antigen, H60. J Immunol. 1998;161:3501. [PubMed] [Google Scholar]
- 13.Diefenbach A, Jensen ER, Jamieson AM, et al. Rae1 and H60 ligands of the NKG2D receptor stimulate tumour immunity. Nature. 2001;413:165. doi: 10.1038/35093109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diefenbach A, Tomasello E, Lucas M, et al. Selective associations with signaling proteins determine stimulatory versus costimulatory activity of NKG2D. Nat Immunol. 2002;3:1142. doi: 10.1038/ni858. [DOI] [PubMed] [Google Scholar]
- 15.Yang J, Jaramillo A, Liu W, et al. Chronic rejection of murine cardiac allografts discordant at the H13 minor histocompatibility antigen correlates with the generation of the H13-specific CD8+ cytotoxic T cells. Transplantation. 2003;76:84. doi: 10.1097/01.TP.0000072013.21336.64. [DOI] [PubMed] [Google Scholar]
- 16.Gittes RF, Russell PS. Male histocompatibility antigens in mouse endocrine tissues: Functional and histologic evidence. J Natl Cancer Inst. 1961;26:283. [PubMed] [Google Scholar]
- 17.He C, Schenk S, Zhang Q, et al. Effects of T cell frequency and graft size on transplant outcome in mice. J Immunol. 2004;172:240. doi: 10.4049/jimmunol.172.1.240. [DOI] [PubMed] [Google Scholar]
- 18.Shatry AM, Roopenian DC, Levy RB. Survival and function of MiHA epitope-specific host CD8 TM cells following ablative conditioning and HCT. Biol Blood Marrow Transplant. 2007;13:293. doi: 10.1016/j.bbmt.2006.12.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takada A, Yoshida S, Kajikawa M, et al. Two novel NKG2D ligands of the mouse H60 family with differential expression patterns and binding affinities to NKG2D. J Immunol. 2008;180:1678. doi: 10.4049/jimmunol.180.3.1678. [DOI] [PubMed] [Google Scholar]
- 20.Gasser S, Orsulic S, Brown EJ, et al. The DNA damage pathway regulates innate immune system ligands of the NKG2D receptor. Nature. 2005;436:1186. doi: 10.1038/nature03884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ye Z, Feng L, Huang S, et al. Expression of H60 on mice heart graft and influence of cyclosporine. Transplant Proc. 2006;38:2168. doi: 10.1016/j.transproceed.2006.06.096. [DOI] [PubMed] [Google Scholar]
- 22.Samarakoon A, Chu H, Malarkannan S. Murine NKG2D ligands: “Double, double toil and trouble. Mol Immunol. 2009;46:1011. doi: 10.1016/j.molimm.2008.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kwun J, Hu H, Schadde E, et al. Altered distribution of H60 minor H antigen-specific CD8 T cells and attenuated chronic vasculopathy in minor histocompatibility antigen mismatched heart transplantation in Cxcr3−/− mouse recipients. J Immunol. 2007;179:8016. doi: 10.4049/jimmunol.179.12.8016. [DOI] [PubMed] [Google Scholar]
- 24.Feng L, Ke N, Ye Z, et al. Expression of NKG2D and its ligand in mouse heart allografts may have a role in acute rejection. Transplant Proc. 2009;41:4332. doi: 10.1016/j.transproceed.2009.08.060. [DOI] [PubMed] [Google Scholar]
- 25.Cheng F, Feng L, Li S, et al. Yisheng injection decreases the expression of H60 and RAE-1 genes in ischemic mice liver. Transplant Proc. 2006;38:2210. doi: 10.1016/j.transproceed.2006.06.100. [DOI] [PubMed] [Google Scholar]
- 26.Ryu SJ, Jung KM, Yoo HS, et al. Cognate CD4 help is essential for the reactivation and expansion of CD8 memory T cells directed against the hematopoietic cell-specific dominant minor histocompatibility antigen, H60. Blood. 2009;113:4273. doi: 10.1182/blood-2008-09-181263. [DOI] [PubMed] [Google Scholar]
- 27.Skoskiewicz MJ, Colvin RB, Schneeberger EE, et al. Widespread and selective induction of major histocompatibility complex-determined antigens in vivo by γ interferon. J Exp Med. 1985;162:1645. doi: 10.1084/jem.162.5.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bui JD, Carayannopoulos LN, Lanier LL, et al. IFN-dependent down-regulation of the NKG2D ligand H60 on tumors. J Immunol. 2006;176:905. doi: 10.4049/jimmunol.176.2.905. [DOI] [PubMed] [Google Scholar]
- 29.de Mattos AM, Bennett WM, Barry JM, et al. HLA-identical sibling renal transplantation—A 21-yr single-center experience. Clin Transplant. 1999;13:158. doi: 10.1034/j.1399-0012.1999.130202.x. [DOI] [PubMed] [Google Scholar]
- 30.Krieger NR, Becker BN, Heisey DM, et al. Chronic allograft nephropathy uniformly affects recipients of cadaveric, nonidentical living-related, and living-unrelated grafts. Transplantation. 2003;75:1677. doi: 10.1097/01.TP.0000063830.60937.06. [DOI] [PubMed] [Google Scholar]
- 31.Billingham RE, Medawar PB. The technique of free skin grafting in mammals. J Exp Biol. 1951;28:385. [Google Scholar]
- 32.Bailey DW, Usama B. A rapid method of grafting skin on tails of mice. Transplant Bull. 1960;7:424. doi: 10.1097/00006534-196004000-00045. [DOI] [PubMed] [Google Scholar]
- 33.Corry RJ, Winn HJ, Russell PS. Primarily vascularized allografts of hearts in mice. The role of H-2D, H-2K and non-H-2 antigen in rejections. Transplantation. 1973;16:343. doi: 10.1097/00007890-197310000-00010. [DOI] [PubMed] [Google Scholar]
- 34.Russell PS, Chase CM, Winn HJ, et al. Coronary atherosclerosis in transplanted mouse hearts. I. Time course, immunogenetic and immunopathological considerations. Am J Path. 1994;144:260. [PMC free article] [PubMed] [Google Scholar]
- 35.Russell PS, Chase CM, Colvin RB. Coronary atherosclerosis in transplanted mouse hearts. IV. Effects of treatment with monoclonal antibodies to intercellular adhesion molecule-1 and leukocyte function-associated antigen-1. Transplantation. 1995;60:724. [PubMed] [Google Scholar]