Male patients greater than 50 years of age and cases performed by low-volume surgeons were found to have a higher rate of conversion to open procedures.
Keywords: Laparoscopic cholecystectomy, Laparoscopic conversion rate, Surgeon-specific factors, Patient-specific factors
Abstract
Background and Objectives:
Now nearly 2 decades into the laparoscopic era, nationwide laparoscopic cholecystectomy conversion rates remain around 5% to 10%. We analyzed patient- and surgeon-specific factors that may impact the decision to convert to open.
Methods:
We retrospectively analyzed 2205 LCs performed at a large tertiary community hospital over a 52-month period (May 2004 through October 2008).
Results:
The overall conversion rate was 4.9%. The most common reason for conversion was adhesions, and the majority of these patients had prior abdominal surgery. Males and patients >50 years old had a significantly higher likelihood of open conversion. The conversion rate of high-volume surgeons (≥100 total cases) in comparison to low-volume surgeons (40 to 99 total cases) was significantly lower. Conversion rates were lower among surgeons with fellowship training and those who completed residency training after 1990. Interestingly, the percentage of conversions due to technical difficulty was lower among those with fellowship training but higher among those who completed training after 1990.
Conclusion:
Conversion occurred in ∼5% of all laparoscopic cholecystectomies. Males, patients >50 years old, and cases performed by low-volume surgeons had a higher likelihood of conversion. Other surgeon-specific factors did not have a significant impact on conversion rate.
INTRODUCTION
It has been more than 2 decades since McKernan performed the first laparoscopic cholecystectomy (LC) in North America1 and 17 years since LC was deemed equivalent to open cholecystectomy (OC) in a consensus statement by the National Institutes of Health (NIH).2 Today, LC has become the procedure of choice for symptomatic biliary disease.3,4 Approximately 75% of all cholecystectomies are performed laparoscopically, and conversion to the open procedure ranges from 5% to 10% nationwide.5 The NIH postulated that the outcome of LCs would be greatly influenced by surgeon-specific factors, such as training, experience, skill and judgment.2 In addition, numerous patient and disease-related factors, such as male gender, obesity, old age (>65), prior abdominal surgery, acute cholecystitis, choledocholithiasis, and anomalous anatomy have been reported as significant risk factors for conversion to the open procedure.5–8
Preliminary reports on the performance of LC suggest that the increased rate of intraoperative complications is attributed to technical difficulty.3 The initial rate of common bile duct (CBD) injury in LC ranged from 0.2% to 3%, or up to 5 times higher than in OC.4,9–11 However, experience with LC and improved laparoscopic principles encouraging the accurate anatomical identification of structures, limited dissection within Calot's triangle, and the judicious use of intraoperative cholangiography have stabilized the CBD injury rate to a range of 0.25% to 0.5% nationwide.12 Furthermore, a 2006 Cochrane review declared there was no significant difference in morbidity, mortality, and operative time between LC and OC.3 More importantly, LC has been shown to have significant advantages over OC by reducing postoperative pain and thereby accelerating recovery and return to work and activity.3,8 Despite this progress, there are still a substantial proportion of patients in whom LC cannot be successfully performed and conversion to open surgery is required.
Patient-specific factors associated with a higher likelihood of conversion have been thoroughly studied.4–7,13–15 In contrast, surgeon-specific factors, aside from case volume, remain uncultivated.6,7,16 We sought to assess our experience with LC, while focusing on surgeon-specific and patient-specific factors that may be associated with a higher rate of conversion over a 52-month study period. This new insight should contribute to a better understanding of predictors for laparoscopic conversion and thus enhance the surgeon's ability to formulate and articulate a patient's unique risk of conversion prior to LC.
MATERIALS AND METHODS
A retrospective chart review of all patients who underwent LC at the Saint Barnabas Medical Center (SBMC) in Livingston, New Jersey, over a 52-month period (May 2004 to October 2008) was performed. The LCs were performed by 35 distinct surgeons. The decision to convert to open was made by the individual surgeon, and the reason for conversion was extracted from the patient's medical record (operative report).
Operative Technique
All operations were performed with the patient under general anesthesia with endotracheal intubation using either a 3- or 4-trocar technique.
Evaluation Criteria and Comparison Factors
Patient-specific characteristics that were extracted included age, sex, and a history of prior abdominal surgery. Surgeon-specific characteristics that were extracted included annual and total case volume over the study period, year of general surgery residency graduation, and whether each of the surgeons obtained additional minimally invasive fellowship training. Based on the total case volume/surgeon over the study period, each surgeon was categorized into either high-volume (≥100 LCs performed) or low-volume (40 to 99 LCs performed) groups. Similarly, surgeons were categorized into those that graduated before or in 1990 versus after 1990, as well as those who completed versus those who did not complete additional minimally invasive fellowship training. CRs and the proportion of conversions due to technical difficulty or iatrogenic injury were calculated for each of the groups to perform a comparative analysis.
Statistical Analysis
Collected data were tabulated, and calculations were performed using Microsoft Excel statistical functions. Statistical analysis included a z-test for comparing proportions, and a Student t test (2-tailed) for comparing means. Significance levels were defined as P<0.05.
RESULTS
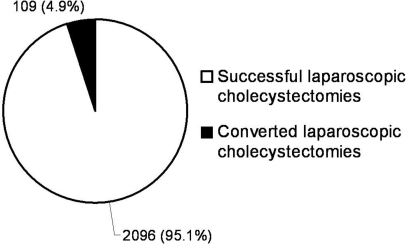
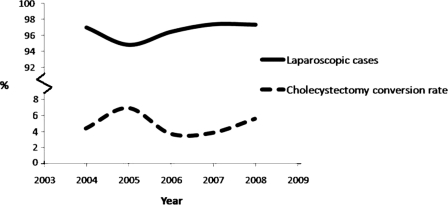
Over the 52-month study period, 2284 patients underwent cholecystectomy. Among patients undergoing cholecystectomy, LC was attempted in 2205 (96.5%) patients, and primary OC was performed in 79 (3.5%) patients. Of the 2205 attempted LCs, 2096 (95.1%) were completed successfully, and 109 (4.9%) were converted to the open procedure (Figure 1). An average of 441 LCs were attempted annually with the greatest number (N=515) performed in 2006. The annual rate of attempted LCs ranged from 94.8% (457 of 482 total cholecystectomies in 2005) to 97.4% (446 of 458 total cholecystectomies in 2007) (P<0.06, Figure 2). The annual CR ranged from 3.7% (19 of 515 attempted LCs in 2006) to 7.2% (33 of 457 attempted LCs in 2005) (P<0.02, Figure 2).
Figure 1.
Attempted laparoscopic cholecystectomies (LCs) and the percentage of laparoscopic cholecystectomy (LC) procedures that were successful and converted to open. Laparoscopic cholecystectomy conversion rate (LCCR) was 4.9% (109/2205).
Figure 2.
The annual rate of attempted laparoscopic cholecystectomies (solid line) ranged from 94.8% in 2005 to 97.4% in 2007 (P<0.06). The annual conversion rate (dashed line) ranged from 3.7% in 2006 to 7.2% in 2005 (P<0.02).
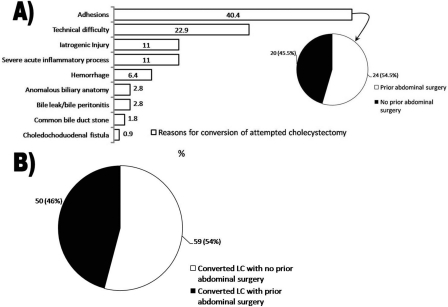
Figure 3A depicts the various reasons for laparoscopic conversion in all 109 patients. Presence of adhesions defined as scar tissue in response to prior surgery, trauma, or inflammation was the most common reason for conversion (44 of 109 patients, 40.4%). Among the 109 patients who underwent laparoscopic conversion to open, 54.1% (N=59) had no prior history of abdominal surgery (Figure 3B). However, among the conversions due to adhesions, 54.5% (N=24) had a history of abdominal surgery, while 45.5% (N=20) had no history of abdominal surgery (Figure 3A). The second most common reason for conversion was technical difficulty (25 of 109 patients, 22.9%), and of these cases 22 surgeons cited inadequate visualization in the absence of hepatomegaly, 2 cited inadequate visualization in the presence of hepatomegaly, and one cited inadequate pneumoperitoneum. Additional reasons for conversion included (in order of most common to least common): iatrogenic injury (N=12), severe acute inflammatory process (N=12), hemorrhage (N=7), anomalous biliary anatomy (N=3), bile leak/bile peritonitis (N=3), CBD stone (N=2), and choledochoduodenal fistula (N=1). Among patients with iatrogenic injury who underwent laparoscopic conversion, 7 patients incurred gallbladder perforation, 2 incurred CBD injury, 1 incurred right posterior sectoral duct injury, and 1 incurred an enterotomy. CBD injury at our institution occurred in 0.1% (2 of 2205) of all attempted LCs and accounted for 1.8% (2 of 109) of converted LCs. Of those patients with bile leak/bile peritonitis, one had perforated (noniatrogenic) cholecystitis, another had sustained erosion of the T-tube into the CBD, and the third leaked bile through a remnant GB infundibulum.
Figure 3.
A) Detailed indications for conversion of laparoscopic cholecystectomy to an open procedure among 109 patients are provided. Among patients with adhesions as the indication for conversion, 54.5% had a history of abdominal surgery (see insert); B) Of the 109 patients converted to open procedures 54% had no prior abdominal surgeries.
Patient-Specific Characteristics
In patients in whom LC was attempted, 74.7% (1647/2205) were females, and 25.3% (558/2205) were males. The mean age of patients in whom LC was attempted was 50.5 years old. The mean age of patients undergoing successful LC was 49.6 years old and 66.1 years old among those converted to OC (P<0.0001). Twenty pediatric (age<18) patients (0.9%, 20/2205) underwent LC, and there were no conversions to OC.
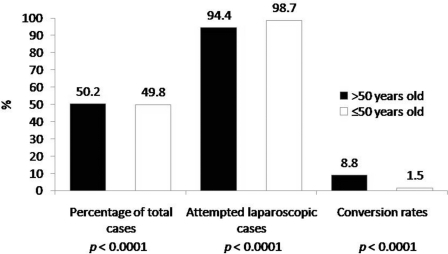
Cholecystectomy was performed slightly more often in patients >50 years of age versus those <50 years of age [50.2% (N=1146) versus 49.8% (N=1138); P<0.06]. LC was attempted in a larger proportion of patients <50 years of age versus those >50 years of age [98.7% (N=1123) versus 94.4% (N=1082); P<0.0001, respectively), and a larger proportion of patients >50 years of age versus those <50 years of age underwent conversion to OC [8.8% (N=92) versus 1.5% (N=17); P<0.0001] (Figure 4).
Figure 4.
Comparison of the proportion of total cholecystectomy cases, attempted laparoscopic cases, and conversion rates in male versus female patients.
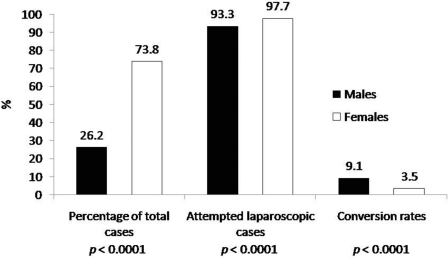
A larger percentage of females versus males underwent cholecystectomies [73.8% (N=1686) versus 26.2% (N=602), P<0.0001), and LCs were attempted in a larger percentage of females versus males [97.7% (N=1647) versus 92.7% (N=558); P<0.0001]. On the contrary, a larger proportion of males versus females underwent conversion to OC [9.1% (N=51) versus 3.5% (N=58), P<0.0001) (Figure 5).
Figure 5.
Comparison of the proportion of total cholecystectomy cases, attempted laparoscopic cases, and conversion rates in patients >50 years old versus ≤50 years old. The mean age of patients in whom laparoscopic cholecystectomy (LC) was attempted was 50.5.
Surgeon-Specific Characteristics
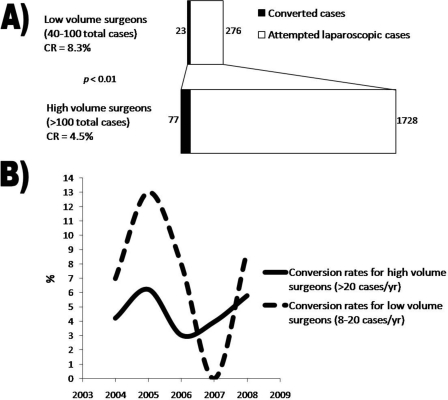
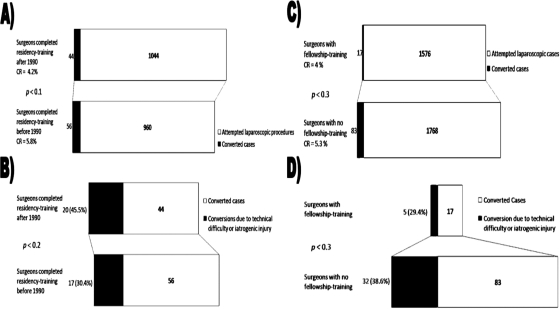
The overall CR of LCs performed by high-volume surgeons was significantly lower in comparison with that of low-volume surgeons [4.5% (N=77) versus 8.3% (N=23); P<0.01) (Figure 6A). The CRs ranged from 1% (1/113) to 6.5% (10/155) in the high-volume group and 0% (0/40) to 15.3% (11/72) in the low-volume group. The average number of cases collectively performed over a year by the high-volume group was 216 (range, 113 to 616), and the number of cases performed by the low-volume group was 69 (range, 41 to 99). The annual CRs among surgeons who performed >20 cases/year and those who performed 8 to 20 cases/year did not differ significantly (P<0.07 to 0.7, Figure 6B). CRs among surgeons who performed >20 cases/year reached their nadir in 2006 at 3.7% (N=515) and peaked in 2005 at 7.2% (N=457). CRs among surgeons who performed 8 to 20 cases/year reached their nadir in 2007 at 0% (N=32) and peaked in 2005 at 13% (N=77). CRs were lower among surgeons who completed residency training after 1990 versus those who completed training before 1990 [4.2% (N = 44) versus 5.8% (N=56); P<0.1) (Figure 7A). In addition, surgeons with fellowship training had lower CRs than those without additional training [4% (N=17) versus 5.3% (N=83); P<0.3) (Figure 7B). Moreover, the percentage of conversions due to technical difficulty/intraoperative complications were lower among surgeons with fellowship training [29.4% (N=5) versus 38.6% (N=32); P<0.3) (Figure 7D) but was higher among those who had completed training after 1990 [45.5% (N=20) versus surgeons trained prior to 1990: 30.4% (N=17); P<0.2) (Figure 7C).
Figure 6.
Comparison of high-volume versus low-volume surgeons. A) Conversion rates and attempted laparoscopic cases for lowvolume surgeons (40 to 100 total cases) versus high-volume surgeons (>100 total cases); B) Annual CR variance between highvolume and low-volume surgeons did not vary significantly (P<0.07 to 0.7).
Figure 7.
A) Difference in conversion rates (CRs) between surgeons who completed residency training after or before 1990 was not significantly different; B) Surgeons who completed residency training after 1990 converted due to technical difficulty or iatrogenic injury more often; however, this was not statistically significant; C) Difference in CRs between surgeons with fellowship training and those without was not significantly different; D) Surgeons with fellowship training converted due to technical difficulty or iatrogenic injury less often; however, this was not statistically significant.
DISCUSSION
The first LC in North America was performed on June 22, 1988 by J. Barry McKernan at the Marietta Surgical Center in Marietta, Georgia, USA.1 Since then, the popularity of LC has grown logarithmically to its current stature as the procedure of choice for symptomatic biliary pathology.3–6 LC has been associated with lower patient morbidity, hospital costs, and a shorter recovery period, but carries with it the inherent risk of conversion to an open procedure and a slightly higher risk of CBD injury.2,3,7,8 In fact, the published national averages for CR ranges between 5% to 10%, which is consistent with the CR of 4.9% in the current study.5 A review of the literature on LC reveals that the risk of laparoscopic conversion is associated with specific patient- and surgeon-specific factors.4,7,17–19 To date, patient-specific risk factors for open conversion have been extensively evaluated,4–7,13–15,20 whereas surgeon-specific factors remain poorly assessed.7,17,18,21–25 While identification of patient-specific factors may assist in carefully selecting patients for laparoscopic procedures, an understanding of surgeon specific factors may assist the patient and surgical team to make better decisions about procedure selection and technique as well as operative considerations.
Patient-specific risk factors, such as male gender, old age, and prior abdominal surgery, have been shown to be predictors of unsuccessful LC. One such risk factor, the male gender, is associated with a higher likelihood of conversion, which may be due to the more frequent association of pathologically severe disease.5,7,19 Data derived from the National Hospital Discharge database by Livingston et al5 revealed that males with acute cholecystitis incurred a higher risk of conversion (2.5%) than their female counterparts (1.5%). Further, Lein et al19 also noted that acute cholecystitis has a disproportionately higher incidence in males compared with the proportion undergoing cholecystectomy. The current report is in agreement with these findings in that 46.8% of those undergoing conversion were male, although males made up only 25.3% undergoing LC. Another patient-specific risk factor for conversion was advanced age.5–8,26 In the current study, the CR in the older patient group (>50 years old) was nearly 6-fold higher than in younger patients. Previous findings have found 2- to 4-fold higher CRs in older patients.5–7,27 Notably, elderly patients have a higher incidence of severe, acute, and gangrenous cholecystitis as well as an increased incidence of comorbid cardiopulmonary disease, choledocholithiasis, and prior abdominal surgery, which may contribute to the higher CR.7,26,27 Finally, prior abdominal surgery has also been associated with a higher likelihood of conversion in numerous studies.7,8,28 Prior surgery, especially in the upper abdomen, may result in adhesions around the gallbladder, limiting visualization, retraction, and dissection.28 Adhesions were the most commonly cited reason for conversion in the present study, and almost half of the patients undergoing conversion had a history of abdominal surgery. While the aforementioned patient factors certainly contribute to unsuccessful LCs, it is generally accepted that the most important factors are surgeon-specific.2
The NIH 1992 consensus statement stated that the outcome of LCs are influenced greatly by the training, experience, skill, and judgment of the surgeon performing the procedure.2 Since then, various studies have demonstrated that case volume significantly affects the proficiency of a surgeon.6,7,17,21–25 Liu et al6 demonstrated that the laparoscopic cholecystectomy conversion rate (LCCR) decreased from 17% in a surgeon's initial 100 cases to 4% when the surgical experience reached between 400 to 500 cases (P<0.05). Synonymous with the learning curve, a “proficiency-gain curve” for surgeons refers to a graphical representation of improving the rate of proficiency in relation to the increasing number of laparoscopic procedures performed.29 This would explain the difference between the CRs of high-volume and low-volume surgeons in the current study (4.5% versus 8.3%; P<0.01). Several studies have attempted to define the actual number of operations a surgeon must perform to attain the stage of consistent efficiency and safe execution of any minimally invasive procedure.4,7,19,20,30 However, results vary widely (10 to 200 operations), and it seems likely that additional surgeon-specific factors may also influence CRs.
Advanced minimally invasive surgical training purportedly increases a surgeon's skill set beyond that gained during surgical residency.31 Hence, it is reasonable to think that acquisition of different perspectives and adopting an effortless approach to tissue dissection in the 2- dimensional realm during advanced surgical training should translate into improved performance of basic minimally invasive procedures such as LC. In our study, the overall CRs and CRs due to technical difficulty or iatrogenic injury among fellowship-trained surgeons were lower than their counterparts (4% versus 5.3%, P<0.3 and 29.4% versus 38.5%, P<0.3, respectively), although not statistically significant. Similarly, Bohacek and Pace16 revealed a lower LCCR in cases performed by a minimally invasive fellowship-trained surgeon (3.2%) in comparison with cases performed by surgeons with no formal advanced laparoscopic training (16.5%) (P<0.05).
Advent of the laparoscopic era, the period of time post-1990 until the present, saw a dramatic transformation of surgical education. LC and endoscopic surgical procedures were included nationwide in surgical training programs in the early 1990s, potentially instilling greater confidence in post-1990 surgical graduates in regards to laparoscopic skills.32,33 Surprisingly, the current study found no difference between CRs of surgeons trained in the laparoscopic era and the prelaparoscopic era. Moreover, there was no significant difference between the percentages of cases converted due to technical difficulty or iatrogenic injury in either of these groups.
CONCLUSIONS
Patient- and surgeon-specific factors are significantly associated with conversion of LC. Specifically, male gender, old age (>50 years old) and low-volume surgeons (40 to 99 cases performed over the 52-month period) were found to have significantly higher CRs than their counterparts. On the contrary, additional fellowship training and residency completion in the laparoscopic era (post-1990) did not reveal significant differences in CRs versus their counterparts nor did it result in a significant decrease in likelihood of conversion due to technical difficulty or iatrogenic injury. An appreciation of these predictors of conversion should permit more appropriate preoperative consent of patients and encourage surgeons to evaluate their own skill set and seek additional training if necessary.
Contributor Information
Sujit Vijay Sakpal, Department of Surgery, Saint Barnabas Medical Center, Livingston, New Jersey, USA..
Supreet Singh Bindra, St. George's University School of Medicine, St. George, Grenada, West Indies..
Ronald S. Chamberlain, Department of Surgery, Saint Barnabas Medical Center, Livingston, New Jersey, USA.; Department of Surgery, University of Medicine & Dentistry of New Jersey, Newark, New Jersey, USA. St. George's University School of Medicine, St. George, Grenada, West Indies.
References:
- 1. McKernan JB. Origin of laparoscopic cholecystectomy in the USA: personal experience. World J Surg. 1999; 23: 332–333 [PubMed] [Google Scholar]
- 2. Gallstones and Laparoscopic Cholecystectomy. NIH Consensus Statement Online 1992; 10: 1–20 [PubMed] [Google Scholar]
- 3. Keus F, de Jong JA, Gooszen HG, van Laarhoven CJ. Laparoscopic versus open cholecystectomy for patients with symptomatic cholecystolithiasis. Cochrane Database Syst Rev. 2006; 18 (4): CD006231 [DOI] [PubMed] [Google Scholar]
- 4. The Southern Surgeons Club. A prospective analysis of 1518 laparoscopic cholecystectomies. N Engl J Med. 1991; 324: 1073–1078 [DOI] [PubMed] [Google Scholar]
- 5. Livingston EH, Rege RV. A nationwide study of conversion from laparoscopic to open cholecystectomy. Am J Surg. 2004; 188: 205–211 [DOI] [PubMed] [Google Scholar]
- 6. Liu CL, Fan ST, Lai EC, Lo CM, Chu KM. Factors affecting conversion of laparoscopic cholecystectomy to open surgery. Arch Surg. 1996; 131: 98–101 [DOI] [PubMed] [Google Scholar]
- 7. Tang B, Cuschieri A. Conversions during laparoscopic cholecystectomy: risk factors and effects on patient outcome. J Gastrointest Surg. 2006; 10: 1081–1091 [DOI] [PubMed] [Google Scholar]
- 8. Ibrahim S, Hean TK, Ho LS, Ravintharan T, Chye TN, Chee CH. Risk factors for conversion to open surgery in patients undergoing laparoscopic cholecystectomy. World J Surg. 2006; 30: 1698–1704 [DOI] [PubMed] [Google Scholar]
- 9. Soper NJ. Laparoscopic cholecystectomy. Curr Probl Surg. 1991; 28: 581–655 Review [DOI] [PubMed] [Google Scholar]
- 10. Strasberg SM, Hertl M, Soper NJ. An analysis of the problem of biliary injury during laparoscopic cholecystectomy. J Am Coll Surg. 1995; 180: 101–25 [PubMed] [Google Scholar]
- 11. Way LW. Bile duct injury during laparoscopic cholecystectomy. Ann Surg. 1992; 215: 195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Connor S, Garden OJ. Bile duct injury in the era of laparoscopic cholecystectomy. Br J Surg. 2006; 93: 158–168 [DOI] [PubMed] [Google Scholar]
- 13. Nachnani J, Supe A. Pre-operative prediction of difficult laparoscopic cholecystectomy using clinical and ultrasonographic parameters. Indian J Gastroenterol. 2005; 24: 16–18 [PubMed] [Google Scholar]
- 14. Polychronidis A, Botaitis S, Tsaroucha A, et al. Laparoscopic cholecystectomy in elderly patients. J Gastrointestin Liver Dis. 2008; 17: 309–313 [PubMed] [Google Scholar]
- 15. Steiner CA, Bass EB, Talamini MA, Pitt HA, Steinberg EP. Surgical rates and operative mortality for open and laparoscopic cholecystectomy in Maryland. N Engl J Med. 1994; 330: 403–408 [DOI] [PubMed] [Google Scholar]
- 16. Bohacek L, Pace DE. Advanced laparoscopic training and outcomes in laparoscopic cholecystectomy. Can J Surg. 2009; 52: 291–294 [PMC free article] [PubMed] [Google Scholar]
- 17. Moore MJ, Bennet CL. The learning curve for laparoscopic cholecystectomy. The Southern Surgeons Club. Am J Surg. 1995; 170: 55–59 [DOI] [PubMed] [Google Scholar]
- 18. Johansson M, Thune A, Nelvin L, Stiernstam M, Westman B, Lundell L. Randomized clinical trial of open versus laparoscopic cholecystectomy in the treatment of acute cholecystitis. Br J Surg. 2005; 92: 44–49 [DOI] [PubMed] [Google Scholar]
- 19. Lein HH, Huang CS. Male gender: Risk factor for severe symptomatic cholelithiasis. World J Surg. 2002; 26: 598:601 [DOI] [PubMed] [Google Scholar]
- 20. Shea JA, Healey MJ, Berlin JA, et al. Mortality and complications associated with laparoscopic cholecystectomy. A meta-analysis. Ann Surg. 1996; 224: 609–620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bingener-Casey J, Lichards ML, Stodel WE, Schwesinger WH, Sirinek KR. Reasons for conversion from laparoscopic to open cholecystectomy: A 10-year review. J Gastrointest Surg. 2002; 6: 800–805 [DOI] [PubMed] [Google Scholar]
- 22. McMahon AJ, Fishbacher CM, Frame SH, MacLeod MC. Impact of laparoscopic cholecystectomy: A population based study. Lancet. 2000; 356: 1632–1637 [DOI] [PubMed] [Google Scholar]
- 23. Cagir B, Rangraj M, Maffuci L, Herz BL. The learning curve for laparoscopic cholecystectomy. J Laparoendosc Surg. 1994; 4: 419–427 [DOI] [PubMed] [Google Scholar]
- 24. Tang B, Hanna GB, Joice P, Cuschieri A. Identification and categorization of technical errors by Observational Clinical Human Reliability Assessment (OCHRA) during laparoscopic cholecystectomy. Arch Surg. 2004. November;139:1215–1220 [DOI] [PubMed] [Google Scholar]
- 25. Voitk AJ, Tsao SGS, Ignatius S. The tail of the learning curve for laparoscopic cholecystectomy. Am J Surg. 2001; 182: 250–253 [DOI] [PubMed] [Google Scholar]
- 26. Brunt LM, Quaseharth MA, Dunnegan DL, Soper NJ. Outcomes analysis of laparoscopic cholecystectomy in the extremely elderly. Surg Endosc. 2001; 15: 700–705 [DOI] [PubMed] [Google Scholar]
- 27. Schrenk P, Woisetschlager R, Wayand WU. Laparoscopic cholecystectomy. Cause of conversions in 1,300 patients and analysis of risk factors. Surg Endosc. 1995; 9: 25–28 [DOI] [PubMed] [Google Scholar]
- 28. Karayiannakis AJ, Polychronidis A, Perente S, Botaitis S, Simopoulos C. Laparoscopic cholecystectomy in patients with previous upper or lower abdominal surgery. Surg Endosc. 2004; 18: 97–101 [DOI] [PubMed] [Google Scholar]
- 29. Cuschieri A. Lest we forget the surgeon. Sem Lap Surg. 2003; 10: 141–148 [DOI] [PubMed] [Google Scholar]
- 30. Deziel DJ, Millikan KW, Economou SG, Doolas A, Ko ST, Airan MC. Complications of laparoscopic cholecystectomy: a national survey of 4,292 hospitals and an analysis of 77,604 cases. Am J Surg. 1993; 165: 9–14 [DOI] [PubMed] [Google Scholar]
- 31. Pace DE, Chiasson PM, Schlachta CM, et al. Laparoscopic splenectomy: does the training of minimally invasive surgical fellows affect outcomes. Surg Endosc. 2002; 16: 954–956 [DOI] [PubMed] [Google Scholar]
- 32. Zucker KA, Bailey RW, Graham SM, Scovil W, Imbembo AL. Training for laparoscopic surgery. World J Surg. 1993; 17: 3–7 [DOI] [PubMed] [Google Scholar]
- 33. Hawasli A, Featherstone R, Lloyd L, Vorhees M. Laparoscopic training in residency program. J Laparoendosc Surg. 1996; 6: 171–174 [DOI] [PubMed] [Google Scholar]