Abstract
The cysteine prodrug N-acetylcysteine (NAC) has been shown to reduce reinstatement of cocaine seeking by normalization of glutamatergic tone. However, enduring inhibition of cocaine seeking produced by NAC has not been explored under different withdrawal conditions. Thus, the present study determined whether chronic NAC administered during daily extinction training or daily abstinence after withdrawal from cocaine self-administration would reduce cocaine seeking. Rats self-administered intravenous cocaine during daily 2-h sessions for 12 days, followed by daily extinction or abstinence sessions. During this period, rats received daily injections of saline or NAC (60 or 100 mg/kg). Subsequently, rats were tested for cocaine seeking via conditioned cue, cue + cocaine-primed, and context-induced relapse. Chronic NAC administration blunted cocaine seeking under multiple experimental protocols. Specifically, NAC attenuated responding during cue and cue + cocaine-primed reinstatement tests after extinction and context, cue, and cue + cocaine relapse tests after abstinence. Protection from relapse by NAC persisted well after treatment was discontinued, particularly when the high dose was combined with extinction trials. The finding that NAC reduced cocaine seeking after drug treatment was discontinued has important implications for the development of effective antirelapse medications. These results support recent preclinical and clinical findings that NAC may serve as an effective treatment for inhibiting relapse in cocaine addicts.
Introduction
A key feature of drug addiction is the inability of addicts to inhibit relapse. Thus, the development of effective antirelapse medication is a critical goal of pharmacotherapy for addiction. Although a large number of pharmacological agents have been assessed in animal models of addiction and clinical studies (Yahyavi-Firouz-Abadi and See, 2009), there are no currently available drugs with established efficacy for the treatment of psychostimulant addiction and relapse.
Drug-induced plasticity in central glutamate function has been increasingly implicated in the process of cocaine addiction and relapse (Kalivas, 2009). For example, chronic cocaine self-administration (SA) reduces basal concentrations of extracellular glutamate, but potentiates nucleus accumbens glutamate release during cocaine-primed reinstatement of extinguished drug seeking (McFarland et al., 2003; Madayag et al., 2007). Basal glutamate concentrations are regulated by the glutamate/cystine antiporter (Baker et al., 2002), which are down-regulated during withdrawal (Baker et al., 2003a; Madayag et al., 2007). N-acetylcysteine (NAC) is a cystine prodrug that restores basal glutamate levels and prevents increased glutamate during reinstatement by reversing glutamate dysregulation after cocaine self-administration in rats (Baker et al., 2002; Madayag et al., 2007; Kalivas, 2009; Moussawi et al., 2009). Acute NAC administration prevents cocaine-induced increases in extinguished drug seeking (Baker et al., 2003b; Madayag et al., 2007; Kau et al., 2008). Chronic NAC given during cocaine self-administration does not affect cocaine intake with daily 2-h access conditions (Madayag et al., 2007; Amen et al., 2011), but does reduce and block escalation of intake in extended 6-h access conditions (Madayag et al., 2007; Kau et al., 2008). Consequently, NAC does not change the reinforcing mechanisms associated with cocaine, but instead prevents drug seeking by a reduction or reversal of the development of neuroplasticity required for cocaine-induced reinstatement (Madayag et al., 2007; Amen et al., 2011; Moussawi et al., 2011).
We demonstrated that repeated NAC administration during daily extinction trials reduces reinstatement triggered by either cocaine-associated conditioned cues or the combination of drug-associated cues and a priming injection of cocaine (Moussawi et al., 2011). Likewise, chronic NAC during extinction produced lasting reductions in heroin seeking in rats (Zhou and Kalivas, 2008), and repeated NAC decreased cocaine-primed reinstatement of drug seeking when tested 24 h after the last injection (Amen et al., 2011). Finally, initial clinical assessments indicate promising results for reduced craving in cocaine addicts after repeated NAC treatment (LaRowe et al., 2007; Amen et al., 2011). These studies suggest that NAC may serve as an effective antirelapse medication.
Extinction occurs when a Pavlovian signal or an instrumental action is repeatedly presented without its associated reinforcer. Ample evidence shows extinction does not result in the “unlearning” of the original association, but instead involves new learning of the altered contingency (Bouton, 2002; Quirk and Mueller, 2008). The extinction-reinstatement model involves a period of maintained drug self-administration for intravenous infusions, followed by extinction training, whereby operant responding decreases in the drug-associated context when lever pressing no longer results in reinforcement. Drug seeking is reinstated by exposure to a priming injection of drug, stress, or previously drug-associated cues. Although most animal models of relapse have implemented extinction-reinstatement procedures (Shaham et al., 2003), most “treatment-as-usual” protocols for human drug addicts do not explicitly extinguish drug-related cues. An alternative approach in animal models has been to assess drug seeking after a period of forced abstinence with no daily extinction trials (Reichel and Bevins, 2009). Rather than extinguishing the operant behavior (e.g., lever pressing), subjects are kept away from the self-administration environment for a prescribed amount of time. At the time of relapse testing, the drug-reinforced associations (i.e., lever presses, environmental context, and conditioned cues) are fully intact, having never been experienced in the absence of the drug. It is important to study both extinction-reinstatement and abstinence-relapse models because the incorporation of extinction training into an abstinence period results in different behavioral profiles (Neisewander et al., 2000; Zavala et al., 2007) and neuroadaptations related to relapse (Schmidt et al., 2001; Sutton et al., 2003; Self, 2004; Fuchs et al., 2006; Knackstedt et al., 2010).
In the current study, we compared the impact of chronic NAC treatment during abstinence versus chronic NAC during daily extinction trials on subsequent cocaine seeking at both early and late withdrawal points. Although extinction and abstinence may involve differing neuroadaptations, the ability of NAC to prevent cocaine seeking relies on the restoration of glutamate homeostasis and the reversal of cocaine-induced synaptic potentiation (Baker et al., 2003a; Kalivas, 2009; Moussawi et al., 2011). Therefore, we hypothesized that cocaine seeking should be reduced by chronic NAC regardless of whether or not explicit extinction training occurred during treatment.
Materials and Methods
Subjects.
Ninety-three male Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA), weighing 250 to 300 g at the time of delivery, were maintained on a reversed 12:12 light/dark cycle in a temperature- and humidity-controlled vivarium. Rats were individually housed and received ad libitum water throughout the study and 25 g of standard rat chow (Harlan, Indianapolis, IN) daily until self-administration stabilized, at which time animals were maintained ad libitum. Procedures were conducted in accordance with the Guide for the Care and Use of Laboratory Rats (Institute of Laboratory Animal Resources on Life Sciences, National Research Council, Washington, DC) and approved by the Institutional Animal Care and Use Committee of the Medical University of South Carolina.
Apparatus.
Self-administration chambers (30 × 20 × 20 cm; Med Associates, St. Albans, VT) were housed inside sound-attenuating cubicles fitted with a fan for airflow. Each chamber contained two retractable levers, two stimulus lights, a speaker for tone delivery, and a house light to provide general illumination. In addition, each chamber was equipped with a balanced metal arm and spring leash attached to a swivel (Instech Laboratories, Plymouth Meeting, PA). Tygon tubing extended through the leash and was connected to a 10-ml syringe mounted on an infusion pump located outside of the sound-attenuating cubicle.
Drugs.
Cocaine hydrochloride dissolved in 0.9% sterile saline (generously provided by the National Institute on Drug Abuse, Research Triangle Park, NC) was administered intravenously at a volume of 50 μg/50-μl infusion. N-acetylcysteine (Sigma, St. Louis, MO) was dissolved in saline, adjusted with NaOH to 7.2 pH, and injected at a volume of 1 ml/kg i.p. Drugs used for anesthesia were ketamine (Vedco Inc., St Joseph, MO), xylazine (Lloyd Laboratories, Shenandoah, IA), equithesin (sodium pentobarbital 4 mg/kg, chloral hydrate 17 mg/kg, and 21.3 mg/kg magnesium sulfate heptahydrate dissolved in 44% propylene glycol, 10% ethanol solution), and ketorolac (Sigma). Catheters were flushed with heparin (Elkins-Sinn, Cherry Hill, NJ) and cephazolin (Schein Pharmaceuticals, Florham Park, NJ). Catheter patency was verified with methohexital sodium (Eli Lilly & Co., Indianapolis, IN).
Surgery.
Anesthesia consisted of intraperitoneal injections of ketamine (66 mg/kg), xylazine (1.3 mg/kg), and equithesin (0.5 ml/kg). Ketorolac (2.0 mg/kg i.p.) was given just before surgery as an analgesic. One end of a silastic catheter was inserted 33 mm into the right jugular and secured with 4.0 silk sutures. The other end ran subcutaneously and exited from a small incision just below the scapula. This end attached to an infusion harness (Instech Laboratories) that provided access to an external port for intravenous drug delivery. An antibiotic solution of cefazolin (10 mg/0.1 ml) was given after surgery and during recovery along with 0.1 ml of 70 U/ml heparinized saline. During self-administration, rats received an intravenous infusion (0.1 ml) of 10 U/ml heparinized saline before each session. After each session, catheters were flushed with cefazolin and 0.1 ml of 70 U/ml heparinized saline. Catheter patency was periodically verified with intravenous methohexital sodium (10 mg/ml dissolved in 0.9% physiological saline), a short-acting barbiturate that produces a rapid loss of muscle tone.
Cocaine Self-Administration.
After at least 5 days of recovery, rats were given daily 2-h sessions to self-administer cocaine on a fixed ratio 1 schedule of reinforcement. The house light always signaled the beginning of a session and remained on throughout the session. During the sessions, a response on the active lever resulted in activation of the pump for a 2-s infusion (50 μg/50 μl bolus infusion) and presentation of a stimulus complex consisting of a 5-s tone (78 dB, 4.5 kHz) and a white stimulus light over the active lever, followed by a 20-s timeout. Responses that occurred during the timeout and on the inactive lever were recorded, but had no scheduled consequences. All sessions took place during the dark cycle and were conducted 6 days/week until each rat reached a criterion of >10 infusions during a session for 12 cumulative days. All ensuing experiments used the same self-administration procedures.
Abstinence, Extinction, and Reinstatement.
The term “reinstatement” connotes responding subsequent to extinction of the lever press response (Bouton, 2002). Adhering to the traditional extinction-reinstatement model (Shaham et al., 2003), tests conducted after extinction are termed reinstatement tests, and tests that occur after abstinence in the absence of daily extinction are referred to as “relapse.” After cocaine self-administration, rats were placed into abstinence and/or extinction before testing. During abstinence, rats were transported, handled, and injected daily with saline or NAC (60 or 100 mg/kg), but were not placed in the self-administration chamber. Clinical studies report dose ranges from 1200 to 3600 mg in a 24-h period (LaRowe et al., 2007; Mardikian et al., 2007; Amen et al., 2010). Using an average body weight of 70 kg, this range converts to 17.14 to 51.43 mg/kg. Keeping in mind species differences and administration routes, our NAC doses can be considered to be in the higher range. After abstinence, relapse tests consisted of placing rats back into the self-administration context for a 2-h test. Responding was recorded, but had no consequences except during cue-induced reinstatement, whereby each response resulted in presentation of the stimulus complex. During extinction, rats were placed in the self-administration chamber for daily 2-h sessions for 12 days, and responding on either lever had no scheduled consequences. To have met extinction criterion, responses on the active (i.e., drug seeking) lever needed to be ≤25 presses for 2 consecutive days. When this criterion was met, rats were tested for cue-induced, cocaine-primed, and/or cue + cocaine-primed reinstatement. For cue-induced reinstatement tests, active lever presses resulted in presentation of the conditioned light + tone in the same manner as during self-administration. For cocaine-primed reinstatement tests, rats received a cocaine injection (10 mg/kg) before testing and responses on both levers were recorded, but had no consequences. The cue + cocaine condition consisted of the cocaine priming injection and presentation of cues. Daily extinction sessions occurred between reinstatement tests until the criterion of ≤25 presses for 2 consecutive days was met.
Experiments 1 and 2: Effects of Chronic NAC (60 and 100 mg/kg) during Extinction on Subsequent Reinstatement.
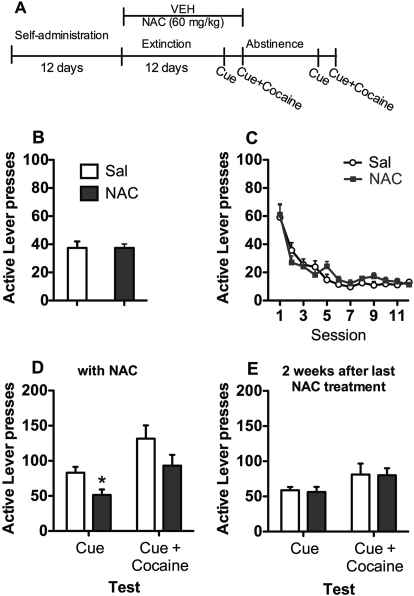
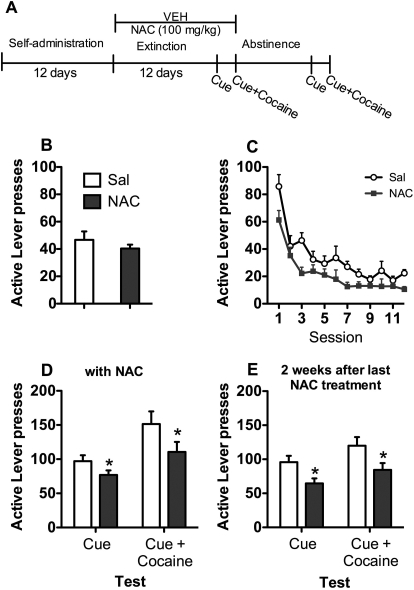
Figures 1A and 2A depict the time line for experiments 1 and 2, respectively. Rats experienced 12 days of cocaine self-administration, followed by at least 12 days of extinction. During extinction, rats received saline, 60 mg/kg NAC (experiment 1), or 100 mg/kg NAC (experiment 2) 2 h before each extinction trial. At the end of extinction, rats were tested for cue and cue + cocaine-primed reinstatement. Between tests, rats were re-extinguished to criterion and NAC or saline treatment continued. After 2 weeks of abstinence, rats were retested in the absence of NAC. A subset of rats used in experiment 2 has been previously included in a published data set (Moussawi et al., 2011).
Fig. 1.
Experiment 1: 60 mg/kg NAC during extinction. A, time course for experiment 1. NAC (60 mg/kg) treatment occurred during daily extinction sessions and on cue-induced and cue + cocaine-primed reinstatement test days. Rats were retested after 2 weeks of abstinence without NAC. B, active lever presses during the last 3 days of self-administration. C, lever responding over the 12 days of extinction. D, reinstatement of cocaine-seeking by cues or cues + cocaine. *, reduced responding by NAC treatment relative to saline treatment; p < 0.05. E, cocaine seeking at 2 weeks after discontinuation of NAC treatment. Sal, saline; VEH, vehicle.
Fig. 2.
Experiment 2: 100 mg/kg NAC during extinction. A, time course for experiment 2. NAC (100 mg/kg) treatment occurred during daily extinction sessions and on cue and cue + cocaine-primed reinstatement testing. Rats were retested after 2 weeks of abstinence without NAC. B, active lever presses during the last 3 days of self-administration. C, lever responding over the 12 days of extinction (main effect of group, p < 0.05). D, reinstatement of cocaine seeking by cues or cues + cocaine. *, reduced responding by NAC treatment relative to saline treatment; p < 0.05. E, cocaine seeking at 2 weeks after discontinuation of NAC treatment. *, reduced responding by NAC treatment relative to saline treatment; p < 0.05. Sal, saline; VEH, vehicle.
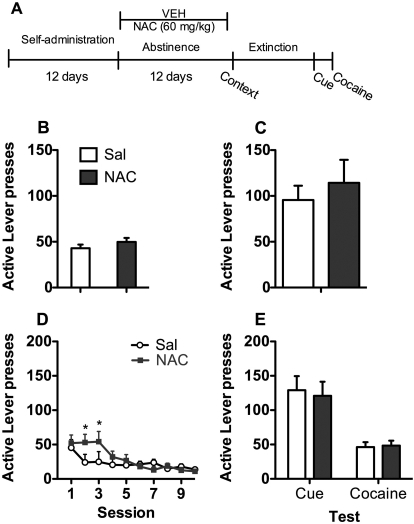
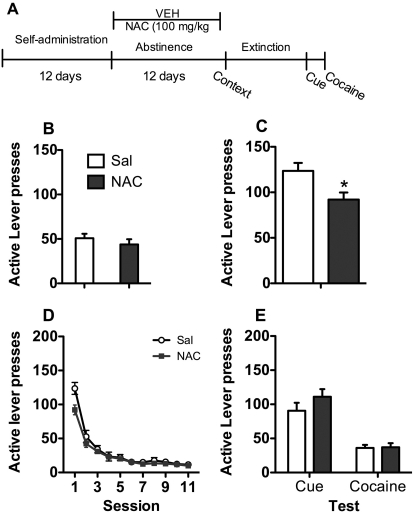
Experiments 3 and 4: Effects of Chronic NAC (60 and 100 mg/kg) during Abstinence on Subsequent Relapse and Reinstatement.
Figures 3A and 4A depict the time line for experiments 3 and 4, respectively. Rats underwent 12 days of cocaine self-administration, followed by 2 weeks of abstinence. During abstinence, rats were transported, handled, and injected daily with saline, 60 mg/kg NAC (experiment 3), or 100 mg/kg NAC (experiment 4), but were not placed back into the self-administration environment. After 2 weeks, rats were tested for relapse to cocaine seeking by placement into the chambers. Responding was subsequently extinguished in daily trials, and rats were tested for cue and cocaine-primed reinstatement.
Fig. 3.
Experiment 3: 60 mg/kg NAC during abstinence. A, time course for experiment 3. NAC (60 mg/kg) treatment occurred daily during abstinence but not on tests or during extinction. Rats were tested for context relapse and went through subsequent extinction and reinstatement testing. B, active lever presses during the last 3 days of self-administration. C, relapse to the cocaine context after abstinence. D, lever responding over the 11 days of extinction. *, increased responding by NAC relative to saline. E, reinstatement of cocaine seeking by cues or cocaine. Sal, saline; VEH, vehicle.
Fig. 4.
Experiment 4: 100 mg/kg NAC during abstinence. A, time course for experiment 4. NAC (100 mg/kg) treatment occurred daily during abstinence, but not on tests or during extinction. Rats were tested for context relapse and went through subsequent extinction and reinstatement testing. B, active lever presses during the last 3 days of self-administration. C, relapse to the cocaine context after abstinence. *, reduced responding by NAC treatment relative to saline treatment; p < 0.05. D, lever responding over the 11 days of extinction. E, reinstatement of cocaine seeking by cues or cocaine. Sal, saline; VEH, vehicle.
Experiment 5: Effects of Chronic NAC (100 mg/kg) during Abstinence on Cue and Cue + Cocaine-Primed Relapse.
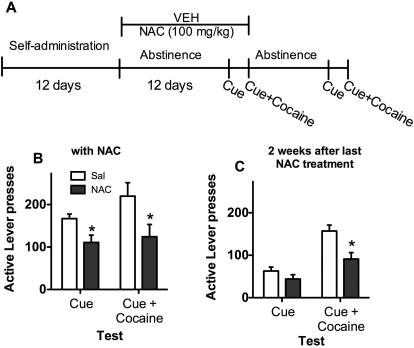
Figure 5A depicts the time line for experiment 5. Rats experienced 12 days of cocaine self-administration followed by 2 weeks of abstinence. During abstinence, rats were transported, handled, and injected daily with saline or 100 mg/kg NAC, but were not placed back into the self-administration environment. After 12 to 14 days, rats were tested for cue and cue + cocaine-primed relapse while receiving NAC. Two weeks after NAC was discontinued, rats were again tested for cue and cue + cocaine-primed relapse.
Fig. 5.
Experiment 5: 100 mg/kg NAC during abstinence. A, time course for experiment 5. NAC (100 mg/kg) treatment occurred daily during abstinence and on cue and cue + cocaine-primed reinstatement testing. Rats were retested after 2 weeks of abstinence without NAC. B, relapse of cocaine seeking by cues or cues + cocaine. *, reduced responding by NAC treatment relative to saline treatment; p < 0.05. C, cocaine seeking at 2 weeks after discontinuation of NAC treatment. *, reduced responding by NAC treatment relative to saline treatment; p < 0.05. Sal, saline; VEH, vehicle.
Data Analysis.
The number of active and inactive lever presses, as well as cocaine intake served as the dependent measures. To determine that groups did not differ before NAC treatment, data from the last 3 days of self-administration were averaged for each variable and analyzed with t tests. The effects of NAC on extinction responding used a mixed analysis of variance, with drug as the between-subjects factor and session as the within-subjects factor. Based on a priori assumptions, planned comparisons (t tests, one-tail) were limited to assessment of reductions in reinstatement responding produced by NAC treatment relative to saline-treated rats. Data points that were two S.D. from the mean were excluded (Hill and Lewicki, 2007). Based on very low responses in all groups, data for inactive lever presses are not presented. All data are presented as mean ± S.E.M., and the α was set at p < 0.05.
Results
Experiments 1 and 2: NAC Attenuates Reinstatement after Extinction
Experiment 1: NAC (60 mg/kg) during Extinction.
Rats given saline or 60 mg/kg NAC during extinction did not differ on cocaine intake (saline = 17.96 ± 1.47; NAC = 18.60 ± 0.87) or lever responding during SA (Fig. 1B). During extinction (Fig. 1C), lever pressing decreased across the 12 sessions for both groups on the active lever (session main effect, F11,154 = 32.96, p < 0.05). However, saline- and NAC-treated rats did not differ, nor did treatment interact with session. Data from the first cue-induced and cue + cocaine-primed reinstatement tests are shown in Fig. 1D, while rats were still receiving NAC. NAC-treated rats had lower responding relative to saline-treated rats on the cue test (t14 = 2.7, p < 0.05) and marginally lower responding on the cue + cocaine test (t14 = 1.7, p = 0.06). During the second series of reinstatement tests at 2 weeks after saline or NAC injections (Fig. 1E), the groups did not differ.
Experiment 2: NAC (100 mg/kg) during Extinction.
Rats given saline or 100 mg/kg NAC during extinction did not differ on cocaine intake (saline = 18.85 ± 1.25 and NAC = 18.08 ± 0.90) or lever responding during SA (Fig. 2B). During extinction (Fig. 2C), lever pressing decreased across the 12 sessions for both groups on the active lever (session main effect, F11,209 = 27.08, p < 0.05). Rats treated with 100 mg/kg NAC pressed the lever significantly less than saline-treated rats throughout the extinction period (group main effect, F1,19 = 7.57, p < 0.05). However, group did not interact with session. Data from the first cue-induced reinstatement and cue + cocaine-primed reinstatement tests are represented in Fig. 2D, while rats were still receiving NAC. NAC-treated rats had lower responding relative to saline-treated rats on the cue test (t19 = 1.8, p < 0.05) and cue + cocaine test (t19 = 1.7, p < 0.05). Likewise, during the second series of reinstatement tests 2 weeks after saline or NAC injections (Fig. 2E), NAC-treated rats had lower responding relative to saline-treated rats on the cue test (t19 = 2.6, p < 0.05) and cue + cocaine test (t19 = 2.2, p < 0.05).
Experiments 3 and 4: NAC (60 and 100 mg/kg) during Abstinence Has Dose-Dependent Effects on Context-Induced Relapse
Experiment 3: NAC (60 mg/kg) during Abstinence.
Rats given saline or 60 mg/kg NAC during abstinence did not differ on cocaine intake (saline = 18.37 ± 1.2 and NAC = 20.04 ± 1.6) or lever responding during SA (Fig. 3B). On the relapse test (Fig. 3C), NAC and saline rats also showed no significant differences. During extinction (Fig. 3D), lever pressing decreased across the 10 sessions for both groups on the active lever (session main effect, F9,126 = 9.39, p < 0.05). NAC-treated rats maintained higher levels of responding on days 2 and 3 of extinction (session × group, F9,126 = 2.93, p < 0.05, and Tukey honestly significant difference test, p < 0.05). During the cued-induced reinstatement and cocaine-primed reinstatement tests (Fig. 3E), there were no group differences.
Experiment 4: NAC (100 mg/kg) during Abstinence.
Rats given saline or 100 mg/kg NAC during abstinence did not differ on cocaine intake (saline = 21.48 ± 1.63 and NAC = 19.63 ± 1.47) or lever responding during SA (Fig. 4B). On the context relapse test (Fig. 4C), NAC-treated rats showed lower responding relative to saline-treated rats on the test day (F1,26 = 7.8, p < 0.05). During extinction (Fig. 4D), lever pressing decreased across the 10 sessions for both groups (session main effect, F9,264 = 27.6, p < 0.05). During the cued-induced reinstatement and cocaine-primed reinstatement tests (Fig. 4E), there were no group differences.
Experiment 5: NAC (100 mg/kg) during Abstinence Attenuates Cue and Cue + Cocaine-Induced Relapse.
Rats given saline or 100 mg/kg NAC during abstinence did not differ on cocaine intake (saline = 16.83 ± 1.66 and NAC = 17.58 ± 1.69) or lever responding during SA (saline = 33.62 ± 2.54 and NAC = 37.95 ± 4.16). Data from the first cue-induced reinstatement and cue + cocaine-primed reinstatement tests are represented in Fig. 5B, while rats were still receiving NAC. NAC-treated rats showed lower responding relative to saline-treated rats on the cue test (t12 = 2.7, p < 0.05) and the cue + cocaine test (t12 = 2.3, p < 0.05). During the second series of reinstatement tests at 2 weeks after saline or NAC injections (Fig. 5C), saline- and NAC-treated rats responded the same on the cue test; however, NAC-treated rats had lower responding on the cue + cocaine test (t12 = 3.2, p < 0.05).
Discussion
In the current study, chronic NAC administration blunted cocaine seeking under multiple experimental protocols of extinction and abstinence after cocaine self-administration. Specifically, NAC decreased responding during cue-induced and cue + cocaine-primed reinstatement tests after extinction, and context, cue, and cue + cocaine relapse tests after abstinence. The reduction of cocaine seeking by NAC persisted well after treatment was discontinued, particularly when the high dose (100 mg/kg) was administered during the extinction trials. These results support and extend earlier findings in animal models suggesting that chronic NAC may act as an effective antirelapse medication (Madayag et al., 2007; Zhou and Kalivas, 2008; Amen et al., 2011).
Evidence suggests that NAC reduces cocaine seeking by restoration of corticostriatal glutamate homeostasis and the reversal of cocaine-induced synaptic potentiation (Baker et al., 2003a; Kalivas, 2009; Moussawi et al., 2011). Thus, it may be expected that NAC should reduce cocaine seeking, regardless of experience during the withdrawal phase (i.e., extinction or no extinction). The current data generally support this contention in several ways. First, chronic NAC (60 and 100 mg/kg) during extinction reduced both cue-induced and cue + cocaine-primed reinstatement, and the reduced responding was still maintained at 2 weeks after final NAC treatment for the high-dose group. Second, specific for the high dose, NAC significantly attenuated cocaine seeking on a context-relapse test. It is noteworthy that in this condition rats never received NAC in the self-administration chamber. Thus, NAC never influenced the learning of a new contingency between lever responding and a lack of reinforcement. The mere exposure to chronic NAC treatment was sufficient to reduce relapse behavior when rats were placed drug-free into the cocaine-associated environment. Finally, chronic NAC (100 mg/kg) administered during abstinence blunted both cue-induced and cue + cocaine relapse (experiment 5), with persistently reduced cocaine seeking during the cue + cocaine test at 2 weeks after final NAC treatment.
It is noteworthy that the ability of NAC to blunt cocaine seeking under multiple experimental conditions is unlikely to result from nonspecific motor alterations. Inactive lever responding remained low and relatively unchanged across all saline- and NAC-treated rats. In our laboratory, we have confirmed that NAC (100 mg/kg) has no impact on activity in a novel environment in cocaine-exposed rats with a previous history of NAC treatment relative to saline-treated rats (unpublished data). Furthermore, NAC has been consistently found to produce neither increases nor decreases in locomotor activity (Ferreira et al., 2008; Prakash and Kumar, 2009). Thus, nonspecific changes in activity are unlikely to contribute to the ameliorative effects of chronic NAC treatment on motivated cocaine seeking.
The NAC-induced reductions in cocaine seeking under conditions of abstinence and extinction are modest relative to some direct pharmacological challenges. For example, systemic administration of metabotropic glutamate receptor (mGluR) receptor antagonists can completely block conditioned cue-induced and cocaine-primed reinstatement (Peters and Kalivas, 2006; Kumaresan et al., 2009). These drugs are administered via one-time injections and act directly on the specific receptor subtypes that mediate reinstatement. Furthermore, intracranial infusions of mGluR antagonists into the nucleus accumbens can completely block cocaine-primed reinstatement (Peters and Kalivas, 2006; Kumaresan et al., 2009). Mechanistically, these studies differ from the current results, because chronic NAC treatment provides an enduring increase in overall glutamatergic tone, resulting in restoration of glutamate homeostasis, rather than directly blocking receptor activity. In addition, clinical studies cannot take advantage of intracranial infusions for drug administration protocols. As such, the chronic dosing paradigm we used here is more akin to clinical treatment protocols, thus enhancing the translational relevance of our findings.
Although NAC effectively reduced reinstatement and relapse after extinction and abstinence, our findings suggest that repeated NAC exerts more profound effects in reducing relapse when NAC modifies the neuroadaptations resulting from explicit extinction training. For example, NAC at 60 mg/kg was effective in combination with extinction training, but not when the contextual associations remained intact after abstinence. In addition, only two treatment circumstances provided enduring protection from relapse: 100 mg/kg NAC administered during extinction (experiment 2) and 100 mg/kg NAC given during abstinence and reinstatement (experiment 5). In both experiments, animals had systemically available NAC while in the self-administration chamber. In experiment 1, NAC was biologically available during the extinction session, and in experiment 5, NAC was present during both the cue and cue + cocaine reinstatement tests (see Fig. 5A). Thus, for abstinent rats in experiment 5, these test sessions would have served as extinction sessions because of the lack of primary reinforcement with cocaine. As such, enduring reductions in cocaine seeking were evident in both conditions when concurrent NAC treatment occurred in combination with extinction training.
Reduced cocaine seeking after discontinuation of pharmacotherapy is a desirable milestone for the successful development of effective antirelapse medications. Although it is difficult to directly extrapolate these findings to the treatment of cocaine-dependent patients, our data suggest that NAC may reduce the probability of relapse in these patients, particularly when combined with extinction. It is conceivable that NAC improves extinction learning of the new contingency between lever pressing and lack of drug reinforcement. Support for this possibility comes from studies on the cognitive-enhancing effects of NAC in rodents. Acute NAC improved memory retention in the Morris water maze in rats exposed to chronic aluminum (Prakash and Kumar, 2009) and improved cognition in aged mice on a T-maze footshock avoidance procedure (Farr et al., 2003; Prakash and Kumar, 2009). Acute NAC also improved memory consolidation in a conditioned place-preference procedure (Achat-Mendes et al., 2007). Future comparisons are warranted to test whether NAC enhances extinction learning and differentially modifies the neural adaptations that occur with or without explicit extinction. For example, extinction-induced up-regulation of Homer 1b/c enhanced internalization and sequestering of mGluR5, a mechanism that probably accounted for a loss of mGluR5-dependent long-term depression in extinguished, but not abstinent, rats (Knackstedt et al., 2010). Cystine-glutamate exchange is down-regulated after chronic cocaine, thereby reducing glutamatergic tone on mGluR5 in the accumbens (Madayag et al., 2007; Moussawi et al., 2009). Restoration of this tone with NAC during extinction training may restore the capacity to induce long-term depression in the nucleus accumbens core and potentiates cocaine-induced reinstatement in the presence of an mGluR5-positive allosteric modulator (Moussawi et al., 2009). Taken together, extinction-specific changes combined with restoration of glutamatergic tone by NAC administration during extinction could enhance protection from relapse.
In summary, the current data suggest that repeated NAC treatment results in enduring attenuation of cocaine seeking under different withdrawal regimens of abstinence and extinction, but that the persisting antirelapse features of NAC are enhanced in conjunction with explicit extinction training. Extinction techniques are integral to intervention approaches, such as guided imagery and cue-exposure therapy (Conklin, 2006; Lee et al., 2007). However, these techniques to date have shown only moderate clinical success (Childress et al., 1993; Marissen et al., 2007). Newer applications of cue exposure show promise in enhancing cue-exposure therapies, including the use of computer-based virtual environments (Lee et al., 2007; Culbertson et al., 2010). In addition, medication-facilitated cue-exposure therapies have begun to emerge in several fields of psychiatry, with the most notable success seen in the area of phobia treatment (Ressler et al., 2004; Davis et al., 2006). Such approaches have also been suggested for addiction treatment (Otto et al., 2004), and NAC may offer a useful approach for restoration of glutamatergic tone during extinction-based therapy in the treatment of cocaine and other drug addictions.
Acknowledgments
We thank Sarah Deptula, Amanda King, and John Yang for technical assistance.
This research was supported by the National Institutes of Health National Institute on Drug Abuse [Grant P50-DA015369] (to P.W.K., R.E.S.); [Grant T32-DA007288] (to C.M.R.); and [Grant F32-DA029344] (to C.M.R.).
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.111.179317.
- SA
- self-administration
- NAC
- N-acetylcysteine
- mGluR
- metabotropic glutamate receptor.
Authorship Contributions
Participated in research design: Reichel, Moussawi, Kalivas, and See.
Conducted experiments: Reichel, Moussawi, and Do.
Performed data analysis: Reichel and See.
Wrote or contributed to the writing of the manuscript: Reichel, Kalivas, and See.
References
- Achat-Mendes C, Anderson KL, Itzhak Y. (2007) Impairment in consolidation of learned place preference following dopaminergic neurotoxicity in mice is ameliorated by N-acetylcysteine but not D1 and D2 dopamine receptor agonists. Neuropsychopharmacology 32:531–541 [DOI] [PubMed] [Google Scholar]
- Amen SL, Piacentine LB, Ahmad ME, Li SJ, Mantsch JR, Risinger RC, Baker DA. (2011) Repeated N-acetyl cysteine reduces cocaine seeking in rodents and craving in cocaine-dependent humans. Neuropsychopharmacology 36:871–878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker DA, McFarland K, Lake RW, Shen H, Tang XC, Toda S, Kalivas PW. (2003a) Neuroadaptations in cystine-glutamate exchange underlie cocaine relapse. Nat Neurosci 6:743–749 [DOI] [PubMed] [Google Scholar]
- Baker DA, McFarland K, Lake RW, Shen H, Toda S, Kalivas PW. (2003b) N-acetyl cysteine-induced blockade of cocaine-induced reinstatement. Ann NY Acad Sci 1003:349–351 [DOI] [PubMed] [Google Scholar]
- Baker DA, Xi ZX, Shen H, Swanson CJ, Kalivas PW. (2002) The origin and neuronal function of in vivo nonsynaptic glutamate. J Neurosci 22:9134–9141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouton ME. (2002) Context, ambiguity, and unlearning: sources of relapse after behavioral extinction. Biol Psychiatry 52:976–986 [DOI] [PubMed] [Google Scholar]
- Childress AR, Hole AV, Ehrman RN, Robbins SJ, McLellan AT, O'Brien CP. (1993) Cue reactivity and cue reactivity interventions in drug dependence. NIDA Res Monogr 137:73–95 [PubMed] [Google Scholar]
- Conklin CA. (2006) Environments as cues to smoke: implications for human extinction-based research and treatment. Exp Clin Psychopharmacol 14:12–19 [DOI] [PubMed] [Google Scholar]
- Culbertson C, Nicolas S, Zaharovits I, London ED, De La Garza R, 2nd, Brody AL, Newton TF. (2010) Methamphetamine craving induced in an online virtual reality environment. Pharmacol Biochem Behav 96:454–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M, Ressler K, Rothbaum BO, Richardson R. (2006) Effects of d-cycloserine on extinction: translation from preclinical to clinical work. Biol Psychiatry 60:369–375 [DOI] [PubMed] [Google Scholar]
- Farr SA, Poon HF, Dogrukol-Ak D, Drake J, Banks WA, Eyerman E, Butterfield DA, Morley JE. (2003) The antioxidants α-lipoic acid and N-acetylcysteine reverse memory impairment and brain oxidative stress in aged SAMP8 mice. J Neurochem 84:1173–1183 [DOI] [PubMed] [Google Scholar]
- Ferreira FR, Biojone C, Joca SR, Guimarães FS. (2008) Antidepressant-like effects of N-acetyl-l-cysteine in rats. Behav Pharmacol 19:747–750 [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Branham RK, See RE. (2006) Different neural substrates mediate cocaine seeking after abstinence versus extinction training: a critical role for the dorsolateral caudate-putamen. J Neurosci 26:3584–3588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill T, Lewicki P. (2007) Statistics Methods and Application. StatSoft, Tulsa, OK [Google Scholar]
- Kalivas PW. (2009) The glutamate homeostasis hypothesis of addiction. Nat Rev Neurosci 10:561–572 [DOI] [PubMed] [Google Scholar]
- Kau KS, Madayag A, Mantsch JR, Grier MD, Abdulhameed O, Baker DA. (2008) Blunted cystine-glutamate antiporter function in the nucleus accumbens promotes cocaine-induced drug seeking. Neuroscience 155:530–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knackstedt LA, Moussawi K, Lalumiere R, Schwendt M, Klugmann M, Kalivas PW. (2010) Extinction training after cocaine self-administration induces glutamatergic plasticity to inhibit cocaine seeking. J Neurosci 30:7984–7992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumaresan V, Yuan M, Yee J, Famous KR, Anderson SM, Schmidt HD, Pierce RC. (2009) Metabotropic glutamate receptor 5 (mGluR5) antagonists attenuate cocaine priming- and cue-induced reinstatement of cocaine seeking. Behav Brain Res 202:238–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaRowe SD, Myrick H, Hedden S, Mardikian P, Saladin M, McRae A, Brady K, Kalivas PW, Malcolm R. (2007) Is cocaine desire reduced by N-acetylcysteine? Am J Psychiatry 164:1115–1117 [DOI] [PubMed] [Google Scholar]
- Lee JH, Kwon H, Choi J, Yang BH. (2007) Cue-exposure therapy to decrease alcohol craving in virtual environment. Cyberpsychol Behav 10:617–623 [DOI] [PubMed] [Google Scholar]
- Madayag A, Lobner D, Kau KS, Mantsch JR, Abdulhameed O, Hearing M, Grier MD, Baker DA. (2007) Repeated N-acetylcysteine administration alters plasticity-dependent effects of cocaine. J Neurosci 27:13968–13976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mardikian PN, LaRowe SD, Hedden S, Kalivas PW, Malcolm RJ. (2007) An open-label trial of N-acetylcysteine for the treatment of cocaine dependence: a pilot study. Prog Neuropsychopharmacol Biol Psychiatry 31:389–394 [DOI] [PubMed] [Google Scholar]
- Marissen MA, Franken IH, Blanken P, van den Brink W, Hendriks VM. (2007) Cue exposure therapy for the treatment of opiate addiction: results of a randomized controlled clinical trial. Psychother Psychosom 76:97–105 [DOI] [PubMed] [Google Scholar]
- McFarland K, Lapish CC, Kalivas PW. (2003) Prefrontal glutamate release into the core of the nucleus accumbens mediates cocaine-induced reinstatement of drug-seeking behavior. J Neurosci 23:3531–3537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moussawi K, Pacchioni A, Moran M, Olive MF, Gass JT, Lavin A, Kalivas PW. (2009) N-acetylcysteine reverses cocaine-induced metaplasticity. Nat Neurosci 12:182–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moussawi K, Zhou W, Shen H, Reichel CM, See RE, Carr DB, Kalivas PW. (2011) Reversing cocaine-induced synaptic potentiation provides enduring protection from relapse. Proc Natl Acad Sci USA 108:385–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neisewander JL, Baker DA, Fuchs RA, Tran-Nguyen LT, Palmer A, Marshall JF. (2000) Fos protein expression and cocaine-seeking behavior in rats after exposure to a cocaine self-administration environment. J Neurosci 20:798–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto MW, Safren SA, Pollack MH. (2004) Internal cue exposure and the treatment of substance use disorders: lessons from the treatment of panic disorder. J Anxiety Disord 18:69–87 [DOI] [PubMed] [Google Scholar]
- Peters J, Kalivas PW. (2006) The group II metabotropic glutamate receptor agonist, LY379268, inhibits both cocaine- and food-seeking behavior in rats. Psychopharmacology 186:143–149 [DOI] [PubMed] [Google Scholar]
- Prakash A, Kumar A. (2009) Effect of N-acetyl cysteine against aluminium-induced cognitive dysfunction and oxidative damage in rats. Basic Clin Pharmacol Toxicol 105:98–104 [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Mueller D. (2008) Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology 33:56–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichel CM, Bevins RA. (2009) Forced abstinence model of relapse to study pharmacological treatments of substance use disorder. Curr Drug Abuse Rev 2:184–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ressler KJ, Rothbaum BO, Tannenbaum L, Anderson P, Graap K, Zimand E, Hodges L, Davis M. (2004) Cognitive enhancers as adjuncts to psychotherapy: use of d-cycloserine in phobic individuals to facilitate extinction of fear. Arch Gen Psychiatry 61:1136–1144 [DOI] [PubMed] [Google Scholar]
- Schmidt EF, Sutton MA, Schad CA, Karanian DA, Brodkin ES, Self DW. (2001) Extinction training regulates tyrosine hydroxylase during withdrawal from cocaine self-administration. J Neurosci 21:RC137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Self DW. (2004) Regulation of drug-taking and -seeking behaviors by neuroadaptations in the mesolimbic dopamine system. Neuropharmacology 47:242–255 [DOI] [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Lu L, De Wit H, Stewart J. (2003) The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology 168:3–20 [DOI] [PubMed] [Google Scholar]
- Sutton MA, Schmidt EF, Choi KH, Schad CA, Whisler K, Simmons D, Karanian DA, Monteggia LM, Neve RL, Self DW. (2003) Extinction-induced up-regulation in AMPA receptors reduces cocaine-seeking behaviour. Nature 421:70–75 [DOI] [PubMed] [Google Scholar]
- Yahyavi-Firouz-Abadi N, See RE. (2009) Anti-relapse medications: preclinical models for drug addiction treatment. Pharmacol Ther 124:235–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavala AR, Biswas S, Harlan RE, Neisewander JL. (2007) Fos and glutamate AMPA receptor subunit coexpression associated with cue-elicited cocaine-seeking behavior in abstinent rats. Neuroscience 145:438–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Kalivas PW. (2008) N-acetylcysteine reduces extinction responding and induces enduring reductions in cue- and heroin-induced drug-seeking. Biol Psychiatry 63:338–340 [DOI] [PMC free article] [PubMed] [Google Scholar]