Abstract
Pharmacological magnetic resonance imaging (phMRI) is increasingly being used in drug discovery and development to speed the translation from the laboratory to the clinic. The two primary methods in phMRI include blood-oxygen-level-dependent (BOLD) contrast and arterial spin-labeled (ASL) perfusion MRI. BOLD contrast has been widely applied in existing phMRI studies. However, because of the lack of absolute quantification and poor reproducibility over time scales longer than hours or across scanning sessions, BOLD fMRI may not be suitable to track oral and other long-term drug effects on baseline brain function. As an alternative method, ASL provides noninvasive, absolute quantification of cerebral blood flow both at rest and during task activation. ASL perfusion measurements have been shown to be highly reproducible over minutes and hours to days and weeks. These two characteristics make ASL an ideal tool for phMRI for studying both intravenous and oral drug action as well as understanding drug effects on baseline brain function and brain activation to cognitive or sensory processing. When ASL is combined with BOLD fMRI, drug-induced changes in cerebral metabolic rate of oxygen may also be inferred. Representative phMRI studies using ASL perfusion MRI on caffeine, remifentanil, and metoclopramide (dopamine antagonist) are reviewed here, with an emphasis on the methodologies used to control for potentially confounding vascular and systemic effects. Both the potentials and limitations of using ASL as an imaging marker of drug action are discussed.
Introduction
Imaging methods are increasingly being used in drug discovery and development to speed the translation from the laboratory to the clinic (Wong et al., 2009). Imaging biomarkers of drug effects can be applied in both preclinical models and human patients and have the potential to demonstrate a proof-of-concept of drug actions in small human cohorts. Imaging is particularly valuable for the evaluation of drug candidates for central nervous system disorders because of the difficulties in quantifying the human brain function in vivo. The use of radioisotope-labeled ligands with positron emission tomography (PET) remains central to the assessment of drug penetration through the blood-brain barrier as well as the engagement of specific receptor targets. However, pharmacological MRI (phMRI), the use of functional MRI to study drug effects on baseline brain function as well as drug modulation of brain activation to cognitive or sensory processing, has been gaining popularity because of its noninvasiveness, lower cost, and relatively wide availability (Wise and Tracey 2006). Although phMRI may never rival PET for demonstrating specificity at a molecular level, its complementary capabilities for assessing brain function may provide greater sensitivity than traditional clinical endpoints for early-phase studies.
Blood-oxygen-level-dependent (BOLD) contrast is the most widely used approach for phMRI because of its technical simplicity and relatively high sensitivity (Iannetti and Wise 2007). The effects of a drug on baseline brain function in the absence of other modulating factors have been demonstrated by BOLD fMRI using a variety of pharmacological agents including cocaine, dopamine agonists, nicotine, and opioidergic and serotonergic agents (Breiter et al., 1997; Chen et al., 1997; Stein et al., 1998; McKie et al., 2005; Becerra et al., 2006; Leppä et al., 2006; Upadhyay et al., 2010). However, because of the noise properties of BOLD fMRI (Aguirre et al., 2002; Wang et al., 2003) and the lack of absolute quantification, BOLD phMRI is in general poorly suited to detecting the effects of oral drugs or drug responses over days or weeks. It is worth noting that resting BOLD fMRI measures (e.g., functional connectivity and independent component analysis) have been applied to characterize drug effects on baseline brain function (Li et al., 2009; Upadhyay et al., 2010).
Instead of directly studying drug-induced changes in baseline brain function, more recent phMRI based on BOLD contrast has focused on pharmacological modulation of the brain response to a cognitive task or sensory stimulation. Drug effects can then be inferred from the modulation of the benchmark BOLD activation associated with task performance or sensorimotor stimulation. Modulation of the BOLD fMRI responses to a painful stimulus has been among the most widely studied applications of BOLD phMRI (Wise et al., 2002; Wise and Tracey, 2006), although a range of sensorimotor or cognitive task effects in response to pharmacological modulation have also been examined (Jokeit et al., 2001; Laurienti et al., 2002; Anderson et al., 2011). A limitation of this approach is that it is difficult to determine whether the modulational effects of the drug on BOLD fMRI are attributable to changes in baseline brain function, the activation state, or both. Conversely, a lack of modulational effects on BOLD fMRI does not exclude the possibility that the drug equally affects the baseline and activation states (i.e., a shift of approximately equal magnitude). These theoretical arguments may be further complicated by the fact that BOLD fMRI reflects a complex interplay between cerebral blood flow (CBF), cerebral blood volume, and oxygen consumption. An observed drug effect on BOLD fMRI may thus be the result of changes in one or more of the above parameters at baseline either regionally or systemically (e.g., blood pressure or vascular tone). There have been several studies demonstrating an interaction between basal physiologic and metabolic states and task-induced BOLD activation (Cohen et al., 2002; Hyder et al., 2002).
Arterial Spin-Labeled Perfusion MRI: Advantages for phMRI
Arterial spin-labeled (ASL) perfusion MRI provides an alternative and complementary tool to BOLD fMRI for phMRI (Detre et al., 2009). Methodologically, ASL is similar to 15O-water PET. Instead of injecting the subject with 15O-labeled water, ASL uses radiofrequency pulses to magnetically label the water molecules in the subject's own arterial blood, usually at the base of the brain. The magnetically labeled blood water is then used as an endogenous tracer for the measurement of CBF, which has a half-life (determined by blood T1) on the order of 1 to 2 s. The effects of ASL are measured by comparing images acquired with active or control labeling, and this signal difference can be quantified using a model that requires a few additional measured or assumed parameters (Buxton et al., 1998a). Several methodological approaches exist for ASL and include continuous ASL (CASL), pulsed ASL (PASL), and pseudo-continuous ASL (pCASL), which differ in their labeling efficiency, ease of implementation, and compatibility with other scanner hardware (Detre et al., 2009).
Although ASL MRI measurements of CBF have been shown to correlate closely with PET CBF both at rest (Ye et al., 2000) and with task activation (Feng et al., 2004), there are also some significant differences between 15O-water PET and ASL perfusion MRI. First, there is large background tissue signal in ASL, which is eliminated through pairwise acquisition and subtraction of label and control signals. This signal-processing step produces beneficial noise characteristics for phMRI studies, as shown below. Second, the arterial input function can be explicitly defined by the duration and amplitude of the labeling radiofrequency pulses in ASL, whereas in general an arterial line has to be inserted to sample arterial input function in PET studies. This factor greatly facilitates the absolute quantification of CBF using ASL. Finally, ASL is entirely noninvasive and can be repeated as often as required to track the dynamic course of pharmacological effects over time.
Compared with BOLD fMRI, ASL offers several appealing features and may be ideally suited for phMRI studies (Detre and Wang, 2002; Aguirre et al., 2005). Because of the pairwise subtraction of label and control acquisitions, which acts as a high-pass filter of the temporal signal, the frequency spectrum of ASL time series is flat (or “white noise”) (Aguirre et al., 2002; Wang et al., 2003). As opposed to BOLD fMRI, which has universally elevated power at low frequency, ASL is suitable for studying slow changes in brain function, including the direct pharmacological effects of drugs that may take effect over hours or days, obviating the need for measuring drug effects indirectly by their modulation of a stimulus or task response. Parenchymal perfusion or CBF (in unit of ml/100 g/min) is also a well characterized physiological parameter and may be considered a more direct marker of neuronal activity compared with the BOLD contrast that reflects “lumped” changes of CBF, cerebral blood volume, and oxygen metabolism (Buxton et al., 1998b). For task effects, ASL offers quantitative CBF measurements both at rest and during task activation, which is critical for separating drug effects on baseline brain function and task-induced activation. An example can be found in figure 5 of Liau et al. (2008). Although both ASL and BOLD fMRI can demonstrate relative signal changes to visual activation before and after caffeine infusion, quantitative baseline CBF offered by ASL is valuable for calculating CBF changes in physiological units. Finally, ASL perfusion contrast can be imaged using pulse sequences that are resistant to magnetic field inhomogeneity (susceptibility) effects (Wang et al., 2004), offering improved visualization of orbitofrontal, inferior temporal, and limbic regions that are linked to major neurotransmitter systems.
These potential advantages of ASL for phMRI studies have already been recognized by the scientific community, and several review articles have noted the value of ASL for assessing drug effects on baseline brain function (Wise and Tracey, 2006). At least one early phMRI study has included both ASL and BOLD fMRI before and after cocaine infusion (Gollub et al., 1998). However, the limited availability, signal-to-noise ratio, and image coverage of ASL techniques have remained major obstacles for application studies. During the past decade, technical advances have greatly improved sensitivity and coverage of ASL techniques. For instance, ASL methods derive a dual benefit from high magnetic field strengths (Wang et al., 2002). In addition to increased sensitivity at high field, T1 increases, allowing more label to accumulate in brain tissue. ASL methods also benefit from parallel imaging and array receiver coils (Wang et al., 2005b). The most recent approach to ASL, pCASL, combines the advantages of PASL, including technical simplicity and compatibility with array receivers and CASL, including a longer tagging bolus and consequently higher signal-to-noise ratio (Wu et al., 2007; Dai et al., 2008). pCASL can be further combined with 3D acquisitions such as GRASE (a hybrid of gradient and spin echo) readout that allows the use of background suppression (BS) of static tissue signals to improve the temporal stability (Fernández-Seara et al., 2007, 2008). As a result, whole-brain perfusion images with isotropic 4 × 4 × 4-mm3 spatial resolution can be achieved within just a few minutes, free of susceptibility artifacts. Figure 1A displays a sample image acquired using pCASL with 3D BS GRASE, showing excellent whole-brain coverage and visualization of orbitofrontal cortex. Paralleling these technical advances, recent progresses in commercialization also facilitate the application of ASL in translational neurosciences.
Fig. 1.
A, representative whole-brain perfusion images acquired using pCASL with 3D GRASE readout. B, scatter plots showing test-retest results of pCASL perfusion measurements acquired 2 to 4 weeks apart and comparison of pCASL perfusion versus global blood flow measurements using PC-MRI in healthy children aged 7 to 17. A comparison of pCASL versus conventional PASL and pCASL can be found in Wu et al. (2007).
Two of the key characteristics determining the viability of a translational imaging tool are its reliability (precision) and accuracy. The timescale of drug action ranges from seconds/minutes (e.g., intravenous injection) and hours (e.g., oral administration) to days and weeks (e.g., treatment cycle). Various test-retest studies have been carried out using ASL across minutes, hours, days, and weeks. The within-subject coefficient of variation is generally on the order of 10% for global CBF and 10 to 15% for regional measures (Floyd et al., 2003; Parkes and Tofts, 2004; Chen et al., 2010; Jain et al., 2010). The variation between repeated ASL measurements increases with lengthened time interval, suggesting increased effects of fluctuations in subjects' physiological states at long scan intervals. In terms of accuracy, global CBF measurements using ASL have been compared with phase-contrast (PC)-MRI, which measures the flow velocity and cross-sectional area of the carotid and vertebral arteries (Chen and Pike, 2010, Jain et al., 2010). Because the product of flow velocity and cross-sectional area of the carotid and vertebral arteries provides total blood flow to the brain, PC-MRI offers a practical and accurate approach [intraclass correlation coefficient (ICC) = 0.98 by Jain et al., 2010] to calibrate the labeling efficiency of ASL between scans, which may be affected by differences in subjects' head position, shimming conditions, and the efficiency of background suppression (Garcia et al., 2005). Alternatively, a phase cycling approach may be applied to calibrate the labeling efficiency of pCASL (Jung et al., 2010). Regional CBF measurements using ASL have also been validated with 15O-water PET both at rest (Ye et al., 2000) and during task activation (Feng et al., 2004). Figure 1, B and C, shows the scatter plot of test-retest pCASL CBF measurements (2–4 weeks apart) and pCASL versus PC-MRI global CBF measurements, respectively. Both excellent precision (ICC = 0.62; within-subject coefficient of variation = 7.5%) and accuracy (ICC = 0.77) are achieved. These reference data also provide the benchmark for designing ASL-based phMRI studies. For instance, detecting a 15% change in CBF with 90% power, given 15% variation between repeated measurements, requires approximately 20 subjects.
Arterial Spin Labeling Perfusion MRI: Challenges for phMRI
Although PET studies may be sensitive to the distribution of radiolabeled drug ligands or the displacement of receptor-specific ligands, phMRI based on ASL measures changes in regional brain function indirectly through the coupling between neuronal activity and blood flow, also termed functional hyperemia. Mounting evidence suggests that phMRI can successfully detect regional changes in brain function in response to pharmacological challenge, reflecting changes in regional neural function. The physiological basis for neurovascular coupling remains incompletely understood, but it is known to involve coordinated activities of the neuron, astrocyte, and the microvasculature (Iadecola and Nedergaard, 2007; Jakovcevic and Harder, 2007). Synaptic activity triggers an increase in the intracellular calcium concentration of adjacent astrocytes, stimulating the release of ATP and glutamate. Astrocytic Ca2+ elevations can lead to secretion of vasodilatory substances from perivascular endfeet, such as epoxyeicosatrienoic acid, adenosine, nitric oxide, and cyclooxygenase-2 metabolites, resulting in increased local blood flow.
For some phMRI studies, pharmacological agents may directly alter CBF or the mediators of neurovascular coupling. For instance, both caffeine (an adenosine antagonist) and indomethacin (an inhibitor of cyclooxygenase) cause vasoconstriction and reduce baseline CBF. Estrogen increases astrocytic Ca2+ levels, which raises the possibility that both BOLD and ASL signals may fluctuate across phases of the menstrual cycle in female subjects (Iadecola and Nedergaard, 2007). Data suggest that a polymorphism of cyclooxygenase-2 may affect the magnitude of hemodynamic responses (Hahn et al., 2010). Drugs may also cause systemic cardiovascular effects, which in turn may affect cerebral circulation. For example, many neurotransmitters, including dopamine, serotonin, and norepinephrine, have receptors distributed within the cardiovascular system. However, systemic effects on CBF are mitigated by cerebrovascular autoregulation and would be expected to produce global rather than focal changes, although optimal study designs would control for such effects by monitoring systemic physiology.
A promising approach to circumvent (vascular) confounding factors in phMRI is to estimate drug-induced changes in cerebral metabolic rate of oxygen (CMRO2) through concurrent or combined ASL and BOLD scanning. For instance, the calibrated BOLD approach (Davis et al., 1998) uses a vasoactive agent such as CO2 that has minimal effect on CMRO2 to “calibrate” the BOLD contrast against known CBF levels using ASL. The derived mathematical relationship between CBF, BOLD, and CMRO2 can then be applied to BOLD and CBF changes from functional data to calculate relative changes in CMRO2. Alternatively, oxygen extraction fraction (OEF) can be derived from quantitative BOLD MRI scans based on the model originally proposed by Yablonskiy and Haacke (1994). The product of OEF and CBF provides an estimate of CMRO2. To date, measurement of CMRO2 is available through PET scanning that involves three boluses of radioactive tracers (Mintun et al., 1984). It is worth noting that MRI measurements of CMRO2 rely on assumptions of minimal changes in CMRO2 during calibration (Davis et al., 1998) and neglectable effects of diffusion (Yablonskiy and Haacke, 1994), which await further validation.
The main challenge for the widespread application of ASL for phMRI is probably the relatively lower sensitivity and image coverage of existing ASL methods compared with BOLD fMRI. The latest pCASL techniques such as the one with background-suppressed 3D GRASE need to be translated into phMRI studies. Concurrent acquisitions of ASL and BOLD fMRI offer another appealing approach to assess changes in both CBF and oxygenation associated with a drug. phMRI studies have generally used a standard double-blind randomized controlled trial design used in clinical trials. But, although conventional clinical trials typically rely on a single primary outcome to determine the efficacy of treatment, the complexity of statistical analysis for phMRI studies is magnified when drug effects on multiple brain regions are being considered simultaneously or when changes over the entire brain are being considered at the voxel level. Appropriate correction for multiple comparisons must be used when determining the significance of observed effects, and wherever possible a priori regions should be specified. A promising approach is to apply multivariate analyses (that simultaneously process all brain pixels) of perfusion-based pharmacological and functional MRI data, often in a data-driven fashion (Wang et al., 2007). The existence of a potential hierarchical brain response to drugs, from regional CBF changes to neurotransmitter system to whole-brain CBF changes to repeated measurements within the same subject, may call for more advanced statistical analysis methods and clinical trial design such as multilevel mixed effects model in conjunction with within-subject crossover design to improve the sensitivity of ASL-based phMRI (Mériaux et al., 2006).
Representative phMRI Studies Using ASL
In the next section, we show representative phMRI studies involving the use of ASL perfusion MRI. Because it is such a new field, many studies are still in the form of meeting abstracts. Nevertheless, we emphasize the design and methodological aspects of these phMRI studies, in particular with respect to the controlling of potential vascular and systemic confounding factors.
Dose-Response Curve: Remifentanil and Caffeine-Induced CBF Responses.
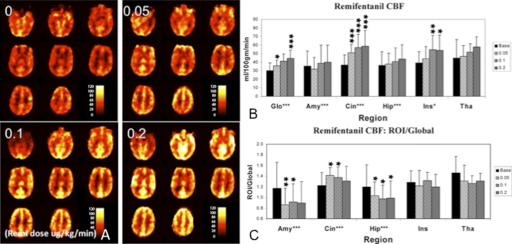
Measuring dose-response curve is typically used in early-phase drug trials to determine the optimal dosage for safety and efficacy. Such methodology can be adapted for phMRI studies to determine the drug-specific CBF response. In one study to investigate opioid effects on brain function (Kofke et al., 2007), healthy volunteers received a remifentanil (μ-opioid) infusion at four sequentially increasing doses: 0, 0.05, 0.1, and 0.2 μg/kg/min while receiving 100% oxygen. Mean CBF values were measured by PASL at each dose globally and in the amygdala, cingulate, hippocampus, insula, and thalamic regions, based on an anatomical template in the SPM Marsbar toolbox (Tzourio-Mazoyer et al., 2002). Significant dose-related CBF increases were detected in all areas (Fig. 2, A and B). This global CBF increase was expected because PaCO2 increases with deepened sedative states induced by remifentanil. To correct for PaCO2 effects, regional CBF values were normalized by global CBF. After normalization, the remifentanil-mediated increased CBF in the cingulate persisted, with decreased flow occurring in the hippocampus and amygdala (Fig. 2C). Kofke et al. (2007) also investigated whether the observed limbic activation was linked to the presence of the ApoE4 genotype. The results showed that all of these PaCO2-corrected effects were reversed in the presence of the ApoE4 polymorphism.
Fig. 2.
A, mean CBF images for the four conditions (baseline and dose levels 1–3) from a representative subject. Remifentanil doses are noted as 0.0, 0.05, 0.1, and 0.2 μg · kg−1 · min−1. B, significant dose effect was observed using absolute CBF values in global (Glo), amygdale (Amy), cingulated (Cin), hippocampus (Hip), and insula (Ins). C, ratio of regional to global CBF at each dose, thus normalizing for effects of PaCO2 shows significant dose effect in the amygdala, cingulate, and hippocampus. [Reproduced from Kofke WA, Blissitt PA, Rao H, Wang J, Addya K, and Detre J (2007) Remifentanil induced cerebral blood flow effects in normal humans: dose and ApoE genotype. Anesth Analg 105:167–175. Copyright © 2007 International Anesthesia Research Society. Used with permission.]
A caveat in ASL phMRI studies is the potential error of CBF quantification caused by changes in arterial transit time (ATT), the time for the labeled blood to flow into brain tissue. To address this issue, a PASL study with 3D BS GRASE readout at multiple delay times (500–2500 ms with 250-ms interval) was carried out to simultaneously measure ATT and CBF during remifentanil infusion (MacIntosh et al., 2008). Administration of remifentanil produced an increase in end-tidal CO2, an increase in CBF from 57 ± 12.0 to 77 ± 18.4 ml/100g/min, and a reduction in ATT from 0.73 ± 0.073 to 0.64 ± 0.076 s.
Caffeine is a popular psychostimulant that improves mental performance and alertness at low to intermediate doses, but may induce negative feelings such as insomnia, anxiety, and nervousness at high doses. Chen and Parrish (2009b) investigated the dose-response curve of caffeine effects on functional activation. Twenty-seven healthy subjects were assigned randomly to four different groups: saline and doses of 1, 2.5, and 5 mg/kg of caffeine. Simultaneous ASL/BOLD time series were collected both before and after an intravenous infusion of saline or caffeine, and task-induced CBF and BOLD percentages of signal changes were compared. The maximum increase in BOLD response was associated with the intermediate caffeine dose of 2.5 mg/kg, whereas the maximum increase in CBF response was associated with the highest caffeine dose of 5 mg/kg. Chen and Parrish attributed the difference in BOLD and ASL responses to a different density of A1 and A2A adenosine receptors in the brain.
Metoclopramide-Induced Systemic and Regional CBF Effects.
Metoclopramide, a substituted benzamide compound with antidopaminergic action, is widely prescribed in the symptomatic treatment of nausea and vomiting, although it can cause adverse neurologic side effects. To determine the effects on brain function, cerebral perfusion changes after a single 10-mg oral dose were assessed in healthy volunteers in a placebo-controlled paradigm (Fernández-Seara et al., 2010). CBF was measured using pCASL before and 1 h after medication intake. PC-MRI was used to measure total blood flow through the carotid and vertebral arteries as a way to calibrate the labeling efficiency of pCASL. PC-MRI showed that metoclopramide caused a significant reduction in mean blood velocity in the internal carotid arteries, which is consistent with its known hypotensive effect (MacDonald, 1991). Whole-brain voxel-wise statistical analysis on the CBF data showed that metoclopramide-induced perfusion increases bilaterally in the striatum, consistent with its dopamine D2 receptor antagonism. In contrast, reduced perfusion was observed in the insular cortices and anterior temporal lobes. In addition, a decrease in functional connectivity was found between the insular cortex and the dorsolateral prefrontal cortex. Fernández-Seara et al. propose that these cortical changes affecting neural circuits between high-order association areas may underlie certain neuropsychiatric conditions occasionally reported after metoclopramide administration.
Caffeine-Induced Metabolic and Physiologic Responses: Multimodal Neuroimaging.
As an adenosine antagonist, caffeine is suspected to alter the coupling between blood flow and oxygen metabolism and has been the research topic in many phMRI studies. To derive the metabolic and neuronal changes induced by caffeine intake, several investigators have used concurrent or combined BOLD/ASL to estimate CMRO2 (Perthen et al., 2008; Chen and Parrish, 2009a). A calibration paradigm is carried out during hypercapnia (e.g., breathing 5% CO2), assuming no significant changes in CMRO2. Using such calibrated fMRI, Perthen et al. (2008) reported that caffeine decreased CBF (−34.5%, ±2.6) with a nonsignificant change in CMRO2 (+5.2%, ±6.4). The coupling between CBF and CMRO2 in response to visual stimulation was significantly different during caffeine consumption. Using visual and motor cortex stimulation tasks, Chen and Parrish (2009a) showed that caffeine decreased the CBF/CMRO2 coupling ratio from 2.58 to 2.33 in motor (p = 0.006) and from 2.45 to 2.23 in visual (p = 0.002) areas, respectively. A similar calibrated BOLD/ASL fMRI study has been carried out using indomethacin (an inhibitor of cyclooxygenase) (St Lawrence et al., 2003). Although indomethacin reduced the CBF increase during activation, it did not significantly affect the CMRO2 increase, suggesting CMRO2 may be a closer marker of neuronal activity compared with CBF. It was later argued that indomethacin may reduce the baseline CMRO2 and there is a coupled response between changes in CBF and CMRO2 (approximately 2:1) with and without indomethacin (Uludag and Buxton, 2004). However, such response may depend on the dose of indomethacin and developmental stages of the species (St Lawrence et al., 2004), highlighting the complexity of phMRI studies.
In a study, we used CASL, quantitative BOLD MRI (a modeling technique to fit oxygen extraction fraction and venous blood volume) (An and Lin, 2000, He and Yablonskiy, 2007) and 18fluorodeoxyglucose PET to study physiological and metabolic responses to a 200-mg caffeine pill (Chen et al., 2009). Figure 3a shows the mean CBF maps before and after caffeine intake (n = 4). Caffeine caused CBF reduction (−10%; p = 0.001) globally (Fig. 3b) and in ROIs based on the SPM Marsbar toolbox template (Tzourio-Mazoyer et al., 2002). Caffeine also decreased CMRGlu (−18%, p < 0.05) (Fig. 3c) and increased OEF (+7%, p = 0.006) and the CMRO2/CMRGlu ratio (+22%, p = 0.003) in all regions. CMRO2, on the other hand, remained unchanged because of compensatory effects of CBF and OEF. As shown in Fig. 3d, anterior cingulate and caudate show significantly different CMRO2/CMRGlu ratio (p = 0.014), which may be associated with heightened alertness after caffeine intake. The results suggest that CBF may be affected by vascular agents (e.g., caffeine) and combined physiological and metabolic MRI can provide more accurate information regarding the underlying neuronal events.
Fig. 3.
Mean CBF maps before and after caffeine intake (a) and ROI results of mean CBF (b), CMRGlu (c), and CMRO2/CMRGlu ratio (d). Anterior cingulate and caudate show significantly different CMRO2/CMRGlu ratio (p = 0.014), which may be associated with heightened alertness after caffeine intake. Errors bars indicate S.D.
Comparison of ASL and BOLD for phMRI and Future Directions
Because of the specific focus on ASL, a comprehensive comparison of ASL and BOLD phMRI studies is out of the scope of this review. Interested readers can refer to earlier reviews addressing the general properties of ASL and BOLD contrast for fMRI studies (Detre and Wang, 2002; Aguirre et al., 2005) as well as reviews on existing BOLD phMRI studies (Honey and Bullmore, 2004; Wise and Tracey 2006). To date, BOLD remains the main contrast for phMRI because of its relatively high sensitivity and technical simplicity. For intravenous drug action that takes place on the order of minutes or even seconds, BOLD is advantageous for tracking the hemodynamic responses associated with drug action, especially when the design includes repeated injections of drug and saline (Leppä et al., 2006). For detecting modulation effects of drug on task performance, several BOLD fMRI paradigms have been proven to be “robust” such as N-back working memory and sensorimotor stimulation. For instance, BOLD activation in the visual cortex has been shown not to be sensitive to baseline flow reduction (Gollub et al., 1998; Liau et al., 2008).
Existing ASL methods are generally suitable for studying (oral) drug actions on the time scale of minutes and hours to days and weeks, although the latest ASL technique allows reliable perfusion measurements within a minute (Fernández-Seara et al., 2008). As a quantitative MRI technique, one key advantage of ASL for phMRI studies is the capability for detecting drug effects on CBF during both resting and task activation states. In clinical populations, direct assessments of baseline or resting CBF may be more advantageous than task activation fMRI studies, which may be confounded by deteriorating performance as well as compensatory processes. Drug-induced global CBF changes generally indicate systemic effects and can be compensated for by normalization with global mean CBF or calibrated using PC-MRI. Regional CBF responses may indicate specific drug action or binding to target regions, although alternative explanations exist. In this sense, resting ASL perfusion MRI provides a noninvasive and alternative approach to PET for assessing target engagement. Indeed, recent industry-sponsored trials have applied ASL CBF as the marker of a single dose of Citalopram in healthy volunteers (Chen et al., 2011) as well as 3-month treatment of Aricept in subjects with mild Alzheimer's disease (Antuono et al., 2009). The capability of ASL for absolute quantification of CBF may also facilitate the uniform exercise of multicenter phMRI studies.
As a way to assess drug effects on brain function and bridge biochemical reaction and clinical endpoints, an fMRI paradigm may be included in addition to baseline CBF measurement in phMRI studies. This fMRI paradigm can be performed with either BOLD or ASL or both contrasts. With knowledge of drug effects on baseline CBF, the observed modulational effects on fMRI activation can be interpreted with greater accuracy. A less explored area is the use of perfusion-based fMRI in phMRI studies, which involves the use of long epochs of behavioral tasks such as cue-induced drug craving (Franklin et al., 2007), emotional distress (Gillihan et al., 2010), and psychological stress (Wang et al., 2005a) etc. that are otherwise difficult to study using BOLD fMRI. Combined or concurrent BOLD and ASL acquisition is a promising approach in phMRI to estimate metabolic changes that are thought to be closely linked to neural activity. Repeated ASL scans can readily be carried out to track the dynamic course of pharmacological action, and regional CBF values can be correlated with the plasma drug concentration (drug exposure) to improve the specificity of ASL in phMRI studies.
Acknowledgments
We thank Drs. Matthias Günther and Hongyu An for sharing the GRASE and quantitative BOLD MRI sequences and Drs. Andrew Kofke and Hengyi Rao for sharing the data of the remifentanil study.
This work was supported by the National Institutes of Health National Institute of Mental Health [Grants MH080892, MH080729]; the National Institutes of Health National Center for Research Resources [Grant RR002305]; the National Institutes of Health National Institute of Neurological Disorders and Stroke [Grant NS058386]; the National Institutes of Health National Institute on Aging [Grant AG01657011A]; and the American Recovery and Reinvestment Act [Grant MH080892S1].
J.A.D. is an inventor on the University of Pennsylvania's patent for ASL MRI and is entitled to institutional royalty sharing for its licensure. D.J.J.W. is a consultant for Pfizer Inc.
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.110.172577.
- PET
- positron emission tomography
- ASL
- arterial spin labeled
- CASL
- continuous ASL
- pCASL
- pseudo-CASL
- PASL
- pulsed ASL
- BOLD
- blood-oxygen-level-dependent
- MRI
- magnetic resonance imaging
- phMRI
- pharmacological MRI
- fMRI
- functional MRI
- CBF
- cerebral blood flow
- 3D
- three-dimensional
- GRASE
- gradient and spin echo
- BS
- background suppression
- PC
- phase-contrast
- ICC
- intraclass correlation coefficient
- CMRO2
- cerebral metabolic rate of oxygen
- CMRGlu
- cerebral metabolic rate of glucose
- OEF
- oxygen extraction fraction
- ATT
- arterial transit time
- ROI
- region of interest
- PaCO2
- partial pressure of carbon dioxide in the arterial blood.
Authorship Contributions
Participated in research design: Wang, Chen, Fernández-Seara, and Detre.
Conducted experiments: Wang, Chen, Fernández-Seara, and Detre.
Performed data analysis: Wang, Chen, and Fernández-Seara.
Wrote or contributed to the writing of the manuscript: Wang, Chen, Fernández-Seara, and Detre.
Other: Wang and Detre acquired funding for the research.
References
- Aguirre GK, Detre JA, Wang J. (2005) Perfusion fMRI for functional neuroimaging. Int Rev Neurobiol 66:213–236 [DOI] [PubMed] [Google Scholar]
- Aguirre GK, Detre JA, Zarahn E, Alsop DC. (2002) Experimental design and the relative sensitivity of BOLD and perfusion fMRI. Neuroimage 15:488–500 [DOI] [PubMed] [Google Scholar]
- An H, Lin W. (2000) Quantitative measurements of cerebral blood oxygen saturation using magnetic resonance imaging. J Cereb Blood Flow Metab 20:1225–1236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson IM, Juhasz G, Thomas E, Downey D, McKie S, Deakin JF, Elliott R. (2011) The effect of acute citalopram on face emotion processing in remitted depression: a pharmacoMRI study. Eur Neuropsychopharmacol 21:140–148 [DOI] [PubMed] [Google Scholar]
- Antuono P, Jones J, Wu Z, McRea T, Franczak M, Ward DB, Li SJ. (2009) Detection of changes in cerebral blood flow perfusion following Aricept treatment in mild Alzheimer subjects. Alzheimers Dement 5 (4 Suppl 1):P66 [Google Scholar]
- Becerra L, Harter K, Gonzalez RG, Borsook D. (2006) Functional magnetic resonance imaging measures of the effects of morphine on central nervous system circuitry in opioid-naive healthy volunteers. Anesth Analg 103:208–216, table of contents [DOI] [PubMed] [Google Scholar]
- Breiter HC, Gollub RL, Weisskoff RM, Kennedy DN, Makris N, Berke JD, Goodman JM, Kantor HL, Gastfriend DR, Riorden JP, et al. (1997) Acute effects of cocaine on human brain activity and emotion. Neuron 19:591–611 [DOI] [PubMed] [Google Scholar]
- Buxton RB, Frank LR, Wong EC, Siewert B, Warach S, Edelman RR. (1998a) A general kinetic model for quantitative perfusion imaging with arterial spin labeling. Magn Reson Med 40:383–396 [DOI] [PubMed] [Google Scholar]
- Buxton RB, Wong EC, Frank LR. (1998b) Dynamics of blood flow and oxygenation changes during brain activation: the balloon model. Magn Reson Med 39:855–864 [DOI] [PubMed] [Google Scholar]
- Chen JJ, Pike GB. (2010) Global cerebral oxidative metabolism during hypercapnia and hypocapnia in humans: implications for BOLD fMRI. J Cereb Blood Flow Metab 30:1094–1099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Newberg AB, Wang J, Rao H, An H, Greenberg J, Wintering N, Tolles V, Detre JA. (2009) Caffeine's effects on resting-state oxygen and glucose metabolism: a combined MR and PET study. Proc Int Soc Magn Reson Med 17:793 [Google Scholar]
- Chen Y, Parrish TB. (2009a) Caffeine's effects on cerebrovascular reactivity and coupling between cerebral blood flow and oxygen metabolism. Neuroimage 44:647–652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Parrish TB. (2009b) Caffeine dose effect on activation-induced BOLD and CBF responses. Neuroimage 46:577–583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Wan HI, O'Reardon JP, Wang DJ, Wang Z, Korczykowski M, Detre JA. (2011) Quantification of cerebral blood flow as biomarker of drug effect: arterial spin labeling phMRI after a single dose of oral citalopram. Clin Pharmacol Ther 89:251–258 [DOI] [PubMed] [Google Scholar]
- Chen Y, Wang J, Korczykowski M, Fernández-Seara MA, Detre JA. (2010) Comparison of reproducibility between continuous pulsed, and pseudo-continuous arterial spin labeling. Proc Int Soc Magn Reson Med 18:4078 [Google Scholar]
- Chen YC, Galpern WR, Brownell AL, Matthews RT, Bogdanov M, Isacson O, Keltner JR, Beal MF, Rosen BR, Jenkins BG. (1997) Detection of dopaminergic neurotransmitter activity using pharmacologic MRI: correlation with PET, microdialysis, and behavioral data. Magn Reson Med 38:389–398 [DOI] [PubMed] [Google Scholar]
- Cohen ER, Ugurbil K, Kim SG. (2002) Effect of basal conditions on the magnitude and dynamics of the blood oxygenation level-dependent fMRI response. J Cereb Blood Flow Metab 22:1042–1053 [DOI] [PubMed] [Google Scholar]
- Dai W, Garcia D, de Bazelaire C, Alsop DC. (2008) Continuous flow-driven inversion for arterial spin labeling using pulsed radio frequency and gradient fields. Magn Reson Med 60:1488–1497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis TL, Kwong KK, Weisskoff RM, Rosen BR. (1998) Calibrated functional MRI: mapping the dynamics of oxydative metabolism. Proc Natl Acad Sci USA 95:1834–1839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detre JA, Wang J. (2002) Technical aspects and utility of fMRI using BOLD and ASL. Clin Neurophysiol 113:621–634 [DOI] [PubMed] [Google Scholar]
- Detre JA, Wang J, Wang Z, Rao H. (2009) Arterial spin-labeled perfusion MRI in basic and clinical neuroscience. Curr Opin Neurol 22:348–355 [DOI] [PubMed] [Google Scholar]
- Feng CM, Narayana S, Lancaster JL, Jerabek PA, Arnow TL, Zhu F, Tan LH, Fox PT, Gao JH. (2004) CBF changes during brain activation: fMRI vs. PET. Neuroimage 22:443–446 [DOI] [PubMed] [Google Scholar]
- Fernández-Seara MA, Aznarez-Sanado M, Mengual E, Irigoyen J, Heukamp F, Pastor MA. (2010) Effects on resting cerebral blood flow and functional connectivity induced by metoclopramide: a perfusion MRI study in healthy volunteers. Br J Pharmacol doi:10.1111/j.1476-5381.2010.01161.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Seara MA, Edlow BL, Hoang A, Wang J, Feinberg DA, Detre JA. (2008) Minimizing acquisition time of arterial spin labeling at 3T. Magn Reson Med 59:1467–1471 [DOI] [PubMed] [Google Scholar]
- Fernández-Seara MA, Wang J, Wang Z, Korczykowski M, Guenther M, Feinberg DA, Detre JA. (2007) Imaging mesial temporal lobe activation during scene encoding: comparison of fMRI using BOLD and arterial spin labeling. Hum Brain Mapp 28:1391–1400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floyd TF, Ratcliffe SJ, Wang J, Resch B, Detre JA. (2003) Precision of the CASL-perfusion MRI technique for the measurement of cerebral blood flow in whole brain and vascular territories. J Magn Reson Imaging 18:649–655 [DOI] [PubMed] [Google Scholar]
- Franklin TR, Wang Z, Wang J, Sciortino N, Harper D, Li Y, Ehrman R, Kampman K, O'Brien CP, Detre JA, et al. (2007) Limbic activation to cigarette smoking cues independent of nicotine withdrawal: a perfusion fMRI study. Neuropsychopharmacology 32:2301–2309 [DOI] [PubMed] [Google Scholar]
- Garcia DM, Duhamel G, Alsop DC. (2005) Efficiency of inversion pulses for background suppressed arterial spin labeling. Magn Reson Med 54:366–372 [DOI] [PubMed] [Google Scholar]
- Gillihan SJ, Rao H, Wang J, Detre JA, Breland J, Sankoorikal GM, Brodkin ES, Farah MJ. (2010) Serotonin transporter genotype modulates amygdala activity during mood regulation. Soc Cogn Affect Neurosci 5:1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollub RL, Breiter HC, Kantor H, Kennedy D, Gastfriend D, Mathew RT, Makris N, Guimaraes A, Riorden J, Campbell T, et al. (1998) Cocaine decreases cortical cerebral blood flow but does not obscure regional activation in functional magnetic resonance imaging in human subjects. J Cereb Blood Flow Metab 18:724–734 [DOI] [PubMed] [Google Scholar]
- Hahn T, Heinzel S, Plichta M, Reif A, Lesch KP, Fallgatter A. (2010) Neurovascular coupling in human visual cortex is modulated by cyclooxygenase-1 (COX-1) gene variant. Cereb Cortex doi:10.1093/cercor/bhq236 [DOI] [PubMed] [Google Scholar]
- He X, Yablonskiy DA. (2007) Quantitative BOLD: mapping of human cerebral deoxygenated blood volume and oxygen extraction fraction: default state. Magn Reson Med 57:115–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honey G, Bullmore E. (2004) Human pharmacological MRI. Trends Pharmacol Sci 25:366–374 [DOI] [PubMed] [Google Scholar]
- Hyder F, Rothman DL, Shulman RG. (2002) Total neuroenergetics support localized brain activity: implications for the interpretation of fMRI. Proc Natl Acad Sci USA 99:10771–10776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadecola C, Nedergaard M. (2007) Glial regulation of the cerebral microvasculature. Nat Neurosci 10:1369–1376 [DOI] [PubMed] [Google Scholar]
- Iannetti GD, Wise RG. (2007) BOLD functional MRI in disease and pharmacological studies: room for improvement? Magn Reson Imaging 25:978–988 [DOI] [PubMed] [Google Scholar]
- Jain V, Giannetta M, Langham M, Xie SX, Licht DJ, Giannetta J, Roberts T, Detre JA, Hurt H, Wehrli FW, et al. (2010) Precision and accuracy of arterial spin labeling perfusion MRI in the pediatric population. Proc Int Soc Magn Reson Med 18:2040 [Google Scholar]
- Jakovcevic D, Harder DR. (2007) Role of astrocytes in matching blood flow to neuronal activity. Curr Top Dev Biol 79:75–97 [DOI] [PubMed] [Google Scholar]
- Jokeit H, Okujava M, Woermann FG. (2001) Carbamazepine reduces memory induced activation of mesial temporal lobe structures: a pharmacological fMRI-study. BMC Neurol 1:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung Y, Wong EC, Liu TT. (2010) Multiphase pseudocontinuous arterial spin labeling (MP-PCASL) for robust quantification of cerebral blood flow. Magn Reson Med 64:799–810 [DOI] [PubMed] [Google Scholar]
- Kofke WA, Blissitt PA, Rao H, Wang J, Addya K, Detre J. (2007) Remifentanil-induced cerebral blood flow effects in normal humans: dose and ApoE genotype. Anesth Analg 105:167–175 [DOI] [PubMed] [Google Scholar]
- Laurienti PJ, Field AS, Burdette JH, Maldjian JA, Yen YF, Moody DM. (2002) Dietary caffeine consumption modulates fMRI measures. Neuroimage 17:751–757 [PubMed] [Google Scholar]
- Leppä M, Korvenoja A, Carlson S, Timonen P, Martinkauppi S, Ahonen J, Rosenberg PH, Aronen HJ, Kalso E. (2006) Acute opioid effects on human brain as revealed by functional magnetic resonance imaging. Neuroimage 31:661–669 [DOI] [PubMed] [Google Scholar]
- Li SJ, Ward DB, Wu Z, Jones J, McRae T, Franczak M, Antuono P. (2009) Detection of changes in functional connectivity following Aricept treatment in mild Alzheimer disease subjects. Alzheimers Dement 5 (4 Suppl 1):P327 [Google Scholar]
- Liau J, Perthen JE, Liu TT. (2008) Caffeine reduces the activation extent and contrast-to-noise ratio of the functional cerebral blood flow response but not the BOLD response. Neuroimage 42:296–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald TM. (1991) Metoclopramide, domperidone and dopamine in man: actions and interactions. Eur J Clin Pharmacol 40:225–230 [DOI] [PubMed] [Google Scholar]
- MacIntosh BJ, Pattinson KT, Gallichan D, Ahmad I, Miller KL, Feinberg DA, Wise RG, Jezzard P. (2008) Measuring the effects of remifentanil on cerebral blood flow and arterial arrival time using 3D GRASE MRI with pulsed arterial spin labelling. J Cereb Blood Flow Metab 28:1514–1522 [DOI] [PubMed] [Google Scholar]
- McKie S, Del-Ben C, Elliott R, Williams S, del Vai N, Anderson I, Deakin JF. (2005) Neuronal effects of acute citalopram detected by pharmacoMRI. Psychopharmacology 180:680–686 [DOI] [PubMed] [Google Scholar]
- Mériaux S, Roche A, Dehaene-Lambertz G, Thirion B, Poline JB. (2006) Combined permutation test and mixed-effect model for group average analysis in fMRI. Hum Brain Mapp 27:402–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mintun MA, Raichle ME, Martin WR, Herscovitch P. (1984) Brain oxygen utilization measured with O-15 radiotracers and positron emission tomography. J Nucl Med 25:177–187 [PubMed] [Google Scholar]
- Parkes LM, Tofts PS. (2004) Normal cerebral perfusion measurements using arterial spin labeling: reproducibility, stability, and age and gender effects. Magn Reson Med 51:736–743 [DOI] [PubMed] [Google Scholar]
- Perthen JE, Lansing AE, Liau J, Liu TT, Buxton RB. (2008) Caffeine-induced uncoupling of cerebral blood flow and oxygen metabolism: a calibrated BOLD fMRI study. Neuroimage 40:237–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Lawrence KS, Ye FQ, Frank JA, McLachlan AC. (2004) Response: measuring the effects of indomethacin on changes in cerebral oxidative metabolism and cerebral blood flow during sensorimotor activation. Magn Reson Med 51:1090. [DOI] [PubMed] [Google Scholar]
- St Lawrence KS, Ye FQ, Lewis BK, Frank JA, McLaughlin AC. (2003) Measuring the effects of indomethacin on changes in cerebral oxidative metabolism and cerebral blood flow during sensorimotor activation. Magn Reson Med 50:99–106 [DOI] [PubMed] [Google Scholar]
- Stein EA, Pankiewicz J, Harsch HH, Cho JK, Fuller SA, Hoffmann RG, Hawkins M, Rao SM, Bandettini PA, Bloom AS. (1998) Nicotine-induced limbic cortical activation in the human brain: a functional MRI study. Am J Psychiatry 155:1009–1015 [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. (2002) Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 15:273–289 [DOI] [PubMed] [Google Scholar]
- Uludag K, Buxton RB. (2004) Measuring the effects of indomethacin on changes in cerebral oxidative metabolism and cerebral blood flow during sensorimotor activation. Magn Reson Med 51:1088–1089; author reply 1090 [DOI] [PubMed] [Google Scholar]
- Upadhyay J, Anderson J, Schwarz AJ, Baumgartner RW, Coimbra A, Nutile L, Bishop J, George E, Robertson B, Iyengar S, et al. (2010) Comparison of BOLD response modulation during pain stimulation and resting-state conditions under intravenous (0.2 mg/70 kg) or sublingual (2 mg) buprenorphine treatment. Proc Int Soc Magn Reson Med 18:1167 [Google Scholar]
- Wang J, Aguirre GK, Kimberg DY, Roc AC, Li L, Detre JA. (2003) Arterial spin labeling perfusion fMRI with very low task frequency. Magn Reson Med 49:796–802 [DOI] [PubMed] [Google Scholar]
- Wang J, Alsop DC, Li L, Listerud J, Gonzalez-At JB, Schnall MD, Detre JA. (2002) Comparison of quantitative perfusion imaging using arterial spin labeling at 1.5 and 4.0 Tesla. Magn Reson Med 48:242–254 [DOI] [PubMed] [Google Scholar]
- Wang J, Li L, Roc AC, Alsop DC, Tang K, Butler NS, Schnall MD, Detre JA. (2004) Reduced susceptibility effect in perfusion fMRI with single-shot spin-echo EPI acquisitions at 1.5 Tesla. Magn Reson Imaging 22:1–7 [DOI] [PubMed] [Google Scholar]
- Wang J, Rao H, Wetmore GS, Furlan PM, Korczykowski M, Dinges DF, Detre JA. (2005a) Perfusion functional MRI reveals cerebral blood flow pattern under psychological stress. Proc Natl Acad Sci USA 102:17804–17809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Childress AR, Wang J, Detre JA. (2007) Support vector machine learning-based fMRI data group analysis. Neuroimage 36:1139–1151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Wang J, Connick TJ, Wetmore GS, Detre JA. (2005b) Continuous ASL (CASL) perfusion MRI with an array coil and parallel imaging at 3T. Magn Reson Med 54:732–737 [DOI] [PubMed] [Google Scholar]
- Wise RG, Rogers R, Painter D, Bantick S, Ploghaus A, Williams P, Rapeport G, Tracey I. (2002) Combining fMRI with a pharmacokinetic model to determine which brain areas activated by painful stimulation are specifically modulated by remifentanil. Neuroimage 16:999–1014 [DOI] [PubMed] [Google Scholar]
- Wise RG, Tracey I. (2006) The role of fMRI in drug discovery. J Magn Reson Imaging 23:862–876 [DOI] [PubMed] [Google Scholar]
- Wong DF, Tauscher J, Gründer G. (2009) The role of imaging in proof of concept for CNS drug discovery and development. Neuropsychopharmacology 34:187–203 [DOI] [PubMed] [Google Scholar]
- Wu WC, Fernández-Seara M, Detre JA, Wehrli FW, Wang J. (2007) A theoretical and experimental investigation of the tagging efficiency of pseudocontinuous arterial spin labeling. Magn Reson Med 58:1020–1027 [DOI] [PubMed] [Google Scholar]
- Yablonskiy DA, Haacke EM. (1994) Theory of NMR signal behavior in magnetically inhomogeneous tissues: the static dephasing regime. Magn Reson Med 32:749–763 [DOI] [PubMed] [Google Scholar]
- Ye FQ, Berman KF, Ellmore T, Esposito G, van Horn JD, Yang Y, Duyn J, Smith AM, Frank JA, Weinberger DR, et al. (2000) H(2)(15)O PET validation of steady-state arterial spin tagging cerebral blood flow measurements in humans. Magn Reson Med 44:450–456 [DOI] [PubMed] [Google Scholar]