Abstract
Darbepoetin alfa (darbEpo) is an erythropoietic glycoprotein that activates the erythropoietin receptor. The aim of our study was to determine whether darbEpo is neuroprotective in a cortical impact injury (CII) model and to determine the characteristics of dose response and time window. To better understand the vascular mechanism of darbEpo neuroprotection, the reactivity of cerebral blood flow (CBF) to l-arginine administration was also studied. Rats were given saline or darbEpo from 2.5 to 50 μg/kg at 5 min after CII or a dose of 25 μg/kg darbEpo at times ranging from 5 min to 24 h after CII. Histological assessment was determined 2 weeks after a severe CII. Other rats were given either darbEpo (25 μg/kg) or saline daily for 3 days before injury. Five minutes after severe CII, they were given either l-arginine or d-arginine. Hemodynamic variables were monitored for 2 h after injury. In the dose-response study, darbEpo in doses of 25 and 50 μg/kg significantly reduced contusion volume from 39.1 ± 6.7 to 8.1 ± 3.1 and 11.2 ± 6.0 mm3, respectively. In the time window study, darbEpo reduced contusion volume when given in a dose of 25 μg/kg at 5 min to 6 h after the impact injury. In animals pretreated with darbEpo, the CBF response to l-arginine was significantly greater than in the animals pretreated with saline. These data demonstrate that darbEpo has neuroprotective effects in traumatic brain injury in a dose- and time-dependent manner and that vascular effects of darbEpo may have a role in neuroprotection.
Introduction
Erythropoietin (Epo) has been reported to have neuroprotective effects against a variety of types of experimental central nervous system injuries, including neonatal hypoxia-ischemia (Aydin et al., 2003), global cerebral ischemia (Catania et al., 2002), middle cerebral artery occlusion (Sirén et al., 2001), experimental traumatic brain injury (TBI) (Brines et al., 2004; Cherian et al., 2007), spinal cord ischemia (Celik et al., 2002), spinal cord trauma (Gorio et al., 2002), and subarachnoid hemorrhage (Alafaci et al., 2000).
Darbepoetin alfa (darbEpo), a novel erythropoiesis-stimulating protein closely related to Epo, consists of 165 amino acids (Elliott et al., 2003). darbEpo contains five N-linked oligosaccharide chains, whereas Epo has only three. The additional carbohydrate chains increase the molecular mass from 30 to 37 kDa and prolong the half-life of darbEpo in the circulation by 3-fold. The longer half-life of darbEpo is a significant advantage in the treatment of anemia caused by chronic kidney disease and chemotherapy because doses, which must be given parenterally, are required less frequently.
The major goal of this study was to determine whether darbEpo has neuroprotective effects in a cortical impact injury (CII) model of TBI and, if so, to determine the characteristics of dose response and time window. To better understand a potential vascular mechanism of darbEpo neuroprotection, the reactivity of cerebral blood flow (CBF) to l-arginine administration was also studied.
Materials and Methods
Experimental Groups
To achieve these goals, three experiments were performed. The first experiment examined the effect of different doses of darbEpo on neurological outcome assessed by histological indices. In the second experiment, the time window for darbEpo at a dose of 25 μg/kg was determined. The third experiment examined the effects of darbEpo administration on l-arginine reactivity of CBF after TBI. All the experiments were approved by the institutional animal protocol review committee, using guidelines for humane care and use of animals developed by the National Institutes of Health. darbEpo (Aranesp) was supplied by Amgen, Inc. (Thousand Oaks, CA).
Dose Response for darbEpo Neuroprotection.
A total of 48 Long Evans rats underwent cortical impact injury (5 m/s velocity, 3-mm deformation) and were randomly assigned to be given darbEpo at different doses (2.5, 5, 10, 25, or 50 μg/kg) subcutaneously or an equal volume of saline at 5 min after injury. The numbers of animals in each group are shown in the figures. The neurological outcome was assessed by measuring contusion volume and neuron density in the CA1 and CA3 regions of the hippocampus at 2 weeks postinjury. The investigators performing the outcome assessments were blinded to the treatment assignment.
Time Window for darbEpo Neuroprotection.
A total of 58 Long Evans rats were subjected to cortical impact injury (5 m/s velocity, 3-mm deformation) and randomly assigned to treatment with either darbEpo (25 μg/kg) or saline subcutaneously at 5 min, 1 h, 3 h, 6 h, 9 h, 12 h, or 24 h postinjury. The numbers of animals in each group are shown in the figures. The neurological outcome was assessed by measuring contusion volume and neuron density in the CA1 and CA3 regions of the hippocampus at 2 weeks postinjury.
l-Arginine Reactivity after darbEpo.
Fifty Long-Evans rats (Harlan, Indianapolis, IN) were randomly assigned to one of four treatment-CBF response groups. Each treatment group received either darbEpo (25 μg/kg) or saline subcutaneously once a day for 3 days before injury. Then to test reactivity to l-arginine, each drug treatment group was randomly assigned to receive a single dose of either l-arginine (300 mg/kg) or d-arginine (300 mg/kg) intravenously 5 min after injury. Cerebral hemodynamics and tissue nitric oxide (NO) concentrations were monitored before and for 2 h after cortical impact injury of 5 m/s velocity and 3-mm deformation.
Anesthesia and Surgical Preparation
Rats, weighing 300 to 400 g and fasted overnight, were anesthetized with 3.5% isoflurane in 100% oxygen in a vented anesthesia chamber. After endotracheal intubation with a 16-gauge Teflon catheter, the rats were mechanically ventilated with 2% isoflurane in 100% oxygen during the surgical preparation and for the impact. Rectal temperature was maintained at 36.5 to 37.5°C by a heating pad, which was controlled by rectal thermistor. Brain temperature was monitored with a thermocouple microprobe placed in the brain parenchyma of the right frontal lobe anterior to the impact site and controlled by a heating lamp directed at the head and kept at 37°C.
Production of Brain Injury
The head of the rat was fixed in a stereotaxic frame by ear bars and incisor bar. An 8-mm-diameter craniotomy was performed on the right side of the skull over the parietal cortex. The impactor tip, which had a diameter of 8 mm, was centered in the craniotomy site perpendicular to the exposed surface of the brain at an angle of approximately 45° to the vertical. The tip was lowered until it just touched the dural surface. The impactor rod was then retracted, and the tip advanced an additional 3 mm to produce a brain deformation of 3 mm during the impact. The gas pressure applied to the impactor was adjusted to 150 psi, giving an impact velocity of approximately 5 m/s and an impact duration of approximately 130 ms.
Measurement of Cerebral Blood Flow
CBF was measured by laser Doppler probes (Perimed, Piscataway, NJ) at the impact site 30 min before injury and continuously for 2 h after injury. Values for the laser Doppler flow (LDF) were expressed as percentage of change from the preinjury baseline.
Measurement of Nitric Oxide
Nitric oxide was measured using NO electrodes (tip diameter 200 μm; ISO-NOP200; World Precision Instruments Inc., Sarasota, FL) inserted into the brain at a depth of 1.5 mm at the center of the impact site. The measurement principle of this type of electrode is the oxidation of NO at a working electrode, which is kept at a constant potential of 0.85 V against an Ag/AgCl reference electrode. Selectivity to NO was maintained by a gas-permeable membrane covering the electrode. The redox current proportional to tissue NO concentration was measured with an ISO-NO meter (World Precision Instruments Inc.). NO electrodes were calibrated before and after each experiment by a standard method of chemical degeneration of NO using S-nitroso-N-acetyl-dl-penicillamine (RSNO) and copper sulfate at 37°C. RSNO decomposes to NO and a disulfide byproduct according to the following equation: 2 RSNO − 2NO + RS-SR.
Brain NO concentration was calculated from the current measured with the probe positioned in the brain by means of the in vitro calibration curve. Changes in brain NO were expressed by the change in concentration (nM) from baseline values.
Postoperative Care
After the impact injury, the surgical wound was sutured closed; the rats were allowed to awaken from anesthesia and extubated. For the first 3 days postinjury, the rats were treated with butrophenol tartrate, 0.05 mg i.m. every 12 h, for analgesia and 2.27% enrofloxacin, 0.1 ml i.m. once a day, to reduce the risk of postoperative infections.
Histopathological Assessment of Outcome
At 2 weeks after the impact, the animals were deeply anesthetized and perfused transcardially with 0.9% saline, followed by 10% phosphate-buffered formaldehyde. The entire brain was removed and fixed in 4% formalin. The fixed brains were examined grossly for the presence of contusion, hematoma, and herniation. The brains were photographed, sectioned at 2-mm intervals, and then embedded in paraffin. Hematoxylin and eosin-stained 9-μ-thick sections were prepared for histologic examination. Particular care was made to include the largest cross-sectional area of cortical injury on the cut surface of the embedded sections. The coronal sections were digitized using a Polaroid Sprint Scanner (Polaroid Corporation, Minnetonka, MN) equipped with a PathScan Enabler (Meyer Instruments, Houston, TX).
The injury volume was measured by determining the cross-sectional area of injury in each coronal image and multiplying by the thickness of the tissue between the slices. This slab volume technique was implemented on the image processing program Optimas 5.2 (Optimas Corporation, Seattle, WA). Neurons in the middle 1-mm segments of the CA1 and CA3 regions of the hippocampus were counted at a magnification of ×200. Neurons were identified by nuclear and cytoplasmic morphology, and individual cells were counted as either normal or damaged. Neurons with cytoplasmic shrinkage, basophilia, eosinophilia, or loss of nuclear detail were regarded as damaged. The regions measured were 1 mm long and 1 mm wide (0.5 mm on either side of the long axis of the segment). The total number of neurons and the number of neurons that appeared normal were expressed as neurons/mm2.
Statistical Analysis
The data for CBF and NO were collected every 30 s using a LabView data logging program (National Instruments, Austin, TX). Because the laser Doppler method for assessing CBF provides a relative measure of blood flow, all preinjury LDF values were normalized to 100% and expressed as percentage of changes from the preimpact baseline values. Changes in brain NO were expressed as the change in concentration (nM) from baseline values. Repeated-measures analysis of variance was used to analyze both nitric-oxide and cerebral blood flow measurements, followed by Tukey's test to adjust for multiple comparisons. Histological outcome measures were compared using one-way analysis of variance followed by the Holm-Sidak test to adjust for multiple comparisons. The values are expressed as mean ± S.E.
Results
Dose Response of darbEpo Neuroprotection.
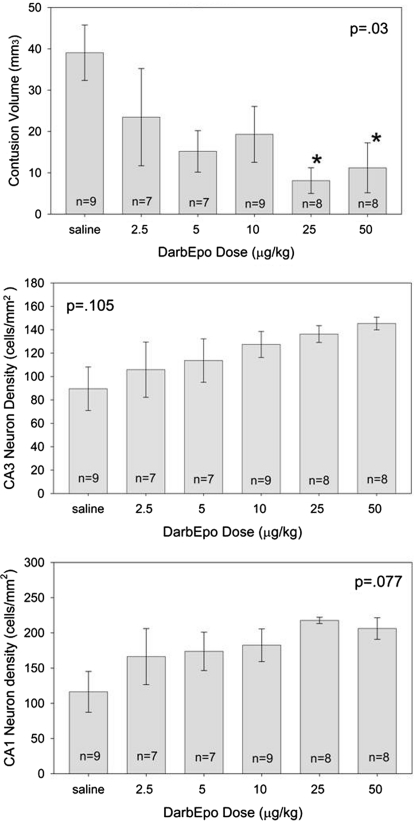
The results of the dose-response study are shown in Fig. 1. There was a significant main effect of treatment on contusion volume (p = 0.03; Fig. 1, top). darbEpo doses of 25 and 50 μg/kg given 5 min after injury resulted in a significantly smaller contusion volume (8.1 ± 3.1 and 11.1 ± 6.0 mm3, respectively) than in the saline-treated animals, where contusion volume averaged 39.1 ± 6.7 mm3. There was a similar trend for the neuron density in the CA3 and CA1 regions of the hippocampus, but the differences were not significant (p = 0.105 and 0.077, respectively).
Fig. 1.
Top, contusion volume was significantly reduced by 25 and 50 μg/kg darbEpo given 5 min after cortical impact injury in rats (F5, 42 = 2.767, p = 0.03). *, those values that are significantly different from the saline-treated value (p < 0.05) by Holm-Sidak test. Middle and bottom, CA3 (middle; F5, 42 = 1.956, p = 0.105) and CA1 (bottom; F5, 42 = 2.162, p = 0.077) followed the same trend but the differences were not significant.
Time Window for darbEpo Neuroprotection.
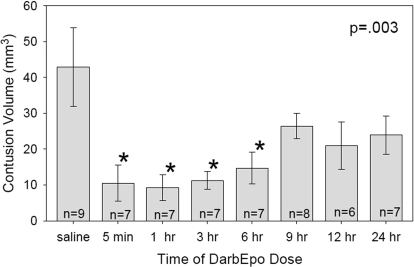
The results of the time-window study are shown in Fig. 2. There was a significant main effect of time for contusion volume (p = 0.003). Treatment with 25 μg/kg darbEpo reduced contusion volume to 10.5 ± 5.0 mm3 when given at 5 min postinjury, 9.2 ± 3.6 mm3 when given at 1 h, 11.3 ± 2.4 mm3 when given at 3 h, and 14.7 ± 4.4 mm3 when given at 6 h postinjury, compared with a contusion volume of 42.9 ± 11.0 mm3 in animals treated with saline. darbEpo did not consistently preserve neuron density in the CA1 and CA3 regions of the hippocampus.
Fig. 2.
Contusion volume was significantly smaller than in the saline-treated group when darbEpo (25 μg/kg) was given 5 min, 1 h, 3 h, or 6 h after cortical impact injury (F7, 50 = 3.586, p = 0.003). *, those values that are significantly different from the saline-treated value (p < 0.05) by Holm-Sidak test.
Cerebrovascular Effects of darbEpo.
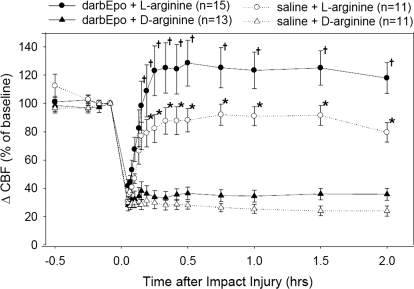
The pertinent results for the cerebral hemodynamic changes are shown in Fig. 3. As previous studies had shown, LDF at the injury site fell immediately after the impact injury and remained low for the duration of the 2-h experiment. Postinjury administration of l-arginine significantly increased LDF, whereas d-arginine had no effect on LDF. In the animals pretreated with darbEpo, the increase in LDF induced by l-arginine administration was even greater than in the animals pretreated with saline (p < 0.05).
Fig. 3.
LDF at the impact site was significantly different among the four experimental groups (time effect, p < 0.001; treatment effect, p < 0.001; time × treatment interaction, p < 0.001). LDF increased in both groups treated with l-arginine, but the increase in LDF was larger in the group pretreated with 25 μg/kg darbEpo. †, different from darbEpo + d-arginine group and different from saline + d-arginine group; *, different from darbEpo + d-arginine group and different from both saline groups.
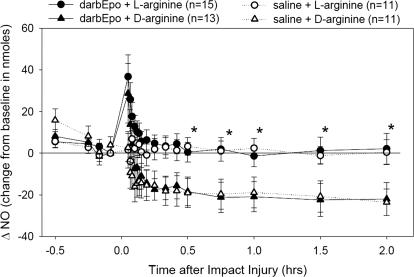
Postinjury l-arginine administration (Fig. 4) also restored brain tissue NO concentration to preinjury levels, compared with treatment with d-arginine where NO levels remained low for the duration of the 2-h experiment. Tissue NO levels in both darbEpo- and saline-treated animals recovered in a similar time frame back to preinjury baseline levels after infusion of l-arginine.
Fig. 4.
Nitric-oxide concentration (NO) at the impact site was significantly different among the four experimental groups (time effect, p < 0.001; treatment effect, p = 0.007; time × treatment interaction, p = 0.001). Both groups treated with l-arginine had better preservation of NO levels in the brain, but there was not a difference between the darbEpo (25 μg/kg)- and saline-pretreated animals, *, different from respective d-arginine group.
Systemic Physiology and Injury Severity Parameters.
There were no significant differences in physiological variables, such as blood pressure and intracranial pressure between treatment groups. The injury severity measured by impact velocity and duration were similar in all of the treatment groups.
In the first two chronic experiments, there was a significant increase in hematocrit in the darbEpo-treated group, averaging 51 ± 2 compared with 42 ± 3 in the saline-treated group (p = 0.025) at the time the brains were examined for histology at 2 weeks postinjury.
Discussion
Epo is a hematopoietic growth factor that until recently was thought to be produced primarily by the kidney in adult animals. Digicaylioglu et al. (1995) detected mRNA for both Epo and EpoR in mouse brain. Hypoxia induced a 20-fold increase in Epo expression. Major Epo binding sites were observed in hippocampus, internal capsule, and midbrain areas. Multiple laboratory studies have reported reduced neurological damage in TBI models with Epo administration (Brines et al., 2000; Yatsiv et al., 2005; Cherian et al., 2007; Grasso et al., 2007; Xiong et al., 2008; Zhang et al., 2009; Zhu et al., 2009b). Epo has also been reported to protect against some of the potentially adverse systemic effects of TBI, including pulmonary, myocardial, and intestinal injury (Emir et al., 2004; Yildirim et al., 2005; Zhu et al., 2009a). These observations that Epo and EpoR are expressed in the brain, Epo expression is increased after injury, and administration of exogenous Epo has neuroprotective activity have suggested a role for Epo in the brain's response to injury.
The receptor mediating the cytoprotective effects of Epo may differ from the homodimer Epo receptor complex [(EPOR2)]. Brines et al. (2004) have proposed that the receptor that promotes tissue protection is a heteromer composed of two EPOR monomers and two molecules of CD131 or the β common receptor. This receptor complex has a lower affinity for Epo, is not usually expressed until after an injury occurs, and requires only a brief exposure to Epo to trigger sustained biological activity (Brines and Cerami, 2008). In contrast, (EPOR)2 is normally expressed in hematopoietic cells and requires sustained levels of Epo to support erythropoiesis.
Much less is known about the potential effects of the Epo derivative darbEpo. The advantage of darbEpo over Epo in the clinical setting is the longer half-life that requires less frequent dosing when treating patients with anemia. A number of reports have reported cytoprotection for darbEpo in organs such as the heart and liver.
Baker et al. (2007) showed that doses of darbEpo as low as 2.5 μg/kg given at the onset of ischemia and at the start of reperfusion provided protection in a myocardial ischemia/reperfusion model. In a similar model, Gao et al. (2007) found high doses (30 μg/kg) of darbEpo protect the myocardium even when given 24 h after ischemia/reperfusion. Studies using a transgenic mouse deficient in the common β subunit suggest that the protective effects of darbEpo given at the onset of reperfusion after 60 min of myocardial ischemia are not mediated by the receptor complex of Epo and the common β subunit (Kanellakis et al., 2010).
Likewise in a renal ischemia/reperfusion model, darbEpo provided cytoprotection equivalent to Epo even with delayed administration 6 h after ischemia (Johnson et al., 2006) and reduced apoptosis in in vivo studies of toxin and hypoxia-induced injury in renal mesangial cells (Fishbane et al., 2004). In contrast to these positive studies, Brendt et al. (2009) did not find preservation of myocardial function with darbEpo in a sepsis model. However, those investigators also reported that their sepsis model induced resistance to erythropoiesis with darbEpo.
Despite the differences in structure, studies of unidirectional blood-to-brain influx rate have shown that both Epo and darbEpo cross the blood-brain barrier at approximately the same rate as albumin (Banks et al., 2004). In these studies, the percentage of the intravenously injected dose that was taken up was 0.05 to 0.1%/g of brain tissue and the tissue levels peaked at 3 h after the injection. Consistent with these pharmacokinetic data, a few studies have demonstrated neuroprotection with darbEpo in various models of central nervous system injury. darbEpo (10 μg/kg) given after the onset of ischemia reduced infarct volume and improved neurological recovery after 2 h of middle cerebral artery occlusion (Belayev et al., 2005). In an intracerebral hemorrhage model where autologous blood was injected into the striatum darbEpo improved neurological recovery and reduced histological signs of injury (Grasso et al., 2009). In spinal cord injury, however, 10 μg/kg darbEpo given at 1 h after a thoracic contusion injury did not improve behavioral outcome or spare histological signs of injury (Mann et al., 2008).
The present study confirms that darbEpo in the doses of 25 and 50 μg/kg given 5 min after traumatic injury resulted in a significantly smaller contusion volume than in the saline-treated animals, suggestive of a neuroprotective effect in a model of TBI. The reduction in contusion volume occurred when darbEpo was given within a time window of 6 h postinjury, which is similar to the time window that has been observed for Epo in this TBI model (Cherian et al., 2007). Unlike Epo, however, significant improvement in hippocampal neuron survival was not observed with darbEpo.
Numerous actions of Epo and darbEpo might be potentially neuroprotective after TBI. Both agents have erythropoietic stimulating effects; however, studies where the increased hematocrit was normalized through hemodilution suggest that the neuroprotective effects of Epo are independent of the changes in hematocrit (Zhang et al., 2009). Other derivatives of Epo, such as carbamylated Epo and Epo-derived peptides, do not stimulate erythropoiesis and yet still have neuroprotective actions (Leist et al., 2004; Adembri et al., 2008; Brines et al., 2008). Other protective effects of Epo may include inhibition of apoptosis, oxidative stress, inflammatory mediators, and preservation of mitochondrial function (Yatsiv et al., 2005).
Vascular effects of Epo may also play a role in neuroprotection, but results are conflicting. Recent studies in mice lacking the neural Epo receptor have shown that administration of Epo still provides neuroprotection, suggesting that at least part of its therapeutic effect after TBI may be mediated through vascular protection (Xiong et al., 2010). Some studies have suggested that these vascular effects of Epo may involve vascular endothelium. The presence of EpoR has been observed in vascular endothelium, although one group using a specific EpoR antibody has failed to identify functional EpoR in endothelium (Sinclair et al., 2010). In vitro studies using isolated middle cerebral artery preparations have shown that abluminal application of Epo induces concentration-dependent dilations and pretreatment with Epo for 24 h in vivo potentiates dilations mediated by endothelium-derived hyperpolarizing factor (Shafi et al., 2008).
In contrast to these studies demonstrating vasodilation and enhanced nitric-oxide production with Epo, chronic administration (1 week) of Epo caused attenuated depressor responses to endothelium-dependent vasodilators that may have suggested inhibition of nitric-oxide synthase activity (Noguchi et al., 2007). Others have suggested that Epo inhibits nitric-oxide synthase protein expression (Wang and Vaziri, 1999) and reduces nitric-oxide concentration in the brain after ischemia (Calapai et al., 2000).
In previous studies in the cortical impact injury model, pretreatment with Epo for 3 days before cortical impact injury resulted in an enhanced recovery of CBF and tissue nitric-oxide concentrations in peri-contusional brain in response to postinjury infusion of l-arginine (Cherian et al., 2007). The present studies show similar enhancement of CBF recovery after infusion of l-arginine with darbEpo. The tissue nitric-oxide levels, however, were not significantly different in the present study, recovering back to preinjury baselines in both the darbEpo- and saline-treated groups after administration of l-arginine. This finding suggests that a mechanism other than or in addition to nitric oxide might be responsible for the enhanced recovery of CBF.
Some limitations of the methods used in the present study should be acknowledged. The assessment of neurological outcome used only histological end-points. Inclusion of behavioral endpoints might have resulted in different conclusions about neuroprotection with darbEpo. The method for assessing CBF is not an absolute measure of blood flow, and measurements must be standardized to a preinjury baseline value. The laser Doppler method for assessing CBF is also affected by hemoglobin concentration. It is unlikely that treatment with darbEpo for only 3 days before injury resulted in a significant increase in hemoglobin concentration at the time of the injury. However, because hemoglobin concentration was not measured at the time of injury in the present studies, this possibility cannot be completely excluded.
The measurement of tissue nitric oxide does not clearly distinguish which isoform of NO synthase is responsible for the changes in nitric oxide that were observed. Because the measurements were performed in the period immediately surrounding the injury, it can probably be concluded that the constitutive isoforms of nitric-oxide synthase are involved. A previous study performed in transgenic mice deficient in the endothelial isoform of nitric-oxide synthase suggested that the endothelial isoform is involved in the reduction in CBF that occurs after injury (Hlatky et al., 2003).
Although darbEpo has different pharmacokinetic characteristics from Epo, postinjury treatment with darbEpo resulted in a similar dose- and time-dependent reduction in contusion volume to Epo. darbEpo also enhanced the CBF response to postinjury infusion of l-arginine, suggesting that vascular effects of darbEpo may play a role in neuroprotection.
This study was supported by the National Institutes of Health National Institute of Neurological Disorders and Stroke [Grant P01-NS38660] and a grant from Amgen, Inc.
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.110.176602.
- Epo
- erythropoietin
- darbEpo
- darbepoetin alfa
- EpoR
- Epo receptor
- EPOR2
- Epo receptor complex
- CBF
- cerebral blood flow
- CII
- cortical impact injury
- TBI
- traumatic brain injury
- LDF
- laser Doppler flow
- NO
- nitric oxide
- RSNO
- S-nitroso-N-acetyl-dl-penicillamine.
Authorship Contributions
Participated in research design: Cherian, Goodman, and Robertson.
Conducted experiments: Cherian, Goodman, and Robertson.
Performed data analysis: Cherian, Goodman, and Robertson.
Wrote or contributed to the writing of the manuscript: Cherian, Goodman, and Robertson.
References
- Adembri C, Massagrande A, Tani A, Miranda M, Margheri M, De Gaudio R, Pellegrini-Giampietro DE. (2008) Carbamylated erythropoietin is neuroprotective in an experimental model of traumatic brain injury. Crit Care Med 36:975–978 [DOI] [PubMed] [Google Scholar]
- Alafaci C, Salpietro F, Grasso G, Sfacteria A, Passalacqua M, Morabito A, Tripodo E, Calapai G, Buemi M, Tomasello F. (2000) Effect of recombinant human erythropoietin on cerebral ischemia following experimental subarachnoid hemorrhage. Eur J Pharmacol 406:219–225 [DOI] [PubMed] [Google Scholar]
- Aydin A, Genç K, Akhisaroglu M, Yorukoglu K, Gokmen N, Gonullu E. (2003) Erythropoietin exerts neuroprotective effect in neonatal rat model of hypoxic-ischemic brain injury. Brain Dev 25:494–498 [DOI] [PubMed] [Google Scholar]
- Baker JE, Kozik D, Hsu AK, Fu X, Tweddell JS, Gross GJ. (2007) Darbepoetin alfa protects the rat heart against infarction: dose-response, phase of action, and mechanisms. J Cardiovasc Pharmacol 49:337–345 [DOI] [PubMed] [Google Scholar]
- Banks WA, Jumbe NL, Farrell CL, Niehoff ML, Heatherington AC. (2004) Passage of erythropoietic agents across the blood-brain barrier: a comparison of human and murine erythropoietin and the analog darbepoetin alfa. Eur J Pharmacol 505:93–101 [DOI] [PubMed] [Google Scholar]
- Belayev L, Khoutorova L, Zhao W, Vigdorchik A, Belayev A, Busto R, Magal E, Ginsberg MD. (2005) Neuroprotective effect of darbepoetin alfa, a novel recombinant erythropoietic protein, in focal cerebral ischemia in rats. Stroke 36:1071–1076 [DOI] [PubMed] [Google Scholar]
- Brendt P, Frey U, Adamzik M, Schäfer ST, Peters J. (2009) Darbepoetin alpha, a long-acting erythropoietin derivate, does not alter LPS evoked myocardial depression and gene expression of Bax, Bcl-Xs, Bcl-XL, Bcl-2, and TNF-α. Shock 31:50–54 [DOI] [PubMed] [Google Scholar]
- Brines M, Cerami A. (2008) Erythropoietin-mediated tissue protection: reducing collateral damage from the primary injury response. J Intern Med 264:405–432 [DOI] [PubMed] [Google Scholar]
- Brines M, Grasso G, Fiordaliso F, Sfacteria A, Ghezzi P, Fratelli M, Latini R, Xie QW, Smart J, Su-Rick CJ, et al. (2004) Erythropoietin mediates tissue protection through an erythropoietin and common β-subunit heteroreceptor. Proc Natl Acad Sci USA 101:14907–14912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brines M, Patel NS, Villa P, Brines C, Mennini T, De Paola M, Erbayraktar Z, Erbayraktar S, Sepodes B, Thiemermann C, et al. (2008) Nonerythropoietic, tissue-protective peptides derived from the tertiary structure of erythropoietin. Proc Natl Acad Sci USA 105:10925–10930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brines ML, Ghezzi P, Keenan S, Agnello D, de Lanerolle NC, Cerami C, Itri LM, Cerami A. (2000) Erythropoietin crosses the blood-brain barrier to protect against experimental brain injury. Proc Natl Acad Sci USA 97:10526–10531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calapai G, Marciano MC, Corica F, Allegra A, Parisi A, Frisina N, Caputi AP, Buemi M. (2000) Erythropoietin protects against brain ischemic injury by inhibition of nitric oxide formation. Eur J Pharmacol 401:349–356 [DOI] [PubMed] [Google Scholar]
- Catania MA, Marciano MC, Parisi A, Sturiale A, Buemi M, Grasso G, Squadrito F, Caputi AP, Calapai G. (2002) Erythropoietin prevents cognition impairment induced by transient brain ischemia in gerbils. Eur J Pharmacol 437:147–150 [DOI] [PubMed] [Google Scholar]
- Celik M, Gökmen N, Erbayraktar S, Akhisaroglu M, Konakc S, Ulukus C, Genc S, Genc K, Sagiroglu E, Cerami A, et al. (2002) Erythropoietin prevents motor neuron apoptosis and neurologic disability in experimental spinal cord ischemic injury. Proc Natl Acad Sci USA 99:2258–2263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherian L, Goodman JC, Robertson C. (2007) Neuroprotection with erythropoietin administration following controlled cortical impact injury in rats. J Pharmacol Exp Ther 322:789–794 [DOI] [PubMed] [Google Scholar]
- Digicaylioglu M, Bichet S, Marti HH, Wenger RH, Rivas LA, Bauer C, Gassmann M. (1995) Localization of specific erythropoietin binding sites in defined areas of the mouse brain. Proc Natl Acad Sci USA 92:3717–3720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott S, Lorenzini T, Asher S, Aoki K, Brankow D, Buck L, Busse L, Chang D, Fuller J, Grant J, et al. (2003) Enhancement of therapeutic protein in vivo activities through glycoengineering. Nat Biotechnol 21:414–421 [DOI] [PubMed] [Google Scholar]
- Emir M, Ozisik K, Cagli K, Misirlioglu M, Ozisik P, Iscan Z, Yildirim E, Kilinc K, Sener E. (2004) Effect of erythropoietin on bcl-2 gene expression in rat cardiac myocytes after traumatic brain injury. Transplant Proc 36:2935–2938 [DOI] [PubMed] [Google Scholar]
- Fishbane S, Ragolia L, Palaia T, Johnson B, Elzein H, Maesaka JK. (2004) Cytoprotection by darbepoetin/epoetin alfa in pig tubular and mouse mesangial cells. Kidney Int 65:452–458 [DOI] [PubMed] [Google Scholar]
- Gao E, Boucher M, Chuprun JK, Zhou RH, Eckhart AD, Koch WJ. (2007) Darbepoetin alfa, a long-acting erythropoietin analog, offers novel and delayed cardioprotection for the ischemic heart. Am J Physiol Heart Circ Physiol 293:H60–H68 [DOI] [PubMed] [Google Scholar]
- Gorio A, Gokmen N, Erbayraktar S, Yilmaz O, Madaschi L, Cichetti C, Di Giulio AM, Vardar E, Cerami A, Brines M. (2002) Recombinant human erythropoietin counteracts secondary injury and markedly enhances neurological recovery from experimental spinal cord trauma. Proc Natl Acad Sci USA 99:9450–9455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grasso G, Graziano F, Sfacteria A, Carletti F, Meli F, Maugeri R, Passalacqua M, Certo F, Fazio M, Buemi M, et al. (2009) Neuroprotective effect of erythropoietin and darbepoetin alfa after experimental intracerebral hemorrhage. Neurosurgery 65:763–769; discussion 769–770 [DOI] [PubMed] [Google Scholar]
- Grasso G, Sfacteria A, Meli F, Fodale V, Buemi M, Iacopino DG. (2007) Neuroprotection by erythropoietin administration after experimental traumatic brain injury. Brain Res 1182:99–105 [DOI] [PubMed] [Google Scholar]
- Hlatky R, Lui H, Cherian L, Goodman JC, O'Brien WE, Contant CF, Robertson CS. (2003) The role of endothelial nitric oxide synthase in the cerebral hemodynamics after controlled cortical impact injury in mice. J Neurotrauma 20:995–1006 [DOI] [PubMed] [Google Scholar]
- Johnson DW, Pat B, Vesey DA, Guan Z, Endre Z, Gobe GC. (2006) Delayed administration of darbepoetin or erythropoietin protects against ischemic acute renal injury and failure. Kidney Int 69:1806–1813 [DOI] [PubMed] [Google Scholar]
- Kanellakis P, Pomilio G, Agrotis A, Gao X, Du XJ, Curtis D, Bobik A. (2010) Darbepoetin-mediated cardioprotection after myocardial infarction involves multiple mechanisms independent of erythropoietin receptor-common β-chain heteroreceptor. Br J Pharmacol 160:2085–2096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leist M, Ghezzi P, Grasso G, Bianchi R, Villa P, Fratelli M, Savino C, Bianchi M, Nielsen J, Gerwien J, et al. (2004) Derivatives of erythropoietin that are tissue protective but not erythropoietic. Science 305:239–242 [DOI] [PubMed] [Google Scholar]
- Mann C, Lee JH, Liu J, Stammers AM, Sohn HM, Tetzlaff W, Kwon BK. (2008) Delayed treatment of spinal cord injury with erythropoietin or darbepoetin–a lack of neuroprotective efficacy in a contusion model of cord injury. Exp Neurol 211:34–40 [DOI] [PubMed] [Google Scholar]
- Noguchi CT, Asavaritikrai P, Teng R, Jia Y. (2007) Role of erythropoietin in the brain. Crit Rev Oncol Hematol 64:159–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafi NI, Andresen J, Marrelli SP, Bryan RM., Jr (2008) Erythropoietin potentiates EDHF-mediated dilations in rat middle cerebral arteries. J Neurotrauma 25:257–265 [DOI] [PubMed] [Google Scholar]
- Sinclair AM, Coxon A, McCaffery I, Kaufman S, Paweletz K, Liu L, Busse L, Swift S, Elliott S, Begley CG. (2010) Functional erythropoietin receptor is undetectable in endothelial, cardiac, neuronal, and renal cells. Blood 115:4264–4272 [DOI] [PubMed] [Google Scholar]
- Sirén AL, Fratelli M, Brines M, Goemans C, Casagrande S, Lewczuk P, Keenan S, Gleiter C, Pasquali C, Capobianco A, et al. (2001) Erythropoietin prevents neuronal apoptosis after cerebral ischemia and metabolic stress. Proc Natl Acad Sci USA 98:4044–4049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XQ, Vaziri ND. (1999) Erythropoietin depresses nitric oxide synthase expression by human endothelial cells. Hypertension 33:894–899 [DOI] [PubMed] [Google Scholar]
- Xiong Y, Lu D, Qu C, Goussev A, Schallert T, Mahmood A, Chopp M. (2008) Effects of erythropoietin on reducing brain damage and improving functional outcome after traumatic brain injury in mice. J Neurosurg 109:510–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Y, Mahmood A, Qu C, Kazmi H, Zhang ZG, Noguchi CT, Schallert T, Chopp M. (2010) Erythropoietin improves histological and functional outcomes after traumatic brain injury in mice in the absence of the neural erythropoietin receptor. J Neurotrauma 27:205–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatsiv I, Grigoriadis N, Simeonidou C, Stahel PF, Schmidt OI, Alexandrovitch AG, Tsenter J, Shohami E. (2005) Erythropoietin is neuroprotective, improves functional recovery, and reduces neuronal apoptosis and inflammation in a rodent model of experimental closed head injury. FASEB J 19:1701–1703 [DOI] [PubMed] [Google Scholar]
- Yildirim E, Ozisik K, Solaroglu I, Kaptanoglu E, Beskonakli E, Sargon MF, Kilinc K, Sakinci U. (2005) Protective effect of erythropoietin on type II pneumocyte cells after traumatic brain injury in rats. J Trauma 58:1252–1258 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Xiong Y, Mahmood A, Meng Y, Qu C, Schallert T, Chopp M. (2009) Therapeutic effects of erythropoietin on histological and functional outcomes following traumatic brain injury in rats are independent of hematocrit. Brain Res 1294:153–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L, Jin W, Pan H, Hu Z, Zhou J, Hang C, Shi J. (2009a) Erythropoietin inhibits the increase of intestinal labile zinc and the expression of inflammatory mediators after traumatic brain injury in rats. J Trauma 66:730–736 [DOI] [PubMed] [Google Scholar]
- Zhu L, Wang HD, Yu XG, Jin W, Qiao L, Lu TJ, Hu ZL, Zhou J. (2009b) Erythropoietin prevents zinc accumulation and neuronal death after traumatic brain injury in rat hippocampus: in vitro and in vivo studies. Brain Res 1289:96–105 [DOI] [PubMed] [Google Scholar]