Abstract
Studies show that kidneys produce 2′,3′-cAMP, 2′,3′-cAMP is exported and metabolized to 2′-AMP and 3′-AMP, 2′-AMP and 3′-AMP are metabolized to adenosine, 2′,3′-cAMP inhibits proliferation of preglomerular vascular smooth muscle cells (PGVSMCs) and glomerular mesangial cells (GMCs), and A2B (not A1, A2A, or A3) adenosine receptors mediate part of the antiproliferative effects of 2′,3′-cAMP. These findings suggest that extracellular 2′,3′-cAMP attenuates proliferation of PGVSMCs and GMCs partly via conversion to corresponding AMPs, which are metabolized to adenosine that activates A2B receptors. This hypothesis predicts that extracellular 2′-AMP and 3′-AMP should exert A2B receptor-mediated antiproliferative effects. Therefore, we examined the antiproliferative effects (cell counts) of 2′-AMP and 3′-AMP. In PGVSMCs and GMCs, 2′-AMP and 3′-AMP exerted concentration-dependent antiproliferative effects. 3′-AMP was equipotent with and 2′-AMP was 3-fold less potent than 5′-AMP (prototypical adenosine precursor). In PGVSMCs, the effects of 2′-AMP and 3′-AMP were mimicked by adenosine, and 8-[4-[((4-cyanophenyl)carbamoylmethyl)oxy]phenyl]-1,3-di(n-propyl)xanthine (MRS-1754) (A2B receptor antagonist) equally blocked the antiproliferative effects of 2′-AMP, 3′-AMP, and adenosine but less effectively blocked the effects of 2′,3′-cAMP. Similar results were obtained in GMCs except that MRS-1754 also incompletely blocked the effects of 3′-AMP. We conclude that in PGVSMCs, 2′-AMP and 3′-AMP are antiproliferative, the antiproliferative effects of 2′-AMP and 3′-AMP are mediated nearly entirely by adenosine/A2B receptors, and some of the antiproliferative effects of 2′,3′-cAMP are independent of adenosine/A2B receptors. Similar conclusions apply to GMCs except that 3′-AMP also has actions independent of adenosine/A2B receptors. Because A2B receptors are renoprotective, 2′-AMP and 3′-AMP may provide renoprotection by generating adenosine that activates A2B receptors.
Introduction
Studies using high-performance liquid chromatography-tandem mass spectrometry demonstrate the production and release into the extracellular compartment of 2′,3′-cAMP, a positional isomer of 3′,5′-cAMP, by intact rat kidneys (Ren et al., 2009). Moreover, experiments in intact rat kidneys show that extracellular 2′,3′-cAMP is metabolized to extracellular 2′-AMP and 3′-AMP and that these AMPs are further metabolized to extracellular adenosine (a mechanism called the extracellular 2′,3′-cAMP-adenosine pathway) (Jackson et al., 2009).
More recent studies indicate that extracellular 2′,3′-cAMP has biological effects on kidney cells (Jackson et al., 2010). For example, extracellular 2′,3′-cAMP inhibits the proliferation of preglomerular vascular smooth muscle cells (PGVSMCs) and glomerular mesangial cells (GMCs). Mechanistically, this inhibition is probably in part due to conversion of 2′,3′-cAMP to adenosine, a conclusion supported by the observations that the antiproliferative effects of 2′,3′-cAMP are accompanied by the metabolism of 2′,3′-cAMP to 2′-AMP and 3′-AMP, which are further metabolized to adenosine (Jackson et al., 2010), and the antigrowth effects of 2′,3′-cAMP are attenuated by antagonism of A2B receptors with 8-[4-[((4-cyanophenyl)carbamoylmethyl)oxy]phenyl]-1,3-di(n-propyl)xanthine (MRS-1754), but not by antagonism of A1 receptors with 1,3-dipropyl-8-cyclopentylxanthine, A2A receptors with 7-(2-phenylethyl)-5-amino-2-(2-furyl)-pyrazolo-[4,3-e]-1,2,4-triazolo[1,5-c]pyrimidine (SCH-58261), or A3 receptors with N-(2-methoxyphenyl)-N′-[2-(3-pyridinyl)-4-quinazolinyl]-urea (VUF-5574) (Jackson et al., 2010). In addition, previous studies demonstrated that adenosine, via A2B receptors, inhibits growth of vascular smooth muscle cells and GMCs (Dubey et al., 1996b, 1998b, 2000, 2005). It seems likely then that extracellular 2′,3′-cAMP inhibits growth of PGVSMCs and GMCs at least in part via the extracellular 2′,3′-cAMP-adenosine pathway, which then activates antiproliferative A2B receptors (solid arrows in Fig. 1). However, this hypothesis predicts that extracellular 2′-AMP and 3′-AMP should also exert antiproliferative effects that are blocked by antagonism of A2B receptors, and this prediction has yet to be tested. Therefore, a major objective of this study was to further test the hypothesis shown in Fig. 1 (solid arrows) by examining the antiproliferative effects of 2′-AMP and 3′-AMP in the absence and presence of a selective and potent A2B receptor antagonist.
Fig. 1.
Our previously published work (Jackson et al., 2009, 2010) indicated that 2′,3′-cAMP exerts antiproliferative effects on PGVSMCs and GMCs at least in part by the mechanism shown by the solid arrows. The dashed and dotted arrows represent possible additional mechanisms. PDE, phosphodiesterase; NT, nucleotidase.
Although extracellular 2′,3′-cAMP probably inhibits growth of PGVSMCs and GMCs in part via the extracellular 2′,3′-cAMP-adenosine pathway and via A2B receptors (Jackson et al., 2010), it is conceivable that extracellular 2′,3′-cAMP has antiproliferative effects that are not adenosine-mediated. In this regard, it is possible that 2′,3′-cAMP exerts nonadenosine-mediated antiproliferative actions that are also independent of 2′-AMP or 3′-AMP (dashed arrows in Fig. 1); it is also conceivable that 2′,3′-cAMP has antiproliferative actions that are due to nonadenosine-mediated effects of 2′-AMP or 3′-AMP (dotted arrows in Fig. 1). Therefore, another goal of the present study was to explore the possibility that extracellular 2′,3′-cAMP inhibits growth of PGVSMCs and GMCs independently of adenosine.
Materials and Methods
Animals.
PGVSMCs and GMCs were harvested from adult male Wistar-Kyoto rats (Taconic Farms, Germantown, NY). The institutional animal care and use committee approved all procedures, and the investigation conforms to the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, 1996).
Drugs.
2′,3′-cAMP, 5′-AMP, 3′-AMP, 2′-AMP, and MRS-1754 [selective A2B receptor antagonist (Jacobson and Knutsen, 2001)] were obtained from Sigma-Aldrich (St. Louis, MO).
Cell Culture.
Rat PGVSMCs and GMCs were harvested and cultured as described by us previously (Dubey et al., 1992; Inoue et al., 1998; Mokkapatti et al., 1998). All experiments were performed in cells in third to fifth passage.
Cell Proliferation Studies.
Cell proliferation studies were conducted as described previously (Dubey et al., 1996a). In brief, PGVSMCs and GMCs were plated at an initial density of 5000 cells/culture in Dulbecco's modified Eagle's medium Nutrient Mixture F-12 (DMEM/F12) containing 2.5% fetal calf serum and were allowed to attach and proliferate overnight. The next day, cells were growth-arrested in DMEM/F12 containing 0.25% albumin for 24 h to synchronize cells. Then DMEM/F12 containing 2.5% fetal calf serum (along with the various treatments) was added to stimulate cell proliferation. Treatments were administered daily, and after 4 days the cell number was determined by counting cells with a Coulter counter.
Statistics.
Data were analyzed by one- or two-factor analysis of variance, with post hoc comparisons using Fisher's least significant difference test if the main effects of the analysis of variance were significant. The criterion of significance was p < 0.05. All values in text and figures are means ± S.E.M.
Results
We first investigated the concentration-dependent effects of 2′-AMP and 3′-AMP on proliferation of PGVSMCs and GMCs. As shown in Fig. 2, both 2′-AMP and 3′-AMP significantly, profoundly, and concentration dependently attenuated the proliferation of PGVSMCs (Fig. 2A) and GMCs (Fig. 2B). Of importance, 3′-AMP was as potent and efficacious in this regard as the prototypical adenosine precursor, 5′-AMP. Although 2′-AMP was approximately 3-fold less potent than 3′-AMP and 5′-AMP, nonetheless, 2′-AMP was an effective inhibitor of PGVSMC and GMC proliferation (Fig. 2, A and B, respectively). 2′-AMP, 3′-AMP, and 5′-AMP did not affect cell morphology or trypan blue exclusion, indicating that the AMPs did not alter cell viability.
Fig. 2.
Line graphs summarize the concentration-dependent effects of 2′-AMP, 3′-AMP, and 5′-AMP on cell number in PGVSMCs (A) and GMCs (B). Values are means ± S.E.M. of n PGVSMC and GMC cultures (S.E.M.s are smaller than symbol size). a, p < 0.05 for “0” levels of AMPs compared with all other concentrations of all other AMPs; b, p < 0.05 for 2′-AMP compared with corresponding concentrations of 3′-AMP and 5′-AMP; c, p < 0.05 for 3′-AMP compared with corresponding concentration of 5′-AMP.
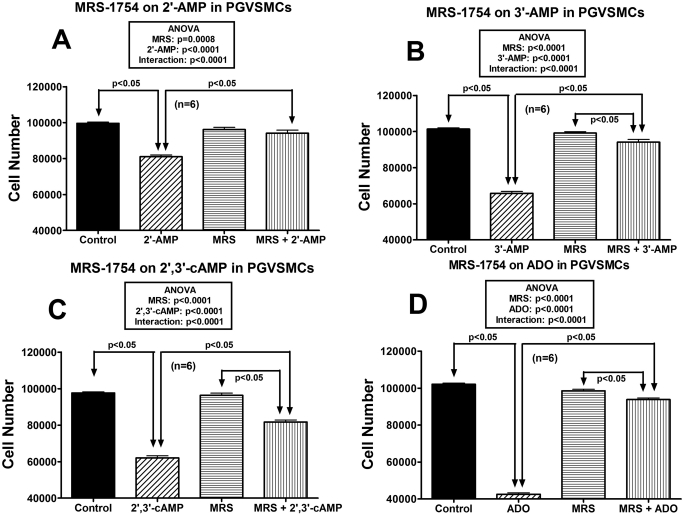
Next, we examined whether the effects of 2′-AMP and 3′-AMP in PGVSMCs could be accounted for by adenosine acting via A2B receptors. The ability of 2′-AMP (30 μM) to inhibit PGVSMC proliferation was abolished by MRS-1754 (100 nM) (Fig. 3A), and the ability of 3′-AMP (30 μM) to inhibit PGVSMC proliferation was nearly abolished (Fig. 3B). In contrast to 2′-AMP and 3′-AMP, although the antiproliferative effects of 2′,3′-cAMP (30 μM) were reduced by MRS-1754 (100 nM), there remained a considerable antiproliferative effect of 2′,3′-cAMP even in the presence of MRS-1754 (Fig. 3C). MRS-1754 (100 nM) inhibited nearly all of the antiproliferative actions of adenosine (Fig. 3D), indicating that the concentration of MRS-1754 was adequate to antagonize the A2B receptor in PGVSMCs.
Fig. 3.
Bar graphs illustrate the effects of 30 μM 2′-AMP (A), 30 μM 3′-AMP (B), 30 μM 2′,3′-cAMP (C), and 30 μM adenosine (ADO) (D) on PGVSMC cell number in the absence and presence of 100 nM MRS-1754 (MRS). Values are means ± S.E.M. of n PGVSMC cultures.
Subsequently, we examined whether the effects of 2′-AMP and 3′-AMP in GMCs could be accounted for by adenosine acting via A2B receptors. The ability of 2′-AMP (30 μM) to inhibit GMC proliferation was abolished by MRS-1754 (100 nM) (Fig. 4A), and the ability of 3′-AMP (30 μM) to inhibit GMC proliferation was attenuated but clearly present (Fig. 4B). In contrast to 2′-AMP, although the antiproliferative effects of 2′,3′-cAMP (30 μM) were reduced by MRS-1754 (100 nM), there remained a considerable antiproliferative effect of 2′,3′-cAMP even in the presence of MRS-1754 (Fig. 4C). As in PGVSMCs, in GMCs, MRS-1754 (100 nM) inhibited nearly all of the antiproliferative actions of adenosine (Fig. 4D), indicating that the concentration of MRS-1754 was adequate to antagonize the A2B receptor in GMCs.
Fig. 4.
Bar graphs illustrate the effects of 30 μM 2′-AMP (A), 30 μM 3′-AMP (B), 30 μM 2′,3′-cAMP (C), and 30 μM adenosine (ADO) (D) on GMC cell number in the absence and presence of 100 nM MRS-1754 (MRS). Values are means ± S.E.M. of n PGVSMC cultures.
The data in Figs. 3 and 4 were used to statistically compare the percent reversal (antagonism) by MRS-1754 of the antiproliferative effects of adenosine, 2′,3′-cAMP, 2′-AMP, and 3′-AMP. As shown in Fig. 5, in PGVSMCs (Fig. 5A) the antagonism by MRS-1754 of the antiproliferative effects of the purines was 1) nearly complete for adenosine, 2′-AMP, and 3′-AMP, 2) not statistically significantly different for adenosine versus 2′-AMP versus 3′-AMP, and 3) statistically significantly less for 2′,3′-cAMP versus those of the other three purines (adenosine, 2′-AMP, and 3′-AMP). As shown in Fig. 5B, similar results were obtained in GMCs with the exception that although MRS-1754 antagonized the effects of 3′-AMP more so than the effects of 2′,3′-cAMP, the antagonism by MRS-1754 was greater against adenosine than against 3′-AMP.
Fig. 5.
Bar graphs directly comparing percent reversal (antagonism) by 100 nM MRS-1754 (MRS) of the antiproliferative effects of adenosine (ADO) (30 μM), 2′,3′-cAMP (30 μM), 2′-AMP (30 μM), and 3′-AMP (30 μM) on PGVSMCs (A) and GMCs (B). Values are means ± S.E.M. of n PGVSMC cultures. a, p < 0.05 versus adenosine, 2′-AMP, and 3′-AMP; b, p < 0.05 versus adenosine and 2′,3′-cAMP.
Discussion
Very little is known about the pharmacology of 2′-AMP or 3′-AMP. However, the discovery that 2′,3′-cAMP is endogenously generated and can be metabolized to 2′-AMP and 3′-AMP (Jackson et al., 2009; Ren et al., 2009) provides strong motivation to explore thoroughly the pharmacology and physiology of these neglected AMPs. The existence of 2′,3′-cyclic nucleotide 3′-phosphodiesterase, an enzyme that can metabolize 2′,3′-cAMP to 2′-AMP (Sprinkle, 1989), along with the important recent discovery by Rao et al. (2010) of six different phosphodiesterases that metabolize 2′,3′-cAMP to 3′-AMP, stimulates additional enthusiasm for investigating the pharmacology and physiology of AMPs generated from 2′,3′-cAMP.
In the current report, we focused on the pharmacological effects of 2′-AMP and 3′-AMP on proliferation of PGVSMCs and GMCs. The incentive for these experiments is that previously published data (Jackson et al., 2010) indicated that 2′,3′-cAMP inhibits PGVSMC and GMC proliferation in part via adenosine A2B receptors and that the mechanism seems to be 2′,3′-cAMP → 2′-AMP + 3′-AMP → adenosine → A2B receptor activation → antiproliferative effect. This model predicts that 2′-AMP or 3′-AMP ought also to inhibit PGVSMC and GMC proliferation and the antiproliferative effects of the AMPs should be at least partially blocked by antagonism of A2B adenosine receptors. Indeed, the present experiments confirm that both 2′-AMP and 3′-AMP potently and efficaciously inhibit proliferation of both PGVSMCs and GMCs via, at least in part, A2B receptors.
Our previous study regarding the antiproliferative effect of 2′,3′-cAMP demonstrates that a portion of the antiproliferative action of 2′,3′-cAMP, as assessed by thymidine incorporation, is not reversed by antagonism of A2B receptors with MRS-1754 (Jackson et al., 2010). This result suggests that either 2′,3′-cAMP has nonadenosine-mediated effects or that some of the antiproliferative effects of the adenosine generated from 2′,3′-cAMP are mediated in part by A1, A2A, or A3 adenosine receptors. However, the second possibility is ruled out by our findings that the antiproliferative effects of 2′,3′-cAMP are not affected whatsoever by antagonism of A1, A2A, or A3 adenosine receptors (Jackson et al., 2010). In addition, our previous studies showed that the antiproliferative effects of adenosine are accompanied by increases in intracellular levels of 3′,5′-cAMP (suggestive of A2B receptor signaling), are inhibited by antisense oligonucleotide-induced down-regulation of A2B receptors, and are not mimicked by selective A1, A2A, or A3 receptor agonists (Dubey et al., 1996b, 1997, 1998a,b, 2000, 2001, 2005, 2010). Thus, we are left with the possibility that 2′,3′-cAMP has effects that are independent of adenosine.
The results of the present study confirm, using cell counting (a more reliable index of antiproliferative effects), that although some of the antiproliferative effects of 2′,3′-cAMP are mediated via adenosine acting on A2B receptors, indeed a portion of the effects are not mediated by adenosine. In this regard, we observe that concentrations of MRS-1754 that nearly abolish the antiproliferative effects of adenosine are statistically less effective in preventing the effects of 2′,3′-cAMP. Because the concentration of MRS-1754 used in the present study effectively prevents the antiproliferative actions of adenosine, the lack of full reversal of 2′,3′-cAMP-induced growth inhibition is not due to inadequate concentrations of MRS-1754. This result is consistent with the fact that the Ki of MRS-1754 for A2B receptors is approximately 2 nM (Jacobson and Knutsen, 2001; Kalla et al., 2009), and the current study uses a concentration of 100 nM (50-fold greater than the Ki). Thus, the present study confirms that 2′,3′-cAMP has adenosine-independent actions.
The adenosine-independent actions of 2′,3′-cAMP could be independent of the antiproliferative effect of its “downstream” AMPs or could be mediated by antiproliferative actions of 2′-AMP or 3′-AMP that are adenosine-independent. The present study demonstrates that in PGVSMCs, MRS-1754 abolishes the antiproliferative effects of 2′-AMP and nearly abolishes the antiproliferative effects of 3′-AMP. Of importance, there is no statistically significant difference between adenosine, 2′-AMP, and 3′-AMP with regard to reversal of their antiproliferative effects by MRS-1754. Thus, in PGVSMCs the evidence supports the conclusion that 2′,3′-cAMP exerts an adenosine-independent antiproliferative effect that bypasses both 2′-AMP and 3′-AMP (dashed arrow in Fig. 1). On the other hand, in GMCs, although MRS-1754 reverses completely the antiproliferative effects of 2′-AMP, MRS-1754 is unable to block the effects of 3′-AMP as efficiently as the effects of adenosine or 2′-AMP. Moreover, MRS-1754 has even less effect on 2′,3′-cAMP than on 3′-AMP. Thus, in GMCs, the evidence supports the conclusion that 2′,3′-cAMP exerts an adenosine-independent antiproliferative effect that bypasses both 2′-AMP and 3′-AMP (dashed arrow in Fig. 1) but also exerts an adenosine-independent antiproliferative effect that is due to the nonadenosine-dependent effects of 3′-AMP.
The present study does not address the mechanisms of the nonadenosine-mediated antiproliferative component of 2′,3′-cAMP or 3′-AMP. Studies by Azarashvili et al. (2009) demonstrated that in isolated mitochondria 2′,3′-cAMP promotes the opening of mitochondrial permeability transition pores, and studies by Johnson et al. (1989) in detergent-solubilized and purified tissue preparations showed that 3′-AMP is a P site inhibitor of adenylyl cyclase. It is conceivable that these reported effects of 2′,3′-cAMP and 3′-AMP are involved in the nonadenosine-mediated antiproliferative actions of these purines. However, in the present study these compounds are applied extracellularly. Although cAMPs are actively pumped out of cells (Barber and Butcher, 1983; Deeley et al., 2006; Li et al., 2007; Cheng et al., 2010), because both 2′,3′-cAMP and 3′-AMP are very hydrophilic, they are not likely to diffuse across cell membranes into cells. Because the effects on mitochondria and the P site of adenylyl cyclase would require an intracellular action of 2′,3′-cAMP and 3′-AMP, it seems unlikely that these mechanisms are involved. Moreover, whereas activation of A2B receptors stimulates adenylyl cyclase and inhibits proliferation; in contrast, interaction of 3′-AMP with the P site inhibits adenylyl cyclase, an effect not predicted to inhibit proliferation.
As mentioned, our recent report showed that 2′,3′-cAMP, 2′-AMP, and 3′-AMP are metabolized to adenosine in PGVSMCs and GMCs (Jackson et al., 2010), and our previous studies (Dubey et al., 1996b, 1998b, 2000, 2005) and the current study demonstrate that adenosine inhibits proliferation of vascular smooth muscle cells and GMCs via A2B receptors. Thus, it is likely that any antiproliferative effects of 2′,3′-cAMP, 2′-AMP, and 3′-AMP prevented by MRS-1754 are mediated by adenosine generated from these purines. Although it is logically possible that 2′,3′-cAMP, 2′-AMP, and 3′-AMP could be acting in part as direct A2B receptor agonists, this possibility is extremely unlikely. The pharmacology and structure-activity analysis of a vast number of adenosine analogs support the conclusion that the 2′-hydroxy and 3′-hydroxy moieties on the ribose ring of adenosine analogs are, with very few exceptions not involving negatively charge substituents, required for activity at adenosine receptors (Jacobson and Knutsen, 2001). However, rigorous testing of this hypothesis must await the availability of inhibitors that can block the conversion of 2′,3′-cAMP, 2′-AMP, and 3′-AMP to adenosine.
Evidence is rapidly accumulating that renovascular A2B receptors play an important role in the renal microcirculation. For example, A2B receptors are strongly expressed in rat preglomerular microvessels (Jackson et al., 2002), and studies in A2B receptor knockout mice provide striking evidence that adenosine (Grenz et al., 2007a,b) via activation of renovascular A2B receptors (Grenz et al., 2008) mediates renal ischemic preconditioning and protects the kidney from ischemia/reperfusion injury. Moreover, renovascular A2B receptors dampen the hypoxia-induced vascular leak in mouse kidneys (Eckle et al., 2008), and recent studies using the rat in vitro blood-perfused juxtamedullary nephron technique show that A2B receptors in the afferent arteriole cause profound vasodilation and counteract A1 receptor-induced vasoconstriction (Feng and Navar, 2010). The fact that 2′,3′-cAMP, 2′-AMP, and 3′-AMP can be metabolized to adenosine and can thereby activate renovascular A2B receptors suggests that the 2′,3′-cAMP-adenosine pathway not only may regulate vascular and glomerular structure via antiproliferative effects but may also participate in renal protection and regulation of renovascular tone.
In summary, the present study shows for the first time that 2′-AMP and 3′-AMP exert antiproliferative effects in both PGVSMCs and GMCs. In PGVSMCs, these effects are completely mediated by adenosine-A2B receptor interactions. In GMCs, all of the antiproliferative effects of 2′-AMP are due to adenosine; however, in GMCs a small component of the antiproliferative effects of 3′-AMP is independent of adenosine. Finally, in both PGVSMCs and GMCs, 2′,3′-cAMP has antiproliferative effects independent of 2′-AMP, 3′-AMP, and adenosine. Because mRNA turnover is a major endogenous source of 2′,3′-cAMP (Jackson et al., 2009; Ren et al., 2009), increased mRNA metabolism stimulated by apoptosis (Del Prete et al., 2002) may inhibit PGVSMC and GMC growth and limit pathological remodeling in renal diseases via production of 2′,3′-cAMP, 2′-AMP, and 3′-AMP. Moreover, part of the pharmacology of rapamycin, which stimulates mRNA turnover (Banholzer et al., 1997; Hashemolhosseini et al., 1998; Albig and Decker, 2001), may be due to effects of 2′,3′-cAMP, 2′-AMP, and 3′-AMP. In this regard, rapamycin is commonly used for renal transplant patients because of its lower renal toxicity relative to that of other immunosuppressants, and inhibition of proliferation of PGVSMCs and GMCs could be involved in this beneficial action.
The work was supported by the National Institutes of Health National Heart, Lung, and Blood Institute [Grant HL069846]; the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases [Grants DK068575, DK079307]; the Swiss National Science Foundation [Grants 3200B0-106098/1, 320000-117998/1]; Oncosuisse [Grant OCS-01551-08-2004]; and EMDO Stiftung.
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.110.178137.
- PGVSMC
- preglomerular vascular smooth muscle cell
- GMC
- glomerular mesangial cell
- MRS-1754
- 8-[4-[((4-cyanophenyl)carbamoylmethyl)oxy]phenyl]-1,3-di(n-propyl)xanthine
- SCH58261
- 7-(2-phenylethyl)-5-amino-2-(2-furyl)-pyrazolo-[4,3-e]-1,2,4-triazolo[1,5-c]pyrimidine
- VUF-5574
- N-(2-methoxyphenyl)-N′-[2-(3-pyridinyl)-4-quinazolinyl]-urea
- DMEM/F12
- Dulbecco's modified Eagle's medium Nutrient Mixture F-12.
Authorship Contributions
Participated in research design: Jackson and Dubey.
Conducted experiments: Gillespie.
Contributed new reagents or analytic tools: Dubey.
Performed data analysis: Jackson.
Wrote or contributed to the writing of the manuscript: Jackson and Dubey.
Other: Jackson acquired funding for the research.
References
- Albig AR, Decker CJ. (2001) The target of rapamycin signaling pathway regulates mRNA turnover in the yeast Saccharomyces cerevisiae. Mol Biol Cell 12:3428–3438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azarashvili T, Krestinina O, Galvita A, Grachev D, Baburina Y, Stricker R, Evtodienko Y, Reiser G. (2009) Ca2+-dependent permeability transition regulation in rat brain mitochondria by 2′,3′-cyclic nucleotides and 2′,3′-cyclic nucleotide 3′-phosphodiesterase. Am J Physiol Cell Physiol 296:C1428–C1439 [DOI] [PubMed] [Google Scholar]
- Banholzer R, Nair AP, Hirsch HH, Ming XF, Moroni C. (1997) Rapamycin destabilizes interleukin-3 mRNA in autocrine tumor cells by a mechanism requiring an intact 3′ untranslated region. Mol Cell Biol 17:3254–3260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber R, Butcher RW. (1983) The egress of cyclic-AMP from metazoan cells. Adv Cyclic Nucleotide Res 15:119–138 [Google Scholar]
- Cheng D, Ren J, Jackson EK. (2010) Multidrug resistance protein 4 mediates cAMP efflux from rat preglomerular vascular smooth muscle cells. Clin Exp Pharmacol Physiol 37:205–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deeley RG, Westlake C, Cole SP. (2006) Transmembrane transport of endo- and xenobiotics by mammalian ATP-binding cassette multidrug resistance proteins. Physiol Rev 86:849–899 [DOI] [PubMed] [Google Scholar]
- Del Prete MJ, Robles MS, Guáo A, Martínez-A C, Izquierdo M, Garcia-Sanz JA. (2002) Degradation of cellular mRNA is a general early apoptosis-induced event. FASEB J 16:2003–2005 [DOI] [PubMed] [Google Scholar]
- Dubey RK, Gillespie DG, Jackson EK. (1998a) Adenosine inhibits collagen and protein synthesis in cardiac fibroblasts: role of A2B receptors. Hypertension 31:943–948 [DOI] [PubMed] [Google Scholar]
- Dubey RK, Gillespie DG, Mi Z, Jackson EK. (1997) Exogenous and endogenous adenosine inhibits fetal calf serum-induced growth of rat cardiac fibroblasts: role of A2B receptors. Circulation 96:2656–2666 [DOI] [PubMed] [Google Scholar]
- Dubey RK, Gillespie DG, Mi Z, Jackson EK. (1998b) Adenosine inhibits growth of human aortic smooth muscle cells via A2B receptors. Hypertension 31:516–521 [DOI] [PubMed] [Google Scholar]
- Dubey RK, Gillespie DG, Mi Z, Jackson EK. (2005) Adenosine inhibits PDGF-induced growth of human glomerular mesangial cells via A2B receptors. Hypertension 46:628–634 [DOI] [PubMed] [Google Scholar]
- Dubey RK, Gillespie DG, Mi Z, Jackson EK. (2010) Extracellular 3′,5′-cyclic AMP-adenosine pathway inhibits glomerular mesangial cell growth. J Pharmacol Exp Ther 333:808–815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubey RK, Gillespie DG, Mi Z, Suzuki F, Jackson EK. (1996a) Smooth muscle cell-derived adenosine inhibits cell growth. Hypertension 27:766–773 [DOI] [PubMed] [Google Scholar]
- Dubey RK, Gillespie DG, Osaka K, Suzuki F, Jackson EK. (1996b) Adenosine inhibits growth of rat aortic smooth muscle cells. Possible role of A2b receptor. Hypertension 27:786–793 [DOI] [PubMed] [Google Scholar]
- Dubey RK, Gillespie DG, Shue H, Jackson EK. (2000) A2B receptors mediate antimitogenesis in vascular smooth muscle cells. Hypertension 35:267–272 [DOI] [PubMed] [Google Scholar]
- Dubey RK, Gillespie DG, Zacharia LC, Mi Z, Jackson EK. (2001) A2B receptors mediate the antimitogenic effects of adenosine in cardiac fibroblasts. Hypertension 37:716–721 [DOI] [PubMed] [Google Scholar]
- Dubey RK, Roy A, Overbeck HW. (1992) Culture of renal arteriolar smooth muscle cells. Mitogenic responses to angiotensin II. Circ Res 71:1143–1152 [DOI] [PubMed] [Google Scholar]
- Eckle T, Faigle M, Grenz A, Laucher S, Thompson LF, Eltzschig HK. (2008) A2B adenosine receptor dampens hypoxia-induced vascular leak. Blood 111:2024–2035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng MG, Navar LG. (2010) Afferent arteriolar vasodilator effect of adenosine predominantly involves adenosine A2B receptor activation. Am J Physiol Renal Physiol 299:F310–F315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenz A, Osswald H, Eckle T, Yang D, Zhang H, Tran ZV, Klingel K, Ravid K, Eltzschig HK. (2008) The reno-vascular A2B adenosine receptor protects the kidney from ischemia. PLoS Med 5:e137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenz A, Zhang H, Eckle T, Mittelbronn M, Wehrmann M, Köhle C, Kloor D, Thompson LF, Osswald H, Eltzschig HK. (2007a) Protective role of ecto-5′-nucleotidase (CD73) in renal ischemia. J Am Soc Nephrol 18:833–845 [DOI] [PubMed] [Google Scholar]
- Grenz A, Zhang H, Hermes M, Eckle T, Klingel K, Huang DY, Müller CE, Robson SC, Osswald H, Eltzschig HK. (2007b) Contribution of E-NTPDase1 (CD39) to renal protection from ischemia-reperfusion injury. FASEB J 21:2863–2873 [DOI] [PubMed] [Google Scholar]
- Hashemolhosseini S, Nagamine Y, Morley SJ, Desrivières S, Mercep L, Ferrari S. (1998) Rapamycin inhibition of the G1 to S transition is mediated by effects on cyclin D1 mRNA and protein stability. J Biol Chem 273:14424–14429 [DOI] [PubMed] [Google Scholar]
- Inoue T, Mi Z, Gillespie DG, Jackson EK. (1998) Cyclooxygenase inhibition reveals synergistic action of vasoconstrictors on mesangial cell growth. Eur J Pharmacol 361:285–291 [DOI] [PubMed] [Google Scholar]
- Institute of Laboratory Animal Resources (1996) Guide for the Care and Use of Laboratory Animals, 7th ed, Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council, Washington, DC [Google Scholar]
- Jackson EK, Ren J, Gillespie DG, Dubey RK. (2010) Extracellular 2′,3′-cyclic adenosine 5′-monophosphate is a potent inhibitor of preglomerular vascular smooth muscle cell and mesangial cell growth. Hypertension 56:151–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson EK, Ren J, Mi Z. (2009) Extracellular 2′,3′-cAMP is a source of adenosine. J Biol Chem 284:33097–33106 (PMC2785151) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson EK, Zhu C, Tofovic SP. (2002) Expression of adenosine receptors in the preglomerular microcirculation. Am J Physiol Renal Physiol 283:F41–F51 [DOI] [PubMed] [Google Scholar]
- Jacobson KA, Knutsen LJS. (2001) P1 and P2 purine and pyrimidine receptor ligands, in Purinergic and Pyrimidinergic Signalling I (Abbracchio MP, Williams M. eds) pp 129–175, Springer-Verlag, Berlin [Google Scholar]
- Johnson RA, Yeung SM, Stübner D, Bushfield M, Shoshani I. (1989) Cation and structural requirements for P site-mediated inhibition of adenylate cyclase. Mol Pharmacol 35:681–688 [PubMed] [Google Scholar]
- Kalla RV, Zablocki J, Tabrizi MA, Baraldi PG. (2009) Recent developments in A2B adenosine receptor ligands. Handb Exp Pharmacol 193:99–122 [DOI] [PubMed] [Google Scholar]
- Li C, Krishnamurthy PC, Penmatsa H, Marrs KL, Wang XQ, Zaccolo M, Jalink K, Li M, Nelson DJ, Schuetz JD, et al. (2007) Spatiotemporal coupling of cAMP transporter to CFTR chloride channel function in the gut epithelia. Cell 131:940–951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mokkapatti R, Vyas SJ, Romero GG, Mi Z, Inoue T, Dubey RK, Gillespie DG, Stout AK, Jackson EK. (1998) Modulation by angiotensin II of isoproterenol-induced cAMP production in preglomerular microvascular smooth muscle cells from normotensive and genetically hypertensive rats. J Pharmacol Exp Ther 287:223–231 [PubMed] [Google Scholar]
- Rao F, Qi Y, Murugan E, Pasunooti S, Ji Q. (2010) 2′,3′-cAMP hydrolysis by metal-dependent phosphodiesterases containing DHH, EAL, and HD domains is non-specific: implications for PDE screening. Biochem Biophys Res Commun 398:500–505 [DOI] [PubMed] [Google Scholar]
- Ren J, Mi Z, Stewart NA, Jackson EK. (2009) Identification and quantification of 2′,3′-cAMP release by the kidney. J Pharmacol Exp Ther 328:855–865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprinkle TJ. (1989) 2′,3′-Cyclic nucleotide 3′-phosphodiesterase, an oligodendrocyte-Schwann cell and myelin-associated enzyme of the nervous system. Crit Rev Neurobiol 4:235–301 [PubMed] [Google Scholar]