Abstract
Positron emission tomography (PET) neuroimaging in nonhuman primates has led to significant advances in our current understanding of the neurobiology and treatment of stimulant addiction in humans. PET neuroimaging has defined the in vivo biodistribution and pharmacokinetics of abused drugs and related these findings to the time course of behavioral effects associated with their addictive properties. With novel radiotracers and enhanced resolution, PET neuroimaging techniques have also characterized in vivo drug interactions with specific protein targets in the brain, including neurotransmitter receptors and transporters. In vivo determinations of cerebral blood flow and metabolism have localized brain circuits implicated in the effects of abused drugs and drug-associated stimuli. Moreover, determinations of the predisposing factors to chronic drug use and long-term neurobiological consequences of chronic drug use, such as potential neurotoxicity, have led to novel insights regarding the pathology and treatment of drug addiction. However, similar approaches clearly need to be extended to drug classes other than stimulants. Although dopaminergic systems have been extensively studied, other neurotransmitter systems known to play a critical role in the pharmacological effects of abused drugs have been largely ignored in nonhuman primate PET neuroimaging. Finally, the study of brain activation with PET neuroimaging has been replaced in humans mostly by functional magnetic resonance imaging (fMRI). There has been some success in implementing pharmacological fMRI in awake nonhuman primates. Nevertheless, the unique versatility of PET imaging will continue to complement the systems-level strengths of fMRI, especially in the context of nonhuman primate drug abuse research.
Introduction
Noninvasive neuroimaging techniques have led to significant advances in our current understanding of the neurobiology and treatment of drug addiction in humans. In positron emission tomography (PET) imaging, ligands of interest are radiolabeled with unstable atomic isotopes (see Phelps and Mazziotta, 1985; Senda et al., 2002; Fowler et al., 2007). Detector arrays and computer algorithms map the source and concentration of the radiotracer. Numerous radiotracers have been developed for use in PET neuroimaging that enable in vivo measurement of brain neurochemistry and physiology (Table 1). PET neuroimaging has defined the in vivo biodistribution and pharmacokinetics of abused drugs and related these findings to the time course of behavioral effects associated with their addictive properties. With novel radiotracers and enhanced resolution, PET neuroimaging techniques have also characterized in vivo drug interactions with specific protein targets in brain, including neurotransmitter receptors and transporters. In vivo determinations of cerebral blood flow and metabolism have begun to localize brain circuits implicated in the effects of abused drugs and drug-associated environmental stimuli. Moreover, documentation of the long-term neurobiological consequences of chronic drug use and potential neurotoxicity has led to novel insights regarding the pathology and treatment of drug addiction.
TABLE 1.
Radiotracers used in previously published nonhuman primate drug abuse research
Isotopes in brackets indicate the isotope most often used to radiolabel the tracer.
| Name/Acronym | Protein Target | Structure | Action | Common Uses | Selected Studies |
|---|---|---|---|---|---|
| [18F]FECNT | Dopamine transporter | 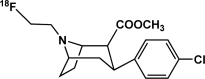 |
Inhibitor | Occupancy; availability | Lindsey et al., 2004; Howell et al., 2007; Fantegrossi et al., 2009; Andersen et al., 2010 |
| [18F]CFT | Dopamine transporter | 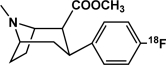 |
Inhibitor | Occupancy; availability | Hashimoto et al., 2007 |
| [18F]ZIENT | Serotonin transporter | 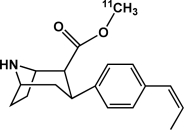 |
Inhibitor | Occupancy; availability | Lindsey et al., 2004 |
| [11C]DASB | Serotonin transporter | 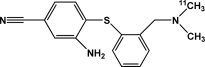 |
Inhibitor | Occupancy; availability | Banks et al., 2008 |
| [11C]McN5652 | Serotonin transporter | 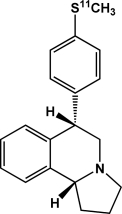 |
Inhibitor | Occupancy; availability | Scheffel et al., 1998 |
| [11C]DTBZ | Vesicular monoamine transporter | 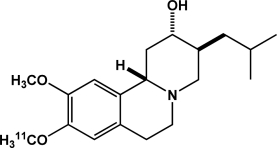 |
Inhibitor | Occupancy; availability | Fantegrossi et al., 2004 |
| [11C]SCH 23390 | Dopamine D1 receptors | 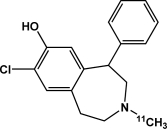 |
Antagonist | Occupancy; availability | Hashimoto et al., 2007 |
| [11C]Raclopride | Dopamine D2 receptors | 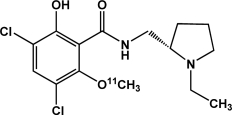 |
Antagonist | Occupancy; availability | Dewey et al., 1992; Villemagne et al., 1999; Volkow et al., 1999; van Berkel et al., 2006; Seneca et al., 2006 |
| [18F]Fallypride | Dopamine D2 receptors | 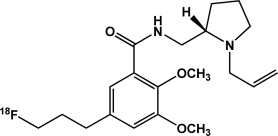 |
Antagonist | Occupancy; availability | Mukherjee et al., 1997; Slifstein et al., 2004 |
| [11C]MNPA | Dopamine D2 receptors | 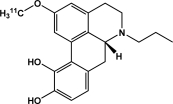 |
Agonist | Occupancy; availability | Seneca et al., 2006; Tokunaga et al., 2009 |
| [18F]FCP | Dopamine D2 receptors | 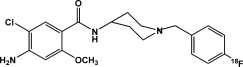 |
Reversible ligand | Occupancy; availability | Mach et al., 1997; Czoty et al., 2004; Nader and Czoty, 2005 |
| [18F]FDG | Mitochondria |  |
Substrate | Metabolism | Henry et al., 2010 |
| [15O]Water | None | Substrate | Blood flow | Howell et al., 2001, 2002, 2010 | |
| Labeled drugs (e.g. cocaine or MDMA) | Various | Various | Various | Occupancy; availability; drug distribution | Fowler et al., 1989, 2007; Kimmel et al., 2008 |
FECNT, 8-(2-fluoroethyl)-2-carbomethoxy-3-(4-chlorophenyl) nortropane; CFT, 2β-carbomethoxy-3β-(4-fluorophenyl)tropane; ZIENT, 2β-carbomethoxy-3β-[4′-((Z)-2-iodoethenyl)phenyl]nortropane; DASB, 3-amino-4-(2-dimethylaminomethyl-phenylsulfanyl)-benzonitrile; McN5652, trans-1,2,3,5,6,10 β-hexahydro-6-[4-(methylthio) phenyl]pyrrolo-[2,1-a]-isoquinolone); DTBZ, (+)-α-dihydrotetrabenazine; SCH23390, 7-chloro-3-methyl-1-phenyl-1,2,4,5-tetrahydro-3-benzazepin-8-ol; raclopride, (S)-(−)-3,5-dichloro-N-[(1-ethyl-2-pyrrolidinyl)]methyl-2-hydroxy-6-methoxybenzamide; fallypride, (S)-N-[(1-allyl-2-pyrrolidinyl)methyl]-5-(3-fluoropropyl)-2,3-dimethoxybenzamide; MNPA, (R)-2-(11)CH(3)O-N-n-propylnorapomorphine; FCP, fluoroclebopride; FDG, fluorodeoxyglucose.
Parallel neuroimaging studies in nonhuman primates and human subjects provide a powerful translational approach that can link findings from humans and laboratory animals. In particular, nonhuman primate models allow for initially drug-naive subjects in longitudinal designs to characterize within-subject changes in aspects of the neurobiology associated with chronic drug use. Moreover, experimental drugs under investigation can be evaluated in subjects with well documented drug histories. As with all animal models, enhanced experimental control is a noted advantage over the necessary restrictions imposed in human clinical research. Nonhuman primates also offer distinct advantages over other laboratory animal species. For example, their longevity is advantageous for many experimental designs. Compared with rodents, nonhuman primates are more similar to humans in the pharmacokinetics and metabolism of several drug classes including 3,4-methylenedioxymethamphetamine (MDMA) (Banks et al., 2007; Weerts et al., 2007). Furthermore, the brain distribution of PET radiotracers exhibits some heterogeneity even within primates, suggesting greater differences may be encountered when comparisons are drawn across orders (Yokoyama et al., 2010). Lastly, nonhuman primates exhibit complex social behaviors that provide unique opportunities for examining the influence of social variables on the abuse-related effects of drugs (Morgan et al., 2002; Nader and Czoty, 2005; Nader et al., 2008). With few exceptions, drug abuse research using PET neuroimaging in nonhuman primates has focused on cocaine and related stimulants. Accordingly, the current review will focus on abused stimulants.
Drug Mechanism of Action
Biodistribution.
PET neuroimaging in nonhuman primates has been critical for understanding the mechanism of action of cocaine and related stimulants. An early study focused on the distribution of cocaine binding in the brain of anesthetized baboons using 11C-labeled cocaine (Fowler et al., 1989). Cocaine binding was heterogeneous but showed some selectivity for dopamine transporter (DAT)-rich striatal regions. Striatal cocaine binding was inhibited by pretreatments with pharmacological doses of cocaine and DAT inhibitors but not by norepinephrine transporter or serotonin transporter (SERT) inhibitors. Direct comparisons in human subjects showed a similar distribution of binding with the highest concentration in the striatum. A subsequent study documented significant overlap in the distributions of binding of 11C-labeled cocaine and methylphenidate (Volkow et al., 1995). It is noteworthy that a direct relationship was established between self-reports of “high” induced by cocaine and the time course of striatal uptake (Volkow et al., 1997). More recently, the brain pharmacokinetics of methamphetamine were compared with cocaine in anesthetized baboons using 11C-labeled d-methamphetamine and (−)cocaine (Fowler et al., 2007). The results indicated that the slower clearance of methamphetamine compared with cocaine probably contributed to its longer-lasting stimulant effects. Finally, the reinforcing effects of several cocaine analogs were compared with the time course of uptake of the 11C-labeled drugs in the putamen of awake rhesus monkeys (Kimmel et al., 2008). The cocaine analogs were reliably self-administered, but the rates of responding were lower than those maintained by cocaine. It is noteworthy that there was a clear trend toward an inverse relationship between the time to peak uptake of 11C-labeled drugs in putamen and the peak number of intravenous infusions received, such that the faster-onset drugs produced greater levels of responding relative to the slower-onset drugs. There was also a close correspondence between the time course of drug uptake in brain and drug-induced increases in extracellular dopamine in caudate (Czoty et al., 2002; Ginsburg et al., 2005; Kimmel et al., 2007, 2008). These studies clearly show that PET biodistribution measures inform our understanding of drug mechanism of action and concomitant behavioral effects, such as reinforcing and subjective effects. However, because these studies exclusively examined the brain biodistribution of stimulants, it remains to be determined whether these techniques will prove to be as useful when applied to other drug classes.
Drug Occupancy.
PET neuroimaging in nonhuman primates has documented the relationship between drug occupancy at monoamine transporters and the behavioral effects of stimulants. For example, PET imaging in rhesus monkeys using 18F-labeled FECNT, a DAT-selective radioligand, showed that FECNT labels a cocaine-sensitive binding site, and high levels of DAT occupancy are associated with behaviorally active cocaine doses (Votaw et al., 2002). More recently, the reinforcing effects of local anesthetics that bind to the DAT in vitro were evaluated for DAT occupancy in vivo in rhesus monkeys (Wilcox et al., 2005). Doses of dimethocaine that maintained maximum response rates produced DAT occupancies between 66 and 82%. These values are highly concordant with results from human PET imaging studies, which found that DAT occupancies were between 60 and 77% for cocaine doses that subjects reported as rewarding (Volkow et al., 1997). They are also concordant with PET imaging data in rhesus monkeys, which revealed that cocaine DAT occupancies between 65 and 76% maintain peak response rates (Wilcox et al., 2002). Unlike dimethocaine, procaine was ineffective in maintaining self-administration and resulted in DAT occupancies between 10 and 41% (Wilcox et al., 2005). However, irrespective of the drug, in vivo microdialysis showed that reinforcing effects and DAT occupancy were closely related to drug-induced increases in extracellular dopamine. These studies illustrate the power of PET imaging to unmask drug mechanisms of action, particularly as it relates to monoamine transporters, and they highlight the utility of the translational nature of PET imaging in nonhuman primates. Consistent with these findings, it has been shown that DAT occupancy by methylphenidate is highly concordant between rhesus monkeys and humans when blood levels of the drug are matched (Wilcox et al., 2008).
PET imaging has also been used to study protein occupancy by other stimulants. The interoceptive and reinforcing effects of the substituted phenethylamine MDMA share some similarity to other stimulants (Fantegrossi et al., 2002; Murnane et al., 2009), but the role of the DAT in the behavioral effects of MDMA is not well documented, especially in primates. Accordingly, a study assessed the role of the DAT in the behavioral effects of racemic MDMA in nonhuman primates (Fantegrossi et al., 2009). A dose of MDMA that suppressed operant behavior had negligible effects on extracellular dopamine in the caudate, and the percentage of DAT occupancy of MDMA was marginal. This work was subsequently corroborated by findings that higher doses of MDMA were required to elicit significant release of dopamine (Murnane et al., 2010). Collectively, these results indicate that the rate-suppressant effects of MDMA are not mediated by the DAT and that MDMA has lower potency at the DAT compared with other stimulants, such as cocaine or amphetamine. Similar to MDMA, the role of the DAT in the behavioral effects of the wake-promoting drug modafinil is not well documented. It is noteworthy that a number of clinical studies suggest that modafinil may improve clinical outcomes for the treatment of cocaine dependence by reducing self-reports of craving and cocaine-induced euphoria (Dackis et al., 2003, 2005; Hart et al., 2008; Anderson et al., 2009) through a possible DAT-mediated mechanism (Volkow et al., 2009; Zolkowska et al., 2009). To this end, a study in rhesus monkeys demonstrated that the in vivo effects of modafinil at the DAT are similar to other stimulants, such as cocaine (Andersen et al., 2010). Modafinil induced nocturnal locomotor-stimulant effects and reinstated previously extinguished cocaine self-administration. An effective dose of modafinil resulted in approximately 60% DAT occupancy in the striatum and significantly increased extracellular dopamine levels, comparable with effects observed after cocaine doses that reliably maintain self-administration (Ito et al., 2002; Votaw et al., 2002; Wilcox et al., 2002, 2005). The results obtained provide important information about the mechanism of action of modafinil and show low-potency DAT-related effects in nonhuman primates. Although these studies collectively demonstrate the power of PET imaging to characterize the transporter-related effects of stimulants, the relationship between presynaptic transporter effects and postsynaptic dopamine receptor effects requires further elucidation.
Neurotransmitter Release.
Competition between radiolabeled ligands and endogenous neurotransmitters provides an effective means of evaluating drug-induced changes in extracellular neurotransmitter concentrations in vivo (see Laruelle, 2000). Similar to other PET measures, findings in nonhuman primates and human subjects exhibit considerable overlap (Table 2). PET neuroimaging with 18F-labeled fluoroclebopride (FCP) as a reversible D2 receptor ligand characterized stimulant-induced dopamine release in rhesus monkeys (Mach et al., 1997). Intravenous administration of cocaine, amphetamine, methylphenidate, and methamphetamine increased rates of FCP washout from the basal ganglia, consistent with the capacity of each drug to elevate extracellular dopamine. 11C-labeled raclopride studies in baboons (Dewey et al., 1992; Villemagne et al., 1998; Volkow et al., 1999) and 18F-labeled fallypride studies in rhesus monkeys (Mukherjee et al., 1997) documented that these effects can be demonstrated with several radioligands and in several primate species. Other studies in baboons and rhesus monkeys have begun to document the usefulness of drug-induced displacement of 18F-labeled fallypride to characterize dopamine release in extrastriatal brain regions (Mukherjee et al., 1997; Slifstein et al., 2004). Taken together, these results validate the use of PET imaging as an in vivo measure of neurotransmitter release in nonhuman primates and provide a solid foundation for human studies (Martinez et al., 2007; Volkow et al., 2008). However, most studies have been limited to dopamine displacement of D2 binding in the striatum. In apparent contrast to the effects of dopamine releasers, the serotonin releaser fenfluramine significantly increased extracellular serotonin in rhesus monkeys without displacing the 18F-labeled serotonin 1A receptor ligand MPPF [4-fluoro-N-[2-[4-(2-methoxyphenyl)-1-piperazinyl]ethyl]-N-(2-pyridyl)benzamide] (Udo de Haes et al., 2006). Moreover, dopamine depletion did not affect the binding potential of the 11C-labeled D2 receptor ligand FLB 457 [(S)-5-bromo-N-[(1-cyclopropylmethyl-2-pyrrolidinyl)methyl]-2,3-dimethoxybenzamide] in human cortex (Frankle et al., 2010). The relative affinity of the radioligand and the endogenous neurotransmitter, protein density in specific brain regions, and direct interactions between the drug and its metabolites with the protein target all are important considerations that may influence the outcome and interpretation of in vivo displacement studies.
TABLE 2.
Comparison of the acute effects of drugs of abuse in nonhuman primate or human subjects as measured by PET or single-photon emission computed tomography neuroimaging
| Effect | Drug | Nonhuman Primates | Humans |
|---|---|---|---|
| Cerebral blood flow | Cocaine | ↑a | ↓b |
| Cerebral metabolism | Cocaine | ↑c | ↓d |
| DAT binding | Cocaine, modafinil | ↑e,f | ↑e |
| Correlation between brain kinetics and reinforcing or interoceptive effects | Cocaine, cocaine analogs | Yesg | Yesh |
| Correlation between protein occupancy and reinforcing or interoceptive effects | Cocaine, modafinil, local anesthetics | Yesf,g | Yesh |
| Displacement of D2 receptor selective ligands | Cocaine, amphetamine, methylphenidate, methamphetamine | ↑i | ↑j |
PET imaging has been used in nonhuman primates to examine the receptor pharmacology influencing dopamine release. In one study, pretreatment with the metabotropic glutamate receptor 1 antagonist 2-methyl-6-(phenylethynyl)pyridine attenuated dopamine release by methamphetamine, as measured by 11C-labeled MNPA (Tokunaga et al., 2009). Likewise, the metabotropic glutamate receptor 2 agonist (2S,4S)-2-amino-4-(4,4-diphenylbut-1-yl)-pentane-1,5-dioic acid (LY354740) potentiated amphetamine-elicited dopamine release, as measured by 11C-labeled raclopride (van Berckel et al., 2006). Similar to biodistribution studies, displacement of radiotracers by drug-induced increases in neurotransmitter levels can be used to study the time course of drug action (Narendran et al., 2007). In addition, the importance of the intrinsic efficacy of PET radioligands has become recognized. For example, the D2 receptor agonist radioligand MNPA is more sensitive than the D2 antagonist radioligand raclopride to amphetamine-elicited increases in dopamine levels (Seneca et al., 2006). This is consistent with prior in vitro competition binding work showing that agonists have a higher apparent affinity for receptors labeled with agonist radioligands than antagonist radioligands (Sleight et al., 1996). Moving forward, these pharmacodynamic and pharmacokinetic studies are likely to yield new insights into mechanisms that modulate the neurochemical and behavioral effects of drugs of abuse.
Cerebral Blood Flow and Metabolism.
The noninvasive measurement of cerebral blood flow with PET neuroimaging and [15O]water provides a useful means to characterize acute drug-induced changes in brain activity. Early studies in human cocaine abusers using single-photon emission computed tomography imaging largely showed regional decreases in cerebral blood flow after acute administration of cocaine (Pearlson et al., 1993; Wallace et al., 1996; Johnson et al., 1998). Functional changes in cerebral blood flow using PET imaging have also been determined in awake, drug-naive rhesus monkeys after acute intravenous cocaine administration (Howell et al., 2001, 2002). In contrast to the deactivations found in humans with a history of cocaine abuse, brain activation maps normalized to global flow showed prominent cocaine-induced activation of prefrontal cortex, especially dorsolaterally. These differences in the direction of the change in blood flow induced by cocaine may have been caused by differences in the methodology implemented, the dose of cocaine tested, the drug history of the subject, or other factors. Nevertheless, importantly, the selective SERT inhibitor alaproclate attenuated the brain activational effects of cocaine, cocaine-induced increases in striatal dopamine, and self-administration of cocaine in rhesus monkeys (Czoty et al., 2002; Howell et al., 2002). Hence, there was close concordance between in vivo measures of behavior, neurochemistry, and functional imaging. Another study was the first to use PET imaging with [15O]water to document acute cocaine-induced changes in brain activity during cocaine self-administration in nonhuman primates (Howell et al., 2010). The area of major activation included the anterior cingulate cortex, a region associated with the extended limbic system. Furthermore, drug-associated stimuli elicited increases in regional cerebral blood flow in the dorsomedial prefrontal cortex, indicating robust cortical activation. Consistent with these findings, a study in cocaine-addicted humans showed that a dose of methylphenidate that increased dopamine levels, as indexed by displacement of 11C-labeled raclopride binding, only elicited drug “craving” when presented in combination with drug-associated stimuli (Volkow et al., 2008). In another study in nonhuman primates, functional changes in glucose metabolism were characterized in rhesus monkeys with 18F-labeled flurodeoxyglucose (FDG) after acute administration of cocaine (Henry et al., 2010). Similar to the results obtained using [15O]water to map cerebral blood flow, metabolic mapping demonstrated acute cocaine-induced activation of extended limbic regions in cocaine-naive rhesus monkeys. Collectively, these studies enhance our understanding of the neurobiological effects of drugs of abuse. However, although extensive PET imaging work has examined dopaminergic mechanisms (Fig. 1) of drugs of abuse in nonhuman primates, a relative paucity of work regarding other mechanisms of action, such as serotonergic effects, has been published.
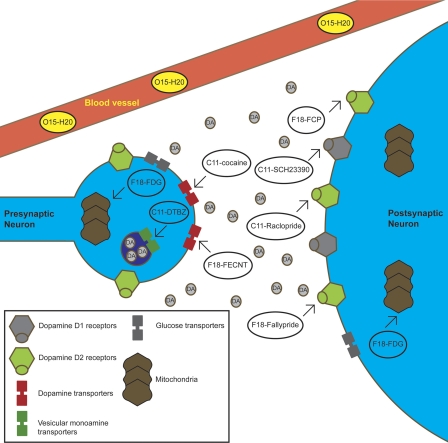
Fig. 1.
Elements of the dopamine neurovascular unit that have been previously imaged using PET imaging in nonhuman primate drug abuse research. As can be seen, numerous radiotracers have been developed and validated for studying a broad range of dopaminergic elements. Inset, the key for the elements.
Biomarkers
Neurobiological Changes.
A major advantage of PET imaging is the ability to use longitudinal designs that involve repeated measures over extended periods of time. This approach has been used effectively in nonhuman primates to characterize enduring changes in brain chemistry associated with chronic drug exposure (Table 3). For example, PET imaging studies conducted in socially housed cynomolgus monkeys characterized the effects of chronic cocaine exposure in dominant and subordinate subjects. Although dominant monkeys initially exhibited higher D2 receptor availability and lower rates of cocaine self-administration (Morgan et al., 2002), chronic exposure to self-administered cocaine resulted in reductions in D2 receptor availability to levels comparable with subordinate monkeys (Czoty et al., 2004). A subsequent study examined D2 receptor availability during extended cocaine abstinence (Nader et al., 2006). In subjects with short-term exposure, D2 receptor availability returned to predrug levels within 3 weeks. In subjects with long-term exposure, some showed complete recovery within 3 months, whereas others did not recover after 1 year of abstinence. However, the rate of recovery did not correlate with total drug intake. Nevertheless, individual differences in D2 receptor availability recovery rates have also been observed after drug-induced increases by D2 receptor antagonists (Czoty et al., 2005). An early study of cocaine self-administration effects on DAT levels largely showed increases in DAT levels, but in a dose-, brain region-, and exposure duration-dependent fashion (Letchworth et al., 2001). A study of DAT availability using 18F-labeled (+)-N-(4-fluorobenzyl)-2β-propanoyl-3β-(4-chlorophenyl)tropane examined the effects of cocaine self-administration in rhesus monkeys under contingencies that resulted in low drug intake (Czoty et al., 2007). Self-administration of a low cocaine dose over 9 weeks did not significantly affect DAT availability in any brain region. After more prolonged histories of cocaine self-administration, significant increases in striatal SERT were observed in experiments using 11C-labeled DASB (Banks et al., 2008). In other work, a noncontingent dosing regimen of morphine decreased DAT availability in rhesus monkeys (Xiao et al., 2006). Collectively, these studies demonstrate substantial, but yet to be fully elucidated, plasticity of the dopaminergic system in response to exposure to drugs of abuse.
TABLE 3.
Long-term consequences of exposure to drugs of abuse in nonhuman primates measured using PET neuroimaging
| Effect | Drug | Radiotracer |
|---|---|---|
| Recruitment of brain activation | Cocaine | [18F]FDGa |
| No change in DAT availability | Cocaine | [18F]FCTb |
| Decreased DAT availability | Methamphetamine | [11C]WIN-35,428c |
| [18F]CFTd | ||
| No change in D1 dopamine receptor availability | Methamphetamine | [11C]SCH23390d |
| Decreased D2 receptor availability | Cocaine | [18F]FCPb,e |
| Increased SERT availability | Cocaine | [11C]DASBf |
| Decreased SERT availability | MDMA | [11C]McN5652g |
| No change in SERT availability | MDMA | [11C]DASBf |
| No change in vesicular monoamine transporter availability | MDMA | [11C]DTBZh |
FCT, (+)-N-(4-fluorobenzyl)-2β-propanoyl-3β-(4-chlorophenyl)tropane; WIN 35,428, ((−)-2-β-carbomethoxy-3-β-(4-fluorophenyl)tropane 1,5-napthalenedisulfonate).
Evaluations of drug-induced changes in protein binding in vivo are complemented by a study that documented cocaine-induced changes in brain metabolic activity as a function of cocaine self-administration history (Henry et al., 2010). Experimentally naive rhesus monkeys were given increasing access to cocaine self-administration. PET neuroimaging with 18F-labeled FDG was used to measure acute cocaine-induced changes in brain metabolism in the cocaine-naive state and in limited- and extended-access conditions. In the cocaine-naive state, cocaine-induced increases in brain metabolism were restricted to the anterior cingulate and medial prefrontal cortex. Increased cocaine exposure from limited through extended access recruited cocaine-induced metabolic effects in additional frontal cortical areas and within the striatum. In apparent contrast, tolerance to cocaine- and amphetamine-induced synaptic release of dopamine in the striatum was observed in these same animals under both access conditions (Kirkland Henry et al., 2009). It is noteworthy that blunting of dopamine release has also been recorded in cocaine-dependent humans, in experiments using 11C-labeled raclopride (Martinez et al., 2007). Furthermore, this blunting of dopamine release was associated with whether the subject would choose cocaine over money. Accordingly, further combined study of the relationship between drug self-administration and both tolerance to the dopaminergic effects of cocaine and its recruitment of cortical activation may be highly relevant toward efforts to develop treatments for cocaine addiction. Indeed, a treatment that reverses these effects may have significant clinical value.
There has been significant interest in the potential neurotoxic effects of amphetamine derivatives, such as methamphetamine and MDMA. Under a variety of conditions, MDMA has selective and enduring effects on markers of brain serotonin systems, which some investigators interpret as neurotoxicity. However, early studies were limited by biochemical and histological analyses that required between-subject comparisons. An early PET imaging study in a baboon characterized the effects of MDMA on in vivo SERT availability using 11C-labeled McN5652 (Scheffel et al., 1998). After treatment with MDMA twice daily for 4 consecutive days, PET scans showed reductions in SERT availability in all brain regions analyzed at 13 to 40 days after drug treatment but regional differences in its apparent recovery at 9 and 13 months. Similar results have been reported for methamphetamine-induced reductions in DAT availability in baboons (Villemagne et al., 1998) and rhesus monkeys (Hashimoto et al., 2007). However, other studies provided more ambiguous results (Melega et al., 2008), including small and transient changes in D1 receptor availability, using 11C-labeled SCH23390 (Hashimoto et al., 2007). Furthermore, behavioral decrements resulting from neurochemical changes induced by exposure to amphetamine derivatives have been much more difficult to establish (Winsauer et al., 2002; Saadat et al., 2006). It is critical to note that studies reporting neurotoxic effects of amphetamine derivatives have relied on noncontingent drug administration and have typically administered large and/or repeated doses. In one of the first studies to characterize the neurochemical effects of self-administered MDMA in nonhuman primates, rhesus monkeys self-administered MDMA for approximately 18 months. PET neuroimaging with 11C-labeled DTBZ was used to quantify vesicular monoamine transporter availability after at least 2 months of drug abstinence (Fantegrossi et al., 2004). The reinforcing effects of MDMA were selectively attenuated by chronic MDMA self-administration but there was no significant change in vesicular monoamine transporter binding potential and no significant changes in serotonin or dopamine levels in postmortem brains. Another study found a similar lack of significant SERT availability changes after MDMA self-administration in rhesus monkeys using 11C-labeled DASB (Banks et al., 2008). Hence, noncontingent drug administration has yielded neurochemical changes in the absence of behavioral correlates, whereas drug self-administration has yielded behavioral alterations in the absence of any significant neurochemical correlates. As such, given the important public health implications of drug-induced neurotoxicity, further study is clearly warranted. In this regard, PET imaging in rhesus monkeys has shown that before or after exposure treatment with the antibiotic minocycline prevents methamphetamine-elicited reductions in DAT availability (Hashimoto et al., 2007). Such an approach may be highly beneficial in the prevention or treatment of any neurotoxic effects of amphetamine derivatives.
Vulnerability Factors.
It has become well accepted that behavior, brain chemistry, and neuronal function can be readily influenced by pharmacological challenges and environmental conditions. Differences in the dominance rank among socially housed nonhuman primates have been associated with differential levels of dopamine D2 receptors as measured with 18F-labeled FCP (see Nader and Czoty, 2005). Social housing of male cynomolgus monkeys increased the availability of D2 receptors in dominant animals without producing any changes in subordinate group members, and these changes seemed to exert significant effects on cocaine self-administration (Morgan et al., 2002). Cocaine reliably functioned as a reinforcer in subordinate subjects but failed to maintain self-administration in dominant monkeys. Likewise, subordinate animals were more sensitive to the reinforcing effects of cocaine evaluated with a choice procedure, such that they would choose a lower dose of cocaine over food compared with dominant animals (Czoty et al., 2005). In addition, the protective effects associated with high D2 receptor density in dominant animals can be attenuated with prolonged exposure to cocaine (Czoty et al., 2004). Hence, a trait variable that is associated with low vulnerability to stimulant abuse may become less important with continued exposure to stimulants. Furthermore, the observation that female cynomolgus monkeys show significant changes in D2 binding potential associated with menstrual cycle phase may have direct relevance to understanding the neurobiological basis of human sex differences in sensitivity to stimulants (Czoty et al., 2009). The success of these studies suggests that the identification of additional vulnerability factors other than D2 levels would be of great benefit to drug abuse research.
Medications Development
Despite extensive efforts directed toward the development of medications to treat cocaine abuse, no effective pharmacotherapy is currently in clinical use. Given the important role of DAT in the addictive properties of cocaine, the development of compounds that target the DAT represents a reasonable approach for the pharmacological treatment of cocaine abuse. A series of studies was conducted in nonhuman primates that evaluated the effectiveness of DAT inhibitors in reducing cocaine self-administration. PET neuroimaging quantified DAT occupancy at behaviorally relevant doses, characterized the time course of drug uptake in brain, and documented drug-induced changes in cerebral blood flow as a model of brain activation. Selective DAT inhibitors were effective in reducing cocaine self-administration but only at high (>70%) levels of DAT occupancy. For example, effective doses of the DAT-selective inhibitor RTI-113 [phenyl 3-(4-chlorophenyl)-8-methyl-8-azabicyclo[3.2.1]octane-2-carboxylate], which dose-dependently reduced cocaine-maintained responding, produced DAT occupancies between 72 and 84% (Wilcox et al., 2002). Similar results were observed with other DAT-selective inhibitors, including the phenyltropane RTI-177 [5-[(1R,2S,3S,5S)-3-(4-chlorophenyl)-8-methyl-8-azabicyclo[3.2.1]octan-2-yl]-3-(4-methylphenyl)-1,2-oxazole] and the phenylpiperazine GBR 12909 [1-[2-[bis-(4-fluorophenyl)methoxy]ethyl]-4-(3-phenylpropyl)piperazine] (Lindsey et al., 2004). It is noteworthy that selective SERT inhibitors were also effective in reducing cocaine self-administration and blocked cocaine-induced brain activation and increases in extracellular dopamine (Czoty et al., 2002; Howell et al., 2002). Likewise, a mixed-action inhibitor of DAT and SERT, RTI-112 [(1R,2S,3S)-methyl 3-(4-chloro-3-methylphenyl)-8-methyl-8-azabicyclo[3.2.1]octane-2-carboxylate], significantly reduced cocaine self-administration by rhesus monkeys at doses producing levels of DAT occupancy below the limit of detection (Lindsey et al., 2004). Furthermore, coadministrations of the selective SERT inhibitors fluoxetine or citalopram and the selective DAT inhibitor RTI-336 [3β-(4-chlorophenyl)-2β-[3-(4′-methylphenyl)isoxazol-5-yl]tropane hydrochloride] produced more robust reductions in cocaine self-administration than RTI-336 alone, even at comparable levels of DAT occupancy by RTI-336 (Howell et al., 2007). Collectively, it seems that serotonergic effects enhance suppression of cocaine self-administration by DAT inhibitors, indicating that duel DAT/SERT inhibitors warrant consideration as viable medications for cocaine addiction.
Translational Value of Nonhuman Primate Neuroimaging
With few exceptions, functional neuroimaging studies in nonhuman primates have used Old World macaques and baboons. These animals offer important advantages over other species of laboratory animals for the study of drug abuse. For example, the nonhuman primate prefrontal cortex is anatomically and functionally homologous to the human prefrontal cortex, which is a brain region that, as discussed previously, has marked relevance for drug abuse. Nonhuman primates have a sophisticated behavioral repertoire, which allows for the study of complex schedules of reinforcement and cognitive processes important for studying the etiology, maintenance, and consequences of drug abuse. Nonhuman primate social behavior, including the development of social hierarchies, has demonstrated relevance for studying drug abuse. Drug metabolic and pharmacokinetics effects in nonhuman primates are arguably more similar to human drug metabolism and pharmacokinetics than those of other laboratory animal species. The life span of nonhuman primates is long and exhibits similarities to humans, such as delayed maturation and prolonged adolescence, that may be relevant for studying drug abuse, and are well suited for long-term longitudinal studies using the relatively noninvasive techniques of neuroimaging. Of these examples, perhaps the area that is most germane to neuroimaging and of greatest translational value is the homology of the nonhuman primate prefrontal cortex to the human prefrontal cortex because of the relevance of drug effects on prefrontal cortical function to human drug addiction. Functional brain imaging in humans has begun to define the neural circuitry underlying the acute pharmacological effects of cocaine, conditioned responses to cocaine cues, and the experience of drug craving in humans. Activation of the anterior cingulate and dorsolateral prefrontal cortex has been observed in response to acute administration of cocaine and related stimulants (Breiter et al., 1997; Volkow et al., 1999; Kufahl et al., 2005) and cocaine-related environmental cues (Maas et al., 1998; Childress et al., 1999; Kilts et al., 2001; Wexler et al., 2001). The anterior cingulate, part of the extended limbic system, is anatomically linked to the prefrontal cortex and nucleus accumbens and serves diverse functions including the integration of mood and cognition (Vogt et al., 1992; Devinsky et al., 1995). The dorsolateral and dorsomedial prefrontal cortices are activated during the performance of a variety of cognitive tasks that require working memory or goal-directed behavior (Fuster 1997). Hence, it is apparent that the effects of cocaine and associated cues extend beyond the limbic system to engage brain areas underlying complex cognitive processes. It is noteworthy that the same neuroanatomical regions as reported in humans subjects are activated during cocaine self-administration and extinction in rhesus monkeys, establishing strong validity for the nonhuman primate model used (Howell et al., 2002, 2010). Elevations in rates of glucose utilization in the same brain areas after cocaine self-administration in rhesus monkeys have also been reported (Porrino et al., 2002; Henry et al., 2010). Obviously, self-reports of drug craving cannot be obtained in animal studies. However, the distinct pattern of brain activation observed in nonhuman primates may provide a novel functional measure to assess interventions designed to attenuate cue-induced changes in brain activity. These translational neuroimaging studies of drug dependence have been complemented by behavioral measures associated with drug use and relapse after periods of drug abstinence. Collectively, neuroimaging studies in nonhuman primates have identified neuronal targets to effectively reduce cocaine use and have characterized underling neurochemical mechanisms associated with potential therapeutic effects. The identification of neural circuits underlying the direct pharmacological and conditioned-stimulus effects of cocaine may be highly relevant toward efforts to develop pharmacological treatments for drug addiction.
Limitations and Future Directions
The use of PET neuroimaging in nonhuman primates has advanced our understanding of the neurobiological basis of stimulant addiction, providing an effective translational approach for medications development and treatment of stimulant abuse in humans. However, similar approaches clearly need to be extended to other drug classes with high abuse liability. Although the importance of dopamine in drug addiction is well recognized, other neurotransmitter systems known to play a critical role in the pharmacological effects of abused drugs have been largely ignored in nonhuman primate PET neuroimaging. There has been some progress in the development of techniques to study serotonergic and glutamatergic systems, and a comprehensive understanding of the neurobiology underlying drug addiction probably will depend on the continued development of such novel approaches. For example, the identification of vulnerability factors other than dopamine D2 receptor availability would be highly beneficial. In addition, in vivo PET measures of neurotransmitter release in nonhuman primates have been limited to dopamine displacement of D2 receptor binding in the striatum. However, it remains to be determined whether neurotransmitters other than dopamine reliably displace PET ligand binding at alternative targets in nonhuman primates, and it will be important to validate these displacement studies with direct measures of neurotransmitter levels derived from in vivo microdialysis. Finally, the study of brain activation by PET imaging with [15O]water and FDG has been replaced in humans mostly by functional magnetic resonance imaging (fMRI) because of the higher temporal and spatial resolution and lack of radiation exposure with this imaging modality. There has been some success in implementing pharmacological fMRI in awake nonhuman primates (Jenkins et al., 2004; Brevard et al., 2006; Murnane and Howell, 2010) (Fig. 2). However, there are significant challenges associated with the conduct of fMRI imaging in awake nonhuman primates because it is inherently more sensitive to subject motion than PET imaging and requires restraint equipment built entirely from nonferrous materials. Despite these challenges, fMRI should prove to be highly effective in characterizing drug-induced changes in brain activity at a systems level. Nevertheless, the unique versatility and specificity of PET imaging will continue to complement the systems level strengths of fMRI, especially in the context of nonhuman primate drug abuse research.
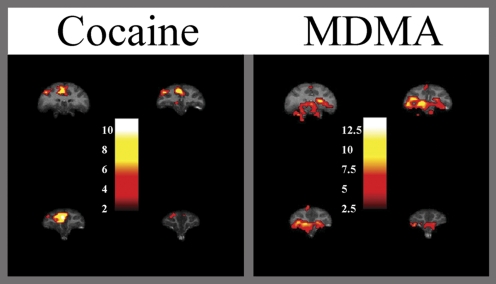
Fig. 2.
Brain activation elicited by cocaine and MDMA in a single nonhuman primate imaged using fMRI. Each drug was administered at 0.3 mg/kg i.v., and the subject was fully conscious during the scan. Images are presented as coronal sections of the prefrontal cortex. Efforts are underway to extend PET brain activation findings using this imaging modality. As can be seen, fMRI finely localizes a different systems-level activation elicited by each drug of abuse. Insets, the statistical value of the change in activation expressed in units of the t statistic value.
This work was funded, in part, by the National Institutes of Health National Institute on Drug Abuse [Grants DA15040 (to K.S.M.), DA10344 (to L.L.H.), DA00517 (to L.L.H.)] and a Yerkes Base Grant [Grant RR00165] (to K.S.M., L.L.H.).
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.108.136689.
- PET
- positron emission tomography
- fMRI
- functional magnetic resonance imaging
- MDMA
- 3,4-methylenedioxymethamphetamine
- DAT
- dopamine transporter
- SERT
- serotonin transporter
- FCP
- fluoroclebopride
- FDG
- fluorodeoxyglucose
- FECNT
- 8-(2-fluoroethyl)-2-carbomethoxy-3-(4-chlorophenyl) nortropane
- CFT
- 2β-carbomethoxy-3β-(4-fluorophenyl)tropane
- ZIENT
- 2β-carbomethoxy-3β-[4′-((Z)-2-iodoethenyl)phenyl]nortropane
- DASB
- 3-amino-4-(2-dimethylaminomethyl-phenylsulfanyl)-benzonitrile
- McN5652
- trans-1,2,3,5,6,10-β-hexahydro-6-[4-(methylthio)phenyl]pyrrolo-[2,1-a]-isoquinolone)
- DTBZ
- (+)-α-dihydrotetrabenazine
- SCH23390
- 7-chloro-3-methyl-1-phenyl-1,2,4,5-tetrahydro-3-benzazepin-8-ol
- raclopride
- (S)-(−)-3,5-dichloro-N-[(1-ethyl-2-pyrrolidinyl)]methyl-2-hydroxy-6-methoxybenzamide
- fallypride
- (S)-N-[(1-allyl-2-pyrrolidinyl)methyl]-5-(3-fluoropropyl)-2,3-dimethoxybenzamide
- MNPA
- (R)-2-(11)CH(3)O-N-n-propylnorapomorphine
- LY354740
- (2S,4S)-2-amino-4-(4,4-diphenylbut-1-yl)-pentane-1,5-dioic acid
- MPPF
- 4-fluoro-N-[2-[4-(2-methoxyphenyl)-1-piperazinyl]ethyl]-N-(2-pyridyl)benzamide
- RTI-113
- phenyl 3-(4-chlorophenyl)-8-methyl-8-azabicyclo[3.2.1]octane-2-carboxylate
- RTI-177
- 5-[(1R,2S,3S,5S)-3-(4-chlorophenyl)-8-methyl-8-azabicyclo[3.2.1]octan-2-yl]-3-(4-methylphenyl)-1,2-oxazole
- GBR 12909
- 1-[2-[bis-(4-fluorophenyl)methoxy]ethyl]-4-(3-phenylpropyl)piperazine
- RTI-112
- (1R,2S,3S)-methyl 3-(4-chloro-3-methylphenyl)-8-methyl-8-azabicyclo[3.2.1]octane-2-carboxylate
- RTI-336
- 3β-(4-chlorophenyl)-2β-[3-(4′-methylphenyl)isoxazol-5-yl]tropane hydrochloride
- WIN 35,428
- ((−)-2-β-carbomethoxy-3-β-(4-fluorophenyl)tropane-1,5-napthalenedisulfonate)
- FLB 457
- (S)-5-bromo-N-[(1-cyclopropylmethyl-2-pyrrolidinyl)methyl]-2,3-dimethoxybenzamide.
Authorship Contributions
Wrote or contributed to the writing of the manuscript: Howell and Murnane.
References
- Andersen ML, Kessler E, Murnane KS, McClung JC, Tufik S, Howell LL. (2010) Dopamine transporter-related effects of modafinil in rhesus monkeys. Psychopharmacology (Berl) 210:439–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson AL, Reid MS, Li SH, Holmes T, Shemanski L, Slee A, Smith EV, Kahn R, Chiang N, Vocci F, et al. (2009) Modafinil for the treatment of cocaine dependence. Drug Alcohol Depend 104:133–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks ML, Czoty PW, Gage HD, Bounds MC, Garg PK, Garg S, Nader MA. (2008) Effects of cocaine and MDMA self-administration on serotonin transporter availability in monkeys. Neuropsychopharmacology 33:219–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks ML, Sprague JE, Kisor DF, Czoty PW, Nichols DE, Nader MA. (2007) Ambient temperature effects on 3,4-methylenedioxymethamphetamine-induced thermodysregulation and pharmacokinetics in male monkeys. Drug Metab Dispos 35:1840–1845 [DOI] [PubMed] [Google Scholar]
- Breiter HC, Gollub RL, Weisskoff RM, Kennedy DN, Makris N, Berke JD, Goodman JM, Kantor HL, Gastfriend DR, Riorden JP, et al. (1997) Acute effects of cocaine on human brain activity and emotion. Neuron 19:591–611 [DOI] [PubMed] [Google Scholar]
- Brevard ME, Meyer JS, Harder JA, Ferris CF. (2006) Imaging brain activity in conscious monkeys following oral MDMA (“ecstasy”). Magn Reson Imaging 24:707–714 [DOI] [PubMed] [Google Scholar]
- Childress AR, Mozley PD, McElgin W, Fitzgerald J, Reivich M, O'Brien CP. (1999) Limbic activation during cue-induced cocaine craving. Am J Psychiatry 156:11–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czoty PW, Gage HD, Nader MA. (2005) PET imaging of striatal dopamine D2 receptors in nonhuman primates: increases in availability produced by chronic raclopride treatment. Synapse 58:215–219 [DOI] [PubMed] [Google Scholar]
- Czoty PW, Ginsburg BC, Howell LL. (2002) Serotonergic attenuation of the reinforcing and neurochemical effects of cocaine in squirrel monkeys. J Pharmacol Exp Ther 300:831–837 [DOI] [PubMed] [Google Scholar]
- Czoty PW, Morgan D, Shannon EE, Gage HD, Nader MA. (2004) Characterization of dopamine D1 and D2 receptor function in socially housed cynomolgus monkeys self-administering cocaine. Psychopharmacology (Berl) 174:381–388 [DOI] [PubMed] [Google Scholar]
- Czoty PW, Reboussin BA, Calhoun TL, Nader SH, Nader MA. (2007) Long-term cocaine self-administration under fixed-ratio and second-order schedules in monkeys. Psychopharmacology (Berl) 191:287–295 [DOI] [PubMed] [Google Scholar]
- Czoty PW, Riddick NV, Gage HD, Sandridge M, Nader SH, Garg S, Bounds M, Garg PK, Nader MA. (2009) Effect of menstrual cycle phase on dopamine D2 receptor availability in female cynomolgus monkeys. Neuropsychopharmacology 34:548–554 [DOI] [PubMed] [Google Scholar]
- Dackis CA, Kampman KM, Lynch KG, Pettinati HM, O'Brien CP. (2005) A double-blind, placebo-controlled trial of modafinil for cocaine dependence. Neuropsychopharmacology 30:205–211 [DOI] [PubMed] [Google Scholar]
- Dackis CA, Lynch KG, Yu E, Samaha FF, Kampman KM, Cornish JW, Rowan A, Poole S, White L, O'Brien CP. (2003) Modafinil and cocaine: a double-blind, placebo-controlled drug interaction study. Drug Alcohol Depend 70:29–37 [DOI] [PubMed] [Google Scholar]
- Devinsky O, Morrell MJ, Vogt BA. (1995) Contributions of anterior cingulate cortex to behaviour. Brain 118:279–306 [DOI] [PubMed] [Google Scholar]
- Dewey SL, Smith GS, Logan J, Brodie JD, Yu DW, Ferrieri RA, King PT, MacGregor RR, Martin TP, Wolf AP. (1992) GABAergic inhibition of endogenous dopamine release measured in vivo with 11C-raclopride and positron emission tomography. J Neurosci 12:3773–3780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantegrossi WE, Bauzo RM, Manvich DM, Morales JC, Votaw JR, Goodman MM, Howell LL. (2009) Role of dopamine transporters in the behavioral effects of 3,4-methylenedioxymethamphetamine (MDMA) in nonhuman primates. Psychopharmacology (Berl) 205:337–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantegrossi WE, Ullrich T, Rice KC, Woods JH, Winger G. (2002) 3,4-Methylenedioxymethamphetamine (MDMA, “ecstasy”) and its stereoisomers as reinforcers in rhesus monkeys: serotonergic involvement. Psychopharmacology (Berl) 161:356–364 [DOI] [PubMed] [Google Scholar]
- Fantegrossi WE, Woolverton WL, Kilbourn M, Sherman P, Yuan J, Hatzidimitriou G, Ricaurte GA, Woods JH, Winger G. (2004) Behavioral and neurochemical consequences of long-term intravenous self-administration of MDMA and its enantiomers by rhesus monkeys. Neuropsychopharmacology 29:1270–1281 [DOI] [PubMed] [Google Scholar]
- Fowler JS, Kroll C, Ferrieri R, Alexoff D, Logan J, Dewey SL, Schiffer W, Schlyer D, Carter P, King P, et al. (2007) PET studies of d-methamphetamine pharmacokinetics in primates: comparison with l-methamphetamine and (−)-cocaine. J Nucl Med 48:1724–1732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler JS, Volkow ND, Wolf AP, Dewey SL, Schlyer DJ, Macgregor RR, Hitzemann R, Logan J, Bendriem B, Gatley SJ. (1989) Mapping cocaine binding sites in human and baboon brain in vivo. Synapse 4:371–377 [DOI] [PubMed] [Google Scholar]
- Frankle WG, Mason NS, Rabiner EA, Ridler K, May MA, Asmonga D, Chen CM, Kendro S, Cooper TB, Mathis CA, et al. (2010) No effect of dopamine depletion on the binding of the high affinity D 2/3 radiotracer [11C]FLB 457 in the human cortex. Synapse 64:879–885 [DOI] [PubMed] [Google Scholar]
- Fuster JM. (1997) Network memory. Trends Neurosci 20:451–459 [DOI] [PubMed] [Google Scholar]
- Ginsburg BC, Kimmel HL, Carroll FI, Goodman MM, Howell LL. (2005) Interaction of cocaine and dopamine transporter inhibitors on behavior and neurochemistry in monkeys. Pharmacol Biochem Behav 80:481–491 [DOI] [PubMed] [Google Scholar]
- Hart CL, Haney M, Vosburg SK, Rubin E, Foltin RW. (2008) Smoked cocaine self-administration is decreased by modafinil. Neuropsychopharmacology 33:761–768 [DOI] [PubMed] [Google Scholar]
- Hashimoto K, Tsukada H, Nishiyama S, Fukumoto D, Kakiuchi T, Iyo M. (2007) Protective effects of minocycline on the reduction of dopamine transporters in the striatum after administration of methamphetamine: a positron emission tomography study in conscious monkeys. Biol Psychiatry 61:577–581 [DOI] [PubMed] [Google Scholar]
- Henry PK, Murnane KS, Votaw JR, Howell LL. (2010) Acute brain metabolic effects of cocaine in rhesus monkeys with a history of cocaine use. Brain Imaging Behav 4:212–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell LL, Carroll FI, Votaw JR, Goodman MM, Kimmel HL. (2007) Effects of combined dopamine and serotonin transporter inhibitors on cocaine self-administration in rhesus monkeys. J Pharmacol Exp Ther 320:757–765 [DOI] [PubMed] [Google Scholar]
- Howell LL, Hoffman JM, Votaw JR, Landrum AM, Jordan JF. (2001) An apparatus and behavioral training protocol to conduct positron emission tomography (PET) neuroimaging in conscious rhesus monkeys. J Neurosci Methods 106:161–169 [DOI] [PubMed] [Google Scholar]
- Howell LL, Hoffman JM, Votaw JR, Landrum AM, Wilcox KM, Lindsey KP. (2002) Cocaine-induced brain activation determined by positron emission tomography neuroimaging in conscious rhesus monkeys. Psychopharmacology (Berl) 159:154–160 [DOI] [PubMed] [Google Scholar]
- Howell LL, Votaw JR, Goodman MM, Lindsey KP. (2010) Cortical activation during cocaine use and extinction in rhesus monkeys. Psychopharmacology (Berl) 208:191–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito R, Dalley JW, Robbins TW, Everitt BJ. (2002) Dopamine release in the dorsal striatum during cocaine-seeking behavior under the control of a drug-associated cue. J Neurosci 22:6247–6253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins BG, Sanchez-Pernaute R, Brownell AL, Chen YC, Isacson O. (2004) Mapping dopamine function in primates using pharmacologic magnetic resonance imaging. J Neurosci 24:9553–9560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson B, Lamki L, Fang B, Barron B, Wagner L, Wells L, Kenny P, Overton D, Dhother S, Abramson D, et al. (1998) Demonstration of dose-dependent global and regional cocaine-induced reductions in brain blood flow using a novel approach to quantitative single photon emission computerized tomography. Neuropsychopharmacology 18:377–384 [DOI] [PubMed] [Google Scholar]
- Kilts CD, Schweitzer JB, Quinn CK, Gross RE, Faber TL, Muhammad F, Ely TD, Hoffman JM, Drexler KP. (2001) Neural activity related to drug craving in cocaine addiction. Arch Gen Psychiatry 58:334–341 [DOI] [PubMed] [Google Scholar]
- Kimmel HL, Negus SS, Wilcox KM, Ewing SB, Stehouwer J, Goodman MM, Votaw JR, Mello NK, Carroll FI, Howell LL. (2008) Relationship between rate of drug uptake in brain and behavioral pharmacology of monoamine transporter inhibitors in rhesus monkeys. Pharmacol Biochem Behav 90:453–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmel HL, O'Connor JA, Carroll FI, Howell LL. (2007) Faster onset and dopamine transporter selectivity predict stimulant and reinforcing effects of cocaine analogs in squirrel monkeys. Pharmacol Biochem Behav 86:45–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkland Henry P, Davis M, Howell LL. (2009) Effects of cocaine self-administration history under limited and extended access conditions on in vivo striatal dopamine neurochemistry and acoustic startle in rhesus monkeys. Psychopharmacology (Berl) 205:237–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kufahl PR, Li Z, Risinger RC, Rainey CJ, Wu G, Bloom AS, Li SJ. (2005) Neural responses to acute cocaine administration in the human brain detected by fMRI. Neuroimage 28:904–914 [DOI] [PubMed] [Google Scholar]
- Laruelle M. (2000) Imaging synaptic neurotransmission with in vivo binding competition techniques: a critical review. J Cereb Blood Flow Metab 20:423–451 [DOI] [PubMed] [Google Scholar]
- Letchworth SR, Nader MA, Smith HR, Friedman DP, Porrino LJ. (2001) Progression of changes in dopamine transporter binding site density as a result of cocaine self-administration in rhesus monkeys. J Neurosci 21:2799–2807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsey KP, Wilcox KM, Votaw JR, Goodman MM, Plisson C, Carroll FI, Rice KC, Howell LL. (2004) Effects of dopamine transporter inhibitors on cocaine self-administration in rhesus monkeys: relationship to transporter occupancy determined by positron emission tomography neuroimaging. J Pharmacol Exp Ther 309:959–969 [DOI] [PubMed] [Google Scholar]
- London ED, Cascella NG, Wong DF, Phillips RL, Dannals RF, Links JM, Herning R, Grayson R, Jaffe JH, Wagner HN., Jr (1990) Cocaine-induced reduction of glucose utilization in human brain. A study using positron emission tomography and [fluorine 18]-fluorodeoxyglucose. Arch Gen Psychiatry 47:567–574 [DOI] [PubMed] [Google Scholar]
- Maas LC, Lukas SE, Kaufman MJ, Weiss RD, Daniels SL, Rogers VW, Kukes TJ, Renshaw PF. (1998) Functional magnetic resonance imaging of human brain activation during cue-induced cocaine craving. Am J Psychiatry 155:124–126 [DOI] [PubMed] [Google Scholar]
- Mach RH, Nader MA, Ehrenkaufer RL, Line SW, Smith CR, Gage HD, Morton TE. (1997) Use of positron emission tomography to study the dynamics of psychostimulant-induced dopamine release. Pharmacol Biochem Behav 57:477–486 [DOI] [PubMed] [Google Scholar]
- Martinez D, Narendran R, Foltin RW, Slifstein M, Hwang DR, Broft A, Huang Y, Cooper TB, Fischman MW, Kleber HD, et al. (2007) Amphetamine-induced dopamine release: markedly blunted in cocaine dependence and predictive of the choice to self-administer cocaine. Am J Psychiatry 164:622–629 [DOI] [PubMed] [Google Scholar]
- Melega WP, Jorgensen MJ, Laćan G, Way BM, Pham J, Morton G, Cho AK, Fairbanks LA. (2008) Long-term methamphetamine administration in the vervet monkey models aspects of a human exposure: brain neurotoxicity and behavioral profiles. Neuropsychopharmacology 33:1441–1452 [DOI] [PubMed] [Google Scholar]
- Morgan D, Grant KA, Gage HD, Mach RH, Kaplan JR, Prioleau O, Nader SH, Buchheimer N, Ehrenkaufer RL, Nader MA. (2002) Social dominance in monkeys: dopamine D2 receptors and cocaine self-administration. Nat Neurosci 5:169–174 [DOI] [PubMed] [Google Scholar]
- Mukherjee J, Yang ZY, Lew R, Brown T, Kronmal S, Cooper MD, Seiden LS. (1997) Evaluation of d-amphetamine effects on the binding of dopamine D-2 receptor radioligand, 18F-fallypride in nonhuman primates using positron emission tomography. Synapse 27:1–13 [DOI] [PubMed] [Google Scholar]
- Murnane KS, Fantegrossi WE, Godfrey JR, Banks ML, Howell LL. (2010) Endocrine and neurochemical effects of MDMA and its stereoisomers in rhesus monkeys. J Pharmacol Exp Ther 334:642–650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murnane KS, Howell LL. (2010) Development of an apparatus and methodology for conducting functional magnetic resonance imaging (fMRI) with pharmacological stimuli in conscious rhesus monkeys. J Neurosci Methods 191:11–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murnane KS, Murai N, Howell LL, Fantegrossi WE. (2009) Discriminative stimulus effects of psychostimulants and hallucinogens in S(+)-3,4-methylenedioxymethamphetamine (MDMA) and R(−)-MDMA trained mice. J Pharmacol Exp Ther 331:717–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nader MA, Czoty PW. (2005) PET imaging of dopamine D2 receptors in monkey models of cocaine abuse: genetic predisposition versus environmental modulation. Am J Psychiatry 162:1473–1482 [DOI] [PubMed] [Google Scholar]
- Nader MA, Czoty PW, Gould RW, Riddick NV. (2008) Review. Positron emission tomography imaging studies of dopamine receptors in primate models of addiction. Philos Trans R Soc London B Biol Sci 363:3223–3232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nader MA, Morgan D, Gage HD, Nader SH, Calhoun TL, Buchheimer N, Ehrenkaufer R, Mach RH. (2006) PET imaging of dopamine D2 receptors during chronic cocaine self-administration in monkeys. Nat Neurosci 9:1050–1056 [DOI] [PubMed] [Google Scholar]
- Narendran R, Slifstein M, Hwang DR, Hwang Y, Scher E, Reeder S, Martinez D, Laruelle M. (2007) Amphetamine-induced dopamine release: duration of action as assessed with the D2/3 receptor agonist radiotracer (−)-N-[11C]propyl-norapomorphine ([11C]NPA) in an anesthetized nonhuman primate. Synapse 61:106–109 [DOI] [PubMed] [Google Scholar]
- Pearlson GD, Jeffery PJ, Harris GJ, Ross CA, Fischman MW, Camargo EE. (1993) Correlation of acute cocaine-induced changes in local cerebral blood flow with subjective effects. Am J Psychiatry 150:495–497 [DOI] [PubMed] [Google Scholar]
- Phelps ME, Mazziotta JC. (1985) Positron emission tomography: human brain function and biochemistry. Science 228:799–809 [DOI] [PubMed] [Google Scholar]
- Porrino LJ, Lyons D, Miller MD, Smith HR, Friedman DP, Daunais JB, Nader MA. (2002) Metabolic mapping of the effects of cocaine during the initial phases of self-administration in the nonhuman primate. J Neurosci 22:7687–7694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saadat KS, Elliott JM, Green AR, Moran PM. (2006) High-dose MDMA does not result in long-term changes in impulsivity in the rat. Psychopharmacology (Berl) 188:75–83 [DOI] [PubMed] [Google Scholar]
- Scheffel U, Szabo Z, Mathews WB, Finley PA, Dannals RF, Ravert HT, Szabo K, Yuan J, Ricaurte GA. (1998) In vivo detection of short- and long-term MDMA neurotoxicity–a positron emission tomography study in the living baboon brain. Synapse 29:183–192 [DOI] [PubMed] [Google Scholar]
- Senda M, Kimura Y, Herscovitch P. (2002) Brain Imaging Using PET. Academic Press, New York [Google Scholar]
- Seneca N, Finnema SJ, Farde L, Gulyás B, Wikström HV, Halldin C, Innis RB. (2006) Effect of amphetamine on dopamine D2 receptor binding in nonhuman primate brain: a comparison of the agonist radioligand [11C]MNPA and antagonist [11C]raclopride. Synapse 59:260–269 [DOI] [PubMed] [Google Scholar]
- Sleight AJ, Stam NJ, Mutel V, Vanderheyden PM. (1996) Radiolabelling of the human 5-HT2A receptor with an agonist, a partial agonist and an antagonist: effects on apparent agonist affinities. Biochem Pharmacol 51:71–76 [DOI] [PubMed] [Google Scholar]
- Slifstein M, Narendran R, Hwang DR, Sudo Y, Talbot PS, Huang Y, Laruelle M. (2004) Effect of amphetamine on [18F]fallypride in vivo binding to D(2) receptors in striatal and extrastriatal regions of the primate brain: single bolus and bolus plus constant infusion studies. Synapse 54:46–63 [DOI] [PubMed] [Google Scholar]
- Tokunaga M, Seneca N, Shin RM, Maeda J, Obayashi S, Okauchi T, Nagai Y, Zhang MR, Nakao R, Ito H, et al. (2009) Neuroimaging and physiological evidence for involvement of glutamatergic transmission in regulation of the striatal dopaminergic system. J Neurosci 29:1887–1896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udo de Haes JI, Harada N, Elsinga PH, Maguire RP, Tsukada H. (2006) Effect of fenfluramine-induced increases in serotonin release on [18F]MPPF binding: a continuous infusion PET study in conscious monkeys. Synapse 59:18–26 [DOI] [PubMed] [Google Scholar]
- van Berckel BN, Kegeles LS, Waterhouse R, Guo N, Hwang DR, Huang Y, Narendran R, Van Heertum R, Laruelle M. (2006) Modulation of amphetamine-induced dopamine release by group II metabotropic glutamate receptor agonist LY354740 in non-human primates studied with positron emission tomography. Neuropsychopharmacology 31:967–977 [DOI] [PubMed] [Google Scholar]
- Villemagne V, Yuan J, Wong DF, Dannals RF, Hatzidimitriou G, Mathews WB, Ravert HT, Musachio J, McCann UD, Ricaurte GA. (1998) Brain dopamine neurotoxicity in baboons treated with doses of methamphetamine comparable to those recreationally abused by humans: evidence from [11C]WIN-35,428 positron emission tomography studies and direct in vitro determinations. J Neurosci 18:419–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villemagne VL, Rothman RB, Yokoi F, Rice KC, Matecka D, Dannals RF, Wong DF. (1999) Doses of GBR12909 that suppress cocaine self-administration in non-human primates substantially occupy dopamine transporters as measured by [11C] WIN35,428 PET scans. Synapse 32:44–50 [DOI] [PubMed] [Google Scholar]
- Vogt BA, Finch DM, Olson CR. (1992) Functional heterogeneity in cingulate cortex: the anterior executive and posterior evaluative regions. Cereb Cortex 2:435–443 [DOI] [PubMed] [Google Scholar]
- Volkow ND, Ding YS, Fowler JS, Wang GJ, Logan J, Gatley JS, Dewey S, Ashby C, Liebermann J, Hitzemann R. (1995) Is methylphenidate like cocaine? Studies on their pharmacokinetics and distribution in the human brain. Arch Gen Psychiatry 52:456–463 [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Gatley SJ, Dewey SL, Wang GJ, Logan J, Ding YS, Franceschi D, Gifford A, Morgan A, et al. (1999) Comparable changes in synaptic dopamine induced by methylphenidate and by cocaine in the baboon brain. Synapse 31:59–66 [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Logan J, Alexoff D, Zhu W, Telang F, Wang GJ, Jayne M, Hooker JM, Wong C, et al. (2009) Effects of modafinil on dopamine and dopamine transporters in the male human brain: clinical implications. Jama 301:1148–1154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fischman MW, Foltin RW, Fowler JS, Abumrad NN, Vitkun S, Logan J, Gatley SJ, Pappas N, et al. (1997) Relationship between subjective effects of cocaine and dopamine transporter occupancy. Nature 386:827–830 [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Childress AR, Jayne M, Ma Y, Wong C. (2008) Dopamine increases in striatum do not elicit craving in cocaine abusers unless they are coupled with cocaine cues. Neuroimage 39:1266–1273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Votaw JR, Howell LL, Martarello L, Hoffman JM, Kilts CD, Lindsey KP, Goodman MM. (2002) Measurement of dopamine transporter occupancy for multiple injections of cocaine using a single injection of [F-18]FECNT. Synapse 44:203–210 [DOI] [PubMed] [Google Scholar]
- Wallace EA, Wisniewski G, Zubal G, vanDyck CH, Pfau SE, Smith EO, Rosen MI, Sullivan MC, Woods SW, Kosten TR. (1996) Acute cocaine effects on absolute cerebral blood flow. Psychopharmacology 128:17–20 [DOI] [PubMed] [Google Scholar]
- Weerts EM, Fantegrossi WE, Goodwin AK. (2007) The value of nonhuman primates in drug abuse research. Exp Clin Psychopharmacol 15:309–327 [DOI] [PubMed] [Google Scholar]
- Wexler BE, Gottschalk CH, Fulbright RK, Prohovnik I, Lacadie CM, Rounsaville BJ, Gore JC. (2001) Functional magnetic resonance imaging of cocaine craving. Am J Psychiatry 158:86–95 [DOI] [PubMed] [Google Scholar]
- Wilcox KM, Kimmel HL, Lindsey KP, Votaw JR, Goodman MM, Howell LL. (2005) In vivo comparison of the reinforcing and dopamine transporter effects of local anesthetics in rhesus monkeys. Synapse 58:220–228 [DOI] [PubMed] [Google Scholar]
- Wilcox KM, Lindsey KP, Votaw JR, Goodman MM, Martarello L, Carroll FI, Howell LL. (2002) Self-administration of cocaine and the cocaine analog RTI-113: relationship to dopamine transporter occupancy determined by PET neuroimaging in rhesus monkeys. Synapse 43:78–85 [DOI] [PubMed] [Google Scholar]
- Wilcox KM, Zhou Y, Wong DF, Alexander M, Rahmim A, Hilton J, Weed MR. (2008) Blood levels and DA transporter occupancy of orally administered methylphenidate in juvenile rhesus monkeys measured by high resolution PET. Synapse 62:950–952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winsauer PJ, McCann UD, Yuan J, Delatte MS, Stevenson MW, Ricaurte GA, Moerschbaecher JM. (2002) Effects of fenfluramine, m-CPP and triazolam on repeated-acquisition in squirrel monkeys before and after neurotoxic MDMA administration. Psychopharmacology (Berl) 159:388–396 [DOI] [PubMed] [Google Scholar]
- Xiao ZW, Cao CY, Wang ZX, Li JX, Liao HY, Zhang XX. (2006) Changes of dopamine transporter function in striatum during acute morphine addiction and its abstinence in rhesus monkey. Chin Med J (Engl) 119:1802–1807 [PubMed] [Google Scholar]
- Yokoyama C, Yamanaka H, Onoe K, Kawasaki A, Nagata H, Shirakami K, Doi H, Onoe H. (2010) Mapping of serotonin transporters by positron emission tomography with [11C]DASB in conscious common marmosets: comparison with rhesus monkeys. Synapse 64:594–601 [DOI] [PubMed] [Google Scholar]
- Zolkowska D, Jain R, Rothman RB, Partilla JS, Roth BL, Setola V, Prisinzano TE, Baumann MH. (2009) Evidence for the involvement of dopamine transporters in behavioral stimulant effects of modafinil. J Pharmacol Exp Ther 329:738–746 [DOI] [PMC free article] [PubMed] [Google Scholar]