Abstract
B-cell lymphoma 6 (BCL6) and PR domain containing 1 (PRDM1) are considered as master regulators for germinal center (GC) formation and terminal B-cell differentiation. Dysregulation of BCL6 and PRDM1 has been associated with lymphomagenesis. Here, we show for the first time that direct cell–cell contact between follicular dendritic cells (FDC) and B-lymphocytes, by influencing the expression of a set of microRNAs (miRNAs), regulates the expression of BCL6 and PRDM1. We identify that, on cell adhesion to FDC, FDC induces upregulation of PRDM1 expression through downregulation of miR-9 and let-7 families and induces downregulation of BCL-6 through upregulation of miR-30 family in B-lymphocytes and lymphoma cells. We further demonstrate that the miR-30 family directly controls BCL-6 expression and miR-9-1 and let-7a directly control PRDM-1 expression through targeting their 3′UTR, mediating the FDC effect. Our studies define a novel regulatory mechanism in which the FDC, through induction of miRNAs in B-lymphocytes, orchestrates the regulation of transcription factors, promotes germinal center B-cell survival and differentiation. Dysregulation of miRNAs may interfere with B-cell survival and maturation, thus representing a novel molecular mechanism, as well as a potential therapeutic target in B-cell lymphomas.
Keywords: lymphoma, BCL-6, PRDM-1, miRNA, cell adhesion
Introduction
In response to antigenic challenge, uncommitted B-progenitor lymphocytes migrate through the follicular dendritic cell (FDC) network, are positively selected and establish highly organized germinal centers (GC) in lymphoid organs. Within the GC, proliferating GC B cells undergo somatic hypermutation, affinity maturation and isotype switch, and finally differentiate into either memory B cells or plasma cells. This B-cell differentiation process is strongly regulated by suppression or induction of specific transcription factors. Among various transcriptional regulators, B-cell lymphoma 6 (BCL6) and PR domain containing 1 (PRDM1) are master regulators for GC formation and terminal B-cell differentiation.1,2 Dysregulation of BCL6 and PRDM1 has been associated with lymphomagenesis.1,2 It has been proposed that, within the GC, B cells closely interact with FDCs, which provide survival signals to protect B cells from apoptosis and are essential for the differentiation of GC B cells.3 However, considerably less is understood about the molecular mechanisms, especially in the GC, that initiate and determine late B-cell differentiation and expression of BCL6 and PRDM1. In this study, we determined whether interaction between FDC and B lymphocytes can regulate the expression of BCL6 and PRDM1 in the context of the GC. Furthermore, we explored the role of microRNA (miRNA) in the expression of BCL6 and PRDM1 in GC B lymphocytes.
miRNAs are a newly discovered class of short (19–25 nucleotides) endogenous small RNAs that negatively regulate gene expression post-transcriptionally.4 There has been an explosion of interest in the miRNA field as these molecules have been found to have key roles in a wide range of biological processes and to be aberrantly expressed in many types of cancer, including lymphomas. 5,6 However, the role of miRNAs in B-lymphocyte development and B-cell lymphomagenesis is largely unknown. More researchers are beginning to address the role of miRNAs in B-cell malignancies, with some suggesting that specific miRNAs have an important role in B-cell function and malignancy. MiR-155 has been shown to regulate GC response through modulation of cytokine production7 and regulation of the activation-induced cytidine deaminase.8 A more recent study has shown that miR-155, by downmodulating Src homology 2 domain-containing inositol-5-phosphatase and Src homology 2 domain-containing inositol-5-phosphatase (C/EBPβ), initiates a chain of events that lead to the accumulation of large pre-B cells and acute lymphoblastic leukemia/high-grade lymphoma.9
FDCs are stromal cells unique to primary and secondary lymphoid follicles. Recirculating resting B cells migrate through the FDC networks, whereas antigen-activated B cells undergo clonal expansion within the FDC networks in a T-cell-dependent manner, thereby generating GCs.3 Only B cells that bind to FDC survive in the GC and differentiate into high-affinity plasma cells and memory B cells.10,11 As physical interactions between B cells and FDCs from the lymphoid tissue microenvironment are critical for B-cell maturation and survival of normal and malignant B cells, we have identified the miRNAs induced by interactions between FDCs and B cells. Furthermore, we explored the regulation of BCL6 and PRDM1 by FDCs and the role of FDC-induced miRNAs in BCL6 and PRDM1 expression using an in vitro GC B-cell-FDC coculture model.
Materials and methods
The details of this section are available online as Supplementary Information.
Results
FDCs regulate expression of BCL6 and PRDM1 via cell–cell contact
We used a previously described FDC-B-cell coculture model12,13 to examine the effects of cell adhesion to FDC on transcription factor (BCL6 and PRDM1) expression. The GC-derived large lymphoma cell line SUDHL-4 (SU-4), Burkitt’s lymphoma cell line Ramos, as well as HK FDCs were used in our study. Examination of the effects of FDC on BCL6 and PRDM1 revealed decreased BCL6 expression and increased PRDM1 expression at 24, 48, 72 and 96 h after coculture of HK and SU-4 cells (Figure 1). After 24 h of adhesion, we observed a dramatic decrease in BCL6 expression at both the protein and mRNA levels in SU-4 lymphoma cells (Figures 1a and b) and Ramos cells (Supplementary Figure S1). PRDM1 protein increased ~2-fold after 24 h of adhesion and remained elevated for up to 96 h (Figure 1d). A similar consistent increase was also detected for PRDM1 mRNA (Figure 1e). To examine the contribution of soluble factors on physical cell–cell contact, we used Transwell inserts to allow lymphoma cells to be juxtaposed to HK cells without cell–cell contact. As shown in Figures 1c and f, lymphoma cells in direct contact with HK cells, but not cells exposed only to soluble factors released by HK cells, were induced to express PRDM1 and downregulate BCL6. This result indicates that cell–cell contact is required for FDC-induced PRDM1 and BCL6 expression changes. To determine whether the BCL6 and PRDM1 changes initiated by adhesion to HK cells were reversible, cells were detached after 24 h of adhesion and then grown in suspension for 12–48 h before reexamination of BCL6 and PRDM1. As shown in Supplementary Figure S2A–C, disruption of adhesion to HK cells resulted in increased BCL6 and decreased PRDM1 expression. This indicates that interaction of HK cells with SU-4 induces a reversible regulation of BCL6 and PRDM1 via cell–cell contact.
Figure 1.
Cell adhesion to FDCs (HK cells) induces cell–cell contact-dependent downregulation of BCL6 and upregulation of PRDM1 at protein and mRNA levels.SU-4 cells (105/ml) were incubated for 24–96 h in the absence of HK cells on a confluent HK monolayer (SU-4+HK), or in the same well with a confluent HK monolayer, but separated by cell-culture inserts (Tanswell). (a) Downregulation of BCL6 protein over time after SU-4 adhesion to HK cells. (b) Downregulation of BCL6 mRNA over time after SU-4 adhesion to HK cells. (c) Direct cell–cell contact but not soluble factor(s) induced downregulation of BCL6. (d) Upregulation of PRDM1 protein over time after SU-4 adhesion to HK cells. (e) Upregulation of PRDM1 mRNA over time after SU-4 adhesion to HK cells. (f) Direct cell–cell contact but not soluble factor(s) induced upregulation of PRDM1. Data are means of three experiments and representative of at least three experiments. For relative mRNA expression, the mRNA level of SU-4 cells in suspension was arbitrarily set as 1. *,**P<0.05.
FDCs regulate expression of B-cell survival and differentiation-related miRNA expression
We aimed to identify which miRNAs were involved in FDC-induced BCL6 and PRDM1 expression changes. For this, we first performed miRNA analysis on SU-4 cells in suspension, and after 24 and 48 h of HK coculture. To ensure that no HK cell contamination occurred, SU-4 cells in suspension and after coculture were purified by CD19-positive magnetic selection, followed by total RNA isolation. Duplicate microarray platforms were used to examine expression levels of 875 validated human miRNAs in these RNA preparations. Cells maintained in suspension were designated as the reference population. Using criteria and statistical analysis described in experimental procedures, we identified miRNAs that were differentially expressed on B-lymphoma cell adhesion to HK cells. As shown in Figures 2a and b, with respect to SU-4 cells in suspension, 38 miRNAs were upregulated and 39 were downregulated after 24 h of coculture (Figure 2a), and 41 were both upregulated and downregulated after 48 h of coculture (Figure 2b) (>twofold change; P≤0.01). It is noteworthy that clusters or paralog families of miRNA precursors, such as miR-17~92, miR-106a~92 and miR-106b~25, were identified in both 24-h and 48-h cocultures with respect to cells in suspension (representative miRNAs shown in Figure 2c). A total of 18 miRNAs were upregulated and 12 were downregulated at both 24 and 48 h of coculture.
Figure 2.
Differential miRNA expression after cell adhesion to HK cells after 24 h (a) and 48 h (b) and representative common differentially expressed miRNAs at both 24 and 48 h (c). SU-4 cells in suspension and after 24 and 48 h of co-culture with HK cells were sorted out by CD19 magnet beads, and isolated total RNA was then applied for miRNA array assay. A duplicate of microarray platform was used to examine expression levels of 875 validated human miRNAs. Total RNA (5 µg) isolated from SU-4 in suspension and after coculture was analyzed by miRNA array analysis. A clustering graph was created using a hierarchical method from miRNAs with signal density of > 1000, showing increased (red) or decreased expression (P<0.05) of individual miRNAs. The column heading indicates the individual sample code in the assay. Each row shows the expression pattern of individual miRNAs as a log 2-transformed expression ratio, with the most closely related expression joined by a branch and clustered by an average-linked algorithm. Of these miRNAs, 38 were upregulated (red in heat map) and 39 were downregulated (green in heat map) after 24 h and 41 were both upregulated (red in heat map) and downregulated after 48 h of coculture. Asterisks (*) indicates the specific associated cluster. (d, e) Validation of cell-adhesion (SU-4+HK)-induced miRNA changes determined using TaqMan MicroRNA qRT-PCR assays; miR-30 family member (d) and let-7a (e). Results in d and e are representative of at least four experiments. For relative miR-30 and let-7a expression, it is relative to the miRNA level of SU-4 in suspension (arbitrarily set as 1).
We next searched for miRNAs that target BCL6 and PRDM1. As miRNAs negatively regulate their target genes through base-pairing interaction between their seed sequence and the 3′-UTR of target genes, we searched the miRNA TargetScan Database and found that a sequence motif of the 3′-UTR of BCL6 matches the miR-30 family, and PRDM1 matches the miRNA let-7a seed sequences. We selected a set of miRNAs, including let-7 miRNAs and the miR-30 family, for initial validation and further functional studies. As shown in Figure 2c, these miRNAs were indeed regulated by FDC, and each is predicted to have target site(s) in either BCL6 or PRDM1 mRNA 3′-UTR. We therefore examined whether FDC induced miRNA let-7 and miR30 expression changes using quantitative real-time RT-PCR. In agreement with miRNA, microarray data shown in Figures 2c, d and e show that adhesion of SU-4 to HK cells induced significant miR-30 family upregulation and let-7a downregulation, respectively, with respect to cells in suspension (arbitrarily set as 1). These effects are cell–cell contact dependent, as let-7a and miR-30s remained unchanged after HK and SU-4 coculture, when the cells were cocultured with Transwell inserts to allow SU-4 cells to be juxtaposed to HK cells without cell–cell contact. The downregulation of let-7a and upregulation of the miR-30 family are cell–cell contact dependent and reversible as resuspension of SU-4 (detach) after 48 h of coculture resulted in reversal of HK-induced miR30 family and let-7a changes (Supplementary Figures S2D–2E).
miRNAs regulate the expression of BCL6 and PRDM1 and are required for FDC-induced changes in BCL6 and PRDM1 expression
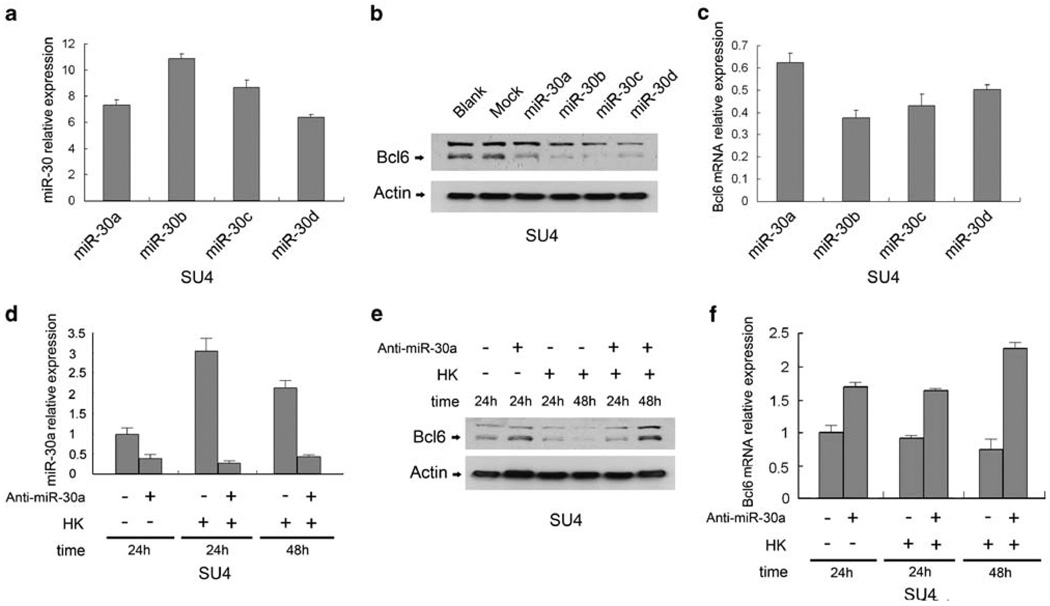
We investigated whether BCL6 and PRDM1 were indeed regulated by miRNAs (let-7 and miR-30 families) and whether the observed increase in PRDM1 and downregulation in BCL6 expression after adhesion to HK cells are mediated through these miRNAs. To examine whether BCL6 was regulated by miR-30, we ectopically expressed each of the miR-30 family members (miR-30a, b, c and d) in SU-4 and Ramos cells by lentiviral transduction. Overexpression of miR-30 family members resulted in a dramatic decrease in expression of BCL6 protein compared with a scrambled control (arbitrarily set as 1; Figures 3a and b). Knockdown of miR-30s by antisense miR-30a using 2′-O-methyl oligonucleotide (2-O-ME) antagonists (anti-miR-30a) partially restored BCL6 in SU-4 cells (Figures 3c and d). In addition, we determined whether depletion of miR-30 by anti-miR30 suppressed HK cell-induced miR-30 expression and, subsequently, HK cell-regulated BCL6 expression. As shown in Figures 3c–e, depletion of miR-30 expression by anti-miR-30s abolished FDC-induced BCL6 downregulation, indicating that the miR-30 family was required for regulation of BCL6 expression by FDCs. The result was further confirmed by similar results in Ramos cells using the same lentiviral vector expressing miR-30a and anti-miR-30a (Figures S3A-C). In addition, we also examined whether PRDM1 was regulated by miR-30, as PRDM1 is another potential target of the moR30 family. We ectopically expressed each of the miR-30 family members (miR-30a, b, c and d) in SU-4 cells by lentiviral transduction. Overexpression of miR-30 family members resulted in a modest decrease (miR-30a-c) or no change (miR-30d) in expression of PRDM1 protein compared with a scrambled control (Supplementary Figure S3D), suggesting miR-30 involvement of fine balanced tuning of regulation on both BCL6 and PRDM1 expression. Furthermore, we performed similar gain- or loss-of-function experiments to examine the effects of overexpression and knockdown of let-7a on PRDM1 expression in SU-4 cells (Figure 4a). SU-4 cells were transduced with the precursor of let-7a (pre-let-7a). Overexpression of let-7a resulted in a consistent decrease in PRDM1 protein and mRNA expression, compared with a non-targeting control miRNA precursor (Figure 4c), whereas let-7a depletion through anti-let-7a partially restored PRDM1 in SU-4 cells (Figure 4b). To further explore the role of let-7 in HK-induced PRDM1 expression, we applied pre-let-7a-transfected cells to our co-culture experiments. Figure 4d shows that overexpression of let-7a partially prevented HK-induced PRDM1 expression. Together, these data support that miR-30 and let-7 regulate expression of BCL6 and PRDM1 and are required for FDC-induced expression of BCL6 and PRDM1.
Figure 3.
The miR-30 family suppresses expression of BCL6 and is required for FDC-induced downregulation of BCL6. (a) miR-30a, 30b, 30c and 30d overexpression by their lentiviral vectors. (b) Overexpression of miR-30 family depleted BCL6 protein (b) and mRNA (c) in SU-4 lymphoma cells. (d–f) Effect of miR-30 on cell-adhesion-induced BCL6 downregulation. Cell adhesion to HK cells induced miR-30 expression (d), and downregulation of miR-30 by anti-miR-30 blocked adhesion-induced miR-30 expression (d), blocked HK adhesion-induced BCL6 protein (e) and mRNA (f) downregulation in SU-4, respectively. SU-4 (a–c) cells were infected with lentivirus encoding GFP alone (empty vector, control) or a fusion protein of GFP and miR-30 members (miR-30s) to ectopically express miR-30s. miR-30s were inhibited (d–f) with antisense 2-O-ME against miR-30 by using Amaxa’s nucleofector system (Anti-miR-30). Results in a–f are representative of at least three experiments. For relative mRNA expression, mRNA levels of SU-4 cells in suspension were arbitrarily set as 1.
Figure 4.
Let-7a negatively regulates expression of PRDM1 and its downregulation is required for FDC-induced upregulation of PRDM1. (a) Pre-let-7a and anti-let-7a increased and decreased levels of let-7a in SU-4 cells. (b) Anti-let-7a enhanced HK adhesion-induced PRDM1 expression. (c) Pre-let-7a blocked HK adhesion-induced PRDM1 mRNA expression. (d) Pre-let-7a blocked HK adhesion-induced PRDM1 protein expression. Results in a–d are representative of at least three experiments. SU-4 cells were co-cultured with HK for 48 h. For relative mRNA or miRNA expression, the mRNA or miRNA level of SU-4 cells in suspension was arbitrarily set as 1. *P<0.05.
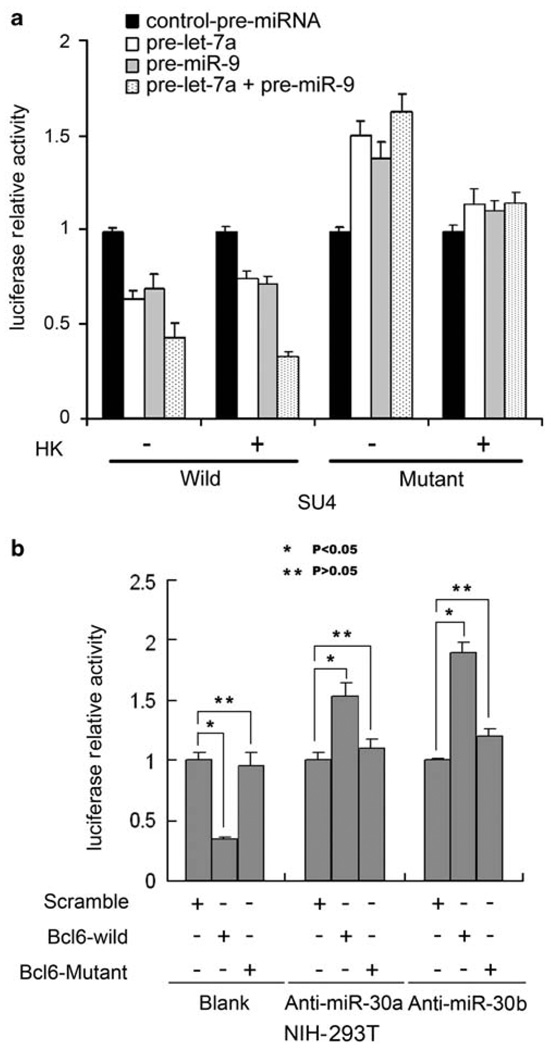
We further used a luciferase reporter with the PRDM1 3′-UTR fused to luciferase (pSIC.PRDM1.3′-UTR.538–2419) to validate the direct regulation of PRDM1 by let-7a as described previously.14 Both wild-type (WT) and mutant reporters with the let-7a binding site mutated were co-transfected with pre-let-7a into SU-4 cells, and a luciferase reporter assay was performed. Figure 5 shows that luciferase activity in pSIC.PRDM1.3′-UTR, but not in mutant reporter plasmids, was significantly suppressed by ectopic expression of let-7a in SU-4 cells. We observed increased reporter activity for pSIC.PRDM1.3′-UTR following cell adhesion to HK cells. Pre-let-7a only moderately suppressed reporter activity for PRDM1.3′-UTR and partially decreased HK-induced reporter activity for PRDM1.3′-UTR, as shown in Figure 5a. Of note, HK cells increased luciferase activity of the let-7a mutant reporter of PRDM1.3′-UTR, indicating that let-7a only partially regulated PRDM1 expression. Because pSIC.PRDM1.3′-UTR.538–2419 contained a binding site for miR-9, another known and predicted miRNA targeting PRDM1, we speculated that miR-9 might be involved in PRDM1 expression, especially in the setting of HK-B cell interaction.
Figure 5.
Let-7a and miR-9 interact with the conserved site of the PRDM1 3′-UTR, and BCL6 is a direct target of miR-30. (a) SU-4 cells were transfected with WT psiC.PRDM1/3′UTR.538–2419 or one of the mutant reporter plasmids harboring point mutations in the target sites for let-7a or miR-9. These reporter plasmids were co-transfected with let-7a precursor (50 nmol/l) or/and miR-9 precursor (50 nmol/l). pcDNA3 plasmid or miRNA negative control oligonucleotides (Applied biosystems Inc., Austin, TX, USA) were used as negative controls. SU-4 cells were transfected with the indicated plasmids, with or without HK coculture for 48 h, and luciferase activities (in triplicate) were measured 36 h after transfection. (b) Anti-miR-30 increased luciferase reporter activity in WT BCL6 3′-UTR reporter but not in mutant reporter plasmids harboring point mutations in the target sites for miR-30. Luciferase reporter assays were conducted in NIH-293T cells co-transfected with luciferase constructs containing BCL6-miR-30a putative binding sites and antisense 2-O-ME against miR-30a (Anti-miR-30) or co-transfected with mutated versions of the BCL6-miR-30a putative binding sites and antisense 2-O-ME against miR-30a. Scrambled antisense 2-O-ME was used as control for anti-miR-30a. For a and b, Renilla luciferase activities were normalized against firefly luciferase activities, and results for normalized Renilla luciferase activities are means (±s.e.) from at least three independent experiments and expressed relative to control values. *,**P<0.05.
Figure 5a shows that miR-9 overexpression of miR-9 precursor transfection decreased PRDM1 reporter activity and partially blocked HK-enhanced PRDM1 reporter activity in SUDH-4 cells. Interestingly, transfection of combined pre-let-7a and miR-9 significantly decreased PRDM1 reporter activity and markedly blocked HK-enhanced PRDM1 reporter activity, indicating that let-7a and miR-9 functioned in a coordinated manner to regulate PRDM1 expression. These results are in line with previously described additive repression effects of let-7a and miR-9 on PRDM1 expression.14 Of note, cell-adhesion-induced miR-9 downregulation is detected by qRT-PCR as shown in Figure 5a but not from our miRNA array experiment. This is likely because of technique sensitivity.
To further validate the direct interaction between BCL6 and miR-30, we constructed luciferase reporters containing the BCL6 3′-UTR that included miR-30 binding sites and a mutant 3′-UTR harboring mutations in the ‘seed pairing’ sequences of the miR-30 binding site. WT and mutant reporters were introduced into NIH-293T cells. As NIH-293T cells contain abundant miR-30s, we transfected these cells with WT or mutant BCL6 3′-UTR reporter with and without anti-miR30s (2-O-ME-miR-30s). Figure 5b shows that reporter activity of WT but not mutant BCL6-3′-UTR was significantly decreased after transfection. This is likely due to the effect of abundant endogenous miR-30s. However, when co-transfecting WT or mutant BCL6-3′-UTR with anti-miR30a and anti-miR30b in NIH-293T cells, reporter activity of WT but not mutant BCL6-3′-UTR was significantly increased, supporting the role of the miR-30 family in the regulation of BCL6 expression.
Collectively, these data support the notion that interaction of HK cells with B cells triggers the expression of a set of miRNAs and that miRNAs regulate expression of differentiation-related transcriptional factors, such as BCL6 and PRDM1.
FDC-regulated expression of BCL6 and PRDM1 and miRNAs let-7 and miR-30 in primary normal B-lymphocytes and lymphoma cells
We next investigated whether the observed changes in BCL6 and PRDM1 and their related miRNAs, let-7 and miR-30, can be validated in primary normal B lymphocytes and lymphoma cells. Figures 6a and b show that all primary B-lymphocyte samples (including one follicular lymphoma, two diffuse large B-cell lymphomas, four benign B lymph nodes) had changes similar to those observed in the lymphoma cell lines (SU-4 and Ramos). The results shown in primary lymphocytes urther support that miRNAs have a role in the regulation of BCL6 and PRDM1 expression and that modulation of miRNAs by interaction between FDCs and B lymphocytes contributes to induction and suppression of BCL6 and PRDM1. BCL6 and PRDM1 have been shown to be important for regulation of the gene expression required for B-cell differentiation. Dysregulation of BCL6 and PRDM1 is often associated with lymphomagenesis.
Figure 6.
Cell adhesion induced downregulation of BCL6 and upregulation of PRDM1 through miR-30s and let-7 in primary B lymphocytes and B-lymphoma cells. (a–d) BCL6, PRDM1, miR-30 and let-7a expression levels in primary follicular center cell lymphoma (FCC), diffuse large B cell (DLBC) and normal B lymphocytes from reactive lymph nodes (NLN) in suspension versus HK adhesion for 24 h were analyzed by qRT-PCR.
Discussion
We show for the first time a function for FDC in BCL6 and PRDM1 regulation that is cell–cell contact dependent. Our findings of downregulation of BCL6 and upregulation of PRDM1 by FDCs imply a role for FDC in late B-cell differentiation. On antigen stimulation, B lymphocytes undergo terminal differentiation into antibody-secreting plasma cells and memory B cells. These changes are the result of silencing of BCL6 and induction of PRDM1. BCL6 is selectively expressed in mature B cells within the GCs15,16 and is required for the formation of GCs.17,18 Independent lines of evidence suggest that down-regulation of BCL6 is necessary for normal B cells to exit the GC, whereas PRDM1 appears to be that key regulator the gene of which serves as a hub for the gene network for B-cell terminal differentiation into plasma cells. Overexpression of PRDM1 induces mature B cells to differentiate into plasma cells.19,20
Our finding of BCL6 downregulation and PRDM1 induction by contact interaction of FDCs and B lymphocytes is in agreement with that of a BCL6/PRDM1 expression pattern, when B cells undergo GC B-cell maturation. Contact interaction of FDCs and B cells in GC may have a critical role in commitment of B-cell terminal development by regulating the balance of BCL6 and PRDM1. Our study also supports kinetic changes of BCL6 and PRDM1 during plasma cell and memory B-cell differentiation, as shown in a recent report.21 Our study used a similar but modified model that did not include CD40L and other cytokines such as interleukin (IL)-4 and IL-10 in our co-culture model for the purpose of exploring the sole effect of FDC on BCL6 and PRDM1 expression. Activated T cells regulate BCL6 and PRDM1 expression through CD40–CD154 (CD40L) and secretion of cytokines. Our study shows that FDCs are directly involved in regulation of BCL/PRDM1 expression through cell–cell contact interaction.
FDCs constitute the backbone of follicles and are a major component of the follicular microenvironment and have an antiapoptotic role in B-cell survival and late B-cell development. Among the miRNAs that we observed to be associated with B-cell survival and proliferation (see Figure 2) are the let-7 family, miR-30 family and three paralog families of miRNA precursors, as well as miR181, miR-15a and miR-16-1, miR-146, and miR-99 families. The coordinated effect of these miRNAs may have a critical role in B-cell survival in GC and in terminal B-cell differentiation. Interestingly, when we compared our in vitro coculture model data with two recent studies that analyzed stage-specific expression of native, GC, as well as memory B cells, we found significant overlap between miRNAs expressed after interaction with FDCs and miRNAs expressed in GC cells identified in these studies. More than half of the 39 GC miRNAs identified in Malumbres’s report22 were also noted in this study. Similarly, a sizeable fraction of sharing miRNAs that were specifically expressed in our study are shown in Supplementary Table S1 of Zhang et al.,23 suggesting highly specialized regulatory functions of FDC in B-cell development. The common miRNAs found in our study and in the other two studies are miR-103, miR-106b, miR-16, miR-17, miR-181 family, miR-18a, miR-19 family, miR-20 family, miR-30 family, and miR-93 (all upregulated) and let-7 family, miR-320, miR-150 and miR-26 (all downregulated). This was further validated by a recent in situ study on intact normal lymphnode.24 miRNA in situ hybridization25 in tonsil tissue sections confirmed that miR-17-5p, -106a and -181b were elevated and that miR-150 was reduced in GC B cells.
Taken together, these findings are in line with our findings and previous reports that lymphoma stromal cells bind to GC B cells and sustain their survival and cell-cycle progression.26 The FDC-induced miRNA changes provide insight into the underlying mechanisms for the enhanced survival provided by coculture with HK cells.27–28 Our experiments show a previously undescribed mechanism of BCL-6 and verify the mechanism of PRDM1 regulation through miRNAs targeting 3′-UTR of their genes. This substantially expands our understanding of miRNA function in B-cell survival, maturation and B lymphomagenesis. The miR-30-mediated downregulation of BCL6 activity represents a new pathway for the FDC-induced inhibition of BCL6 function. The signals and effectors inducing miRNA changes are likely from cell–cell contact. Identification of the FDC factors that determine the pathways controlling miRNA expression will be critical to understanding the mechanisms that regulate GC formation and lymphomagenesis. When the regulation of B-cell maturation and the intricate machinery are disrupted, B-cell lymphomas can occur. Dysregulation of miRNAs may interfere with normal B-cell terminal differentiation, thus revealing a novel molecular lesion and a potential therapeutic target in B-cell lymphomas.
Supplementary Material
Acknowledgements
We thank Rasa Hamilton for editorial assistance. Grant support was provided by the National Cancer Institutes was provided by the (R01CA137123, to JT), Leukemia Research Foundation (to JT) and the Maher Fund (to JT).
Footnotes
Conflict of interest
The authors declare no conflict of interest.
Supplementary Information accompanies the paper on the Leukemia website (http://www.nature.com/leu)
References
- 1.Klein U, Dalla-Favera R. Germinal centres: role in B-cell physiology and malignancy. Nat Rev Immunol. 2008;8:22–33. doi: 10.1038/nri2217. [DOI] [PubMed] [Google Scholar]
- 2.Shaffer AL, Rosenwald A, Staudt LM. Lymphoid malignancies: the dark side of B-cell differentiation. Nat Rev Immunol. 2002;2:920–932. doi: 10.1038/nri953. [DOI] [PubMed] [Google Scholar]
- 3.Park CS, Choi YS. How do follicular dendritic cells interact intimately with B cells in the germinal centre? Immunology. 2005;114:2–10. doi: 10.1111/j.1365-2567.2004.02075.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Visone R, Croce CM. MiRNAs and cancer. Am J Pathol. 2009;174:1131–1138. doi: 10.2353/ajpath.2009.080794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Croce CM. MicroRNAs and lymphomas. Ann Oncol. 2008;19 Suppl 4:iv39–iv40. doi: 10.1093/annonc/mdn192. [DOI] [PubMed] [Google Scholar]
- 6.Fabbri M, Croce CM, Calin GA. MicroRNAs in the ontogeny of leukemias and lymphomas. Leuk Lymphoma. 2009;50:160–170. doi: 10.1080/10428190802535114. [DOI] [PubMed] [Google Scholar]
- 7.Thai TH, Calado DP, Casola S, Ansel KM, Xiao C, Xue Y, et al. Regulation of the germinal center response by microRNA-155. Science. 2007;316:604–608. doi: 10.1126/science.1141229. [DOI] [PubMed] [Google Scholar]
- 8.Teng G, Hakimpour P, Landgraf P, Rice A, Tuschl T, Casellas R, et al. MicroRNA-155 is a negative regulator of activation-induced cytidine deaminase. Immunity. 2008;28:621–629. doi: 10.1016/j.immuni.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Costinean S, Sandhu SK, Pedersen IM, Tili E, Trotta R, Perrotti D, et al. Src homology 2 domain-containing inositol-5-phosphatase and CCAAT enhancer-binding protein beta are targeted by miR-155 in B cells of Emicro-MiR-155 transgenic mice. Blood. 2009;114:1374–1382. doi: 10.1182/blood-2009-05-220814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.MacLennan IC. Germinal centers. Annu Rev Immunol. 1994;12:117–139. doi: 10.1146/annurev.iy.12.040194.001001. [DOI] [PubMed] [Google Scholar]
- 11.Kim HS, Zhang X, Choi YS. Activation and proliferation of follicular dendritic cell-like cells by activated T lymphocytes. J Immunol. 1994;153:2951–2961. [PubMed] [Google Scholar]
- 12.Choe J, Kim HS, Armitage RJ, Choi YS. The functional role of B cell antigen receptor stimulation and IL-4 in the generation of human memory B cells from germinal center B cells. J Immunol. 1997;159:3757–3766. [PubMed] [Google Scholar]
- 13.Choe J, Choi YS. IL-10 interrupts memory B cell expansion in the germinal center by inducing differentiation into plasma cells. Eur J Immunol. 1998;28:508–515. doi: 10.1002/(SICI)1521-4141(199802)28:02<508::AID-IMMU508>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 14.Nie K, Gomez M, Landgraf P, Garcia JF, Liu Y, Tan LH, et al. MicroRNA-mediated down-regulation of PRDM1/Blimp-1 in Hodgkin/Reed-Sternberg cells: a potential pathogenetic lesion in Hodgkin lymphomas. Am J Pathol. 2008;173:242–252. doi: 10.2353/ajpath.2008.080009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cattoretti G, Chang CC, Cechova K, Zhang J, Ye BH, Falini B, et al. BCL-6 protein is expressed in germinal-center B cells. Blood. 1995;86:45–53. [PubMed] [Google Scholar]
- 16.Parekh S, Polo JM, Shaknovich R, Juszczynski P, Lev P, Ranuncolo SM, et al. BCL6 programs lymphoma cells for survival and differentiation through distinct biochemical mechanisms. Blood. 2007;110:2067–2074. doi: 10.1182/blood-2007-01-069575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ye BH, Cattoretti G, Shen Q, Zhang J, Hawe N, de Waard R, et al. The BCL-6 proto-oncogene controls germinal-centre formation and Th2-type inflammation. Nat Genet. 1997;16:161–170. doi: 10.1038/ng0697-161. [DOI] [PubMed] [Google Scholar]
- 18.Seyfert VL, Allman D, He Y, Staudt LM. Transcriptional repression by the proto-oncogene BCL-6. Oncogene. 1996;12:2331–2342. [PubMed] [Google Scholar]
- 19.Shaffer AL, Lin KI, Kuo TC, Yu X, Hurt EM, Rosenwald A, et al. Blimp-1 orchestrates plasma cell differentiation by extinguishing the mature B cell gene expression program. Immunity. 2002;17:51–62. doi: 10.1016/s1074-7613(02)00335-7. [DOI] [PubMed] [Google Scholar]
- 20.Turner A, Jr, Mack DH, Davis MM. Blimp-1, a novel zinc finger-containing protein that can drive the maturation of B lymphocytes into immunoglobulin-secreting cells. Cell. 1994;77:297–306. doi: 10.1016/0092-8674(94)90321-2. [DOI] [PubMed] [Google Scholar]
- 21.Kuo TC, Shaffer AL, Haddad J, Jr, Choi YS, Staudt LM, Calame K. Repression of BCL-6 is required for the formation of human memory B cells in vitro. J Exp Med. 2007;204:819–830. doi: 10.1084/jem.20062104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Malumbres R, Sarosiek KA, Cubedo E, Ruiz JW, Jiang X, Gascoyne RD, et al. Differentiation stage-specific expression of microRNAs in B lymphocytes and diffuse large B-cell lymphomas. Blood. 2009;13:3754–3764. doi: 10.1182/blood-2008-10-184077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang J, Jima DD, Jacobs C, Fischer R, Gottwein E, Huang G, et al. Patterns of microRNA expression characterize stages of human B-cell differentiation. Blood. 2009;13:4586–4594. doi: 10.1182/blood-2008-09-178186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tan LP, Wang M, Robertus JL, Schakel RN, Gibcus JH, Diepstra A, et al. miRNA profiling of B-cell subsets: specific miRNA profile for germinal center B cells with variation between centroblasts and centrocytes. Lab Invest. 2009;89:708–716. doi: 10.1038/labinvest.2009.26. [DOI] [PubMed] [Google Scholar]
- 25.Basso K, Sumazin P, Morozov P, Schneider C, Maute RL, Kitagawa Y, et al. Identification of the human mature B cell miRNome. Immunity. 2009;30:744–752. doi: 10.1016/j.immuni.2009.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lwin T, Hazlehurst LA, Dessureault S, Lai R, Bai W, Sotomayor E, et al. Cell adhesion induces p27Kip1-associated cell-cycle arrest through down-regulation of the SCFSkp2 ubiquitin ligase pathway in mantle-cell and other non-Hodgkin B-cell lymphomas. Blood. 2007;110:1631–1638. doi: 10.1182/blood-2006-11-060350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li L, Zhang X, Kovacic S, Long AJ, Bourque K, Wood CR, et al. Identification of a human follicular dendritic cell molecule that stimulates germinal center B cell growth. J Exp Med. 2000;191:1077–1084. doi: 10.1084/jem.191.6.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schwarz YX, Yang M, Qin D, Wu J, Jarvis WD, Grant S, et al. Follicular dendritic cells protect malignant B cells from apoptosis induced by anti-Fas and antineoplastic agents. J Immunol. 1999;163:6442–6447. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.