Abstract
BACKGROUND
Short stature and ovarian failure are characteristic features of Turner’s syndrome. Although recombinant human growth hormone is commonly used to treat the short stature associated with this syndrome, a randomized, placebo-controlled trial is needed to document whether such treatment increases adult height. Furthermore, it is not known whether childhood estrogen replacement combined with growth hormone therapy provides additional benefit. We examined the independent and combined effects of growth hormone and early, ultra-low-dose estrogen on adult height in girls with Turner’s syndrome.
METHODS
In this double-blind, placebo-controlled trial, we randomly assigned 149 girls, 5.0 to 12.5 years of age, to four groups: double placebo (placebo injection plus childhood oral placebo, 39 patients), estrogen alone (placebo injection plus childhood oral low-dose estrogen, 40), growth hormone alone (growth hormone injection plus childhood oral placebo, 35), and growth hormone–estrogen (growth hormone injection plus childhood oral low-dose estrogen, 35). The dose of growth hormone was 0.1 mg per kilogram of body weight three times per week. The doses of ethinyl estradiol (or placebo) were adjusted for chronologic age and pubertal status. At the first visit after the age of 12.0 years, patients in all treatment groups received escalating doses of ethinyl estradiol. Growth hormone injections were terminated when adult height was reached.
RESULTS
The mean standard-deviation scores for adult height, attained at an average age of 17.0±1.0 years, after an average study period of 7.2±2.5 years were −2.81±0.85, −3.39±0.74, −2.29±1.10, and −2.10±1.02 for the double-placebo, estrogen-alone, growth hormone–alone, and growth hormone–estrogen groups, respectively (P<0.001). The overall effect of growth hormone treatment (vs. placebo) on adult height was a 0.78±0.13 increase in the height standard-deviation score (5.0 cm) (P<0.001); adult height was greater in the growth hormone–estrogen group than in the growth hormone–alone group, by 0.32±0.17 standard-deviation score (2.1 cm) (P = 0.059), suggesting a modest synergy between childhood low-dose ethinyl estradiol and growth hormone.
CONCLUSIONS
Our study shows that growth hormone treatment increases adult height in patients with Turner’s syndrome. In addition, the data suggest that combining childhood ultra-low-dose estrogen with growth hormone may improve growth and provide other potential benefits associated with early initiation of estrogen replacement. (Funded by the National Institute of Child Health and Human Development and Eli Lilly; ClinicalTrials.gov number, NCT00001221.)
Turner’s syndrome, which results from partial or complete X-chromosome monosomy, occurs in about 1 in 2000 live female births1 and encompasses diverse clinical features, including short stature, ovarian dysgenesis, and neurocognitive problems.2 The marked short stature in Turner’s syndrome (an average, untreated adult height 20 cm below that of the general female population3,4) can be ameliorated by treatment with recombinant human growth hormone. Although there is substantial evidence that growth hormone treatment increases adult stature in patients with Turner’s syndrome,5–13 data from randomized, double-blind, placebo-controlled studies have been lacking.13
Ovarian failure, the second major problem associated with Turner’s syndrome, presents important treatment challenges because of uncertainty about the appropriate timing, route, formulation, and dosage for estrogen-replacement therapy.2,14,15 Indirect evidence, including elevated gonadotropin concentrations and delayed skeletal maturation, indicates that estrogen deficiency in Turner’s syndrome begins in infancy.16,17 Despite such evidence, as well as reports of positive behavioral and neurocognitive effects of estrogen,18–21 common clinical practice has been to postpone estrogen-replacement therapy until the mid-teens because of the widely held view that estrogen reduces adult height by accelerating epiphyseal fusion.8,22,23
On the basis of the observations of childhood estrogen deficiency in Turner’s syndrome16,17 and the biphasic nature of the estrogen-mediated growth response,24–27 we postulated that lower, more physiologic estrogen replacement during childhood might increase adult height and have other potential benefits.18,21,28 To test this hypothesis, we conducted a placebo-controlled trial to assess the effects on adult height of growth hormone treatment alone and in combination with childhood ultra-low-dose estrogen, followed by pubertal estrogen-replacement therapy.
METHODS
PATIENTS
Patients were recruited from referring physicians and pediatric endocrine clinics to the National Institutes of Health (NIH) and Thomas Jefferson University. Criteria for study entry included a karyotype diagnosis of Turner’s syndrome (without Y-chromosome material), an age of 5.0 to 12.9 years, a bone age of 12 years or less, breast development at Tanner stage 1 to 2 (with 1 representing prepubertal status and 5 representing maturity),29 and height at or below the 10th percentile of the general population standard for age,30 with measurements of height obtained within 6 months before study entry. Additional criteria were adequate thyroid hormone–replacement therapy for at least 3 months in patients with hypothyroidism, and no recent or concurrent treatment that might influence growth.
STUDY DESIGN
The study (conducted between 1987 and 2003, with enrollment closed in November 1996) was designed by the authors in accord with the directives of a Food and Drug Administration (FDA) advisory committee regarding growth hormone studies in children without growth hormone deficiency and was approved by the human subjects committees of the participating centers. Written informed consent was obtained from the patients’ parents or guardians, and yearly assent was obtained from the patients when appropriate. After FDA approval of growth hormone treatment for Turner’s syndrome (in December 1996), we again obtained assent from all active participants and consent from their parents or guardians for continued participation. Beginning in 1993, an independent data and safety monitoring board reviewed annual interim analyses; in 2002, the board recommended that the study be terminated because of the relatively advanced ages of the seven remaining participants and because estimates of the treatment effect had remained virtually unchanged for the previous 2 years.
The data were collected by three of the academic authors and three authors who were employed by Eli Lilly; one of the academic authors and two of the authors employed by Eli Lilly wrote the statistical analysis plan. All the authors contributed to data analysis and vouch for its accuracy and for adherence to the study protocol (available with the full text of this article at NEJM.org). The study was cosponsored by the National Institute of Child Health and Human Development (NICHD) and Eli Lilly. One of the academic authors and one author employed by Eli Lilly wrote the first draft of the manuscript, and all the authors contributed to subsequent revisions and made the decision to submit the manuscript for publication. The sponsors of this study did not impose any impediment, directly or indirectly, on the publication of the study results.
TREATMENTS AND PROCEDURES
All patients received an oral liquid medication (either placebo or estrogen [ethinyl estradiol]) once daily and a subcutaneously injected medication (either placebo or growth hormone) three times per week. Patients were randomly assigned to one of four treatment groups: placebo injection plus childhood oral placebo, placebo injection plus childhood oral low-dose estrogen, growth hormone injection plus childhood oral placebo, and growth hormone injection plus childhood oral low-dose estrogen (Fig. 1). The dose of growth hormone (somatropin, recombinant DNA origin [Humatrope], Eli Lilly) was 0.1 mg per kilogram three times per week (0.3 mg per kilogram per week). The ethinyl estradiol solution (1 µg per milliliter) and its placebo equivalent were prepared by the Clinical Center at the NIH in protocol-specified daily doses of 25 ng per kilogram per day for children 5.0 to 8.0 years of age and 50 ng per kilogram per day for those older than 8.0 and up to 12.0 years of age (see the Supplementary Appendix, available at NEJM.org). From the time of the first study visit after the 12th birthday, all patients were to receive pubertal estrogen-replacement therapy according to an escalating dosage regimen: 100 ng per kilogram per day for patients older than 12.0 and up to 14.0 years of age, 200 ng per kilogram per day for those older than 14.0 and up to 15.0 years of age, 400 ng per kilogram per day for those older than 15.0 and up to 16.0 years of age, and 800 ng per kilogram per day for those older than 16 years of age. For the orally administered drugs, the dose was reduced as needed on the basis of an individualization regimen (Fig. 2). Cyclic therapy with ethinyl estradiol and progestin (with the addition of medroxyprogesterone acetate for 10 days per month or by changing to an oral contraceptive containing 30 µg of ethinyl estradiol) was introduced after estradiol-induced menarche.
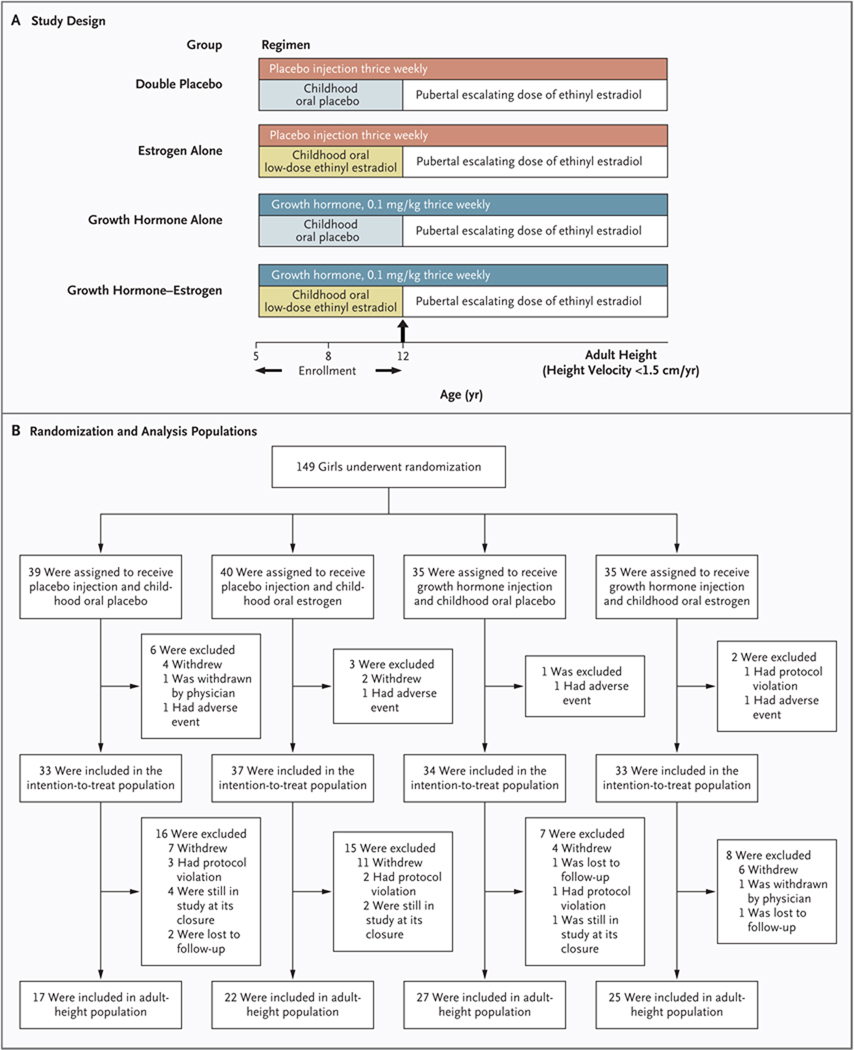
Figure 1. Study Design, Randomization, and Analysis Populations.
The factorial study design is shown in Panel A. As shown in Panel B, a total of 149 patients were randomly assigned to four treatment groups: placebo injection plus oral placebo, placebo injection plus childhood oral low-dose estrogen, growth hormone injection plus childhood oral placebo, and growth hormone injection plus childhood oral low-dose estrogen. Patients were stratified according to their baseline height into upper, middle, and lower thirds of the Turner’s syndrome height standards for age3 and were randomly assigned to treatment in a 1:1:1:1 ratio with the use of a computer-generated randomization table. The intention-to-treat population consisted of 137 girls whose height measurement was available 120 days or more after randomization; the adult-height population consisted of 91 girls whose height measurement was available after their height velocity was less than 1.5 cm per year.
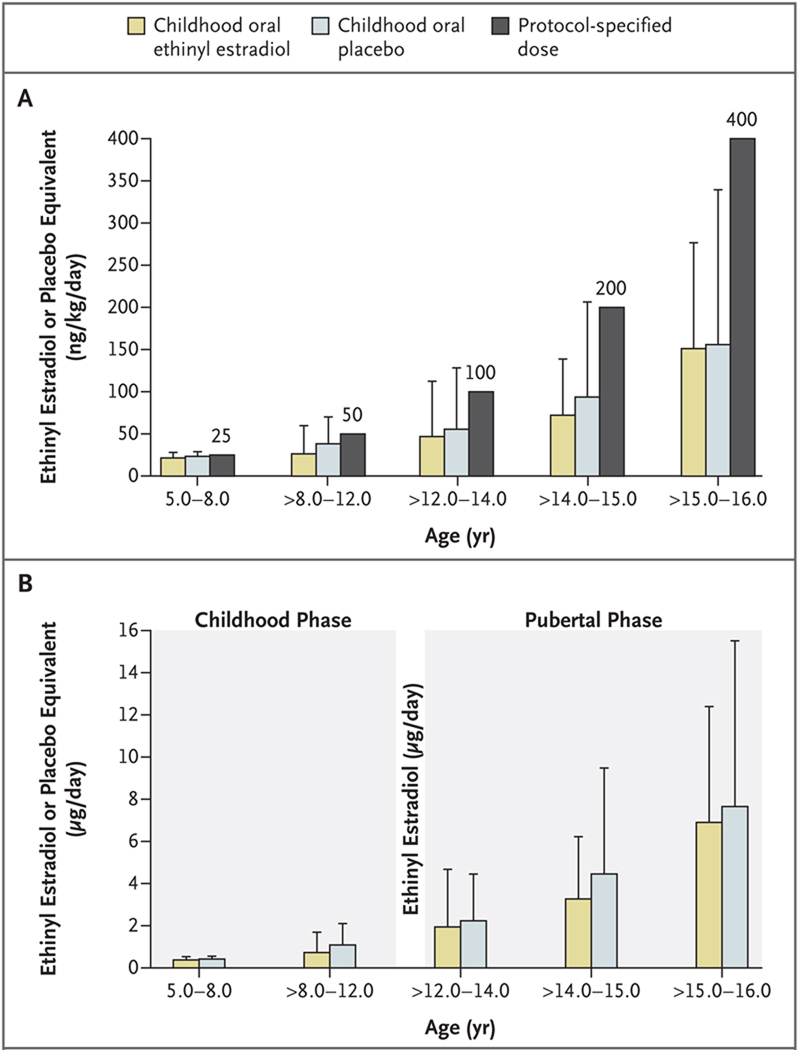
Figure 2. Oral Doses of Ethinyl Estradiol (or Oral Placebo Equivalent) during the Childhood Phase, as Compared with Protocol-Specified Doses, and Average Daily Doses in the Intention-to-Treat Population, According to Age Group.
Panel A shows the mean (±SD) dose of estrogen or placebo for each age group, as compared with the protocol-specified dose. During the childhood phase of the study (until the age of 12.0 years), patients were randomly assigned to receive either oral low-dose ethinyl estradiol or oral placebo. Starting at the first visit after 12.0 years of age, all the study groups received pubertal estrogen-replacement therapy in escalating doses. The protocol-specified doses of estradiol (or placebo during the childhood phase of the study) were 25 ng per kilogram of body weight per day from study entry until the age of 8.0 years and 50 ng per kilogram per day after the age of 8.0 years until 12.0 years of age. Pubertal-phase doses were 100 ng per kilogram per day after the age of 12.0 to 14.0 years of age, 200 ng per kilogram per day after the age of 14.0 to 15.0 years of age, 400 ng per kilogram per day after the age of 15.0 to 16.0 years of age, and 800 ng per kilogram per day after the age of 16.0 years. To individualize the oral dosage regimen, a protocol-specified dose-reduction schedule was used, whereby the dose could be reduced by 50% at the discretion of the investigator for any of the following reasons: breast development reached Tanner stage 2 or higher before the age of 12.0 years (premature breast development), vaginal bleeding occurred before the age of 14.0 years (premature vaginal bleeding), bone age advanced by 2 years within 1 year, or bone age exceeded chronologic age up to the age of 14.0 years. If the dose was reduced, it was doubled at the next protocol-specified dose increase, but thereafter, the dose remained below the protocol-specified dose according to age. Panel B shows the mean ±SD total daily dose in micrograms according to age group.
Patients were assessed at 6-month intervals until their annualized height velocity was less than 1.5 cm per year, which indicated that they had reached the protocol-specified adult height (i.e., protocol completion). An additional height measurement was obtained approximately 1 year after study completion or after height velocity was less than 1.5 cm per year for patients who withdrew from the study before protocol completion.
EFFICACY AND SAFETY OUTCOME MEASURES
The following evaluations were performed every 6 months: height (by stadiometer), weight, Tanner stage, and bone age (read centrally31). The primary outcome measure was adult height, defined as the last height measured once the height velocity was less than 1.5 cm per year. Height and midparental (target) height standard-deviation scores were based on data from the Centers for Disease Control and Prevention.32,33 Safety was evaluated at each visit by means of physical examination, laboratory testing, and assessment for adverse events (see the Supplementary Appendix).
STATISTICAL ANALYSIS
We hypothesized that the mean standard-deviation score for adult height would be significantly higher for patients treated with growth hormone than for those given placebo injections. The target sample size of 160 patients provided at least 80% power to detect a between-group height difference of 0.5 standard-deviation scores for the main-effects comparison between growth hormone and placebo injections. The efficacy analyses focused on two prospectively defined populations: the adult-height population included all patients with a height measurement available after height velocity was less than 1.5 cm per year, either at protocol completion or at post-study follow-up; the modified intention-to-treat population comprised all patients whose height had been measured 120 days or more after randomization, irrespective of treatment duration.
The primary efficacy evaluation used an analysis-of-covariance (ANCOVA) model for the adult-height population, with the standard-deviation score for adult height as the response variable, the main effects of growth hormone and ethinyl estradiol as fixed effects, and the baseline standard-deviation score for height and baseline age as covariates. The same model was used to evaluate the last available standard-deviation score for height in the intention-to-treat population. In addition, we performed a mixed-model, repeated-measures analysis as a sensitivity analysis,34 using all available measured heights at ages 10 to 18 years, to estimate the adult height of patients in the intention-to-treat population. Explanatory variables included treatment group, age group (rounded to the nearest year), baseline age, baseline standard-deviation score for height, and an interaction term for age and treatment. The effect of treatment on adult height was estimated on the basis of the difference between treatment groups for the least-squares mean for height standard-deviation score at the age of 18.0 years. To account for repeated measurements at different ages, a first-order autoregressive covariance structure was assumed.
A key secondary objective was to determine the efficacy of childhood low-dose estrogen as adjunctive therapy in improving adult height. ANCOVA models, as described above, were used to assess the effects of growth hormone (in the two growth hormone groups combined), of ethinyl estradiol (in the two childhood estradiol groups combined), and of the interaction of childhood ethinyl estradiol with growth hormone (in the group that received growth hormone plus childhood estradiol vs. the group that received growth hormone alone) on the standard-deviation score for height. Model terms included treatment (growth hormone and childhood ethinyl estradiol) and the interaction between treatments, with baseline standard-deviation score for height and baseline age as covariates.
Unless otherwise specified, data are presented as means ±SD or as least-squares means ±SE. Hypothesis testing was two-sided, with a type I error rate of 5%. Analyses were performed with the use of SAS software, version 8.0 and higher (SAS Institute). Statistical methods used for safety and laboratory analyses are described in the Supplementary Appendix.
RESULTS
STUDY PARTICIPANTS
The 149 girls (5.0 to 12.5 years of age) were randomly assigned to placebo injection plus childhood oral placebo (39 girls), placebo injection plus childhood oral low-dose estrogen (40), growth hormone injection plus childhood oral placebo (35), and growth hormone injection plus childhood oral low-dose estrogen (35). Adult-height data were available for 91 patients (61%): 84 met adult-height criteria while in the study and 7 withdrew from the study before completing the protocol but met the adult-height definition on the basis of measurements obtained after they withdrew (average age, 15.8 years; and average years in the study, 5.6); none of these 7 patients had received growth-promoting medication in the intervening period. Apart from modest differences in chronologic age and bone age, the baseline characteristics were similar among the four study groups (Table 1). Karyotype distribution overall was 45,X, 73%; 45,X/46,XXiq, 8%; 45,X/46,XX, 5%; other, 14% (P = 0.63 among groups). Information on pubertal development and skeletal maturation is summarized in the Supplementary Appendix.
Table 1.
Baseline and End-Point Characteristics of the Intention-to-Treat and Adult-Height Populations.*
| Characteristic | Intention-to-Treat Population (N = 137) |
Adult-Height Population (N = 91) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Double- Placebo Group (N = 33) |
Estrogen- Alone Group (N = 37) |
GH-Alone Group (N = 34) |
GH–Estrogen Group (N = 33) |
P Value | Double- Placebo Group (N = 17) |
Estrogen- Alone Group (N = 22) |
GH-Alone Group (N = 27) |
GH–Estrogen Group (N = 25) |
P Value | |
| Baseline | ||||||||||
| Chronologic age (yr) | 7.5±2.3 | 8.5±2.7 | 8.2±2.6 | 9.3±2.5 | 0.041 | 8.2±2.6 | 9.5±2.4 | 8.4±2.7 | 9.6±2.6 | 0.14 |
| Bone age (yr) | 6.0±2.3 | 7.0±2.8 | 7.1±2.8 | 7.9±2.4 | 0.043 | 6.7±2.5 | 8.0±2.4 | 7.4±2.8 | 8.2±2.2 | 0.25 |
| Bone-age delay (yr) | 1.5±1.1 | 1.5±1.0 | 1.1±1.2 | 1.3±1.3 | 0.55 | 1.5±1.4 | 1.5±1.1 | 0.9±1.1 | 1.4±1.1 | 0.20 |
| Height (cm) | 109.7±12.2 | 112.1±13.6 | 112.5±13.2 | 117.3±11.3 | 0.10 | 113.5±13.1 | 117.0±11.9 | 114.0±13.3 | 119.1±11.1 | 0.39 |
| Height SDS | −2.59±0.96 | −3.01±0.74 | −2.65±0.91 | −2.71±0.81 | 0.18 | −2.50±0.98 | −2.96±0.72 | −2.50±0.91 | −2.66±0.82 | 0.25 |
| Target height SDS | 0.29±0.94 | 0.08±0.82 | 0.07±0.92 | 0.18±0.93 | 0.73 | 0.23±0.91 | 0.15±0.92 | 0.23±0.92 | 0.35±0.91 | 0.89 |
| Weight (kg) | 21.5±7.8 | 23.5±10.5 | 24.5±10.6 | 26.8±10.9 | 0.19 | 23.7±9.1 | 25.0±8.9 | 26.0±11.3 | 28.2±11.9 | 0.55 |
| Weight SDS | −1.41±1.58 | −1.75±1.32 | −1.12±1.25 | −1.20±1.24 | 0.21 | −1.29±1.27 | −1.82±1.21 | −0.90±1.22 | −1.16±1.30 | 0.09 |
| Body-mass index† | 17.4±4.0 | 17.8±4.2 | 18.6±4.3 | 18.8±4.7 | 0.49 | 17.6±3.0 | 18.3±4.3 | 19.1±4.6 | 19.3±5.3 | 0.60 |
| End point | ||||||||||
| Chronologic age (yr) | 15.2±2.9 | 14.9±3.7 | 16.2±2.4 | 16.5±2.4 | 0.07 | 16.8±1.0 | 17.0±1.3 | 16.8±0.9 | 17.3±1.0 | 0.33 |
| Bone age (yr) | 13.8±3.4 | 13.7±3.6 | 14.9±2.8 | 15.1±2.6 | 0.15 | 15.9±1.0 | 15.7±1.3 | 15.6±1.3 | 15.9±1.0 | 0.72 |
| Treatment duration (yr) | 7.3±2.8 | 5.9±2.9 | 6.5±3.4 | 6.4±2.5 | 0.28 | 8.2±2.8 | 6.8±1.9 | 7.4±2.8 | 6.8±2.1 | 0.23 |
| Height (cm) | 138.1±12.3 | 134.4±14.0 | 144.8±11.2 | 146.8±11.3 | <0.001 | 144.6±5.5 | 140.8±5.0 | 147.9±7.2 | 149.3±6.6 | <0.001 |
| Height SDS | −3.08±0.95 | −3.40±0.74 | −2.45±1.13 | −2.18±1.00 | <0.001 | −2.81±0.85 | −3.39±0.74 | −2.29±1.10 | −2.10±1.02 | <0.001 |
| Height SDS at 18 yr of age‡ | −2.94±0.12 | −2.97±0.10 | −2.37±0.11 | −2.00±0.10 | <0.001 | NA | NA | NA | NA | NA |
| Change in height SDS from baseline§ | −0.41±0.10 | −0.41±0.10 | 0.22±0.10 | 0.49±0.10 | <0.001 | −0.23±0.15 | −0.42±0.13 | 0.26±0.12 | 0.58±0.12 | <0.001 |
| Weight (kg)¶ | 45.8±14.3 | 43.3±14.1 | 51.1±18.6 | 53.6±18.8 | 0.040 | 51.8±11.9 | 48.3±8.6 | 57.1±15.3 | 55.4±16.3 | 0.13 |
| Weight SDS¶ | −0.92±1.66 | −1.14±1.47 | −0.38±1.44 | −0.36±1.40 | 0.07 | −0.71±1.51 | −1.06±1.52 | −0.01±1.29 | −0.32±1.27 | 0.058 |
| Body-mass index¶ | 23.5±5.1 | 23.4±5.2 | 24.7±6.6 | 24.8±7.2 | 0.69 | 24.8±5.0 | 24.5±3.8 | 26.3±6.3 | 24.8±6.9 | 0.66 |
Plus–minus values are means ±SD, except as otherwise noted. P values for comparisons of mean values among the four study groups are based on analysis of variance. GH denotes growth hormone, NA not applicable, and SDS standard-deviation score.
The body-mass index is the weight in kilograms divided by the square of the height in meters.
Data are least-squares means (±SE), based on repeated-measures analysis.
Data are least-squares means (±SE), based on analysis of covariance, with baseline age and baseline height SDS as covariates.
Weight measurements were not obtained at the post-study follow-up visit, so data shown for weight and body-mass index represent the last available measurements during the study; on average, these measurements were obtained approximately 0.8 years earlier than the last height measurement. Mid-parental height (target height) was calculated as (father’s height − 13 cm + mother’s height) ÷ 2; the resulting value was converted to SDS with the use of normative data for 20-year-old women.32
TREATMENTS
Because the regimen for the oral drug dose was individualized, one or more reductions in the protocol-specified doses of ethinyl estradiol or placebo were made during the course of the study (the childhood and pubertal phases combined) for 95 of the 149 girls (64%) (43 of the 74 girls who received the childhood oral placebo [58%] and 52 of the 75 girls who received childhood oral ethinyl estradiol [69%], P = 0.18). Information about treatment compliance and reasons for the reductions in estradiol and placebo doses are provided in the Supplementary Appendix. Figure 2 shows the mean prescribed estradiol dosages as compared with the mean protocol-specified dosages.
EFFECT OF GROWTH HORMONE TREATMENT ON THE STANDARD-DEVIATION SCORE FOR HEIGHT
Adult-Height Population
The primary efficacy ANCOVA showed that the patients treated with growth hormone had greater adult height than did those who received placebo injections (least-squares mean [±SE] difference in standard-deviation score, 0.78±0.13, approximately 5.0 cm; P<0.001). This difference resulted from the overall decline in height standard-deviation score of 0.39 for the placebo-injection groups and the gain of 0.39 for the growth hormone–treated groups (Fig. 3A). The mean standard-deviation scores for adult height attained at 17.0±1.0 years after a mean of 7.2±2.5 years in the study (range, 1.0 to 12.1) were −2.81±0.85 (144.6±5.5 cm) for the double-placebo group, −3.39±0.74 (140.8±5.0 cm) for the estrogen-alone group, −2.29±1.10 (147.9±7.2 cm) for the growth hormone–alone group, and −2.10±1.02 (149.3±6.6 cm) for the growth hormone–estrogen group (P<0.001 among the four groups); least-squares mean (±SE) changes in standard-deviation scores from baseline, as estimated by means of ANCOVA, ranged from −0.42±0.13 to 0.58±0.12 for the estrogen-alone group and the growth hormone–estrogen group, respectively (P<0.001) (Table 1). The treatment effect accrued gradually, as shown by the progressive increases in the standard-deviation score for height in the growth hormone–treated groups versus the progressive declines in the corresponding placebo groups (Fig. 3B).
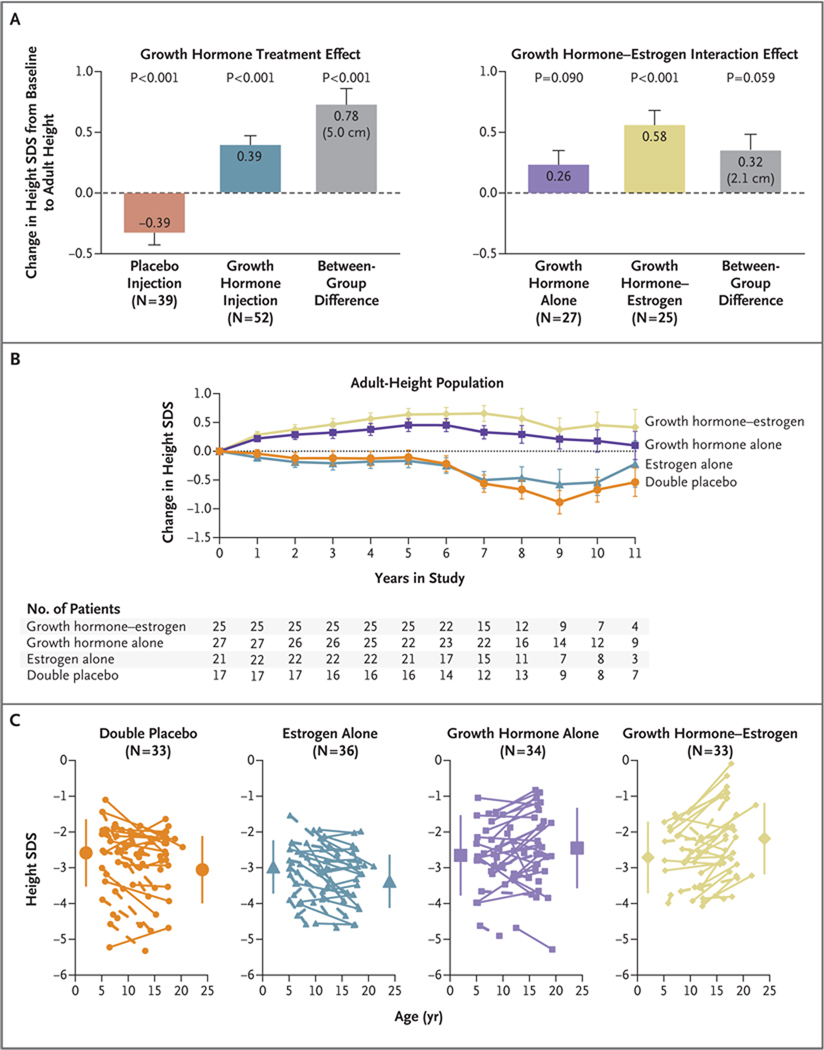
Figure 3. Effects of Treatment on Adult Height.
In Panel A, the graph on the left shows the growth hormone treatment effect on adult height. The observed treatment effect (least-squares mean [±SE] difference in the change in standard-deviation score [SDS] from baseline to adult height, 0.78±0.13 [equivalent to 5.0 cm], on the basis of analysis of covariance [ANCOVA]) results from the combined effects of the gain in the height SDS observed in the groups treated with growth hormone and prevention of the height SDS loss observed in the placebo injection groups. The graph on the right shows the effects of the interaction of growth hormone and childhood estrogen therapy. The mean between-group difference (growth hormone plus estrogen vs. growth hormone plus placebo) of 0.32±0.17 in the SDS change (equivalent to 2.1 cm) represents the incremental effect of childhood low-dose estrogen in combination with growth hormone. The graph in Panel B shows the change in the height SDS from baseline for the adult-height population according to number of years in the study. Annual data points represent the ANCOVA least-squares mean (±SE) values, with baseline age and baseline height SDS as covariates. Changes above the horizontal line represent gains in the SDS for height; changes below the horizontal line represent decreases in the SDS for height. In the adult-height population, the groups treated with growth hormone had consistent increases in height for the first 5 years of the study, whereas the groups that received placebo injections had progressive declines in height SDS. Panel C shows the changes in the SDS for height from baseline to the last available height measurement for individual patients in the intention-to-treat population. Solid lines represent patients with adult-height measurements, and dashed lines patients without adult-height measurements. One patient in the estrogen-alone group who received surreptitious growth hormone during the study is not included. Large symbols represent the group means (±SD) at baseline and at the time of the last height measurement. Mean baseline SDS and end-point SDS in the four groups were as follows: double-placebo group, −2.59±0.96 and −3.08±0.95; estrogen-alone group, −3.01±0.74 and −3.40±0.74; growth hormone–alone group, −2.65±0.91 and −2.45±1.13; and growth hormone–estrogen group, −2.71±0.81 and −2.18±1.00 (P<0.001 for the comparison among the four groups). Individual gains in the height SDS for patients with adult-height measurements (i.e., change in height SDS from baseline to adult height >0) were observed for 15% of patients in the double-placebo group, 32% in the estrogen-alone group, 65% in the growth hormone–alone group, and 79% in the growth hormone– estrogen group (P<0.001 for the comparison among the four groups).
Intention-to-Treat Population
Two prospectively planned analyses were performed to assess the treatment effects in all patients for whom a height measurement was available 120 days or more after randomization (137 patients in the intention-to-treat population) (Table 1). Although adult measurements were unavailable for 58 of the 149 randomly assigned patients (39%), many nevertheless remained in the study until they were close to adult height, so the results of these analyses were similar to those for the adult-height population. At the time of the last available height measurement, the patients in the combined growth hormone groups were taller than those in the combined placebo injection groups by 0.77±0.10 standard-deviation score, or 5.0 cm (P<0.001 by ANCOVA). Furthermore, repeated-measures analysis revealed a similar effect of growth hormone treatment, both on an annual basis and overall (an increase of 0.78±0.10 in the standard-deviation score [5.0 cm], P<0.001). Gains in height (i.e., individual patients’ increases in the standard-deviation score from the baseline measurement to the last available measurement) were observed for 15% of the double-placebo group, 32% of the estrogen-alone group, 65% of the growth hormone–alone group, and 79% of the growth hormone–estrogen group (P<0.001 for differences among groups) (Fig. 3C). Adult height was within the normal range (greater than −2 SD) for 27 of the 67 growth hormone–treated patients (40%) versus 3 of the 70 placebo-treated patients (4%) (P<0.001, by Fisher’s exact test).
COMBINED EFFECTS OF GROWTH HORMONE AND CHILDHOOD ETHINYL ESTRADIOL
Adult-Height Population
The efficacy of childhood low-dose estrogen as adjunctive therapy for increasing adult height was examined with the use of ANCOVA models to compare height gains in patients treated with growth hormone alone with those in patients who received low-dose estrogen during childhood in addition to growth hormone. The height gain was greater for the growth hormone–estrogen group than for the growth hormone–alone group by 0.32±0.17 standard-deviation score (P = 0.059; P = 0.051 for growth hormone–estrogen interaction) (Fig. 3A) and by 2.3±1.1 cm (P = 0.04; P = 0.04 for growth hormone–estrogen interaction). This difference resulted from the consistently greater height gain from baseline in the group that received growth hormone and estrogen during childhood than in the group that received growth hormone alone (Fig. 3B).
Intention-to-Treat Population
The growth hormone–estrogen interaction in the intention-to-treat population was similar to that in the adult-height population — that is, the last available height measurement in the growth hormone–estrogen group was 0.26±0.15 standard-deviation score greater (approximately 1.7 cm) than that in the group that received growth hormone alone during childhood (P = 0.07 for the least-squares mean difference). By repeated-measures analysis, the patients who received growth hormone plus childhood estrogen were taller than those who received growth hormone and placebo, by 0.36±0.15 standard-deviation score, or approximately 2.3 cm (P = 0.01 for the least-squares mean difference). Thus, the sensitivity analyses support a modest synergy between growth hormone and estrogen treatments.
ADVERSE EVENTS
No deaths occurred during the study. Serious adverse events were reported for 27 of the 149 patients (Table 3 in the Supplementary Appendix). Adverse events occurring during treatment were reported in all the patients (Table 2, and Table 4 in the Supplementary Appendix). Gynecologic disorders, pain, otitis media, scoliosis, and thyroid disorders were commonly reported adverse events. A slipped capital femoral epiphysis occurred in one patient, and the fasting blood glucose level was reported to be elevated in one patient, both of whom were in the growth hormone–estrogen group. Insulin-like growth factor 1 (IGF-1) and other laboratory data are provided in the Supplementary Appendix. Overall, there were no new or unexpected safety findings with respect to growth hormone or estrogen treatment in this study.
Table 2.
Relevant Adverse Events That Occurred during Treatment among All Randomly Assigned Patients.*
| Event | Double- Placebo Group (N = 39) |
Estrogen- Alone Group (N = 40) |
GH-Alone Group (N = 35) |
GH– Estrogen Group (N = 35) |
Total (N = 149) |
P Value† | |
|---|---|---|---|---|---|---|---|
| GH vs. Placebo Injection |
Estrogen vs. Oral Placebo |
||||||
| percent of patients | |||||||
| Any event | 100 | 100 | 100 | 100 | 100 | 1.00 | 1.00 |
| Gynecologic disorders‡ | 43.6 | 60.0 | 54.3 | 60.0 | 54.4 | 0.62 | 0.20 |
| Pain (no precipitant reported) | 33.3 | 52.5 | 37.1 | 54.3 | 44.3 | 0.87 | 0.03 |
| Otitis media | 43.6 | 45.0 | 42.9 | 42.9 | 43.6 | 0.87 | 1.00 |
| Scoliosis | 30.8 | 40.0 | 48.6 | 40.0 | 39.6 | 0.27 | 1.00 |
| Thyroid disorders | 35.9 | 35.0 | 37.1 | 34.3 | 35.6 | 1.00 | 0.87 |
| Edema | 33.2 | 22.5 | 20.0 | 28.6 | 26.2 | 0.71 | 0.85 |
| Lipid abnormalities | 33.3 | 25.0 | 14.3 | 11.4 | 21.5 | 0.02 | 0.43 |
| Nevi | 25.6 | 17.5 | 14.3 | 28.6 | 21.5 | 1.00 | 0.84 |
| Cardiac disorders | 25.6 | 25.0 | 20.0 | 11.4 | 20.8 | 0.16 | 0.55 |
| Lymphoid enlargement | 15.4 | 12.5 | 17.1 | 5.7 | 12.8 | 0.81 | 0.23 |
| Hearing disturbance | 15.4 | 17.5 | 8.6 | 5.7 | 12.1 | 0.13 | 1.00 |
| Bone disorders | 15.4 | 10.0 | 2.9 | 5.7 | 8.7 | 0.09 | 0.78 |
| Neoplasia§ | 5.1 | 12.5 | 8.6 | 2.9 | 7.4 | 0.54 | 1.00 |
| Elevated blood glucose | 0.0 | 0.0 | 0.0 | 2.9 | 0.7 | 0.47 | 1.00 |
| Slipped growth plate | 0.0 | 0.0 | 0.0 | 2.9 | 0.7 | 0.47 | 1.00 |
Events are listed in descending order of frequency for the total patient group. Serious adverse events were defined according to regulatory criteria as any event that resulted in death, was life-threatening, required hospitalization or prolonged the hospital stay, resulted in permanent disability, resulted in a congenital anomaly in an offspring of a study patient, or was otherwise serious in the judgment of the investigator. Serious adverse events were reported for nine patients in the double-placebo group, seven patients in the estrogen-alone group, four patients in the growth hormone (GH)–alone group, and seven patients in the GH–estrogen group (for details, see Table 3 in the Supplementary Appendix). The majority of these events were hospitalizations for procedures or illnesses that are common in patients with Turner’s syndrome. Only one serious adverse event, dysfunctional uterine bleeding, was considered likely to have been related to exposure to the study drug, and it occurred in a patient in the estrogen-alone group.
Significance testing was performed with the use of Fisher’s exact test for the two GH groups combined as compared with the two placebo-injection groups combined and the two estrogen groups combined as compared with the two oral-placebo groups combined. All P values were nonsignificant when adjusted for false discovery rate (multiple-hypothesis testing).
This category includes menstrual problems (e.g., irregular, intermittent, or heavy bleeding); vaginal discharge; and itching, discomfort, or redness in the genital area.
This category includes lipoma, mucocele, pilonidal swelling, “skin benign neoplasm,” “tumor recurrence” (for details, see the Supplementary Appendix), and unspecified growths, lumps, or nodules.
DISCUSSION
This randomized trial provides objective evidence concerning the efficacy and safety of growth hormone treatment initiated in mid-childhood for short stature associated with Turner’s syndrome. As compared with placebo, growth hormone treatment given at a dose of 0.1 mg per kilogram three times per week increased adult height by approximately 5.0 cm over an average period of 7.2 years. Data on adult height were not available for 39% of the study participants, an observation that reflects the long and complex nature of the study. However, a prospectively planned repeated-measures analysis of the intention-to-treat population showed treatment effects that were highly consistent with those in the adult-height analyses, indicating the robustness of the efficacy data.
There are probably multiple reasons for the somewhat smaller gain in height in our study than in some previous studies.5–13,35,36 Most previous studies have used either historical controls or the patient’s own baseline predicted or projected height5,8,10,11,13 to assess the treatment effect, possibly leading to systematic errors in the efficacy estimates.35 In addition, the growth hormone regimen in our study, which was designed in the mid-1980s, may be considered suboptimal by current standards. We used a dose of 0.3 mg per kilogram per week, which is 20% lower than the currently approved dose for children with Turner’s syndrome (0.375 mg per kilogram per week), and the thrice-weekly injection schedule in our study is less effective than daily administration.36 This difference in injection frequency may explain the greater mean height gain (7.2 cm during an average of 5.7 years) in a randomized, open-label study (the only prior study to include an untreated control group whose members were followed until they reached adult height) in which the total weekly dose of growth hormone given in divided doses 6 times per week was the same as in our study.12
Our results showed a trend toward a synergistic growth benefit from childhood low-dose estrogen combined with growth hormone, as indicated by the greater height gain in the growth hormone–estrogen group than in the group given growth hormone alone and by the lack of height gain in the group that received childhood low-dose estrogen without growth hormone. Because differences in the pubertal induction regimen initiated after the age of 12.0 years were minor (owing to protocol-defined individualization of doses), the between-group difference (growth hormone–estrogen vs. growth hormone alone) appears to have resulted from the childhood treatment regimen administered between 5.0 and 12.0 years of age — a finding that is consistent with our original hypothesis that treatment with a sufficiently low dose of estrogen would optimize growth in girls with Turner’s syndrome.24 The modest synergy observed between growth hormone and ultra-low doses of estrogen in childhood is not explained by differential effects of these two agents on systemic IGF-1, because the mean IGF-1 concentrations were similar throughout the study in patients treated with growth hormone plus childhood estrogen and those treated with growth hormone alone. Rather, the synergy might be due to a local increase in responsiveness to IGF-1 or growth hormone mediated by ultra-low estradiol concentration acting directly at the skeletal growth plate.37
Although estrogen replacement during mid-childhood (prepuberty) may seem counterintuitive, this approach has a physiological rationale: the normal mid-childhood ovary is not entirely quiescent — plasma estradiol concentrations in healthy prepubertal girls, albeit low, are up to eight times as high as those in prepubertal boys.38,39 Furthermore, low-dose estrogen administration in childhood has beneficial effects on cognition and self-image in patients with Turner’s syndrome, as reported previously in a subgroup of patients from this study.20,21 Nonverbal processing speed, motor performance, and verbal and nonverbal memory were significantly better in estrogen-treated girls 5.0 to 12.0 years of age than in placebo recipients of the same age.20,21
In addition to the timing, the dosage, type, and route of estrogen administration appear to influence its tissue-specific effects.40,41 We previously reported differential effects of varying doses of estrogen with respect to linear growth, vaginal maturation, and IGF-1 production.24 In the present study, we used an ultra-low-dose estrogen regimen derived from the initial study,24 aiming to approximate the estrogen milieu in healthy prepubertal girls. However, because our study was designed more than 20 years ago, the estrogen regimen has some limitations as a guide to current therapy. The protocol-specified dosages were excessive for most girls, with dose reductions required to minimize premature pubertal development and undue skeletal maturation. Dosage individualization based on protocol-defined criteria was a unique aspect of the present study; finding the lowest beneficial estrogen dose for each patient is an important goal, not only to minimize premature feminization but also to avoid potential late deleterious effects. Finally, some girls with Turner’s syndrome do not require estrogen supplementation, at least initially, because spontaneous pubertal development may occur, as evidenced by breast development observed in 13% of girls who received oral placebo during the childhood phase of this study (see the Supplementary Appendix).
In conclusion, this placebo-controlled trial shows that growth hormone treatment initiated at an average age of 9 years increases adult height in girls with Turner’s syndrome. Furthermore, the modest growth benefit observed with the combination of ultra-low-dose childhood estrogen replacement and growth hormone suggests that the practice of delaying estrogen therapy should be reconsidered. A regimen combining carefully individualized childhood estrogen replacement with growth hormone in girls with Turner’s syndrome has the potential not only to optimize adult height but also to provide the neurocognitive and behavioral benefits of early estrogen administration.
Supplementary Material
Acknowledgments
Supported by the National Institute of Child Health and Human Development and Eli Lilly.
We thank the patients and their families for their participation in this study, Mike Massa and Xiaohai Wan for technical assistance, and Jeff Baron and Werner Blum for helpful discussions.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
REFERENCES
- 1.Nielsen J, Wohlert M. Chromosome abnormalities found among 34,910 newborn children: results from a 13-year incidence study in Arhus, Denmark. Hum Genet. 1991;87:81–83. doi: 10.1007/BF01213097. [DOI] [PubMed] [Google Scholar]
- 2.Bondy CA. Care of girls and women with Turner syndrome: a guideline of the Turner Syndrome Study Group. J Clin Endocrinol Metab. 2007;92:10–25. doi: 10.1210/jc.2006-1374. [DOI] [PubMed] [Google Scholar]
- 3.Lyon AJ, Preece MA, Grant DB. Growth curve for girls with Turner syndrome. Arch Dis Child. 1985;60:932–935. doi: 10.1136/adc.60.10.932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davenport ML, Punyasavatsut N, Stewart PW, Gunther DF, Savendahl L, Sybert VP. Growth failure in early life: an important manifestation of Turner syndrome. Horm Res. 2002;57:157–164. doi: 10.1159/000058376. [DOI] [PubMed] [Google Scholar]
- 5.Rosenfeld RG, Attie KM, Frane J, et al. Growth hormone therapy of Turner’s syndrome: beneficial effect on adult height. J Pediatr. 1998;132:319–324. doi: 10.1016/s0022-3476(98)70452-4. [DOI] [PubMed] [Google Scholar]
- 6.Vanderschueren-Lodeweyckx M, Massa G, Maes M, et al. Growth promoting effect of growth hormone and low dose ethinyl estradiol in girls with Turner’s syndrome. J Clin Endocrinol Metab. 1990;70:122–126. doi: 10.1210/jcem-70-1-122. [DOI] [PubMed] [Google Scholar]
- 7.Carel JC, Mathivon L, Gendrel C, Ducret JP, Chaussain JL. Near normalization of final height with adapted doses of growth hormone in Turner’s syndrome. J Clin Endocrinol Metab. 1998;83:1462–1466. doi: 10.1210/jcem.83.5.4777. [DOI] [PubMed] [Google Scholar]
- 8.Chernausek SD, Attie KM, Cara JF, Rosenfeld RG, Frane J. Growth hormone therapy of Turner syndrome: the impact of age of estrogen replacement on final height. J Clin Endocrinol Metab. 2000;85:2439–2445. doi: 10.1210/jcem.85.7.6684. [DOI] [PubMed] [Google Scholar]
- 9.Quigley CA, Crowe BJ, Anglin DG, Chipman JJ. Growth hormone and low dose estrogen in Turner syndrome: results of a United States multi-center trial to near-final height. J Clin Endocrinol Metab. 2002;87:2033–2041. doi: 10.1210/jcem.87.5.8477. [DOI] [PubMed] [Google Scholar]
- 10.Ranke MB, Partsch CJ, Lindberg A, et al. Adult height after GH therapy in 188 Ullrich-Turner syndrome patients: results of the German IGLU Follow-up Study 2001. Eur J Endocrinol. 2002;147:625–633. doi: 10.1530/eje.0.1470625. [DOI] [PubMed] [Google Scholar]
- 11.van Pareren YK, de Muinck Keizer-Schrama SM, Stijnen T, et al. Final height in girls with Turner syndrome after long-term growth hormone treatment in three dosages and low dose estrogens. J Clin Endocrinol Metab. 2003;88:1119–1125. doi: 10.1210/jc.2002-021171. [DOI] [PubMed] [Google Scholar]
- 12.Stephure DK. Impact of growth hormone supplementation on adult height in Turner syndrome: results of the Canadian randomized controlled trial. J Clin Endocrinol Metab. 2005;90:3360–3366. doi: 10.1210/jc.2004-2187. [DOI] [PubMed] [Google Scholar]
- 13.Baxter L, Bryant J, Cave CB, Milne R. Recombinant growth hormone for children and adolescents with Turner syndrome. Cochrane Database Syst Rev. 2007;1 doi: 10.1002/14651858.CD003887.pub2. CD003887. [DOI] [PubMed] [Google Scholar]
- 14.Carel JC, Elie C, Ecosse E, et al. Self-esteem and social adjustment in young women with Turner syndrome — influence of pubertal management and sexuality: population-based cohort study. J Clin Endocrinol Metab. 2006;91:2972–2979. doi: 10.1210/jc.2005-2652. [DOI] [PubMed] [Google Scholar]
- 15.Donaldson MD, Gault EJ, Tan KW, Dunger DB. Optimising management in Turner syndrome: from infancy to adult transfer. Arch Dis Child. 2006;91:513–520. doi: 10.1136/adc.2003.035907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Conte FA, Grumbach MM, Kaplan SL. A diphasic pattern of gonadotropin secretion in patients with the syndrome of gonadal dysgenesis. J Clin Endocrinol Metab. 1975;40:670–674. doi: 10.1210/jcem-40-4-670. [DOI] [PubMed] [Google Scholar]
- 17.Cutler GB., Jr The role of estrogen in bone growth and maturation during childhood and adolescence. J Steroid Biochem Mol Biol. 1997;61:141–144. [PubMed] [Google Scholar]
- 18.Ross JL, McCauley E, Roeltgen D, et al. Self-concept and behavior in adolescent girls with Turner syndrome: potential estrogen effects. J Clin Endocrinol Metab. 1996;81:926–931. doi: 10.1210/jcem.81.3.8772552. [Erratum, J Clin Endocrinol Metab 1996;81:2191.] [DOI] [PubMed] [Google Scholar]
- 19.Sherwin BB, Tulandi T. “Add-back” estrogen reverses cognitive deficits induced by a gonadotropin-releasing hormone agonist in women with leiomyomata uteri. J Clin Endocrinol Metab. 1996;81:2545–2549. doi: 10.1210/jcem.81.7.8675575. [DOI] [PubMed] [Google Scholar]
- 20.Ross JL, Roeltgen D, Feuillan P, Kushner H, Cutler GB., Jr Effects of estrogen on nonverbal processing speed and motor function in girls with Turner’s syndrome. J Clin Endocrinol Metab. 1998;83:3198–3204. doi: 10.1210/jcem.83.9.5087. [DOI] [PubMed] [Google Scholar]
- 21.Idem. Use of estrogen in young girls with Turner syndrome: effects on memory. Neurology. 2000;54:164–170. doi: 10.1212/wnl.54.1.164. [DOI] [PubMed] [Google Scholar]
- 22.Tanner JM, Whitehouse RH, Hughes PC, Carter BS. Relative importance of growth hormone and sex steroids for the growth at puberty of trunk length, limb length, and muscle width in growth hormone-deficient children. J Pediatr. 1976;89:1000–1008. doi: 10.1016/s0022-3476(76)80620-8. [DOI] [PubMed] [Google Scholar]
- 23.Saenger P, Wikland KA, Conway GS, et al. Recommendations for the diagnosis and management of Turner syndrome. J Clin Endocrinol Metab. 2001;86:3061–3069. doi: 10.1210/jcem.86.7.7683. [DOI] [PubMed] [Google Scholar]
- 24.Ross JL, Cassorla FG, Skerda MC, Valk IM, Loriaux DL, Cutler GB., Jr A preliminary study of the effect of estrogen dose on growth in Turner’s syndrome. A N Engl J Med. 1983;309:1104–1106. doi: 10.1056/NEJM198311033091806. [DOI] [PubMed] [Google Scholar]
- 25.Caruso-Nicoletti M, Cassorla F, Skerda M, Ross JL, Loriaux DL, Cutler GB., Jr Short term, low dose estradiol accelerates ulnar growth in boys. J Clin Endocrinol Metab. 1985;61:896–898. doi: 10.1210/jcem-61-5-896. [DOI] [PubMed] [Google Scholar]
- 26.Ross JL, Long LM, Skerda M, et al. Effect of low doses of estradiol on 6-month growth rates and predicted height in patients with Turner syndrome. J Pediatr. 1986;109:950–953. doi: 10.1016/s0022-3476(86)80274-8. [DOI] [PubMed] [Google Scholar]
- 27.Ross JL, Cassorla F, Carpenter G, et al. The effect of short term treatment with growth hormone and ethinyl estradiol on lower leg growth rate in girls with Turner’s syndrome. J Clin Endocrinol Metab. 1988;67:515–518. doi: 10.1210/jcem-67-3-515. [DOI] [PubMed] [Google Scholar]
- 28.Mauras N, Rogol AD, Veldhuis JD. Increased hGH production rate after low-dose estrogen therapy in prepubertal girls with Turner’s syndrome. Pediatr Res. 1990;28:626–630. doi: 10.1203/00006450-199012000-00018. [DOI] [PubMed] [Google Scholar]
- 29.Tanner JM. Growth at adolescence. 2nd ed. Oxford, England: Blackwell Scientific; 1962. [Google Scholar]
- 30.National Center for Health Statistics. Rockville, MD: Health Resources Administration; 1976. NCHS growth charts. 1976 http://www.cdc.gov/nchs/data/mvsr/supp/mv25_03sacc.pdf. [Google Scholar]
- 31.Greulich WW, Pyle SI. Radiographic atlas of skeletal development of the hand and wrist. 2nd ed. Stanford, CA: Stanford University Press; 1959. [Google Scholar]
- 32.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, et al. Advance data from vital and health statistics. Hyattsville, MD: National Center for Health Statistics; 2000. CDC growth charts: United States. No. 246. [Google Scholar]
- 33.Tanner JM, Whitehouse RH, Hughes PC, Vince FP. Effect of human growth hormone treatment for 1 to 7 years on growth of 100 children, with growth hormone deficiency, low birthweight, inherited smallness, Turner’s syndrome, and other complaints. Arch Dis Child. 1971;46:745–782. doi: 10.1136/adc.46.250.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Siddiqui O, Hung HMJ, O’Neill R. MMRM vs. LOCF: a comprehensive comparison based on simulation study and 25 NDA datasets. J Biopharm Stat. 2009;19:227–246. doi: 10.1080/10543400802609797. [DOI] [PubMed] [Google Scholar]
- 35.Sacks H, Chalmers TC, Smith H., Jr Randomized versus historical controls for clinical trials. Am J Med. 1982;72:233–240. doi: 10.1016/0002-9343(82)90815-4. [DOI] [PubMed] [Google Scholar]
- 36.Hopwood NJ, Hintz RL, Gertner JM, et al. Growth response of children with non-growth-hormone deficiency and marked short stature during three years of growth hormone therapy. J Pediatr. 1993;123:215–222. doi: 10.1016/s0022-3476(05)81691-9. [DOI] [PubMed] [Google Scholar]
- 37.Nilsson O, Marino R, De Luca F, Phillip M, Baron J. Endocrine regulation of the growth plate. Horm Res. 2005;64:157–165. doi: 10.1159/000088791. [DOI] [PubMed] [Google Scholar]
- 38.Klein KO, Baron J, Colli MJ, McDonnell DP, Cutler GB., Jr Estrogen levels in childhood determined by an ultrasensitive recombinant cell bioassay. J Clin Invest. 1994;94:2475–2480. doi: 10.1172/JCI117616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Courant F, Aksglaede L, Antignac J-P, et al. Assessment of circulating sex steroid levels in prepubertal and pubertal boys and girls by a novel ultrasensitive gas chromatography-tandem mass spectrometry method. J Clin Endocrinol Metab. 2010;95:82–92. doi: 10.1210/jc.2009-1140. [DOI] [PubMed] [Google Scholar]
- 40.Rosenfield RL, Devine N, Hunold JJ, Mauras N, Moshang T, Jr, Root AW. Salutary effects of combining early very low-dose systemic estradiol with growth hormone therapy in girls with Turner syndrome. J Clin Endocrinol Metab. 2005;90:6424–6430. doi: 10.1210/jc.2005-1081. [DOI] [PubMed] [Google Scholar]
- 41.Soriano-Guillen L, Coste J, Ecosse E, et al. Adult height and pubertal growth in Turner syndrome after treatment with recombinant growth hormone. J Clin Endocrinol Metab. 2005;90:5197-–5204. doi: 10.1210/jc.2005-0470. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.