Abstract
Carbon monoxide (CO) is produced endogenously by heme oxygenase (HO) enzymes. HO-1 is highly expressed in many inflammatory disease states, where it is broadly protective. The protective effects of HO-1 expression can be largely mimicked by the exogenous application of CO and CO-releasing molecules (CORMs). Despite a dearth of pharmacological tools for their study, molecular methodologies have identified P2X4 receptors as a potential anti-nociceptive drug target. P2X4 receptors are up-regulated in animal models of inflammatory pain, and their knock-down reduces pain behaviours. In these same animal models, HO-1 expression is anti-nociceptive, and we therefore investigated whether P2X4 was a target for CO and tricarbonyldichlororuthenium (II) dimer (CORM-2). Using conventional whole-cell and perforated-patch recordings of heterologously expressed human P2X4 receptors, we demonstrate that CORM-2, but not CO gas, is an inhibitor of these channels. We also investigated the role of soluble guanylate cyclase and mitochondria-derived reactive oxygen species using pharmacological inhibitors but found that they were largely unable to affect the ability of CORM-2 to inhibit P2X4 currents. A control breakdown product of CORM-2 was also without effect on P2X4. These results suggest that P2X4 receptors are not a molecular target of endogenous CO production and are, therefore, unlikely to be mediating the anti-nociceptive effects of HO-1 expression in inflammatory pain models. However, these results show that CORM-2 is an effective antagonist at human P2X4 receptors and represents a useful pharmacological tool for the study of these receptors given the current dearth of antagonists.
Keywords: Carbon monoxide, P2X4 receptors, ATP, CORM-2, Neuropathic pain, Inflammatory pain, Antagonist
Introduction
P2X receptors are trimeric ionotropic ion channels gated by the binding of extracellular ATP. There are seven mammalian P2X homologs (nomenclature follows [1]) of which P2X4 is the most widely expressed. First cloned from rat brain [2, 3], P2X4 subunits are expressed throughout the central nervous system, for example, in Purkinje cells [4], hippocampus [2] and dorsal horn of the spinal cord [5]. P2X4 is also widely expressed in non-neuronal tissues and can be found in the kidney, liver, pancreas [6], heart and lungs [7] oesteoclasts [8], and immune cells such as B lymphocytes [9] and macrophages [10]. In a great deal of these tissues and cells, the role of P2X4 receptors is not well understood. There are several reasons for this, which include (a) their fast recycling to the plasma membrane, the physiological significance of which is not well understood; (b) when the membrane is disrupted, as during whole-cell recordings, membrane currents appear to run down due to an unknown mechanism [11]; and (c) a lack of potent antagonists and other pharmacological tools for discriminating P2X4 receptors. Currently, ivermectin is the primary tool for positive discrimination of P2X4 receptors. Ivermectin is an allosteric modulator of P2X4 currents, potentiating the peak current and increasing the duration of currents evoked by brief ATP applications [12, 13]. There are no specific agonists, and broad-spectrum P2X antagonists are relatively insensitive at P2X4 receptors [3, 14]. Recently, antidepressant drugs such as paroxetine, fluoxetine, desipramine and amitriptyline, which are used to treat neuropathic pain, have been proposed to act as P2X4 antagonists [15], though it now appears that they are not true antagonists and have no effect on currents mediated by P2X4 receptors [16, 17]. Rather, their mechanism of action is to modulate lysosomal function and, as a consequence, indirectly to affect the trafficking of P2X4 receptors to the cell surface [17], thus reducing the P2X4 response in activated cells.
Despite the dearth of pharmacological agents able to discriminate between P2X4 and other family members, molecular techniques and animal models have shed some insight into the role of P2X4, leading to the hypothesis that P2X4 may be an attractive drug target for the treatment of neuropathic and inflammatory pain. Tsuda et al. [18] showed that P2X4 was up-regulated in spinal cord microglia following L5 nerve ligation injury, an established model of neuropathic pain. Similarly, Guo et al. [19] showed that P2X4 was up-regulated in spinal cord microglia following hind-paw formalin injection, an established model of inflammatory pain.
Carbon monoxide (CO) is endogenously produced during the hydrolysis of heme by heme oxygenase (HO) enzymes in an oxygen-dependent process (for review, see [20]). Originally considered a by-product of heme breakdown, CO is increasingly recognised as an important signalling molecule in physiological and pathophysiological processes and has been shown to be responsible for many of the protective effects observed when HO-1 is up-regulated following cellular stress [21]. Increased HO-1 expression induced by cobalt protoporphyrin [22] or epibatidine [23] intraperitoneal injection causes a reduction in nociceptive behaviours in the formalin injection inflammatory pain model. Injection of heme oxygenase inhibitors, or minimising HO-1 upregulation with a dominant negative Nrf2 transcription factor, negates the protective effect of HO-1 upregulation [22, 23]. There are many proposed cellular targets of CO including soluble guanylate cyclase (sGC) [24] and mitogen-activated protein kinases (for review, see [25]). Recent experiments have also shown CO to play important roles in cardiovascular function [26] and O2 sensing [27, 28] via an activation of large-conductance, calcium-activated potassium channel (BKCa). Other ion channels have also been shown to be modulated by CO such as L-type calcium channels (Cav1.2) [29], TREK-1 [30] and ENaC channels [31]. Recently, we observed that short applications (10 s) of CO or a CO donor molecule were able to potentiate ATP-evoked currents at rat P2X2 receptors, whilst causing a small but significant decrease in P2X4 currents [32]. Here, we extend the study of this inhibition and show that the CO donor, CORM-2, but not CO gas, is a non-competitive inhibitor of P2X4 receptors, by a mechanism which is independent of the production of cGMP and cellular reactive oxygen species (ROS). That CORM-2 inhibits P2X4 receptors at all ATP concentrations means that this compound can be used to distinguish between P2X4 and P2X2, P2X2/3 and P2X3 receptors where CORM-2 either potentiates or has no effect [32].
Methods
Cell culture
Unless otherwise stated, all reagents were obtained from Sigma-Aldrich Company Ltd, Dorset, UK. A human embryonic kidney (HEK) 293 cell line stably expressing human P2X4 receptors was used throughout these studies (kindly provided by Prof. RA North, The University of Manchester, UK). Cells were maintained in Dulbecco’s modified Eagle’s medium/Ham’s F12 (1:1) supplemented with 10% foetal calf serum, 2 mM l-glutamine, 100 U mL−1 penicillin G, 100 μg mL−1 streptomycin, 250 ng mL−1 amphotericin B and 150 μg mL−1 Geneticin (all purchased from Invitrogen, Paisley, Strathclyde, UK). Cells lacking mitochondria (ρ0 cells) were generated as previously described by us and others [29, 33–35] by growth in media containing ethidium bromide (50 ng mL−1), sodium pyruvate (1 mM) and uridine (50 μg mL−1) for 2 months prior to experimentation. Cells were incubated at 37°C in a humidified incubator gassed with 5% CO2/95% air. Cells were passaged every 3–4 days using 2.5 g L−1 trypsin dissolved in Mg2+- and Ca2+-free phosphate-buffered saline. For electrophysiological recordings, cells were plated onto glass coverslips and incubated as above for 18–36 h before recording.
Electrophysiology
Whole-cell patch-clamp recordings were made at room temperature using borosilicate glass pipettes (World Precision Instruments, Sarasota, FL, USA) which had resistances of 4–6 MΩ when filled with a solution containing the following (in mM): 117 KCl, 10 NaCl, 2 MgCl2, 1 CaCl2, 11 EGTA, 2 Na2.ATP and 11 HEPES, with the pH adjusted to 7.2 with KOH. The recording chamber was continually perfused with a saline solution containing (in mM): 135 NaCl, 5 KCl, 1.2 MgCl2, 2.5 CaCl2, 5 HEPES and 10 glucose, with the pH adjusted to 7.4 with NaOH. Working solutions of all drugs used were made up in this solution daily from frozen aliquots to the concentrations indicated in the text and figure legends.
Current recordings were made at a holding potential of −60 mV using an Axon Instruments Axopatch Multiclamp 700A amplifier and Digidata 1322A A/D interface (Molecular Devices, Sunnyvale, CA, USA). Whole-cell capacitance was measured and compensated for using the Multiclamp auto compensation feature. In all cases, agonist applications were made at 90-s intervals. Compounds were applied to patch-clamped cells by means of a rapid perfusion system (RSC-160, Biologic, Claix, France), allowing solution exchange times in the range 20–100 ms.
For perforated-patch recordings, a 40 mg mL−1 solution of nystatin was made up daily in dimethyl sulfoxide (DMSO) and kept in the dark. A working solution (400 μg mL−1) was made up hourly from this stock in a pipette solution containing (in mM): 117 KCl, 10 HEPES and 10 EGTA to pH 7.3 with KOH. This solution was kept on ice in the dark and was vortexed immediately before back-filling a patch pipette which had been tip-filled with a solution containing no nystatin. A GΩ seal was generated as quickly as possible, the negative pressure was then released and the access resistance was continually monitored. Recordings only took place when access resistance was <50 MΩ.
CO gas solutions were generated by bubbling CO gas (99.5%) (BOC gases, Guilford, UK) through the bath solution under positive pressure for at least 2 h. This saturated solution (0.93 mM) was diluted 1:4, to obtain a 20% CO gas solution which was used within 2 h. CO applications were also made using a well-characterised CO donor molecule, tricarbonyldichlororuthenium (II) dimer [Ru(CO3)Cl2]2, also known as CORM-2 [27, 32, 36]. Stock solutions of CORM-2 were made up in DMSO at 100 mM and stored at −20°C for up to 1 month. Working solutions were made up daily at the concentrations described in the text and figures, and RuCl2(DMSO)4 + 0.1% DMSO was used as the breakdown product control and was synthesised as described by Williams et al. [27].
Data analysis
Data are shown as mean ± standard error of the mean (s.e.m.). Electrophysiological traces were analysed with the pCLAMP 9.0 suite of software. Statistical comparisons of means were made with Student’s paired t test. Concentration–response curves for ATP and for CORM-2 were each fitted with the Hill equation using an iterative fitting routine in Microcal Origin 6.0. In all cases, the figures show mean ± s.e.m., although concentration–response curves were fitted to the whole data set.
Results
CORM-2 inhibits human P2X4 receptors in perforated-patch recordings
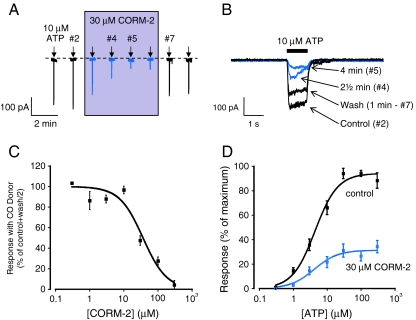
In agreement with previous studies, we observed stable, repeatable P2X4 currents evoked by ATP in the perforated-patch configuration. Application of 30 μM CORM-2 caused a robust inhibition of ATP-evoked currents through P2X4 receptors. This inhibition was reversible upon washout of the CO donor (Fig. 1a, b). Thus, 30 μM CORM-2 applied for 2.5 min was able to inhibit the currents evoked by 10 μM ATP to 45.3 ± 3.4% of control (n = 8) (Fig. 1a, b) and was fully reversible within 2.5 min of donor washout to 95.6 ± 12.6% of control (n = 8) (Fig. 1a, b). The action of CORM-2 was concentration dependent (Fig. 1c) with an apparent mean IC50 value of 36.6 ± 8.7 μM and mean Hill coefficient of −1.3 ± 0.3 (n = 2–8). The ATP concentration–response (Fig. 1d) showed that the inhibition by CORM-2 was non-competitive with ATP since the maximal response was inhibited to 33.3 ± 5.5% of control (n = 5). However, the mean EC50 for ATP was not significantly altered by the presence of the CO donor (5.8 ± 1.6 μM, control and 6.5 ± 1.7 μM, + 30 μM CORM-2; n = 5).
Fig. 1.
CORM-2 inhibits human P2X4 currents in perforated-patch configuration. a Typical time course of currents evoked by repeated applications of ATP (10 μM, 2 s) at 90-s intervals, as indicated by the arrows. CORM-2 (30 μM) was applied for 1 min prior to second application of ATP and continuously perfused until 30 s after the sixth application, as shown by the shaded box. b Superimposed currents shown of a fast time-base taken from the record shown in a, highlighting the effect of 2.5- and 4-min 30 μM CORM-2 applications and the recovery following 1 min wash out. c Concentration–inhibition curve for CORM-2 inhibition of human P2X4 responses. Data shown are mean ± s.e.m. CORM-2 was applied for 2.5 min, and inhibition was expressed as a percentage of the average of the control and wash responses. The Hill equation was used to calculate an apparent IC50 (n = 4–8). d Mean ± s.e.m., paired ATP concentration–response curves in the presence and absence of 30 μM CORM-2. The responses from each individual cell were normalised such that the largest current observed in the absence of CORM-2 was considered to be 100% (n = 5)
The action of CORM-2 and carbon monoxide gas on P2X4 receptors recorded in the conventional whole-cell configuration
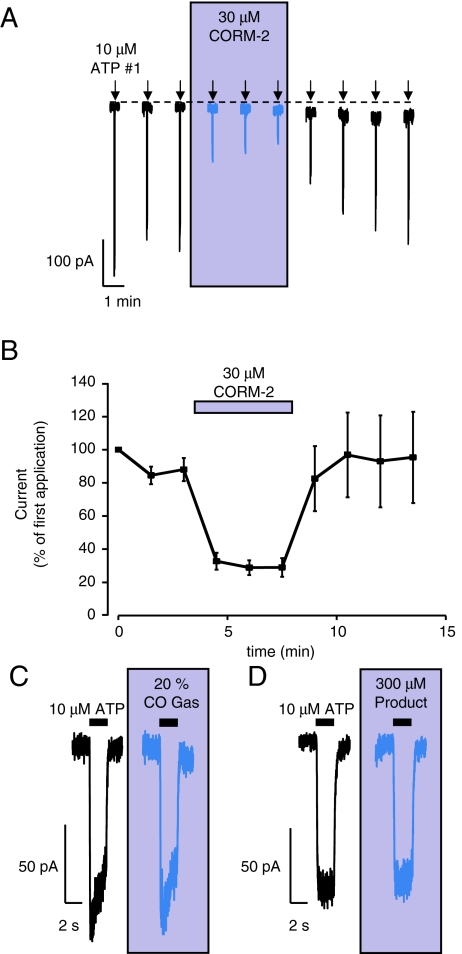
Toulme et al. [17] have recently shown that the anti-depressants paroxetine, fluoxetine, desipramine and amitriptyline affect the surface trafficking of P2X4 receptors in activated microglia and have no acute effect on whole-cell P2X4 recordings, suggesting that they are not true antagonists of P2X4 receptors. These findings also suggest that the trafficking of P2X4 receptors is impaired in conventional whole-cell recordings at room temperature. Therefore, we decided to investigate whether the inhibition of P2X4 by CORM-2 was maintained in the whole-cell configuration where receptor trafficking is unlikely to occur and in the presence of amitriptyline which would impair receptor trafficking if it were occurring [17]. In whole-cell patch clamp, application of CORM-2 was still able to inhibit P2X4 currents in a reversible manner (Fig. 2a, b), and maximal inhibition occurred more rapidly in this configuration than with perforated patches. Thus, at 1 min, 30 μM CORM-2 inhibited currents evoked by 10 μM ATP to 31.1 ± 3.8% of first ATP application (n = 23). Even after a 4-min application of 30 μM CORM-2, the inhibition was reversible to 81.5 ± 18% of the first ATP application. In agreement with Sim and North [16], we found that 10 μM amitriptyline had no effect on human P2X4-mediated currents in HEK cells (data not shown) and that 30 μM CORM-2 was still able to inhibit ATP-evoked currents in the presence of 10 μM amitriptyline to 30.9 ± 2.5% of currents evoked in amitriptyline alone (n = 3, Fig. 3a). Given that CORM-2 inhibition of P2X4 currents was maintained in whole-cell patch clamp, for ease of experimentation, further pharmacological investigations into the mechanism of action were performed in the conventional whole-cell configuration.
Fig. 2.
Inhibition of human P2X4 currents by CORM-2 using conventional whole-cell patch configuration. a Typical time-course of currents evoked by repeated applications of ATP (10 μM, 2 s) at 90-s intervals, as indicated by the arrows. CORM-2 (30 μM) was applied for 1 min prior to the fourth application of ATP and continuously perfused until 30 s after the sixth application, as indicated by the shaded box. b Mean (±s.e.m.) inhibition by 30 μM CORM-2 in experiments exemplified in a. Mean responses are shown as percentage of first response (n = 24). c Typical currents, shown on a faster time-base, of the lack of effect of a 20% saturated CO gas solution applied for 1 min on currents evoked by 10 μM ATP (2 s; black bar). On the left is control response and on the right is response during CO gas application (shaded box). d Typical currents, shown on a faster time-base, of the lack of effect of 300 μM RuCl2(DMSO)4, a control breakdown product of CORM-2, which is unable to donate CO, applied for 1 min on currents evoked by 10 μM ATP (2 s; black bar). On the left is control response and on the right is response during 300 μM RuCl2(DMSO)4 application
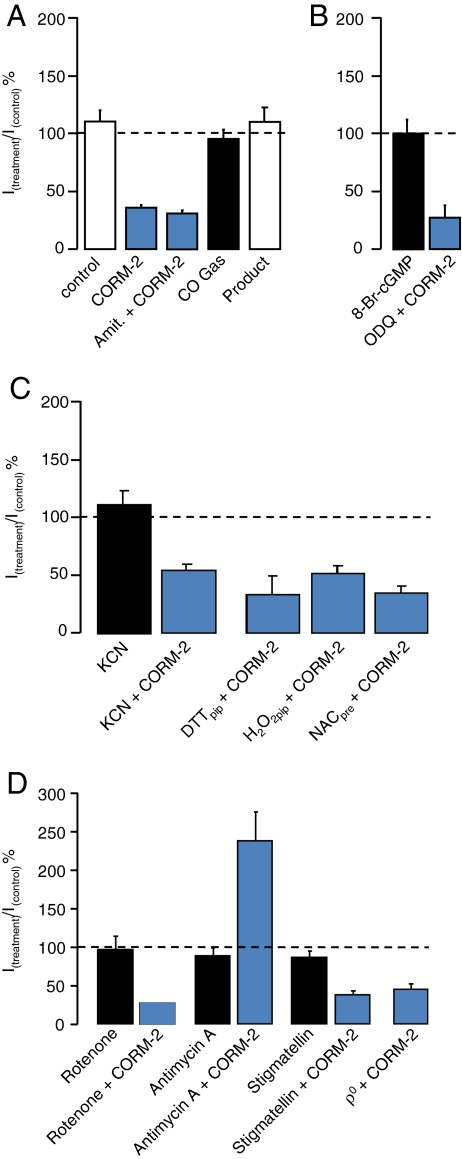
Fig. 3.
Effect of various pharmacological interventions on P2X4 responses and on the inhibition mediated by CORM-2. Data in this figure were acquired in the conventional whole-cell patch-clamp configuration. In all cases, protocols were as those shown in Fig. 1a, with three control 10 μM ATP applications followed by 1-min pre-incubation of drugs before a fourth ATP application. In each case, mean responses shown were obtained by expressing the fourth response in the presence of drug as a percentage of the third control response. In all cases, data shown represent mean (±s.e.m.). a Effect on P2X4 currents of applications of the following: 30 μM CORM-2 (n = 24), 10 μM amitriptyline + 30 μM CORM-2 (n = 3), 20% CO gas (n = 6), and 300 μM product control (n = 3). b Effect on P2X4 currents of 100 μM 8-Br-cGMP (n = 3) and 10 μM ODQ + 30 μM CORM-2 (n = 3). c Effect on P2X4 currents of 100 μM KCN (n = 8) and 100 μM KCN + 30 μM CORM-2 (n = 6). Also shown are the effects of 30 μM CORM-2 following the inclusion of 2 mM DTT (n = 3) or 100 μM H2O2 (n = 5) within the patch pipette and of pre-loading of cells with 1 mM N-acetylcysteine (NAC) for 1 h before patching (n = 4). d Effect on P2X4 currents of 2 μM rotenone (n = 3), 2 μM rotenone + 30 μM CORM-2 (n = 2), 3 μM antimycin A (n = 5), 3 μM antimycin A + 30 μM CORM-2 (n = 8), 30 nM stigmatellin (n = 4), 30 nM stigmatellin + 30 μM CORM-2 (n = 5), and the effect of 30 μM CORM-2 on HEK-P2X4 ρ0 cells (n = 5)
Incubation for up to 4 min with 20% CO gas had no significant effect on currents evoked by 10 μM ATP (at 1 min, currents were 95 ± 7.9% of control, n = 6; Figs. 2c and 3a). The breakdown product of CORM-2 (RuCl2(DMSO)4) was utilised as a control, and we found that a 2.5-min pre-incubation of this compound also did not inhibit currents evoked by 10 μM ATP, even at 300 μM (+0.1% DMSO) (115 ± 7.7% of control, n = 3; Figs. 2d and 3a). These conflicting data leave open the possibilities that either (a) CORM-2 is a P2X4 antagonist which acts independently of its ability to release CO or (b) that the effect of CORM-2 is dependent upon CO release and that delivery of CO to the site of action using a 20% solution is insufficient to produce an effect. With such possibilities in mind, we investigated the potential involvement of known targets of CO.
The action of CORM-2 is independent of cGMP and soluble guanylate cyclase
CO is a well-established activator of sGC [24], activation of which leads to an increase in cGMP, which may regulate ion channel function at the membrane. The action of cGMP on P2X4 currents was studied using a membrane-permeable cGMP analogue, 8-Br-cGMP (100 μM), which was found to have no effect on P2X4 currents during a 4-min application (n = 3) (Fig. 3b). To investigate further the involvement of sGC, we co-applied the specific sGC inhibitor, ODQ [37], with CORM-2. CORM-2 (30 μM) mediated inhibition of P2X4 currents was still observed during co-application with 10 μM ODQ (n = 3) (Fig. 3b); taken together, these results suggest that sGC activation plays no role in the CORM-2-mediated inhibition of P2X4 receptors.
Role of cellular redox state
Previous studies have implicated the generation of ROS from mitochondria in the action of CORM-2 on L-type voltage-gated calcium channels [29]. CO inhibits complex IV, which would lead to an augmentation of superoxide anion generation from complex III. Increased ROS formation alters the redox state of cysteines within the L-type calcium channel and inhibits channel activity. Thus, we have investigated whether P2X4 receptors are sensitive to mitochondria-derived ROS.
Potassium cyanide (KCN) was used to mimic the generation of cellular ROS, also via inhibition of complex IV. Pre-application of KCN alone (100 μM, up to 4 min) did not inhibit ATP-evoked currents (111 ± 12% of control; n = 8, Fig. 3c), and CORM-2 co-application with 100 μM KCN was still able to inhibit the currents (n = 6, Fig. 3c). The inclusion of the reducing agent, dithiolthreitol (DTT, 2 mM) in the patch pipette solution, also did not affect the ability of CORM-2 to inhibit P2X4 currents (n = 3; Fig. 3c). Similarly, the inhibition by CORM-2 was unaffected by the inclusion of 100 μM hydrogen peroxide (H2O2) in the patch pipette (n = 5; Fig. 3c). Finally, pre-loading cells with the anti-oxidant N-acetylcysteine (NAC, 1 mM, 1 h) did not prevent CORM-2 inhibition of ATP-evoked currents (n = 4; Fig. 3c). These results strongly suggest that the effect of CORM-2 on P2X4 is not mediated by the generation of cellular ROS.
Role of mitochondrial signalling
The investigation into mitochondrial ROS was extended by using specific inhibitors of electron transport. Application of the complex I inhibitor, rotenone (2 μM, 4 min), was not able to mimic the inhibitory effect of CORM-2 (n = 3), had no effect on currents evoked by ATP, and did not effect the ability of CORM-2 to inhibit these currents (Fig. 3d). Pre-application of antimycin A (3 μM), which acts via the Qi site of complex III and is predicted to increase ROS within the cytosol [38], had no effect on currents evoked by 10 μM ATP (90 ± 10% of control; n = 5). However, co-application of 3 μM antimycin A and 30 μM CORM-2 caused a robust potentiation of currents evoked by 10 μM ATP (241 ± 38% of control; n = 8; P < 0.01). Pre-application of stigmatellin (30 nM, 1 min), which acts via the Qo site, and is predicted to decrease cytosolic ROS generation [38], had no effect on currents evoked by 10 μM ATP and was unable to inhibit the action of 30 μM CORM-2 on P2X4 currents (Fig. 3d). Finally, employing P2X4-HEK cells which lacked mitochondria (ρ0 cells), 30 μM CORM-2, was still able to inhibit P2X4 responses (45.5 ± 7.9% of control; n = 5; Fig. 3d).
Discussion
This study shows that CORM-2, a well-established CO-releasing molecule, is a potent, reversible and non-competitive inhibitor of recombinant human P2X4 receptors. This inhibition was not mimicked by the application of a 20% CO gas solution and did not involve the generation of mitochondria-derived ROS or activation of soluble guanylate cyclase, two established mechanisms by which CO can exert cellular effects. The simplest explanation for these observations is that the effect of CORM-2 is not dependent on its ability to release CO. Whilst this explanation would suggest that there is no physiological role for CO regulation of P2X4 receptors, it would still identify CORM-2 as a useful pharmacological tool for the inhibition of P2X4 receptors. We have previously shown that CORM-2 is able to potentiate ATP-evoked responses at P2X2 receptors at low agonist concentrations, and although a small inhibition was observed at high ATP concentrations, this was not as powerful as the inhibition observed here at P2X4 receptors [32]. We have also previously observed that CORM-2 was without effect at P2X3 and P2X2/3 receptors [32], though it is currently untested at other P2X receptors. Although the CO-releasing properties of CORM-2 would likely lead to many off-target effects when used as a P2X4 receptor antagonist, this molecule might represent a useful lead compound in the future generation of specific P2X4 receptor antagonists.
There is a clinical and research need for potent P2X4 antagonists, given their highlighted role in neuropathic and inflammatory pain animal models. However, there is a current dearth of such molecules. P2X4 receptors are relatively insensitive to broad-spectrum P2X antagonists, suramin and PPADS [3, 14], and although a specific P2X4 antagonist has been reported [39], data regarding its effectiveness and specificity have not yet been published. Tri-cyclic antidepressant drugs such as paroxetine, fluoxetine, desipramine and amitriptyline have been prescribed for many years for the treatment of neuropathic pain [40] and were recently shown to inhibit P2X4 receptor function [15], a mechanism which may contribute to their analgesic properties. However, recent studies by Toulme et al. [17] have shown that, with the exception of paroxetine which has some direct effects on P2X4 currents, these drugs act by disrupting lysosomal function and the trafficking of P2X4 receptors to the plasma membrane and are, therefore, not true P2X4 antagonists. Despite this, these drugs remain useful pharmacological tools and serve to highlight the importance of P2X4 receptors as a drug target for the treatment of neuropathic pain, and the clinical and research need for P2X4 antagonists. Here, we utilised amitriptyline and conventional whole-cell recordings to show that the action of CORM-2 is unlikely to be mediated by altered trafficking of P2X4 receptors, given that CORM-2 can still inhibit ATP-evoked currents in whole-cell recordings (Fig. 2) and in the presence of 10 μM amitriptyline (Fig. 3a).
The observation that CORM-2 can exert effects on ion channels independently of its ability to release CO is not without precedent [41]. That study showed that CORM-2 was able to activate a non-selective cation current in human endothelial cells, whilst CO gas and tricarbonylchloro(glycinato)ruthenium (II) (CORM-3), a water-soluble CO donor, were unable to mimic CO. These authors concluded that CORM-2 exerted its effects independently of CO release. It is possible that the target for CORM-2 binding in both human endothelium and P2X4-HEK cells is a common protein which interacts with both P2X4 and the unidentified cation channel, or it may that this exogenous compound is able to exert effects at two different ion channels. However, we propose a third possible explanation for why CO cannot mimic the effect of CORM-2, namely that CO and CORM-2 have different diffusion properties across the plasma membrane. Since CORM-2 is highly lipophilic, it will diffuse rapidly across the membrane and might be expected to deliver CO to intracellular motifs of membrane proteins at much higher concentrations than can be achieved with membrane diffusion of CO gas. Of course, there is also the possibility that CO, like CO2 [42] and O2 [43], requires a specific permeation pathway, which is absent in these cells. Such an idea is supported by recent experiments in Escherichia coli, which show that CORM-3, the water-soluble CO donor molecule, is taken up and exerts CO-specific growth inhibitory effects, whilst the addition of dissolved CO into the growth medium is without effect [44].
Acknowledging the possibility that the observed inhibition of P2X4 receptors by CORM-2 was dependent on intracellular CO release, we investigated the involvement of known CO-modulating pathways. The immediate cellular targets for CO are not particularly well established, although the chemistry of CO suggests it is suited to the binding of heme proteins. One such protein, sGC, has been shown to be activated by CO [24] and would produce rapid rises in intracellular cGMP that may cause changes to ion channel function. We have ruled out the involvement of this pathway in P2X4 inhibition since a cell-permeable analogue of cGMP was unable to mimic the inhibition of P2X4, and ODQ, an inhibitor of sGC, was unable to block the inhibition of P2X4 by CORM-2 (Fig. 3b).
CO and CO donor molecules have also been proposed to inhibit electron transport and subsequently cause the generation of cellular ROS. This is the proposed mechanism by which CO and CORM-2 exert an inhibitory effect on L-type calcium channels [29]. Incubation of cells with 100 μM KCN, which would also inhibit complex IV, did not mimic the inhibitory effect of CORM-2, and we found that the inclusion of reducing (DTT) or oxidising (H2O2) agents in the patch pipette had no effect on the ability of CORM-2 to inhibit P2X4 currents (Fig. 3c). Similarly, pre-loading the cells with an anti-oxidant (NAC) also did not inhibit the effect of CORM-2. We also investigated the effects of other mitochondrial transport inhibitors (Fig. 3d) and found that complex I inhibition was without effect on P2X4 currents or their modulation by CORM-2. Similarly, inhibition of the complex III Qo site, which would be expected to decrease cytosolic ROS [38] and has been shown to block the CORM-2 inhibition of L-type calcium channels [29], had no effect on P2X4 currents or their inhibition by CORM-2. Results obtained with the complex III inhibitor, antimycin A, were intriguing. Antimycin A acts at the Qi site and would be expected to increase cytosolic ROS [38]. Applied alone, antimycin A had no effect on P2X4 currents, but when co-applied with CORM-2, it caused a potentiation of ATP-evoked currents. Although we have no experimental clues as to the mechanism of this augmentation, the fact that CORM-2 is not inhibitory reinforces the idea that the action of CORM-2 is not dependent on mitochondrial ROS production, a notion fully supported by our data employing ρ0 cells (Fig. 3d). Together, these results suggest that there is no role for cellular ROS production in the mechanism by which CORM-2 inhibits P2X4 receptors.
Although the modus operandi remains elusive, we have demonstrated that CORM-2 is a robust, non-competitive inhibitor of P2X4 receptors. Since it either potentiates or has no effect on P2X2, P2X2/3 and P2X3 receptors, CORM-2 is a unique pharmacological tool for distinguishing between these receptors in physiology and disease.
Acknowledgment
This work was funded by the Medical Research Council (BB/DO1/59X1), and the authors declare no conflicting interests.
References
- 1.Alexander SP, Mathie A, Peters JA. Guide to receptors and channels (GRAC), 3rd edition. Br J Pharmacol. 2008;153(Suppl 2):S1–S209. doi: 10.1038/sj.bjp.0707746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bo X, Zhang Y, Nassar M, Burnstock G, Schoepfer R. A P2X purinoceptor cDNA conferring a novel pharmacological profile. FEBS Lett. 1995;375:129–133. doi: 10.1016/0014-5793(95)01203-Q. [DOI] [PubMed] [Google Scholar]
- 3.Buell G, Lewis C, Collo G, North RA, Surprenant A. An antagonist-insensitive P2X receptor expressed in epithelia and brain. EMBO J. 1996;15:55–62. [PMC free article] [PubMed] [Google Scholar]
- 4.Rubio ME, Soto F. Distinct localization of P2X receptors at excitatory postsynaptic specializations. J Neurosci. 2001;21:641–653. doi: 10.1523/JNEUROSCI.21-02-00641.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Collo G, North RA, Kawashima E, Merlo-Pich E, Neidhart S, Surprenant A, Buell G. Cloning of P2X5 and P2X6 receptors and the distribution and properties of an extended family of ATP-gated ion channels. J Neurosci. 1996;16:2495–2507. doi: 10.1523/JNEUROSCI.16-08-02495.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bo X, Kim M, Nori SL, Schoepfer R, Burnstock G, North RA. Tissue distribution of P2X4 receptors studied with an ectodomain antibody. Cell Tissue Res. 2003;313:159–165. doi: 10.1007/s00441-003-0758-5. [DOI] [PubMed] [Google Scholar]
- 7.Soto F, Garcia-Guzman M, Gomez-Hernandez JM, Hollmann M, Karschin C, Stuhmer W. P2X4: an ATP-activated ionotropic receptor cloned from rat brain. Proc Natl Acad Sci USA. 1996;93:3684–3688. doi: 10.1073/pnas.93.8.3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Naemsch LN, Weidema AF, Sims SM, Underhill TM, Dixon SJ. P2X4 purinoceptors mediate an ATP-activated, non-selective cation current in rabbit osteoclasts. J Cell Sci. 1999;112(Pt 23):4425–4435. doi: 10.1242/jcs.112.23.4425. [DOI] [PubMed] [Google Scholar]
- 9.Sluyter R, Barden JA, Wiley JS. Detection of P2X purinergic receptors on human B lymphocytes. Cell Tissue Res. 2001;304:231–236. doi: 10.1007/s004410100372. [DOI] [PubMed] [Google Scholar]
- 10.Stokes L, Surprenant A. Dynamic regulation of the P2X4 receptor in alveolar macrophages by phagocytosis and classical activation. Eur J Immunol. 2009;39:986–995. doi: 10.1002/eji.200838818. [DOI] [PubMed] [Google Scholar]
- 11.Fountain SJ, North RA. A C-terminal lysine that controls human P2X4 receptor desensitization. J Biol Chem. 2006;281:15044–15049. doi: 10.1074/jbc.M600442200. [DOI] [PubMed] [Google Scholar]
- 12.Khakh BS, Proctor WR, Dunwiddie TV, Labarca C, Lester HA. Allosteric control of gating and kinetics at P2X4 receptor channels. J Neurosci. 1999;19:7289–7299. doi: 10.1523/JNEUROSCI.19-17-07289.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Priel A, Silberberg SD. Mechanism of ivermectin facilitation of human P2X4 receptor channels. J Gen Physiol. 2004;123:281–293. doi: 10.1085/jgp.200308986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jones CA, Chessell IP, Simon J, Barnard EA, Miller KJ, Michel AD, Humphrey PP. Functional characterization of the P2X(4) receptor orthologues. Br J Pharmacol. 2000;129:388–394. doi: 10.1038/sj.bjp.0703059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nagata K, Imai T, Yamashita T, Tsuda M, Tozaki-Saitoh H, Inoue K. Antidepressants inhibit P2X4 receptor function: a possible involvement in neuropathic pain relief. Mol Pain. 2009;5:20. doi: 10.1186/1744-8069-5-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sim JA, North RA. Amitriptyline does not block the action of ATP at human P2X4 receptor. Br J Pharmacol. 2010;160:88–92. doi: 10.1111/j.1476-5381.2010.00683.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Toulme E, Garcia A, Samways D, Egan TM, Carson MJ, Khakh BS. P2X4 receptors in activated C8-B4 cells of cerebellar microglial origin. J Gen Physiol. 2010;135:333–353. doi: 10.1085/jgp.200910336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsuda M, Shigemoto-Mogami Y, Koizumi S, Mizokoshi A, Kohsaka S, Salter MW, Inoue K. P2X4 receptors induced in spinal microglia gate tactile allodynia after nerve injury. Nature. 2003;424:778–783. doi: 10.1038/nature01786. [DOI] [PubMed] [Google Scholar]
- 19.Guo LH, Trautmann K, Schluesener HJ. Expression of P2X4 receptor by lesional activated microglia during formalin-induced inflammatory pain. J Neuroimmunol. 2005;163:120–127. doi: 10.1016/j.jneuroim.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 20.Maines MD, Gibbs PE. 30 some years of heme oxygenase: from a “molecular wrecking ball” to a “mesmerizing” trigger of cellular events. Biochem Biophys Res Commun. 2005;338:568–577. doi: 10.1016/j.bbrc.2005.08.121. [DOI] [PubMed] [Google Scholar]
- 21.Ryter SW, Alam J, Choi AM. Heme oxygenase-1/carbon monoxide: from basic science to therapeutic applications. Physiol Rev. 2006;86:583–650. doi: 10.1152/physrev.00011.2005. [DOI] [PubMed] [Google Scholar]
- 22.Rosa AO, Egea J, Lorrio S, Rojo AI, Cuadrado A, Lopez MG. Nrf2-mediated haeme oxygenase-1 up-regulation induced by cobalt protoporphyrin has antinociceptive effects against inflammatory pain in the formalin test in mice. Pain. 2008;137:332–339. doi: 10.1016/j.pain.2007.09.015. [DOI] [PubMed] [Google Scholar]
- 23.Egea J, Rosa AO, Lorrio S, Barrio L, Cuadrado A, Lopez MG. Haeme oxygenase-1 overexpression via nAChRs and the transcription factor Nrf2 has antinociceptive effects in the formalin test. Pain. 2009;146:75–83. doi: 10.1016/j.pain.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 24.Stone JR, Marletta MA. Soluble guanylate cyclase from bovine lung: activation with nitric oxide and carbon monoxide and spectral characterization of the ferrous and ferric states. Biochemistry. 1994;33:5636–5640. doi: 10.1021/bi00184a036. [DOI] [PubMed] [Google Scholar]
- 25.Kim HP, Ryter SW, Choi AM. CO as a cellular signaling molecule. Annu Rev Pharmacol Toxicol. 2006;46:411–449. doi: 10.1146/annurev.pharmtox.46.120604.141053. [DOI] [PubMed] [Google Scholar]
- 26.Wang R, Wu L. The chemical modification of KCa channels by carbon monoxide in vascular smooth muscle cells. J Biol Chem. 1997;272:8222–8226. doi: 10.1074/jbc.272.13.8222. [DOI] [PubMed] [Google Scholar]
- 27.Williams SE, Wootton P, Mason HS, Bould J, Iles DE, Riccardi D, Peers C, Kemp PJ. Hemoxygenase-2 is an oxygen sensor for a calcium-sensitive potassium channel. Science. 2004;306:2093–2097. doi: 10.1126/science.1105010. [DOI] [PubMed] [Google Scholar]
- 28.Williams SE, Brazier SP, Baban N, Telezhkin V, Muller CT, Riccardi D, Kemp PJ. A structural motif in the C-terminal tail of slo1 confers carbon monoxide sensitivity to human BK Ca channels. Pflugers Arch. 2008;456:561–572. doi: 10.1007/s00424-007-0439-4. [DOI] [PubMed] [Google Scholar]
- 29.Scragg JL, Dallas ML, Wilkinson JA, Varadi G, Peers C. Carbon monoxide inhibits L-type Ca2+ channels via redox modulation of key cysteine residues by mitochondrial reactive oxygen species. J Biol Chem. 2008;283:24412–24419. doi: 10.1074/jbc.M803037200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dallas ML, Scragg JL, Peers C. Modulation of hTREK-1 by carbon monoxide. NeuroReport. 2008;19:345–348. doi: 10.1097/WNR.0b013e3282f51045. [DOI] [PubMed] [Google Scholar]
- 31.Althaus M, Fronius M, Buchackert Y, Vadasz I, Clauss WG, Seeger W, Motterlini R, Morty RE. Carbon monoxide rapidly impairs alveolar fluid clearance by inhibiting epithelial sodium channels. Am J Respir Cell Mol Biol. 2009;41:639–650. doi: 10.1165/rcmb.2008-0458OC. [DOI] [PubMed] [Google Scholar]
- 32.Wilkinson WJ, Gadeberg HC, Harrison AW, Allen ND, Riccardi D, Kemp PJ. Carbon monoxide is a rapid modulator of recombinant and native P2X2 ligand-gated ion channels. Br J Pharmacol. 2009;158:862–871. doi: 10.1111/j.1476-5381.2009.00354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.King MP, Attardi G. Human cells lacking mtDNA: repopulation with exogenous mitochondria by complementation. Science. 1989;246:500–503. doi: 10.1126/science.2814477. [DOI] [PubMed] [Google Scholar]
- 34.Waypa GB, Chandel NS, Schumacker PT. Model for hypoxic pulmonary vasoconstriction involving mitochondrial oxygen sensing. Circ Res. 2001;88:1259–1266. doi: 10.1161/hh1201.091960. [DOI] [PubMed] [Google Scholar]
- 35.Searle GJ, Hartness ME, Hoareau R, Peers C, Kemp PJ. Lack of contribution of mitochondrial electron transport to acute O2 sensing in model airway chemoreceptors. Biochem Biophys Res Commun. 2002;291:332–337. doi: 10.1006/bbrc.2002.6428. [DOI] [PubMed] [Google Scholar]
- 36.Motterlini R, Mann BE, Foresti R. Therapeutic applications of carbon monoxide-releasing molecules. Expert Opin Investig Drugs. 2005;14:1305–1318. doi: 10.1517/13543784.14.11.1305. [DOI] [PubMed] [Google Scholar]
- 37.Garthwaite J, Southam E, Boulton CL, Nielsen EB, Schmidt K, Mayer B. Potent and selective inhibition of nitric oxide-sensitive guanylyl cyclase by 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one. Mol Pharmacol. 1995;48:184–188. [PubMed] [Google Scholar]
- 38.Raha S, McEachern GE, Myint AT, Robinson BH. Superoxides from mitochondrial complex III: the role of manganese superoxide dismutase. Free Radic Biol Med. 2000;29:170–180. doi: 10.1016/S0891-5849(00)00338-5. [DOI] [PubMed] [Google Scholar]
- 39.Donnelly-Roberts D, McGaraughty S, Shieh CC, Honore P, Jarvis MF. Painful purinergic receptors. J Pharmacol Exp Ther. 2008;324:409–415. doi: 10.1124/jpet.106.105890. [DOI] [PubMed] [Google Scholar]
- 40.Sindrup SH, Otto M, Finnerup NB, Jensen TS. Antidepressants in the treatment of neuropathic pain. Basic Clin Pharmacol Toxicol. 2005;96:399–409. doi: 10.1111/j.1742-7843.2005.pto_96696601.x. [DOI] [PubMed] [Google Scholar]
- 41.Dong DL, Chen C, Huang W, Chen Y, Zhang XL, Li Z, Li Y, Yang BF. Tricarbonyldichlororuthenium (II) dimer (CORM2) activates non-selective cation current in human endothelial cells independently of carbon monoxide releasing. Eur J Pharmacol. 2008;590:99–104. doi: 10.1016/j.ejphar.2008.05.042. [DOI] [PubMed] [Google Scholar]
- 42.Nakhoul NL, Davis BA, Romero MF, Boron WF. Effect of expressing the water channel aquaporin-1 on the CO2 permeability of Xenopus oocytes. Am J Physiol. 1998;274:C543–C548. doi: 10.1152/ajpcell.1998.274.2.C543. [DOI] [PubMed] [Google Scholar]
- 43.Echevarria M, Munoz-Cabello AM, Sanchez-Silva R, Toledo-Aral JJ, Lopez-Barneo J. Development of cytosolic hypoxia and hypoxia-inducible factor stabilization are facilitated by aquaporin-1 expression. J Biol Chem. 2007;282:30207–30215. doi: 10.1074/jbc.M702639200. [DOI] [PubMed] [Google Scholar]
- 44.Davidge KS, Sanguinetti G, Yee CH, Cox AG, McLeod CW, Monk CE, Mann BE, Motterlini R, Poole RK. Carbon monoxide-releasing antibacterial molecules target respiration and global transcriptional regulators. J Biol Chem. 2009;284:4516–4524. doi: 10.1074/jbc.M808210200. [DOI] [PubMed] [Google Scholar]