Abstract
The role of the interaction between neurons and glial cells in the pathogenesis of neurodegenerative diseases is gaining more attention. Neuroinflammation participates in the progressive nature of diverse neurologic diseases including Parkinson's disease, Alzheimer's disease and multiple sclerosis. Activated microglia release neurotoxic molecules, which take part in the neuroinflammatory responses. Astrocytes are also key players in these responses. Reactive astrocytes secrete inflammatory factors, including tumor necrosis factor-α (TNF-α). This secretion can be regulated by extracellular ATP mediated through P2X7 receptors. However, whether the activity of astrocytic P2X7 receptors changes in Parkinson’s disease and whether these changes would influence the secretion of inflammatory factors in astrocytes are still unclear. In our study, through immunocytochemistry, whole-cell patch clamp and ELISA assay, we found that P2X7 receptors were expressed in midbrain astrocytes, and that, rotenone, a Parkinson’s disease model used at a low concentration (2–20 nM) for 48 h increased the P2X7 receptor current density and thereby inhibited the secretion of TNF-α. Our research suggests that rotenone can regulate cytokine secretion of astrocytes through elevated P2X7 channel current density and, in turn, take part in the neuroinflammatory process in the rotenone Parkinson’s disease model.
Electronic supplementary material
The online version of this article (doi:10.1007/s11302-011-9218-y) contains supplementary material, which is available to authorized users.
Keywords: Astrocytes, Rotenone, Purinergic P2X7 receptor, Tumor necrosis factor-α
Introduction
There has been a consensus that neuroinflammation participates in the progressive nature of diverse neurologic diseases including Parkinson's disease (PD), Alzheimer's disease and multiple sclerosis. Microglia can be chronically activated in response to dopaminergic neuron death fuelling reactive microgliosis in PD [1]. Activated microglia release neurotoxic molecules such as tumor necrosis factor (TNF)-α, interleukin (IL)-1β, IL-6, nitric oxide and reactive oxygen species [2]. Besides microglia, astrocytes are other key players in neuroinflammatory responses and are intimately involved in virtually every form of neuropathology [3]. Reactive astrogliosis is generally mild or moderate in autopsy specimens of substantia nigra from PD patients [4, 5]. In reactive astroglia there is variable upregulation of expression of glial fibrillary acid protein (GFAP) and other proteins, including cytokines, growth factors and transcriptional regulators [6]. The secretion of cytokines is regulated by extracellular ATP, which is elevated after CNS injury [7, 8].
P2X7, a ligand-gated purinergic receptor, has been reported in astrocytes [9]. Both 1 mM ATP and 3′-O-(4-benzoyl)benzoyl-ATP (BzATP), a P2X7 receptor agonist, have been shown to attenuate TNF-α release in lipopolysaccharide (LPS)-stimulated astrocytes [8]. On the other hand, ATP-induced microparticle shedding and IL-1β release are markedly reduced by the inhibition of acid sphingomyelinase which is rapidly activated by activation of the ATP receptor P2X7 in astrocytes [10]. Therefore, P2X7 receptor activity may influence the secretion of inflammatory factors in astrocytes. However, whether the activity of P2X7 receptors in astrocytes changes in PD and whether these changes influence the secretion of inflammatory factors in astrocytes are still unclear.
In this study, we used electrophysiological methods to investigate the effect of rotenone, a classic mitochondrial complex I inhibitor which has been widely used as a PD model, on P2X7 activity in astrocytes. Furthermore, we used enzyme-linked immunosorbent assay (ELISA) to evaluate TNF-α secretion and thereby infer the possible role(s) of glial P2X7 receptors.
Experimental procedures
Astrocyte-purified cultures
Astrocytes were harvested from neonatal rats (P2-3) as previously described [11, 12]. The ventral portion of the midbrain was removed, demembranated, chopped, and then incubated with 0.125% trypsin at 37°C for 30 min. The mixture was then triturated in triturating solution and the cells were centrifuged, resuspended in serum, and then plated onto PDL-coated culture flasks, and incubated in DMEM containing 10% FBS. After 6–10 days, as soon as a monolayer of astrocytes was formed, microglia and cells of the oligodendrocyte lineage were removed by shaking the culture at 150 rpm at 37°C overnight. This protocol resulted in highly pure astrocyte cultures (>96%) as assessed by immunocytochemical staining using primary antibodies raised against GFAP. Finally, astrocytes were harvested by treating the culture flasks with 0.125% trypsin solution. Collected cells were plated onto coverslips in 24-well plates for electrophysiological recordings and immunocytochemistry assay at a density of 1 × 103/cm2. Cells were cultured for 24 h before being treated with drugs.
Immunocytochemistry
Immunocytochemistry of cells cultured on coverslips was performed as previously described [13]. Briefly, coverslips were fixed with 4% paraformaldehyde in PBS for 20 min, washed with 0.1 M PBS (pH 7.4), then incubated overnight at 4°C with monoclonal antibodies to GFAP (Sigma) and rabbit polyclonal antibodies to P2X7 receptors (Alomone, APR-004) diluted in antibody diluent containing 10% normal goat serum. After washing three times with PBS, cells were incubated with FITC-conjugated goat anti-mouse or Cy3 conjugated goat anti-rabbit antibodies (Jackson ImmunoResearch Laboratories, West Grove, PA, USA) for 90 min at room temperature. After washing, astrocytes were incubated with 1 μg/ml Hoechst dye in PBS for 10 min at room temperature, washed with PBS and mounted using PBS with glycerol (3:1, v/v). Fluorescence was visualised using a Nikon digital camera DXM1200 (Nikon, Japan) adapted to a Nikon Eclipse E600 fluorescence microscope (Nikon, Japan).
Electrophysiological recordings
A whole-cell patch clamp technique was used to record ionic currents. The pipette solution contained 130 mM KCl, 1 mM CaCl2, 2 mM MgCl2, 10 mM EGTA, and 10 mM HEPES. The pH was adjusted to 7.2 with KOH. The external solution contained 140 mM NaCl, 5 mM KCl, 2.5 mM CaCl2, 1 mM MgCl2, 10 mM glucose, and 10 mM HEPES. The pH was adjusted to 7.4 with NaOH. The pipette resistance was 6 ~ 8 MΩ. Whole-cell voltage-clamp recordings were performed at room temperature (21–24°C) with a MultiClamp 700A amplifier (Axon Instruments, Foster City, CA, USA). A membrane potential was held at −70 mV, and those with series resistance above 20 MΩ were rejected. Analogue signals were filtered at 2 kHz, sampled at 10 kHz. Drug solutions were delivered by OCTAFLOW system (ALA Scientific Instruments, Westbury, NY, USA). BzATP was applied for 5 s. One barrel was used to apply a drug-free solution to enable rapid termination of the drug application. Experiments were controlled by Clampex 8.1 software (Axon Instruments).
ELISA
Astrocytes were cultured at a density of 1 × 105/cm2. LPS (10 μg/ml) was added to the culture medium. After treatment of the cells, they were grown in 24-well plates, with the indicated concentrations of drugs for the times indicated, in triplicate. The culture medium was collected from each well and centrifuged at 600 g for 10 min. Supernatants were assayed for TNF-α by ELISA according to the manufacturer’s instructions (R&D Systems Europe). The sensitivity limit for this procedure is 15 pg TNF-α/ml. The absorbance at 450 nm was read with a Bio-Rad model 680 microplate spectrophotometer (Bio-Rad, California, USA).
Drugs
Rotenone, LPS, BzATP and Brilliant blue G (BBG) were purchased from Sigma (Shanghai, China). All chemicals were dissolved in water or dimethyl sulfoxide and then diluted in the recording physiological solution or cell culture medium just before use.
Statistical analysis
Data were analysed by Clampfit 8.1 software (Axon Instruments) and Origin 7.02 (OriginLab, Northampton, MA, USA). All results are expressed as mean ± SEM. A Fisher’s least significance difference t-test was used to compare data between the two groups. A difference was accepted as significant if the probability was less than 5% (P < 0.05).
Results
Expression of P2X7 receptors in cultured astroglia
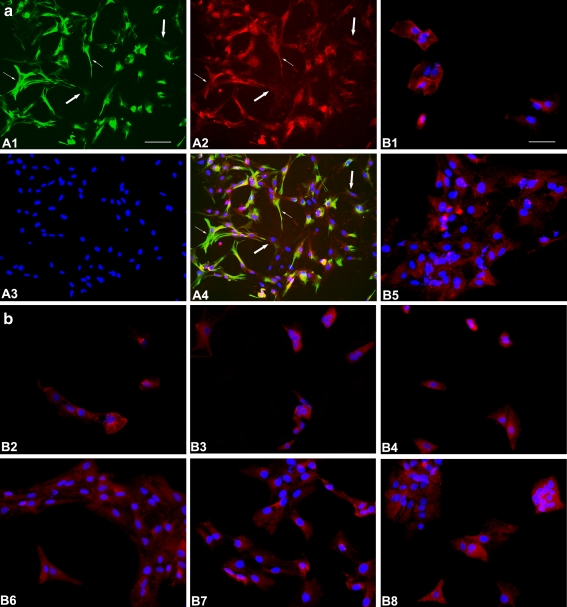
The glial cells exhibited various shapes 72 h after secondary plating. Some showed flat and polygonal structures and some hypertrophy with long processes (Fig. 1a and Supplementary Fig. 1). The immunocytochemical studies revealed that 96% of the glial cell population exhibited GFAP-immunoreactivity, but the immunofluorescence signal intensity of GFAP was much stronger in the hypertrophy type than in the polygonal type cells. These features suggest that the former type of cells might be reactive astrocytes. Further detailed immunocytochemical studies on the glial cell population revealed that the signal intensity of P2X7 receptors expressed in the reactive astrocytes was stronger than that in flat and polygonal cells. We further observed the effect of rotenone treatment on the expression of P2X7 receptors. Astrocytes were cultured for 24 h before 0, 2, 20 and 200 nM rotenone was added to the culture medium. As the culture period increased, the astrocytes clustered into islands, which might be the result of cell proliferation (Fig. 1b5–8). Rotenone (200 nM) treatment caused significant cell death. Thus fewer cells remained on the coverslips than in the control groups (Fig. 1b4, 8). After cell treatment with 2, 20 or 200nM rotenone for 24 or 48 h, P2X7 receptor expression of astrocytes did not show any significant difference from that of the control groups (Fig. 1b).
Fig. 1a, b.
Expression of P2X7 receptors in cultured astroglia. a Representative pictures of astrocytes which were stained by GFAP (green, A1), P2X7 receptor (red, A2) antibody and Hoechst dye (blue, A3) after 72 h in culture. A4 Merged picture. Immunofluorescence signal intensity of GFAP was stronger in the cells showing hypertrophy with long processes (thin arrows) than in the polygonal type cells (thick arrows). P2X7 receptors were preferentially expressed in the hypertrophic cells, while the signal intensity was relatively low, or sometimes could not be detected in the polygonal type cells. Bar = 100 μm. b Representative pictures of astrocytes which were treated by various concentrations of rotenone for various time periods. B1 0 nM rotenone (control) for 24 h, B2 2 nM for 24 h, B3 20 nM for 24 h, B4 200 nM for 24 h, B5 0 nM rotenone (control) for 48 h, B6 2 nM for 48 h, B7 20 nM for 48 h, B8 200 nM for 48 h. Cells clustered into islands after 72 h in culture (B5–8). The signal intensity of P2X7 receptors in astrocytes that remained in the treatment group (B2–4, 6–8) was not significantly different from that of the control group (B1, 5). Bar = 50 μm
Rotenone increases BzATP-sensitive current density in astrocytes
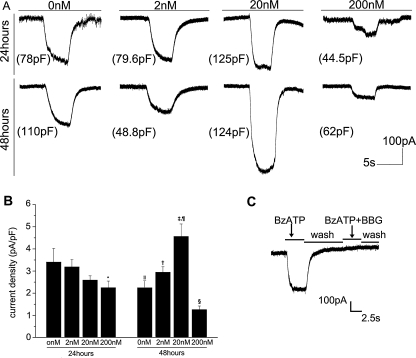
Recordings from astrocytes were made within 3 days after secondary plating. Application of BzATP (300 μM) to the astrocytes, voltage clamped at −70 mV, evoked an inward current in 142 of 157 cells tested (Fig. 2a). The current rapidly ceased after removal of the agonist. The current amplitude varied greatly, from 8 to 553 pA. BzATP failed to induce any inward current in flat and polygonal cells, while large amplitude currents were always detected in the hypertrophy cells. These results are consistent with those of the immunocytochemical studies. Therefore, in the following experiments, we mainly investigated the P2X7 receptor currents in these reactive astrocytes characterised by long processes.
Fig. 2a, b, c.
Rotenone influences the P2X7 receptor current densities in a time- and concentration-dependent way. a Sample traces of inward currents induced by 300 μM BzATP in astrocytes after treatment with 0, 2, 20 or 200 nM rotenone for 24 or 48 h. Numbers in parentheses represent the membrane capacitance of astrocytes investigated. b Histogram of P2X7 receptor current densities after different treatments. *P200 vs. 0 nM, 24 h < 0.05, n = 18. †P2 vs. 0 nM, 48 h < 0.05, n = 16. ‡P20 vs. 0 nM, 48 h < 0.01, n = 16. §P200 vs. 0 nM, 48 h < 0.05, n = 15. ¶P20 vs. 2 nM, 48 h < 0.05, n = 16. ‖P0 nM, 48 vs. 24 h < 0.05, n = 17. c The inward current elicited by 100 μM BzATP can be blocked by 10 μM BBG
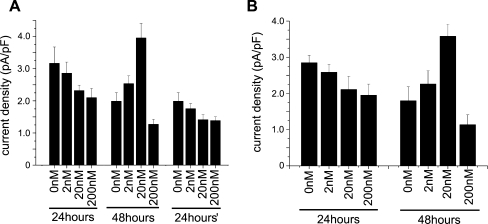
Before the astrocytes were treated by the following drugs, they were cultured for 24 h after secondary plating. In order to investigate whether rotenone influenced the P2X7 receptor currents of astrocytes, we treated the cells with 0, 2, 20 or 200 nM rotenone in the culture medium for 24 or 48 h separately before the currents were recorded. As the shapes and sizes of the cells differed greatly (Cm = 82.35 ± 44.4 pF), we used current density as a comparison index (Fig. 2b). After 24 h treatment, P2X7 receptor current densities were inhibited as the rotenone concentration increased. But only the 200 nM group reached statistical significance. After 48 h, the current density of the 0 nM group was lower than that of the 0 nM group 24 h before, while the current densities of the 2 and 20 nM groups increased. In contrast, the current density of the 200 nM group was substantially decreased (Fig. 2b, Table 1). The currents elicited by BzATP could be abolished by 10 μM BBG (Fig. 2c). After 48 h recovery, cells were treated for 24 h with 2 or 20 nM rotenone, which failed to increase the current densities (Fig. 3a). LPS (10 μg/ml) pretreatment decreased the current densities but did not influence the effects of rotenone (Fig. 3b).
Table 1.
Current densities (I) of P2X7 receptors
| 24 hours | 48 hours | |||||||
|---|---|---|---|---|---|---|---|---|
| 0 nM | 2 nM | 20 nM | 200 nM | 0 nM | 2 nM | 20 nM | 200 nM | |
| I (pA/pF) | 3.41 ± 0.62 | 3.19 ± 0.34 | 2.59 ± 0.19 | 2.25 ± 0.30* | 2.25 ± 0.32‖ | 2.95 ± 0.24† | 4.56 ± 0.56‡¶ | 1.26 ± 0.16§ |
*P200 vs. 0 nM, 24 h < 0.05. †P2 vs. 0 nM, 48 h < 0.05. ‡P20 vs. 0 nM, 48 h < 0.01. §P200 vs. 0 nM, 48 h < 0.05. ¶P20 vs. 2 nM, 48 h < 0.05. ‖P0 nM, 48 vs. 24 h < 0.05
Fig. 3a, b.
Rotenone influences the P2X7 receptor current densities, which are related to cell age or LPS pretreatment. a After 24 h recovery, astrocytes were treated with rotenone for 24 or 48 h (groups 24hours and 48hours) before the currents were tested by 100 μM BzATP. After 48 h recovery, astrocytes were treated with 2 and 20 nM rotenone for 24 h (group 24hours′), and the current densities did not increase. b After 24 h recovery, astrocytes were treated with rotenone and 10 μg/ml LPS for 24 or 48 h (groups 24hours and 48hours) before the currents were tested by 100 μM BzATP
We also investigated whether the kinetic characteristics of the P2X7 receptor were affected by rotenone. We fitted the inactivation curve of the P2X7 receptor with a single exponential equation (Eq. 1):
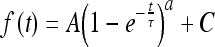 |
1 |
where a = 1 and τ is time constant, which reflects the inactivation speed of P2X7 receptors. According to our results (Table 2), there was no significant difference between the time constants of each rotenone concentration group after the first 24 h of treatment, although that of the 2 nM group showed a slight upward trend. After 48 h of treatment, the time constants of all groups increased except for the 200 nM group. The time constant of the 2 nM group increased further significantly, while that of the 20 nM group still did not show any difference from the 0 nM group.
Table 2.
τ values of the inactivation curves of P2X7 receptors
| 24 hours | 48 hours | |||||||
|---|---|---|---|---|---|---|---|---|
| 0 nM | 2 nM | 20 nM | 200 nM | 0 nM | 2 nM | 20 nM | 200 nM | |
| τ (ms) | 552.77 ± 41.20 | 617.34 ± 61.05 | 545.90 ± 30.42 | 531.02 ± 47.47 | 659.07 ± 36.64* | 883.12 ± 80.68*† | 679.16 ± 48.91* | 527.39 ± 36.30 |
*P48 vs. 24 h < 0.05. †P2 vs. 0 nM, 48 h < 0.05
Rotenone inhibits secretion of TNF-α
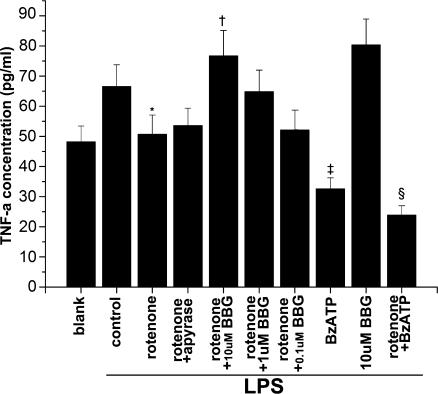
According to the previous electrophysiological results, the P2X7 receptor current density did not increase significantly until astrocytes had been treated with rotenone for over 48 h. Further, 20 nM rotenone had the most significant effect. Therefore, in the ELISA experiments, we chose 20 nM as the working concentration of rotenone and collected the supernatant after 48 h treatment. In addition, the P2X7 receptor agonist BzATP (100 μM) and the antagonist BBG (10 μM) were used to investigate the action of rotenone (Fig. 4). Rotenone (20 nM) significantly reduced the TNF-α concentration in the culture supernatant, while apyrase (5 U/ml) abolished the rotenone effect. BBG also reversed this effect in a dose-dependent manner. Meanwhile, BzATP mimicked the inhibitiory effect of rotenone. When BBG was applied alone, the TNF-α concentration slightly increased but was not significantly different from the control. The TNF-α levels were much less when the cells were treated with rotenone and BzATP than with BzATP alone (Fig. 4, Table 3).
Fig. 4.
Rotenone inhibits secretion of TNF-α from astrocytes. Except group blank, the cells of all groups were treated with 10 μg/ml LPS for 48 h along with respective treatments. The cells of group blank were cultured for 48 h without any treatment. Control LPS alone, rotenone 20 nM, apyrase 5 U/ml, BzATP 100 μM. *Protenone vs. control < 0.05. †Protenone+BBG vs. rotenone < 0.05. ‡PBzATP vs. control < 0.01. §Protenone+BzATP vs. rotenone < 0.05
Table 3.
TNF-α concentration (C) in the culture supernatant of astrocytes
| Control | Rotenone | Rotenone and BBG | BzATP | BBG | Rotenone and BzATP | |
|---|---|---|---|---|---|---|
| C (pg/ml) | 66.56 ± 7.19 | 50.69 ± 6.37* | 76.70 ± 8.43† | 32.60 ± 3.62‡ | 80.37 ± 8.56 | 23.91 ± 3.03§ |
*Protenone vs. control < 0.05. †Protenone+BBG vs. rotenone < 0.05. ‡PBzATP vs. control < 0.01. §Protenone+BzATP vs. rotenone < 0.05
Discussion
According to previous reports, the expression of P2X7 receptor subunits in astrocytes is very heterogeneous [3]. Immunocytochemical studies have shown the localisation of P2X7 receptor subunits on astrocytes in various hippocampal subregions [14], in the cerebellum [15], in the nucleus accumbens after mechanical damage [16] and in the cerebral cortex after ischaemia [17]. In the latter two brain regions, P2X7 receptors were localised in GFAP-labelled reactive astrocytes [16, 17]. This preference of P2X7 receptor expression is consistent with our results. Furthermore, the subcellular distributions of P2X7 receptors are different in those brain regions. P2X7 receptors were primarily localised along astrocyte processes in the hippocampus [14], while P2X7 receptors were observed on the bodies of GFAP-labelled reactive astrocytes in the nucleus accumbens after mechanical damage, but no labelling was detected on the processes [16]. In the cerebral cortex after ischaemia, P2X7 receptor immunoreactivity was equally present on cell bodies and processes of astrocytes [17]. In our study, astrocytes were harvested from the ventral portion of the midbrain. Reactive astrocytes have long processes, but the expression of P2X7 receptors was not significantly different between cell bodies and processes. This pattern is similar to that reported in the cerebral cortex. In our system, BzATP induced inward currents in most of the astrocytes. This indicates that the P2X7 receptors we detected by immunocytochemistry were functionally expressed. This is inconsistent with the reports about astrocytes in the hippocampal CA1 subfield [18].
In order to study the influence of rotenone on P2X7 receptors in astrocytes of the midbrain, we treated the cells with different concentrations of rotenone for different time periods. It has been reported that the concentration of rotenone is about 20–30 nM in brains of rotenone PD models [19, 20]. Therefore, we chose 0, 2, 20 and 200 nM as working concentrations of rotenone. If the concentration was higher than 200 nM (e.g. we also tested 2 μM), most of the astrocytes died. Even at 200 nM, some of the cells still died and detached from the coverslip. This might be due to the cell toxicity of high concentrations of rotenone. P2X7 receptor expression was observed continuously for 72 h, including 24 h of cell recovery and 48 h of drug treatment. According to our results, 48-h rotenone treatment did not significantly affect the immunofluorescence signal intensity of P2X7 receptors. This suggests that rotenone may not affect the expression of P2X7 receptors. But this does not rule out the possibility that the function of P2X7 receptors was affected. Therefore, we used the patch clamp method to observe the P2X7 receptor currents. In our system, if astrocytes were cultured over 3 days after secondary plating, the majority of cells connected with each other. In the electrophysiological experiments, we found the existence of gap junctions, which resulted in inaccurate determination of capacitance and current. Therefore, we did not continue to observe the pattern of P2X7 receptor activity after 72 h.
According to our electrophysiological results, P2X7 receptor current density decreased after treatment with different concentrations of rotenone for 24 h. This may be the composite result of the following actions: First, as the culture time was prolonged, the astrocytes gradually returned to the resting state, with decreased P2X7 receptor expression. However, we did not see significant differences in the fluorescence signal density in the immunocytochemistry experiments. Western-blot analysis may more accurately determine changes in protein content. Second, although only the 200 nM group was significantly different from the 0 nM group, the decrease in current density still appeared to be concentration-dependent. This implies that rotenone might inhibit the current density of P2X7 receptors, but this effect was very limited. Third, 24 h of treatment may still not be long enough. Therefore, we investigated P2X7 receptor current density after 48 h treatment. Interestingly, current densities of the 2 and 20 nM groups actually increased. In brains of the rotenone PD model, the concentration of rotenone is between 20 and 30 nM [19, 20]. This concentration is known to partially inhibit mitochondrial complex I activity [21], and this level of complex I inhibition was insufficient to inhibit glutamate-supported respiration in brain mitochondria [22]. The activation of microglia was promoted by this concentration of rotenone [23], but not suppressed. So we speculate that the increase in P2X7 receptor current density might be associated with the appearance of re-activated astrocytes. We also found that there were many apoptotic cells in the groups treated with 200 nM rotenone for 48 h. The current data of these groups were obtained only from the cells with relatively normal resting membrane potentials (−50 ~ −68 mV). Therefore we cannot rule out the possibility that the decrease in current density of these groups was the result of toxicity due to high concentrations of rotenone. In addition, we compared the inactivation speed of P2X7 receptors in different groups and found that long-term treatment with rotenone not only increased current density, but also extended channel inactivation time.
P2X7 receptors play a regulatory role in the secretion of many cytokines [24]. For example, activation of P2X7 receptors in astrocytes inhibits TNF-α secretion [8]. We carried out ELISA experiments in order to investigate whether the enhancement of P2X7 receptor current density by 20 nM rotenone would affect the secretion of cytokines in astrocytes, such as TNF-α. The results showed that rotenone inhibited the secretion of TNF-α, and this effect could be reversed by the P2X7 receptor antagonist BBG. However, compared with the control group, BBG not only reversed the inhibition of rotenone, but even promoted the secretion. An explanation for this phenomenon may be that in the control group, astrocytes could secrete ATP without cell damage [25, 26]. Such ATP would inhibit TNF-α secretion from astrocytes themselves through native P2X7 receptors. When we treated the cells with rotenone and BBG, BBG blocked the P2X7 receptors, including both those induced by rotenone and the native receptors. Therefore, the secreted TNF-α concentration of this group was higher than that of the control group. Data from the BzATP group showed that direct activation of P2X7 receptors mimicked the inhibitiory effect of rotenone. This further indicates that rotenone inhibited the secretion of TNF-α by, at least partly, promoting P2X7 receptor activation.
Conclusions
P2X7 receptors were expressed in astrocytes cultured from the midbrain. Rotenone (20 nM) does not significantly affect the expression of P2X7 receptors following 48 h treatment, but did increase P2X7 receptor current density and extend channel inactivation time. Furthermore, TNF-α secretion can be inhibited by rotenone through P2X7 receptor activation.
Electronic supplementary material
Below is the link to the electronic supplementary material.
The contrast pictures of cultured astrocytes. a The arrow indicates the hypertrophic astrocyte with long process. b The arrow indicates the astrocyte with flat and polygonal structures. Bar = 50 μm (GIF 25 kb)
High resolution image file (TIFF 4451 kb)
Acknowledgements
Funding National Natural Science Foundation (30800430), National Key Basic Research Program (2006CB500702, 2007CB947100), The Ministry of Science and Technology of China (2009ZX09311-001).
Study design Cheng He, Zheng-Hua Xiang, Xiao-Fei Gao
Collection, analysis, and interpretation of data Xiao-Fei Gao, Wei Wang, Qiang Yu
Writing of the report Xiao-Fei Gao
Writing assistance Geoffrey Burnstock
Decision to submit Cheng He, Zheng-Hua Xiang
Contributor Information
Zheng-Hua Xiang, Email: zhxiang@hotmail.com.
Cheng He, Email: chenghe@smmu.edu.cn.
References
- 1.Levesque S, Wilson B, Gregoria V, Thorpe LB, Dallas S, Polikov VS, Hong JS, Block ML. Reactive microgliosis: extracellular micro-calpain and microglia-mediated dopaminergic neurotoxicity. Brain. 2010;133(Pt 3):808–821. doi: 10.1093/brain/awp333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sugama S, Takenouchi T, Cho BP, Joh TH, Hashimoto M, Kitani H. Possible roles of microglial cells for neurotoxicity in clinical neurodegenerative diseases and experimental animal models. Inflamm Allergy Drug Targets. 2009;8:277–284. doi: 10.2174/187152809789352249. [DOI] [PubMed] [Google Scholar]
- 3.Verkhratsky A, Krishtal OA, Burnstock G. Purinoceptors on neuroglia. Mol Neurobiol. 2009;39:190–208. doi: 10.1007/s12035-009-8063-2. [DOI] [PubMed] [Google Scholar]
- 4.Forno LS, DeLanney LE, Irwin I, Monte D, Langston JW. Astrocytes and Parkinson's disease. Prog Brain Res. 1992;94:429–436. doi: 10.1016/S0079-6123(08)61770-7. [DOI] [PubMed] [Google Scholar]
- 5.Mirza B, Hadberg H, Thomsen P, Moos T. The absence of reactive astrocytosis is indicative of a unique inflammatory process in Parkinson's disease. Neuroscience. 2000;95:425–432. doi: 10.1016/S0306-4522(99)00455-8. [DOI] [PubMed] [Google Scholar]
- 6.Sofroniew MV. Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci. 2009;32:638–647. doi: 10.1016/j.tins.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duan S, Neary JT. P2X7 receptors: properties and relevance to CNS function. Glia. 2006;54:738–746. doi: 10.1002/glia.20397. [DOI] [PubMed] [Google Scholar]
- 8.Kucher BM, Neary JT. Bi-functional effects of ATP/P2 receptor activation on tumor necrosis factor-alpha release in lipopolysaccharide-stimulated astrocytes. J Neurochem. 2005;92:525–535. doi: 10.1111/j.1471-4159.2004.02885.x. [DOI] [PubMed] [Google Scholar]
- 9.Duan S, Anderson CM, Keung EC, Chen Y, Chen Y, Swanson RA. P2X7 receptor-mediated release of excitatory amino acids from astrocytes. J Neurosci. 2003;23:1320–1328. doi: 10.1523/JNEUROSCI.23-04-01320.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bianco F, Perrotta C, Novellino L, Francolini M, Riganti L, Menna E, Saglietti L, Schuchman EH, Furlan R, Clementi E, Matteoli M, Verderio C. Acid sphingomyelinase activity triggers microparticle release from glial cells. EMBO J. 2009;28:1043–1054. doi: 10.1038/emboj.2009.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McNaught KS, Jenner P. Extracellular accumulation of nitric oxide, hydrogen peroxide, and glutamate in astrocytic cultures following glutathione depletion, complex I inhibition, and/or lipopolysaccharide-induced activation. Biochem Pharmacol. 2000;60:979–988. doi: 10.1016/S0006-2952(00)00415-9. [DOI] [PubMed] [Google Scholar]
- 12.McCarthy KD, Vellis J. Preparation of separate astroglial and oligodendroglial cell cultures from rat cerebral tissue. J Cell Biol. 1980;85:890–902. doi: 10.1083/jcb.85.3.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fernandes A, Falcão AS, Silva RF, Brito MA, Brites D. MAPKs are key players in mediating cytokine release and cell death induced by unconjugated bilirubin in cultured rat cortical astrocytes. Eur J Neurosci. 2007;25:1058–1068. doi: 10.1111/j.1460-9568.2007.05340.x. [DOI] [PubMed] [Google Scholar]
- 14.Kukley M, Barden JA, Steinhäuser C, Jabs R. Distribution of P2X receptors on astrocytes in juvenile rat hippocampus. Glia. 2001;36:11–21. doi: 10.1002/glia.1091. [DOI] [PubMed] [Google Scholar]
- 15.Carrasquero LM, Delicado EG, Bustillo D, Gutiérrez-Martín Y, Artalejo AR, Miras-Portugal MT. P2X7 and P2Y13 purinergic receptors mediate intracellular calcium responses to BzATP in rat cerebellar astrocytes. J Neurochem. 2009;110:879–889. doi: 10.1111/j.1471-4159.2009.06179.x. [DOI] [PubMed] [Google Scholar]
- 16.Franke H, Grosche J, Schädlich H, Krügel U, Allgaier C, Illes P. P2X receptor expression on astrocytes in the nucleus accumbens of rats. Neuroscience. 2001;108:421–429. doi: 10.1016/S0306-4522(01)00416-X. [DOI] [PubMed] [Google Scholar]
- 17.Franke H, Günther A, Grosche J, Schmidt R, Rossner S, Reinhardt R, Faber-Zuschratter H, Schneider D, Illes P. P2X7 receptor expression after ischaemia in the cerebral cortex of rats. J Neuropathol Exp Neurol. 2004;63:686–699. doi: 10.1093/jnen/63.7.686. [DOI] [PubMed] [Google Scholar]
- 18.Jabs R, Matthias K, Grote A, Grauer M, Seifert G, Steinhäuser C. Lack of P2X receptor mediated currents in astrocytes and GluR type glial cells of the hippocampal CA1 region. Glia. 2007;55:1648–1655. doi: 10.1002/glia.20580. [DOI] [PubMed] [Google Scholar]
- 19.Betarbet R, Sherer TB, MacKenzie G, Garcia-Osuna M, Panov AV, Greenamyre JT. Chronic systemic pesticide exposure reproduces features of Parkinson's disease. Nat Neurosci. 2000;3:1301–1306. doi: 10.1038/81834. [DOI] [PubMed] [Google Scholar]
- 20.Porter RH, Greene JG, Higgins DS, Jr, Greenamyre JT. Polysynaptic regulation of glutamate receptors and mitochondrial enzyme activities in the basal ganglia of rats with unilateral dopamine depletion. J Neurosci. 1994;14:7192–7199. doi: 10.1523/JNEUROSCI.14-11-07192.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davey GP, Clark JB. Threshold effects and control of oxidative phosphorylation in nonsynaptic rat brain mitochondria. J Neurochem. 1996;66:1617–1624. doi: 10.1046/j.1471-4159.1996.66041617.x. [DOI] [PubMed] [Google Scholar]
- 22.Sims NR. Rapid isolation of metabolically active mitochondria from rat brain and subregions using Percoll density gradient centrifugation. J Neurochem. 1990;55:698–707. doi: 10.1111/j.1471-4159.1990.tb04189.x. [DOI] [PubMed] [Google Scholar]
- 23.Ling Z, Chang QA, Tong CW, Leurgans SE, Lipton JW, Carvey PM. Rotenone potentiates dopamine neuron loss in animals exposed to lipopolysaccharide prenatally. Exp Neurol. 2004;190:373–383. doi: 10.1016/j.expneurol.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 24.Mingam R, Smedt V, Amédée T, Bluthé RM, Kelley KW, Dantzer R, Layé S. In vitro and in vivo evidence for a role of the P2X7 receptor in the release of IL-1 beta in the murine brain. Brain Behav Immun. 2008;22:234–244. doi: 10.1016/j.bbi.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bianco F, Pravettoni E, Colombo A, Schenk U, Möller T, Matteoli M, Verderio C. Astrocyte-derived ATP induces vesicle shedding and IL-1 beta release from microglia. J Immunol. 2005;174:7268–7277. doi: 10.4049/jimmunol.174.11.7268. [DOI] [PubMed] [Google Scholar]
- 26.Burnstock G. Physiology and pathophysiology of purinergic neurotransmission. Physiol Rev. 2007;87:659–797. doi: 10.1152/physrev.00043.2006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Below is the link to the electronic supplementary material.
The contrast pictures of cultured astrocytes. a The arrow indicates the hypertrophic astrocyte with long process. b The arrow indicates the astrocyte with flat and polygonal structures. Bar = 50 μm (GIF 25 kb)
High resolution image file (TIFF 4451 kb)