Abstract
G-protein-coupled receptor homo-oligomerization has been increasingly reported. However, little is known regarding the relationship between activation of the receptor and its association/conformational states. The mammalian olfactory receptors (ORs) belong to the G protein-coupled receptor superfamily. In this study, the homo-oligomerization status of the human OR1740 receptor and its involvement in receptor activation upon odorant ligand binding were addressed by co-immunoprecipitation and bioluminescence resonance energy transfer approaches using crude membranes or membranes from different cellular compartments. For the first time, our data clearly show that mammalian ORs constitutively self-associate into homodimers at the plasma membrane level. This study also demonstrates that ligand binding mediates a conformational change and promotes an inactive state of the OR dimers at high ligand concentrations. These findings support and validate our previously proposed model of OR activation/inactivation based on the tripartite odorant-binding protein-odorant-OR partnership.
Keywords: Biophysics, G-protein-coupled Receptors (GPCR), Membrane Proteins, Protein-Protein Interactions, Receptor Structure/Function, Bioluminescence Resonance Energy Transfer (BRET), Olfactory Receptor, Pharmacology
Introduction
The sense of smell endows mammals with the capacity to recognize and discriminate a large number of odorants. Animals rely on olfactory clues as an essential means for survival through food searching, avoidance of danger, and reproduction. The first critical step in odorant detection consists of odorant interaction with olfactory receptors (ORs),2 which are expressed in olfactory sensory neurons within the olfactory mucosa. Olfactory perception involves a combinatorial code in which one OR recognizes multiple odorants, and different odorants are recognized by different combinations of ORs (1). It is widely accepted that a single olfactory sensory neuron expresses only one OR gene (2). Besides this complex combinatorial coding of odors, involving odorant binding with various affinities, little is known about the molecular mechanisms underlying the initial step in odorant signal transduction. ORs belong to the large superfamily of the G protein-coupled receptors (GPCRs). In olfactory sensory neurons, the odorant-activated OR couples mainly to the Golf subunit of a heterotrimeric G protein, which initiates cellular signaling to generate the olfactory message (3). Odorants are generally volatile hydrophobic molecules and reach ORs through the aqueous olfactory mucus bathing the mucosa. This thin layer contains large amounts of odorant-binding proteins (OBPs), members of the lipocalin family (4). In insects, OBPs are thought to interact with ORs and play a scavenger role to maintain ORs in an active conformation (5). We have recently demonstrated a tripartite functional interaction between OBPs, odorants, and ORs in mammals (6).
GPCRs are widely documented to exist as self-associated dimers or higher order oligomers (7–9). GPCR oligomerization may play an important role in receptor trafficking to the cell surface and intracellular signaling. Kaupmann et al. (10) have thoroughly demonstrated that heterodimerization is required for GABAB receptor-1 and GABAB receptor-2 to ensure both plasma membrane targeting and functionality. In contrast, oligomerization occurs only upon ligand interaction at the cell surface for other GPCRs (11). In efforts to identify the GPCR association state, the relationship between activation and oligomerization remains to be understood for many GPCRs, including ORs.
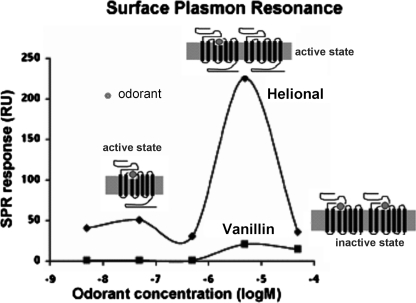
To date, no study has provided clear and undisputable evidence of OR dimerization, even though heterodimerization of ORs has been reported in rodents and insects (12–14). Some ORs, including human OR1740, were reported not to heterodimerize with a non-olfactory GPCR, the β2-adrenergic receptor (15). Our previous studies (6, 16, 17), in line with others (18, 19), showed a bell-shaped OR dose-response curve upon odorant stimulation. Several assumptions have been raised to elucidate the decreased OR response at high ligand concentrations (20–22). This observation could result either from a nonspecific inhibition of the response or from various association/activation states and conformational changes of ORs. Because protomer association can modulate GPCR functionality, we suggested that the activity of potential OR dimers could depend on the number of bound odorant ligands. Indeed, one bound odorant could activate the OR dimer, whereas two bound odorants, one on each protomer, could hinder signaling due to an inappropriate dimer conformation. We therefore proposed a model for OBP-odorant-OR ligand interactions based on two hypotheses, competitive binding of OBP and odorant to the OR and association of ORs into homodimers. This model explains the bell-shaped dose-response curve observed for receptor interaction with odorants in the absence of OBPs (Fig. 1). On the one hand, in support of this model, we already demonstrated the functional role of OBP in maintaining OR activity at high odorant concentrations (6). On the other hand, investigation of the association states of the OR is crucial to validate this whole mechanistic model of odorant detection by ORs.
FIGURE 1.
OR1740 functional response to Helional or vanillin stimulation as a function of odorant concentration (redrawn from Ref. 6). Shown is the differential SPR response to odorants relative to controls obtained by replacing odorant with water. RU, response units.
In this study, our goal was to determine whether mammalian ORs are present as homo-oligomers in living cells. For this purpose, we heterologously expressed the human OR1740 receptor under optimized experimental conditions in the yeast Saccharomyces cerevisiae (17, 23) and investigated its homo-oligomerization status. Besides a biochemical approach, we used bioluminescence resonance energy transfer (BRET), a well developed biophysical technology employed largely in the past decade to explore GPCR oligomerization to monitor not only OR homodimerization but also the odorant ligand-mediated conformational changes in the receptors. For the first time, our data show unambiguously that a human OR can form homodimers. Interestingly, the BRET approach also allowed us to establish that odorant ligand binding induces conformational changes in the OR dimers, which are compatible with their decreased functional response at high odorant concentrations.
MATERIALS AND METHODS
Odorant Solution Preparation
Stock solutions of octanal, vanillin (Sigma), and Helional (a gift from Givaudan-Roure, courtesy of B. Schilling, Dübendorf, Switzerland) were extemporaneously prepared in dimethyl sulfoxide at 10−1 m. 10−4 m odorant dilutions were performed in water or BRET buffer (1× PBS, 0.01% (w/v) MgSO4, and 0.1% (w/v) glucose) directly from the stock solution. Further dilutions (10−5–10−8 m) were prepared by successive dilutions in water or BRET buffer.
Plasmid Constructs
The yeast expression vectors pESC-URA and pESC-TRP (Stratagene) were modified as follows. Two multicloning sites containing restriction sites and a tag (c-Myc for BcMBAN/NABMcB and HA for BBSHAN/NAHSBB) were designed and synthesized as single strands (Eurofins MWG): BcMBAN, 5′-cccggatccatggaacagaagttgatttccgaagaagacctcctcagatctgcgatcgctagcccc-3′; NABMcB, 5′-ggggctagcgatcgcagatctgaggaggtcttcttcggaaatcaacttctgttccatggatccggg-3′; BBSHAN, 5′-cccggatccctgagatctactgcgatcgcatacccatacgatgttccagattacgcttaagctagcccc-3′; and NAHSBB, 5′-ggggctagcttaagcgtaatctggaacatcgtatgggtatgcgatcgcagtagatctcagggatccggg-3′. BcMBAN and NABMcB single-stranded DNAs or BBSHAN and NAHSBB single-stranded DNAs were heated to 94 °C for 5 min and then cooled to room temperature for annealing. pESC plasmids were digested by ClaI and NaeI, and protruding ends were filled in. Both pESC-URA and pESC-TRP plasmids were ligated and amplified in Escherichia coli. The new plasmids (pESC-del-URA and pESC-del-TRP) were double-digested with EcoRI and SpeI. The GAL4 gene was released by EcoRI and NheI from the pJH2 vector (24) and inserted into open pESC-del plasmids. After ligation, the plasmids were amplified in E. coli. The resulting plasmids (pESC-del-GAL4-URA and pESC-del-GAL4-TRP) were linearized by BamHI and NheI. The BcMBAN/NABMcB or BBSHAN/NAHSBB double-stranded DNA was ligated to the linearized plasmids to obtain cassette expression vectors for c-Myc-ORs or HA-ORs in the yeast S. cerevisiae.
OR1740 Fusion to Renilla Luciferase (Rluc) or Enhanced YFP (EYFP)
The fusion sequences of OR1740-Rluc and OR1740-EYFP were constructed and inserted into the pESC-URA or pESC-TRP yeast expression vector as follows. All primers used are listed in Table 1. OR1740 cDNA without a stop codon was PCR-amplified using the primer 1 sense and antisense sequences, introducing the unique BglII restriction site and a linker sequence extension onto which the EYFP or Rluc sequence could be added. The EYFP gene was site-mutated by a A206K replacement as described by Zacharias et al. (25) to avoid self-association of the proteins. EYFP and Rluc sequences to be fused to the OR1740 receptor were generated using primers 2 and 3, respectively, harboring the linker sequence and the unique AsiSI restriction site. OR1740-Rluc and OR1740-EYFP fusion constructs were generated by PCR amplification using mixtures of DNA fragments to be fused and primers 4 and 5, respectively. These fusions were digested by BglII and AsiSI and cloned into the BglII/AsiSI cloning site of the yeast expression vectors to obtain pESC-URA-c-Myc-OR1740-Rluc and pESC-TRP-c-Myc-OR1740-EYFP.
TABLE 1.
Primers used for PCR amplifications
The restriction sites are underlined, and the linker sequences are in boldface.
| Sequences (5′ to 3′) | Restriction site | |
|---|---|---|
| Primer 1 | ||
| Sense | GGACCAAGATCTCAGCCAGAATCTGGGGCCAATGGA | BglII |
| Antisense | GCACCGTCACCAGCCAGTGACCGTCTCCCTGTGA | |
| Primer 2 | ||
| Sense | ACTGGCTGGTGACGGTGCTGGTTTATTGGTGAGCAAGGGCGAGGA | |
| Antisense | ACCTCGGCGATCGCTTACTTGTACAGCTCGTCCATGCC | AsiSI |
| Primer 3 | ||
| Sense | ACTGGCTGGTGACGGTGCTGGTTTATTGACTTCGAAAGTTTATGATCCA | |
| Antisense | ACCTCGGCGATCGCTTATTGTTCATTTTTGAGAACTCGCTC | AsiSI |
| Primer 4 | ||
| Sense | GGACCAAGATCTCAGCCAGAATCTGGGGCCAATGGA | BglII |
| Antisense | ACCTCGGCGATCGCTTACTTGTACAGCTCGTCCATGCC | AsiSI |
| Primer 5 | ||
| Sense | GGACCAAGATCTCAGCCAGAATCTGGGGCCAATGGA | BglII |
| Antisense | ACCTCGGCGATCGCTTATTGTTCATTTTTGAGAACTCGCTC | AsiSI |
Transformation and Yeast Growth
The S. cerevisiae yeast strain MC18 (17) was transformed with expression vectors pESC-URA-c-Myc-OR1740-Rluc, pESC-TRP-c-Myc-OR1740-EYFP, and pRGP-Golf (26) for BRET experiments or with pESC-TRP-c-Myc-OR1740, pESC-TRP-HA-OR1740, and pRGP-Golf for co-immunoprecipitation (27). The transformed cells were grown and induced at 15 °C for 108 h for protein production as described previously (17).
Crude Membrane Preparation
All steps were performed at 4 °C unless indicated otherwise. Transformed yeast cells were harvested, washed twice with ice-cold water, and resuspended in ice-cold lysis buffer A (50 mm Tris-HCl (pH 7.5), 1 mm EDTA, 0.1 mm PMSF, and 250 mm sorbitol) and Complete protease inhibitor mixture (Roche Applied Science) prior to membrane preparation as described previously (6, 17). The protein concentration of the preparation was determined using the BCA reagent (Pierce) with bovine serum albumin as a standard.
Subcellular Fractionation
Crude membranes containing 2–5 mg/ml protein in 500 μl of 10% sucrose buffer B (10 mm Tris-HCl (pH 7.5), 1 mm EDTA, 1 mm DTT, and Complete protease inhibitor mixture) were applied on top of a 11-ml 30–70% (w/v) continuous sucrose gradient according to the method of Eraso et al. (28) with slight modifications. After centrifugation for 16 h at 30,000 rpm in a Beckman SW 41 Ti rotor at 4 °C, a total of 12 subfraction samples were successively collected from the top (light membrane vesicular fractions) to the bottom (plasma membrane) of the tube.
Samples of identical volumes taken from the fractions were immunoblotted to check the efficiency of the sucrose gradient to sort the various membranes, notably the endoplasmic reticulum (ER) and plasma membranes. To perform BRET assays, sucrose was removed from the subfractions by dilution in buffer B without sucrose and additional centrifugation at 30,000 rpm for 1 h at 4 °C. Each pellet was resuspended in buffer B. Protein concentration was measured in each fraction as described above.
Co-immunoprecipitation
500 μg of crude membranes prepared from yeast cells expressing c-Myc-OR1740 alone or with HA-OR1740 were immunoprecipitated using anti-c-Myc antibody (Roche Diagnostics) at 10 μg/ml according to the manufacturer's instructions (Pierce Classic IP kit). 10 μl of the anti-c-Myc antibody-immunoprecipitated samples were analyzed by immunoblotting using anti-HA antibody (Cell Signaling). The same experiment was done using crude membranes prepared from yeast cells expressing HA-OR1740 alone or with c-Myc-OR1740 and anti-HA antibody for immunoprecipitation and anti-c-Myc antibody for immunoblotting.
Immunoblotting
Homogenized crude membranes (5 μg of proteins), subfractionated membranes (5 μg of proteins) from a sucrose gradient, or immunoprecipitates were separated by 10% SDS-PAGE and electrotransferred onto Immobilon-P transfer membranes (Millipore). Membranes were blocked with 4.5% non-fat dry milk in buffer C (1× PBS and 0.1% Tween 20). Membranes were hybridized with anti-c-Myc antibody (for crude and subfractionated membranes), anti-HA antibody (for immunoprecipitates), or antibody targeting the yeast plasma membrane marker Pma1 (Abcam) or the yeast ER marker Dpm1p (Molecular Probes). Peroxidase-conjugated anti-mouse and anti-rabbit IgG (Sigma) were used as secondary antibodies. The enhanced chemiluminescence reaction was performed using the ECL Plus reagent (PerkinElmer Life Sciences).
Surface Plasmon Resonance (SPR) Measurements
Prior to SPR experiments, membrane fractions were sonicated for 15 min at 2 × 160 watts and 35 kHz (Sonoclean S2600 sonicator, LABO-MODERNE, Paris, France) in ice-cold water. This yields membrane nanosomes of uniform size of ∼50 nm in diameter (29). Real-time binding kinetic experiments were conducted on a Biacore 3000 system (GE Healthcare). 0.03 mg/ml yeast nanosomes carrying ORs were immobilized via their lipid bilayer on an L1 sensor chip (GE Healthcare). For this, nanosomes diluted at 0.3 μg/ml total protein in buffer C (10 mm HEPES (pH 7.4) containing 150 mm NaCl, 3 mm EDTA, 0.005% surfactant P20 (GE Healthcare), and 0.005% dimethyl sulfoxide) were injected for 20 min at 1 μl/min. The immobilized layer obtained was washed with buffer C (standby procedure) over several hours to obtain a stable signal. For functional tests, a solution of Helional at 5 μm and GTPγS at 10 μm in HEPES-buffered saline was injected at 10 μl/min over the captured nanosomes for 4 min, and dissociation was registered for 15 min after the end of odorant injection. In control experiments, stimulation was carried out using solutions in which the odorant had been replaced with water. Regeneration of the chip surface was achieved by several 2-min injections of 20 mm CHAPS. The sensorgram observed in the experiment with Helional was corrected by subtracting the response observed in the control experiment with water. All measurements were performed at 20 °C. Sensorgrams were analyzed using BIAevaluation software.
BRET Assays
3 × 107 yeast cells expressing c-Myc-OR1740-Rluc (BRET donors) alone or with c-Myc-OR1740-EYFP (BRET acceptors) were pelleted and disrupted with a 3-min incubation successively in an isopropyl alcohol/dry ice bath (−25 °C) and a 25 °C water bath. Disrupted cells were then resuspended in BRET buffer. Crude membranes from the same yeast cells or subfractionated membranes were also used for BRET assays. Disrupted yeast cells or membranes carrying 10 μg of proteins were then distributed in a white 96-well microplate (Nunc). Coelenterazine-h substrate (Promega) was added at a final concentration of 5 μm in the BRET buffer. Emitted luminescence and fluorescence were measured simultaneously using a TriStar LB 941 multimode reader (Berthold), with emission filters at 485 and 530 nm, respectively. Luminescence emission was checked and is similar for the various samples tested (membranes from yeast expressing OR1740-Rluc alone or coexpressing OR1740-Rluc and various amounts of OR1740-EYFP). The BRET ratio was expressed as the emission at 530 nm to the emission at 485 nm (30), and the normalized BRET ratio was deduced as the BRET ratio for OR1740-Rluc and OR1740-EYFP minus the BRET ratio for OR1740-Rluc alone (mBRET = (530/485 (OR-Rluc + OR-YFP) − 530/485 (OR1740-Rluc)) × 1000). Immunoblot analysis using anti-c-Myc antibody was performed with membranes carrying c-Myc-OR1740-Rluc and c-Myc-OR1740-EYFP to evaluate the OR1740-EYFP/OR1740-Rluc ratio for the BRET saturation assay.
RESULTS
OR Homodimerization Assessment by Co-immunoprecipitation
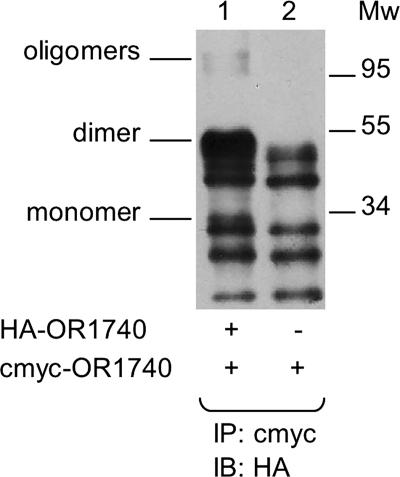
Immunoblots of membranes from yeast cells expressing c-Myc-OR1740 show two bands with apparent molecular masses of 27 and 49 kDa (Fig. 2). These bands have been suggested to correspond to the monomer and dimer forms of the OR1740 receptor, respectively (31). To investigate OR homodimerization, we then co-immunoprecipitated potential OR1740 oligomers using crude membranes prepared from yeast cells coexpressing c-Myc-OR1740 and HA-OR1740. As shown in Fig. 3, nonspecific bands appeared common to both the co-immunoprecipitated proteins (lane 1) and the control sample (lane 2), but additional bands corresponding to the monomer, dimer, and oligomer forms of the OR1740 receptor were specifically present in the co-immunoprecipitated proteins. Thus, these results demonstrate that HA-OR1740 co-immunoprecipitated with c-Myc-OR1740, supporting the hypothesis that ORs exist as homodimers in the cells. We performed the cross-study using anti-HA antibody for immunoprecipitation and anti-c-Myc antibody to reveal the immunoblots. Again, we found that c-Myc-OR1740 co-immunoprecipitated with HA-OR1740, but we could not prevent the presence of nonspecific bands (data not shown).
FIGURE 2.
Visualization by immunoblotting of the c-Myc-OR1740 receptor heterologously expressed in S. cerevisiae. Expression of the receptor was induced by galactose for 108 h at 15 °C. The receptor was detected using anti-c-Myc monoclonal antibody. The bands near 34 and 55 kDa correspond to the monomer and dimer forms of the OR1740 receptor, respectively.
FIGURE 3.
OR1740 receptor homo-oligomerization monitored by co-immunoprecipitation. Crude membranes from S. cerevisiae coexpressing c-Myc-OR1740 and HA-OR1740 (lane 1) or expressing c-Myc-OR1740 alone (lane 2) were immunoprecipitated (IP) using anti-c-Myc antibody. The immunoprecipitated proteins were resolved by SDS-PAGE and immunoblotted (IB) using anti-HA antibody. Molecular mass markers are shown in kilodaltons.
OR Homo-oligomerization Assessment by BRET
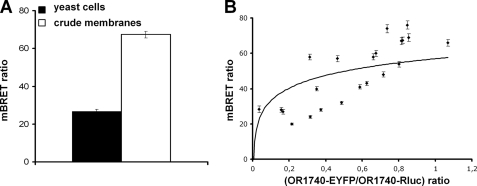
Our initial results based on immunoblotting and co-immunoprecipitation analyses support the hypothesis that ORs self-associate. However, these qualitative techniques suffer from a lack of precision as to the nature of this association, either promiscuity-driven or specific dimerization. We therefore took advantage of the BRET technique, a tool based on energy transfer, to elucidate the specificity of the association of ORs heterologously expressed in yeast cells. BRET assays were conducted either with disrupted yeast cells coexpressing OR1740-Rluc (donors) and OR1740-EYFP (acceptors) or with crude membranes prepared from these cells. We checked by SPR measurement upon ligand stimulation (6, 17) that the OR1740 receptor fused to Rluc and the OR1740 receptor fused to EYFP are functional as OR1740 (data not shown). BRET signals are reported in Fig. 4A. Significant BRET signals were measured, providing evidence that ORs exist in homo-association form. Furthermore, the BRET level was higher for experiments carried out with membranes compared with experiments using whole disrupted yeast cells.
FIGURE 4.
Investigation of OR1740 receptor homo-oligomerization by BRET. A, the BRET level was measured either in disrupted yeast cells coexpressing OR1740-Rluc (donor) and OR1740-EYFP (acceptor) or in crude membranes prepared from these cells with 5 μm coelenterazine-h. The data are shown as the normalized BRET ratio. B, BRET assays were performed with clones with variable acceptor (OR1740-EYFP)/donor (OR1740-Rluc) ratios. BRET levels are plotted as a function of the acceptor/donor ratio, estimated from immunoblots as described under “Materials and Methods.” Data are representative of at least three independent experiments.
Random clustering of the ORs arising from an excessive expression level could result in a nonspecific bystander energy transfer interaction and thus in a nonspecific BRET signal (32, 33). To address this question, BRET measurements were performed as a function of the amount of acceptor (OR1740-EYFP) relative to the donor (OR1740-Rluc), starting from many different yeast clonal populations. Immunoblotting was performed to evaluate the relative amount of OR1740-Rluc and OR1740-EYFP proteins within crude membranes from these clones. The BRET signal increased as a function of the OR1740-EYFP/OR1740-Rluc ratio, with a trend toward saturation at high ratios (Fig. 4B), suggesting a specific interaction of the receptors.
Homo-oligomerization of the OR1740 Receptor at the Subcellular Level
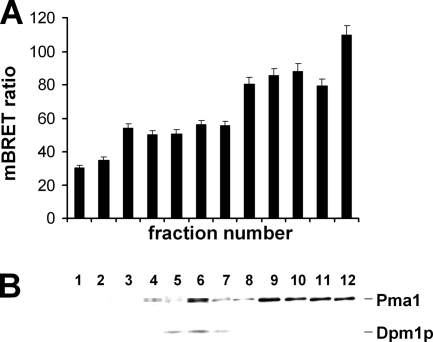
OR oligomerization may occur at different levels of OR expression. It may be required for OR maturation, folding, and plasma membrane targeting or to allow or modulate the response of the OR to its odorant ligand. We thus investigated whether OR oligomerization takes place as early as the ER level or later, at the plasma membrane level. For this purpose, BRET was measured in subcellular membrane fractions obtained from crude membranes of yeast cells coexpressing OR1740-Rluc and OR1740-EYFP and fractionated following the sucrose gradient procedure described under “Materials and Methods.” The subcellular fractions were analyzed by immunoblotting with antibodies targeting the yeast ER and plasma membrane markers Dpm1p and Pma1 (Fig. 5B) to qualify their cellular origin. A BRET signal was measurable in all fractions from the ER to the plasma membrane (Fig. 5A). Interestingly, BRET levels significantly increased from inner fractions to plasma membrane fractions.
FIGURE 5.
Investigation of OR1740 receptor homo-oligomerization at the subcellular level by BRET. A, the BRET level was measured in each cellular membrane subfraction, obtained by sucrose gradient fractionation from crude membranes of yeast cells coexpressing OR1740-Rluc and OR1740-EYFP. B, all subfractions were analyzed by immunoblotting with antibodies targeting the yeast ER and plasma membrane markers Dpm1p (30 kDa) and Pma1 (100 kDa), respectively.
Ligand-mediated Conformational Change in OR1740 Dimers
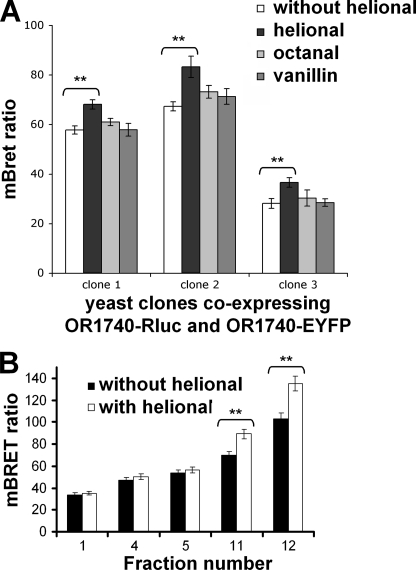
Because the self-association of the OR1740 receptor was demonstrated to take place without ligand, it is constitutive and not ligand-induced. The BRET technique has already been employed to investigate a possible conformational change induced by ligand binding on receptor dimers (34). We thus investigated whether Helional, the preferential agonist of OR1740, induced a conformational change in the OR1740 dimer resulting in a change in the BRET level. For this purpose, we performed BRET experiments with crude membrane preparations in the presence or absence of Helional. Helional at 10−5 m was used because this concentration was previously shown to induce a large response from the OR1740 receptor (35). As shown in Fig. 6A, Helional stimulation of the OR1740 receptor significantly increased the BRET level as compared with the pre-existing level. In contrast, octanal and vanillin, which do not activate the OR1740 receptor (36), did not promote such a change in the BRET signal (Fig. 6A). The Helional-induced increase in resonance energy transfer may result from an OR conformational change bringing the BRET donors and acceptors closer to each other or in a favorable orientation. To further investigate the ligand-mediated conformational changes at the subcellular level, BRET was measured in membrane subfractions with or without Helional stimulation. In the presence of Helional, the BRET level exhibited a significant increase only in plasma membrane fractions, whereas it remained unchanged at a basal level in inner and ER fractions (Fig. 6B). It thus seems that only the mature receptors having reached the plasma membrane exhibit a ligand-induced BRET signal modification due to their interaction with a ligand. This correlates with the functional activity of the receptor at this location (34).
FIGURE 6.
Odorant-induced BRET modulation. A, crude membranes from three different clones coexpressing OR1740-Rluc and OR1740-EYFP were used to perform BRET assays without or with odorants (Helional as an OR1740 agonist and octanal and vanillin as negative controls). B, the BRET level was measured in membrane subfractions 1, 4, and 5 (ER) and membrane subfractions 11 and 12 (plasma membrane) from clone 2 with Helional and without odorant as a reference. **, significant differences estimated by Student's t test (p < 0.05). Data are representative of three independent experiments.
Ligand-induced BRET Modulation Correlates with the Ligand-induced OR Activity Level
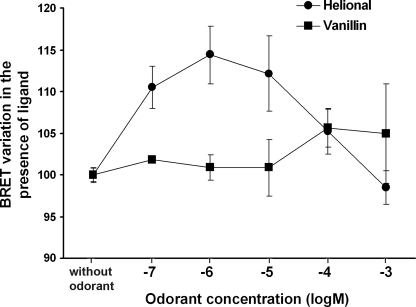
Our group previously demonstrated a bell-shaped dose response of OR1740 to its odorant ligand (17) using measurements of the functional interaction of the ligand with the receptor by SPR experiments. We proposed a model in which the decrease in the OR1740 response at high Helional concentrations was ascribed to a ligand-induced conformational change in the OR1740 dimer resulting in receptor inactivation, whereas the response reached a maximum at intermediate Helional concentrations (6). Here, we monitored the variation in the BRET level upon odorant stimulation with various concentrations of Helional. As was observed in our previous SPR experiments (6), the variation in the BRET level upon odorant stimulation, plotted as a function of Helional concentration, exhibits a bell-shaped curve (Fig. 7). It therefore appears that the OR1740 dimer conformational changes induced by various Helional concentrations elicit an evolution in the BRET level that correlates with that of the functional response. The most functionally active dimer states, i.e. at intermediate Helional concentrations (10−6–10−5 m), result in the largest BRET increase, whereas the inactive dimer states, at the highest Helional concentrations, induce no change in the basal BRET level. These observations tend to confirm our model, according to which dimer activity or inactivity depends on ligand concentration, through the level of ligand-binding site occupancy, which mediates conformational changes toward active or inactive states.
FIGURE 7.
BRET level variation upon OR1740 stimulation with various Helional concentrations. BRET measurements were performed using crude membranes from yeast cells coexpressing OR1740-Rluc and OR1740-EYFP or expressing OR1740-Rluc alone. Receptors were stimulated with various concentrations of Helional. Results are expressed as the relative variation in the BRET level at the various odorant concentrations. Data are representative of three independent experiments.
DISCUSSION
A review by Gurevich and Gurevich (37), elegantly titled “How and Why Do GPCRs Dimerize?,” has tackled a question of great importance. In the past decade, a corpus of studies (GPCR-OKB Database) has documented GPCR oligomerization states, which are thought to play a crucial role in the maturation pathway and plasma membrane targeting of receptors. Some GPCRs oligomerize upon ligand binding, whereas others, such as GABAB and CCR5 receptors, associate earlier during the maturation pathway. Controversial statements have been issued over the proper expression, localization, and function of GPCR oligomers (38, 39). Many assumptions have been made, but few studies thoroughly demonstrate that GPCR oligomerization takes place early in the biosynthetic process and that GPCRs are targeted to the plasma membrane as constitutive oligomers (40–43). Up to now, no consensus has emerged from the literature about the relationship between GPCR oligomerization status and receptor activation (20, 43, 44). In particular, little is presently known about OR self-association, although ORs constitute the largest GPCR subfamily. However, some authors have pointed out the implication of the association of some ORs with other non-olfactory GPCRs to reach the cell surface (12, 14, 45). Some reports support a strong relationship between ligand binding and GPCR oligomer signaling (46, 47). We have proposed a model based on two hypotheses: (i) a tripartite interaction between OBPs, odorants, and ORs (6) and (ii) an activation state of ORs depending on their dimerization status and the number of bound ligands. Indeed, one odorant ligand binding to the dimers would favor an active form of the OR. In contrast, OR dimers with two odorant molecules, each binding to a receptor protomer, would blunt signaling due to a dimer conformation inappropriate for signaling. The first assumption was investigated in the framework of this model and validated (6). It was concluded that OBP plays a crucial role in maintaining OR activity at high odorant concentrations.
Our present results using co-immunoprecipitation and BRET approaches demonstrate that the human OR1740 receptor exists as a non-ligand-induced homodimer in yeast cells. Indeed, both disrupted yeast cells heterologously expressing OR1740 fused to Rluc and OR1740 fused to EYFP and the membrane fraction prepared therefrom exhibited BRET signals demonstrating OR1740 receptor self-association. Interestingly, the highest BRET level (by a factor of 2.5) was reached with the membrane preparations relative to disrupted yeast cells (Fig. 4A). Rather than ascribing this phenomenon to some receptor enrichment in the membrane fraction, we infer that clearing disrupted cells of debris and cytoplasm content could result in a decrease in the occurrence of phenomena that quench the bioluminescence emission and lower the BRET signal.
Reaching a sufficient expression level in heterologous systems is crucial for monitoring GPCR oligomerization, but overexpression can elicit random clustering of the receptors and their nonspecific self-association at densities high enough to elicit nonspecific bystander energy transfer and thus nonspecific BRET signals (11, 32–34). It is possible to distinguish between these bystander effects and those due to specific protomer association by undertaking systematic studies of BRET or FRET levels against donor and acceptor densities (32, 48). Indeed, specific BRET (or FRET) depends on the acceptor/donor ratio but not on donor and acceptor surface densities, whereas nonspecific BRET arising from random clustering varies with acceptor density and is insensitive to the acceptor/donor ratio. In addition, the increase in specific BRET with acceptor/donor ratio should saturate at a level determined by the intrinsic stoichiometry of the receptor complex. We therefore performed BRET measurements as a function of the amount of acceptor (OR1740-EYFP) relative to the donor (OR1740-Rluc). For this purpose, we faced a major difficulty: because yeast cells self-regulate gene expression, they could not be made to express increasing amounts of acceptors while the donor amount remained fixed. Thus, the saturation plateau investigation was limited by the natural ratio observed in the various clones studied. This limitation is often bypassed in other studies through the use of mammalian cells, which allows modulation of expression levels by varying the amount of transfected cDNA (39, 43). However, despite these limitations, the BRET assay showed a saturation curve tendency as a function of the OR1740-EYFP/OR1740-Rluc ratio (Fig. 4B). This indicates the presence of a specific component in the interaction of receptors (33, 43).
In our previous work (6), we demonstrated a tripartite interaction between OBPs, odorants, and ORs, in which OBPs play a crucial role in preserving OR activity at high odorant concentrations. However, the OBP-odorant-OR partnership is not sufficient to fully explain the complexity related to the molecular mechanisms of OR-odorant interactions and signaling. One of the major events in GPCR activation is the receptor conformational change occurring upon ligand binding. This conformational change inducing signaling downstream of the receptors has been reviewed recently (49). BRET studies have been used to prove that ligands can mediate conformational changes in constitutive receptor oligomers such as the MT2 receptor, CCR5, and δ-opioid receptors (34, 39, 50). In an additional approach to prove the specificity of the receptor interaction to form dimers, we thus monitored the BRET signals upon odorant ligand stimulation (Fig. 6). Stimulation with Helional significantly increased the BRET level compared with the pre-existing BRET (Fig. 6A). In contrast, vanillin and octanal, which do not activate the OR1740 receptor (36), did not significantly change the BRET signal. This result further confirms the specificity of the self-association of the OR1740 receptor in the yeast cells through the conformational change upon ligand binding. Interestingly, the BRET levels measured increased from ER membrane fractions to the plasma membrane fractions. Our observation differs from that reported by Issafras et al. (39), who detected comparable BRET signals from the CCR5 receptor expressed in ER and plasma membrane fractions resolved on a sucrose gradient. The effect we observed may be interpreted in terms of maturation and/or localization. The BRET increase suggests that the ratio of OR dimers relative to monomeric species could be larger at the plasma membrane level or that the conformation of the dimers could evolve from the ER to the plasma membrane, being more suitable for BRET at the plasma membrane level. Receptor maturation along the expression pathway may participate in this evolution. However, it must be stressed that, as the receptor density is probably higher in the ER fractions than at the plasma membrane, the lower BRET signals in the ER may well correspond, in whole or in part, to a nonspecific component. In all cases, it is difficult to make conclusions regarding the oligomerization state of the receptor at the early stages of its biosynthesis. We observed that only the BRET measured in the plasma membrane fractions increased in the presence of Helional, whereas that measured in the ER fractions remained unchanged (Fig. 6B). The increase in the BRET level in the plasma membrane fraction upon ligand exposure may be ascribed to the ORs being mature at the cell surface, a compulsory condition for adequate signaling. Both the oligomerization and ligand-induced conformational changes in GPCRs are known to significantly modulate (increase or decrease) the FRET and BRET signals (9, 11, 43, 44, 51). Here, ligand binding clearly induced a conformational change in OR1740 receptors, thus eliciting changes in distance or relative orientation between the donor and acceptor, enhancing energy transfer efficiency.
The bell-shaped curve of BRET level variation upon stimulation with increasing Helional concentrations is compatible with the bell-shaped curve exhibited by the OR functional response in our previous experiments (6, 16, 17). Ligand-mediated conformational rearrangement of the ORs within the dimers is a plausible explanation that fits into the two-state model (active and inactive states) of OR dimers depending on ligand concentration. Indeed, the decrease in the functional response at high Helional concentrations can be ascribed to ligand-induced conformational change in the protomers within the OR1740 dimer resulting in dimer inactivation and is corroborated by ligand-induced BRET variation.
In conclusion, in this work, we have demonstrated that the human OR1740 receptor exists as a constitutive homo-oligomer, which expands the growing list of GPCR oligomers. As for other receptors (GABAB, human luteinizing hormone receptor, and CCR5), OR1740 receptor dimers seem to be formed early in the ER, which may play a role in quality control of receptor biosynthesis, trafficking to the cell surface, and functional signaling. Our results also show that a ligand-mediated conformational rearrangement occurs at the level of the receptor dimers, modulating the pre-existing BRET signals. Although we associate the decrease in the response at high odorant concentrations with the homo-oligomerization status of the OR1740 receptor, the physiological relevance of such an association remains to be fully elucidated.
Acknowledgments
We are grateful to Ralf Jockers and Jean-Louis Banères for fruitful advice.
This work was supported by the Institut National de la Recherche Agronomique (INRA) and Agence Nationale de la Recherche (New Olfactory SEnsors) Grant ANR-07-PCVI-0027-01.
- OR
- olfactory receptor
- GPCR
- G protein-coupled receptor
- OBP
- odorant-binding protein
- BRET
- bioluminescence resonance energy transfer
- Rluc
- Renilla luciferase
- EYFP
- enhanced YFP
- ER
- endoplasmic reticulum
- SPR
- surface plasmon resonance
- GTPγS
- guanosine 5′-3-O-(thio)triphosphate.
REFERENCES
- 1. Malnic B., Hirono J., Sato T., Buck L. B. (1999) Cell 96, 713–723 [DOI] [PubMed] [Google Scholar]
- 2. Mombaerts P. (2004) Curr. Opin. Neurobiol. 14, 31–36 [DOI] [PubMed] [Google Scholar]
- 3. Firestein S. (2001) Nature 413, 211–218 [DOI] [PubMed] [Google Scholar]
- 4. Steinbrecht R. A. (1998) Ann. N.Y. Acad. Sci. 855, 323–332 [DOI] [PubMed] [Google Scholar]
- 5. Xu P. (2005) Science 310, 798–799 [DOI] [PubMed] [Google Scholar]
- 6. Vidic J., Grosclaude J., Monnerie R., Persuy M. A., Badonnel K., Baly C., Caillol M., Briand L., Salesse R., Pajot-Augy E. (2008) Lab. Chip 8, 678–688 [DOI] [PubMed] [Google Scholar]
- 7. Park P. S., Filipek S., Wells J. W., Palczewski K. (2004) Biochemistry 43, 15643–15656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Duncan R. R., Bergmann A., Cousin M. A., Apps D. K., Shipston M. J. (2004) J. Microsc. 215, 1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pfleger K. D., Eidne K. A. (2005) Biochem. J. 385, 625–637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kaupmann K., Malitschek B., Schuler V., Heid J., Froestl W., Beck P., Mosbacher J., Bischoff S., Kulik A., Shigemoto R., Karschin A., Bettler B. (1998) Nature 396, 683–687 [DOI] [PubMed] [Google Scholar]
- 11. Milligan G., Wilson S., López-Gimenez J. F. (2005) J. Mol. Neurosci. 26, 161–168 [DOI] [PubMed] [Google Scholar]
- 12. Hague C., Hall R. A., Minneman K. P. (2004) Mol. Interv. 4, 321–322 [DOI] [PubMed] [Google Scholar]
- 13. Neuhaus E. M., Gisselmann G., Zhang W., Dooley R., Störtkuhl K., Hatt H. (2005) Nat. Neurosci. 8, 15–17 [DOI] [PubMed] [Google Scholar]
- 14. Bush C. F., Jones S. V., Lyle A. N., Minneman K. P., Ressler K. J., Hall R. A. (2007) J. Biol. Chem. 282, 19042–19051 [DOI] [PubMed] [Google Scholar]
- 15. Hague C., Uberti M. A., Chen Z., Bush C. F., Jones S. V., Ressler K. J., Hall R. A., Minneman K. P. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 13672–13676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sanz G., Schlegel C., Pernollet J. C., Briand L. (2005) Chem. Senses 30, 69–80 [DOI] [PubMed] [Google Scholar]
- 17. Minic J., Persuy M. A., Godel E., Aioun J., Connerton I., Salesse R., Pajot-Augy E. (2005) FEBS J. 272, 524–537 [DOI] [PubMed] [Google Scholar]
- 18. Araneda R. C., Peterlin Z., Zhang X., Chesler A., Firestein S. (2004) J. Physiol. 555, 743–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ko H. J., Park T. H. (2006) Biol. Chem. 387, 59–68 [DOI] [PubMed] [Google Scholar]
- 20. Springael J. Y., Urizar E., Costagliola S., Vassart G., Parmentier M. (2007) Pharmacol. Ther. 115, 410–418 [DOI] [PubMed] [Google Scholar]
- 21. Han Y., Moreira I. S., Urizar E., Weinstein H., Javitch J. A. (2009) Nat. Chem. Biol. 5, 688–695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chabre M., Deterre P., Antonny B. (2009) Trends Pharmacol. Sci. 30, 182–187 [DOI] [PubMed] [Google Scholar]
- 23. Pajot-Augy E., Crowe M., Levasseur G., Salesse R., Connerton I. (2003) J. Recept. Signal Transduct. Res. 23, 155–171 [DOI] [PubMed] [Google Scholar]
- 24. Price L. A., Kajkowski E. M., Hadcock J. R., Ozenberger B. A., Pausch M. H. (1995) Mol. Cell. Biol. 15, 6188–6195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zacharias D. A., Violin J. D., Newton A. C., Tsien R. Y. (2002) Science 296, 913–916 [DOI] [PubMed] [Google Scholar]
- 26. Crowe M. L., Perry B. N., Connerton I. F. (2000) J. Recept. Signal Transduct. Res. 20, 61–73 [DOI] [PubMed] [Google Scholar]
- 27. Schiestl R. H., Dominska M., Petes T. D. (1993) Mol. Cell. Biol. 13, 2697–2705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Eraso P., Mazón M. J., Portillo F. (2006) Biochim. Biophys. Acta 1758, 164–170 [DOI] [PubMed] [Google Scholar]
- 29. Vidic J., Pla-Roca M., Grosclaude J., Persuy M. A., Monnerie R., Caballero D., Errachid A., Hou Y., Jaffrezic-Renault N., Salesse R., Pajot-Augy E., Samitier J. (2007) Anal. Chem. 79, 3280–3290 [DOI] [PubMed] [Google Scholar]
- 30. Angers S., Salahpour A., Joly E., Hilairet S., Chelsky D., Dennis M., Bouvier M. (2000) Proc. Natl. Acad. Sci. U.S.A. 97, 3684–3689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cook B. L., Ernberg K. E., Chung H., Zhang S. (2008) PLoS One 3, e2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Milligan G., Bouvier M. (2005) FEBS J. 272, 2914–2925 [DOI] [PubMed] [Google Scholar]
- 33. Bacart J., Corbel C., Jockers R., Bach S., Couturier C. (2008) Biotechnol. J. 3, 311–324 [DOI] [PubMed] [Google Scholar]
- 34. Ayoub M. A., Couturier C., Lucas-Meunier E., Angers S., Fossier P., Bouvier M., Jockers R. (2002) J. Biol. Chem. 277, 21522–21528 [DOI] [PubMed] [Google Scholar]
- 35. Vidic J. M., Grosclaude J., Persuy M. A., Aioun J., Salesse R., Pajot-Augy E. (2006) Lab. Chip 6, 1026–1032 [DOI] [PubMed] [Google Scholar]
- 36. Jacquier V., Pick H., Vogel H. (2006) J. Neurochem. 97, 537–544 [DOI] [PubMed] [Google Scholar]
- 37. Gurevich V. V., Gurevich E. V. (2008) Trends Pharmacol. Sci. 29, 234–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Margeta-Mitrovic M., Jan Y. N., Jan L. Y. (2000) Neuron 27, 97–106 [DOI] [PubMed] [Google Scholar]
- 39. Issafras H., Angers S., Bulenger S., Blanpain C., Parmentier M., Labbé-Jullié C., Bouvier M., Marullo S. (2002) J. Biol. Chem. 277, 34666–34673 [DOI] [PubMed] [Google Scholar]
- 40. Terrillon S., Durroux T., Mouillac B., Breit A., Ayoub M. A., Taulan M., Jockers R., Barberis C., Bouvier M. (2003) Mol. Endocrinol. 17, 677–691 [DOI] [PubMed] [Google Scholar]
- 41. Salahpour A., Angers S., Mercier J. F., Lagacé M., Marullo S., Bouvier M. (2004) J. Biol. Chem. 279, 33390–33397 [DOI] [PubMed] [Google Scholar]
- 42. Herrick-Davis K., Weaver B. A., Grinde E., Mazurkiewicz J. E. (2006) J. Biol. Chem. 281, 27109–27116 [DOI] [PubMed] [Google Scholar]
- 43. Guan R., Feng X., Wu X., Zhang M., Zhang X., Hébert T. E., Segaloff D. L. (2009) J. Biol. Chem. 284, 7483–7494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hébert T. E., Galé C., Rebois R. V. (2006) Cell Biochem. Biophys. 45, 85–109 [DOI] [PubMed] [Google Scholar]
- 45. Larsson M. C., Domingos A. I., Jones W. D., Chiappe M. E., Amrein H., Vosshall L. B. (2004) Neuron 43, 703–714 [DOI] [PubMed] [Google Scholar]
- 46. Armstrong D., Strange P. G. (2001) J. Biol. Chem. 276, 22621–22629 [DOI] [PubMed] [Google Scholar]
- 47. Baker J. G., Hill S. J. (2007) Trends Pharmacol. Sci. 28, 374–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kenworthy A. (2002) Trends Biochem. Sci. 27, 435–437 [DOI] [PubMed] [Google Scholar]
- 49. Kobilka B. K., Deupi X. (2007) Trends Pharmacol. Sci. 28, 397–406 [DOI] [PubMed] [Google Scholar]
- 50. Audet N., Galés C., Archer-Lahlou E., Vallières M., Schiller P. W., Bouvier M., Pineyro G. (2008) J. Biol. Chem. 283, 15078–15088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Cheng Z. J., Miller L. J. (2001) J. Biol. Chem. 276, 48040–48047 [DOI] [PubMed] [Google Scholar]