Abstract
In addition to the classical function of estrogen receptors (ER) as transcription factors, evidence continues to accumulate that they mediate non-nuclear processes in numerous cell types, including the endothelium, in which they activate endothelial NO synthase. Non-nuclear ER signaling entails unique post-translational modifications and protein-protein interactions of the receptor with adaptor molecules, kinases, and G proteins. Recent in vitro and in vivo studies in mice using an estrogen-dendrimer conjugate that is excluded from the nucleus indicate that non-nuclear ER activation underlies the migration and growth responses of endothelial cells to estrogen but not the growth responses of endometrial or breast cancer cells to the hormone. In this minireview, the features of ERα and protein-protein interactions that enable it to invoke extranuclear signaling in the endothelium and the consequences of that signaling are discussed.
Keywords: Breast Cancer, Endothelium, Estrogen, G Proteins, Hormone Receptors, Nitric-oxide Synthase
Introduction
Although estrogen receptors (ER)2 function classically as transcription factors, more recently, it has become apparent that ER also have the novel capacity to activate non-nuclear signaling in a variety of cell types (1–3). An understanding of the basis for non-nuclear ER signaling has been derived from the study of ER associated with plasmalemmal caveolae/lipid rafts in endothelial cells (4), which is the focus of this minireview. After a brief discussion of the actions of estrogen on the endothelium that highlights non-nuclear processes, we will summarize the nature of non-nuclear ER function in the endothelium. We will then discuss the signaling events that non-nuclear ER mediate in the endothelium, the mechanisms by which they are coupled to kinases and other signaling or adaptor molecules, and recent work interrogating these processes in vivo. Finally, we will highlight the current questions in the field.
Endothelial Actions of Estrogen
Estrogen has potentially potent cardiovascular protective actions, and these are primarily through direct effects on endothelial and vascular smooth muscle (VSM) cells (5–7), which express the two primary ER isoforms, ERα and ERβ (4, 5). In endothelial cells in culture, estrogen up-regulates the expression of endothelial NO synthase (eNOS) (4), cyclooxygenase-1 (8), MMP-2 (9), and ERα (10), and it blunts the expression of endothelin (11) and the type 1 angiotensin II receptor (12). Along with these actions that involve the classical functions of ER as transcription factors, short-term effects of estrogen on the vasculature were demonstrated in humans, and they provided the initial suggestion that there are non-nuclear actions of the hormone and its receptors. In early studies, ethinyl estradiol acutely attenuated abnormal coronary vasoconstrictor responses to acetylcholine (ACh) in postmenopausal women, and it also increased basal coronary blood flow and decreased coronary vascular resistance (13). Other studies of estradiol (E2) administered to yield premenopausal serum levels showed no change in basal coronary vasomotor tone, but there were greater vasodilatory responses to ACh when ACh and E2 were give simultaneously (14). In experiments in men using intravenous conjugated estrogens and phytoestrogens, there was rapid NO-dependent vasodilation 15 min after treatment, suggesting that estrogen promotes the bioavailability of NO and that these effects may be comparable in men and women (15). In 1997, it was reported by two laboratories that estrogen rapidly stimulates eNOS enzymatic activity in an ER-dependent manner (16, 17). NO derived from eNOS has diverse potential beneficial vascular actions, including the promotion of endothelial cell growth and migration; the attenuation of VSM cell growth and migration; and the antagonism of platelet activation, thrombus formation, and leukocyte-endothelial cell adhesion (18). Using genetically modified mice, numerous pioneering studies begun in the late 1990s then revealed that the basis for estrogen-related protection from atherosclerosis and vascular injury lies in direct actions of the hormone on ER expressed in vascular cells and that, along with other processes, there is likely a primary role for E2/ER influencing eNOS activity (4).
Nature of Non-nuclear ER in the Endothelium
Estrogen activates non-nuclear ER, resulting in rapid signaling in cell types in culture as diverse as oocytes, osteoblasts, osteoclasts, breast cancer cells, adipocytes, and endothelial cells (1–3, 19, 20). The vast majority of studies in these various model systems have indicated that the rapid actions of estrogen originate at the cell surface and not in the nucleus. In the endothelium, ERα was first implicated in non-nuclear signaling by the findings that overexpression of the receptor enhanced the acute activation of eNOS by E2 and that eNOS stimulation by the endogenous receptor was inhibited by both ICI 182,780 and tamoxifen and by transfection with an ERα mutant lacking coding sequence distal to amino acid 271, which excludes the hormone-binding domain (21). Initial evidence of the potential involvement of endothelial cell ERβ in non-nuclear signaling was obtained from studies in which the response to endogenous ER activation was inhibited by the ERβ-selective antagonist RR-tetrahydrochrysene (22). The localization of ER functional coupling to eNOS in isolated plasma membranes provided additional clarity of membrane receptor identity, with immunoidentification studies directed at multiple epitopes detecting the same 67-kDa ERα protein and the same 54-kDa ERβ protein in purified plasma membrane fractions and in the nucleus (22, 23). The requirement for ER and eNOS colocalization on the plasma membrane was further evident in reconstitution studies in COS-7 cells in which plasma membranes from cells expressing eNOS and ERα displayed rapid ER-mediated eNOS stimulation (24). The majority of studies in the endothelium have identified full-length ERα (67 kDa) as the predominant ERα form associated with the plasma membrane involved in non-nuclear signaling (21, 23, 25). However, an N terminus-deleted splice variant, ERα46, has been found in certain human endothelial cell lines, and it has been shown to colocalize with caveolin-1 in caveolae and to effectively transduce membrane-initiated responses to E2 (26, 27). Interestingly, the transcriptional activity of ERα46 is greatly compromised compared with that of full-length ERα (28).
The G protein-coupled receptor GPR30 has been proposed to be a third form of ER, and it has been localized to the plasma membrane in breast cancer cells and to the endoplasmic reticulum when overexpressed in COS-7 cells (29, 30). As a result of varying phenotypes in three mouse lines with diminished GPR30 expression and other conflicting findings, it is controversial whether GPR30 is a biologically relevant ER or a collaborator in non-nuclear functions of the classical ER in certain contexts (31). The cell types expressing GPR30 have been evaluated in a mouse model with a lacZ reporter in the GPR30 locus. Regarding vascular expression, LacZ-positive vascular cells were present in a number of arterial structures, but their distribution was restricted to specific vascular beds, including arterioles in the kidney and the vasa vasorum of the aorta, and the final branches of mesenteric arteries. The aorta and carotid, intercostal, renal, and superior mesenteric arteries were negative for LacZ staining. In the CNS vasculature, the LacZ-positive cells were VSM cells and pericytes, and staining was absent in the endothelium, whereas in the kidney, LacZ staining colocalized with the endothelial cell marker PECAM-1 (32). Although GPR30 expression was not detected in the carotid arteries in the lacZ reporter mice, carotid arteries isolated from GPR30+/+ mice displayed relaxation in response to the GPR30 agonist G-1, whereas relaxation was absent in arteries from GPR30−/− mice (33). In recent studies of isolated precontracted rat carotid arteries, G-1 and another GPR30 agonist, 5408-0877, both caused endothelium-dependent, NO-dependent relaxation (34). However, whereas E2 binds to whole cells or membranes in primary endothelial cells from wild-type mice, E2 binding is absent in endothelial cells from ERα−/−/ERβ−/− mice, in which GPR30 protein expression is demonstrable and unaltered. In parallel, whereas wild-type endothelial cells display rapid signaling in response to E2, cells from ERα/ERβ double knock-out mice do not, despite unchanged GPR30 expression (25). The additional key experiment is a test of responses to E2 by GPR30+/+ versus GPR30−/− endothelium, but this has not yet been reported. Thus, although GPR30 is expressed in certain endothelial cells, there is currently a lack of clear evidence of a role for the receptor in estrogen action in the endothelium. Instead, classical ERα and ERβ, and perhaps truncated forms of ERα (i.e. ERα46), mediate the non-nuclear actions of the hormone in endothelial cells.
ER Localization to Plasma Membrane Microdomains
Caveolae are specialized cholesterol-rich plasma membrane organelles that are a subset of lipid rafts that compartmentalize signal transduction molecules on the cell surface (35), and eNOS is targeted to caveolae via myristoylation and palmitoylation (24, 36). Fractionation of endothelial cell plasma membranes has revealed both ERα and ERβ association with caveolar membranes. The proteins were also found associated with the non-caveolar plasma membrane fraction. Importantly, studies in isolated caveolar membranes demonstrated functional coupling of both ERα and ERβ to eNOS, and eNOS activation in caveolae in response to E2 was comparable with that observed with the classical eNOS agonist ACh, which signals through M2 muscarinic receptors (22, 23).
The basis for ER localization to the plasma membrane/caveolae has been evaluated by mutagenesis, primarily of ERα. Although not observed in all studies, there is evidence that ERα interacts with caveolin-1; that the interaction requires Ser-522 of the receptor, which is located within the ligand-binding domain (E domain) (Fig. 1); and that the interaction facilitates the trafficking of ERα to caveolae (23, 37). The participation of caveolin-1 in ER function has been evaluated in caveolin-1-deficient mice, which are viable and fertile but display major changes in the structure and function of the heart, lung, and blood vessels that may be due to the uncoupling of eNOS from caveolae (38, 39). The mammary glands of caveolin-1−/− mice are hyper-responsive to estrogen and develop dysplastic lesions with adjacent stromal angiogenesis (40), suggesting cross-talk between caveolin-1 and ER in the tumor microvasculature in vivo.
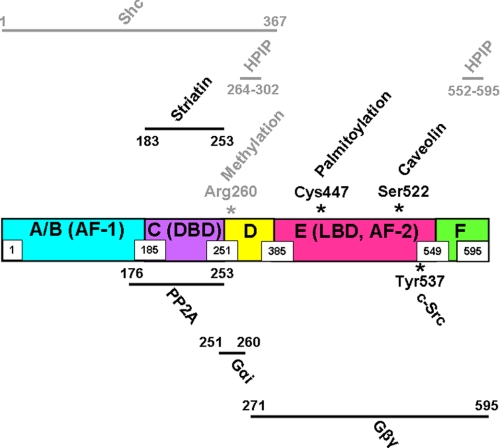
FIGURE 1.
Post-translational modifications and protein-protein interactions involved in non-nuclear ERα signaling. The structure of ERα is shown in linear fashion, with the N- to C-terminal domains designated A–F. The amino acid spans of the domains are indicated by boxed numbers. The N-terminal A/B domain contains a hormone-independent transcription function (AF-1); the C domain contains the DNA-binding domain (DBD); the D domain contains nuclear localization signals; the E domain contains the ligand-binding domain (LBD), which also possesses hormone-dependent activation function (AF-2); and the F domain, which modulates the ability of ERα to respond to tamoxifen in a cell-specific manner. Post-translational modifications and adaptor proteins that interact directly with ERα are shown above the receptor, and kinases, phosphatases, and G proteins with direct interaction are shown below the receptor, with the relevant residues or regions of ERα that contain the interaction domains indicated numerically. Modifications or interacting proteins that regulate ERα non-nuclear functions in the endothelium are shown in black, and those thus far identified in non-endothelial cells are shown in gray.
In addition to potential participation of caveolin-1 in ERα targeting to the plasma membrane, Cys-447 of ERα, which is also within the ligand-binding domain, is a palmitoylation site that is important for membrane localization of the receptor (Fig. 1) (41), and additional residues flanking Cys-447 are also required for plasma membrane targeting via palmitoylation (42). A role for ERα palmitoylation in endothelial cell receptor regulation is further supported by the observations in EA.hy926 immortalized human endothelial cells that the N-terminally truncated variant ERα46 is palmitoylated and that palmitoylation is required for E2-enhanced plasma membrane recruitment of the receptor (27). The best available information regarding the topology of plasma membrane-associated ER is limited to studies of N-terminally Myc-tagged ERα46 expressed in COS-7 cells. Without permeabilization before staining, fluorescence-activated cell sorting detected cells only with antibody to the C terminus of ERα46, whereas a signal with anti-c-Myc antibody occurred only following permeabilization (27). These findings suggest that ERα46 possesses a C-terminal ectodomain, but the basis for such possible membrane orientation remains enigmatic, and studies of the plasma membrane topography of endogenous receptor populations are needed. However, it is now well established that caveolae are the key plasma membrane microdomain in which ERα and ERβ reside to regulate extranuclear events in endothelial cells and that their localization to caveolae most likely depends on interaction with caveolin and/or receptor palmitoylation.
Signaling by Non-nuclear ER in the Endothelium
Following the initial discovery of rapid eNOS activation by E2 (16, 17), the signaling underlying ER coupling to eNOS received considerable attention. In early studies, roles for tyrosine kinases and MAPKs were implicated by the findings that genistein and the MAPKK inhibitor PD98059 prevented eNOS activation by E2 (21). Further work employing pharmacologic intervention or detection of changes in protein phosphorylation upon E2 treatment in arteries or cultured endothelial cells indicated requirements for PI3K, Akt kinase, and ERK1/2 and enhanced phosphorylation of Ser-1177 of eNOS (Fig. 2) (25, 44–46). The enhancement of eNOS phosphorylation by Akt induces activation of the enzyme by a variety of agonists besides E2 (18), and the signaling events immediately proximal to the Ser-1177 phosphorylation invoked by E2 are similar to the upstream events by which a number of plasma membrane receptors regulate eNOS activity. However, in a unique manner, ERα binds in a ligand-dependent fashion to the p85α regulatory subunit of PI3K, and the interaction is direct (Fig. 2) and is not mediated by the Src homology SH2/SH3 domains of p85α (46). The activation of PI3K and its substrate Akt in response to E2 binding to non-nuclear ER is mediated by c-Src kinase, whose SH2 domain interacts with phosphorylated Tyr-537 of ERα (Figs. 1 and 2), and this interaction may facilitate the plasma membrane recruitment of ERα (26, 47, 48). In studies primarily in MCF-7 breast cancer cells, it has been demonstrated that the methylation of Arg-260 in the ERα DNA-binding domain by the arginine methyltransferase PRMT1 (Fig. 1) triggers the interaction of the receptor with the p85 subunit of PI3K and with c-Src and that this process occurs in the cytoplasm and participates in non-nuclear downstream signaling (49). Whether Arg-260 methylation is required for non-nuclear ERα signaling in endothelial cells has yet to be determined.
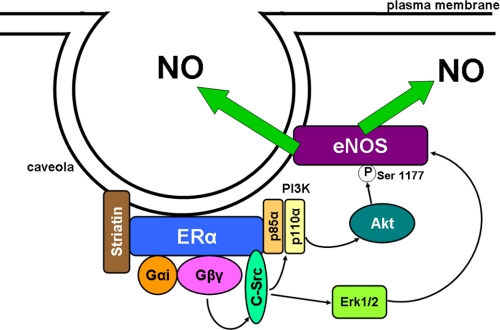
FIGURE 2.
Non-nuclear ERα resides in a signaling complex associated with endothelial cell caveolae/lipid rafts. Upon estrogen binding, Gαi and Gβγ disassociate from ERα, and liberated Gβγ activates c-Src. This leads to the activation of PI3K, which activates Akt to phosphorylate eNOS Ser-1177, and to the activation of ERK1/2, which is also required to yield an increase in eNOS enzymatic activity. The resulting NO that is produced has both autocrine and paracrine actions.
In addition to activating kinases in endothelial cells, there is evidence that non-nuclear ER stimulation alters intracellular calcium homeostasis. In studies of human internal thoracic artery endothelium in situ and human arterial endothelial cells in culture, E2 caused a rapid increase in intracellular calcium at physiologically relevant concentrations of the hormone (10−9 m); this was attenuated following intracellular calcium store depletion, and concurrent NO release was demonstrable. The ER antagonist tamoxifen blunted these responses. Similar findings were obtained using E2 conjugated to BSA, which has been employed to evaluate the role of cell-surface receptors (50). However, observations with E2-BSA should be interpreted with caution because freshly prepared solutions of E2-BSA contain free immunoassayable E2; E2-BSA binds to ER only poorly because the E2 is linked to BSA through chemical groups in E2 that are important for ER binding; and certain E2-BSA preparations are of very high molecular weight, suggesting extreme protein cross-linking (51, 52). In cultured rat endothelial cells, E2 and E2-BSA also caused comparable calcium transients that were attenuated by the ER antagonist ICI 182,780 (53). However, other studies has shown that E2 does not induce a discernible acute increase in intracellular calcium in endothelial cells (17, 54) despite clear evidence that the non-nuclear activation of eNOS by E2 is calcium-dependent (16, 55). These disparities are potentially related to the involvement of highly localized, caveola-associated calcium pools and caveola-associated proteins that regulate calcium homeostasis (56) because E2 activation of the enzyme in isolated endothelial cell plasma membranes and caveolae occurs in the absence of added calcium but is completely prevented by calcium chelation (23).
The localization of ERα to endothelial cell caveolae and both the physical and functional interaction of the receptor with other signaling molecules in that domain suggest that adaptor proteins may also participate in non-nuclear actions of ERα in endothelial cells. One such candidate is the ERα-binding protein striatin, which is a member of the WD repeat protein family. In studies limited to the endothelial cell line EA.hy926, striatin and ERα were co-immunoprecipitated, and overexpression of striatin increased the abundance of the receptor at the plasma membrane. In addition, E2 stimulated the formation of a complex containing ERα, striatin, and Gαi, which is critically involved in non-nuclear ERα function in endothelial cells (see below). Pulldown experiments with purified proteins identified direct interaction between striatin and ERα involving amino acids 183–253 of the receptor (Fig. 1), and a peptide representing amino acids 176–253 of ERα prevented E2 activation of ERK1/2, Akt, and eNOS (57). These findings support a role for striatin in non-nuclear ERα function in endothelial cells, but further genetic evidence and studies in primary endothelial cells and in vivo models are needed to strengthen such a conclusion. There are three additional molecules with direct interactions with ERα that are of consequence to non-nuclear signaling and that have been studied in non-endothelial cells. Work in HeLa and MCF-7 cells has revealed that ERα interacts with hematopoietic PBX-interacting protein (HPIP), which mediates the binding of the receptor with tubulins and also recruits the p85 subunit of PI3K and Src kinases to an ERα complex upon E2 binding, leading to the stimulation of Akt and ERK1/2. There is a direct interaction of ERα with HPIP that requires amino acids 264–302 and 552–595 of the receptor (Fig. 1) (58). The protein Shc (Src homology and collagen homology), which has a direct interaction with ERα that requires residues between amino acids 1 and 367 of the receptor, is critically involved in linking E2 binding to non-nuclear ERα with morphologic changes and the growth of breast cancer cells (59). Furthermore, a scaffolding protein designated as MNAR (modulator of nongenomic activity of ER) has been described that promotes E2-induced interaction between ERα and Src family kinase(s) in non-endothelial cells (60). ERα-related functions of HPIP, Shc, and MNAR in the endothelium have yet to be queried.
G Protein Coupling of Non-nuclear ER in the Endothelium
As illustrated above, our deepest understanding of non-nuclear ER signaling in the endothelium resides in the realm of the modulation of eNOS. The participation of c-Src, PI3K, Akt, and ERK1/2 mimics the involvement of these kinases in eNOS modulation by numerous other agonist-receptor pairs (18). However E2-ER regulation of eNOS entails not only the unique direct interactions between ERα and c-Src and PI3K but also novel G protein coupling of the receptor. The potential involvement of G proteins was initially revealed in studies demonstrating that E2 activation of eNOS is pertussis toxin-sensitive and that ERα and Gαi can be co-immunoprecipitated from endothelial cell plasma membranes, with pertussis toxin preventing the co-immunoprecipitation (61). Pulldown experiments then revealed that ERα binds directly to Gαi and Gβγ, and mutagenesis and experiments with a blocking peptide showed that this occurs via amino acids 251–260 and 271–595 of human ERα, respectively (Fig. 1). Studies of ERα complexed with heterotrimeric G proteins further showed that E2 causes the release of both Gαi and Gβγ without stimulating guanine nucleotide exchange. Moreover, in COS-7 cells, the disruption of ERα-Gαi interaction by deletion mutagenesis of the Gαi-binding domain of ERα, expression of a blocking peptide, or Gβγ sequestration with the β-adrenergic receptor kinase C terminus fully attenuates Src kinase and ERK1/2 activation by E2. In endothelial cells, the disruption of ERα-Gαi interaction prevents eNOS activation and also the blunting of monocyte adhesion and the stimulation of cell migration caused by E2 (62). Thus, direct ERα-G protein interaction underlies the functional coupling of the receptor to kinase cascades and the resulting downstream cellular responses in the endothelium (Fig. 2).
Cross-talk between Non-nuclear ER Signaling and Nuclear Processes
Along with the rapid cellular responses (such as activation of NO production) that result from the immediate signaling caused by non-nuclear ER activation in endothelial cells, there are important potential consequences on endothelial cell gene expression. Studies in nonvascular cells indicate that ERα Ser-104, Ser-106, and Ser-118 are potential targets for phosphorylation by the cyclin-dependent protein kinases CDK2 (Ser-104 and Ser-106) and CDK7 (Ser-118), with CDK7 actions occurring in response to E2. In addition, ERα Ser-118 can be phosphorylated by ERK1/2 in a ligand-independent manner. It is further known that these events modify the regulation of gene transcription by ERα (63–65). The activation of NO production by non-nuclear E2 and ERα signaling also potentially influences nuclear actions of the hormone. S-Nitrosylation of cysteine residues within ERα has been detected upon NO donor treatment of MCF-7 breast cancer cells, resulting in the inhibition of ERα DNA binding at specific estrogen response elements. Thus, although the studies to date have been carried out in non-endothelial cells, the phosphorylation and also the S-nitrosylation of ERα invoked by its own non-nuclear signaling may have an important influence on the nuclear actions of E2 and ER in the endothelium.
Direct evidence of cross-talk between non-nuclear ER signaling and nuclear processes has been obtained in studies in cultured endothelial cells. In human umbilical vein endothelial cells, E2 treatment for 40 min up-regulates the expression of 250 genes, and their up-regulation is PI3K-dependent. Cyclooxygenase-2 is one such target gene, and its up-regulation results in increased prostacyclin and prostaglandin E2 secretion (66). The inhibitor of angiogenesis thrombospondin-1 is transiently down-regulated by E2 in human umbilical vein endothelial cells via ERK1/2 and JNK/SAPK signaling (67). Furthermore, E2 activation of eNOS leads to the up-regulation of telomerase activity via the stimulation of transcriptional transactivation of hTERT, the catalytic subunit of human telomerase (68). Additional studies have demonstrated that these processes participate in the promigratory response of endothelial cells to E2 (66–68), to which the non-nuclear activation of the small GTPase RhoA, Rho kinase, and moesin also contributes (69). It has also been demonstrated that both the promigratory response to E2 and the stimulation of endothelial cell growth by the hormone are Gαi-, Src-, and eNOS-dependent (70). Furthermore, the ability of E2 to preserve endothelial cell stress fiber formation and actin and membrane integrity under metabolic stress and to attenuate hypoxia-induced apoptosis is dependent on p38 MAPK activation and the resulting activation of MAPKAP-2 kinase and HSP27 phosphorylation (71). These diverse processes take place in endothelial cells concurrently with the classical actions of ER serving as transcription factors binding directly to DNA via estrogen response elements or indirectly via tethered processes occurring via Sp1 or AP-1. Interestingly, the protein phosphatase PP2A interacts directly with ERα via amino acids 176–253 of the receptor (Fig. 1), and the phosphatase dephosphorylates ERα Ser-118. Okadaic acid inhibition of PP2A activates ERα-mediated gene transcription, and in endothelial cells, this leads to eNOS protein up-regulation (72). Because PP2A also dephosphorylates eNOS Ser-1177 (73), which is phosphorylated by Akt to yield greater eNOS enzymatic activity upon E2 treatment, it is interesting to envision that a member of the complex of proteins interacting with non-nuclear ER may counter-regulate the activating function of that complex. It is becoming more and more apparent that the ultimate physiologic responses of the endothelium to E2 entail complex combinations of non-nuclear and nuclear processes and the influences of the diverse modes of cross-talk that occur between them.
In Vivo Interrogation of Non-nuclear ER Actions
Until recently, our ability to rigorously distinguish nuclear from non-nuclear ER actions in the endothelium and other cell types was limited, and in vivo interrogation was not feasible. E2-BSA was initially used in studies of cultured endothelial cells, and it caused the stimulation of eNOS phosphorylation (74). However, as noted above, the selectivity of E2-BSA for non-nuclear ER activation and its stability are uncertain. An estrogen-dendrimer conjugate (EDC) was then created in which estrogen was attached to a large positively charged non-degradable poly(amido)amine dendrimer via hydrolytically stable linkages, thereby excluding EDC from the nucleus (43). EDC stimulates cultured endothelial cell proliferation and migration via ERα, Gαi, and the activation of Src kinase and eNOS that they invoke. Studies of estrogen response element-luciferase reporter mice and ER target gene expression in the uterus further demonstrated that EDC causes the selective activation of non-nuclear ER signaling when administered in vivo. In mice, E2 and EDC equally stimulate carotid artery re-endothelialization (Fig. 3A), and this is dependent on ER-Gαi coupling (70); both agents attenuate the development of neointimal hyperplasia that occurs following endothelial injury in the setting of hypercholesterolemia (Fig. 3B). However, whereas uterine and MCF-7 breast cancer cell xenograft growth in vivo (Fig. 3, C and D, respectively) is stimulated by E2, it is not promoted by EDC (70). Further studies in cultured endometrial carcinoma cells and MCF-7 breast cancer cells demonstrated that although non-nuclear signaling and ERK1/2 activation indeed occur upon treatment with EDC, in contrast to the response of endothelial cells, the selective activation of non-nuclear ER signaling does not stimulate growth of the cancer cells (43, 70). Thus, EDC is a non-nuclear selective ER modulator in vivo, and non-nuclear ER signaling provides potent cardiovascular protection without promoting uterine or breast cancer tumor growth. These processes can potentially be harnessed to provide vascular benefit without increasing cancer risk.
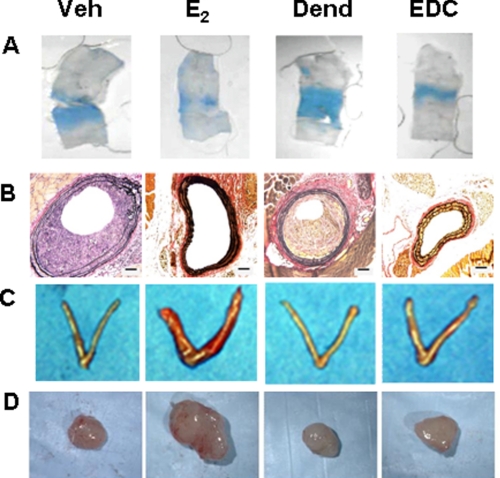
FIGURE 3.
Non-nuclear ER activation in mice promotes re-endothelialization and provides protection from neointima formation, but it does not promote uterine or breast cancer growth. Experiments were performed in ovariectomized female mice administered vehicle (Veh), E2, control dendrimer (Dend), or EDC. A, in studies of carotid artery re-endothelialization following perivascular electric injury, the remaining area of endothelial denudation that has incorporated Evans blue dye 3 days post-injury is shown. B, neointima formation was evaluated in ApoE−/− mice 2 weeks following carotid artery endothelial denudation. C, uterotrophic responses to the treatments were also assessed. D, following the establishment of MCF-7 cell tumor xenografts in SCID mice, the growth responses of the tumors to 21 days of treatment were determined. This figure was reprinted with permission (70).
Conclusions and Current Questions
The study of non-nuclear ER signaling in the endothelium has revealed novel mechanisms that provide greater diversity of function of the receptor. In its extranuclear locale, ERα has novel actions related to unique post-translational modifications and interactions with adaptor proteins, kinases, and phosphatases and also G proteins. The studies in mice using EDC revealed that non-nuclear ERα coupling to Gαi is operative in vivo, and a beneficial impact on neointima formation has been demonstrated. We do not yet know whether non-nuclear selective ER activation affords atheroprotection, and although non-nuclear ERα activation in the endothelium is likely of importance, the cell types in which non-nuclear ERα influences vascular disease pathogenesis have yet to be identified. It is also unclear how non-nuclear ER function contributes to estrogen modulation of metabolism. Considering the prevalence of cross-talk between non-nuclear and nuclear ER actions, it is important to recognize that although EDC provides a selective gain-of-function intervention targeting non-nuclear ER, loss-of-function strategies to specifically negate non-nuclear ER function are also needed. It is only through loss of function that new knowledge will be gained about the role of non-nuclear ER signaling in the actions of endogenous estrogens, whose levels are highly dynamic. The structural features of ERα involved in non-nuclear signaling (Fig. 1) can potentially be modified to yield non-nuclear selective loss of function. Such approaches will be important in future studies attempting to understand the disparate roles of non-nuclear ER activation in the regulation of cell growth, as observed in endothelial cells compared with endometrial or breast cancer cells (70). Numerous mysteries also remain about the topology of plasma membrane-associated ER and the basis on which trafficking to the plasma membrane versus nucleus is determined. It is only through further investigation of this unique aspect of endocrinology that we will increase our understanding of how non-nuclear ER signaling influences the behavior of the endothelium, as well as other cell types, under normal or pathologic conditions.
Acknowledgments
We thank many colleagues and collaborators who have contributed to the effort to better understand non-nuclear ER signaling in the endothelium.
This work was supported, in whole or in part, by National Institutes of Health Grants HD030276 and HL087564. This work was also supported by the Lowe Foundation and the Crystal Charity Ball Center for Pediatric Critical Care Research. This minireview will be reprinted in the 2011 Minireview Compendium, which will be available in January, 2012
- ER
- estrogen receptor(s)
- VSM
- vascular smooth muscle
- eNOS
- endothelial NO synthase
- ACh
- acetylcholine
- E2
- estradiol
- SH
- Src homology
- HPIP
- hematopoietic PBX-interacting protein
- EDC
- estrogen-dendrimer conjugate.
REFERENCES
- 1. Norman A. W., Mizwicki M. T., Norman D. P. (2004) Nat. Rev. Drug Discov. 3, 27–41 [DOI] [PubMed] [Google Scholar]
- 2. Osborne C. K., Schiff R. (2005) J. Clin. Oncol. 23, 1616–1622 [DOI] [PubMed] [Google Scholar]
- 3. Levin E. R. (2005) Mol. Endocrinol. 19, 1951–1959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chambliss K. L., Shaul P. W. (2002) Endocr. Rev. 23, 665–686 [DOI] [PubMed] [Google Scholar]
- 5. Mendelsohn M. E., Karas R. H. (1999) N. Engl. J. Med. 340, 1801–1811 [DOI] [PubMed] [Google Scholar]
- 6. Hodgin J. B., Maeda N. (2002) Endocrinology 143, 4495–4501 [DOI] [PubMed] [Google Scholar]
- 7. Mendelsohn M. E. (2000) J. Steroid Biochem. Mol. Biol. 74, 337–343 [DOI] [PubMed] [Google Scholar]
- 8. Jun S. S., Chen Z., Pace M. C., Shaul P. W. (1998) J. Clin. Invest. 102, 176–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wingrove C. S., Garr E., Godsland I. F., Stevenson J. C. (1998) Biochim. Biophys. Acta 1406, 169–174 [DOI] [PubMed] [Google Scholar]
- 10. Ihionkhan C. E., Chambliss K. L., Gibson L. L., Hahner L. D., Mendelsohn M. E., Shaul P. W. (2002) Circ. Res. 91, 814–820 [DOI] [PubMed] [Google Scholar]
- 11. Akishita M., Kozaki K., Eto M., Yoshizumi M., Ishikawa M., Toba K., Orimo H., Ouchi Y. (1998) Biochem. Biophys. Res. Commun. 251, 17–21 [DOI] [PubMed] [Google Scholar]
- 12. Nickenig G., Strehlow K., Wassmann S., Bäumer A. T., Albory K., Sauer H., Böhm M. (2000) Circulation 102, 1828–1833 [DOI] [PubMed] [Google Scholar]
- 13. Reis S. E., Gloth S. T., Blumenthal R. S., Resar J. R., Zacur H. A., Gerstenblith G., Brinker J. A. (1994) Circulation 89, 52–60 [DOI] [PubMed] [Google Scholar]
- 14. Gilligan D. M., Quyyumi A. A., Cannon R. O., 3rd (1994) Circulation 89, 2545–2551 [DOI] [PubMed] [Google Scholar]
- 15. Walker H. A., Dean T. S., Sanders T. A., Jackson G., Ritter J. M., Chowienczyk P. J. (2001) Circulation 103, 258–262 [DOI] [PubMed] [Google Scholar]
- 16. Lantin-Hermoso R. L., Rosenfeld C. R., Yuhanna I. S., German Z., Chen Z., Shaul P. W. (1997) Am. J. Physiol. 273, L119–L126 [DOI] [PubMed] [Google Scholar]
- 17. Caulin-Glaser T., García-Cardeña G., Sarrel P., Sessa W. C., Bender J. R. (1997) Circ. Res. 81, 885–892 [DOI] [PubMed] [Google Scholar]
- 18. Shaul P. W. (2002) Annu. Rev. Physiol 64, 749–774 [DOI] [PubMed] [Google Scholar]
- 19. Cheskis B. J. (2004) J. Cell. Biochem. 93, 20–27 [DOI] [PubMed] [Google Scholar]
- 20. Kelly M. J., Levin E. R. (2001) Trends Endocrinol. Metab. 12, 152–156 [DOI] [PubMed] [Google Scholar]
- 21. Chen Z., Yuhanna I. S., Galcheva-Gargova Z., Karas R. H., Mendelsohn M. E., Shaul P. W. (1999) J. Clin. Invest. 103, 401–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chambliss K. L., Yuhanna I. S., Anderson R. G., Mendelsohn M. E., Shaul P. W. (2002) Mol. Endocrinol. 16, 938–946 [DOI] [PubMed] [Google Scholar]
- 23. Chambliss K. L., Yuhanna I. S., Mineo C., Liu P., German Z., Sherman T. S., Mendelsohn M. E., Anderson R. G., Shaul P. W. (2000) Circ. Res. 87, E44–E52 [DOI] [PubMed] [Google Scholar]
- 24. Shaul P. W., Smart E. J., Robinson L. J., German Z., Yuhanna I. S., Ying Y., Anderson R. G., Michel T. (1996) J. Biol. Chem. 271, 6518–6522 [DOI] [PubMed] [Google Scholar]
- 25. Pedram A., Razandi M., Levin E. R. (2006) Mol. Endocrinol. 20, 1996–2009 [DOI] [PubMed] [Google Scholar]
- 26. Haynes M. P., Li L., Sinha D., Russell K. S., Hisamoto K., Baron R., Collinge M., Sessa W. C., Bender J. R. (2003) J. Biol. Chem. 278, 2118–2123 [DOI] [PubMed] [Google Scholar]
- 27. Li L., Haynes M. P., Bender J. R. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 4807–4812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Figtree G. A., McDonald D., Watkins H., Channon K. M. (2003) Circulation 107, 120–126 [DOI] [PubMed] [Google Scholar]
- 29. Revankar C. M., Cimino D. F., Sklar L. A., Arterburn J. B., Prossnitz E. R. (2005) Science 307, 1625–1630 [DOI] [PubMed] [Google Scholar]
- 30. Filardo E. J., Quinn J. A., Bland K. I., Frackelton A. R., Jr. (2000) Mol. Endocrinol. 14, 1649–1660 [DOI] [PubMed] [Google Scholar]
- 31. Levin E. R. (2009) Endocrinology 150, 1563–1565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Isensee J., Meoli L., Zazzu V., Nabzdyk C., Witt H., Soewarto D., Effertz K., Fuchs H., Gailus-Durner V., Busch D., Adler T., de Angelis M. H., Irgang M., Otto C., Noppinger P. R. (2009) Endocrinology 150, 1722–1730 [DOI] [PubMed] [Google Scholar]
- 33. Haas E., Bhattacharya I., Brailoiu E., Damjanović M., Brailoiu G. C., Gao X., Mueller-Guerre L., Marjon N. A., Gut A., Minotti R., Meyer M. R., Amann K., Ammann E., Perez-Dominguez A., Genoni M., Clegg D. J., Dun N. J., Resta T. C., Prossnitz E. R., Barton M. (2009) Circ. Res. 104, 288–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Broughton B. R., Miller A. A., Sobey C. G. (2010) Am. J. Physiol. Heart Circ. Physiol. 298, H1055–H1061 [DOI] [PubMed] [Google Scholar]
- 35. Shaul P. W., Anderson R. G. W. (1998) Am. J. Physiol. 275, L843–L851 [DOI] [PubMed] [Google Scholar]
- 36. García-Cardeña G., Oh P., Liu J., Schnitzer J. E., Sessa W. C. (1996) Proc. Natl. Acad. Sci. U.S.A. 93, 6448–6453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Razandi M., Alton G., Pedram A., Ghonshani S., Webb P., Levin E. R. (2003) Mol. Cell. Biol. 23, 1633–1646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Le Lay S., Kurzchalia T. V. (2005) Biochim. Biophys. Acta 1746, 322–333 [DOI] [PubMed] [Google Scholar]
- 39. Rahman A., Sward K. (2009) Acta Physiol. 195, 231–245 [DOI] [PubMed] [Google Scholar]
- 40. Mercier I., Casimiro M. C., Zhou J., Wang C., Plymire C., Bryant K. G., Daumer K. M., Sotgia F., Bonuccelli G., Witkiewicz A. K., Lin J., Tran T. H., Milliman J., Frank P. G., Jasmin J. F., Rui H., Pestell R. G., Lisanti M. P. (2009) Am. J. Pathol. 174, 1172–1190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Acconcia F., Ascenzi P., Fabozzi G., Visca P., Marino M. (2004) Biochem. Biophys. Res. Commun. 316, 878–883 [DOI] [PubMed] [Google Scholar]
- 42. Pedram A., Razandi M., Sainson R. C., Kim J. K., Hughes C. C., Levin E. R. (2007) J. Biol. Chem. 282, 22278–22288 [DOI] [PubMed] [Google Scholar]
- 43. Harrington W. R., Kim S. H., Funk C. C., Madak-Erdogan Z., Schiff R., Katzenellenbogen J. A., Katzenellenbogen B. S. (2006) Mol. Endocrinol. 20, 495–502 [DOI] [PubMed] [Google Scholar]
- 44. Florian M., Lu Y., Angle M., Magder S. (2004) Steroids 69, 637–645 [DOI] [PubMed] [Google Scholar]
- 45. Guo X., Razandi M., Pedram A., Kassab G., Levin E. R. (2005) J. Biol. Chem. 280, 19704–19710 [DOI] [PubMed] [Google Scholar]
- 46. Simoncini T., Hafezi-Moghadam A., Brazil D. P., Ley K., Chin W. W., Liao J. K. (2000) Nature 407, 538–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Migliaccio A., Castoria G., Di Domenico M., de Falco A., Bilancio A., Lombardi M., Barone M. V., Ametrano D., Zannini M. S., Abbondanza C., Auricchio F. (2000) EMBO J. 19, 5406–5417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Li L., Hisamoto K., Kim K. H., Haynes M. P., Bauer P. M., Sanjay A., Collinge M., Baron R., Sessa W. C., Bender J. R. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 16468–16473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Le Romancer M., Treilleux I., Leconte N., Robin-Lespinasse Y., Sentis S., Bouchekioua-Bouzaghou K., Goddard S., Gobert-Gosse S., Corbo L. (2008) Mol. Cell 31, 212–221 [DOI] [PubMed] [Google Scholar]
- 50. Stefano G. B., Prevot V., Beauvillain J. C., Cadet P., Fimiani C., Welters I., Fricchione G. L., Breton C., Lassalle P., Salzet M., Bilfinger T. V. (2000) Circulation 101, 1594–1597 [DOI] [PubMed] [Google Scholar]
- 51. Stevis P. E., Deecher D. C., Suhadolnik L., Mallis L. M., Frail D. E. (1999) Endocrinology 140, 5455–5458 [DOI] [PubMed] [Google Scholar]
- 52. Temple J. L., Wray S. (2005) Endocrinology 146, 558–563 [DOI] [PubMed] [Google Scholar]
- 53. Rubio-Gayosso I., Sierra-Ramirez A., García-Vazquez A., Martinez-Martinez A., Muñoz-García O., Morato T., Ceballos-Reyes G. (2000) J. Cardiovasc. Pharmacol. 36, 196–202 [DOI] [PubMed] [Google Scholar]
- 54. Miller V. M., Li L., Sieck G. C. (2002) Vascul. Pharmacol. 38, 109–113 [DOI] [PubMed] [Google Scholar]
- 55. Goetz R. M., Thatte H. S., Prabhakar P., Cho M. R., Michel T., Golan D. E. (1999) Proc. Natl. Acad. Sci. U.S.A. 96, 2788–2793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Murata T., Lin M. I., Stan R. V., Bauer P. M., Yu J., Sessa W. C. (2007) J. Biol. Chem. 282, 16631–16643 [DOI] [PubMed] [Google Scholar]
- 57. Lu Q., Pallas D. C., Surks H. K., Baur W. E., Mendelsohn M. E., Karas R. H. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 17126–17131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Manavathi B., Acconcia F., Rayala S. K., Kumar R. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 15981–15986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Song R. X., McPherson R. A., Adam L., Bao Y., Shupnik M., Kumar R., Santen R. J. (2002) Mol. Endocrinol. 16, 116–127 [DOI] [PubMed] [Google Scholar]
- 60. Barletta F., Wong C. W., McNally C., Komm B. S., Katzenellenbogen B., Cheskis B. J. (2004) Mol. Endocrinol. 18, 1096–1108 [DOI] [PubMed] [Google Scholar]
- 61. Wyckoff M. H., Chambliss K. L., Mineo C., Yuhanna I. S., Mendelsohn M. E., Mumby S. M., Shaul P. W. (2001) J. Biol. Chem. 276, 27071–27076 [DOI] [PubMed] [Google Scholar]
- 62. Kumar P., Wu Q., Chambliss K. L., Yuhanna I. S., Mumby S. M., Mineo C., Tall G. G., Shaul P. W. (2007) Mol. Endocrinol. 21, 1370–1380 [DOI] [PubMed] [Google Scholar]
- 63. Rogatsky I., Trowbridge J. M., Garabedian M. J. (1999) J. Biol. Chem. 274, 22296–22302 [DOI] [PubMed] [Google Scholar]
- 64. Chen D., Washbrook E., Sarwar N., Bates G. J., Pace P. E., Thirunuvakkarasu V., Taylor J., Epstein R. J., Fuller-Pace F. V., Egly J. M., Coombes R. C., Ali S. (2002) Oncogene 21, 4921–4931 [DOI] [PubMed] [Google Scholar]
- 65. Cheng J., Zhang C., Shapiro D. J. (2007) Endocrinology 148, 4634–4641 [DOI] [PubMed] [Google Scholar]
- 66. Pedram A., Razandi M., Aitkenhead M., Hughes C. C., Levin E. R. (2002) J. Biol. Chem. 277, 50768–50775 [DOI] [PubMed] [Google Scholar]
- 67. Sengupta K., Banerjee S., Saxena N. K., Banerjee S. K. (2004) Mol. Cancer Res. 2, 150–158 [PubMed] [Google Scholar]
- 68. Grasselli A., Nanni S., Colussi C., Aiello A., Benvenuti V., Ragone G., Moretti F., Sacchi A., Bacchetti S., Gaetano C., Capogrossi M. C., Pontecorvi A., Farsetti A. (2008) Circ. Res. 103, 34–42 [DOI] [PubMed] [Google Scholar]
- 69. Simoncini T., Scorticati C., Mannella P., Fadiel A., Giretti M. S., Fu X. D., Baldacci C., Garibaldi S., Caruso A., Fornari L., Naftolin F., Genazzani A. R. (2006) Mol. Endocrinol. 20, 1756–1771 [DOI] [PubMed] [Google Scholar]
- 70. Chambliss K. L., Wu Q., Oltmann S., Konaniah E. S., Umetani M., Korach K. S., Thomas G. D., Mineo C., Yuhanna I. S., Kim S. H., Madak-Erdogan Z., Maggi A., Dineen S. P., Roland C. L., Hui D. Y., Brekken R. A., Katzenellenbogen J. A., Katzenellenbogen B. S., Shaul P. W. (2010) J. Clin. Invest. 120, 2319–2330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Razandi M., Pedram A., Levin E. R. (2000) J. Biol. Chem. 275, 38540–38546 [DOI] [PubMed] [Google Scholar]
- 72. Lu Q., Surks H. K., Ebling H., Baur W. E., Brown D., Pallas D. C., Karas R. H. (2003) J. Biol. Chem. 278, 4639–4645 [DOI] [PubMed] [Google Scholar]
- 73. Greif D. M., Kou R., Michel T. (2002) Biochemistry 41, 15845–15853 [DOI] [PubMed] [Google Scholar]
- 74. Haynes M. P., Sinha D., Russell K. S., Collinge M., Fulton D., Morales-Ruiz M., Sessa W. C., Bender J. R. (2000) Circ. Res. 87, 677–682 [DOI] [PubMed] [Google Scholar]