Abstract
A large fraction of mutations causing cystic fibrosis impair the function of the cystic fibrosis transmembrane conductance regulator (CFTR) chloride channel by causing reduced channel activity (gating defect) and/or impaired exit from the endoplasmic reticulum (trafficking defect). Such defects need to be treated with separate pharmacological compounds termed potentiators and correctors, respectively. Here, we report the characterization of aminoarylthiazoles (AATs) as compounds having dual activity. Cells expressing mutant CFTR were studied with functional assays (fluorescence-based halide transport and short circuit current measurements) to assess the effect of acute and chronic treatment with compounds. We found that AATs are effective on F508del, the most frequent cystic fibrosis mutation, which is associated with both a gating and a trafficking defect. AATs are also effective on mutations like G1349D and G551D, which cause only a gating defect. Evaluation of a panel of AAT analogs identified EN277I as the most effective compound. Incubation of cells expressing mutant CFTR with EN277I caused a strong stimulation of channel activity as demonstrated by single channel recordings. Compounds with dual activity such as AATs may be useful for the development of effective drugs for the treatment of cystic fibrosis.
Keywords: Chloride Channels, Cystic Fibrosis, Drug Action, Fluorescence, Trafficking, Pharmacotherapy
Introduction
Cystic fibrosis (CF)2 is one of the most frequent inherited diseases affecting 1 in ∼2,500 Caucasian individuals (1). CF is caused by mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene, which codes for a plasma membrane protein expressed at the apical surface of epithelial cells in the airways, intestine, pancreas, and other organs. CFTR functions as a chloride channel, activated through cAMP-dependent phosphorylation of the regulatory domain by protein kinase A (2). Until now, more than 1500 mutations have been described. They have been grouped into five classes according to the mechanisms through which they cause CFTR loss of function (3, 4). In particular, class II mutations cause a mistrafficking of CFTR that remains trapped in the endoplasmic reticulum and is subsequently degraded. Conversely, class III mutations (like G551D and G1349D) do not impair protein trafficking but severely decrease CFTR channel opening in response to cAMP elevation. The deletion of phenylalanine 508 (F508del), the most frequent CF mutation (∼70%), causes both a trafficking (5–7) and a channel gating defect (8, 9), although the latter one is less severe than that of class III mutants. In fact, when the F508del-CFTR protein is induced to traffic to the plasma membrane by incubation at low temperature or by overexpression, it shows a maximal open channel probability (Po) that is nearly 3-fold lower than that of the wild-type CFTR (8, 9).
Several studies have demonstrated the importance of class II and class III mutants as a target for pharmacotherapy. Indeed, mutant CFTR defects can be corrected in vitro using specific small molecules. In particular, the gating defect displayed by F508del and class III mutants can be ameliorated by small molecules called “potentiators” that have been found by screening libraries of chemical compounds (10–14) or by hypothesis-driven research (15, 16). Potentiators do not activate mutant CFTR by themselves but enhance channel activity above the level elicited by cAMP stimulation alone. In other words, maximal stimulation of mutant CFTR with a cAMP agonist evokes only a fraction (20–40% for F508del, less than 5–10% for G551D and G1349D) of the total activity obtained by also adding a potentiator (10, 11, 17–19). Mechanistic insight into the action of potentiators have been provided by several groups. For example, Cai and Sheppard (15) demonstrated that the CFTR potentiator phloxine B enhances the ATP dependence of CFTR channel gating by increasing ATP affinity and Po, whereas by studying the ATPase activity of purified F508del-CFTR, Wellhauser et al. (20) demonstrated that VRT-532 decreases ATP turnover, providing an explanation for how this agent enhances burst duration. Importantly, Ai et al. (16) used the ATP-driven NBD dimerization model of CFTR channel gating to explain how CFTR potentiators work. Typically, potentiators work with a fast mechanism of action. Maximal effect is reached within minutes of compound application.
High throughput screenings have also revealed the existence of small molecules, named correctors, that appear to correct the defective processing caused by F508del (21–23). Indeed, cell incubation with correctors rescues, at least partially, the mutant protein from the endoplasmic reticulum, allowing its targeting to the plasma membrane. However, the rescued F508del protein still shows a gating defect, thus requiring a potentiator to reach maximal activity. In contrast to potentiators, correctors act with a slow mechanism requiring several hours to obtain maximal effect.
It is evident that the pharmacotherapy of the basic defect for CF patients will need to be tailored according to patient genotype. Indeed, a corrector will be useful for all patients carrying the F508del mutation. Such patients may also require co-treatment with a potentiator. Conversely, individuals with class III mutation need only treatment with a potentiator. Considering the efforts and financial resources needed to develop each single drug, it would be highly desirable to identify molecules having a dual activity, i.e. acting at the same time as a potentiator and as a corrector.
In a previous study (21), we found that corr-2b, belonging to the chemical class of aminoarylthiazoles (AATs), acts as a corrector of F508del mistrafficking. However, this compound has also the interesting ability to improve F508del activity after long term incubation, a mechanism that seems different from that of classical CFTR potentiators. Here we describe the effects of chronic treatment with corr-2b and other AATs on F508del-CFTR and report, for the first time, that these compounds also act on class III mutants.
EXPERIMENTAL PROCEDURES
Cell Culture
Fischer rat thyroid (FRT) cells were stably transfected with F508del, G551D, or G1349D-CFTR (12). A549 were stably transfected with F508del-CFTR (24). The CF bronchial epithelial cell line CFBE41o-, with stable expression of F508del-CFTR, was obtained by Dr. J. P. Clancy (25). The three cell types were also transfected with the halide-sensitive yellow fluorescent protein (HS-YFP) YFP-H148Q/I152L (26). The culture media were: Coon's modified Ham's F-12 medium for FRT cells, DMEM/Ham's F12 (1:1) for A549 cells, and minimum essential medium for CFBE41o-. All of the media were supplemented with 10% fetal calf serum, 2 mm l-glutamine, 100 units/ml penicillin, and 100 μg/ml streptomycin. Primary cultures of human bronchial epithelial cells were cultured as described elsewhere (11, 21). For fluorescence assays of CFTR activity, FRT, A549, and CFBE41o- cells were plated (50,000 cells/well) on clear-bottomed 96-well black microplates (Corning Life Sciences, Acton, MA). For short circuit current experiments, 500,000 FRT or primary bronchial epithelial cells were seeded into Snapwell permeable supports (Corning Life Sciences).
Compound Synthesis
The pyridine (EN275 analogs) and aniline (EN277 analogs) derivatives were dissolved in anhydrous tetrahydrofuran at room temperature, and NaH (60% dispersion) was added. When the bubbling stopped, 2-Cl-4(4Br-phenyl)thiazole was added, and the reaction was heated to reflux. The reaction was monitored by TLC, using an n-hexane/ethyl acetate/acetic acid 8/3/0.1 mixture. After 2–24 h (according to the derivative), tetrahydrofuran was removed in vacuo, and the resulting solution was suspended in acidic water. The mixture was then extracted with CH2Cl2, dried with MgSO4, and filtrated. The organic phase was evaporated, and the precipitate was dissolved in tetrahydrofuran and lyophilized. The analysis of the final product was performed by HPLC-electrospray ionization-MS using an Agilent 1100 series LC/MSD ion trap instrument. The molecular structure was checked and confirmed by tandem mass analysis. Products were then purified by reverse phase HPLC on a Shimadzu LC-9A preparative HPLC equipped with a Phenomenex C18 Luna column. The solvent program was a gradient starting with 40% (for EN275 analogs) or 50% (for EN277 analogs) solvent A (0.1% TFA in water) for 5 min, linearly increasing to 90% solvent B (0.1% TFA in acetonitrile) in 30 min and up to 100% B in 5 min. The purity of the final products was further checked by electrospray ionization-MS. All of the compounds were synthesized according to the chemistry described by Bilodeau et al. (27), with substantial modifications.
HS-YFP Assay for CFTR Activity
Measurements of CFTR activity were carried out on FRT, A549, and CFBE41o cells expressing mutant CFTR and the HS-YFP 48 h after plating on microplates. Twenty-four hours after plating, the cells were incubated with test compounds at 37 °C for 20–24 h. At the time of assay, the cells were washed with PBS (containing 137 mm NaCl, 2.7 mm KCl, 8.1 mm Na2HPO4, 1.5 mm KH2PO4, 1 mm CaCl2, and 0.5 mm MgCl2) and stimulated for 30 min with forskolin (20 μm) in the absence or presence of genistein (50 μm). Then the cells were transferred to a microplate reader (FluoStar Galaxy; BMG Labtech GmbH, Offenburg, Germany) for CFTR activity determination. The plate reader was equipped with high quality excitation (HQ500/20X: 500 ± 10 nm) and emission (HQ535/30M: 535 ± 15 nm) filters for YFP (Chroma Technology Corp., Brattleboro, VT). Each assay consisted of a continuous 14-s fluorescence reading with 2 s before and 12 s after injection of an iodide-containing solution (PBS with Cl− replaced by I−; final I− concentration in the well, 100 mm). The data were normalized to the initial background-subtracted fluorescence. To determine fluorescence quenching rate (QR) associated with I− influx, the final 11 s of the data for each well were fitted with an exponential function to extrapolate initial slope (dF/dt).
Short Circuit Current Recordings
Experiments on FRT and bronchial epithelial cells were performed 7–10 days after seeding, when the transepithelial resistance was >1,000 Ω·cm2, by mounting the Snapwell inserts in a self-contained Ussing chamber system (vertical diffusion chamber; Corning Life Sciences). Transepithelial currents of FRT cells were measured using a transepithelial Cl− gradient. Accordingly, the basolateral solution contained 130 mm NaCl, 2.7 mm KCl, 1.5 mm KH2PO4, 1 mm CaCl2, 0.5 mm MgCl2, 10 mm Na-Hepes (pH 7.3), and 10 mm glucose. For the apical side, this solution was modified by replacing half of the NaCl with sodium gluconate and increasing CaCl2 to 2 mm to compensate for calcium buffering caused by gluconate. During experiments, solutions in both chambers were continuously bubbled with air. For bronchial epithelial cells, the apical and basolateral side contained the same solution (126 mm NaCl, 0.38 mm KH2PO4, 2.1 mm K2HPO4, 1 mm MgSO4, 1 mm CaCl2, 24 mm NaHCO3, and 10 mm glucose) bubbled with 5% CO2 in air. The hemichambers were connected to DVC-1000 voltage clamps (World Precision Instruments, Inc., Sarasota, FL) via Ag/AgCl electrodes and 1 m KCl agar bridges. Transepithelial currents were digitized using PowerLab 4/25 data acquisition systems and stored on a personal computer. All of the measurements were done at 37 °C.
Drug Uptake Experiments
FRT cells expressing F508del-CFTR were plated at high density on six-well plates. After 24 h, when cells reached 100% confluency, the cells were washed with PBS and then incubated with PBS (750 μl) containing Me2SO, genistein (50 μm), or EN277I (50 μm). After 30 s, 10 min, and 30 min, the PBS was removed, and the cells were briefly washed with 10% methanol in PBS (to remove completely not internalized compound) and then lysed in methanol (750 μl). Concentration of compounds in lysates was measured by HPLC. Briefly, the samples were centrifuged at 14,000 rpm for 5 min, and 100 μl of supernatant were loaded on a 4.6 × 250 mm C18 reversed phase Luna column packed with 3.5-mm particle size stationary phase (Phenomenex, Torrance, CA). The analysis was performed in isocratic mode with a water/acetonitrile 45/55 mobile phase added with formic acid 0.1% v/v. The flow rate was set to 0.8 ml/min. The 250-nm absorbance peaks corresponding to genistein and EN277I were identified on the basis of elution times. The peak areas were used to calculate concentrations in the original lysate. Intracellular concentration of compounds was determined considering a cell volume calculated from the surface and average thickness of the confluent cell monolayer (thickness measured with a confocal microscope).
Immunoprecipitation and Western Blots
The cells grown on 100-mm-diameter dishes were lysed in lysis buffer (20 mm Hepes, pH 7, 150 mm NaCl, 1 mm EGTA, 1% Igepal) containing Complete Protease Inhibitor Mixture (Roche Applied Science). After preclearing of lysates with pansorbin (Calbiochem), CFTR was immunoprecipitated in radioimmune precipitation assay buffer (50 mm Tris, pH 8.0, 150 mm NaCl, 1% Triton X-100, 1% sodium deoxycholate, 0.1% SDS) containing 2 mm Mg-ATP, using a mouse monoclonal anti-CFTR C-terminal antibody (24-1; R & D System) and pansorbin. The immunoprecipitates were subjected to SDS-PAGE and analyzed by Western blotting. Proteins were immunodetected by a mouse monoclonal anti-CFTR antibody (M3A7; Millipore) followed by HRP-conjugated anti-mouse IgG and visualized by chemiluminescence with the LiteAblot Turbo kit (Euroclone). Direct recording of the chemiluminescence was performed using the Molecular Imager ChemiDoc XRS system and quantification using the Image J software (National Institutes of Health).
Patch Clamp Experiments
Single channel analysis was done on FRT cells stably expressing CFTR with G1349D mutation in cell-attached and inside-out configurations. Borosilicate glass pipettes were pulled on a vertical two-step puller to a final resistance of 4–6 MΩ measured in the working solution. The pipette solution contained 150 mm N-methyl-d-glucamine chloride, 3 mm CaCl2, 2 mm MgCl2, 10 mm Na-Hepes (pH 7.3). The bath solution in cell attached experiments was 150 mm NaCl, 1 mm CaCl2, 1 mm MgCl2, 10 mm glucose, 10 mm mannitol, and 10 mm Na-Hepes (pH 7.4). The bath solution in inside-out patch experiments was instead 150 mm N-methyl-d-glucamine chloride, 2 mm MgCl2, 10 mm EGTA, 10 mm Hepes, 1 mm ATP (pH 7.3). The experiments were done at room temperature (22–24 °C). To activate CFTR in cell-attached patches, the bath was supplemented with 500 μm CPT-cAMP. To activate CFTR in inside-out configurations, the bath solution was supplemented with 125 nm catalytic subunit of protein kinase A (recombinant; Promega). The cell membrane was voltage-clamped at −60 mV using an EPC-7 patch clamp amplifier (List Medical). The data were low pass-filtered at 400 Hz and digitized at 1000 Hz using an Instrutech ITC-16 AD/DA interface and the PULSE software (Heka).
The experiments were analyzed using IgorPro (Wavemetrics, Lake Oswego, OR). The amplitude of single-channel currents was estimated from the fit of Gaussian distributions of the current amplitude histograms. The apparent number of channels present on a membrane patch was assumed to be equal to the maximal number of simultaneously open channels (28). Open channel probability (Po), mean burst duration, and interburst interval were calculated as described previously (29), by ignoring closures shorter than 20 ms, to discard the brief closures within a burst. Analysis was done on traces lasting 250–1500 s (mode ∼750 s). To analyze mean burst duration and interburst interval on patches containing more than one channel, we utilized an algorithm (30) previously used for K+ channels (31) and for CFTR (32). Briefly, the mean burst duration in multichannels was obtained from the following equation,
 |
where tj is the time that j channels open at the same time, and n is the number of open to close transitions. The interburst interval was obtained from the following equation.
 |
Data Analysis
The data for all of the experiments are presented as representative traces or as the means ± S.E. The statistical analyses were done using the Student's t test for unpaired data. The differences between two groups of data were considered statistically significant when p < 0.05.
RESULTS
AATs Correct Both the Trafficking and the Gating Defects of F508del-CFTR
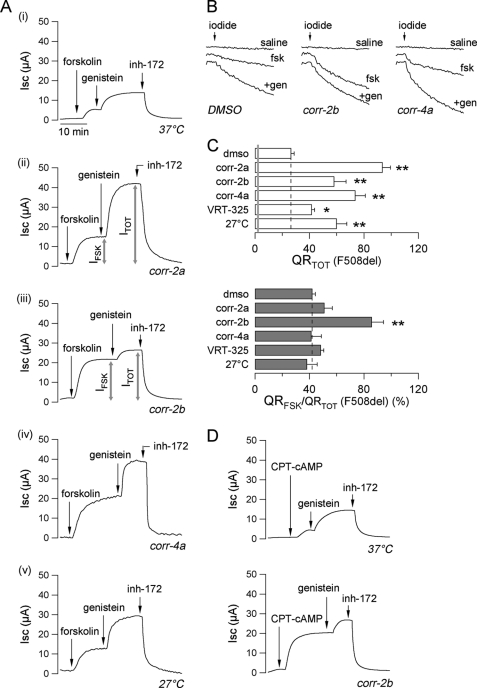
Fig. 1A shows results obtained by measuring transepithelial Cl− currents in FRT cells expressing the F508del-CFTR mutant. The cells were kept under control conditions or exposed to different treatments including correctors or incubation at low temperature (27 °C). Untreated cells (Fig. 1A, trace i) had small responses to the cAMP-elevating agent forskolin (an adenylate cyclase activator, 20 μm) and to the subsequent addition of the potentiator genistein (50 μm). At the end of the experiment, the addition of CFTRinh-172 (10 μm), a selective CFTR inhibitor (33), caused a full block of the transepithelial Cl− currents elicited by forskolin plus genistein. The size of the current blocked by CFTRinh-172 (corresponding to the total CFTR-mediated current evoked by stimulation with forskolin plus genistein, named ITOT) may be considered a parameter reflecting the total amount of CFTR that is available for activation in the plasma membrane. Treatment with correctors (Fig. 1A, traces ii and iv) or with low temperature (Fig. 1A, trace v) increased ITOT by 2–4-fold. Instead, corr-2b (Fig. 1A, trace iii) displayed a peculiar behavior. Compared with the other types of treatment, this compound enhanced the fraction of the total current that is activated by forskolin alone (IFSK/ITOT, expressed as a percentage). Although for the other treatments IFSK/ITOT was ∼30–40% (not different from untreated cells), for corr-2b this parameter was ∼80% (Fig. 1A). This behavior was evident also when CFTR activity was evaluated using the HS-YFP assay (Fig. 1, B and C). In this assay, the rate of fluorescence quenching (QR) caused by I− influx reflects the extent of CFTR activity in the plasma membrane (Fig. 1B). All of the rescue maneuvers, i.e. low temperature and correctors, caused an increase in the total CFTR activity elicited by forskolin plus genistein (QRTOT; see Fig. 1C, top panel). However, corr-2b was the only compound having the ability to enhance the fraction of halide transport that is stimulated by forskolin alone (QRFSK/QRTOT, expressed as a percentage; see Fig. 1C, bottom panel). To verify whether corr-2b modulates the cAMP signaling, for example by up-regulating adenylate cyclase or down-regulating phosphodiesterases, we repeated the experiments using 8-chlorophenylthio-cAMP (CPT-cAMP) instead of forskolin. CPT-cAMP is a membrane-permeable cAMP analog that is not hydrolyzable by phosphodiesterases. Therefore, it acts directly on the cAMP-dependent protein kinase A, bypassing the steps controlling cAMP levels. Representative short circuit current traces are shown in Fig. 1D. The AAT corr-2b was able to enhance the relative response to CPT-cAMP, to an extent similar to that observed using forskolin.
FIGURE 1.
Corrector activity on F508del-CFTR. A, representative traces of transepithelial chloride currents measured in Ussing chambers on F508del-CFTR-expressing FRT epithelia incubated for 24 h at 37 °C with the indicated compounds (corr-2b, 50 μm; other correctors, 10 μm) or at 27 °C. The concentrations were: forskolin, 20 μm; genistein, 50 μm; CFTRinh-172, 10 μm. B, representative traces recorded in FRT cells showing iodide influx after 24 h of incubation at 37 °C with vehicle alone (Me2SO, DMSO), corr-4a (10 μm), or corr-2b (50 μm) and under nonstimulated condition (saline) or upon acute stimulation with forskolin (fsk, 20 μm) alone or plus genistein (+gen, 50 μm). C, total CFTR activity elicited by forskolin (20 μm) plus genistein (50 μm) (QRTOT, top panel) and relative activity stimulated by forskolin alone (QRFSK/QRTOT, bottom panel) in FRT cells treated for 24 h at 37 °C with the indicated compounds (corr-2b, 50 μm; other correctors, 10 μm) or at 27 °C (means ± S.E., n = 8). The gray line indicates the activity measured under nonstimulated condition. *, p < 0.05; **, p < 0.01 versus Me2SO. D, representative Ussing traces recorded on F508del-CFTR-expressing FRT epithelia incubated for 24 h at 37 °C in the absence or presence of corr-2b (50 μm). The concentration of CPT-cAMP was 200 μm.
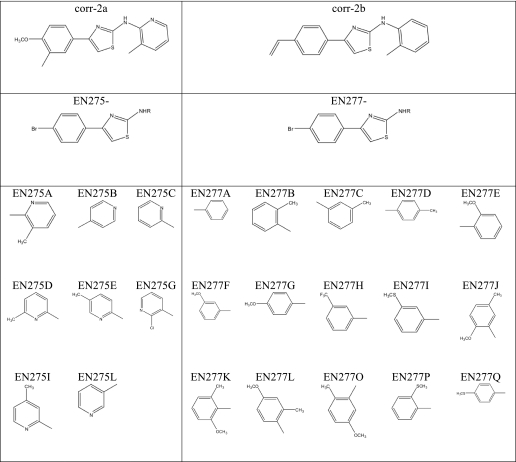
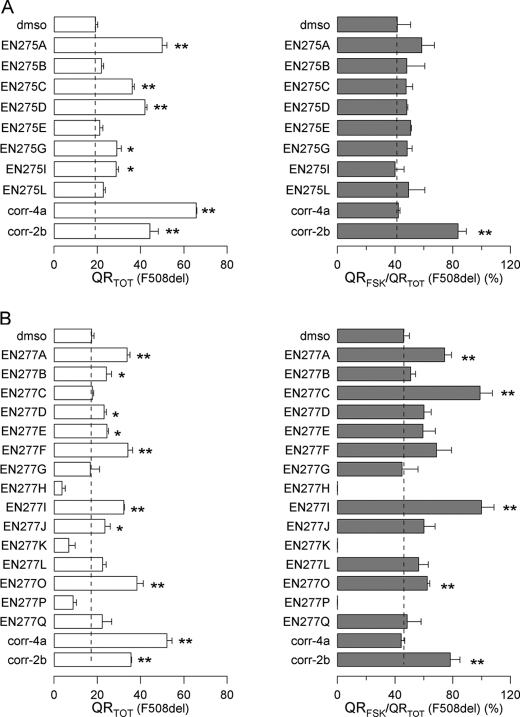
To explore the structure-activity relationship of AATs as dual acting compounds, we synthesized two small panels of analogs, based on the scaffold of corr-2b and corr-2a (the progenitor of the class, described in Ref. 21). The structures are displayed in Table 1. The new compounds were tested on FRT cells expressing F508del-CFTR using the HS-YFP assay (Fig. 2). Many corr-2a analogs were able to rescue the mutant CFTR to the plasma membrane, causing an increase in total activity, defined as QRTOT (Fig. 2A, left panel). However, none of them was able to enhance the relative response to forskolin, i.e. QRFSK/QRTOT (Fig. 2A, right panel). The results obtained from the panel of corr-2b analogs were more interesting. Indeed, some compounds enhanced total F508del-CFTR activity (Fig. 2B, left panel) and, at the same time, improved the QRFSK/QRTOT, similarly to corr-2b (Fig. 2B, right panel). Particularly interesting was compound EN277I, which was comparable with corr-2b as a corrector but was better as an enhancer of forskolin response (Fig. 2B). In particular, EN277I acted at lower concentrations (25 μm versus 50 μm). Other compounds, such as EN277C, were effective mainly on the forskolin response. In contrast, a classical corrector, corr-4a, was effective only on total activity. Such results indicate that the “dual activity” is not a general feature of the AAT chemical scaffold but is instead linked to particular substituents.
TABLE 1.
Analogs of corr-2a and corr-2b
The table shows, for each analog, the chemical group that substitutes the group indicated as R in the parent structure.
FIGURE 2.
Activity of analogs of corr-2a and corr-2b on F508del-CFTR. A and B, total CFTR activity elicited by forskolin (20 μm) plus genistein (50 μm) (QRTOT, left panels) and relative activity stimulated by forskolin alone (QRFSK/QRTOT, right panels) in FRT cells treated for 24 h at 37 °C with analogs of corr-2a (A) or analogs of corr-2b (B) (means ± S.E., n = 8). The concentrations were: corr-4a, 10 μm; corr-2b, 50 μm; other compounds, 25 μm. *, p < 0.05; **, p < 0.01 versus Me2SO (dmso).
Activity of AATs on F508del-CFTR Is Independent of Cell Background and Additive to Low Temperature Rescue
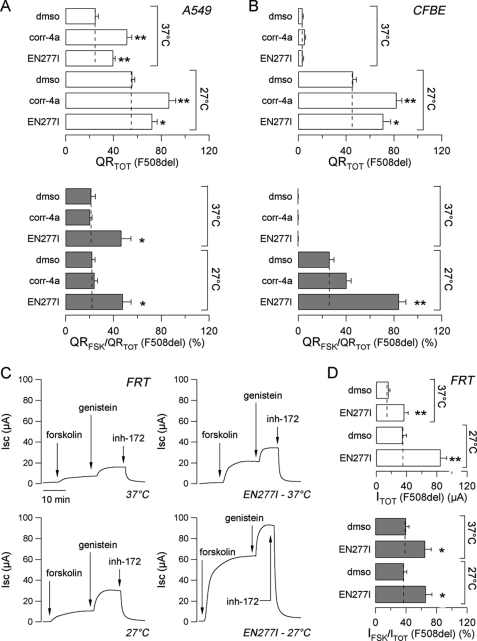
To verify whether AATs are effective also on a different cell background, we used the HS-YFP assay to test compound EN277I on other two cell lines, A549 and CFBE41o-, expressing F508del-CFTR (Fig. 3, A and B). On A549 cells, long term treatment with EN277I caused a significant increase in QRTOT. The corrector activity of EN277I, as well as that of corr-4a, was additive to that of low temperature (Fig. 3A, top panel). The additivity of correctors and low temperature has been already shown previously (21, 24). At the same time, EN277I, but not corr-4a, enhanced the relative response to forskolin, QRFSK/QRTOT (Fig. 3A, bottom panel). In CFBE41o- cells, the activity detected at 37 °C was almost negligible, even in the presence of correctors. However, when the cells were grown at 27 °C, there was a strong rescue of total F508del activity that was further increased by co-treatment with corr-4a or EN277I (Fig. 3B, top panel). Under these conditions, i.e. after low temperature rescue, EN277I strongly enhanced QRFSK/QRTOT (Fig. 3B, bottom panel).
FIGURE 3.
AAT activity on F508del-CFTR in different cell backgrounds. A and B, total CFTR activity elicited by forskolin (20 μm) plus genistein (50 μm) (QRTOT, top panel) and relative activity stimulated by forskolin alone (QRFSK/QRTOT, bottom panel) in A549 (A) and CFBE41o- (B) cells treated for 24 h at 37 or 27 °C with the indicated compounds (means ± S.E., n = 8) measured using the HS-YFP assay. The concentrations were: corr-4a, 10 μm; EN277I, 30 μm. *, p < 0.05; **, p < 0.01 versus Me2SO (dmso). C, representative traces of chloride current measured in Ussing chambers on F508del-CFTR-expressing FRT epithelia incubated for 24 h at 37 or 27 °C in the absence or presence of EN277I (30 μm). The concentrations were: forskolin, 20 μm; genistein, 50 μm; CFTRinh-172 (inh-172), 10 μm. D, total CFTR activity elicited by forskolin (20 μm) plus genistein (50 μm) (ITOT, top panel) and relative activity stimulated by forskolin alone (IFSK/ITOT, bottom panel) in Ussing chamber experiments performed on FRT cells. *, p < 0.05; **, p < 0.01 versus Me2SO.
The additive effect induced by combined treatment with low temperature, and EN277I was confirmed also in FRT cells expressing F508del-CFTR using the HS-YFP assay (not shown) and in short circuit current recordings (Fig. 3, C and D). Indeed, incubation of FRT epithelia for 24 h at 27 °C or with EN277I caused a 2-fold increase in ITOT, as compared with control condition (Fig. 3D, top panel). At the same time, long term treatment with EN277I, but not low temperature incubation, enhanced the fraction of the total current activated by forskolin alone, with the IFSK/ITOT percentage increasing from ∼20–30% to ∼60–70% (Fig. 3D, bottom panel). Combination of low temperature and EN277I caused a 4–5-fold increase in the ITOT value (Fig. 3D, top panel), along with an enhancement of IFSK/ITOT (Fig. 3D, bottom panel).
Correction of F508del-CFTR Gating Defect Is Not Due to Potentiator-like Activity and Requires New Protein Synthesis
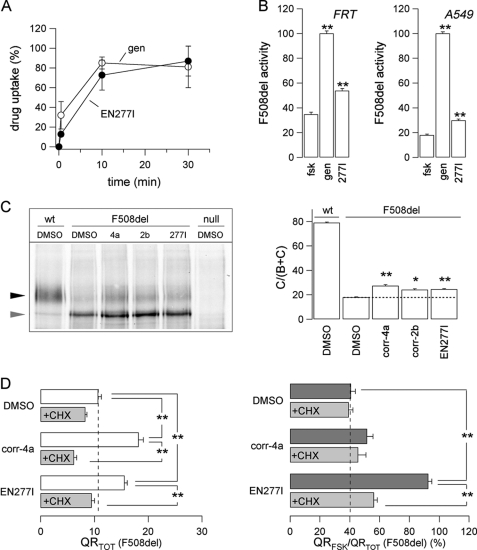
We have shown previously that corr-2b does not behave as a classical potentiator because acute application is ineffective in enhancing CFTR activity (21). However, we considered the possibility that AATs are indeed potentiators but with a low membrane permeability, thus requiring a long incubation to enter the cells and potentiate CFTR channel activity. To verify this hypothesis, we measured drug uptake in FRT cells, comparing the uptake of EN227I to that of genistein, the reference compound for potentiators (Fig. 4A). We found that the uptake time courses of the two compounds were very similar. Although the uptake of EN277I was slightly slower than that of genistein, it was almost complete by 10 min after drug exposure. At this time, the intracellular concentration of the compounds was ∼80% of the extracellular concentration, indicating that AAT permeability through the plasma membrane is relatively high. We tested also the ability of EN277I to act as a classical F508del-CFTR potentiator, i.e. by treating the FRT and A549 cells for only 30–45 min (Fig. 4B). These experiments, carried out with the HS-YFP assay, demonstrated that EN277I can actually cause a significant increase in the activity elicited by forskolin alone. However, this effect was by far smaller than that elicited by genistein (Fig. 4B).
FIGURE 4.
Analysis of AAT mechanism of action. A, time course of drug uptake of indicated compounds measured in FRT cells (means ± S.E., n = 3). B, maximal effect obtained for EN277I and genistein (50 μm for both compounds) tested as potentiators (30–45 min of incubation) of F508del-CFTR in the presence of forskolin (20 μm) in FRT (left panel) and A549 (right panel) cells, normalized to genistein effect (means ± S.E., n = 8). **, p < 0.01 versus forskolin alone. C, biochemical analysis of activity of the indicated correctors on the expression pattern of F508del-CFTR in A549 cells. The cells were cultured for 24 h at 37 °C with or without correctors. The concentrations were: corr-4a, 10 μm; corr-2b, 50 μm; EN277I, 30 μm. CFTR was visualized using anti-CFTR primary and HRP-conjugated secondary antibodies (left panel). Black arrowhead, complex-glycosylated form (band C); gray arrowhead, core-glycosylated form (band B). The right panel shows the quantification of CFTR maturation. The data are expressed as relative abundance of band C, i.e. as band C/(band B + band C) (means ± S.E., n = 5). *, p < 0.05; **, p < 0.01 versus Me2SO (DMSO). D, total CFTR activity elicited by forskolin (20 μm) plus genistein (50 μm) (QRTOT, left panel) and relative activity stimulated by forskolin alone (QRFSK/QRTOT, right panel) in FRT cells treated for 24 h at 37 °C with indicated compounds in the absence or presence of CHX. The concentrations were: corr-4a, 10 μm; EN277I, 30 μm; CHX, 150 μm (means ± S.E., n = 8). **, p < 0.01 versus indicated condition.
The efficacy of EN277I and corr-2b to act as correctors of the F508del trafficking defect was also evaluated at the biochemical level by determining the pattern of electrophoretic mobility of the CFTR protein (Fig. 4C). Wild-type CFTR is detected in Western blots as two bands, named B and C, of nearly 150 and 170 kDa, respectively. Band B corresponds to partially glycosylated CFTR residing in the endoplasmic reticulum. Band C is instead the mature fully processed CFTR that has passed through the Golgi. The prevalent form in cells expressing wild-type CFTR is band C, as shown for A549 cells in Fig. 4C. In contrast, cells expressing F508del-CFTR have mostly band B, in agreement with the severe trafficking defect caused by the mutation. Treatment of F508del cells with corr-4a, EN277I, or corr-2b caused a modest but significant increase in the relative abundance of mature CFTR (Fig. 4C), i.e. the ratio of band C intensity to that of total CFTR (band C + band B). Correctors also increased the absolute intensity of band B.
The activity of EN277I and, as a positive control, of corr-4a was examined in F508del-CFTR FRT cells upon inhibition of protein synthesis by cycloheximide (CHX), a translation elongation inhibitor (Fig. 4D). When the cells were treated chronically with corr-4a or EN277I in the presence of CHX, the increase in total activity, QRTOT, was abolished (Fig. 4D, left panel). Similarly, CHX also abolished activity of EN277I as a gating enhancer, as reflected by the decrease of the QRFSK/QRTOT value (Fig. 4D, right panel). These results indicate that not only the rescue of trafficking but also the correction of the gating defect by the AAT requires newly synthesized protein.
Evaluation of AATs in Primary Cells
Primary bronchial epithelial cells from a F508del homozygote patient were used for short circuit current recordings. The cells were treated for 18–24 h with vehicle (Me2SO), 25 μm EN277I, or 5 μm corr-4a. During recordings, the cells were sequentially treated with amiloride (10 μm), to block Na+ absorption, followed by CPT-cAMP (200 μm) plus genistein (50 μm). The activity of F508del-CFTR was estimated from the amplitude of current blocked by 10 μm CFTRinh-172 (not shown). In vehicle-treated cells, this current was 0.77 ± 0.14 μA/cm2 (n = 8). Treatment with EN277I increased the current by 48% (1.14 ± 0.08 μA/cm2, n = 6), but the effect did not reach statistical significance (p > 0.05). The current with corr-4a was instead 1.69 ± 0.18 μA/cm2 (n = 5; p < 0.05).
AATs Can Correct the Gating Defect of Class 3 Mutants upon Chronic Treatment
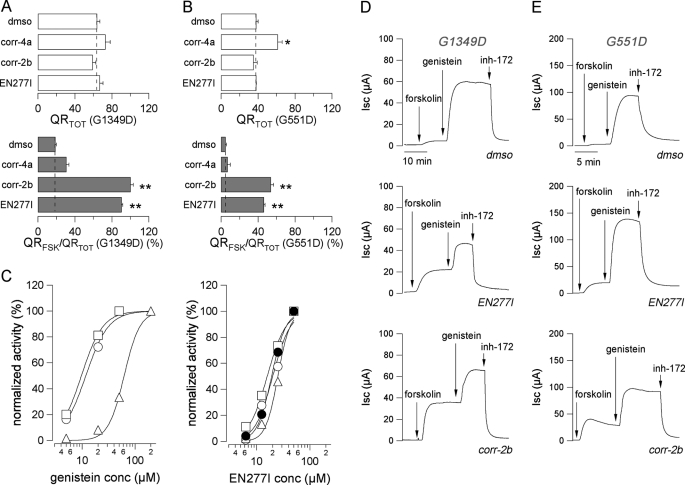
Given the effect of AATs on F508del-CFTR channel gating, we asked whether they are effective also on class 3 mutants. Therefore, we tested corr-2b and EN277I on FRT cells expressing G1349D-CFTR or G551D-CFTR mutant, using the HS-YFP assay and short circuit current recordings (Fig. 5). On G1349D-CFTR cells, long term treatment with AATs or corr-4a did not change QRTOT (Fig. 5A, top panel). However, AATs caused a marked increase in QRFSK/QRTOT (Fig. 5A, bottom panel). Similarly, on G551D-CFTR FRT cells, AATs were ineffective on the absolute QRTOT value (Fig. 5B, top panel), whereas they significantly increased QRFSK/QRTOT (Fig. 5B, bottom panel). In contrast, corr-4a slightly increased total activity but did not affect the relative response to forskolin (Fig. 5B).
FIGURE 5.
Effect of AATs on class 3 mutants. A and B, the bar graphs show the total CFTR activity elicited by forskolin (20 μm) plus genistein (50 μm for G1349D and 200 μm for G551D) (QRTOT, top panels) and the relative activity stimulated by forskolin alone (QRFSK/QRTOT, bottom panels) in FRT cells expressing G1349D-CFTR (A) or G551D-CFTR (B) treated for 24h at 37 °C with the indicated compounds (means ± S.E., n = 8). The concentrations were: corr-4a, 10 μm; corr-2b, 50 μm; EN277I, 30 μm. *, p < 0.05; **, p < 0.01 versus Me2SO (DMSO). C, comparison between dose-response relationships of genistein (left panel) and EN277I (right panel) in correcting F508del-CFTR trafficking defect (●, for EN277I only) or in increasing the relative forskolin response of F508del-CFTR (○), G1349-CFTR (□), or G551D-CFTR (△). The error bars are smaller than symbol size. Each dose-response relationship derives from four experiments. D and E, representative traces of chloride current measured in Ussing chambers on G1349D-CFTR (D) and G551D-CFTR (E) FRT epithelia incubated for 24 h at 37 °C in the absence or presence of EN277I (30 μm) or corr-2b (50 μm). The concentrations were: forskolin, 20 μm; genistein, 50 μm for G1349D and 200 μm for G551D; CFTRinh-172 (inh-172), 10 μm.
Using the HS-YFP assay, we compared the concentrations of EN277I and genistein that are effective in improving the relative forskolin response of F508del-, G1349D-, and G551D-CFTR mutants (Fig. 5C). In parallel, we also determined the dose response of EN277I as a corrector of F508del-CFTR mistrafficking. As expected from previous studies (10, 11, 17), we found that the concentrations of genistein required to activate G551D-CFTR were 5–10 times higher than those effective on the other two mutants (Fig. 5C, left panel). Indeed the Kd values for genistein were: 11.8 ± 1.5 μm for F508del; 9.7 ± 0.7 μm for G1349D; and 62.4 ± 5.3 μm for G551D. Although the net potentiating effect of genistein at very high concentrations may be partially diminished by open channel block (34), it has been repeatedly found that G551D responds to genistein and other potentiators with significantly reduced affinity (10, 11, 17). This behavior may be interpreted as a direct or allosteric alteration of the binding site for potentiators caused by the G551D mutation. In contrast to genistein, EN277I enhanced the forskolin response of the three mutants and corrected the mistrafficking of F508del-CFTR with comparable potency (Fig. 5C, right panel). The Kd values were as follows: 19.2 ± 0.8 and 20.1 ± 2.2 μm for F508del (correction of trafficking and gating defects, respectively); 15.8 ± 1.1 μm for G1349D; and 25.1 ± 1.9 μm for G551D. These results may suggest that EN277I acts on the different defects through a common mechanism of action that is different from that of classical potentiators.
The ability of AATs to correct the gating defect of class 3 mutants after long term treatment was confirmed performing short circuit current recordings on FRT cells expressing G1349D- or G551D-CFTR (Fig. 5, D and E). CFTR activity in untreated cells was very small or even undetectable (in the case of G551D), despite the use of a maximal forskolin concentration. As expected, subsequent addition of genistein elicited a large current increase caused by CFTR potentiation. Incubation of G1349D cells for 24 h with EN277I or corr-2b did not change the absolute ITOT value, as compared with control condition but enhanced the percentage of the total current activated by forskolin alone, with IFSK/ITOT increasing from <10% to ∼50%, as shown in Fig. 5D (control, IFSK = 8.0 ± 1.6 μA; corr-2b, IFSK = 35.5 ± 1.5 μA, p < 0.01; EN277I, IFSK = 31.5 ± 1.8 μA, p < 0.01; control, ITOT = 70 ± 6 μA; corr-2b, ITOT = 69 ± 8 μA, not significant; EN277I, ITOT = 72 ± 7 μA, not significant). The effect of AATs on G551D cells was qualitatively similar, although the increase in IFSK/ITOT was smaller (from <5% to ∼20–30%; see Fig. 5E) (control, IFSK = 3.9 ± 0.5 μA; corr-2b, IFSK = 29 ± 3 μA, p < 0.01; EN277I, IFSK = 22 ± 4 μA, p < 0.01; control, ITOT = 79 ± 4 μA; corr-2b, ITOT = 80 ± 8 μA, not significant; EN277I, ITOT = 82 ± 9 μA, not significant).
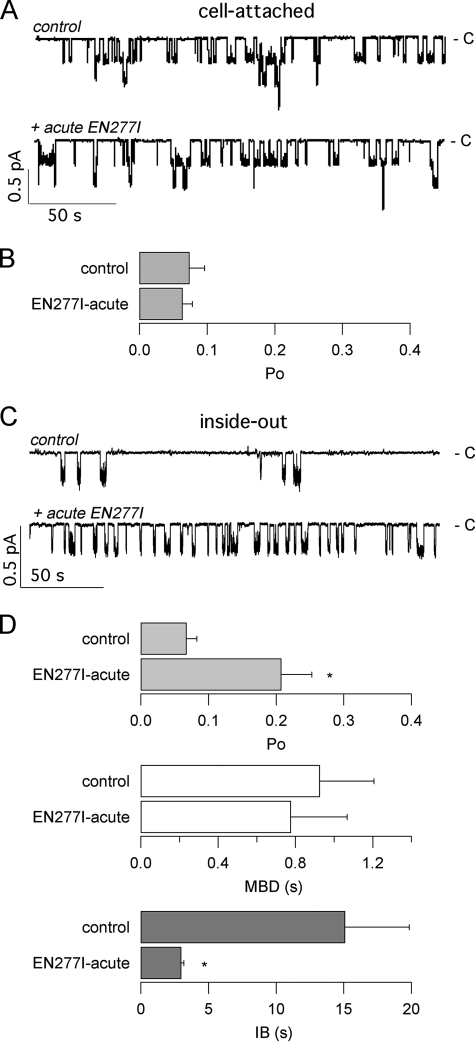
The activity of EN277I on G1349D-CFTR expressed in FRT cells was examined in patch clamp experiments, in cell-attached and in inside-out patch configurations following acute or chronic exposure. Fig. 6A shows representative recordings in the cell-attached configuration. The low channel activity measured under control conditions (i.e. in the presence of 500 μm CPT-cAMP) was not modified by acute treatment with EN277I (Fig. 6, A and B). EN277I was also tested on inside-out patches, in the presence of the catalytic subunit of the protein kinase A to induce maximal phosphorylation (Fig. 6, C and D). Under this condition, acute stimulation with EN277I caused a ∼3-fold increase in the Po value. Analysis of gating kinetics indicated that the increase in Po was caused by a reduction in the interburst time rather than by an increase in the mean burst duration (Fig. 6D). It is worth noting that EN277I reduced the current amplitude of the channel from 0.28 ± 0.03 to 0.23 ± 0.02 in the presence of 30 μm EN277I (n = 4; p < 0.05). This effect, which is similar to those of other CFTR potentiators like genistein and phloxine B, which act as open channel blockers (15, 34), suggests that EN277I is also a weak blocker of the CFTR pore.
FIGURE 6.
Patch clamp analysis of the acute effect of compound EN277I on G1349D-CFTR. A, representative traces recorded at −60 mV in cell-attached configuration before (control) and after acute application of 30 μm EN277I to FRT cells stimulated with 500 μm CPT-cAMP. The closed channel level is indicated (c), and downward deflections reflect channel opening. The apparent number of channels in this patch was three. B, mean open channel probability (Po) from eight and six cell-attached patches in the absence and presence and EN277I, respectively. C, representative recordings in inside-out configuration before (control) and after the addition of 30 μm EN277I. The bath (intracellular) solution contained 1 mm ATP and 125 nm PKA. D, mean Po, mean burst duration (MBD), and interburst interval (IB) (and S.E.) obtained from five different experiments in inside-out patches. The asterisks indicate values that were statistically different from control conditions (p < 0.05).
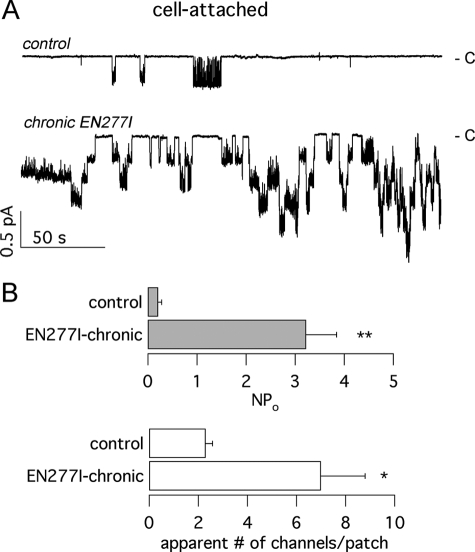
We examined also the effect of chronic treatment of G1349D-CFTR cells with EN277I (Fig. 7). Representative patch clamp recordings in cell-attached configuration are presented in Fig. 7A showing a dramatic increase in the activity following long term incubation with the AAT compound. Cells treated with the vehicle alone showed low G1349D-CFTR activity, with rare openings separated by long channel closings. Cells treated chronically with EN277I displayed a markedly increased activity, with a large occurrence of simultaneous multichannel openings (Fig. 7). In particular, we found that EN277I increased NPo by nearly 25-fold and the apparent number of channels by 3-fold (Fig. 7B).
FIGURE 7.
Patch clamp analysis of the chronic effect of compound EN277I on G1349D-CFTR. A, representative traces obtained at −60 mV in cell-attached configuration in the presence of 500 μm CPT-cAMP. The cells were in control conditions or incubated for ∼24 h with 30 μm EN277I. The closed level is indicated on the right. B, mean value and S.E. of NPo (top) and apparent number of channels (bottom) from eight and five experiments in control conditions and after treatment with EN277I, respectively. * and **, statistically significant differences between conditions (p < 0.05 and 0.01, respectively).
DISCUSSION
In the last years, there has been an increasing effort to identify small molecules able to correct the phenotypic effects of CF mutations. At the molecular level, most CF mutations impair the function of CFTR protein by altering the protein targeting to the plasma membrane and/or by causing an abnormally low open channel probability (3, 4). A number of structurally unrelated CFTR chemical modulators, namely correctors (to correct the protein mistrafficking) and potentiators (to restore the normal open channel probability), have been already discovered (10–14, 21–23). Until now, the ability to correct either the processing or the gating defect seemed to be exerted by distinct chemical entities. To treat the F508del-CFTR mutant, which has both the trafficking and gating defects, CF patients should receive two distinct drugs. This may be a problem given the amount of work and the financial resources needed for the preclinical and clinical evaluation of each single drug. A possible way to address this problem is the identification of dual acting drugs, i.e. molecules able to correct the protein mistrafficking and to stimulate mutant channel activity.
AATs were previously identified in a high throughput search for F508del correctors (21). Typically, correctors enhance the total F508del-CFTR activity that can be elicited by combined treatment with a cAMP-elevating agent (e.g. forskolin) plus a potentiator but do not change the fraction of activity that is elicited by the cAMP signal alone. This effect is interpreted as an increase in the number of CFTR channels in the plasma membrane because of rescue of the mutant protein from the endoplasmic reticulum. However, these rescued channels still maintain a gating defect so that a potentiator is required to further increase mutant CFTR function. In contrast to “conventional” correctors, AATs improve both trafficking and gating. In the present study, we have further evaluated the properties of AATs as dual acting compounds. First, we have found that AAT dual activity is not affected by cell background because it was detected in FRT, A549, as well as in CFBE41o- cells expressing F508del-CFTR. Second, AATs do not act as classical potentiators because they require long term incubation to maximally improve gating. The mechanism of action appears to require new protein synthesis because it is blocked by cycloheximide. Actually, we found that AATs have some acute effect on channel activity, but this effect is markedly smaller than that obtained after chronic treatment. Indeed channel activity increased by ∼3- and ∼25-fold after acute and chronic treatment, respectively. Third, AATs also improve F508del-CFTR trafficking to the plasma membrane as demonstrated by functional and biochemical assays. Although the extent of F508del rescue is comparable with that of a “classical” corrector such as corr-4a, it is still far from full correction of trafficking defect.
We have also tested AATs on pure class III mutants, namely G1349D and G551D, the latter being the one with the most severe gating defect. Interestingly, we found that dual acting AATs also enhance the fraction of G1349D and G551D activity that is evoked by cAMP, in the absence of the potentiator. The extent of gating enhancement is larger for G1349D relative to the other mutant. In contrast, the total activity for these two mutants, which have no trafficking defect, was not altered by AATs. The effect of AATs on class III mutants is very intriguing and never reported before for other types of F508del correctors such as corr-4a.
At the moment, the mechanism of action of AATs is unclear. F508del correctors may in theory act as pharmacological chaperones or proteostasis regulators (35). The former class of molecules is predicted to bind directly to the CFTR protein favoring folding and trafficking. The latter class of molecules instead modulates the activity of other proteins affecting CFTR processing. We do not have enough information to make a conclusion about the mechanism of action of AATs as dual acting compounds. We can speculate that AATs interact with CFTR itself or other proteins promoting long lasting effects on CFTR protein conformation or post-translational modifications leading to improved trafficking and gating. In this respect, we have found that AATs have a mild potentiatior effect, as shown by the increase in channel activity after acute exposure (Figs. 4B and Fig. 6, C and D). It is probable that this acute effect is indeed an indication of a direct interaction with CFTR protein. A prolonged interaction at the same binding site may be responsible for the long term effects that we observe on CFTR trafficking and gating. Interestingly, this site may be different from that of classical potentiators because it shows no difference in affinity between G551D and the other mutants. A different site would explain why AATs have an effect on F508del-CFTR trafficking, whereas most potentiators have no corrector activity, even after long term treatment (10). Actually, the potentiator VRT-532 has been reported as a compound also having dual activity (36). However, VRT-532 acts as a full potentiator when applied acutely (22), whereas AATs require long term treatment to achieve full gating improvement. AAT action is also reminiscent of revertant mutations that not only enhance F508del-CFTR trafficking to the cell surface, but also rescue, albeit incompletely, the mutant channel gating defect (37).
Summarizing, we speculate that AATs bind to a site, possibly in CFTR, that causes a mild potentiation of channel activity. Prolonged interaction at this site, or at another location, would cause a further improvement in gating as well as an improvement in trafficking. The general activity of AATs on different CF mutations is interesting for the possible development of novel drugs aiming at overcoming the CF basic defect. Our results represent a proof of principle concerning the possibility of a monotherapy for most CF patients. However, a larger correction of the F508del trafficking defect needs to be obtained. The relatively moderate efficacy of EN277I was probably the reason for the lack of a significant rescue in primary bronchial epithelial cells, because these cells are a much more stringent system compared with cell lines. Indeed, corr-4a is also modestly effective in primary cells (this study and Ref. 21). The goal of improving AATs efficacy may be reached by further synthesis and testing of new AAT analogs and a better understanding of the underlying mechanism(s) of action.
This work was supported by funds from Cystic Fibrosis Foundation Therapeutics, Telethon Foundation Grant GGP10026, and Fondazione Italiana Fibrosi Cistica Grants FFC#2/2009 and FFC#3/2009 with the contribution of Delegazione FFC di Vicenza, Anna Iacomini, Gruppo di Sostegno FFC “Rita” Verona, Delegazione FFC di Como.
- CF
- cystic fibrosis
- CFTR
- CF transmembrane conductance regulator
- AAT
- aminoarylthiazole
- FRT
- Fischer rat thyroid
- HS-YFP
- halide-sensitive yellow fluorescent protein
- QR
- quenching rate
- CPT-cAMP
- 8-chlorophenylthio-cAMP
- CHX
- cycloheximide.
REFERENCES
- 1. Bobadilla J. L., Macek M., Jr., Fine J. P., Farrell P. M. (2002) Hum. Mutat. 19, 575–606 [DOI] [PubMed] [Google Scholar]
- 2. Sheppard D. N., Welsh M. J. (1999) Physiol. Rev. 79, S23–S45 [DOI] [PubMed] [Google Scholar]
- 3. Welsh M. J., Smith A. E. (1993) Cell 73, 1251–1254 [DOI] [PubMed] [Google Scholar]
- 4. McAuley D. F., Elborn J. S. (2000) Paediatr. Respir. Rev. 1, 93–100 [DOI] [PubMed] [Google Scholar]
- 5. Denning G. M., Anderson M. P., Amara J. F., Marshall J., Smith A. E., Welsh M. J. (1992) Nature 358, 761–764 [DOI] [PubMed] [Google Scholar]
- 6. Lukacs G. L., Mohamed A., Kartner N., Chang X. B., Riordan J. R., Grinstein S. (1994) EMBO J. 13, 6076–6086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cheng S. H., Gregory R. J., Marshall J., Paul S., Souza D. W., White G. A., O'Riordan C. R., Smith A. E. (1990) Cell 63, 827–834 [DOI] [PubMed] [Google Scholar]
- 8. Dalemans W., Barbry P., Champigny G., Jallat S., Dott K., Dreyer D., Crystal R. G., Pavirani A., Lecocq J. P., Lazdunski M. (1991) Nature 354, 526–528 [DOI] [PubMed] [Google Scholar]
- 9. Haws C. M., Nepomuceno I. B., Krouse M. E., Wakelee H., Law T., Xia Y., Nguyen H., Wine J. J. (1996) Am. J. Physiol. 270, C1544–C1555 [DOI] [PubMed] [Google Scholar]
- 10. Pedemonte N., Sonawane N. D., Taddei A., Hu J., Zegarra-Moran O., Suen Y. F., Robins L. I., Dicus C. W., Willenbring D., Nantz M. H., Kurth M. J., Galietta L. J., Verkman A. S. (2005) Mol. Pharmacol. 67, 1797–1807 [DOI] [PubMed] [Google Scholar]
- 11. Pedemonte N., Diena T., Caci E., Nieddu E., Mazzei M., Ravazzolo R., Zegarra-Moran O., Galietta L. J. (2005) Mol. Pharmacol. 68, 1736–1746 [DOI] [PubMed] [Google Scholar]
- 12. Pedemonte N., Boido D., Moran O., Giampieri M., Mazzei M., Ravazzolo R., Galietta L. J. (2007) Mol. Pharmacol. 72, 197–207 [DOI] [PubMed] [Google Scholar]
- 13. Van Goor F., Hadida S., Grootenhuis P. D., Burton B., Cao D., Neuberger T., Turnbull A., Singh A., Joubran J., Hazlewood A., Zhou J., McCartney J., Arumugam V., Decker C., Yang J., Young C., Olson E. R., Wine J. J., Frizzell R. A., Ashlock M., Negulescu P. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 18825–18830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Noel S., Faveau C., Norez C., Rogier C., Mettey Y., Becq F. (2006) J. Pharmacol. Exp. Ther. 319, 349–359 [DOI] [PubMed] [Google Scholar]
- 15. Cai Z., Sheppard D. N. (2002) J. Biol. Chem. 277, 19546–19553 [DOI] [PubMed] [Google Scholar]
- 16. Ai T., Bompadre S. G., Wang X., Hu S., Li M., Hwang T. C. (2004) Mol. Pharmacol. 65, 1415–1426 [DOI] [PubMed] [Google Scholar]
- 17. Zegarra-Moran O., Romio L., Folli C., Caci E., Becq F., Vierfond J. M., Mettey Y., Cabrini G., Fanen P., Galietta L. J. (2002) Br. J. Pharmacol. 137, 504–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cai Z., Taddei A., Sheppard D. N. (2006) J. Biol. Chem. 281, 1970–1977 [DOI] [PubMed] [Google Scholar]
- 19. Bompadre S. G., Sohma Y., Li M., Hwang T. C. (2007) J. Gen. Physiol. 129, 285–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wellhauser L., Kim Chiaw P., Pasyk S., Li C., Ramjeesingh M., Bear C. E. (2009) Mol. Pharmacol. 75, 1430–1438 [DOI] [PubMed] [Google Scholar]
- 21. Pedemonte N., Lukacs G. L., Du K., Caci E., Zegarra-Moran O., Galietta L. J., Verkman A. S. (2005) J. Clin. Invest. 115, 2564–2571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Van Goor F., Straley K. S., Cao D., González J., Hadida S., Hazlewood A., Joubran J., Knapp T., Makings L. R., Miller M., Neuberger T., Olson E., Panchenko V., Rader J., Singh A., Stack J. H., Tung R., Grootenhuis P. D., Negulescu P. (2006) Am. J. Physiol. Lung Cell Mol. Physiol 290, L1117–L1130 [DOI] [PubMed] [Google Scholar]
- 23. Robert R., Carlile G. W., Liao J., Balghi H., Lesimple P., Liu N., Kus B., Rotin D., Wilke M., de Jonge H. R., Scholte B. J., Thomas D. Y., Hanrahan J. W. (2010) Mol. Pharmacol. 77, 922–930 [DOI] [PubMed] [Google Scholar]
- 24. Pedemonte N., Tomati V., Sondo E., Galietta L. J. (2010) Am. J. Physiol. Cell Physiol. 298, C866–C874 [DOI] [PubMed] [Google Scholar]
- 25. Bebok Z., Collawn J. F., Wakefield J., Parker W., Li Y., Varga K., Sorscher E. J., Clancy J. P. (2005) J. Physiol. 569, 601–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Galietta L. J., Haggie P. M., Verkman A. S. (2001) FEBS Lett. 499, 220–224 [DOI] [PubMed] [Google Scholar]
- 27. Bilodeau M. T., Balitza A. E., Koester T. J., Manley P. J., Rodman L. D., Buser-Doepner C., Coll K. E., Fernandes C., Gibbs J. B., Heimbrook D. C., Huckle W. R., Kohl N., Lynch J. J., Mao X., McFall R. C., McLoughlin D., Miller-Stein C. M., Rickert K. W., Sepp-Lorenzino L., Shipman J. M., Subramanian R., Thomas K. A., Wong B. K., Yu S., Hartman G. D. (2004) J. Med. Chem. 47, 6363–6372 [DOI] [PubMed] [Google Scholar]
- 28. Horn R. (1991) Biophys. J. 60, 433–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Taddei A., Folli C., Zegarra-Moran O., Fanen P., Verkman A. S., Galietta L. J. (2004) FEBS Lett. 558, 52–56 [DOI] [PubMed] [Google Scholar]
- 30. Fenwick E. M., Marty A., Neher E. (1982) J. Physiol. 331, 599–635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hosoya Y., Yamada M., Ito H., Kurachi Y. (1996) J. Gen. Physiol. 108, 485–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wang F., Zeltwanger S., Yang I. C., Nairn A. C., Hwang T. C. (1998) J. Gen. Physiol. 111, 477–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ma T., Thiagarajah J. R., Yang H., Sonawane N. D., Folli C., Galietta L. J., Verkman A. S. (2002) J. Clin. Invest. 110, 1651–1658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lansdell K. A., Cai Z., Kidd J. F., Sheppard D. N. (2000) J. Physiol. 524, 317–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mu T. W., Ong D. S., Wang Y. J., Balch W. E., Yates J. R., 3rd, Segatori L., Kelly J. W. (2008) Cell 134, 769–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wang Y., Bartlett M. C., Loo T. W., Clarke D. M. (2006) Mol. Pharmacol. 70, 297–302 [DOI] [PubMed] [Google Scholar]
- 37. Roxo-Rosa M., Xu Z., Schmidt A., Neto M., Cai Z., Soares C. M., Sheppard D. N., Amaral M. D. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 17891–17896 [DOI] [PMC free article] [PubMed] [Google Scholar]