Abstract
We previously described a non-classical mechanism that arrests FcγRIIa signaling in human neutrophils once engaged by immune complexes or opsonized pathogens. The engagement of FcγRIIa leads to its ubiquitination by the ubiquitin ligase c-Cbl and degradation by the proteasome. Herein, we further examined some of the events regulating this novel pathway. The adaptor protein CIN85 was described in other systems to be involved in the regulation of the c-Cbl-dependent pathway. We found that CIN85 is expressed in human neutrophils and that it translocates like c-Cbl from the cytosol to the plasma membrane following receptor cross-linking. CIN85 was also recruited to the same subset of high density detergent-resistant membrane fractions in which stimulated FcγRIIa partitioned with c-Cbl. The integrity of these microdomains is essential to the FcγRIIa degradation process because the cholesterol-depleting agent methyl-β-cyclodextrin inhibits this event. Silencing the expression of CIN85 by siRNA in dibutyryl cyclic AMP-differentiated PLB 985 cells prevented FcγRIIa degradation and increased IgG-mediated phagocytosis. Confocal microscopy revealed that the presence of CIN85 is essential to the proper sorting of FcγRIIa during endocytosis. We also provide direct evidence that CIN85 is a substrate of serine/threonine kinase PKCs. Classical PKCs positively regulate FcγRIIa ubiquitination and degradation because these events were inhibited by Gö6976, a classical PKC inhibitor. We conclude that the ubiquitination and degradation of stimulated FcγRIIa mediated by c-Cbl are positively regulated by the adaptor protein CIN85 in a PKC-dependent manner and that these events contribute to the termination of FcγRIIa signaling.
Keywords: E3 Ubiquitin Ligase, Lipid Raft, Neutrophil, Phagocytosis, Protein Kinase C (PKC), CIN85, Fcγ Receptors, c-Cbl
Introduction
Fcγ receptors (FcγRs)3 represent a family of membrane proteins involved in the recognition of the Fc portion of immunoglobulin G. They act as sensors for opsonized pathogens or immune complexes, and their engagement initiates intracellular signals that lead to multiple cell functions, such as degranulation, activation of the respiratory burst, and phagocytosis (1–3). FcγR isoforms either express an immunoreceptor tyrosine-based activating motif (ITAM) in their intracellular portion or associate with ITAM-containing accessory proteins with the exception of FcγRIIb, the unique inhibitory member of the FcγR family that transmits inhibitory signals through an immunoreceptor tyrosine-based inhibition motif (ITIM) (4, 5). In the case of antibody-mediated responses, such as IgG-dependent phagocytosis, neutrophils and other immune effectors, such as macrophages, have the capacity to trigger strong proinflammatory responses, which must be tightly controlled. On human neutrophils, only two FcγRs, namely FcγRIIa (CD32a) and FcγRIIIb (CD16b), are constitutively expressed, neither of which possess an ITIM. The available data indicate that in contrast to other human phagocytes, including macrophages, human neutrophils express very little (6) or no (7) FcγRIIb.
Several lines of evidence indicate that FcγRIIa is directly involved in the phagocytic process (8–10). The structure of FcγRIIa is unique among FcγRs in that it contains a single transmembrane domain, two extracellular immunoglobulin domains, and a short cytoplasmic segment with an ITAM in which the signature tyrosines are 12 amino acids apart rather than 8 as in canonical ITAMs (11, 12). It should be noted that this receptor is not expressed in mice, precluding the use of murine models to directly study its function and regulation.
We recently described a novel mechanism involved in the down-regulation of FcγRIIa expression and function, namely the c-Cbl-dependent proteasomal degradation of FcγRIIa (13). This event appears to be essential to maintaining a homeostatic regulation of immunity because disruption of mechanisms controlling FcγRs contributes to autoimmune diseases (14). In rheumatoid arthritis (15), the activation of FcγRs on neutrophils by IgG-containing immune complexes plays an important role in the chronicity and the severity of the disease, leading to exacerbated inflammation and joint destruction. Furthermore, in rheumatoid arthritis and in systemic lupus erythematosus (16), the efficiency of anti-TNF therapy (17) or methotrexate treatment (18) is related to a loss of function of FcγRIIa. Thus, understanding the mechanism of activation of neutrophils via FcγRIIa at the molecular level is clearly important for the development of strategies aimed at modulating their involvement in host defense as well as in chronic inflammation.
The earliest events following the cross-linking of FcγRIIa on human neutrophils include the translocation of the receptor to detergent-resistant membrane domains (DRMs) (13, 19, 20) that act as signaling platforms (21–23) and an enhancement of the tyrosine phosphorylation profile of the activated neutrophil (19, 20, 24). Among the tyrosine-phosphorylated substrates, the rapid and transient tyrosine phosphorylation of the FcγRIIa itself (8, 25) has been described. Our previous studies have shown that cross-linking of FcγRIIa on human neutrophils led to a rapid Src-dependent loss of immunoreactivity of the fraction of the receptor that translocates to DRMs (20, 26). We also investigated the biological and biochemical basis of the loss of this subpopulation of FcγRIIa in human neutrophils as well as its functional impact. In human neutrophils, c-Cbl is rapidly phosphorylated following the cross-linking of FcγRIIa (27). The tyrosine phosphorylation of c-Cbl plays a critical role in the regulation of its ubiquitin ligase activity (28, 29). We also showed that, in human neutrophils, FcγRIIa is ubiquitinated by c-Cbl, leading to the down-regulation of its surface expression, function, and degradation probably via the proteasomal pathway (13). We proposed that this stimulated degradation represents a non-ITIM-dependent termination step following the engagement of the FcγRIIa and could play an important role in the regulation of the activation of FcγRIIa in human neutrophils.
The three homologues of the Cbl family (c-Cbl, Cbl-b, and Cbl-3) were initially described as adaptor proteins because they can interact via their tyrosine kinase-binding domains with numerous signaling proteins (30). More recently, these proteins were described to possess ubiquitin ligase (E3) activity that is mediated through their RING finger domain. Hence, they can negatively regulate signaling by directing the ubiquitination and the degradation of activated receptors (31). Because these regulatory processes are based on the assembly of signaling complexes of multiple types of proteins, adaptor proteins are required to stabilize the protein modules. The Cbl-interacting protein of 85 kDa (CIN85) was described to interact with c-Cbl (32). Its protein domains, three Src homology 3 domains, and multiple proline-rich domains confer the capacity to bind a variety of proteins (33, 34). Furthermore, a coiled-coil domain permits the recruitment of CIN85 to cellular membranes through its affinity to phosphatidic acid (32, 35). As illustrated by the CIN85 interactome, the majority of partners are localized or dynamically recruited to plasma membranes and/or associated with the cytoskeleton (36). These properties suggest that CIN85 could play a major role in functions that require membrane remodeling by participating in the formation of efficient signaling complexes. CIN85 was previously described to be essential for the internalization of EGF receptors. CIN85 not only stabilizes the interaction between c-Cbl and the EGF receptor but also acts as a linker between the receptor complex and the endocytic machinery (reviewed in Ref. 33).
In this study, we describe a novel regulatory pathway that controls the activity of the ubiquitin ligase c-Cbl in FcγRIIa-dependent responses in human neutrophils. We describe for the first time the presence of the adaptor protein CIN85 in this cell, and we provide evidence that CIN85 is essential for the ubiquitination and the degradation steps of FcγRIIa. The engagement of FcγRIIa leads to the serine phosphorylation of CIN85 by a member of the PKC family. This novel signaling pathway regulates the ubiquitination of FcγRIIa by the ubiquitin ligase c-Cbl, leading to the down-regulation of the receptor.
EXPERIMENTAL PROCEDURES
Antibodies
Two different antibodies against FcγRIIa were used in this study. (i) The monoclonal antibody, IV.3, was purified from ascites of mice inoculated with the hybridoma HB-217 obtained from the American Type Culture Collection (Manassas, VA). This antibody recognizes a native extracellular epitope of FcγRIIa. It was used for all of the cross-linking experiments. Confocal microscopy experiments were performed with phycoerythrin-conjugated IV.3 (catalogue no. IM1935) from Beckman Coulter (Mississauga, Canada). (ii) The CT10 antibody is an IgG fraction of a polyclonal rabbit serum raised against the cytoplasmic domain of FcγRIIa previously described by Ibarrola et al. (37). It was used for immunoblotting.
Three different antibodies against CIN85 were used. The monoclonal mouse anti-CIN85 antibody (catalogue no. 05-731) purchased from Millipore (Billerica, MA) was used for immunoprecipitation experiments. Immunoprecipitations were analyzed by immunoblots using the polyclonal mouse anti-CIN85 antibody (catalogue no. H00030011-B01) from Abnova (Walnut, CA). For the other immunoblot analysis, we used the polyclonal rabbit anti-CIN85 antibody (catalogue no. sc-48746) from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA).
Goat anti-mouse F(ab′)2 (anti-Fc, catalogue no. 115-006-071 or anti-F(ab′)2 catalogue no. 115-006-072), FITC-conjugated F(ab′)2 fragment goat anti-mouse IgG (Fc fragment-specific, catalogue no. 115-096-071), and horseradish peroxidase-labeled donkey anti-rabbit IgGs (catalogue no. 711-035-152) were purchased from Jackson ImmunoResearch Laboratories (West Grove, PA). Horseradish peroxidase-labeled sheep anti-mouse IgGs (catalogue no. NXA931) were obtained from GE Healthcare. The anti-phosphotyrosine (4G10, catalogue no. 05-321) antibody was purchased from Upstate Biotechnology (Lake Placid, NY), and the polyclonal rabbit anti-ubiquitin antibody (catalogue no. Z0458) was from DakoCytomation (Mississauga, Canada). Anti-c-Cbl (catalogue no. sc-170) was purchased from Santa Cruz Biotechnology, Inc., and the monoclonal anti-flotillin-1 (catalogue no. 610820) and anti-PKCα/β (catalogue no. 610107) antibodies were from BD Transduction Laboratories (Mississauga, Canada). The anti-phospho-(Ser)PKC substrate antibody (catalogue no. 2261) was obtained from Cell Signaling (Danvers, MA).
Reagents
Sodium orthovanadate (Na3VO4), soybean trypsin inhibitor, Bt2cAMP, PMSF, DMSO, methyl-β-cyclodextrin (mβCD), OptiPrep density gradient medium, Triton X-100, and IgGs from human serum were obtained from Sigma-Aldrich Canada (Oakville, Canada). Dextran T-500 was obtained from U.S. Biological (Swampscott, MA). Percoll and Protein A-Sepharose were purchased from GE Healthcare. CHAPS, aprotinin, and leupeptin were purchased from Roche Applied Science (Laval, Canada). Western Lightning Chemiluminescence Plus was obtained from PerkinElmer Life Sciences. Ficoll-Paque and Hepes were obtained from Wisent (St-Bruno, Canada). Diisopropyl fluorophosphate (DFP) was purchased from Serva Electrophoresis (Heidelberg, Germany). Gelatin was obtained from Fisher. RPMI 1640, Alexa488-conjugated Zymosan A bioparticles, calcein-AM, and IgG-free fetal bovine serum were obtained from Invitrogen. Gö6976 was purchased from Calbiochem. Calyculin was obtained from Biomol International (Plymouth, PA), and phorbol 12-myristate 13-acetate was from Enzo Life Sciences (Plymouth Meeting, PA).
siRNA
The CIN85 siRNA ON-TARGETplus SMARTpool (catalogue no. L-014748-00) and negative control (catalogue no. D-001210-01) were purchased from Dharmacon Inc. (Lafayette, CO).
Cells
Neutrophils were collected from healthy adult volunteers and sterilely isolated as previously described (38). They were resuspended in Mg2+-free HBSS containing 1.6 mm CaCl2.
The myeloid cell line PLB-985 obtained from the German Collection of Microorganisms and Cell Cultures (DSMZ), was cultured in RPMI 1640 medium containing 10% FBS, 10 mm Hepes, 1 mm sodium pyruvate at 37 °C in a 5% CO2 humidified atmosphere. The cells were maintained in culture for 12 passages before new batches were thawed. To induce differentiation to a neutrophil-like phenotype, PLB-985 cells were cultured in medium supplemented with 0.3 mm Bt2cAMP for 3 days before each experiment.
Transfection of Bt2cAMP-differentiated PLB-985 Cells
The day following the initiation of differentiation by the addition of Bt2cAMP (0.3 mm), PLB-985 cells were transiently transfected using the Nucleofector system from Amaxa Biosystems. After centrifugation, 2 × 106 cells were suspended in 100 μl of nucleofection buffer (25 mm Hepes, pH 7.4, 120 mm KCl, 2 mm MgCl2, 10 mm K2HPO4, 5 mm l-cysteine) containing 3 μg of siRNA. The samples were transferred into an electroporation cuvette, and transfections were performed with the program setting U-002. After nucleofection, the cells were immediately gently transferred into prewarmed complete RPMI 1640 medium containing 0.3 mm Bt2cAMP, 10% FBS, 10 mm Hepes, 1 mm sodium pyruvate and maintained at 37 °C in a 5% CO2 humidified atmosphere. Two days after nucleofection, cells were harvested and resuspended in Mg2+-free HBSS containing 1.6 mm CaCl2 for analysis.
Cell Stimulation
Freshly purified human neutrophils or PLB-985 cells were suspended in HBSS at 20 × 106 cells/ml except when indicated. The cells were then incubated with the IV.3 antibody (1 μg/ml for 20 × 106 cells/ml) for 5 min followed by cross-linking with the goat anti-mouse F(ab′)2 anti-Fc (25 μg/ml) antibody for the times and temperatures indicated in the figure legends. For the negative control, cells were incubated in HBSS. For the immunoprecipitation experiments, see “Immunoprecipitation.” The stimulations were stopped at the indicated times by transferring aliquots of the cell suspensions directly in the same volume of 2× boiling modified Laemmli's sample buffer (composition of 1×: 62.5 mm Tris-HCl (pH 6.8), 4% (w/v) SDS, 5% (v/v) β-mercaptoethanol, 8.5% (v/v) glycerol, 2.5 mm orthovanadate, 10 mm para-nitrophenylphosphate, 10 μg/ml leupeptin, 10 μg/ml aprotinin, and 0.025% bromphenol blue) and boiled for 7 min. Where indicated, cells were incubated with Gö6976 (1 μm) for 10 min before the addition of the IV.3 antibody. For cholesterol depletion essays, neutrophils were preincubated with 10 mm mβCD for 15 min before the stimulation.
Immunoprecipitation
FcγRIIa
Neutrophils (20 × 106 cells/ml, 1 ml for each immunoprecipitation) were preincubated with 1 mm DFP for 10 min at room temperature and in the presence or absence of Gö6976 (1 μm) for a further 10 min prior to FcγRIIa cross-linking at room temperature. A goat anti-mouse F(ab′)2 anti-F(ab′)2 was used for the cross-linking of FcγRIIa in order to leave the Fc portion of IV.3 free. The stimulations were stopped by transferring the tubes containing the cells to an ice bath, following which the neutrophils were quickly centrifuged (10 s at 15 000 × g). The cell pellets were lysed by adding 1 ml of cold lysis buffer (20 mm Hepes, pH 7.4, 137.2 mm NaCl, 1% Triton, 2 mm orthovanadate, 10 μg/ml leupeptin, 10 μg/ml aprotinin, 3 mm DFP) for 2 min on ice. The cell lysates were sonicated on ice for 2 s at power level 1 in a Branson Sonifier 450 sonicator, and the insoluble material was discarded by two successive centrifugations at 400 × g for 2 min at 4 °C. The supernatants (700 μl) were then collected, and FcγRIIa bound to IV.3 was immunoprecipitated by adding protein A-Sepharose beads for 2 h at 4 °C. The beads were then collected and washed three times with cold lysis buffer, and 50 μl of Laemmli's sample buffer was added prior to boiling for 7 min.
CIN85
Neutrophils (20 × 106 cells/ml, 1 ml for each immunoprecipitation) were preincubated with 1 mm DFP for 10 min at room temperature, and FcγRIIa was cross-linked using the F(ab′)2 IV.3 (0.5 μg/ml for 20 × 106 cells/ml) and a goat anti-mouse F(ab′)2 anti-F(ab′)2 (25 μg/ml) antibody. The cell pellets were lysed in 1 ml of CHAPS buffer (10 mm Tris-HCl, 140 mm NaCl, 1 mm EDTA, 0,6% CHAPS, 10 μg/ml aprotinin, 10 μg/ml leupeptin, 2 mm sodium orthovanadate, 250 μg/ml soybean trypsin inhibitor, 3 mm DFP, 1 mm PMSF) for 9 min on ice, and the insoluble material was discarded by two successive centrifugations at 400 × g for 2 min at 4 °C. The supernatants (700 μl) were then collected, and the anti-CIN85 antibody from Millipore (1 μg/condition) was added for 2 h at 4 °C. CIN85 was immunoprecipitated by adding protein A-Sepharose beads for 1 h at 4 °C. The beads were then collected and washed three times with cold lysis buffer, and 50 μl of Laemmli's sample buffer was added prior to boiling for 7 min.
Plasma Membrane Isolation
One ml of cell suspension (40 × 106 cells/ml) was preincubated with 1 mm DFP for 10 min, followed by FcγRIIa cross-linking at room temperature. The tubes containing the stimulated cells were transferred to an ice bath to stop the stimulations. The cells were then quickly centrifuged (15,000 × g) and resuspended in modified relaxation buffer (100 mm KCl, 3 mm NaCl, 10 mm Hepes, pH 7.4, 10 μg/ml aprotinin, 10 μg/ml leupeptin, 2 mm sodium orthovanadate, 250 μg/ml soybean trypsin inhibitor, 1 mm PMSF, 3 mm DFP). In the PKC translocation experiments, 0.5 mm EGTA and 1 mm MgCl2 were added in the relaxation buffer. The cells were sonicated on ice for 22 s at power level 1 in a Branson Sonifier 450 sonicator, and the lysates were centrifuged at 400 × g for 2 min. The supernatants (900 μl) were added to the top of a two-step Percoll gradient composed of an equal volume (1.4 ml) of a 1.12 g/ml Percoll solution layered beneath a 1.05 g/ml Percoll solution, as described previously by Kjeldsen et al. (39). The Percoll gradients were centrifuged for 30 min at 37,000 × g at 4 °C in a fixed angle rotor (Beckman TLA100.4). The plasma membranes partitioned to the upper portion of the gradient underneath the cytosol fractions. An aliquot of the cytosolic fractions was collected and boiled for 7 min in Laemmli's buffer. The plasma membrane fractions were collected and centrifuged at 100,000 × g for 45 min at 4 °C to remove the Percoll. Plasma membranes formed a visible disc above the Percoll pellet. They were collected, resuspended in relaxation buffer, and stored at −80 °C. An aliquot was boiled for 3 min in Laemmli's buffer for immunoblot analysis.
Isolation of DRMs
Isolated plasma membranes from control or FcγRIIa-cross-linked neutrophils (40 × 106 cell equivalents/ml) were solubilized in 1% Nonidet P-40 buffer (137 mm NaCl, 20 mm Hepes, pH 7.4, 10 μg/ml aprotinin, 10 μg/ml leupeptin, 2 mm sodium orthovanadate, 250 μg/ml soybean trypsin inhibitor, 1 mm PMSF, 3 mm DPF) for 20 min on ice. Solubilized membranes were adjusted to 40% (v/v) OptiPrep with a stock solution of 59.4% OptiPrep in 10 mm Hepes (pH 7.4), and 700 μl of this plasma membrane preparation was transferred to 4-ml centrifuge tubes and overlaid with 700 μl of ice-cold solutions of 35, 30, 25, 20, and 0% OptiPrep successively. The gradients were centrifuged at 380,000 × g for 3 h at 4 °C in a TLA 100.4 rotor. Thirteen fractions of 300 μl were collected from the top of the gradient, and proteins were chloroform/methanol-precipitated as described previously (40). The precipitates were resuspended in 60 μl of Laemmli's buffer and boiled for 7 min.
Electrophoresis and Immunoblotting
Proteins were separated by SDS-PAGE on 10% acrylamide gels and transferred to PVDF membranes. For the anti-ubiquitin blots, the PVDF membranes were boiled in water for 30 min following the transfer step (41). Blocking agents and antibodies were diluted in a TBS-Tween solution (25 mm Tris-HCl, pH 7.8, 190 mm NaCl, 0.15% (v/v) Tween 20). Blotto solution (5% w/v) was used to block nonspecific sites prior to immunoblotting with the anti-flotillin-1, anti-ubiquitin, anti-phospho-(Ser)PKC substrate, polyclonal mouse anti-CIN85, and anti-FcγRIIa (CT10 antibody) antibodies. Gelatin solution (2%, w/v) was used to block nonspecific sites before anti-c-Cbl, anti-CIN85, anti-PKCα/β, and anti-phosphotyrosine (4G10) immunoblotting. Anti-ubiquitin and anti-phosphotyrosine antibodies were diluted 1:4000; CT10 antibody, anti-c-Cbl, anti-CIN85, anti-PKCα/β, and anti-flotillin antibodies were diluted 1:1000. Anti-phospho-(Ser)PKC substrate was diluted 1:500 with 5% BSA. Polyclonal mouse anti-CIN85 antibody was diluted 1:2000 in blotto solution (1%, w/v). Horseradish peroxidase-labeled donkey anti-rabbit IgGs and sheep anti-mouse IgGs were diluted 1:20,000 in TBS-Tween solution. Chemiluminescence reagents were used to detect antibodies within a maximal exposure time of 5 min. Equal protein loading was controlled by immunoblotting against the structural protein flotillin-1 or c-Cbl.
Phagocytosis Assay
Alexa 488-conjugated zymosan A bioparticles were opsonized by incubation with 10 mg/ml human IgGs at 37 °C for 1 h and washed twice in HBSS as described (13). To initiate phagocytosis, particles were added to transfected PLB-985 (10 × 106 cells/ml preincubated as described in the corresponding legend) at a ratio of 10:1. To synchronize the interaction between the particles and the cells, the mixture was centrifuged at 400 × g for 15 s prior to an incubation at 37 °C for 10 min. To quench the fluorescence of the non-ingested Alexa 488-conjugated zymosan, 2 mg/ml trypan blue in PBS was added 2 min before flow cytometry analysis with a BD FACSCanto II flow cytometer from BD Biosciences. A phagocytic index was calculated by multiplying the number of cells having internalized particles by the mean fluorescence intensity, which corresponds to the mean number of particles inside each positive cell (calibration: 10 units/zymosan particles).
Confocal Microscopy Analysis
Transfected Bt2cAMP-differentiated PLB-985 cells (20 × 106 cells/ml) were resuspended in RPMI supplemented with 10% IgG-free fetal bovine serum and prestained with 5 μg/ml calcein-AM for 30 min at 37 °C. The cells were centrifuged for 2 min at 1500 × g at room temperature and plated on a glass slide coated with IgG-free, 100% fetal bovine serum. This purified serum was used to avoid neutrophil activation by the glass slide and FcγRs activation by serum IgGs. Neutrophils were stimulated as described in the corresponding legend of Fig. 4 and visualized live at 37 °C in an environment chamber with 5% CO2 with a spinning disc confocal microscope using a ×63 objective (Quorum Spinning Disc Wave FX, Quorum Technologies, Guelph, Canada).
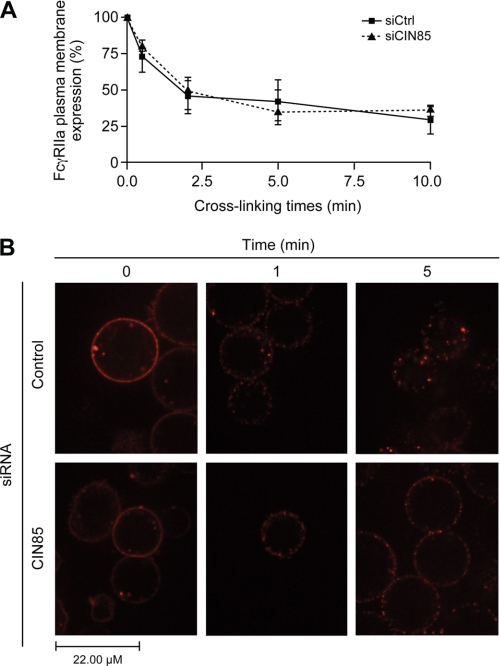
FIGURE 4.
CIN85 is involved in the late steps of FcγRIIa internalization. Bt2cAMP-differentiated PLB-985 cells were transfected with a negative control siRNA or an siRNA against CIN85, as described under “Experimental Procedures. A, the membrane expression of FcγRIIa was monitored by flow cytometry. Transfected cells were stimulated for the indicated times by cross-linking FcγRIIa with a goat anti-mouse F(ab′)2 anti- F(ab′)2 (25 μg/ml) at 37 °C. The stimulations were terminated by transferring the tubes to an ice bath, followed by centrifugation at 400 × g for 2 min at 4 °C. The cell pellets were washed in cold HBSS containing 0.005% BSA and incubated with FITC-labeled goat anti-mouse Fcγ specific IgG (diluted 1:100 in HBSS/BSA) for 30 min on ice. Cells were then washed twice in HBSS/BSA and analyzed by flow cytometry. IgG2b was used as an isotype-matched negative control. The data shown are the quantification of the mean fluorescence intensity of three independent experiments where FcγRIIa levels were normalized to samples without IV.3 cross-linking (100% expression level). B, transfected PLB-985 (107/ml) were incubated with phycoerythrin-conjugated IV.3 (1 μg/ml) for 10 min, washed and plated as described under “Experimental Procedures.” Anti-mouse F(ab′)2 was added in the environment chamber, and cells were visualized live by confocal microscopy for the indicated times. For each condition, at least 25 cells were analyzed. These pictures are representative of three independent cross-linking experiments. Error bars, S.E.
Statistical Analysis
Statistical analyses were performed using Student's paired t test (two-tailed) with GraphPad Prism4 software. Significance was considered to be attained at a value of p < 0.05 (*), p < 0.01 (**), or p < 0.001 (***).
RESULTS
Translocation of CIN85 to DRM-Hs of the Plasma Membranes following FcγRIIa Cross-linking
We have previously shown that the ubiquitin ligase c-Cbl is recruited to the plasma membrane following the engagement of FcγRIIa in an Src-dependent manner in human neutrophils (13). Because CIN85 is described to be associated with the active form of c-Cbl in other cellular models (32, 42, 43), we postulated that this adaptor protein could also be recruited to plasma membranes in response to FcγRIIa cross-linking and be involved in the FcγRIIa degradation pathway that we identified (13). Neutrophil plasma membranes from resting cells or FcγRIIa-stimulated cells were thus isolated as described under “Experimental Procedures” and analyzed by immunoblotting for the presence of CIN85 and flotillin-1 (a plasma membrane marker (44, 45)). We first observed that CIN85 was expressed in human neutrophils and, in contrast to flotillin-1, was mostly present in the cytosol of resting neutrophils (Fig. 1A). The addition of the IV.3 antibody alone did not induce any translocation of CIN85 to the plasma membrane (0 s of cross-linking time) (Fig. 1B). On the other hand, we observed a rapid increase in the levels of CIN85 in the plasma membrane following FcγRIIa cross-linking. This response was observed as early as 10 s following stimulation and was maintained up to 30 s. The anti-flotillin-1 immunoblots indicated that equal amounts of membranes were loaded in each lane. The same results were obtained in response to stimulation of human neutrophils by heat-aggregated IgGs (data not shown). These results indicate that CIN85, as c-Cbl, is recruited to plasma membranes in response to the cross-linking of FcγRIIa on human neutrophils.
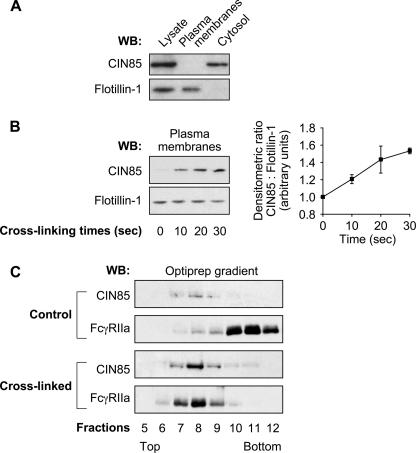
FIGURE 1.
CIN85 translocates to DRM-Hs of the plasma membrane following FcγRIIa cross-linking. Neutrophils (40 × 106 cells/ml) were preincubated with 1 mm DFP for 10 min before FcγRIIa cross-linking at room temperature. Plasma membranes were prepared as described under “Experimental Procedures.” A, aliquots of total cell lysates, plasma membranes, and cytosol fractions from unstimulated cells were analyzed by immunoblots for the presence of CIN85. The loading of each cell fraction corresponded to equal cell equivalents. The same blot was reprobed for the plasma membrane marker flotillin-1. B, plasma membranes from resting or FcγRIIa-cross-linked cells were analyzed by immunoblotting for the presence of CIN85. The same blot was reprobed for flotillin-1, the protein loading control. A densitometric representation of the means of four independent experiments is shown in the histogram. C, plasma membranes of resting or FcγRIIa cross-linked (30 s) neutrophils were prepared as described under “Experimental Procedures” and solubilized in 1% Nonidet P-40. These samples were subject to ultracentrifugation on OptiPrep density gradients as described under “Experimental Procedures.” Thirteen gradient fractions were collected, and the proteins were precipitated and analyzed by immunoblotting with anti-CIN85 or anti-FcγRIIa antibodies. These data are representative of three independent experiments. WB, Western blot. Error bars, S.E.
Our previous studies provided evidence that FcγRIIa and c-Cbl were recruited to high density DRMs (DRM-Hs) of the plasma membrane after cross-linking (13, 20). In the present study, we used the same OptiPrep gradient conditions (20, 46) to examine the distribution of CIN85 within the plane of the plasma membrane. After FcγRIIa cross-linking, plasma membranes were isolated and solubilized in Nonidet P-40 and DRM-H prepared as described under “Experimental Procedures.” An analysis of the gradients derived from control or FcγRIIa-cross-linked membranes is presented in Fig. 1C. Although little FcγRIIa and CIN85 was detected in fractions 7–9 (those corresponding to DRM-H) in resting cells, their presence in these fractions increased significantly following cross-linking of FcγRIIa. The translocation of FcγRIIa and c-Cbl to these fractions has been described previously (13). These results indicate that FcγRIIa, c-Cbl, and CIN85 co-fractionated in the same DRM-H fractions in neutrophils stimulated by cross-linking FcγRIIa. The distribution of flotillin-1, a structural raft component (44, 45) remained the same before and after FcγRIIa cross-linking (data not shown) (20). Taken together, these data are consistent with a model suggesting that CIN85 could potentially represent the adaptor protein that is involved in the regulation of the ubiquitin ligase activity of c-Cbl responsible for the ubiquitination of the FcγRIIa.
Intact DRM-Hs Are Required for the Stimulated Down-regulation of FcγRIIa
Because FcγRIIa (20) (Fig. 1C), c-Cbl (13), and CIN85 (Fig. 1C) are recruited to DRM-Hs, we postulated that these microdomains serve as signaling platforms for the regulation of molecular events leading to the ubiquitination and degradation of FcγRIIa. To test this hypothesis, neutrophils were preincubated with a 10 mm concentration of the cholesterol-depleting agent mβCD or HBSS for 15 min at 37 °C. Following this treatment, FcγRIIa was cross-linked on neutrophils, and whole cell lysates were analyzed by immunoblotting for the presence of FcγRIIa. Cross-linking led to the degradation of FcγRIIa as evidenced by a loss of immunoreactivity (Fig. 2A) as described previously (13). Cells treated with mβCD presented a defect in FcγRIIa degradation because the amount of FcγRIIa remained unchanged over the entire time frame examined (up to 5 min) in contrast to the control condition, where a decrease of immunoreactivity was observed after 2 min (Fig. 2A). This result suggests that the integrity of DRM-Hs is essential to the efficient degradation of FcγRIIa in stimulated neutrophils.
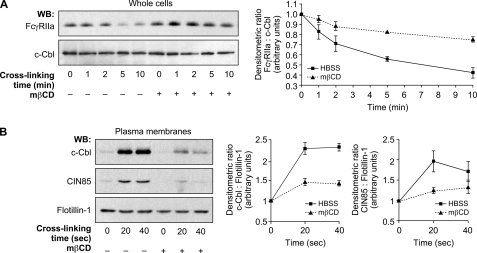
FIGURE 2.
DRM-Hs integrity is essential for FcγRIIa degradation and CIN85 recruitment. Neutrophils (20 × 106 cells/ml) were preincubated in the presence or absence of 10 mm mβCD for 15 min at 37 °C before FcγRIIa cross-linking. A, whole cell lysates were probed by immunoblotting for FcγRIIa (CT10 antibody) and c-Cbl as a loading control. B, following mβCD preincubation, neutrophils were centrifuged and resuspended at 40 × 106 cells/ml. Plasma membranes were prepared as described under “Experimental Procedures” and analyzed by immunoblotting for the presence of CIN85 and c-Cbl. The same blot was reprobed for flotillin-1 (loading control). The line graphs represent the means of densitometric ratios of three independent experiments. WB, Western blot. Error bars, S.E.
To investigate the importance of DRM-Hs as signaling platforms in the FcγRIIa down-regulation pathway, we analyzed the effect of mβCD on the translocation of c-Cbl and CIN85 in response to FcγRIIa engagement. Neutrophils were preincubated with HBSS or mβCD as in Fig. 2A, and FcγRIIa was cross-linked as described under “Experimental Procedures.” Plasma membranes were isolated and immunoblotted with anti-CIN85, anti-c-Cbl, and anti-flotillin-1 antibodies. As shown in Fig. 2B, mβCD significantly reduced the translocation of CIN85 and c-Cbl to the plasma membrane, as observed following the cross-linking of FcγRIIa. This result indicates that the recruitment of c-Cbl and CIN85 to the plasma membrane in response to cross-linking of FcγRIIa in human neutrophils required the integrity of DRM-Hs. Taken together, these data suggest that DRMs could represent a down-regulation signaling platform for the degradation of the FcγRIIa.
Involvement of CIN85 in the Down-regulation of FcγRIIa
Due to the difficulties associated with the transfection of human neutrophils, we opted for the use of a human neutrophil-like cellular model, the Bt2cAMP-differentiated PLB-985 (dPLB-985) cell line, to further test the involvement of CIN85 in the degradation of FcγRIIa. The responses of these cells to FcγRIIa cross-linking were previously characterized and found to closely resemble those of primary neutrophils, including the degradation of the FcγRIIa receptor (13). To confirm the role of CIN85 in the down-regulation of FcγRIIa expression, the expression of endogenous CIN85 was silenced using an siRNA strategy in dPLB-985 cells. The expression level of CIN85 in dPLB-985 (Fig. 3A) was significantly decreased following nucleofection with specific siRNAs. The decreased levels of expression of CIN85 correlated with an inhibition of the degradation of FcγRIIa (Fig. 3A, top). c-Cbl immunoblots were used as loading controls (Fig. 3A, bottom). The same samples were immunoblotted with anti-phosphotyrosine antibodies. A stronger and more sustained tyrosine phosphorylation pattern was observed in response to FcγRIIa cross-linking in cells transfected with the siRNA against CIN85 than in those in which the negative control siRNA was introduced (Fig. 3B). These results indicate that CIN85 contributes to the down-regulation of FcγRIIa activation. They also indicate that the inhibition of FcγRIIa degradation is not due to a lack of stimulation or to a loss of viability of the cells.
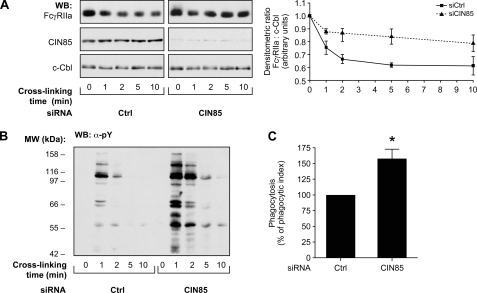
FIGURE 3.
CIN85 positively regulates the degradation of FcγRIIa. Bt2cAMP-differentiated PLB-985 cells were transfected with a negative control siRNA or a siRNA against CIN85 as described under “Experimental Procedures.” Forty-eight h post-transfection, FcγRIIa was cross-linked for the indicated times. A, whole cell lysates were immunoblotted using antibodies recognizing FcγRIIa (CT10 antibody), CIN85, or c-Cbl (loading control). The line graph represents the compilation of densitometric ratios derived from three independent experiments. B, the same samples were probed for tyrosine phosphorylation residues (α-pY). These results are representative of three independent experiments. C, transfected cells were incubated with fluorescent IgG-opsonized zymosan as described under “Experimental Procedures.” Fluorescence was measured by flow cytometry. The phagocytic index was calculated by multiplying the number of phagocytic cells by the number of internalized zymosan particles/cell (mean fluorescence intensity). This graph is a quantification of the means of four independent experiments. WB, Western blot. Error bars, S.E.
Functional Importance of the FcγRIIa Down-regulation Pathway
To show that FcγRIIa degradation is relevant to dampen neutrophil activation, we analyzed the consequence of CIN85 silencing on one of the main FcγRIIa-dependent functions of neutrophils (i.e. phagocytosis) (Fig. 3C). We monitored the phagocytic capacity of the cells using fluorescent zymosan particles opsonized with human IgGs. We observed a 50% increase in the phagocytic index (percentage of cells having internalized zymosan particles multiplied by mean fluorescence intensity) when CIN85 expression was inhibited. These results (Fig. 3, B and C) illustrate that the CIN85-dependent degradation of FcγRIIa plays an important role in dampening the signaling and functional responses downstream of this receptor.
Role of CIN85 in FcγRIIa Internalization
To measure the internalization of the receptor following its cross-linking (13), we quantified the membrane expression of FcγRIIa by flow cytometry. An siRNA approach was utilized to test for the possible implication of CIN85 in this event. CIN85-silenced dPLB-985 or control cells were stimulated as described above by cross-linking FcγRIIa, and the stimulation was stopped on ice after 0.5, 2, 5, and 10 min. Following incubation with fluorescent anti-mouse antibodies, neutrophils were analyzed by flow cytometry. As illustrated in Fig. 4A, silencing CIN85 had no effect on the internalization of FcγRIIa. In both conditions, the fluorescence intensity detected with the anti-mouse antibody (a measure of surface expression of FcγRIIa) decreased in a time-dependent manner. This effect was obvious within the first minute of stimulation and increased for the next 10 min, when up to 60% of FcγRIIa is internalized. We next compared the intracellular distribution of the receptor in control and CIN85-silenced cells by confocal microscopy. As shown in Fig. 4B, before cross-linking (0 min), the receptor is uniformly distributed at the periphery of control cells as in CIN85-silenced cells. One minute following cross-linking, in both conditions, a punctate distribution of FcγRIIa is clearly observed at the inner side of the plasma membrane. This membrane staining pattern is characteristic of the early stages of receptor internalization following receptor cross-linking (13). At later time points (5 min), CIN85 was detected in the cytoplasm of control cells. In contrast, in CIN85-silenced cells, FcγRIIa staining remained localized to the inner side of plasma membrane (submembrane localization). No FcγRIIa staining was detectable in the cytoplasm of CIN85-silenced cells.
PKC-dependent Ser Phosphorylation of CIN85
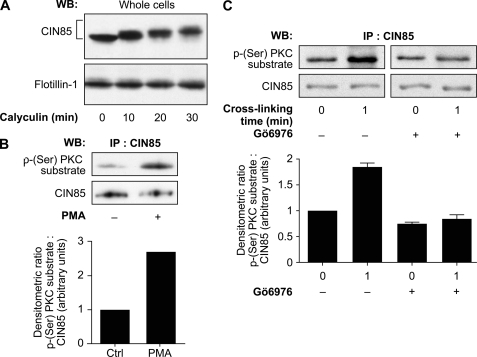
Numerous signaling proteins downstream of the engagement of FcγRIIa (including c-Cbl) are tyrosine-phosphorylated (27). CIN85 does not possess any tyrosine phosphorylation sites but has potential sites of serine/threonine phosphorylation (47). However, these protein modifications on CIN85 are poorly documented in the literature (48). We examined whether this post-translational modification of CIN85 could be detected in stimulated human neutrophils. We first performed a time course of incubation of resting neutrophils with 1 μm calyculin, a potent inhibitor of type 1 and type 2A serine/threonine phosphatases that has been exploited to provide evidence of serine/threonine phosphorylation responses in several cell types, including neutrophils (49–54). Whole cell lysates were analyzed by immunoblotting with anti-CIN85 antibody. As shown in Fig. 5A, a marked retardation of electrophoretic mobility of CIN85 was observed in a time-dependent manner following incubation with calyculin. In contrast, the electrophoretic mobility of the structural protein flotillin-1 remained the same under all the conditions. Changes in electrophoretic migration are often caused by hyperphosphorylation, which results in an increased apparent molecular weight of the protein (49). This effect of calyculin on the elecrophoretic migration of CIN85 suggests that CIN85 could be serine/threonine-phosphorylated. We thus next examined the possibility that this modification could regulate the activity of this adaptor protein.
FIGURE 5.
CIN85 is a phosphosubstrate of classical PKCs in response to FcγRIIa cross-linking. A, neutrophils (20 × 106 cells/ml) were incubated with 1 μm calyculin for the indicated times at room temperature. Whole cell lysates were analyzed by SDS-PAGE and immunoblotted for CIN85 and flotillin-1 (loading control). B, neutrophils (20 × 106 cells/ml) were stimulated with 100 nm phorbol 12-myristate 13-acetate (PMA) or DMSO for 45 s at room temperature. CIN85 was immunoprecipitated as described under “Experimental Procedures,” and the immunoprecipitates were probed for a phospho-(Ser)PKC substrate or CIN85. The graph represents the densitometric ratio of the phospho-(Ser)PKC substrate signal over the total CIN85 signal. C, neutrophils (20 × 106 cells/ml) were incubated in the presence or absence of Gö6976 (1 μm) for 10 min before FcγRIIa cross-linking at room temperature for 1 min. CIN85 was immunoprecipitated as described under “Experimental Procedures,” and the immunoprecipitates were probed for phospho-(Ser)PKC substrates or CIN85. The histogram represents the quantification of the mean densitometric readings of the phospho-(Ser)PKC substrate signal (relative to the total CIN85 signal) observed in three independent experiments. WB, Western blot; IP, immunoprecipitation; Error bars, S.E.
Protein kinases C (PKCs) represent one of the well described serine-threonine kinase families in human neutrophils. In order to examine the involvement of the PKC family in the regulation of CIN85, we probed CIN85 immunoprecipitates with an anti-phospho-(Ser)PKC substrate antibody. This antibody recognizes specific motifs of PKC-dependent phosphorylation composed of a serine residue flanked by arginine or lysine at the −3-, −2-, and +2-positions and hydrophobic amino acids at position +1 (55). As shown in Fig. 5B, the incubation of human neutrophils with phorbol 12-myristate 13-acetate led to the PKC-dependent serine phosphorylation of CIN85, indicating that CIN85 is a candidate phosphorylated substrate of PKCs in the neutrophil. The same PKC-dependent serine phosphorylation of CIN85 is observed in the context of the cross-linking of FcγRIIa on human neutrophils (Fig. 5C, left). This result provides evidence that CIN85 is a substrate for PKC in FcγRIIa-cross-linked human neutrophils. To further support this observation, we next monitored the potential effect of the pharmacological inhibitor of classical PKCs, Gö6976 (56), on this phosphorylation event. We observed that neutrophil preincubation with 1 μm Gö6976 completely abolished the stimulation of the serine phosphorylation of CIN85 (Fig. 5C, right) following FcγRIIa cross-linking.
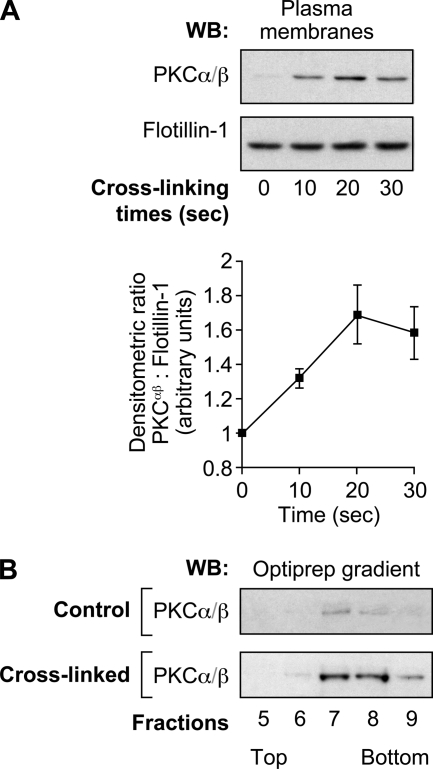
Translocation of Classical PKCs to Plasma Membranes in DRM-Hs
The translocation of PKCs to the plasma membrane of stimulated cells is a widely used index of their activation status (57). We therefore investigated the effect of FcγRIIa cross-linking on the translocation of classical PKCs to the plasma membrane of human neutrophils. Following FcγRIIa engagement, plasma membranes were purified as described under “Experimental Procedures.” They were then immunoblotted with anti-PKCα/β (the classical members of the PKC family present in human neutrophils) and anti-flotillin-1 (a plasma membrane marker used as loading control) antibodies. We first observed that the addition of IV.3 alone did not induce any translocation of PKCα/β to the plasma membrane (0 s of cross-linking time). Following FcγRIIa cross-linking, however, we observed a rapid increase in the levels of PKCα/β at the plasma membrane (Fig. 6A). This response was observed as early as 10 s following stimulation and was maintained for up to 30 s. We next analyzed these membranes on Optiprep gradients as in Fig. 1 to monitor the translocation of PKCs to DRM-Hs. Following cross-linking of FcγRIIa, we observed the recruitment of classical PKCs to the fractions that correspond to DRM-Hs (Fig. 6B). Moreover, the presence of PKCs in DRM-Hs correlated in time with the detection of CIN85 in the DRM-H fractions (Fig. 1C). These results indicate that classical PKCs cofractionate with FcγRIIa, c-Cbl, and CIN85 in the same DRM-H fractions. PKCs are absent in control samples as they are localized in the cytosol of unstimulated cells. Taken together, these data suggest that classical PKCs are recruited in the same way as CIN85 and c-Cbl and could represent key activators of the FcγRIIa down-regulation pathway in human neutrophils.
FIGURE 6.
PKCs translocate to the plasma membranes in DRM-Hs following FcγRIIa cross-linking. Neutrophils (40 × 106 cells/ml) were preincubated with 1 mm DFP for 10 min prior to FcγRIIa cross-linking at room temperature. A, plasma membranes were prepared as described under “Experimental Procedures” and analyzed by immunoblotting for the presence of PKCα/β. The same blot was reprobed for flotillin-1 (protein loading control). A densitometric quantification of the means of three independent experiments is shown in the line graph. B, plasma membranes of resting or FcγRIIa-cross-linked (30 s) neutrophils were prepared as described under “Experimental Procedures” and solubilized in 1% Nonidet P-40. These samples were subjected to ultracentrifugation on OptiPrep density gradients as described under “Experimental Procedures.” The 13 gradient fractions were collected, and the proteins were precipitated and analyzed by immunoblotting using the anti-PKCα/β antibody. These data are representative of three independent experiments. WB, Western blot. Error bars, S.E.
Involvement of Classical PKCs in the Down-regulation of FcγRIIa Expression
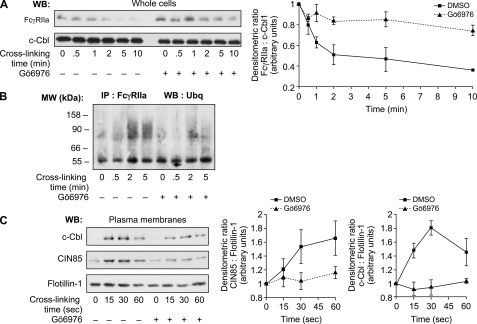
To confirm that classical PKCs are directly involved in the FcγRIIa degradation pathway, we analyzed the effect of Gö6976 on this event. Preincubation of neutrophils with Gö6976 significantly inhibited the degradation of FcγRIIa (Fig. 7A). To further define the role of classical PKCs in this process, we tested the effect of Gö6976 on the ubiquitination of FcγRIIa, a step that we previously described as preceding the degradation of FcγRIIa (13). Following FcγRIIa cross-linking, the receptor was immunoprecipitated, and its ubiquitination was examined by immunoblotting as described under “Experimental Procedures.” Ubiquitinated forms of a protein typically appear as a smear of bands at molecular weights larger than that of the non-ubiquitinated protein. As described previously (13), a smear of bands in the 60–120 kDa region were detected only very weakly 30 s post-stimulation but were clearly evident following 2 min of FcγRIIa cross-linking (Fig. 7B). No ubiquitinated bands were observed in unstimulated cells. This ubiquitination pattern persisted for up to 5 min following receptor cross-linking. We observed a decrease in the ubiquitination pattern of the immunoprecipitated receptor in the presence of Gö6976 for up to 5 min of cross-linking. The ability of Gö6976 to decrease the amount of ubiquitinated forms of FcγRIIa suggests that classical PKCs are involved in the signaling events leading to the activation of the FcγRIIa down-regulation pathway. This result also correlates with the inhibition of the degradation of the receptor (Fig. 7A). Furthermore, the translocations of the ubiquitin ligase c-Cbl and the adaptor protein CIN85 to the plasma membranes following FcγRIIa cross-linking were also decreased in the presence of Gö6976 (Fig. 7C). These results illustrate the importance of PKCs in the activation of the ubiquitination signaling pathway that leads to FcγRIIa degradation and the arrest of signals induced by the activation of this receptor.
FIGURE 7.
Classical PKCs positively regulate the degradation of FcγRIIa. Neutrophils (20 × 106 cells/ml) were incubated in the presence or absence of Gö6976 (1 μm) for 10 min before FcγRIIa cross-linking at room temperature for the indicated times. A, whole cell lysates were probed by immunoblotting for FcγRIIa (CT10) and c-Cbl (loading control). The densitometric values of the CT10 immunoblots were normalized with respect to those of the corresponding values for c-Cbl. These data are representative of four independent experiments. B, FcγRIIa was immunoprecipitated, and immunoprecipitates were probed for ubiquitin as described under “Experimental Procedures.” These data are representative of three independent experiments. C, neutrophils (40 × 106 cells/ml) were preincubated with 1 mm DFP for 10 min at room temperature before incubating with Gö6976 and cross-linking FcγRIIa at room temperature. Plasma membranes were prepared as described under “Experimental Procedures” and analyzed by immunoblotting for the presence of c-Cbl, CIN85, and flotillin-1 (loading control). The line graphs represent densitometric quantifications of the means of three independent experiments. WB, Western blot; IP, immunoprecipitation. Error bars, S.E.
DISCUSSION
We previously described a novel model of the down-regulation of FcγRIIa in human neutrophils in which the engagement of this receptor leads to its ubiquitination by the ubiquitin ligase c-Cbl, thereby targeting it to a proteasome-dependent degradation pathway (13). Herein, we further characterized the c-Cbl-mediated down-regulation of FcγRIIa. We found that the adaptor protein CIN85 is recruited to the plasma membrane, where it cofractionated to the same plasma membrane domains as the receptor. Disturbing these DRMs inhibited FcγRIIa-mediated functions as well as the recruitment of c-Cbl and CIN85 to the plasma membrane, indicating that the integrity of these signaling platforms is essential for the efficient down-regulation of FcγRIIa in human neutrophils. Silencing CIN85 in differentiated PLB-985 cells provided direct evidence that this adaptor protein played a prominent role in the degradation of the receptor and the arrest of FcγRIIa signals. Finally, we showed that CIN85 is phosphorylated by PKCs in response to FcγRIIa engagement and that classical PKCs positively regulated FcγRIIa ubiquitination and degradation. Taken together, our results illustrate that the c-Cbl-mediated degradation of stimulated FcγRIIa is positively regulated by the adaptor protein CIN85 in a PKC-dependent manner, thus contributing to the termination of FcγRIIa signaling.
We previously identified the ubiquitin ligase c-Cbl as responsible for the targeting of the neutrophil's FcγRIIa to the proteasomal degradation. In the present study, we investigated the molecular mechanisms that control this novel down-regulation pathway. Indeed, to rapidly remove a receptor from the cell surface, proteins involved in the ubiquitination process as well as accessory endocytic proteins are required. In the case of tyrosine kinase receptors, c-Cbl was described to bind to and ubiquitinate activated receptors and also to recruit endocytic machinery proteins (47). Adaptor proteins are essential for molecular events that are dependent on the close proximity between c-Cbl and these families of proteins. One such adaptor protein is CIN85, the first described to interact with c-Cbl (32) and to be associated with endophilin (58). To our knowledge, the expression of CIN85 has not been reported in human neutrophils. The results of our experiments provide evidence for a rapid recruitment and a sustained localization of CIN85 from the cytosol to membrane fractions following FcγRIIa cross-linking. The translocation of CIN85, as we previously observed for c-Cbl (13), precedes the degradation of the receptor and correlates with that of the ubiquitination of FcγRIIa. Furthermore, the stimulated recruitment of CIN85 to the plasma membrane is, as previously shown for c-Cbl, specifically localized to DRM-Hs. We have previously shown that FcγRIIa cross-linking of isolated plasma membranes leads to the tyrosine phosphorylation of the receptor but not to its degradation, in contrast to FcγRIIa cross-linking of whole neutrophils (20). This lack of degradation is consistent with the requirement of cytosolic elements. The results of this study as well as those described by Marois et al. (13) indicate that the cytosolic proteins, c-Cbl and CIN85, are involved in the down-regulation of the surface expression of this receptor. Taken together, these data confirm the importance of the relocalization of CIN85 to the plasma membrane and more specifically within DRM-Hs, leading to receptor degradation.
DRMs are cholesterol- and sphingolipid-enriched domains that represent platforms serving to concentrate signaling proteins (59). Our previous data indicate that DRMs are heterogeneous in human neutrophils and include subpopulations with low (DRM-L) and high (DRM-H) densities (60). The functionally relevant subpopulation of DRMs in FcγR-stimulated human neutrophils appears to be DRM-Hs because both FcγRIIIb and FcγRIIa are present in these fractions and not in DRM-Ls (13, 20, 26, 60). We have now observed that cross-linking of FcγRIIa results in the translocation of CIN85 to the same DRM-Hs as the receptor itself and c-Cbl. We also observed that disruption of DRMs inhibits FcγRIIa degradation (19, 61) and the recruitment of c-Cbl and CIN85 to the plasma membrane following FcγRIIa cross-linking with specific monoclonal antibodies. We previously observed that the integrity of these microdomains was essential for optimal FcγR-dependent phagocytosis and transduction of FcγR-mediated signaling pathways (tyrosine phosphorylation pattern and calcium mobilization) in response to IgG-containing immune complexes (61). These results support a model of signaling platforms for DRM-Hs that play a role in the coordination between activation of FcγR-dependent functions and down-regulation mechanisms.
Nucleofection of siRNA oligomers directed at CIN85 very effectively silenced the expression of CIN85 in dPLB-985 cells. This resulted in an inhibition of the degradation of FcγRIIa following its cross-linking, leading to a qualitative and quantitative increase in the tyrosine phosphorylation profile. These results are consistent with the interpretation that CIN85 is involved in the termination of the signals initiated upon engagement of this receptor by directing FcγRIIa toward a degradation pathway. Consistent with this observation, we provide evidence for a functional significance of the degradation of FcγRIIa. CIN85-silenced cells exhibit an increase in IgG-dependent phagocytosis, reflecting a hyperactivation of the cells in the context of an inhibition of the degradation of FcγRIIa. Moreover, both qualitative and quantitative differences in the tyrosine phosphorylation profile were observed, including the phosphorylation of additional substrates and an increase in the intensity of signal detected in resting cells. FcγRIIa internalization, however, was not affected in dPLB-985 cells in which CIN85 was silenced. The mechanisms underlying the internalization of this receptor may differ from those of the EGF receptor, where CIN85 is essential for its internalization (42). It is noteworthy that microscopic analysis of FcγRIIa routing following its cross-linking showed differences in FcγRIIa distribution in CIN85-silenced cells and indicated that the latter is required for the proper targeting of FcγRIIa to proteasomes. These differences in intracellular cycling could be due to a lack of interactions between the endocytic machinery and the cytoskeleton, leading to an accumulation of submembrane localization of FcγRIIa. These results underline that the specific role of CIN85 in the context of FcγRIIa down-regulation in the human neutrophil differs from that previously described in the context of tyrosine kinase receptors (42). This specificity may be explained by the scaffolding properties of CIN85 that could result in multiple distinct interactions for each receptor type. Taken together, these results are in agreement with a specific role for CIN85 in the molecular mechanisms that occur between the internalization and proteosomal targeting of FcγRIIa in human neutrophils.
Proteomic sequence analysis predicted potential sites of serine/threonine phosphorylation on CIN85 (47). Indeed, we detected the PKC-mediated serine phosphorylation of CIN85 following FcγRIIa cross-linking that appears to be mediated by classical PKCs because Gö6976 inhibits this post-tranductional modification. Gö6976 also inhibits FcγRIIa ubiquitination and degradation. The lack of translocation of the ubiquitin ligase c-Cbl and CIN85 in the presence of Gö6976 may explain these observations. Taken together, these data highlight the importance of classical PKCs in the initiation of the FcγRIIa down-regulation pathway and are in accordance with our previous data indicating that FcγRIIa cross-linking induced a mobilization of calcium (a cofactor for classical PKCs) in human neutrophils (26). To our knowledge, these observations represent the first indications for a role of the activation of classical PKCs in the cellular responses to FcγRs, and FcγRIIa in particular, in human neutrophils. Our results indicate that classical PKCs are recruited to DRM-Hs, as are other regulating proteins (c-Cbl and CIN85), to take part in this down-regulation pathway. Further studies are, however, required to precisely identify the principal PKC isoforms involved in this pathway as human neutrophils express three classical PKC isoforms (α, β1, and β2).
The functional role of CIN85 in ubiquitin-dependent endocytosis and the down-regulation of ITAM-containing receptors remains a challenging subject and has not been previously documented in human neutrophils. To our knowledge, this study is the first to describe the involvement of CIN85 in the down-regulation of FcγR, and FcγRIIa in particular, in human neutrophils. Other immunoreceptors have been shown to be controlled by CIN85. In the RBL-2H3 cell line, FcϵRI activation leads to the recruitment of CIN85 to c-Cbl-containing complexes, which controls endosomal sorting of the receptor and lysosome delivery. Furthermore, overexpression of CIN85 in this context decreases mastocyte degranulation in response to FcϵRI engagement (62). This observation suggests that the acceleration of the kinetics of FcϵRI internalization due to the abundance of CIN85 perturbs intracellular signaling and leads to an inappropriate activation of mastocytes. The role of CIN85 in the attenuation of the immune response can be extended to additional immunoreceptors, such as the TCR or TNF receptor 1 (48). Coupled to its role in the down-regulation of receptor activation or of other proteins like Syk (63), CIN85 appears to play a central role in the coordination between intracellular signaling, down-regulation pathways, and sorting downstream of receptor engagement (48). These functions of CIN85, in the particular context of the immune response, highlight the importance of adaptor proteins in the activation and regulation of cell functions dependent on receptor activation.
Our present results complement our previous data on the ITIM-independent pathway of FcγRIIa down-regulation following its engagement on human neutrophils. First, we observed that CIN85 is expressed in human neutrophils and is recruited from the cytosol to DRM-Hs of the plasma membrane following FcγRIIa engagement. This relocalization leads to a cofractionation of CIN85 with FcγRIIa that is also recruited from detergent-soluble regions of the plasma membrane to DRM-Hs (20, 26) with the ubiquitin ligase c-Cbl (13). Our data are consistent with the hypothesis that DRM-Hs act as essential signaling platforms that coordinate transduction of activating and inhibiting signals to optimize FcγR-dependent neutrophil responses and to prevent overactivation of the cell. Afterward, FcγRIIa is then internalized and is ubiquitinated by c-Cbl and probably moves to the proteasome complex, where it is degraded. The inhibition of the expression of CIN85 illustrates an essential role for this adaptor protein in the fate of FcγRIIa. The modified tyrosine phosphorylation profile and increase in IgG-dependent phagocytosis that we observed in the absence of CIN85 both indicate that this mechanism leading to FcγRIIa degradation is likely to allow the neutrophil, which does not possess a classical ITIM-dependent (FcγRIIb) mechanism for FcγRIIa down-regulation, to avoid overactivation during clearance of IgG complexes. Neutrophil activation through FcγRs is exacerbated in systemic autoimmune diseases, such as rheumatoid arthritis or anti-neutrophil cytoplasmic antibody-associated vasculitis (Wegener granulomatosis) (7). In the present study, we characterized a PKC-dependent mechanism involved in the regulation of the expression of the opsonic receptor FcγRIIa in human neutrophils and the role of PKC in the functional responses elicited upon the occupation and activation of this receptor. A concerted investigation of the different mechanisms involved in the regulation of the signaling events that follow FcγRs stimulation is required to better understand autoimmune pathologies.
Acknowledgment
We thank Dr. Maurice Dufour for expert technical assistance with the flow cytometric analyses.
This work was supported in part by grants from the Canadian Institutes of Health Research (CIHR).
- FcγR
- Fcγ receptor
- Bt2cAMP
- dibutyryl cyclic AMP
- DRM
- detergent-resistant microdomain
- mβCD
- methyl-β-cyclodextrin
- DRM-H and DRM-L
- detergent-resistant microdomain of high and low density, respectively
- DFP
- diisopropyl fluorophosphate
- ITAM
- immunoreceptor tyrosine-based activating motif
- ITIM
- immunoreceptor tyrosine-based inhibition motif
- HBSS
- Hanks' balanced saline solution.
REFERENCES
- 1. Huizinga T. W., van Kemenade F., Koenderman L., Dolman K. M., von dem Borne A. E., Tetteroo P. A., Roos D. (1989) J. Immunol. 142, 2365–2369 [PubMed] [Google Scholar]
- 2. Indik Z., Kelly C., Chien P., Levinson A. I., Schreiber A. D. (1991) J. Clin. Invest. 88, 1766–1771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hundt M., Schmidt R. E. (1992) Eur. J. Immunol. 22, 811–816 [DOI] [PubMed] [Google Scholar]
- 4. Salmon J. E., Pricop L. (2001) Arthritis Rheum. 44, 739–750 [DOI] [PubMed] [Google Scholar]
- 5. Kimberly R. P., Salmon J. E., Edberg J. C. (1995) Arthritis Rheum. 38, 306–314 [DOI] [PubMed] [Google Scholar]
- 6. Su K., Yang H., Li X., Li X., Gibson A. W., Cafardi J. M., Zhou T., Edberg J. C., Kimberly R. P. (2007) J. Immunol. 178, 3272–3280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Marois L., Rollet-Labelle E., Naccache P. H. (2011) in Advances in Medicine and Biology, Vol. 15 (Berhardt L. V. ed) pp. 123–152, Nova Publishers, Hauppauge, NY [Google Scholar]
- 8. Indik Z. K., Park J. G., Hunter S., Schreiber A. D. (1995) Blood 86, 4389–4399 [PubMed] [Google Scholar]
- 9. Hunter S., Kamoun M., Schreiber A. D. (1994) Proc. Natl. Acad. Sci. U.S.A. 91, 10232–10236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lowry M. B., Duchemin A. M., Robinson J. M., Anderson C. L. (1998) J. Exp. Med. 187, 161–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cambier J. C. (1995) Immunol. Today 16, 110. [DOI] [PubMed] [Google Scholar]
- 12. Reth M. (1989) Nature 338, 383–3842927501 [Google Scholar]
- 13. Marois L., Vaillancourt M., Marois S., Proulx S., Paré G., Rollet-Labelle E., Naccache P. H. (2009) J. Immunol. 182, 2374–2384 [DOI] [PubMed] [Google Scholar]
- 14. Hogarth P. M. (2002) Curr. Opin. Immunol. 14, 798–802 [DOI] [PubMed] [Google Scholar]
- 15. Firestein G. S. (2003) Nature 423, 356–361 [DOI] [PubMed] [Google Scholar]
- 16. Tan Sardjono C., Mottram P. L., Hogarth P. M. (2003) Immunol. Cell Biol. 81, 374–381 [DOI] [PubMed] [Google Scholar]
- 17. Belostocki K., Park M. S., Redecha P. B., Masuda E., Salmon J. E., Pricop L. (2005) Clin. Immunol. 117, 78–86 [DOI] [PubMed] [Google Scholar]
- 18. Wijngaarden S., van Roon J. A., van de Winkel J. G., Bijlsma J. W., Lafeber F. P. (2005) Rheumatology 44, 729–734 [DOI] [PubMed] [Google Scholar]
- 19. Barabé F., Rollet-Labelle E., Gilbert C., Fernandes M. J., Naccache S. N., Naccache P. H. (2002) J. Immunol. 168, 4042–4049 [DOI] [PubMed] [Google Scholar]
- 20. Rollet-Labelle E., Marois S., Barbeau K., Malawista S. E., Naccache P. H. (2004) Biochem. J. 381, 919–928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Danielsen E. M., Hansen G. H. (2006) Mol. Membr. Biol. 23, 71–79 [DOI] [PubMed] [Google Scholar]
- 22. Mañes S., Viola A. (2006) Mol. Membr. Biol. 23, 59–69 [DOI] [PubMed] [Google Scholar]
- 23. Kabouridis P. S. (2006) Mol. Membr. Biol. 23, 49–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Richard S., Shaw A. S., Showell H. J., Connelly P. A. (1994) Biochem. Biophys. Res. Commun. 199, 653–661 [DOI] [PubMed] [Google Scholar]
- 25. Dusi S., Donini M., Della Bianca V., Gandini G., Rossi F. (1994) Biochem. Biophys. Res. Commun. 201, 30–37 [DOI] [PubMed] [Google Scholar]
- 26. Barabé F., Paré G., Fernandes M. J., Bourgoin S. G., Naccache P. H. (2002) J. Biol. Chem. 277, 13473–13478 [DOI] [PubMed] [Google Scholar]
- 27. Naccache P. H., Gilbert C., Barabé F., Al-Shami A., Mahana W., Bourgoin S. G. (1997) J. Leukoc. Biol. 62, 901–910 [DOI] [PubMed] [Google Scholar]
- 28. Levkowitz G., Waterman H., Ettenberg S. A., Katz M., Tsygankov A. Y., Alroy I., Lavi S., Iwai K., Reiss Y., Ciechanover A., Lipkowitz S., Yarden Y. (1999) Mol. Cell 4, 1029–1040 [DOI] [PubMed] [Google Scholar]
- 29. Melander F., Andersson T., Dib K. (2004) Biochem. Biophys. Res. Commun. 317, 1000–1005 [DOI] [PubMed] [Google Scholar]
- 30. Schmidt M. H., Dikic I. (2005) Nat. Rev. Mol. Cell Biol. 6, 907–918 [DOI] [PubMed] [Google Scholar]
- 31. Swaminathan G., Tsygankov A. Y. (2006) J. Cell. Physiol. 209, 21–43 [DOI] [PubMed] [Google Scholar]
- 32. Take H., Watanabe S., Takeda K., Yu Z. X., Iwata N., Kajigaya S. (2000) Biochem. Biophys. Res. Commun. 268, 321–328 [DOI] [PubMed] [Google Scholar]
- 33. Kowanetz K., Szymkiewicz I., Haglund K., Kowanetz M., Husnjak K., Taylor J. D., Soubeyran P., Engstrom U., Ladbury J. E., Dikic I. (2003) J. Biol. Chem. 278, 39735–39746 [DOI] [PubMed] [Google Scholar]
- 34. Kurakin A. V., Wu S., Bredesen D. E. (2003) J. Biol. Chem. 278, 34102–34109 [DOI] [PubMed] [Google Scholar]
- 35. Gout I., Middleton G., Adu J., Ninkina N. N., Drobot L. B., Filonenko V., Matsuka G., Davies A. M., Waterfield M., Buchman V. L. (2000) EMBO J. 19, 4015–4025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jensen L. J., Kuhn M., Stark M., Chaffron S., Creevey C., Muller J., Doerks T., Julien P., Roth A., Simonovic M., Bork P., von Mering C. (2009) Nucleic Acids Res. 37, D412–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ibarrola I., Vossebeld P. J., Homburg C. H., Thelen M., Roos D., Verhoeven A. J. (1997) Biochim. Biophys. Acta 1357, 348–358 [DOI] [PubMed] [Google Scholar]
- 38. Rollet E., Caon A. C., Roberge C. J., Liao N. W., Malawista S. E., McColl S. R., Naccache P. H. (1994) J. Immunol. 153, 353–363 [PubMed] [Google Scholar]
- 39. Kjeldsen L., Sengelov H., Borregaard N. (1999) J. Immunol. Methods 232, 131–143 [DOI] [PubMed] [Google Scholar]
- 40. Wessel D., Flügge U. I. (1984) Anal. Biochem. 138, 141–143 [DOI] [PubMed] [Google Scholar]
- 41. Swerdlow P. S., Finley D., Varshavsky A. (1986) Anal. Biochem. 156, 147–153 [DOI] [PubMed] [Google Scholar]
- 42. Szymkiewicz I., Kowanetz K., Soubeyran P., Dinarina A., Lipkowitz S., Dikic I. (2002) J. Biol. Chem. 277, 39666–39672 [DOI] [PubMed] [Google Scholar]
- 43. Kirsch K. H., Georgescu M. M., Shishido T., Langdon W. Y., Birge R. B., Hanafusa H. (2001) J. Biol. Chem. 276, 4957–4963 [DOI] [PubMed] [Google Scholar]
- 44. Slaughter N., Laux I., Tu X., Whitelegge J., Zhu X., Effros R., Bickel P., Nel A. (2003) Clin. Immunol. 108, 138–151 [DOI] [PubMed] [Google Scholar]
- 45. Shao D., Segal A. W., Dekker L. V. (2003) FEBS Lett. 550, 101–106 [DOI] [PubMed] [Google Scholar]
- 46. Nebl T., Pestonjamasp K. N., Leszyk J. D., Crowley J. L., Oh S. W., Luna E. J. (2002) J. Biol. Chem. 277, 43399–43409 [DOI] [PubMed] [Google Scholar]
- 47. Dikic I. (2002) FEBS Lett. 529, 110–115 [DOI] [PubMed] [Google Scholar]
- 48. Havrylov S., Redowicz M. J., Buchman V. L. (2010) Traffic 11, 721–731 [DOI] [PubMed] [Google Scholar]
- 49. Rollet-Labelle E., Gilbert C., Naccache P. H. (2000) J. Immunol. 164, 1020–1028 [DOI] [PubMed] [Google Scholar]
- 50. Artçanuthurry V., Grelac F., Maclouf J., Martin-Cramer E., Levy-Tolédano S. (1996) Semin. Thromb. Hemost. 22, 317–326 [DOI] [PubMed] [Google Scholar]
- 51. Qiu W., Cobb R. R., Scholz W. (1998) J. Leukoc. Biol. 63, 631–635 [DOI] [PubMed] [Google Scholar]
- 52. Brumell J. H., Grinstein S. (1994) Am. J. Physiol. 267, C1574–C1581 [DOI] [PubMed] [Google Scholar]
- 53. Liang L., Huang C. K. (1995) Biochem. J. 306, 489–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Mayer A. M., Choudhry M. A., Sayeed M. M., Spitzer J. A. (1997) Life Sci. 61, 199–204 [DOI] [PubMed] [Google Scholar]
- 55. Nishikawa K., Toker A., Johannes F. J., Songyang Z., Cantley L. C. (1997) J. Biol. Chem. 272, 952–960 [DOI] [PubMed] [Google Scholar]
- 56. Martiny-Baron G., Kazanietz M. G., Mischak H., Blumberg P. M., Kochs G., Hug H., Marmé D., Schächtele C. (1993) J. Biol. Chem. 268, 9194–9197 [PubMed] [Google Scholar]
- 57. Popa-Nita O., Proulx S., Paré G., Rollet-Labelle E., Naccache P. H. (2009) J. Immunol. 183, 2104–2114 [DOI] [PubMed] [Google Scholar]
- 58. Soubeyran P., Kowanetz K., Szymkiewicz I., Langdon W. Y., Dikic I. (2002) Nature 416, 183–187 [DOI] [PubMed] [Google Scholar]
- 59. Simons K., Toomre D. (2000) Nat. Rev. Mol. Cell Biol. 1, 31–39 [DOI] [PubMed] [Google Scholar]
- 60. Fernandes M. J., Rollet-Labelle E., Paré G., Marois S., Tremblay M. L., Teillaud J. L., Naccache P. H. (2006) Biochem. J. 393, 351–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Marois L., Paré G., Vaillancourt M., Rollet-Labelle E., Naccache P. H. (2011) J. Biol. Chem. 286, 3509–3519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Molfetta R., Belleudi F., Peruzzi G., Morrone S., Leone L., Dikic I., Piccoli M., Frati L., Torrisi M. R., Santoni A., Paolini R. (2005) J. Immunol. 175, 4208–4216 [DOI] [PubMed] [Google Scholar]
- 63. Peruzzi G., Molfetta R., Gasparrini F., Vian L., Morrone S., Piccoli M., Frati L., Santoni A., Paolini R. (2007) J. Immunol. 179, 2089–2096 [DOI] [PubMed] [Google Scholar]