Abstract
Rheumatoid arthritis (RA) is an autoimmune disease characterized by synovial inflammation and articular damage. Proinflammatory cytokines, antibodies, and matrix-degrading enzymes orchestrate the pathogenic events in autoimmune arthritis. Accordingly, these mediators of inflammation are the targets of several anti-arthritic drugs. However, the prolonged use of such drugs is associated with severe adverse reactions. This limitation has necessitated the search for less toxic natural plant products that possess anti-arthritic activity. Furthermore, it is imperative that the mechanism of action of such products be explored before they can be recommended for further preclinical testing. Using the rat adjuvant-induced arthritis model of human RA, we demonstrate that celastrol derived from Celastrus has potent anti-arthritic activity. This suppression of arthritis is mediated via modulation of the key proinflammatory cytokines (IL-17, IL-6, and IFN-γ) in response to the disease-related antigens, of the IL-6/IL-17-related transcription factor STAT3, of antibodies directed against cyclic citrullinated peptides and Bhsp65, and of the activity of matrix metalloproteinase-9 and phospho-ERK. Most of the clinical and mechanistic attributes of celastrol are similar to those of Celastrus extract. Several studies have addressed the antitumor activity of celastrol. Our study highlights the anti-arthritic activity of Celastrus-derived celastrol and the underlying mechanisms. These results provide a strong rationale for further testing and validation of the use of celastrol and the natural plant extract from Celastrus as an adjunct (with conventional drugs) or alternative modality for the treatment of RA.
Keywords: Antibodies, Cellular Immune Response, Cytokine, Inflammation, MAP Kinases (MAPKs), Matrix Metalloproteinase, Animal Models, Arthritis, Autoimmunity, Plant Products
Introduction
Rheumatoid arthritis (RA)2 is a chronic debilitating autoimmune disease affecting millions of people all over the world (1–3). Both cell-mediated and humoral immune reactions participate in the pathogenesis of RA (1, 4). Several proinflammatory cytokines secreted by immune cells, including TNF-α, IL-1β, IL-15, IL-18, and IFN-γ, have been shown to play a role in the initiation and progression of RA (4, 5). Over the past decade, a new subset of T helper cells producing IL-17 (Th17) has become the focus of RA pathogenesis (6, 7). Induction of a Th17 response involves IL-6 and TGF-β, as well as the transcription factors STAT3 and retinoid related orphan receptor-γt (ROR-γt) (8, 9). In regard to the humoral response, serum levels of anti-cyclic citrullinated protein/peptide (aCCP) antibodies correlate well with the disease severity in RA patients and serve as the specific autoantibody marker for the diagnosis of RA (10, 11). In view of the above, modulation of the proinflammatory cytokines and aCCP antibody levels is a desired goal for the management of RA.
A variety of drugs are available for the treatment of RA. These include corticosteroids, nonsteroidal anti-inflammatory drugs, disease-modifying antirheumatic drugs, and biologics (1, 12). However, besides their high cost, the use of these drugs is associated with severe adverse reactions (1, 12–15). Therefore, adjunct/alternative treatments based on less toxic natural plant products are eagerly been sought for RA and other disorders (16–22). On the one hand, natural plant products have the advantage of limited or no toxicity, but on the other hand, there is skepticism about their use in part because of the lack of information about their mechanism of action.
In this study, we describe the anti-arthritic activity and the underlying mechanisms of the ethanol extract of Celastrus aculeatus Merrill (Celastrus) (23), a Chinese medicinal herb, and celastrol, a bioactive component of Celastrus (23–26), using a well established model of RA, adjuvant-induced arthritis (AA) (27, 28). Most of the literature on celastrol is devoted to its anticancer properties (29). In contrast, our study highlights the anti-arthritic attribute of this compound and the immunological and biochemical mechanisms involved in this activity. Our results offer the scientific rationale for considering the preclinical testing of Celastrus and celastrol in RA patients.
EXPERIMENTAL PROCEDURES
Animals
Male Lewis rats (LEW/Hsd, RT.11; 5–6 weeks old, ∼150–200 g) were purchased from Harlan Sprague-Dawley (Indianapolis, IN) and housed in the vivarium of the University of Maryland School of Medicine. All experimental procedures on these rats were performed following the guidelines of the Institutional Animal Care and Use Committee.
Celastrus Extract and Purified Celastrol
Celastrus
Ethanol extract of the roots and stem of C. aculeatus Merrill (Celastrus) was prepared as described previously (23). Briefly, the roots and stem of this plant were dried, powdered, and then extracted with 75% ethanol. The extract was collected and re-extracted with 75% ethanol. The final extract was concentrated and dried. The extract was subjected to reverse-phase high performance liquid chromatography. The three major groups of compounds, namely triterpenes (celastrol), flavonoids (epiafzelechin) and sesquiterpenes (orbiculin F), were identified (23).
Celastrol
Celastrol ((9β,13α,14β,20α)-3-hydroxy-9,13-dimethyl-2-oxo-24,25,26-trinoroleana-1(10),3,5,7-tetraen-29-oic acid), Mr = 450.6) is one of the bioactive components of Celastrus. Purified celastrol isolated from Celastrus scandens was purchased from Calbiochem.
Antigens
Heat-killed Mycobacterium tuberculosis H37Ra (Mtb) was obtained from Difco (Detroit, MI). Bhsp65 (mycobacterial heat shock protein 65) was prepared by transforming BL21(DE3)pLysS cells (Novagen, Madison, WI) with the pET23b-GroEL2 vector (Colorado State University, Fort Collins, CO) (30). Ovalbumin and keyhole limpet hemocyanin were obtained from Sigma.
Induction and Evaluation of AA
Mtb was ground to a powder form and suspended in mineral oil (Sigma). Lewis rats were immunized subcutaneously with 200 μl of Mtb suspension (1.5 mg/rat) at the base of the tail. Following immunization, these rats were observed regularly for erythema, swelling, and induration in each paw. The severity of arthritis was graded on a scale of 0–4 as described previously (27, 28). The maximum attainable arthritic score per paw was 4 and per rat was 16. In a typical disease course of AA in Lewis rats, four different phases are evident: incubation, onset, peak, and recovery.
Treatment of Arthritic Lewis Rats with Celastrus Extract/Celastrol
Celastrus
Celastrus extract was finely suspended in water using a mortar and pestle and fed to Lewis rats (3 g/kg of body weight in a 2-ml volume) daily using a gavage needle (FNC-16-3, Kant Scientific Corp., Torrington, CT). Celastrus feeding was started from the onset of AA and then continued uninterrupted for the entire duration of the observation period. The control rats received vehicle (water). All rats were graded regularly for the severity of arthritis following Mtb challenge.
Celastrol
A stock solution of celastrol (20 mg in 0.6 ml of dimethyl sulfoxide (DMSO; Sigma) was prepared and frozen at −20 °C in small aliquots until needed, following the method described previously (31, 32). The dose of celastrol (1 mg/kg) used in vivo in our study matched that used previously in different (non-arthritic) model systems (31, 32). The stock solution of celastrol was diluted in PBS (6-μl stock in 500 μl of PBS/rat) and injected intraperitoneally. Celastrol was administered daily to Mtb-challenged rats starting from the onset of AA and then continued uninterrupted for the entire duration of the observation period. The control rats received the vehicle, DMSO (1.2%), in PBS. All rats were observed and graded regularly for the signs of arthritis.
Preparation of Lymph Node Cells (LNC) and Their Restimulation with Bhsp65 for Cytokine Testing
LNC of Rats Treated with Celastrus/Celastrol
Mtb-immunized Lewis rats treated with either Celastrus/celastrol or vehicle only were killed on day 19 of the disease course, and their draining lymph nodes (para-aortic, inguinal, and popliteal) were harvested. Thereafter, a single cell suspension of LNC was prepared, and the cells were washed three times with Hanks' balanced salt solution (Invitrogen). These LNC were cultured (5 × 105 cells/well) in a flat-bottomed 96-well plate in HL-1 serum-free medium (Ventrex Laboratories, Portland, ME) supplemented with 2 mm l-glutamine, 100 units/ml penicillin G sodium, and 100 μg/ml streptomycin sulfate in an atmosphere of 95% air and 5% CO2 (33). The cells were restimulated with Bhsp65 for 24 h. The specificity of the cytokine response induced by Bhsp65 was tested by showing the lack of response of Mtb-primed LNC to a control antigen, keyhole limpet hemocyanin (supplemental Fig. 1).
LNC Treated with Celastrol in Vitro
LNC were prepared from an untreated rat on day 19 after Mtb challenge. These cells were restimulated with Bhsp65 for 24 h in the presence or absence of different concentrations (0.05 and 0.1 μm) of celastrol. The dosage of celastrol used for the in vitro treatment of LNC was optimized such that there was no significant effect on cell viability as measured by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay (supplemental Fig. 2). LNC tolerated celastrol up to 0.5 μm without any effect on their viability. However, increasing the concentration of celastrol from 0.5 to 1 or 2 μm resulted in a decrease in percent viability of LNC to <60%.
Preparation of Spleen Adherent Cells (SAC) and Their Restimulation with Mtb for Cytokine Testing
SAC of Rats Treated with Celastrus/Celastrol
Spleens were harvested from experimental/control rats on day 19 of the disease course. A single cell suspension of spleen cells was prepared as described above for LNC. These spleen cells were placed in a 6-well plate (Corning, Corning, NY) at 37 °C in RPMI 1640 medium supplemented with 5% FBS, 2 mm l-glutamine, 100 units/ml penicillin G sodium, and 100 μg/ml streptomycin sulfate. After 90 min, the non-adherent cells were removed by washing, yielding the SAC (27). These SAC (1.5–2.0 × 106 cells/well) were restimulated with sonicated Mtb (10 μg/ml) for 6 h. The specificity of the cytokine response induced by Mtb was tested by showing the lack of response of the SAC to a control antigen, ovalbumin (supplemental Fig. 1).
SAC Treated with Celastrol in Vitro
SAC from untreated arthritic rats were restimulated for 6 h with sonicated Mtb in the presence or absence of different concentrations (0.05 and 0.1 μm) of celastrol.
Measurement of Cytokine mRNA Expression in LNC and SAC Using Quantitative RT-PCR
RNA was isolated from LNC and SAC using TRIzol reagent (Invitrogen), and then cDNA was prepared from RNA using an iScript cDNA synthesis kit (Bio-Rad). The cDNA prepared was amplified in an ABI PRISM 7900HT cycler (Applied Biosystems, Foster City, CA) by quantitative RT-PCR using SYBR Green PCR Master Mix (Applied Biosystems) and appropriate primers (33). The primers for the detection of mRNAs for different cytokines and hypoxanthine-guanine phosphoribosyltransferase were designed using the Primer Express 2.0 Program (Applied Biosystems) and synthesized at the Biopolymer Genomics Core Facility of the University of Maryland School of Medicine. The mRNA levels of specific genes were normalized to those of the hypoxanthine-guanine phosphoribosyltransferase gene, and the relative gene expression levels were determined (33). The results are expressed as -fold increase.
Testing the Effect of Celastrus/Celastrol on Cytokine-related Transcription Factors by Western Blotting
LNC of Rats Treated with Celastrus/Celastrol
LNC obtained from Lewis rats treated with either Celastrus/celastrol or the corresponding vehicle were restimulated with antigen for 24 h in a 6-well plate. These cells were lysed using cell lysis buffer (Cell Signaling Technology, Danvers, MA) and then centrifuged at 16,000 × g for 10 min. The protein concentration of the lysate supernatant was measured using a BCA protein assay kit (Pierce). The proteins (20 μg) in the lysate supernatant were separated by SDS-PAGE (4–20%). Thereafter, the proteins were transferred onto a PVDF membrane. The membrane was first blocked with 5% milk in Tris-buffered saline containing 0.05% Tween, followed by probing with anti-phospho-STAT3 (pSTAT3) or anti-ROR-γt antibodies (Abcam and Cell Signaling Technology) and then with secondary anti-rabbit antibody (Pierce) (33). The membrane probed with anti-pSTAT3 was reprobed with an antibody against total STAT3 (Santa Cruz Biotechnology, Santa Cruz, CA). GAPDH was used as a protein loading control. Protein bands were visualized with SuperSignal West Dura chemiluminescent detection reagents (Pierce) following the manufacturer's directions.
LNC of Rats Treated with Celastrol in Vitro
To study the in vitro effect of celastrol on pSTAT3 and ROR-γt, LNC obtained from Mtb-immunized Lewis rats were restimulated for 24 h with antigen in the presence or absence of celastrol. Cell lysates were used to study the level of STAT3 and ROR-γt using Western blotting as described above.
Testing the Culture Supernatant of Celastrol-treated Synovial Fibroblasts (Rat Fibroblast-like Synoviocytes)
Synovial fibroblasts (rat fibroblast-like synoviocytes) were cultured as described previously (33), and the cells in passage 4 were incubated in serum-free medium in a 6-well plate (2 × 105 cells/well). These cells were stimulated for 24 h with Mtb (10 μg/ml) in the presence or absence of different concentrations of celastrol. The culture supernatant was collected and used to analyze matrix metalloproteinases (MMPs), IL-6, and VEGF by ELISA (Cytokine Core Facility, University of Maryland School of Medicine).
Gelatin Zymogram Assay for the Detection of MMP Activity
MMP activity in the culture supernatants of synovial fibroblasts (rat fibroblast-like synoviocytes) stimulated with Mtb in the presence or absence of celastrol was determined using a gelatin zymogram assay as described previously (34). Briefly, culture supernatants were loaded onto a gelatin-coated precast polyacrylamide gel (Bio-Rad) and subjected to SDS-PAGE under nonreducing conditions. SDS was removed to renature the gel by incubation with 2.5% Triton X-100 for 1–2 h, followed by three to four washings with water. The gel was then incubated overnight at 37 °C in Tris-HCl buffer (pH 7.4) containing CaCl2, NaCl, and Brij 35. The gel was stained with Coomassie Brilliant Blue R-250 to visualize MMP activity.
Testing the Effect of Celastrol on Cell Signaling by Western Blotting
To study the effect of celastrol on signaling events, synovial fibroblasts (HFLS-RA (human fibroblast-like synoviocytes-rheumatoid arthritis), Cell Applications, Inc., San Diego, CA) were incubated for 6 h in serum-free DMEM, followed by stimulation with IL-1β in the presence or absence of celastrol for 30 min. Cells were lysed in cell lysis buffer as described above and processed for Western blot analysis using rabbit polyclonal antibodies specific for phospho-p38, phospho-ERK (pERK), and phospho-JNK (Cell Signaling Technology). The membrane was reprobed with antibody against total p38, ERK, and JNK. GAPDH was used as a protein loading control. Protein bands were visualized with SuperSignal West Dura chemiluminescent detection reagents following the manufacturer's directions.
Assays for Measuring Serum Levels of aCCP and anti-Bhsp65 Antibodies
Control and test groups of Lewis rats were bled from the tail 1 day before Mtb immunization (day −1) and once a week (days 7, 14, 21, and 28) thereafter. Sera were separated and stored at −80 °C until used. The levels of serum aCCP antibodies were determined using commercial kits (CCP3 IgG ELISA kit, INOVA Diagnostics, Inc., San Diego, CA) with a minor modification of the procedure. Sera (diluted 1:75) were added to wells coated with CCP and incubated at room temperature for 1 h. The wells were then washed thoroughly with washing buffer, followed by the addition to the wells of HRP-conjugated mouse anti-rat total Ig (1:1500) as the second antibody. The plate was incubated for 45 min at room temperature. After washing, color was developed, and the absorbance was read. Negative and positive controls for quality control as well as a 5-point standard curve for quantitation were also included in the assay. The results are expressed as unit values corresponding to 1:100 serum dilution following the manufacturer's instructions. An aCCP antibody level equal to or higher than 20 units was considered a positive response. The assay for antibodies against Bhsp65 was performed as described previously (30). HRP-conjugated mouse anti-rat total Ig was used as a secondary antibody (1:1500) in this assay.
Statistics
Two-way analysis of variance with the Bonferroni post-test was carried out for in vivo clinical experiments. Student's t test was used to analyze cytokine mRNA expression data. A p value <0.05 was considered as significant.
RESULTS
Celastrus Extract and Celastrol Suppress AA in Lewis Rats
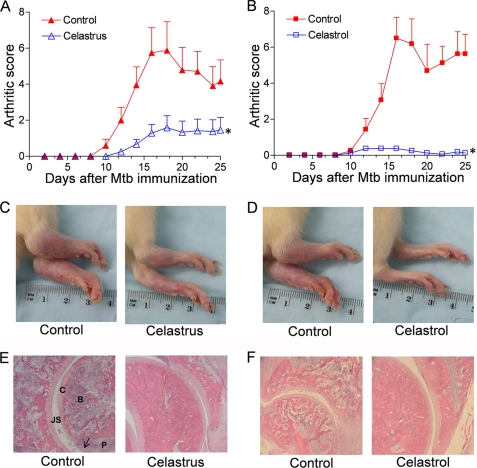
A group each of Mtb-immunized Lewis rats was fed daily with Celastrus extract or injected intraperitoneally with celastrol starting at the onset of AA and then continued for 25 days. The control rats received water orally or PBS/DMSO (1.2%) intraperitoneally. Both experimental groups of rats showed significant reduction in the severity of AA compared with the control rats (p < 0.05) (Fig. 1, A–D). The mean arthritic scores of Celastrus-fed and control rats at the peak phase of the disease course were 1.6 and 5.9, respectively (Fig. 1A). In comparison, celastrol-treated and control rats displayed mean arthritic score of <1.0 and 6.2, respectively (Fig. 1B). The anti-arthritic activity of Celastrus and celastrol was further confirmed by histological examination of the hind paws (Fig. 1, E and F). Synovial mononuclear cell infiltration, pannus formation, and cartilage and bone destruction were significantly reduced in the joints of Celastrus- and celastrol-treated rats compared with the respective control rats. These results show that both Celastrus and its bioactive component, celastrol, have anti-arthritic activity and that the suppression effect of celastrol is much more pronounced than that of Celastrus extract.
FIGURE 1.
Suppression of AA in Lewis rats by Celastrus extract/celastrol. Mtb-immunized Lewis rats were treated with Celastrus extract (n = 6 per group) (A) or celastrol in DMSO (n = 4 per group) (B) beginning at the onset of the disease. The control rats received water or PBS/DMSO. All rats were scored regularly for the severity of arthritis. Photographs of the hind paws of a representative Lewis rat from each group on day 18 after Mtb immunization are shown in C and D. Also shown are the sections of hematoxylin/eosin-stained hind paws (E and F). B, bone; C, cartilage; JS, joint space; P, pannus. The arrow indicates mononuclear cell infiltrates. *, p < 0.05 when comparing experimental and control groups from days 12 to 22 (A) and from days 14 to 25 (B).
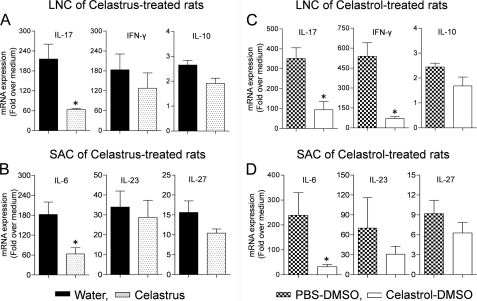
Inhibition of IL-17, IFN-γ, and IL-6 Responses in Arthritic Rats Treated with Celastrus Extract/Celastrol
The draining LNC were harvested from Celastrus/celastrol-treated rats and then restimulated in vitro with the disease-related antigen Bhsp65. The expression of cytokines in LNC was measured by quantitative RT-PCR. There was a significant decrease (p < 0.05) in the expression of IL-17 mRNA in Celastrus/celastrol-treated rats compared with their respective controls (Fig. 2, A and C). The expression of IL-17 mRNA was decreased by 3.4-fold in Celastrus-treated rats and 3.7-fold in celastrol-treated rats compared with the respective control groups. Similarly, the IFN-γ mRNA level was significantly reduced (p < 0.05) in celastrol-treated rats. However, the decline in the IFN-γ mRNA level in Celastrus-treated rats was not significant. Furthermore, neither Celastrus nor celastrol had any measurable effect on the expression of IL-10.
FIGURE 2.
Cytokine expression in LNC and SAC of Lewis rats treated with Celastrus extract/celastrol. The draining LNC from Mtb-immunized rats treated with Celastrus extract or celastrol were harvested on day 19 and restimulated in vitro for 24 h with Bhsp65 (5 μg/ml; n = 3 each) (A and C). SAC from the same rats were restimulated in vitro for 6 h with sonicated Mtb (10 μg/ml; n = 3 each) (B and D). LNC/SAC of control groups of rats were processed in the same manner. The cytokine mRNA expression in these cells was quantified by quantitative RT-PCR. Results are expressed as -fold over medium after normalization to hypoxanthine-guanine phosphoribosyltransferase. *, p < 0.05 when comparing experimental and control samples.
In parallel, SAC obtained from Celastrus/celastrol-treated rats were stimulated with sonicated Mtb, and the cytokine expression was measured. IL-6 mRNA expression in rats treated with Celastrus or celastrol was decreased by 2.8- or 7.3-fold, respectively, compared with their respective controls (Fig. 2, B and D). However, no significant change was observed in the expression of IL-23 and IL-27. These results show that both Celastrus and celastrol inhibit the expression of critical cytokines related to arthritis, namely IL-17, IFN-γ, and IL-6.
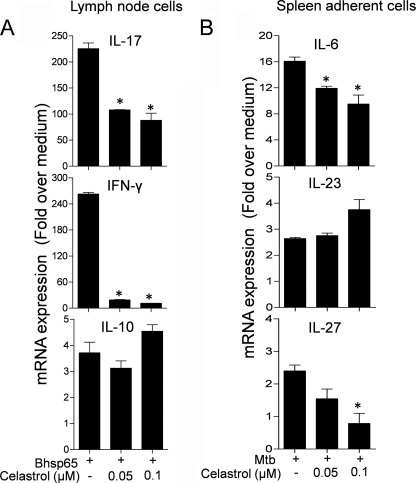
Celastrol Treatment of LNC in Vitro Suppresses Antigen (Bhsp65)-induced IL-17 and IFN-γ
To examine the effect of celastrol on the expression of cytokines in LNC in vitro, LNC were stimulated with Bhsp65 in the presence or absence of celastrol, and the cytokine expression was then measured. Celastrol decreased Bhsp65-induced expression of IL-17 mRNA by 2.1- and 2.5-fold when tested at concentrations of 0.05 and 0.1 μm, respectively (p < 0.05) (Fig. 3A). Similarly, the IFN-γ mRNA level was reduced by 13.9- and 23.2-fold, respectively (p < 0.05). However, no significant change in the levels of IL-10 mRNA expression was observed. We also studied the effect of celastrol on the expression of cytokines in SAC in vitro. The cytokine expression was measured after stimulating SAC with sonicated Mtb in the presence or absence of celastrol. The results show that celastrol significantly decreases Mtb-induced expression of IL-6 and IL-27 mRNAs in SAC (Fig. 3B). Although there was a minor increase in the levels of IL-23 at 0.1 μm celastrol, the change was not significant.
FIGURE 3.
Cytokine expression in LNC and SAC treated with celastrol in vitro. LNC harvested from a Lewis rat on day 19 after Mtb challenge were restimulated in vitro for 24 h with Bhsp65 (A), and SAC from the same rats were restimulated in vitro for 6 h with sonicated Mtb (B) in the presence or absence of celastrol (0.05 or 0.1 μm). The cytokine mRNA expression in these cells was analyzed by quantitative RT-PCR. Values were normalized to the respective hypoxanthine-guanine phosphoribosyltransferase mRNA levels and are expressed as -fold over medium. The results of a representative experiment from a set of three independent experiments done in triplicates are shown. *, p < 0.05 versus Bhsp65/Mtb alone.
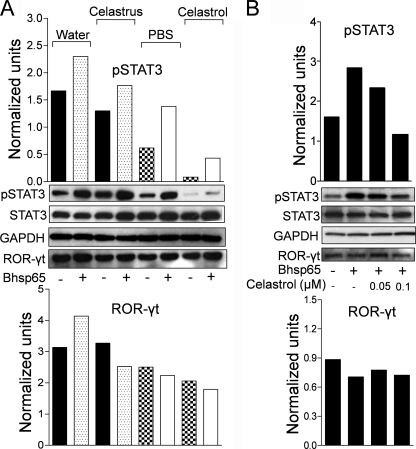
Celastrus and Celastrol Modulate the Expression of pSTAT3 and ROR-γt Induced by the Disease-related Antigen
As described above, both Celastrus and celastrol suppress IL-17 and IFN-γ ex vivo as well as in vitro. To further validate suppression of the IL-17 response, LNC from Celastrus/celastrol-treated and control rats were cultured in the presence or absence of Bhsp65, and the expression of two Th17-related transcription factors (pSTAT3 and ROR-γt) was measured (Fig. 4A). Celastrus/celastrol-treated rats showed reduced expression of Bhsp65-induced pSTAT3 compared with their respective controls. A similar pattern was observed with LNC cultured without Bhsp65, showing the ex vivo expression profile of LNC. In the case of ROR-γt, the reduction was observed in LNC restimulated with Bhsp65 but not in unstimulated LNC from Celastrus-treated rats. The effect was less marked in the celastrol-treated group. Similar results were obtained when the effect of celastrol on LNC was tested in vitro (Fig. 4B).
FIGURE 4.
Effect of Celastrus extract/celastrol on cytokine-related transcription factors. A, LNC from Mtb-immunized rats treated with Celastrus extract/celastrol or the vehicle (water or PBS/DMSO) were harvested on day 19 of AA and restimulated in vitro for 24 h with or without Bhsp65. B, LNC were treated as described in the legend to Fig. 3A. Lysates from these cells were prepared, and the levels of pSTAT3 and ROR-γt were analyzed by Western blotting. GAPDH served as the control. The intensity of the bands was quantified by densitometric analysis and normalized against the appropriate controls. The results of a representative experiment from a set of three independent experiments are shown.
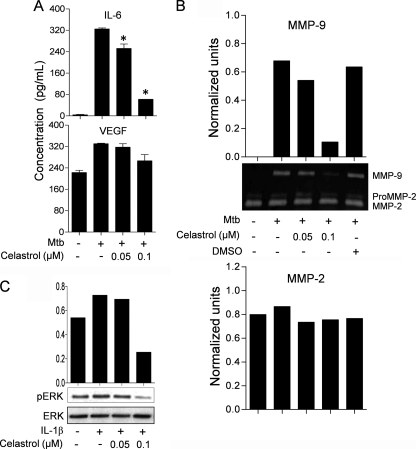
Celastrol Treatment of Synovial Fibroblasts in Vitro Selectively Suppresses the Secretion of IL-6 and MMP-9 and Inhibits pERK
We further examined the effect of celastrol on the secretion of mediators of inflammation and tissue damage by rat fibroblast-like synoviocytes obtained from arthritic joints of Lewis rats. Celastrol significantly suppressed Mtb-induced IL-6 production in a concentration-dependent manner (Fig. 5A). However, there was no significant change in the levels of the angiogenic factor VEGF. In addition, celastrol inhibited the secretion of MMP-9 induced by Mtb, whereas, the activity of pro-MMP-2 and MMP-2 was unaltered by Mtb as well as celastrol (Fig. 5B). Interestingly, celastrol reduced the levels of pERK (Fig. 5C). However, the levels of phosphorylated p38 and JNK remained unaltered (supplemental Fig. 3).
FIGURE 5.
Celastrol modulates the production of mediators of inflammation and tissue damage by synovial fibroblasts. Synovial fibroblasts were stimulated for 24 h with sonicated Mtb (10 μg/ml) in the presence or absence of celastrol (0.05 or 0.1 μm). Culture supernatants from these cells were analyzed for IL-6 and VEGF (n = 3 each) (A) and MMP-9 and MMP-2 activity (B). The graph in B represents the normalized units following densitometric analysis. Synovial fibroblasts were stimulated with IL-1β in the presence or absence of celastrol, and the levels of pERK and total ERK were determined by Western blotting (C). The results shown in B and C are from a representative experiment each from a set of two to three independent experiments.
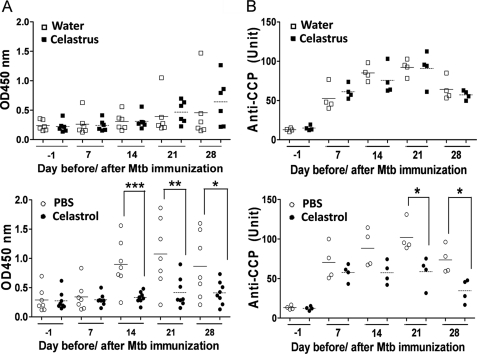
Anti-Bhsp65 and aCCP Antibody Response in Celastrus/Celastrol-treated Rats
All arthritic rats produced antibodies to Bhsp65 (Fig. 6A). Celastrol-treated rats had reduced levels of anti-Bhsp65 antibodies after treatment (on days 14, 21, and 28) compared with the control rats. However, no detectable change in the level of anti-Bhsp65 antibodies was observed in Celastrus-fed rats compared with water-fed rats.
FIGURE 6.
Celastrol treatment modulates anti-Bhsp65 and aCCP antibody responses in arthritic rats. Serum levels of anti-Bhsp65 total Ig (A) and aCCP total Ig (B) in Celastrus-treated (upper panel) and celastrol-treated (lower panel) rats. In A and B, the mean values of each group are indicated by horizontal lines (n = 6–7 per group for anti-Bhsp65 antibody, and n = 4 per group for aCCP antibody. *, p < 0.05; **, p < 0.01; ***, <0.001).
In a recent study, we showed that Lewis rats with AA but not naive rats develop aCCP antibodies during the natural course of the disease (35). The level of aCCP antibodies correlates with the disease severity, thus serving as a reliable biomarker for disease activity. In this study, we observed that arthritic Lewis rats but not unimmunized Lewis rats developed aCCP antibodies (Fig. 6B). However, the level of aCCP antibodies was significantly reduced (p < 0.05) on days 21 and 28 in celastrol-injected rats compared with control rats. In contrast, the aCCP antibody level was unchanged in Celastrus-fed rats compared with water-fed control rats.
DISCUSSION
In this study based on an experimental model of autoimmune arthritis (the AA model), we have described the mechanisms underlying the anti-arthritic activity of C. aculeatus Merrill (23) and celastrol, one of the bioactive components of Celastrus (23–26). Most of the studies on celastrol are based on its antitumor activity (29). Our study has unraveled the immunological basis of the anti-arthritic property of this naturally occurring plant compound.
Lewis rats immunized with Mtb (controls) developed severe AA, whereas rats similarly immunized with Mtb but then treated with celastrol beginning at the onset of AA were significantly protected against arthritis. Celastrol was much more efficient in suppressing AA than the natural plant extract (Celastrus extract) apparently due to the higher molar amount of celastrol in the pure preparation compared with the natural extract. The objective of this experiment was to show that a defined compound (celastrol) derived from the plant Celastrus has anti-arthritic activity, as does the natural plant extract. This information provided us the rationale for examining the mechanism of action of celastrol, which then could be extrapolated to that of the natural Celastrus extract. As such, it is difficult to perform advanced mechanistic and specificity of action studies using a crude plant extract, which is sparingly soluble and possesses multiple components. These limitations can be overcome by using a purified soluble component such as celastrol that possesses the disease-modulating attribute of the natural plant extract. In our previous study, we showed the arthritis-protective effect of Celastrus using a preventive regimen (feeding Celastrus before onset of AA) (21). In this study, we have tested celastrol compared with Celastrus using a therapeutic regimen (after onset of AA) and examined the mechanisms involved in a comprehensive manner.
The pathogenesis of autoimmune arthritis involves both cellular and humoral immune responses (1, 4). CD4+ T cells and macrophages infiltrating into the joint tissue and the synovial fibroblasts represent the critical cellular mediators of inflammatory arthritis. These cells secrete cytokines (e.g. IL-17, IL-6, and IFN-γ) and biochemical mediators such as MMPs, which initiate and propagate arthritic inflammation and tissue damage (4, 5, 36). IL-17 is produced by Th17 cells, which are distinct from the IFN-γ-producing Th1 cells (6, 8). ROR-γt induces the differentiation of Th17 cells, whereas STAT3 plays an important role in IL-17 production (6, 8, 9). In the above context, we hypothesized that the anti-arthritic activity of Celastrus/celastrol involves the down-modulation of the major immunological and biochemical mediators of inflammatory arthritis. In fact, as described below, our results fully supported this proposition.
The draining LNC of Mtb-immunized Lewis rats treated with Celastrus or celastrol showed a significantly reduced expression of IL-17 and IL-6 (in addition, IFN-γ in the case of celastrol) in response to the disease-related antigen Bhsp65, but without much effect on the anti-inflammatory cytokine IL-10. The inhibition of IL-17 and IL-6 expression was further supported by significantly reduced expression of pSTAT3. The relative lack of effect on ROR-γt suggested that Celastrus/celastrol had a major effect on the activity of existing Th17 cells rather than on the differentiation of naive T cells into Th17 cells. Furthermore, it has been shown that IL-6 can induce the phosphorylation of STAT3 in certain cell lines (37). In this regard, it is likely that the reduced pSTAT3 observed in our study might be attributable both to a direct effect of celastrol on STAT3 and to an indirect effect via reduced IL-6 expression. The precise mechanisms involved remain to be tested. Besides the T cell-derived cytokines, the SAC obtained from Celastrus/celastrol-treated rats showed a significantly reduced expression of IL-6. Furthermore, the results regarding the expression of cytokines and the corresponding transcription factors in the LNC or SAC from Lewis rats treated with Celastrus/celastrol were corroborated by parallel sets of in vitro experiments in which the same cell type obtained from Mtb-immunized rats (but not treated in vivo with Celastrus/celastrol) was treated in vitro with celastrol in the presence of the appropriate antigen (Bhsp65 or sonicated Mtb). Taken together, these results show that Celastrus/celastrol reduced the levels of proinflammatory cytokines without enhancing the expression of anti-inflammatory cytokines. The relative increase in anti-inflammatory cytokines was achieved only indirectly by shifting the balance of pro- versus anti-inflammatory cytokines compared with that in the control rats.
MMPs represent one of the major groups of tissue-degrading enzymes in the course of inflammatory arthritis (36, 38). In this regard, we examined the effect of celastrol on the activity of two members of the MMP family, MMP-9 and MMP-2, in synovial fibroblasts stimulated by Mtb in vitro. Our results show that Mtb stimulated MMP-9 activity. These results are similar to those of others showing the induction of MMP-9 in macrophages following mycobacterial infection (39). Furthermore, we observed that celastrol had an inhibitory effect on MMP-9 but not MMP-2 activity. At present, we do not know the precise reasons for this differential effect on MMP-9 over MMP-2. Also, it is not clear if the effect of celastrol on MMP-9 is a direct effect or an indirect effect via the inhibition of IL-6. As clear from our results, celastrol treatment of Mtb-stimulated synovial fibroblasts reduced not only MMP-9 activity but also IL-6 production. IL-6 is known to attract phagocytes to the site of infection as well as to regulate the production and activity of MMPs (40). Furthermore, a linear correlation between the level of IL-6 and MMP-9 activity under inflammatory conditions such as wound healing has been reported by other investigators (40). Therefore, it is logical to invoke the celastrol-mediated suppression of IL-6 as one of the factors leading to the inhibition of MMP-9 activity. MMP-9 has been shown to be a critical matrix-degrading enzyme under inflammatory conditions, and it is involved in cellular migration and tissue remodeling (36, 38, 40). Therefore, the inhibition of MMP-9 by celastrol in vitro explains in part the limited tissue damage in the joints of Celastrus/celastrol-treated rats compared with control rats. In addition to reducing MMP-9 and IL-6, the anti-inflammatory activity of celastrol involved a significant reduction in pERK levels. However, the levels of both phosphorylated p38 and JNK were not affected much. Our results of celastrol-induced reduction of pERK are supported by those from other studies conducted on cell lines (41, 42).
Besides the cell-mediated immune effector mechanisms, antibodies also play an important role in the pathogenesis of arthritis (1, 11). Studies in RA patients (1, 10, 11) and animal models of arthritis (43) have revealed the pathogenic effects of antibodies. Antibodies against type II collagen (43, 44) and glucose-6-phosphate isomerase (45) are examples of pathogenic antibodies as revealed in mice with collagen-induced arthritis and K/BXN mice, respectively. Over the past decade, aCCP antibodies have been the major focus of attention in regard to the pathogenesis of arthritis (1, 10, 11). The aCCP antibodies show a strong correlation with smoking, and they serve as a reliable biomarker for the diagnosis of RA. In a recent study, we have shown that Lewis rats with AA develop aCCP antibodies during the natural course of the disease and that the levels of these antibodies correlated well with disease severity (35). Therefore, in this study, we examined the effect of Celastrus/celastrol treatment of Lewis rats on the levels of aCCP antibodies. Our results show that celastrol-treated rats had significantly reduced levels of aCCP antibodies compared with control rats, whereas Celastrus-treated and control rats had comparable levels of aCCP antibodies. A similar pattern of response was observed for anti-Bhsp65 antibodies in the Celastrus- versus celastrol-treated rats. These results suggest that although both Celastrus extract and celastrol suppressed arthritis significantly, significant reduction in aCCP and anti-Bhsp65 antibodies was observed only in the case of the latter. These results may be interpreted to mean that a drastic reduction in disease severity is accompanied by this change in antibody response. Alternatively, the inhibitory effect on antibody response may be a property of celastrol alone, and this effect may be counterbalanced by other constituents in Celastrus extract. Regardless, these results demonstrate that celastrol has a notable influence on the humoral immune response against the disease-related antigen (Bhsp65) and against citrullinated proteins whose identity in AA remains unknown. It is plausible that Bhsp65 may be among the citrullinated proteins in arthritic Lewis rats and that a subset of aCCP antibodies may be directed against citrullinated Bhsp65.
In summary, our results show that both Celastrus and celastrol attenuate the severity of ongoing AA and suppress the proinflammatory cytokine (e.g. IL-17 and IL-6) response to the disease-related antigen Bhsp65. In addition, both these products significantly suppress serum levels of aCCP antibodies as well as MMP-9 activity. We injected celastrol into rats intraperitoneally, but the same product is effective (against airway inflammation) when delivered to mice orally (44). This has relevance for translational studies. We believe that our results would form the foundation for the preclinical testing of Celastrus and celastrol in RA patients.
Supplementary Material
Acknowledgments
We thank Dr. Muraly Puttabyatappa for help in experimental work and Dr. S. N. Vogel for providing the real-time PCR facility.
This work was supported, in whole or in part, by National Institutes of Health Grant R01AT004321.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1–3.
- RA
- rheumatoid arthritis
- ROR-γt
- retinoid related orphan receptor-γt
- aCCP
- anti-cyclic citrullinated protein/peptide
- AA
- adjuvant-induced arthritis
- Mtb
- heat-killed M. tuberculosis H37Ra
- DMSO
- dimethyl sulfoxide
- LNC
- lymph node cell(s)
- SAC
- spleen adherent cell(s)
- pSTAT3
- phospho-STAT3
- MMP
- matrix metalloproteinase
- pERK
- phospho-ERK.
REFERENCES
- 1. Lipsky P. E. (2005) in Harrison's Principles of Internal Medicine (Kasper D., Braunwald E., Fauci A., Hauser S., Longo D., Jameson J. eds) pp. 1968–1977, 16th Ed., McGraw-Hill Book Co., New York [Google Scholar]
- 2. Backman C. L. (2004) Curr. Opin. Rheumatol. 16, 148–152 [DOI] [PubMed] [Google Scholar]
- 3. Scott D. L., Smith C., Kingsley G. (2003) Clin. Exp. Rheumatol. 21, S20–S27 [PubMed] [Google Scholar]
- 4. Gorman C. L., Cope A. P. (2008) Best Pract. Res. Clin. Rheumatol. 22, 221–238 [DOI] [PubMed] [Google Scholar]
- 5. Brennan F. M., McInnes I. B. (2008) J. Clin. Invest. 118, 3537–3545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gaffen S. L. (2009) Curr. Rheumatol. Rep. 11, 365–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. van den Berg W. B., Miossec P. (2009) Nat. Rev. Rheumatol. 5, 549–553 [DOI] [PubMed] [Google Scholar]
- 8. Bettelli E., Oukka M., Kuchroo V. K. (2007) Nat. Immunol. 8, 345–350 [DOI] [PubMed] [Google Scholar]
- 9. Manel N., Unutmaz D., Littman D. R. (2008) Nat. Immunol. 9, 641–649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pruijn G. J., Wiik A., van Venrooij W. J. (2010) Arthritis Res. Ther. 12, 203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Song Y. W., Kang E. H. (2010) Qjm 103, 139–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kremers H. M., Nicola P., Crowson C. S., O'Fallon W. M., Gabriel S. E. (2004) J. Rheumatol. 31, 2366–2373 [PubMed] [Google Scholar]
- 13. Fitzgerald G. A. (2004) N. Engl. J. Med. 351, 1709–1711 [DOI] [PubMed] [Google Scholar]
- 14. Stiel D. (2000) Am. J. Ther. 7, 91–98 [DOI] [PubMed] [Google Scholar]
- 15. Weir M. R. (2002) Cleve. Clin. J. Med. 69, SI53–SI58 [DOI] [PubMed] [Google Scholar]
- 16. Barnes P. M., Powell-Griner E., McFann K., Nahin R. L. (2004) Adv. Data 343, 1–19 [PubMed] [Google Scholar]
- 17. Taibi D. M., Bourguignon C. (2003) Fam. Community Health 26, 41–52 [DOI] [PubMed] [Google Scholar]
- 18. Kageyama-Yahara N., Wang P., Wang X., Yamamoto T., Kadowaki M. (2010) Biol. Pharm. Bull. 33, 142–145 [DOI] [PubMed] [Google Scholar]
- 19. Lainer-Carr D., Brahn E. (2007) Nat. Clin. Pract. 3, 434–442 [DOI] [PubMed] [Google Scholar]
- 20. Shen C. L., Yeh J. K., Samathanam C., Cao J. J., Stoecker B. J., Dagda R. Y., Chyu M. C., Dunn D. M., Wang J. S. (2011) Osteoporos Int. 22, 327–337 [DOI] [PubMed] [Google Scholar]
- 21. Venkatesha S. H., Berman B. M., Moudgil K. D. (2011) Bioorg. Med. Chem. 19, 21–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Venkatesha S. H., Rajaiah R., Berman B. M., Moudgil K. D. (2011) Evid. Based Complement. Alternat. Med. 2011, 986797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tong L., Moudgil K. D. (2007) Arthritis Res. Ther. 9, R70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wu S., Sun C., Wang K., Pan Y. (2004) J. Chromatogr. 1028, 171–174 [DOI] [PubMed] [Google Scholar]
- 25. Luo D. Q., Wang H., Tian X., Shao H. J., Liu J. K. (2005) Pest Manag. Sci. 61, 85–90 [DOI] [PubMed] [Google Scholar]
- 26. Kim D. H., Shin E. K., Kim Y. H., Lee B. W., Jun J. G., Park J. H., Kim J. K. (2009) Eur. J. Clin. Invest. 39, 819–827 [DOI] [PubMed] [Google Scholar]
- 27. Durai M., Gupta R. S., Moudgil K. D. (2004) J. Immunol. 172, 2795–2802 [DOI] [PubMed] [Google Scholar]
- 28. Moudgil K. D., Chang T. T., Eradat H., Chen A. M., Gupta R. S., Brahn E., Sercarz E. E. (1997) J. Exp. Med. 185, 1307–1316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gupta S. C., Kim J. H., Prasad S., Aggarwal B. B. (2010) Cancer Metastasis Rev. 29, 405–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kim H. R., Kim E. Y., Cerny J., Moudgil K. D. (2006) J. Immunol. 177, 6634–6641 [DOI] [PubMed] [Google Scholar]
- 31. Hu H., Straub A., Tian Z., Bassler N., Cheng J., Peter K. (2009) J. Cardiovasc. Pharmacol. 54, 240–245 [DOI] [PubMed] [Google Scholar]
- 32. Yang H., Chen D., Cui Q. C., Yuan X., Dou Q. P. (2006) Cancer Res. 66, 4758–4765 [DOI] [PubMed] [Google Scholar]
- 33. Rajaiah R., Puttabyatappa M., Polumuri S. K., Moudgil K. D. (2011) J. Biol. Chem. 286, 2817–2825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hembry R. M., Atkinson S. J., Murphy G. (2007) Methods Mol. Med. 135, 227–238 [DOI] [PubMed] [Google Scholar]
- 35. Yu H., Yang Y.-H., Rajaiah R., Moudgil K. D. (2011) Arthritis Rheum. 63, 981–991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chang Y. H., Lin I. L., Tsay G. J., Yang S. C., Yang T. P., Ho K. T., Hsu T. C., Shiau M. Y. (2008) Clin. Biochem. 41, 955–959 [DOI] [PubMed] [Google Scholar]
- 37. Berishaj M., Gao S. P., Ahmed S., Leslie K., Al-Ahmadie H., Gerald W. L., Bornmann W., Bromberg J. F. (2007) Breast Cancer Res. 9, R32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Eck S. M., Blackburn J. S., Schmucker A. C., Burrage P. S., Brinckerhoff C. E. (2009) J. Autoimmun. 33, 214–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Quiding-Järbrink M., Smith D. A., Bancroft G. J. (2001) Infect. Immun. 69, 5661–5670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pajulo O. T., Pulkki K. J., Alanen M. S., Reunanen M. S., Lertola K. K., Mattila-Vuori A. I., Viljanto J. A. (1999) Wound Repair Regen. 7, 453–457 [DOI] [PubMed] [Google Scholar]
- 41. Jung H. W., Chung Y. S., Kim Y. S., Park Y. K. (2007) Exp. Mol. Med. 39, 715–721 [DOI] [PubMed] [Google Scholar]
- 42. Kim Y., Kim K., Lee H., Han S., Lee Y. S., Choe J., Kim Y. M., Hahn J. H., Ro J. Y., Jeoung D. (2009) Eur. J. Pharmacol. 612, 131–142 [DOI] [PubMed] [Google Scholar]
- 43. Uysal H., Nandakumar K. S., Kessel C., Haag S., Carlsen S., Burkhardt H., Holmdahl R. (2010) Immunol. Rev. 233, 9–33 [DOI] [PubMed] [Google Scholar]
- 44. Wooley P. H. (1988) Methods Enzymol. 162, 361–373 [DOI] [PubMed] [Google Scholar]
- 45. Korganow A. S., Ji H., Mangialaio S., Duchatelle V., Pelanda R., Martin T., Degott C., Kikutani H., Rajewsky K., Pasquali J. L., Benoist C., Mathis D. (1999) Immunity 10, 451–461 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.