Abstract
In response to blood vessel injury, hemostasis is initiated by platelet activation, advanced by thrombin generation, and tempered by fibrinolysis. The primary fibrinolytic protease, plasmin, can be activated either on a fibrin-containing thrombus or on cells. Annexin A2 (A2) heterotetramer (A2·p11)2 is a key profibrinolytic complex that assembles plasminogen and tissue plasminogen activator and promotes plasmin generation. We now report that, in endothelial cells, plasmin specifically induces activation of conventional PKC, which phosphorylates serine 11 and serine 25 of A2, triggering dissociation of the (A2·p11)2 tetramer. The resulting free p11 undergoes ubiquitin-mediated proteasomal degradation, thus preventing further translocation of A2 to the cell surface. In vivo, pretreatment of A2+/+ but not A2−/− mice with a conventional PKC inhibitor significantly reduced thrombosis in a carotid artery injury model. These results indicate that augmentation of fibrinolytic vascular surveillance by blockade of serine phosphorylation is A2-dependent. We also demonstrate that plasmin-induced phosphorylation of A2 requires both cleavage of A2 and activation of Toll-like receptor 4 on the cell surface. We propose that plasmin can limit its own generation by triggering a finely tuned “feedback” mechanism whereby A2 becomes serine-phosphorylated, dissociates from p11, and fails to translocate to the cell surface.
Keywords: Annexin, Fibrinolysis, Plasminogen, Protein Kinase C (PKC), Thrombosis
Introduction
The human hemostatic system represents a coordinated balance between procoagulant and anticoagulant/profibrinolytic activities. Together, these systems prevent blood loss while maintaining blood flow. Plasmin, the major protease of the fibrinolytic system, acts to digest insoluble fibrin into soluble fibrin degradation products. Plasmin generation is modulated by plasminogen activators and plasminogen activator inhibitors and is further regulated by binding of plasminogen and its activators to cell surface receptors (1). Membrane-associated annexin A2 (A2)3 is part of a heterotetrameric complex in which the N termini of two copies of A2 bind to two copies of the S100 family protein, S100A10 (p11) (2). A2, a member of the annexin superfamily, consists of a highly conserved C-terminal phospholipid-binding core domain and an N-terminal ligand-interacting domain (3). Complex formation with p11 increases the affinity of A2 for calcium and phospholipids, thereby directing it to cellular membranes (4). Components of the (A2·p11)2 tetramer, moreover, specifically bind tissue plasminogen activator (t-PA) and plasminogen and strongly enhance plasmin generation (5–8, 52).
Several lines of evidence identify the (A2·p11)2 complex as a significant regulator of fibrin balance in vivo. First, A2−/− microvascular endothelial cells lack t-PA cofactor activity, and the fibrin content of highly perfused A2−/− tissues is ∼2-fold greater than that observed in wild type controls (9). In addition, arterial injury in A2−/− mice is followed by a 2-fold increase in thrombotic vascular occlusion with an equivalent reduction in blood flow recovery (9). Third, injury-induced carotid artery thrombosis in rats can be averted by pretreatment with recombinant A2 (10). Fourth, cerebral infarct size can be reduced and cerebral blood flow can be increased, without affecting systemic hemostatic parameters, upon infusion of recombinant A2 alone or in combination with t-PA in a rat model of embolic stroke (11, 12). Fifth, A2 is strikingly overexpressed in blast cells and is associated with hyperfibrinolytic hemorrhage in patients with acute promyelocytic leukemia (13). Sixth, autoantibodies directed against A2 are prevalent and associated with clinical thrombosis in patients with the anti-phospholipid syndrome (14). Finally, infusion of recombinant A2 can reverse microvascular fibrin accumulation and restore perivascular fibrinolysis in hyperhomocysteinemic mice, which are susceptible to arterial thrombosis (15).
Although there is abundant evidence to support a role for the (A2·p11)2 system in modulating hemostasis, relatively little is known about how the (A2·p11)2 system itself is governed. It is clear that translocation of the (A2·p11)2 complex to the cell surface is initiated by signals, such as heat stress and thrombin exposure, which activate a Src-like kinase that phosphorylates A2 at tyrosine 23 (16, 17). It is also known that trafficking of A2 to the cell surface requires the presence of p11 (16). Additional data indicate that p11 probably serves as a cellular rheostat for A2 translocation; endothelial cell p11 is unstable unless it is associated with A2, suggesting that its intracellular level can never exceed that of A2 in the endothelial cell. A2 stabilizes p11 by blocking a polyubiquitination site, which, once unmasked, signals the proteasomal degradation of unpartnered p11 (18).
But how does the endothelial cell control the quantity of plasmin produced on its surface? Excessive plasmin activity could lead to concomitant damage to surrounding tissue and/or hemorrhage. Although plasmin's major inhibitor, α2-plasmin inhibitor, circulates in excess in plasma, receptor-bound plasmin appears to be resistant to α2-plasmin inhibitor (19), and there is no evidence that active plasmin is internalized by the cell. Here, we suggest that plasmin formed on the cell surface may limit its own generation by reducing the rate of (A2·p11)2 translocation from the intracellular compartment to the cell surface. We report that plasmin induces a Toll-like receptor 4-dependent signal that activates conventional protein kinase C (cPKC), which, in turn, phosphorylates A2 at serines 11 and 25. Serine-phosphorylated A2 dissociates from p11 (20), preventing its translocation to the cell surface, a p11-dependent process. Our findings, therefore, define a novel feedback mechanism by which plasmin activity is controlled at the endothelial cell surface. The data imply, furthermore, that the therapeutic usage of a selective cPKC inhibitor might be helpful in some settings of clinical thrombosis.
EXPERIMENTAL PROCEDURES
Materials
The following antibodies were purchased from the indicated vendors: mouse monoclonal anti-annexin A2 and anti-S100A10 IgG (BD Biosciences); goat anti-CD47 IgG, mouse anti-PAR1 IgG (ATAP2 or C-18), and rabbit anti-A2 IgG (Santa Cruz Biotechnology, Inc., Santa Cruz, CA); monoclonal anti-PAR1 IgG (WEDE15) (Beckman Coulter, Fullerton, CA); rabbit anti-S100A10 IgG (ProteinTech Group, Chicago, IL); rabbit anti-human TLR4 IgG (HTA125) and mouse IgG2a isotype control (eBioscience, San Diego, CA); mouse monoclonal anti-phosphotyrosine IgG (Tyr(P)-100), phospho-MAPK family antibody sampler kit, and rabbit pan-phospho-PKC IgG (βII Ser-660) (Cell Signaling Technology, Danvers, MA); mouse monoclonal anti-ubiquitin IgG (Invitrogen); sheep anti-mouse or donkey anti-rabbit IgG and peroxidase-linked species-specific whole antibodies (Amersham Biosciences); and anti-phosphoserine-agarose (Sigma). Other reagents, purchased from Sigma unless otherwise indicated, were as follows. Purified human Lys-plasmin and human thrombin were from American Diagnostica (Greenwich, CT); recombinant tissue-type plasminogen activator was provided by Innovative Research Inc. (Novi); a PAR4 peptide (H-Gly-Tyr-Pro-Gly-Lys-Phe-NH2) was purchased from Peptide International (Louisville, KY); human thrombin, collagen, and ADP were from Chronolog (Havertown, PA); d-Val-Phe-Lys chloromethyl ketone and GÖ6976 were from Calbiochem; and sulfosuccinimidyl-2-(biotinamido)-ethyl-1.3-dithiopropionate and streptavidin-agarose were from Pierce.
Cell Culture
Human umbilical vein endothelial cells (HUVECs) and mouse cardiac microvascular endothelial cells (CMECs) were isolated and propagated as described previously (9, 21), and studied at passages 2–8.
Mice
A2+/+ or A2−/− mice on the C57BL/6 background have been described previously (9). Procedures and animal care were approved by the IACUC at Weill Cornell Medical College and were in accordance with the “Guide for the Care and Use of Laboratory Animals.”
Stable Cell Transfections
The wild type human A2 cDNA was cloned in frame into the eukaryotic expression vector pcDNA3.1(+)/Myc-His (Invitrogen) as described previously (18). The resulting vector was modified by site-directed mutagenesis (QuikChange, Stratagene), substituting serines at position 11 and/or 25 with alanines, resulting in S11A, S25A, and S11A/S25A mutants. The integrity of both vectors was verified by full-length cDNA sequence analysis. Transformed human embryonic kidney cells (HEK293) were transfected with either wild type or S11A, S25A, or S11A/S25A A2 mutant cDNAs using DNA-calcium phosphate precipitation and 1–2 μg of plasmid (22). Following selection with 1 mg/ml G418 for 7–10 days, stable clones were isolated, expanded, and analyzed by immunoblotting for A2 expression.
Immunoblotting and Co-immunoprecipitation
Cells were preincubated with saline or GÖ6976 and then treated with or without 0.3 unit/ml of either plasmin or equivalent amounts of inactive plasmin (25 μm d-Val-Phe-Lys chloromethyl ketone-treated) (23) for the indicated time intervals at 37 °C. The cells were then washed in cold PBS and disrupted in lysis buffer (50 mm Tris, pH 7.4, 150 mm NaCl, 1% Nonidet P-40, 10% glycerol, 2 mm EDTA, 1 mm NaF, 25 mm β-glycerophosphate, 20 mm sodium pyrophosphate, 1 mm sodium orthovanadate, 5 μg/ml aprotinin, 5 μg/ml leupeptin, 5 μg/ml pepstatin, 1 mm PMSF). The lysates were resolved by SDS-PAGE and immunoblotted with various antibodies. The cell lysates were also incubated with anti-phosphoserine or anti-phosphotyrosine-coupled agarose for 12–16 h and then washed 3–4 times in lysis buffer. The resulting immunoprecipitates were separated by SDS-PAGE and immunoblotted with anti-annexin A2 IgG. Detection of ubiquitinated p11 and cell surface A2 or p11 was performed as previously described (16, 18). Blots representative of at least three independent experiments are displayed as figures (Figs. 1–3, 5, and 6).
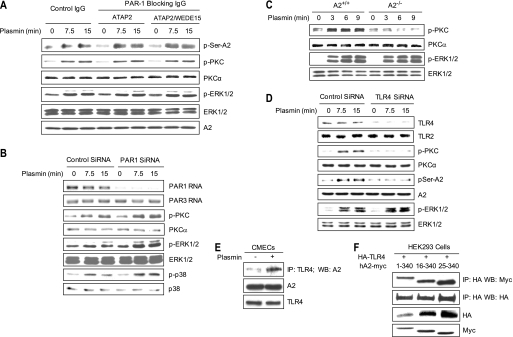
FIGURE 1.
Phosphorylation of endothelial cell PKC induced by plasmin. A, HUVECs were treated with human plasmin for the indicated times and at the indicated doses. Phosphorylation of PKC and MAPK (ERK1/2 and p38) was detected by immunoblot using anti-phospho-PKC, anti-PKCα, anti-phospho-ERK1/2, anti-phospho-p38, or anti-A2. β-Tubulin was employed as a loading control. B, CMECs were stimulated with ionomycin (1 μm) for the indicated time periods. Activation of PKC, ERK1/2, and p38 was assessed as in A. C, CMECs were pretreated with EDTA (1 mm) or 1,2-bis(o-Amino-5′-methylphenoxy)ethane-N,N,N′,N′-tetraacetic acid tetraacetoxymethyl ester (MAPTAM) (100 μm) for 30 min and then treated with plasmin (0.3 unit/ml) for the indicated time period. Cell lysates were then resolved by SDS-PAGE and analyzed by p-PKC, PKCα, and actin immunoblotting.
FIGURE 2.
Plasmin-induced serine phosphorylation of A2 is specifically mediated by classical PKC. A, CMECs were treated with either plasmin (0.3 unit/ml, 15 min; left), trypsin (2.76 units/ml, 3 min; middle), or thrombin (2 units/ml, 7 min; right). Total serine- or tyrosine-phosphorylated proteins (1000, 1000, and 550 μg, respectively) were immunoprecipitated with anti-phosphoprotein IgGs and then immunoblotted with monoclonal anti-A2 IgG. Total A2 and phospho-PKC were also evaluated by immunoblotting. B, HUVECs were transfected with siRNA directed against PKCα (1 μm) or control siRNA for 72 h, washed, and then treated with plasmin for the indicated time periods. Expression of phospho-PKC, serine phospho-A2, and phospho-ERK1/2 was monitored by immunoblotting. Specificity of the knockdown was determined by immunoblot analysis of total PKCα and PKCδ protein.
FIGURE 3.
Plasmin induces serine phosphorylation of A2 at positions 11 and 25. A, HEK293 cells were stably transfected with either a Myc-tagged annexin A2 wild type plasmid or Myc-tagged S11A, S25A, or S11A/S25A mutant plasmids. Then total cell lysates were examined either by phosphoserine immunoprecipitation followed by anti-A2 immunoblot analysis or by immunoblotting with anti-phospho-PKC-, anti-PKCα-, anti-phospho-ERK1/2-, or anti-ERK1/2-specific IgGs. Total transfected A2 was estimated by immunoblotting with an anti-Myc IgG, and β-tubulin was employed as a loading control. B, CMECs were stimulated with plasmin (15 min). Total lysates were immunoprecipitated (IP) with anti-p11 IgG and immunoblotted with anti-A2 IgG or anti-p11. Total lysates were also immunoprecipitated with anti-ubiquitin and immunoblotted with anti-p11, anti-phospho-PKC, and anti-phospho-ERK1/2 IgGs. Cell surface A2, p11, and CD47 were analyzed by cell surface biotinylation. C, the levels of cytoplasmic (A2·p11)2 complex and of surface A2 were quantified by densitometry and presented as the percentage of the signal detected in control cells (defined as 100%). Shown are the mean ± S.D. (error bars) for three separate experiments. D, HUVECs grown on glass coverslips were treated with plasmin for the indicated time periods and washed. Permeabilized cells were stained for cytoplasmic p11 (green), A2 (red), and DAPI (blue) (left two panels). Non-permeabilized cells were stained for surface A2 (red), DAPI (blue), and β-tubulin (green) (right two panels). The absence of signal in control IgG-stained cells indicated the specific staining. E and F, immunofluorescence intensity of cytoplasmic p11 and A2 signals was quantified using ImageJ and presented as the percentage of the signal detected in untreated cells (defined as 100%). G, the percentage of the fluorescence intensity of surface A2 after plasmin treatment, as compared with untreated cells, was estimated as indicated for E and F. H, HUVECs grown in 48-well culture plates were treated with plasmin for the indicated time periods, washed, and then assayed for t-PA-dependent plasmin generation, expressed as relative fluorescence units/min2.
FIGURE 5.
Plasmin-induced PKC activation requires its protease activity. A, CMECs were treated with either active or d-Val-Phe-Lys chloromethyl ketone (VPLCK)-inactivated plasmin for the indicated time periods. Phospho-PKC, -ERK1/2, and -p38 were evaluated by immunoblot using specific antibodies. β-Tubulin served as a loading control. B, CMECs were treated with plasmin at 0.3 unit/ml for 7.5, 15, or 30 min. A2, p11, and CD47 levels were examined by surface biotinylation followed by immunoblot analysis using rabbit polyclonal anti-A2 directed at the N-terminal tail domain of A2, monoclonal anti-p11, and goat anti-CD47 IgGs. Total A2, p11, and phospho-PKC were assessed by immunoblot analysis.
FIGURE 6.
Plasmin-induced PKC activation is mediated by A2 and TLR4 but not PAR1. A, HUVECs were preincubated with either isotype control IgG, the PAR-blocking antibody ATAP2 (20 μg/m), or ATAP2 (10 μg/ml) plus a second PAR-blocking antibody, WEDE15 (25 μg/ml), for 30 min and then stimulated with plasmin (0.3 unit/ml; 7.5 or 15 min). Serine phosphorylation of A2 was assessed by coimmunoprecipitation. Total levels of phospho-ERK1/2, -PKC, and -A2 were analyzed by immunoblotting. B, HUVECs were transfected with Accell siRNA directed against human PAR1, treated as indicated, and blotted with anti-phospho-PKC, -PKCα, -phospho-ERK1/2, -ERK1/2, -phospho-p38, and -p38. Steady state levels of PAR1 and PAR3 mRNA, as detected by RT-PCR, are shown in the first two panels. C, primary A2+/+ and A2−/− CMECs were treated with plasmin for 3, 6, or 9 min, after which phospho-PKC and -ERK1/2 were compared. D, HUVECs were transfected with Accell siRNA for human TLR4, treated as indicated. The levels of phospho-PKC, phospho-ERK1/2, serine phosphorylation of A2, and A2 were measured by immunoblotting. Specificity of the knockdown was shown by immunoblot analysis of TLR4 and TLR2 protein. E, membrane fractions were isolated from plasmin-treated CMECs and immunoprecipitated (IP) with anti-mouse-TLR4 IgG, and the precipitates were immunoblotted with anti-A2 IgG. Total cell lysates were also blotted with anti-A2 and anti-TLR4 antibody. F, HEK293 cells were transiently transfected with plasmids encoding HA-tagged TLR4 and either full-length (residues 1–340) or two N-terminal deletion versions of Myc-tagged A2 (residues 16–340 and 25–340). Two days later, total cell lysates were immunoprecipitated with anti-HA and then blotted with anti-Myc or anti-HA IgG. Total expression of HA-TLR4 and Myc-A2 mutants was assessed by immunoblot analysis.
RT-PCR
RT-PCR was carried out by using mouse p11, A2, and β-actin gene-specific primers as described previously (18) or human PAR1 or PAR3 at sequence targets at 340–683 and 202–687, respectively.
Thrombosis Model
Ferric chloride injury of the carotid artery, adjusted to induce complete arterial occlusion in 4–5-month-old fully backcrossed A2+/+ and A2−/− mice, was performed as described (9). Mice, of both sexes and 16–20 weeks of age, were anesthetized with Avertin and treated with either saline or GÖ6976 (1 mg/kg) before the procedure. The left carotid artery was exposed and fitted with a Transonic flow probe (model 0.5VB; Transonic Systems, Ithaca, NY) adjusted to record base-line flow of ≥0.5 ml/min for at least 1 min. Then, a 0.5 × 1-mm piece of filter paper saturated with 10% FeCl3 was placed on the dried adventitial surface of the vessel. After 1 min, the filter paper was removed, and blood flow was recorded for 30 min using WinDaq data acquisition software (DataQ Instruments, Akron, OH). Complete thrombotic occlusion was considered to occur once flow had decreased to 0.0 ± 0.05 ml/min.
Hematologic Assays
Blood samples were obtained from mice by cardiac puncture. Complete blood counts and differentials were obtained using a Cell Dyn 3700 analyzer (Abbott) in the Laboratory of Comparative Pathology at Weill Cornell Medical College. Platelet poor plasma or platelet-rich plasma was prepared as described (24). Plasma clot lysis was performed as described previously (25). ELISAs, including D-dimer (Diagnostica Stago) and mouse plasmin·antiplasmin complexes (Molecular Innovations), were carried out according to the manufacturers' instructions. Mouse platelet aggregation and ATP release assays were measured simultaneously in a Lumi-Aggregometer (model 700, four channels, Chrono-Log) with stirring at 37 °C, upon stimulation of platelet-rich plasma (3.3 × 108 cells/ml) over a range of concentrations of either thrombin, PAR4 peptide (GYPGKF), collagen, or ADP. The extent of aggregation and ATP release was assessed using Aggro/Link software (version 8, Chrono-Log).
Immunofluorescence
Plasmin-treated HUVECs plated on glass coverslips were fixed for 20 min in methanol at −20 °C and then incubated with rabbit anti-A2 (1:250; Santa Cruz Biotechnology, Inc.) or mouse anti-p11 mAb (1:1000; BD Biosciences) at room temperature for 1 h. The primary antibodies were probed with Cy3-conjugated donkey anti-rabbit IgG (1:300) or FITC-conjugated goat anti-mouse IgG (1:500). Nuclei were stained with DAPI (1:10,000; Molecular Probes), and cells were washed with PBS prior to mounting on Fluoromount G (Electron Microscopy Sciences). Micrographs were acquired using a Nikon ECLIPSE 80i epifluorescence microscope, and images were captured by QCapture software using identical acquisition parameters (Morrel Instrument Company, Inc.). Fluorescence intensity was quantified using the ImageJ program (National Institutes of Health).
To detect cell surface A2, mock- and plasmin-treated cells were lightly fixed with 2% paraformaldehyde for 8 min and incubated with rabbit anti-A2 (1:50) for 1 h on ice. The presence of β-microtubulin was used as an index of inadvertent permeabilization of cells. The cells were then probed with Cy3-conjugated donkey anti-rabbit IgG (1:300) or FITC-conjugated goat anti-mouse IgG (1:500) for 60 min on ice. Stained cells were fixed and stained with DAPI. The slides were mounted and imaged as described above.
Transfection of siRNA
Gene-specific Accell siRNA oligonucleotides (SMARTpool) were purchased from Dharmacon and used at final concentrations of 1 μm for human PKCα, PAR1, and TLR4. HUVECs seeded on 12-well plates were transfected at 30% confluence using Accell delivery medium according to the manufacturer's instructions. After 72 h, cells were treated and harvested. The effects of siRNA on gene expression were assessed by either immunoblotting or RT-PCR.
Cell Surface t-PA-dependent Plasmin Generation
HUVECs were grown in 48-well culture plates, treated as indicated, and assayed for t-PA-dependent plasmin generation as described previously (6, 26).
Statistical Analyses
All data were expressed as mean ± S.E. Comparisons between groups were carried out using the unpaired Student's t test. A p value of <0.05 was considered statistically significant.
RESULTS
Plasmin Induces Activation of Classical PKC in Endothelial Cells
We first investigated whether plasmin could elicit activation of PKC within endothelial cells. Stimulation of HUVECs with plasmin (0.3 unit/ml, 645 nm) led to the time-dependent activation of PKC, evident as early as 7.5 min and reaching a maximal level at 12–15 min (Fig. 1A, left). Activation of PKC was readily observed with plasmin at concentrations as low as 0.075 unit/ml (161 nm) and maximal at 0.3 unit/ml (645 nm; Fig. 1A, right). These doses are consistent with the ambient circulatory plasminogen level (1–2 μm) and plasmin binding affinity (Kd = 74 nm) (6). Interestingly, physiological concentrations of plasminogen neither activated PKC (supplemental Fig. S1) nor antagonized plasmin-induced PKC activation (data not shown). When plasminogen-bearing endothelial cells were treated with t-PA, however, time-dependent activation of PKC was clearly observed, beginning at 5 min and peaking at about 10 min (supplemental Fig. S2). Plasmin also stimulated the time-dependent phosphorylation of components of the mitogen-activated protein kinase (MAPK) system (ERK1/2 and p38). Expression of total A2 did not change upon plasmin stimulation.
Ionomyocin, a calcium ionophore, also triggered the time-dependent phosphorylation of PKC and MAPK in mouse CMECs (Fig. 1B). To evaluate the role of [Ca2+] in plasmin-stimulated PKC phosphorylation, cells were treated with either an extracellular calcium chelator (EDTA) or a cell-permeable intracellular Ca2+ chelator 1,2-bis(o-Amino-5′-methylphenoxy)ethane-N,N,N′,N′-tetraacetic acid tetraacetoxymethyl ester (MAPTAM) prior to the addition of plasmin (Fig. 1C). Blockade of either calcium influx or calcium mobilization from internal stores markedly inhibited PKC activation in plasmin-stimulated endothelial cells, suggesting that plasmin-induced PKC activation is calcium-dependent.
Plasmin Induces cPKC-dependent Serine Phosphorylation of A2
The N terminus of A2 contains several potential phosphorylation targets for PKCs and tyrosine kinases (27). Therefore, we asked whether plasmin induces phosphorylation of A2. Indeed, the serine phosphorylation of A2 increased significantly following stimulation with plasmin, whereas we found no change in total serine- or tyrosine-phosphorylated proteins (Fig. 2A, left). At the same time, interestingly, tyrosine phosphorylation of A2, which is required for its translocation to the cell surface (16), was dramatically reduced. This result is consistent with the previously proposed notion that serine and tyrosine phosphorylation of A2 do not occur simultaneously (27). Because plasmin displays broad trypsin-like substrate specificity, we also treated endothelial cells with trypsin (Fig. 2A, middle) but found no induction of serine phosphorylation of A2. On the other hand and under the same conditions, thrombin induced tyrosine, but not serine, phosphorylation of A2 (Fig. 2A, right). Taken together, these data indicate that plasmin specifically elicits serine phosphorylation of A2 in endothelial cells and that tyrosine and serine phosphorylation of A2 are mutually exclusive events.
To further determine whether plasmin-induced phosphorylation of PKC leads to serine phosphorylation of A2, we first measured protein levels of a series of PKC isoforms in endothelial cells (data not shown). Of the three major classes of PKCs (conventional PKCs (α, β, and γ isoforms), novel PKCs (δ, ϵ, η, and θ isoforms), and the atypical PKCs (λ and τ isoforms)), all nine members examined were expressed in endothelial cells. Only conventional PKCs are dependent upon calcium and phospholipid. However, although α and γ isoforms were abundantly expressed, β PKC was detected only in trace amounts. We then transfected HUVECs with PKCα siRNA (Fig. 2B). This agent decreased the protein level of PKCα by 92% but had no effect on other PKC isoforms, such as PKCδ. Knockdown of PKCα, moreover, effectively blocked plasmin-induced activation of PKC but not phosphorylation of ERK1/2. More importantly, it also blocked the serine phosphorylation of A2. These data further support the hypothesis that A2 is a specific substrate for PKCα (28).
Plasmin Triggers A2 Phosphorylation at Serines 11 and 25, A2 Dissociation from p11, and Ubiquitin-mediated Degradation of p11
Two potential serine phosphorylation sites are located within the N-terminal region of A2. In order to identify those that are specifically phosphorylated in response to plasmin, we generated three vectors for expression of Myc-tagged S11A, S25A, and S11A/S25A mutants in HEK293 cells. Among the four stably transfected wild type- or mutant A2-expressing clones (Fig. 3A), plasmin induced robust serine phosphorylation of wild type A2, whereas no increment in phosphorylation was observed in any of the three A2 mutants, although these mutants bound plasmin(ogen) in a manner indistinguishable from wild type A2 (supplemental Fig. S3). In addition, we observed no increase in activation of PKC in these mutants, although activation of ERK1/2 was preserved. Similar to a recent report illustrating the cooperative nature of phosphorylation of closely associated serine or threonine residues, this result suggested that mutation of one serine may prevent phosphorylation of another in the same region (29). These data suggest that plasmin induces phosphorylation of both serine 11 and serine 25 of A2 in HEK293 cells.
Serine 11 lies within the A2 tail region (residues 1–14), which mediates its binding to p11. Phosphorylation of serine 11 has been suggested to interfere with p11 binding (20, 30, 31). To test this hypothesis, we isolated a membrane fraction of endothelial cells following treatment with or without plasmin (Fig. 3B). Interestingly, upon plasmin treatment, we noted a significant decrease in the abundance of the (A2·p11)2 complex. In addition, there was a concomitant increase in p11 ubiquitination and degradation as well as a reduction in surface expression of A2 and p11. By densitometry scanning of the first and eighth panels of Fig. 3B, the levels of (A2·p11)2 complex and of surface A2 after plasmin stimulation were estimated to be reduced by 47 ± 2.7% and 33 ± 1.2% (mean ± S.D., n = 3 independent experiments), respectively (Fig. 3C). Total mRNAs for p11, A2, and β-actin were not altered during the experiment (not shown).
In addition, we employed immunofluorescence microscopy to evaluate the effect of plasmin on cytoplasmic p11 protein expression. Within the first few min of exposure, plasmin decreased intracellular p11 protein by 47.3 ± 7.8% compared with vehicle-treated cells (mean ± S.D. from seven low power fields, each containing 50–60 cells) (Fig. 3, D and E). Longer exposures failed to reduce p11 expression further, and the amount of intracellular A2 detected did not change significantly (Fig. 3, D and F). Similarly, plasmin decreased surface-associated A2 by 23.5 ± 4.2% within 7.5 min of exposure (mean ± S.D. from 10 low power fields, each containing 40–50 cells) (Fig. 3, D and G). Beyond this interval, plasmin down-regulated surface A2 by 31.7 ± 3.3% and 34.1 ± 5.2% at 15 and 30 min, respectively. Cell surface plasmin generation by t-PA, furthermore, was diminished by 20, 28, and 40% after plasmin treatment for 7.5, 15, and 30 min (Fig. 3H). Taken together, these data suggest that plasmin-induced phosphorylation of serine 11 of A2 serves a regulatory role by initiating intracellular dissociation of p11 from the (A2·p11)2 complex with subsequent p11 degradation. This disruption curtails translocation of the complex to the cell surface and reduces surface associated proteolytic activity.
Inhibition of cPKC in Vivo Protects against Injury-induced Arterial Thrombosis
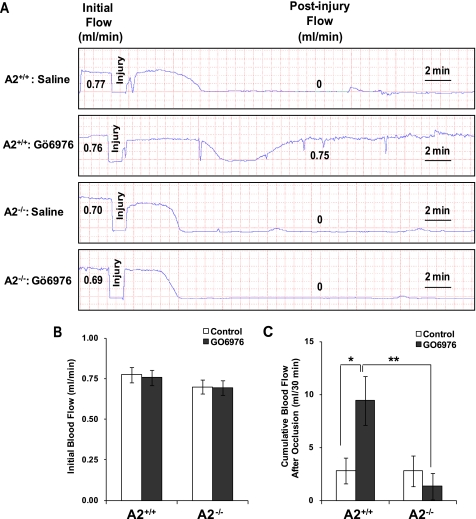
To assess more critically the regulatory role of cPKC on the fibrinolytic activity of A2 in vivo, we employed the FeCl3-induced mouse carotid artery thrombosis model (32). Topical application of ferric chloride to the vascular adventitia induces a reproducible injury with endothelial damage leading to the formation of a thrombus, whose cellular composition and fibrin content approximate those formed in atherosclerotic human coronary arteries. In our system, we employed conditions where 10% FeCl3 induced complete thrombotic occlusion of the carotid artery in both A2+/+ and A2−/− mice in a reproducible manner (Fig. 4A). The initial base-line blood flow did not differ between A2+/+ and A2−/− mice pretreated either with saline (0.77 ± 0.07 (n = 11) versus 0.70 ± 0.07 ml/min (n = 10), mean ± S.E.) or GÖ6976, a classical PKC inhibitor (0.76 ± 0.05 (n = 13) versus 0.69 ± 0.04 ml/min (n = 10)) (Fig. 4B). In addition, the time to complete arterial occlusion after injury was similar, regardless of pretreatment (5.09 ± 0.69 (n = 11) versus 5.24 ± 0.51 min (n = 10), mean ± S.E., for A2+/+ and A2−/− mice pretreated with saline, respectively; 7.08 ± 0.63 (n = 13) versus 6.41 ± 0.94 min (n = 10) for A2+/+ and A2−/− mice injected with GÖ6976, respectively). However, continuous measurement of carotid blood flow over a 30-min period using a Doppler flow probe revealed that administration of 1 mg/kg GÖ6976, but not the vehicle control, was associated with increased blood flow in the injured arteries of A2+/+ (5.25 ± 1.22 ml (n = 11), mean ± S.E., for saline; 12.55 ± 1.91 ml (n = 13) for GÖ6976) but not A2−/− mice (4.96 ± 1.52 ml for saline; 4.26 ± 1.38 ml for GO6976 (n = 10)) (Fig. 4C). These data indicate that the effect of the PKC inhibitor in preventing arterial thrombosis was A2-dependent.
FIGURE 4.
Inhibition of PKC activity with GÖ6976 protects against FeCl3-induced carotid arterial thrombosis. A, representative tracings of carotid blood flow in response to FeCl3 injury after tail vein injection of 1 mg/kg GÖ6976. Values for average initial and postinjury blood flow are shown for representative mice. B, mean initial blood flow rates for A2+/+ (mean ± S.E.; n = 11 for saline, n = 13 for GÖ6976) and A2−/− mice (mean ± S.E., n = 10). C, average cumulative blood flow following arterial occlusion over a 30-min period, calculated as the area under the flow curve, for A2+/+ (mean ± S.E.; n = 11 for saline, n = 13 for GÖ6976) and A2−/− mice (mean ± S.E.; n = 10). *, p < 0.01 GÖ6976 versus saline of A2+/+ mice.
To further evaluate the enhanced recovery from vascular thrombosis induced by inhibition of PKC, we examined several additional hemostatic parameters (Table 1). First, GÖ6976 had no significant effect on erythrocyte, leukocyte, monocyte, or platelet counts in either A2+/+ or A2−/− mice. Second, the degree of inhibition of platelet secretion and aggregation by GÖ6976 was similar between A2+/+ and A2−/− mice. Also, expression levels of PKC isoforms in washed platelets isolated from in A2+/+ and A2−/− mice remained unchanged (data not shown). Third, plasma D-dimer levels and α2-plasmin·antiplasmin complex levels were not different between the two groups, treated with or without GÖ6976. Fourth, we found no differences in plasma clotting times and t-PA-dependent plasma clot lysis times between saline- and GÖ6976-treated A2+/+ and A2−/− mice. Together, these data demonstrate that GÖ6976 enhances clearance of thrombi in an A2-dependent manner that is independent of plasma-based coagulation and fibrinolysis and unrelated to platelet function.
TABLE 1.
Hematologic parameters
AnxA2+/+ or AnxA2−/− mice were injected by tail vein with either saline or GÖ6976 (1 mg/kg). 20–30 min later, the mice underwent FeCl3-induced carotid artery thrombosis, after which blood was withdrawn. Hematologic parameters were assayed and differences among groups were compared using unpaired Student's t test.
A Either AnxA2+/+ or AnxA2−/− mice treated with saline or GÖ6976. NS, not significant.
B % inhibition by GÖ6976. Values represent mean ± S.E.
Plasmin “Kringles” and Protease Activity Are Required for Activation of PKC
To determine whether the lysine-binding kringle structures of plasmin are required for PKC activation in endothelial cells, we employed two lysine analogs, tranexamic acid and ϵ-aminocaproic acid, in PKC activation experiments. Both agents completely inhibited plasmin-induced PKC activation but not activation of MAPK (supplemental Fig. S4) (data not shown). These data suggest that lysine-binding sites are essential for plasmin-mediated PKC activation.
To examine the role of plasmin protease activity in PKC activation, we treated HUVECs with either untreated or d-Val-Phe-Lys chloromethyl ketone-inactivated plasmin (Fig. 5A). Active site-blocked plasmin failed to induce significant PKC phosphorylation in HUVECs. In addition, activation of both ERK1/2 and p38 was also inhibited. These data indicate that catalytic activity of plasmin is a prerequisite for both MAP kinase and PKC activation.
To ascertain whether plasmin, once activated on the endothelial cell surface, modifies A2, as previously suggested for monocytes (33), we carried out immunoblot analyses using polyclonal rabbit anti-A2 IgG (Fig. 5B). Starting at 7.5 min and continuing through 30 min of plasmin treatment, a decline in total surface A2 was associated with the appearance of a 33-kDa fragment that cross-reacted with anti-A2 IgG. A likely explanation for this modification event would be plasmin cleavage of endothelial A2 at lysine 27, as previously reported (33). This event would remove the N-terminal “tail” domain of A2, thus interrupting further plasmin formation. These data indicate that plasmin induction of PKC is associated with A2 cleavage and the progressive loss of A2 and p11 from the cell surface.
Plasmin-mediated A2 Serine Phosphorylation Requires Cell Surface A2 and TLR4
To determine whether plasmin might signal through one or more PAR receptors, which have been reported to be activated by plasmin (34, 35), we examined the effect of two PAR1 signal-blocking antibodies, ATAP2 and WEDE15. Preincubation of HUVECs with these antibodies had no effect on plasmin-induced phosphorylation of PKC or A2 (Fig. 6A). Moreover, when we decreased HUVEC levels of PAR1 mRNA by 92–95% using siRNA, PAR3 mRNA levels remained unchanged (supplemental Figs. S5 and S6B). Although knockdown of endothelial PAR1 completely abolished thrombin-triggered phosphorylation of Src and ERK1/2 (supplemental Fig. S5), it had no effect upon plasmin-induced activation of either PKC or MAPK (Fig. 6B). This result suggested that PAR1 is not the primary receptor for initiating the plasmin-induced serine phosphorylation pathway, although thrombin is an effective PKC activator in the endothelial cell.
We next determined whether PKC activation and serine phosphorylation of A2 require the direct interaction of plasmin with cell membrane-associated (A2·p11)2 (33, 36). In primary CMECs derived from A2−/− mice, phosphorylation of PKC but not ERK1/2 was significantly reduced. This result strongly suggested that ERK1/2 activation is A2-independent (Fig. 6C). The failure of PKC activation in A2−/− endothelial cells was not due to an intrinsic defect in PKC activation itself, because PMA, a direct PKC activator, triggered activation of PKC and ERK1/2 equally in A2−/− and A2+/+ cells (supplemental Fig. S6). Moreover, we found no direct association of cPKC with A2 in pull-down assays in which either the cPKCα or -γ isoform or A2 was immunoprecipitated and then immunoblotted with either anti-A2 or -cPKC isoform- specific IgGs (supplemental Fig. S7). Therefore, our data indicate that PKC activation does not require its direct interaction with A2.
Our data strongly suggest that A2 is the receptor that mediates plasmin-induced PKC activation and its downstream effects in the endothelial cell. However, A2 lacks a transmembrane signaling motif, which implicates the participation of an adaptor molecule. It has been shown that plasmin triggers the release of a number of proinflammatory cytokines and chemokines in human monocytes (23, 37, 38), possibly through the action of Toll-like receptor 4 (TLR4). The signaling elements downstream of TLR4 have been studied primarily in monocyte/macrophage lineage cells. Endothelial cells, furthermore, do not express CD14, which is necessary for LPS signaling via TLR4 in monocytoid cells (39, 40). Consistent with these data, HUVECs and CMECs responded only very weakly to a high dose but not to a low dose of LPS in comparison with either primary or transformed macrophages (data not shown). Nevertheless, transfection of endothelial cells with TLR4 siRNA specifically down-regulated TLR4 protein by 82% and prevented phosphorylation of PKC and serine phosphorylation of A2 but not MAPK by plasmin (Fig. 6D). These data suggest that plasmin utilizes TLR4 to deliver its phosphorylation signal.
Finally, we examined the potential physical interaction between TLR4 and A2 in resting endothelial cells. Immunoblot analysis of anti-TLR4 immunoprecipitates confirmed the presence of A2 and showed that the TLR4-A2 interaction was enhanced upon plasmin treatment (Fig. 6E). Moreover, transfection of HEK293 cells with truncated versions of Myc-tagged A2 and HA-tagged TLR4 with subsequent immunoblot-immunoprecipitation revealed that the tail domain of A2 was not required for this association (Fig. 6F). One might postulate that the increased association between A2 and TLR4 induced by plasmin may reflect a conformational change in TLR4 that obviates the need for CD14, which appears to be absent in the endothelial cell.
DISCUSSION
The normal equilibrium between procoagulant and profibrinolytic activities is precisely regulated, and disruption of hemostatic balance can lead to either thrombosis or hemorrhage. Acute myocardial infarction and thrombotic stroke, for example, are commonly treated with infusion of a thrombolytic agent, but these therapies are often complicated by life-threatening bleeding, which limits their utility. A better understanding of the regulation of the fibrinolytic system may lead to the identification of new therapeutic targets that might enhance endogenous fibrinolytic activity without inducing hemorrhagic sequelae. Recently, in a rat model of embolic stroke, infusion of recombinant A2 significantly reduced cerebral infarct size and increased cerebral blood flow without affecting systemic hemostatic parameters (11). A second recent report indicates that A2 infused in combination with t-PA in a rat model of embolic stroke reduces the effective dose of t-PA, thus preventing hemorrhage (12). In the present work, we delineate, for the first time, a previously unknown regulatory mechanism, whereby cell surface plasmin curtails its own generation through cPKC-dependent serine phosphorylation of endothelial A2 and prevention of A2 translocation to the cell surface. This plasmin-initiated signaling mechanism is clearly distinct from activation of the MAPK pathway, which, while still requiring the active site of plasmin, is cPKC-independent, A2-independent, and plasmin kringle-independent (Figs. 2B, 3A, 5A, and 6C).
Endothelial cells support plasminogen activation by expressing (A2·p11)2, a co-receptor for t-PA and plasminogen, which promotes plasmin generation on the cell surface (5, 7). We and others have shown previously that thrombin, the primary procoagulant protease, paradoxically limits fibrin deposition by triggering PAR1-mediated tyrosine 23 phosphorylation of A2, cell surface translocation of (A2·p11)2, and accelerated t-PA-dependent plasmin generation (16, 17). In the present study, we found that plasmin, the primary profibrinolytic protease, paradoxically limits its own activation by cleaving cell surface A2 and eliciting cPKC activation and serine phosphorylation of intracellular A2. We show that serine phosphorylation of A2 at serine 11 and 25 leads to dissociation of p11 from A2, and subsequent ubiquitination and degradation of p11 by the proteasome. This mechanism prevents further translocation of (A2·p11)2 from the cytoplasm to the cell surface, thereby limiting plasmin generation. In support of the hypothesis that this mechanism applies in vivo, blockade of PKC activation in A2+/+ but not A2−/− mice significantly increased carotid artery blood flow following FeCl3-induced thrombosis without affecting systemic hemostatic parameters. This A2-dependent feedback system may be pivotal in preventing tissue damage or hemorrhage due to excessive production of plasmin.
Plasmin signaling has been recognized for some time in the context of proinflammatory activation of monocytes (41). Plasmin serves as a significant chemoattractant, inducing release of the powerful monocyte chemotactic and chemokinetic leukotriene B4 (42–44). Plasmin may also initiate expression of tissue factor and release of cytokines via the NF-κB pathway (23) and enhance expression of MCP-1 and CD40 via the p38 MAPK and JAK/STAT pathways (37). Plasmin-induced monocyte chemotaxis and release of TNFα appears to be annexin A2-dependent, involving cleavage of A2 at lysine 27 (33). These in vitro studies support the notion that plasmin regulates the monocyte's state of activation through a variety of signaling pathways.
Additional evidence suggests that annexin A2 may participate in plasma membrane-based signaling mechanisms. Activation of endothelial cells by anti-β2GPI antibodies in the antiphospholipid syndrome seems to require cross-linking of β2GPI to annexin A2 (45). The procoagulant response appears to require the participation of myeloid differentiation protein 88 (MyD88), which initiates activation of NF-κB (46). Furthermore, activation of macrophages via the A2 tetramer may be mediated by TLR4 (47), and signaling via TLR2 and TLR4, which play a major role in the innate immune response, seems to be potentiated by plasmin (47). Because TLRs employ MyD88 as a transmembrane adapter molecule (48), it is reasonable to hypothesize that plasmin might communicate to the cell's interior via annexin A2, TLR, and ultimately MyD88.
It is interesting to note that thrombin and plasmin, two serine proteases with opposing actions, function to promote and inhibit, respectively, endothelial cell surface plasmin formation by modulating cell surface expression of (A2·p11)2. This is achieved by activating distinct tyrosine or serine phosphorylation “switches.” As we have shown here, plasmin induces PKC activation and serine phosphorylation of intracellular A2, resulting in dissociation of the A2 tetramer in less than 10 min. In the resting endothelial cell, biosynthetically labeled A2 is translocated to the cell surface within 16 h (49). However, upon thrombin stimulation, this process is greatly accelerated, and new A2 tetramer translocates to the cell surface within 5–10 min.4 The rate at which A2 reappears on the cell surface depends mainly upon 1) the ambient level of the p11 (18), 2) the degree of Src kinase activation (16), and 3) the degree to which phospho-A2 may be dephosphorylated (no data are available). Therefore, under conditions where thrombin (and hence intravascular fibrin) is generated, A2 would be replenished at the cell surface within minutes. In the resting state, on the other hand, turnover would probably be much slower, on the order of hours.
These two systems mirror insulin receptor signaling, where tyrosine phosphorylation of the receptor transduces downstream signaling, whereas serine phosphorylation of the insulin receptor substrate attenuates insulin signaling (50). In NF-κB pathway activation, similarly, serine phosphorylation of IκBα at residues 32 and 36 leads to its dissociation from NF-κB and subsequent ubiquitination and proteasomal degradation, leaving NF-κB free to translocate into the nucleus, where it binds to the promoter region of target genes (51). In a similar manner, we conclude that endothelial p11 is stabilized by its interaction with A2 (18) until plasmin-directed phosphorylation of serines 11 and 25 of A2 triggers its dissociation and subsequent proteasomal degradation.
We show further that endothelial cell TLR4 but not PAR1 mediates activation of PKC by plasmin. TLR4, the receptor for LPS signaling, appears to serve as a platform for the recruitment of downstream signaling molecules. Consistent with other studies, we were unable to demonstrate strong, direct LPS signaling in endothelial cells, probably due to the absence of CD14, the co-receptor for LPS. Nevertheless, TLR4 is readily detected in resting endothelial cells and also interacts with A2. Plasmin appears to enhance this association, possibly by inducing a conformational change in TLR4 that facilitates the recruitment of downstream molecules that activate cPKC. We show here that calcium, which stimulates binding of A2 to membrane phospholipids, also triggers cPKC activation. The A2-dependent nature of the plasmin-induced activation of PKC was further confirmed by the absence of PKC activation in primary A2−/− endothelial cells. Our study suggests that plasmin utilizes the cellular context to precisely adjust its specific function within a given cell type. In endothelial cells, the main action of plasmin is to regulate fibrinolytic activity without inducing tissue damage, whereas, in monocytes and macrophages, its function might be to promote inflammation.
The intrinsic feedback regulation of endothelial cell surface plasmin generation is an elegant example of the kinetic and spatial coordination of signaling through phosphorylation- and ubiquitin-controlled protein-protein interactions, stabilization, and localization (Fig. 7). This mechanism assures that vascular fibrinolysis will be managed in a timely, location-specific manner. More importantly, our data also suggest a rationale for the development of therapeutic agents that target the plasmin-(A2·p11)2 pathway in the setting of thrombosis.
FIGURE 7.
Working model for feedback regulation of endothelial cell surface plasmin generation. Endothelial cell surface (A2·p11)2 tetramer promotes plasmin generation by local assembly of t-PA and plasminogen. Plasmin cleaves surface A2 within its N terminus at lysine 27, separating it from p11 and preventing further plasmin generation. A2 remains bound to membrane phospholipids through its C-terminal core domain. Association of modified A2 with TLR4 activates phosphatidylinositol-specific phospholipase C (PLC), leading to hydrolysis of phospholipids and generation of second messengers, inositol 1,4,5-trisphosphate (IP3) and diacylglycerol (DAG). Inositol 1,4,5-trisphosphate triggers mobilization of intracellular Ca2+, whereas diacylglycerol serves as a cofactor for activation of cPKC. Activation of cPKC leads to dual serine phosphorylation of A2 at residues 11 and 25. Free p11 subsequently dissociates from serine phosphorylated A2, becomes ubiquitinated, and is degraded by the proteasome. In addition, tyrosine phosphorylation of A2 at residue 23 is inhibited, preventing translocation of A2 to the cell surface and inhibiting further plasmin generation. PIP2, phosphatidylinositol 4,5-bisphosphate.
Supplementary Material
Acknowledgments
We thank Ralph L. Nachman for critical reading of the manuscript; Emil Lev, Andrew T. Jacovina, Caroline B. Greenberg, and Dena Almeida for expert technical assistance; and Geri Kreitzer for helpful discussions.
Note Added in Proof
Subsequent to the completion of our paper, O'Connell et al. reported a central role for protein S100A10 (p11) in macrophage recruitment in response to inflammatory stimuli (O'Connell, P. A., Surette, A. P., Liwski, R. S., Svenningsson, P., and Waisman, D. M. (2010) Blood 116, 1136–1146).
This work was supported, in whole or in part, by National Institutes of Health, NHLBI, Grants HL042493 and HL090895 (to K. A. H.), HL046403 (to K. A. H., A. J. M., and M. J. B.), and HL047073 and HL089521 (to A. J. M. and M. J. B.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S7.
A. B. Deora and K. A. Hajjar, unpublished observations.
- A2
- annexin A2
- CMEC
- cardiac microvascular endothelial cell
- HUVEC
- human umbilical vein endothelial cell
- PAR
- protease-activated receptor
- TLR
- Toll-like receptor
- t-PA
- tissue plasminogen activator
- cPKC
- conventional protein kinase C.
REFERENCES
- 1. Hajjar K. A., Ruan J. (2010) in Williams Hematology, 8th Ed. (Kaushansky K., Lichtman M. A., Beutler E., Kipps T. J., Prchal J., Seligsohn U. eds) pp. 2219–2244, McGraw-Hill, New York [Google Scholar]
- 2. Gerke V., Moss S. E. (2002) Physiol. Rev. 82, 331–371 [DOI] [PubMed] [Google Scholar]
- 3. Gerke V., Creutz C. E., Moss S. E. (2005) Nat. Rev. Mol. Cell Biol. 6, 449–461 [DOI] [PubMed] [Google Scholar]
- 4. Thiel C., Osborn M., Gerke V. (1992) J. Cell Sci. 103, 733–742 [DOI] [PubMed] [Google Scholar]
- 5. Hajjar K. A., Jacovina A. T., Chacko J. (1994) J. Biol. Chem. 269, 21191–21197 [PubMed] [Google Scholar]
- 6. Cesarman G. M., Guevara C. A., Hajjar K. A. (1994) J. Biol. Chem. 269, 21198–21203 [PubMed] [Google Scholar]
- 7. Kassam G., Choi K. S., Ghuman J., Kang H. M., Fitzpatrick S. L., Zackson T., Zackson S., Toba M., Shinomiya A., Waisman D. M. (1998) J. Biol. Chem. 273, 4790–4799 [DOI] [PubMed] [Google Scholar]
- 8. Dassah M., Deora A. B., He K., Hajjar K. A. (2009) Gen. Physiol. Biophys. 28, F20–F28 [PMC free article] [PubMed] [Google Scholar]
- 9. Ling Q., Jacovina A. T., Deora A., Febbraio M., Simantov R., Silverstein R. L., Hempstead B., Mark W. H., Hajjar K. A. (2004) J. Clin. Invest. 113, 38–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ishii H., Yoshida M., Hiraoka M., Hajjar K. A., Tanaka A., Yasukochi Y., Numano F. (2001) Circ. Res. 89, 1240–1245 [DOI] [PubMed] [Google Scholar]
- 11. Tanaka Y., Ishii H., Hiraoka M., Miyasaka N., Kuroiwa T., Hajjar K. A., Nagaoka T., Duong T. Q., Ohno K., Yoshida M. (2007) Brain Res. 1165, 135–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhu H., Fan X., Yu Z., Liu J., Murata Y., Lu J., Zhao S., Hajjar K. A., Lo E. H., Wang X. (2010) J. Cereb. Blood Flow. Metab. 30, 1137–1146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Menell J. S., Cesarman G. M., Jacovina A. T., McLaughlin M. A., Lev E. A., Hajjar K. A. (1999) N. Engl. J. Med. 340, 994–1004 [DOI] [PubMed] [Google Scholar]
- 14. Cesarman-Maus G., Ríos-Luna N. P., Deora A. B., Huang B., Villa R., Cravioto Mdel C., Alarcón-Segovia D., Sánchez-Guerrero J., Hajjar K. A. (2006) Blood 107, 4375–4382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jacovina A. T., Deora A. B., Ling Q., Broekman M. J., Almeida D., Greenberg C. B., Marcus A. J., Smith J. D., Hajjar K. A. (2009) J. Clin. Invest. 119, 3384–3394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Deora A. B., Kreitzer G., Jacovina A. T., Hajjar K. A. (2004) J. Biol. Chem. 279, 43411–43418 [DOI] [PubMed] [Google Scholar]
- 17. Peterson E. A., Sutherland M. R., Nesheim M. E., Pryzdial E. L. (2003) J. Cell Sci. 116, 2399–2408 [DOI] [PubMed] [Google Scholar]
- 18. He K. L., Deora A. B., Xiong H., Ling Q., Weksler B. B., Niesvizky R., Hajjar K. A. (2008) J. Biol. Chem. 283, 19192–19200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hall S. W., Humphries J. E., Gonias S. L. (1991) J. Biol. Chem. 266, 12329–12336 [PubMed] [Google Scholar]
- 20. Jost M., Gerke V. (1996) Biochim. Biophys. Acta 1313, 283–289 [DOI] [PubMed] [Google Scholar]
- 21. Hajjar K. A., Hamel N. M. (1990) J. Biol. Chem. 265, 2908–2916 [PubMed] [Google Scholar]
- 22. Wigler M., Pellicer A., Silverstein S., Axel R. (1978) Cell 14, 725–731 [DOI] [PubMed] [Google Scholar]
- 23. Syrovets T., Jendrach M., Rohwedder A., Schüle A., Simmet T. (2001) Blood 97, 3941–3950 [DOI] [PubMed] [Google Scholar]
- 24. Kopp H. G., Hooper A. T., Broekman M. J., Avecilla S. T., Petit I., Luo M., Milde T., Ramos C. A., Zhang F., Kopp T., Bornstein P., Jin D. K., Marcus A. J., Rafii S. (2006) J. Clin. Invest. 116, 3277–3291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nagashima M., Yin Z. F., Zhao L., White K., Zhu Y., Lasky N., Halks-Miller M., Broze G. J., Jr., Fay W. P., Morser J. (2002) J. Clin. Invest. 109, 101–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hajjar K. A., Harpel P. C., Jaffe E. A., Nachman R. L. (1986) J. Biol. Chem. 261, 11656–11662 [PubMed] [Google Scholar]
- 27. Gould K. L., Woodgett J. R., Isacke C. M., Hunter T. (1986) Mol. Cell. Biol. 6, 2738–2744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dubois T., Oudinet J. P., Russo-Marie F., Rothhut B. (1995) Biochem. J. 310, 243–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Weinman E. J., Steplock D., Zhang Y., Biswas R., Bloch R. J., Shenolikar S. (2010) J. Biol. Chem. 285, 25134–25138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Johnsson N., Nguyen Van P., Söling H. D., Weber K. (1986) EMBO J. 5, 3455–3460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Regnouf F., Sagot I., Delouche B., Devilliers G., Cartaud J., Henry J. P., Pradel L. A. (1995) J. Biol. Chem. 270, 27143–27150 [DOI] [PubMed] [Google Scholar]
- 32. Farrehi P. M., Ozaki C. K., Carmeliet P., Fay W. P. (1998) Circulation 97, 1002–1008 [DOI] [PubMed] [Google Scholar]
- 33. Laumonnier Y., Syrovets T., Burysek L., Simmet T. (2006) Blood 107, 3342–3349 [DOI] [PubMed] [Google Scholar]
- 34. Kuliopulos A., Covic L., Seeley S. K., Sheridan P. J., Helin J., Costello C. E. (1999) Biochemistry 38, 4572–4585 [DOI] [PubMed] [Google Scholar]
- 35. Loew D., Perrault C., Morales M., Moog S., Ravanat C., Schuhler S., Arcone R., Pietropaolo C., Cazenave J. P., van Dorsselaer A., Lanza F. (2000) Biochemistry 39, 10812–10822 [DOI] [PubMed] [Google Scholar]
- 36. MacLeod T. J., Kwon M., Filipenko N. R., Waisman D. M. (2003) J. Biol. Chem. 278, 25577–25584 [DOI] [PubMed] [Google Scholar]
- 37. Burysek L., Syrovets T., Simmet T. (2002) J. Biol. Chem. 277, 33509–33517 [DOI] [PubMed] [Google Scholar]
- 38. Li Q., Laumonnier Y., Syrovets T., Simmet T. (2007) Arterioscler. Thromb. Vasc. Biol. 27, 1383–1389 [DOI] [PubMed] [Google Scholar]
- 39. Pugin J., Schürer-Maly C. C., Leturcq D., Moriarty A., Ulevitch R. J., Tobias P. S. (1993) Proc. Natl. Acad. Sci. U.S.A. 90, 2744–2748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hailman E., Lichenstein H. S., Wurfel M. M., Miller D. S., Johnson D. A., Kelley M., Busse L. A., Zukowski M. M., Wright S. D. (1994) J. Exp. Med. 179, 269–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Syrovets T., Simmet T. (2004) Cell Mol. Life Sci. 61, 873–885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Weide I., Römisch J., Simmet T. (1994) Blood 83, 1941–1951 [PubMed] [Google Scholar]
- 43. Weide I., Tippler B., Syrovets T., Simmet T. (1996) Thromb. Haemost. 76, 561–568 [PubMed] [Google Scholar]
- 44. Syrovets T., Tippler B., Rieks M., Simmet T. (1997) Blood 89, 4574–4583 [PubMed] [Google Scholar]
- 45. Ma K., Simantov R., Zhang J. C., Silverstein R., Hajjar K. A., McCrae K. R. (2000) J. Biol. Chem. 275, 15541–15548 [DOI] [PubMed] [Google Scholar]
- 46. Raschi E., Testoni C., Bosisio D., Borghi M. O., Koike T., Mantovani A., Meroni P. L. (2003) Blood 101, 3495–3500 [DOI] [PubMed] [Google Scholar]
- 47. Swisher J. F., Burton N., Bacot S. M., Vogel S. N., Feldman G. M. (2010) Blood 115, 549–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Medzhitov R. (2001) Nat. Rev. Immunol. 1, 135–145 [DOI] [PubMed] [Google Scholar]
- 49. Hajjar K. A., Guevara C. A., Lev E., Dowling K., Chacko J. (1996) J. Biol. Chem. 271, 21652–21659 [DOI] [PubMed] [Google Scholar]
- 50. Sun X. J., Liu F. (2009) Vitam. Horm. 80, 351–387 [DOI] [PubMed] [Google Scholar]
- 51. Karin M., Ben-Neriah Y. (2000) Annu. Rev. Immunol. 18, 621–663 [DOI] [PubMed] [Google Scholar]
- 52. Kwon M., MacLeod T. J., Zhang Y., Waisman D. M. (2005) Front. Biosci. 10, 300–325 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.