Abstract
Parkinson disease is caused by the death of midbrain dopamine neurons from oxidative stress, abnormal protein aggregation, and genetic predisposition. In 2003, Bonifati et al. (23) found that a single amino acid mutation in the DJ-1 protein was associated with early-onset, autosomal recessive Parkinson disease (PARK7). The mutation L166P prevents dimerization that is essential for the antioxidant and gene regulatory activity of the DJ-1 protein. Because low levels of DJ-1 cause Parkinson, we reasoned that overexpression might stop the disease. We found that overexpression of DJ-1 improved tolerance to oxidative stress by selectively up-regulating the rate-limiting step in glutathione synthesis. When we imposed a different metabolic insult, A53T mutant α-synuclein, we found that DJ-1 turned on production of the chaperone protein Hsp-70 without affecting glutathione synthesis. After screening a number of small molecules, we have found that the histone deacetylase inhibitor phenylbutyrate increases DJ-1 expression by 300% in the N27 dopamine cell line and rescues cells from oxidative stress and mutant α-synuclein toxicity. In mice, phenylbutyrate treatment leads to a 260% increase in brain DJ-1 levels and protects dopamine neurons against 1-methyl 4-phenyl 1,2,3,6-tetrahydropyridine (MPTP) toxicity. In a transgenic mouse model of diffuse Lewy body disease, long-term administration of phenylbutyrate reduces α-synuclein aggregation in brain and prevents age-related deterioration in motor and cognitive function. We conclude that drugs that up-regulate DJ-1 gene expression may slow the progression of Parkinson disease by moderating oxidative stress and protein aggregation.
Keywords: Neurodegeneration, Oxidative Stress, Parkinson Disease, Transcription Regulation, Transgenic, Dopamine Neuron, Gene Up-regulation, Neuroprotection, Phenylbutyrate, Protein Aggregation
Introduction
Dopamine cell death in Parkinson disease (PD)3 results from both genetic and environmental factors (1–5). Six genes have been linked to PD including α-synuclein, Parkin, UCHL1, DJ-1, PINK1, and LRRK2 (6–7). α-Synuclein mutations (A53T, A30P, and E46K) cause autosomal dominant forms of PD (8–10). Even in sporadic cases of PD, aggregated α-synuclein has been found to be a major component of Lewy bodies (11–13). The toxicity of mutant forms of α-synuclein results from increased formation of oligomeric and fibrillar aggregates (14–17). We and others (18–22) have demonstrated that expression of A53T mutant α-synuclein results in protein aggregation and cell death in cultured dopamine neurons.
Mutations in the DJ-1 gene (PARK7) lead to early-onset, autosomal recessive Parkinson disease (23–26). Ordinarily, DJ-1 protects cells by a number of mechanisms. The protein can self-oxidize by forming cysteine-sulfinic acid under oxidizing conditions, thereby shifting its pI from 6.1 to 5.8 (27–28). DJ-1 can sequester the cell death protein Daxx and prevent Daxx-induced apoptosis after oxidative stress (29). DJ-1 can stabilize Nrf2 (nuclear factor erythroid 2-related factor) by preventing association with its inhibitor protein, Keap1, thereby blocking the subsequent ubiquitination of Nrf2 (30).
Previously, we have reported that overexpression of WT DJ-1 can protect dopamine neurons from oxidative stress by increasing cellular glutathione (GSH) levels through selective up-regulation of the rate-limiting step in GSH synthesis, glutamate cysteine ligase (GCL) (31). We also discovered that overexpression of WT DJ-1 inhibits A53T human α-synuclein protein aggregation and reduces neural toxicity in N27 cells by up-regulating heat shock protein 70 (Hsp70) without changing glutathione synthesis. Therefore, DJ-1 acts through independent, distinct mechanisms to protect cells from different metabolic challenges.
Because DJ-1 is important for both the oxidative stress response and the elimination of abnormal protein aggregates, we hypothesized that overexpression of DJ-1 in the brain may provide broad protection from metabolic insults. Recently, histone deacetylase inhibitors (HDACi), such as sodium phenylbutyrate (PB) and sodium butyrate (SB), have shown neuroprotective function in several neurodegenerative disease animal models (32–35). We now report that phenylbutyrate and sodium butyrate can increase DJ-1 expression and prevent cell death following oxidative stress. In the MPTP mouse model, we have found that elevating DJ-1 expression by PB administration reduced the toxicity of MPTP to dopamine neurons. Using our newly established Y39C α-synuclein transgenic mouse model of age-related diffuse Lewy body disease (36), we have demonstrated that PB can prevent mutant α-synuclein-induced protein aggregation and improve motor and cognitive function.
EXPERIMENTAL PROCEDURES
Culture of N27 Cells, HDAC Inhibitor Treatment, and Oxidative Stress Treatment
Dopaminergic cells derived from embryonic day 12 rat mesencephalon and immortalized with the SV40 large T antigen designated 1RB3AN27, (N27 cells) were used (37). N27 cells were cultured in 6-well or 24-well plates in RPMI 1640 medium containing 10% fetal bovine serum. Cells were treated with sodium phenylbutyrate (PB, Scandinavian Formulas) and sodium butyrate (SB, Sigma) at different concentrations for 48 h, followed by exposure to varying doses of toxin for 24 h.
Culture of N27 Cells, A53T α-Synuclein Adenovirus Transduction, shDJ-1 Knockdown of Endogenous DJ-1, and Oxidative Stress Treatment
N27 cells were cultured in 6-well or 24-well plates in RPMI 1640 medium containing 10% fetal bovine serum and treated with HDAC inhibitor for 48 h. To transduce cells, A53T α-synuclein adenovirus was mixed with culture medium and incubated with cells for 24 h at a concentration of 200 plaque forming units (pfu)/cell. To knock down endogenous DJ-1, N27 cells were incubated with adenovirus expressing single hairpin rat DJ-1 (Ad-shDJ-1) at 200 pfu/cell as described previously (31). Two days after adenovirus transduction, cells were exposed to drugs at varying doses for 24 h: H2O2 (0–100 μm) and 6-OHDA (0–100 μm).
Immunocytochemistry
Cultured cells were fixed with 4% paraformaldehyde and processed for immunocytochemistry as described (31, 38). The antibodies included mouse anti-α-synuclein (1:300, Transduction Laboratories); mouse anti- human DJ-1 (1:500, Stressagen); rabbit anti-DJ-1 (1:500, Chemicon); and rabbit anti-TH (tyrosine hydroxylase, 1:200, PelFreez).
MTT Assay and Apoptosis Evaluation
At the end of each experiment, methylthiazoletetrazolium (MTT) was added to the culture medium (final concentration 0.4 mg/ml) and incubated for 2 h. Cell viability was measured by a microplate reader as described (31, 38). The nuclear dye Hoechst 33258 (10 μg/ml) was used to visualize and count apoptotic cells.
Western Blotting
N27 cells were cultured in 6-well plates and treated with compounds as described above. Cells were lysed in a dissociation buffer containing 50 mm Tris-HCl, 10 mm NaCl, 0.1% Triton X-100 plus protease inhibitor mixture (Roche). The mouse brain tissues were dissected and quickly frozen in dry ice. Tissues were thawed on ice and homogenized in dissociation buffer with protease inhibitors (Roche). Protein concentration was determined by the BCA method (Pierce). 50 μg of protein was separated on 10% SDS-PAGE gel and transferred to a nitrocellulose membrane. After blocking nonspecific binding, membranes were incubated with antibodies to DJ-1 (1:5000, Chemicon), α-synuclein (1:3000, Transduction Laboratories), TH (1;2000, PelFreez), and β-actin (1:4000, Sigma). Blots were incubated with HRP-conjugated secondary antibody (1:10,000; Jackson Immuno Research), followed by chemiluminescent detection (Perkin Elmer Life Sciences) (31, 38).
Luciferase Assay
The luciferase assay was based on the pGLuc-Basic vector (NEB). The human DJ-1 2 kb promoter area was amplified by PCR using genomic DNA from HEK293 cells. The human DJ-1 promoter was then cloned into the pGLuc-Basic vector, and that reporter vector was transfected into HEK293 cells. G418 (200 μg/ml) was applied to the cultures, and resistant clones were selected and assayed for luciferase activity. Stable cell lines expressing pDJ1-Luciferase were treated with PB and SB for 48 h at various concentrations in 24-well plates. Culture medium samples (20 μl) were incubated with Gaussia luciferase assay substrate (NEB), and luminescence was measured in a 96-well plate reader (BioTek Synergy HT Multi-Mode microplate reader).
Phenylbutyrate and Butyrate Treatment in Mice
All animal procedures were approved by Institutional Animal Care and Use Committee (IACUC) at the University of Colorado Denver. Adult C57BL/6 mice (4–6 months old) were treated with PB and SB in drinking water for 14 days. The PB and SB were dissolved in water at concentrations of 500, 1000, 1500, and 2000 mg/liter. Mice typically drink 4–5 ml of water per day, and their drinking volumes were not affected by the addition of PB or SB. Control animals received water with sodium chloride added to the same molarity as the sodium in the drug-treated animals. Brain tissues were dissected and immediately frozen in dry ice for Western blot analysis using DJ-1, α-synuclein, and β-actin antibodies.
MPTP Injection into Mice
Adult C57BL/6 mice (4–6 months old) were treated with PB in drinking water (1000 mg/liter, 5.4 mm) for 2 weeks, followed by injection of 1-methyl 4-phenyl 1,2,3,6-tetrahydropyridine (MPTP-HCl, dissolved in sterile saline, Sigma-Aldrich) four times at 2-h intervals (20 mg/kg, intraperitoneal). Treatment with PB continued after MPTP injection. Control animals received identical injections of MPTP, but had only sodium chloride added to drinking water as described above. One week after MPTP lesioning, mouse brain tissues were dissected for Western blot and HPLC analysis.
HPLC Analysis of Dopamine and DOPAC
Mouse striatum was frozen in dry ice, sonicated in ice-cold 0.2 m perchloric acid, and centrifuged at 15,000 × g for 15 min at 4 °C. An aliquot (5 μl) of the supernatant solution was analyzed by HPLC equipped with an electrochemical detector (CoulArray system ESA Model 5600; ESA, Boston, MA), a pump (ESA Model 580) set at 1.5 ml/min, and a reverse-phase C18 column (3 μm, 100 × 4.6 mm, Waters, Milford, MA). The mobile phase was composed of 100 mm citric acid, 2% methanol, 1 mm EDTA, and 5 mg/liter sodium octyl sulfate (pH 3.0).
Transgenic Mice Expressing Human Y39C α-Synuclein under Mouse Thy-1 Promoter
Our Y39C human α-synuclein transgenic mouse model has been described (36). Briefly, human Y39C α-synuclein cDNA was cloned into the mouse pThy-1 vector at the NotI site. The construct was micro-injected into mouse oocytes, and founder mice were identified by PCR and Southern blotting analysis. Mice were bred to establish stable transgenic lines. Expression of human Y39C α-synuclein in these transgenic mice was determined by immunostaining and Western blotting with antibodies specific to human α-synuclein (LB509).
Drug Treatment in α-Synuclein Transgenic Mice
The Y39C α-synuclein transgenic mice were divided into younger (6–8 months = Young Tg) and older (10–12 months = Old Tg) groups. Each group received PB (1000 mg/liter, 5.4 mm) or vehicle (NaCl) in the drinking water for 3 months. Animals were tested for rotarod and water maze performance after 6 weeks and 12 weeks of treatment.
Rotarod Test
Mice were tested for their ability to run on a 3-cm diameter rotating rod (rotarod) at speeds ranging from 3 to 33 rpm. The protocol consisted of two phases: habituation (Day 1) and rotarod training/testing (Days 2–5). During habituation on Day 1, the mice were trained to remain on the rotarod at 3 rpm. During training/testing on Days 2–5, mice were placed on the rotating rod at a constant speed for three one-minute trials with a 5-min rest interval between trials. Each test day, the speed was increased, reaching 33 rpm by Day 5. The time the mice spent on the rotarod without falling was recorded for each trial.
Morris Water Maze Testing
Spatial learning was assessed using the Morris water maze. The maze included a circular tank (120 cm in diameter) filled to 10 cm below the edge of the tank with 27 °C water that was made opaque by the addition of non-toxic black ink. A circular escape platform (10 cm in diameter) was located 1 cm below the surface of the water in a constant location in the northwest quadrant of the tank. Mice were first acclimated to the maze during three trial habituation sessions. Each testing session consisted of three consecutive days with four trials per day. The platform was invisible in the pool, and mice were allowed to swim for 60 s before being returned to the home cage. The latency from all training and testing sessions was collected.
Immunohistochemistry and α-Synuclein Staining
The mice were sacrificed by deep anesthesia followed by intracardiac perfusion with saline and 4% paraformaldehyde. The mouse brains were cryosectioned at 40 μm. Immunohistochemistry was performed using antibodies to human α-synuclein (LB509, 1:500). Immunostaining was developed with diaminobenzidine (DAB). Tissue sections were examined for the Lewy body-like inclusions.
Statistics
All experiments were repeated at least three times. Data were analyzed using multivariate ANOVA and the Fisher LSD post hoc test. Significance was set at p < 0.05. Values are shown as mean ± S.E.
RESULTS
Sodium Phenylbutyrate (PB) and Sodium Butyrate (SB) Increase DJ-1 Expression in N27 Cells and HEK293 Cells
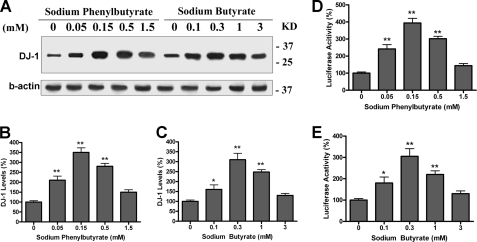
Using the N27 rat dopaminergic cell line, we screened a number of compounds for their ability to increase DJ-1 expression. From this screen, we found that sodium phenylbutyrate (PB) and sodium butyrate (SB) increased DJ-1 protein levels to 300% of control after 2 days of treatment as shown in Fig. 1A. Both compounds had peak effects at concentrations of 0.15–0.3 mm (**, p < 0.01 compared with control, Fig. 1, B and C).
FIGURE 1.
PB and SB increase DJ-1 expression in N27 and HEK293 cells. A–C, N27 cells were incubated with sodium phenylbutyrate or sodium butyrate at various concentrations for 48 h. The cell lysates were separated in 12% SDS-PAGE and probed with DJ-1 and β-actin antibody. Duplicate treatments in 6-well plates were used, and experiments were repeated three times. A typical Western blot image is shown in A. B and C, quantitative data from Western blot images are shown (*, p < 0.05; **, p < 0.01 compared with control, n = 6). (D and E) HEK293 reporter cells expressing human DJ-1 promoter-Luciferase were treated with PB or SB at indicated doses for 48 h, followed by luciferase assay. Duplicate treatments in 24-well plates were used, and experiments were repeated three times. The average luciferase activity is shown. (*, p < 0.05; **, p < 0.01 compared with control, n = 6.)
To test if PB and SB directly up-regulated DJ-1 gene transcription, we created an HEK293 reporter cell line which stably expressed the human DJ-1 promoter-luciferase construct. The HEK293 reporter cells were treated with PB and SB for 48 h, followed by luciferase assay. We found that both PB and SB increased luciferase activity as well as DJ-1 protein levels (*, p < 0.05; **, p < 0.01 compared with control, Fig. 1, D and E). These results indicate that enhanced DJ-1 gene transcription is responsible for the higher DJ-1 protein levels seen after PB and SB treatments.
Sodium Phenylbutyrate Protects N27 Cells from Oxidative Stress and α-Synuclein Induced Toxicity
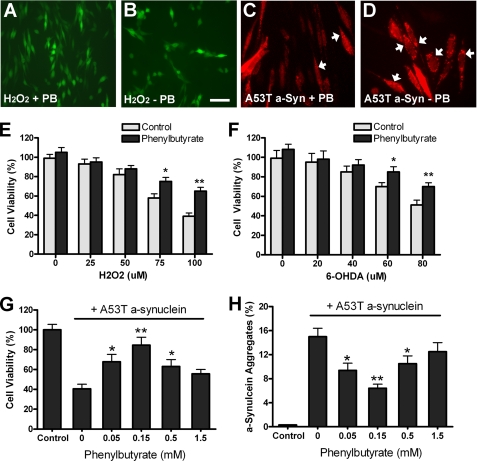
We have previously shown that overexpression of the DJ-1 gene through adenoviral transduction can make N27 cells more resistant to oxidative stress and mutant α-synuclein toxicity (31). To see if phenylbutyrate could replicate these protective effects, we treated N27 cells with 0.15 mm sodium phenylbutyrate for 2 days and then subjected cells to oxidative stress for 24 h. Fig. 2, A and B showed sample images of N27 cells with or without PB followed by H2O2 treatment, in which cells were identified by GFP adenovirus expression. Quantitative results showed that PB treatment significantly increased cell viability after exposure to hydrogen peroxide (H2O2) and 6-hydroxydopamine (6-OHDA) compared with controls (*, p < 0.05; **, p < 0.01; Fig. 2, E and F).
FIGURE 2.
Sodium phenylbutyrate protects N27 cells from oxidative stress and mutant α-synuclein toxicity. A–B and E–F, N27 cells were incubated with PB (0.15 mm) for 48 h, followed by 24 h treatment with H2O2 or 6-OHDA at various concentrations. Sample images were shown in A-B with cells identified by GFP adenovirus expression. Cell viability was determined by MTT assays and shown in E–F. C–D and G–H, N27 cells were incubated with various doses of PB for 48 h, followed by 48 h treatment of adenovirus expressing A53T human α-synuclein (200 pfu/cell). N27 cells with α-synuclein aggregates were identified by α-synuclein antibody LB509 staining, with sample images shown in C–D. Three random fields (150–200 cells per field) were examined to determine the percentage of cells with α-synuclein aggregates (arrows in G–H). Triplicate treatments in 24-well plates were used, and experiments were repeated three times. (*, p < 0.05; **, p < 0.01 compared with control, n = 9.)
We have earlier demonstrated that expression of A53T mutant human α-synuclein in N27 cells led to cell death with α-synuclein-positive cytoplasmic aggregates (31, 38). In the present experiments, we treated N27 cells with PB for 2 days. Cells were then exposed to adenovirus expressing A53T mutant α-synuclein for another 2 days. Fig. 2, C and D showed sample images of N27 cells with or without PB treatment followed by A53T α-synuclein expression, in which cells were identified by α-synuclein immunostaining. We found that PB treatment increased cell viability and reduced the number of cells with α-synuclein aggregates (*, p < 0.05; **, p < 0.01 compared with control, Fig. 2, G and H). Our data indicate that phenylbutyrate can protect dopamine cells from oxidative stress and mutant α-synuclein toxicity.
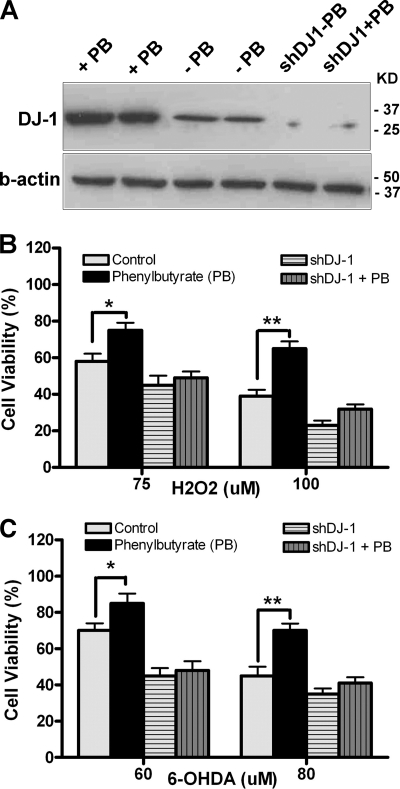
Knocking Down DJ-1 Blocks Phenylbutyrate Effects in N27 Cells
To test whether DJ-1 is needed for the protective actions of PB, we have used knock-down technology. With an adenovirus expressing shDJ-1 (31), we knocked down DJ-1 gene expression in N27 cells (Fig. 3A) and then tested whether PB could rescue cell death resulted from H2O2 (75 or 100 μm) and 6-OHDA (60 or 80 μm). Results showed that DJ-1 knockdown in N27 cells effectively abolished the neuroprotective effect of PB against H2O2 and 6-OHDA-induced toxicity (compare columns with horizontal and vertical bars to control in Fig. 3, B and C), while naïve N27 cells with PB treatment significantly improved cell viability (*, p < 0.05; **, p < 0.01 compared with control, Fig. 3, B and C). These in vitro experiments demonstrate that phenylbutyrate can turn on expression of the DJ-1 gene and protect N27 cells from oxidative stress and mutant α-synuclein toxicity. Importantly, activation of DJ-1 is required for the phenylbutyrate effect.
FIGURE 3.
Knockdown of endogenous DJ-1 abolishes neuroprotection from sodium phenylbutyrate. The N27 cells were transduced with adenovirus expressing shDJ-1 or Ad-GFP at concentration of 200 pfu/cell for 2 days. The cells were then added with or without PB treatment for 48 h, followed by exposure to hydrogen peroxide (H2O2) or 6-hydroxydopamine (6-OHDA) for 24 h. A, sample Western blot showed more than 85% reduction of endogenous DJ-1 treated by shDJ-1 adenovirus. B and C, cell viability was measured by MTT assay. Results showed that knockdown of DJ-1 abolished neuroprotective effects of PB in N27 cells (compare vertical and horizontal bars to white or black bars). Triplicate treatments in 24-well plates were used, and experiments were repeated three times. (*, p < 0.05; **, p < 0.01 compared with control, n = 9.)
Sodium Phenylbutyrate and Sodium Butyrate Increase DJ-1 Expression in Mice
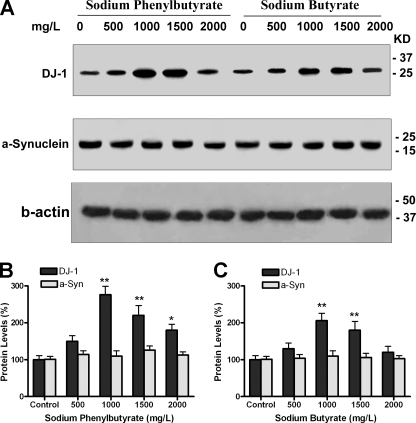
With the hypothesis that PB and SB may provide a way to turn on DJ-1 gene expression in vivo and thereby protect brain from neurotoxic stress, we treated mice with these drugs. Because phenylbutyrate is stable in solution and has a very short half-life (50 min) in vivo, we chose to deliver drugs in drinking water. Adult C57BL/6 mice were treated with PB or SB at various concentrations in drinking water for 2 weeks. Control mice received water with equimolar concentrations of sodium chloride. We found that all treatment and control groups consumed similar volumes of water, and the intake for all was in the range expected for normal daily fluid consumption (data not shown). Animals were sacrificed by an overdose of anesthetic, and brains removed and frozen on dry ice. Brain tissues were analyzed for DJ-1 protein levels by Western blotting. Results showed PB and SB significantly increased DJ-1 levels (*, p < 0.05; **, p < 0.01 compared with control, Fig. 4, A–C).
FIGURE 4.
Sodium phenylbutyrate and sodium butyrate increased DJ-1 expression in mouse brain. A–C, adult C57BL/6 mice (ages 4–6 months old) were treated with PB or SB at different doses in drinking water for 2 weeks. Brain tissues (cortex) were used for Western blotting with DJ-1, α-synuclein, and β-actin antibodies. Four mice were used for each dose, and an equal amount of protein was loaded in each lane after being normalized to β-actin. Typical Western blot images of DJ-1 and α-synuclein are shown in A. B–C, quantitative data of DJ-1 and α-synuclein levels after PB and SB treatment (*, p < 0.–05; **, p < 0.01 compared with control, n = 4.)
To test whether the increase in DJ-1 was caused by a global increase in gene and protein expression, endogenous α-synuclein protein levels were also measured as shown in Fig. 4, A–C. α-Synuclein concentrations were not changed after drug treatment, suggesting that PB and SB showed selectivity in producing an increase in DJ-1 expression in brain.
To study the time course of DJ-1 expression during chronic drug administration, we examined DJ-1 levels in mice treated with PB in the drinking water for 1, 2, and 3 months. We found that brain DJ-1 reached plateau values after 2 weeks of PB, and those levels were sustained for 1–3 months of treatment (data not shown). These data indicate that PB can provide sustained elevation of DJ-1 expression in mouse brain.
Sodium Phenylbutyrate Protects Dopamine Neurons from MPTP-induced Neurotoxicity
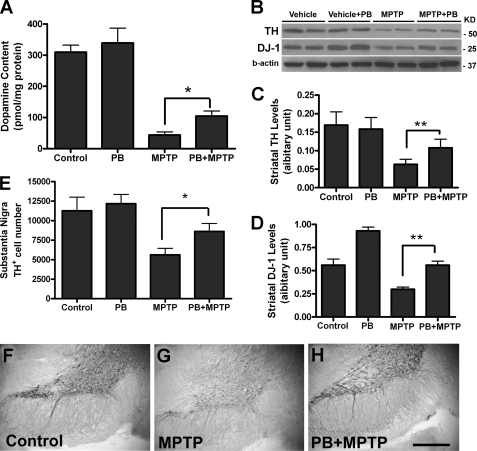
To evaluate whether pretreatment with PB can protect dopamine neurons in the MPTP mouse model of Parkinson disease, adult C57BL/6 mice were treated with PB in the drinking water (1000 mg/liter) for 2 weeks. They were then injected with MPTP 4 times at 2-h intervals (20 mg/kg, intraperitoneal, each injection). One week after MPTP injections, mice were sacrificed for biochemical and histological analysis. We found that PB pretreatment significantly increased striatal dopamine levels as measured by HPLC (*, p < 0.05 compared with MPTP alone, Fig. 5A). PB treatment also increased tyrosine hydroxylase (TH) protein levels in striatum (Western blot, **, p < 0.01 compared with MPTP alone, Fig. 5, B and C). Furthermore, DJ-1 levels in striatum were significantly increased in PB-treated mice (Western blot, **, p < 0.01 compared with MPTP alone, Fig. 5, B and D). We also found that PB-treated mice had significant higher number of TH-positive dopamine neurons in the substantia nigra (*, p < 0.05 compared with MPTP alone, Fig. 5E). Sample immunohistochemical images showing increased survival of TH-positive dopamine neurons in substantia nigra are presented in Fig. 5, F–H. These data indicate that phenylbutyrate can protect dopamine neurons from MPTP neurotoxicity.
FIGURE 5.
Sodium phenylbutyrate prevented mouse dopamine neuron death after MPTP lesion. A–H, adult C57BL/6 mice (4–6 months old) were treated with PB (1000 mg/liter, 5.4 mm) in drinking water for 2 weeks, followed by 4 injections of MPTP at 2-h intervals (20 mg/kg, intraperitoneal). The mice were sacrificed 7 days after MPTP injections. A, dopamine content in mouse striatum tissues were measured by HPLC (6 mice per group, *, p < 0.05 compared with MPTP alone). B–D, striatal TH and DJ-1 protein levels were measured by Western blotting, with sample images shown in B. C–D, quantitative data of TH and DJ-1 levels (n = 6, **, p < 0.01 compared with MPTP alone). E, mouse brains were fixed, and sections were immunostained with TH antibody. The number of dopamine neurons in the substantia nigra was determined using unbiased counting methods (n = 5, *, p < 0.05 compared with MPTP alone). F–H, sample images are shown with TH immunostaining in the substantia nigra in three groups of mice. Bar length, 1 mm for F–H.
Sodium Phenylbutyrate Prevents Age-related Motor and Cognitive Decline in Mice with Diffuse Lewy Body Disease
We have created a transgenic mouse model expressing a tyrosine-to-cysteine (Y39C) mutant human α-synuclein (36). Because the transgene is expressed under control of the Thy1 promoter, mutant protein accumulates throughout the brain in all neurons. These animals have progressive, age-related decline in motor and cognitive function. Histopathology shows Lewy body-like α-synuclein inclusions in neurons. The age-related behavioral and neuropathologic phenotypes have similarities to PD and diffuse Lewy body disease.
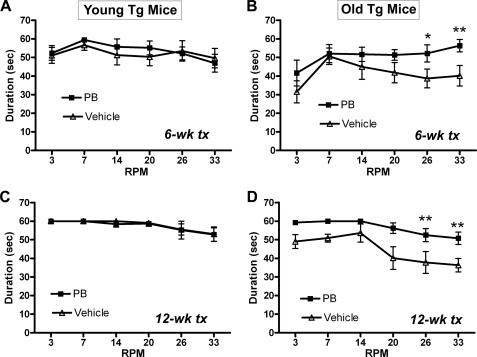
To test whether PB treatment can prevent the development of behavioral and neuropathological deficits in this transgenic mouse model, we divided transgenic mice into a Young Tg group (age 6–8 months) and an Old Tg group (age 10–12 months). Mice were treated with PB (1000 mg/liter, 5.4 mm) or vehicle (NaCl, 310 mg/liter) in drinking water for three months. Mice were tested for motor function after 6 weeks and 12 weeks of treatment using a rotarod with increasing speed (3–33 rpm). Results showed that PB treatment did not change motor function in Young Tg mice at 6-weeks or 12-weeks of therapy when animals were up to 11 months of age (Fig. 6, A and C). By contrast, in Old Tg mice, motor function deteriorated progressively with age in animals that were 13–15 months old at the end of the study. PB treatment prevented the decline and significantly improved motor function in Old Tg mice at both 6-week and 12-week tests (*, p < 0.05; **, p < 0.01 compared with vehicle, Fig. 6, B and D). The Old Tg mice treated with PB performed similarly to Young Tg mice (compare Fig. 6, C and D).
FIGURE 6.
Sodium phenylbutyrate improved motor function in aged α-synuclein transgenic mice. The Y39C transgenic mice were divided into two age groups: 6–8 months old (Young Tg, n = 20, A, C) and 10–12 months old (Old Tg, n = 20, B, D). Half of the transgenic mice in each age group (n = 10) received PB (1000 mg/liter, 5.4 mm) in drinking water, while the other mice (n = 10) were treated with water containing sodium chloride in equal molarity (vehicle). At 6 weeks and 12 weeks of drug treatment, all mice were tested for motor function using a rotarod (speed 3–33 rpm). A and C, Young Tg mice had no differences in rotarod performance between PB and vehicle treatments. B and D, PB treatment in Old Tg mice led to significant improvement in rotarod performance compared with vehicle-treated transgenic mice at both 6 week and 12 week tests. (*, p < 0.05; **, p < 0.01, n = 10.)
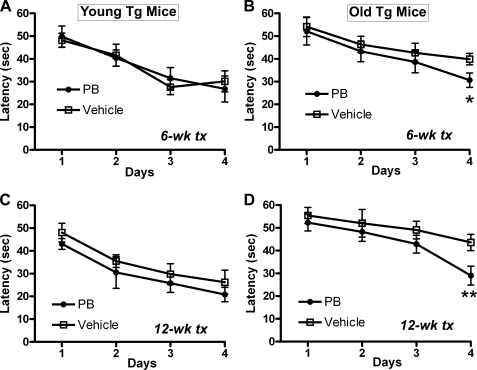
Mice were tested for cognitive function at 6 weeks and 12 weeks of treatments using a Morris water maze. In these transgenic mice, we have reported that water maze performance deteriorates as mice age (36). In the current studies, we have found that Young Tg mice perform well in the Morris water maze, and PB treatment did not change water maze performance at 6-week and 12-week tests (Fig. 7, A and C). In Old Tg mice, PB treatment prevented the age-related decline in water maze function at both 6-week and 12-week tests (*, p < 0.05; **, p < 0.01 compared with vehicle, Fig. 7, B–D). These results show that sodium phenylbutyrate can improve motor and cognitive function in aged transgenic mice.
FIGURE 7.
Sodium phenylbutyrate improved cognitive function in aged α-synuclein transgenic mice. The Y39C transgenic mice were divided into two age groups: 6–8 months old (Young Tg, n = 20, A, C) and 10–12 months old (Old Tg, n = 20, B, D). Half of the transgenic mice in each age group (n = 10) received PB (1000 mg/liter, 5.4 mm) in drinking water, while the other mice (n = 10) were treated with water containing sodium chloride in equal molarity (vehicle). After 6 weeks and 12 weeks of drug treatment, all mice were tested for cognitive function using the Morris water maze. A, C, Young Tg mice had no differences in learning ability between PB and vehicle treatments. B, D, PB treatment in Old Tg mice significantly improved learning ability in the last day of water maze testing compared with vehicle-treated transgenic mice at both 6-week and 12-week tests. (*, p < 0.05; **, p < 0.01, n = 10.)
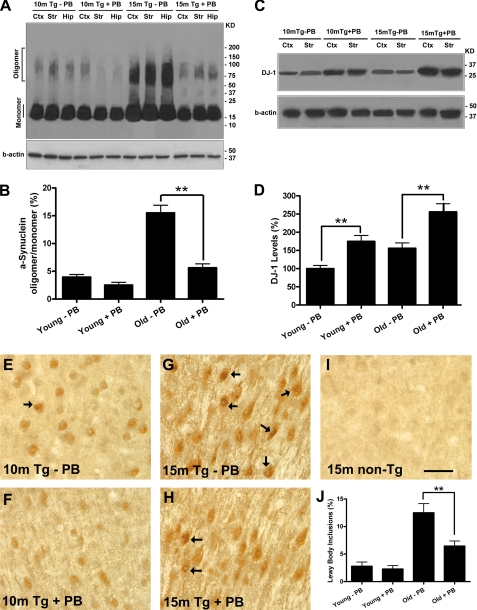
Sodium Phenylbutyrate Reduces α-Synuclein Aggregation and Increases Expression of DJ-1 in Old Transgenic Mouse Brain
We examined α-synuclein aggregation in transgenic mice treated with PB. Brain tissue lysates were separated in SDS-PAGE and probed with α-synuclein antibody. Fig. 8A shows Western blot images from young and old mice. Brain from a 15-month-old transgenic mouse (15m Tg-PB) shows intense α-synuclein oligomer fractions in cortex, striatum, and hippocampus. PB treatment dramatically reduced oligomer formation in an age-matched transgenic mouse (15m Tg+PB) (Fig. 8A). In 10-month-old Young transgenic animals, oligomer accumulation had not yet occurred, and the effect of PB was minimal (10m Tg-PB and +PB) (Fig. 8A). Fig. 8B presents the ratios of α-synuclein oligomer to monomer. The very high level of oligomer in old transgenic mice (Old w/o PB) is dramatically reduced by treatment (**, p < 0.01, Fig. 8B). In these same treatment groups, we found that brain DJ-1 protein levels were significantly increased in both young and old transgenic mice treated with PB compared with age-matched mice without PB treatment (*, p < 0.05; Fig. 8, C and D).
FIGURE 8.
Sodium phenylbutyrate increased DJ-1 expression and reduced α-synuclein oligomer formation and aggregation in aged transgenic mice. The Y39C transgenic mice were divided into two age groups: 6–8 months old (Young Tg, n = 20) and 10–12 months old (Old Tg, n = 20). Half of the transgenic mice in each age group (n = 10) received PB (1000 mg/liter) in drinking water, while the other mice (n = 10) were given water with equimolar of sodium chloride (vehicle). After 6 weeks and 12 weeks of drug treatment, all mice were tested for motor and cognitive function. After the last behavioral test, half of the mice (n = 5 for each age and treatment group) were sacrificed for biochemical analysis; the remaining half (n = 5 for each age and treatment group) were sacrificed for histology. A, brain tissues (cortex, striatum, and hippocampus) from Young and Old transgenic mice with or without PB treatment were analyzed for α-synuclein aggregation. Western blots showed that PB dramatically reduced α-synuclein oligomer formation in old transgenic mice compared with mice of the same age not receiving PB treatment. A sample blot shows 15 month Tg with (+PB) or without (−PB) treatment), while α-synuclein monomer levels were not changed. B, ratio of α-synuclein oligomer to monomer is shown in Young and Old transgenic mice with or without PB treatment (n = 5, **, p < 0.01). Old transgenic mice had high levels of oligomer (15%). After three months of PB treatment, old mice had much lower levels of oligomer which were similar to the ratio seen in Young transgenic mice. C-D, brain tissues (cortex) from Young and Old transgenic mice with or without PB treatment were analyzed for DJ-1 protein levels using Western blot (C). Results showed that PB treatment significantly increased brain DJ-1 levels in both young and old transgenic mice compared with mice without PB treatment (D; *, p < 0.05, n = 5). E-I, brain sections from Young and Old transgenic mice with or without PB treatment were immunostained with human α-synuclein antibody (LB509). Sections were examined for α-synuclein-positive Lewy body-like inclusions. Sample images from Young and Old transgenic mice with or without PB treatment are shown in E–H. A sample image from 15-month-old non-transgenic mouse is shown in I. J, results showed that the percentage of α-synuclein-positive neurons with LB-like inclusions (arrows) was significantly reduced in Old transgenic mice with PB treatment compared with old mice without PB (*, p < 0.05, n = 5), while there was no significant change in Young transgenic mice after PB treatment. Bar length, 25 μm for E–I.
We performed α-synuclein immunostaining in mouse brain sections using LB509 antibody. The PB treatment had little effect on the number of neurons with Lewy body-like inclusions at young mice (sample images from 10m Tg mice with and without PB are shown in Fig. 8, E and F). However, in old mice, the PB treatment greatly reduced the number of neurons with Lewy body-like inclusions (sample images from 10m Tg mice with and without PB are shown in Fig. 8, G and H). Control staining from 15-month-old non-Tg mice is shown in Fig. 8I. The percentage of α-synuclein positive neurons with Lewy body-like inclusions is shown in Fig. 8J (**, p < 0.01, PB compared with Vehicle). These data indicate that phenylbutyrate can increase DJ-1 expression, reduce α-synuclein oligomer formation, and prevent age-related decline in motor and cognitive function in a transgenic mouse model of diffuse Lewy body disease.
DISCUSSION
In this report, we have described the neuroprotective effects of phenylbutyrate in both cell culture and in mouse models. We have found that phenylbutyrate can up-regulate DJ-1 mRNA and protein levels in rat dopaminergic N27 cells and HEK293 cells. Increased expression of DJ-1 renders cells more resistant to oxidative stress and α-synuclein-induced toxicity. Blocking DJ-1 activation with antisense-DJ-1 interferes with the physiologic protection. In mice, we have discovered that phenylbutyrate given through drinking water can increase brain DJ-1 levels. Up-regulation of DJ-1 resulted in neuroprotection for dopamine neurons against MPTP toxicity. Moreover, phenylbutyrate given to transgenic mice that overexpress a mutant form of α-synuclein prevented oligomer formation in brain and stopped the age-related decline in motor and cognitive function.
Phenylbutyrate is a histone deacetylase inhibitor (HDACi) and has been shown to be neuroprotective in animal models of Huntington disease, spinal muscular atrophy, and amyotrophic lateral sclerosis (33–34, 39–43). The drug has been shown to protect dopamine neurons from death in the MPTP mouse model of Parkinson disease (35). Phenylbutyrate can protect dopamine neurons from rotenone-induced cell death. In transgenic mice expressing both A53T and A30P human α-synuclein, treatment with phenylbutyrate can improve behavioral function and reduce neuropathology (44–45). These prior studies did not propose a link between phenylbutyrate and the activation of DJ-1 gene expression.
As a histone deacetylase inhibitor, phenylbutyrate can increase acetylation levels of histones H3 and H4, thereby promoting transcriptional activation (33, 46). Previous studies have demonstrated that phenylbutyrate can increase expression of many genes including anti-apoptotic genes, components of ubiquitin-proteosomal pathways, nuclear factor NF-κB p50, survival motor neuron 1 (SMN1), and adrenoleukodystrophy-related gene (ALD) (40–41, 46–47). Other reports have shown that histone deacetylase inhibitors can activate INK4d and DR5 genes through the Sp1 binding site in the promoters (48–50). Because the DJ-1 gene promoter contains Sp1 binding sequences (51), it is possible that phenylbutyrate increases DJ-1 gene expression by increased binding of Sp1 to the DJ-1 promoter. Our DJ-1 promoter-luciferase reporter assay in HEK 293 cells has provided additional evidence that phenylbutyrate can increase DJ-1 gene expression. We have found that blocking DJ-1 expression with shDJ-1 blocks the neuroprotective effects of phenylbutyrate.
Increased DJ-1 levels protect against oxidative stress and other biochemical toxicity through multiple pathways. DJ-1 can stabilize Nrf2, a master regulator of antioxidant transcriptional responses, by blocking association with its inhibitor protein Keap1, thereby preventing ubiquitination of Nrf2 (30). DJ-1 can also sequester the cell death protein Daxx in the nucleus and prevent Daxx-induced apoptosis after oxidative stress (29). Recent reports show that DJ-1 can work in parallel with the PINK1/parkin pathway to maintain mitochondrial function in the presence of an oxidative environment (52–53). In addition, DJ-1 can act as a redox-dependent molecular chaperone to inhibit α-synuclein aggregate formation (54–55). We have shown that DJ-1 can increase glutathione synthesis after oxidative stress and can up-regulate heat shock protein 70 (Hsp70) to block α-synuclein aggregation (31). While the majority of PD patients do not carry DJ-1 gene mutations, our results indicate that increasing DJ-1 expression to supra-normal levels can make dopamine neurons resistant to neurotoxic insults. Drugs that enhance DJ-1 gene expression may be neuroprotective for all Parkinson disease patients.
Phenylbutyrate has additional metabolic effects. It can be a chaperone molecule. As a chemical chaperone, phenylbutyrate can bind and mask surface-exposed hydrophobic segments of unfolded proteins and thereby stabilize protein structure in the native conformation, reducing endoplasmic reticulum (ER) stress (56–58). In our α-synuclein transgenic mouse model, it is possible that phenylbutyrate directly stabilizes mutant α-synuclein and prevents the formation of high molecular weight oligomers and fibrils.
In summary, we have found that phenylbutyrate can up-regulate DJ-1 activity and prevent progression of motor and cognitive complications in a transgenic mouse model of diffuse Lewy body disease. Phenylbutyrate may be a useful drug for preventing progression of disease in patients with idiopathic Parkinson or diffuse Lewy body disease. Drugs that can increase DJ-1 expression could provide a new strategy for treating Parkinson disease by stopping the underlying disease process.
This work was supported by Charles and Joanne Ackerman, the Leopold Korn and Michael Korn Professorship in Parkinson Disease at the University of Colorado School of Medicine, and the Parkinson Disease Foundation.
- PD
- Parkinson disease
- ALD
- adrenoleukodystrophy-related gene
- ER
- endoplasmic reticulum
- GSH
- glutathione
- GCL
- glutamate cysteine ligase
- HDACi
- histone deacetylase inhibitors
- HEK
- human embryonic kidney
- H2O2
- hydrogen peroxide
- Hsp70
- heat shock protein 70
- LB
- Lewy body
- MPTP
- 1-methyl 4-phenyl 1,2,3,6-tetrahydropyridine
- MTT
- methylthiazoletetrazolium
- 6-OHDA
- 6-hydroxydopamine
- PB
- sodium phenylbutyrate
- SB
- sodium butyrate
- SMN
- survival motor neuron
- Tg
- transgenic
- nTg
- non-transgenic
- TH
- tyrosine hydroxylase.
REFERENCES
- 1. Cookson M. R., Bandmann O. (2010) Hum. Mol. Genet. 19, R21–R27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Huang Y., Cheung L., Rowe D., Halliday G. (2004) Brain Res. Brain Res. Rev. 46, 44–70 [DOI] [PubMed] [Google Scholar]
- 3. Allam M. F., Del Castillo A. S., Navajas R. F. (2005) Neurol. Res. 27, 206–208 [DOI] [PubMed] [Google Scholar]
- 4. Brown R. C., Lockwood A. H., Sonawane B. R. (2005) Environ. Health Perspect. 113, 1250–1256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Morris H. R. (2005) Ann. Med. 37, 86–96 [DOI] [PubMed] [Google Scholar]
- 6. Bonifati V. (2005) Minerva Med. 96, 175–186 [PubMed] [Google Scholar]
- 7. Cookson M. R. (2005) Annu. Rev. Biochem. 74, 29–52 [DOI] [PubMed] [Google Scholar]
- 8. Polymeropoulos M. H., Lavedan C., Leroy E., Ide S. E., Dehejia A., Dutra A., Pike B., Root H., Rubenstein J., Boyer R., Stenroos E. S., Chandrasekharappa S., Athanassiadou A., Papapetropoulos T., Johnson W. G., Lazzarini A. M., Duvoisin R. C., Di Iorio G., Golbe L. I., Nussbaum R. L. (1997) Science 276, 2045–2047 [DOI] [PubMed] [Google Scholar]
- 9. Krüger R., Kuhn W., Müller T., Woitalla D., Graeber M., Kösel S., Przuntek H., Epplen J. T., Schöls L., Riess O. (1998) Nat. Genet. 18, 106–108 [DOI] [PubMed] [Google Scholar]
- 10. Zarranz J. J., Alegre J., Gómez-Esteban J. C., Lezcano E., Ros R., Ampuero I., Vidal L., Hoenicka J., Rodriguez O., Atarés B., Llorens V., Gomez Tortosa E., del Ser T., Muñoz D. G., de Yebenes J. G. (2004) Ann. Neurol. 55, 164–173 [DOI] [PubMed] [Google Scholar]
- 11. Spillantini M. G., Schmidt M. L., Lee V. M., Trojanowski J. Q., Jakes R., Goedert M. (1997) Nature 388, 839–840 [DOI] [PubMed] [Google Scholar]
- 12. Baba M., Nakajo S., Tu P. H., Tomita T., Nakaya K., Lee V. M., Trojanowski J. Q., Iwatsubo T. (1998) Am. J. Pathol. 152, 879–884 [PMC free article] [PubMed] [Google Scholar]
- 13. Trojanowski J. Q., Lee V. M. (1998) Arch. Neurol. 55, 151–152 [DOI] [PubMed] [Google Scholar]
- 14. Conway K. A., Harper J. D., Lansbury P. T. (1998) Nat. Med. 4, 1318–1320 [DOI] [PubMed] [Google Scholar]
- 15. Hashimoto M., Rockenstein E., Masliah E. (2003) Ann. N.Y. Acad. Sci. 991, 171–188 [PubMed] [Google Scholar]
- 16. Narhi L., Wood S. J., Steavenson S., Jiang Y., Wu G. M., Anafi D., Kaufman S. A., Martin F., Sitney K., Denis P., Louis J. C., Wypych J., Biere A. L., Citron M. (1999) J. Biol. Chem. 274, 9843–9846 [DOI] [PubMed] [Google Scholar]
- 17. Wood S. J., Wypych J., Steavenson S., Louis J. C., Citron M., Biere A. L. (1999) J. Biol. Chem. 274, 19509–19512 [DOI] [PubMed] [Google Scholar]
- 18. Zhou W., Hurlbert M. S., Schaack J., Prasad K. N., Freed C. R. (2000) Brain Res. 866, 33–43 [DOI] [PubMed] [Google Scholar]
- 19. Alves Da Costa C., Paitel E., Vincent B., Checler F. (2002) J. Biol. Chem. 277, 50980–50984 [DOI] [PubMed] [Google Scholar]
- 20. Zhou W., Schaack J., Zawada W. M., Freed C. R. (2002) Brain Res. 926, 42–50 [DOI] [PubMed] [Google Scholar]
- 21. Kaul S., Anantharam V., Kanthasamy A., Kanthasamy A. G. (2005) Brain Res. Mol. Brain Res. 139, 137–152 [DOI] [PubMed] [Google Scholar]
- 22. Kim R. H., Smith P. D., Aleyasin H., Hayley S., Mount M. P., Pownall S., Wakeham A., You-Ten A. J., Kalia S. K., Horne P., Westaway D., Lozano A. M., Anisman H., Park D. S., Mak T. W. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 5215–5220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bonifati V., Rizzu P., van Baren M. J., Schaap O., Breedveld G. J., Krieger E., Dekker M. C., Squitieri F., Ibanez P., Joosse M., van Dongen J. W., Vanacore N., van Swieten J. C., Brice A., Meco G., van Duijn C. M., Oostra B. A., Heutink P. (2003) Science 299, 256–259 [DOI] [PubMed] [Google Scholar]
- 24. Hague S., Rogaeva E., Hernandez D., Gulick C., Singleton A., Hanson M., Johnson J., Weiser R., Gallardo M., Ravina B., Gwinn-Hardy K., Crawley A., St. George-Hyslop P. H., Lang A. E., Heutink P., Bonifati V., Hardy J., Singleton A. (2003) Ann. Neurol. 54, 271–274 [DOI] [PubMed] [Google Scholar]
- 25. Ibáñez P., De Michele G., Bonifati V., Lohmann E., Thobois S., Pollak P., Agid Y., Heutink P., Dürr A., Brice A. (2003) Neurology 61, 1429–1431 [DOI] [PubMed] [Google Scholar]
- 26. Clark L. N., Afridi S., Mejia-Santana H., Harris J., Louis E. D., Cote L. J., Andrews H., Singleton A., Wavrant De-Vrieze F., Hardy J., Mayeux R., Fahn S., Waters C., Ford B., Frucht S., Ottman R., Marder K. (2004) Mov. Disord. 19, 796–800 [DOI] [PubMed] [Google Scholar]
- 27. Taira T., Saito Y., Niki T., Iguchi-Ariga S. M., Takahashi K., Ariga H. (2004) EMBO Rep. 5, 213–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Canet-Avilés R. M., Wilson M. A., Miller D. W., Ahmad R., McLendon C., Bandyopadhyay S., Baptista M. J., Ringe D., Petsko G. A., Cookson M. R. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 9103–9108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Junn E., Jang W. H., Zhao X., Jeong B. S., Mouradian M. M. (2009) J. Neurosci. Res 87, 123–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Clements C. M., McNally R. S., Conti B. J., Mak T. W., Ting J. P. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 15091–15096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhou W., Freed C. R. (2005) J. Biol. Chem. 280, 43150–43158 [DOI] [PubMed] [Google Scholar]
- 32. Ying M., Xu R., Wu X., Zhu H., Zhuang Y., Han M., Xu T. (2006) J. Biol. Chem. 281, 12580–12586 [DOI] [PubMed] [Google Scholar]
- 33. Gardian G., Browne S. E., Choi D. K., Klivenyi P., Gregorio J., Kubilus J. K., Ryu H., Langley B., Ratan R. R., Ferrante R. J., Beal M. F. (2005) J. Biol. Chem. 280, 556–563 [DOI] [PubMed] [Google Scholar]
- 34. Minamiyama M., Katsuno M., Adachi H., Waza M., Sang C., Kobayashi Y., Tanaka F., Doyu M., Inukai A., Sobue G. (2004) Hum. Mol. Genet. 13, 1183–1192 [DOI] [PubMed] [Google Scholar]
- 35. Gardian G., Yang L., Cleren C., Calingasan N. Y., Klivenyi P., Beal M. F. (2004) Neuromolecular Med. 5, 235–241 [DOI] [PubMed] [Google Scholar]
- 36. Zhou W., Milder J. B., Freed C. R. (2008) J. Biol. Chem. 283, 9863–9870 [DOI] [PubMed] [Google Scholar]
- 37. Adams F. S., La Rosa F. G., Kumar S., Edwards-Prasad J., Kentroti S., Vernadakis A., Freed C. R., Prasad K. N. (1996) Neurochem Res 21, 619–627 [DOI] [PubMed] [Google Scholar]
- 38. Zhou W., Freed C. R. (2004) J. Biol. Chem. 279, 10128–10135 [DOI] [PubMed] [Google Scholar]
- 39. Hogarth P., Lovrecic L., Krainc D. (2007) Mov. Disord. 22, 1962–1964 [DOI] [PubMed] [Google Scholar]
- 40. Brahe C., Vitali T., Tiziano F. D., Angelozzi C., Pinto A. M., Borgo F., Moscato U., Bertini E., Mercuri E., Neri G. (2005) Eur. J. Hum. Genet. 13, 256–259 [DOI] [PubMed] [Google Scholar]
- 41. Andreassi C., Angelozzi C., Tiziano F. D., Vitali T., De Vincenzi E., Boninsegna A., Villanova M., Bertini E., Pini A., Neri G., Brahe C. (2004) Eur. J. Hum. Genet. 12, 59–65 [DOI] [PubMed] [Google Scholar]
- 42. Cudkowicz M. E., Andres P. L., Macdonald S. A., Bedlack R. S., Choudry R., Brown R. H., Jr., Zhang H., Schoenfeld D. A., Shefner J., Matson S., Matson W. R., Ferrante R. J. (2009) Amyotroph Lateral Scler. 10, 99–106 [DOI] [PubMed] [Google Scholar]
- 43. Tremolizzo L., Rodriguez-Menendez V., Sala G., Di Francesco J. C., Ferrarese C. (2005) Amyotroph Lateral Scler. Other Motor Neuron Disord. 6, 185–186 [DOI] [PubMed] [Google Scholar]
- 44. Inden M., Kitamura Y., Takeuchi H., Yanagida T., Takata K., Kobayashi Y., Taniguchi T., Yoshimoto K., Kaneko M., Okuma Y., Taira T., Ariga H., Shimohama S. (2007) J. Neurochem. 101, 1491–1504 [DOI] [PubMed] [Google Scholar]
- 45. Ono K., Ikemoto M., Kawarabayashi T., Ikeda M., Nishinakagawa T., Hosokawa M., Shoji M., Takahashi M., Nakashima M. (2009) Parkinsonism Relat. Disord. 15, 649–654 [DOI] [PubMed] [Google Scholar]
- 46. Ryu H., Smith K., Camelo S. I., Carreras I., Lee J., Iglesias A. H., Dangond F., Cormier K. A., Cudkowicz M. E., Brown R. H., Jr., Ferrante R. J. (2005) J. Neurochem. 93, 1087–1098 [DOI] [PubMed] [Google Scholar]
- 47. Gondcaille C., Depreter M., Fourcade S., Lecca M. R., Leclercq S., Martin P. G., Pineau T., Cadepond F., ElEtr M., Bertrand N., Beley A., Duclos S., De Craemer D., Roels F., Savary S., Bugaut M. (2005) J. Cell Biol. 169, 93–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kim S., Kang J. K., Kim Y. K., Seo D. W., Ahn S. H., Lee J. C., Lee C. H., You J. S., Cho E. J., Lee H. W., Han J. W. (2006) Biochem. Biophys. Res. Commun. 342, 1168–1173 [DOI] [PubMed] [Google Scholar]
- 49. Yokota T., Matsuzaki Y., Miyazawa K., Zindy F., Roussel M. F., Sakai T. (2004) Oncogene 23, 5340–5349 [DOI] [PubMed] [Google Scholar]
- 50. Kim Y. H., Park J. W., Lee J. Y., Kwon T. K. (2004) Carcinogenesis 25, 1813–1820 [DOI] [PubMed] [Google Scholar]
- 51. Taira T., Takahashi K., Kitagawa R., Iguchi-Ariga S. M., Ariga H. (2001) Gene 263, 285–292 [DOI] [PubMed] [Google Scholar]
- 52. Thomas K. J., McCoy M. K., Blackinton J., Beilina A., van der Brug M., Sandebring A., Miller D., Maric D., Cedazo-Minguez A., Cookson M. R. (2011) Hum. Mol. Genet. 20, 40–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Irrcher I., Aleyasin H., Seifert E. L., Hewitt S. J., Chhabra S., Phillips M., Lutz A. K., Rousseaux M. W., Bevilacqua L., Jahani-Asl A., Callaghan S., MacLaurin J. G., Winklhofer K. F., Rizzu P., Rippstein P., Kim R. H., Chen C. X., Fon E. A., Slack R. S., Harper M. E., McBride H. M., Mak T. W., Park D. S. (2010) Hum. Mol. Genet. 19, 3734–3746 [DOI] [PubMed] [Google Scholar]
- 54. Shendelman S., Jonason A., Martinat C., Leete T., Abeliovich A. (2004) PLoS Biol 2, e362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Zhou W., Zhu M., Wilson M. A., Petsko G. A., Fink A. L. (2006) J. Mol. Biol. 356, 1036–1048 [DOI] [PubMed] [Google Scholar]
- 56. Yam G. H., Gaplovska-Kysela K., Zuber C., Roth J. (2007) Invest. Ophthalmol. Vis. Sci. 48, 1683–1690 [DOI] [PubMed] [Google Scholar]
- 57. Perlmutter D. H. (2002) Pediatr Res 52, 832–836 [DOI] [PubMed] [Google Scholar]
- 58. Papp E., Csermely P. (2006) Handb. Exp. Pharmacol. 172, 405–416 [DOI] [PubMed] [Google Scholar]