Abstract
We have reported that α1 Na/K-ATPase regulates the trafficking of caveolin-1 and consequently alters cholesterol distribution in the plasma membrane. Here, we report the reciprocal regulation of α1 Na/K-ATPase by cholesterol. Acute exposure of LLC-PK1 cells to methyl β-cyclodextrin led to parallel decreases in cellular cholesterol and the expression of α1 Na/K-ATPase. Cholesterol repletion fully reversed the effect of methyl β-cyclodextrin. Moreover, inhibition of intracellular cholesterol trafficking to the plasma membrane by compound U18666A had the same effect on α1 Na/K-ATPase. Similarly, the expression of α1, but not α2 and α3, Na/K-ATPase was significantly reduced in the target organs of Niemann-Pick type C mice where the intracellular cholesterol trafficking is blocked. Mechanistically, decreases in the plasma membrane cholesterol activated Src kinase and stimulated the endocytosis and degradation of α1 Na/K-ATPase through Src- and ubiquitination-dependent pathways. Thus, the new findings, taken together with what we have already reported, revealed a previously unrecognized feed-forward mechanism by which cells can utilize the Src-dependent interplay among Na/K-ATPase, caveolin-1, and cholesterol to effectively alter the structure and function of the plasma membrane.
Keywords: Cholesterol, Membrane, Na/K-ATPase, Src, Trafficking
Introduction
Na/K-ATPase was originally discovered as an active ion transporter residing in the plasma membrane (1). The functional Na/K-ATPase consists of α and β subunits. The α subunit is the catalytic subunit, and four isoforms have been identified. Although the α1 isoform is found in all cells, the expression of other isoforms is tissue-specific (2, 3). Na/K-ATPase is a centrally important ion transporter that is essential for many cellular activities, including maintaining membrane potential and excitability in neurons. Moreover, recent studies indicate that Na/K-ATPase performs many non-pumping functions (4–8). For example, Na/K-ATPase interacts with Src kinase, forming a functional signaling complex capable of transducing extracellular signals into activation of intracellular kinase cascades (9). Interestingly, the signaling Na/K-ATPase mainly resides in the specialized membrane microdomains called caveolae and interacts with caveolin-1, a caveolae protein marker (7).
Caveolin-1 is a 22-kDa protein. In addition to its role in biogenesis of caveolae, caveolin-1 is involved in cholesterol trafficking to and from the plasma membrane (10). Reciprocally, the plasma membrane cholesterol controls the mobility and trafficking of caveolin-1 (11, 12). Interestingly, we have reported that the plasma membrane pool of Na/K-ATPase directly interacts with caveolin-1 (6). Moreover, this interaction not only regulates the mobility and trafficking of caveolin-1 but also plays an important role in maintaining the plasma membrane cholesterol content and in regulating intracellular cholesterol trafficking (8). Thus, to understand the interplay among Na/K-ATPase, cholesterol, and caveolin-1, we examined whether cholesterol regulates the expression of α1 Na/K-ATPase. Our new findings indicate that a decrease in plasma membrane cholesterol could stimulate a Src-dependent pathway, leading to the endocytosis and degradation of α1 Na/K-ATPase. Moreover, these regulations appear to be operational in vivo because the expression of α1 Na/K-ATPase is significantly reduced in the target organs of NPC13 mice.
EXPERIMENTAL PROCEDURES
Materials
The antibodies and their sources are as follows: The mouse monoclonal anti-Na/K-ATPase α1 antibody (α6F) for Western blot analysis was purchased from the Developmental Studies Hybridoma Bank at the University of Iowa. The mouse monoclonal anti-Na/K-ATPase α1 antibody for immunocytochemistry was from Upstate Biotechnology, Inc. (Lake Placid, NY). The rabbit polyclonal anti-pY418-Src antibody was bought from Invitrogen. The rabbit polyclonal anti-insulin receptor β subunit antibody, the rabbit polyclonal anti-caveolin-1 antibody, the mouse monoclonal anti-α-tubulin antibody, the mouse monoclonal anti-c-Src antibody, and all secondary antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA). The rabbit polyclonal anti-Na/K-ATPase α2 antibody and the rabbit polyclonal anti-Na/K-ATPase α3 antibody were gifts from Dr. Thomas A. Pressley (Texas Tech University). Filipin, Mβ-CD, and cycloheximide were obtained from Sigma-Aldrich (St. Louis, MO). The U18666A compound and MG-132 were from Caymen Chemical (Ann Arbor, M I). The Amplex Red cholesterol assay kit was purchased from Molecular Probes, Inc. (Eugene, OR).
Cell Culture
The LLC-PK1 cells were obtained from the American Type Culture Collection. Cells were cultured in DMEM containing 10% fetal bovine serum, penicillin (100 units/ml)/streptomycin (100 μg/ml) in a 5% CO2-humidified incubator. After cells reached full confluence, they were serum-starved for 24 h and used for experiments unless indicated otherwise.
Experimental Animals
The BALB/c +/npcnih mice were purchased from The Jackson Laboratory. BALB/c +/+ (NPC1+/+) and BALB/c npcnih/npcnih (NPC1−/−) mice were produced by mating BALB/c +/npcnih mice. Genomic DNA was obtained from tail biopsies and used for PCR-based genotyping. All mice were kept in a 12-h dark/light cycle and fed standard chow ad libitum. Littermates were euthanized at the age of 10 weeks, and organs, including brain, liver, heart, and kidney, were carefully dissected and weighed. All tissues were immediately frozen in liquid nitrogen and stored at −80 °C for Western blot analysis. All procedures were approved by the Institutional Animal Care and Use Committee of the University of Toledo, Health Science Campus.
Plasmid Constructs and Transfection
The RFP-Rab7 plasmid was requested from Addgene (Cambridge, MA). For transfection, cells were grown to about 70% confluence and transfected with the corresponding plasmids by Lipofectamine 2000 as described previously (8). Experiments were performed 24 h after transfection.
Other Assays
Protein concentrations of cell lysates or tissue homogenates were measured by a protein assay kit from Bio-Rad. For Western blotting, equal amounts of protein were loaded onto the gel, separated on 10% SDS-PAGE, transferred to an Optitran membrane, and probed with corresponding antibodies. Protein signals were detected with an ECL kit and quantified by Image J software. To study cellular distribution of the Na/K-ATPase α1, cells were cultured on coverslips and treated. Afterward, cells were fixed with ice-cold methanol for 30 min, immunostained, and imaged as described previously (13). After immunostaining of the Na/K-ATPase α1, the total cellular signals and intracellular signals were quantified using the Image J software. Specifically, Na/K-ATPase α1 immunofluorescence within 1 μm of the cell surface is considered plasma membrane and its proximity, and immunofluorescence internal and away from this zone is considered intracellular region. The ratio between intracellular and total signals was calculated by intracellular signals/total cellular signals. To quantify the mRNA, cells were cultured in 6-cm dishes and serum-starved for 24 h after reaching confluence. Then, cells were treated with U18666A (10 μg/ml) for 24 h. Total RNA was extracted using TRIzol and subjected to quantitative RT-PCR analysis of the Na/K-ATPase α1 and GAPDH as described previously (13). To study the cellular distribution of cholesterol, cells were stained by filipin as described previously (8). Total cellular cholesterol was measured using the Amplex Red cholesterol assay kit as instructed.
Statistical Analysis
Data are given as mean ± S.E. Statistical analysis was performed using the Student's t test, and significance was accepted at p < 0.05.
RESULTS
Reduction in the Membrane Cholesterol Decreases Cellular α1 Na/K-ATPase
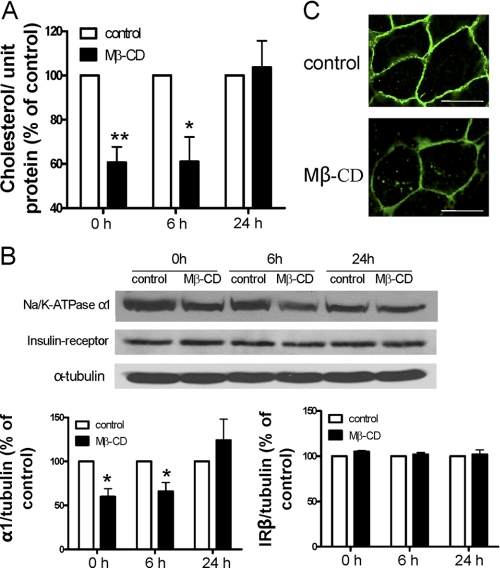
Because of its high affinity for cholesterol, Mβ-CD is able to specifically extract cholesterol from the plasma membrane (14). Thus, to begin addressing whether the plasma membrane cholesterol regulates the expression of Na/K-ATPase, we assessed the effects of Mβ-CD on the cellular content of the α1 subunit of Na/K-ATPase. LLC-PK1 cells are derived from pig kidney proximal tubules and express only the α1 Na/K-ATPase (15). As depicted in Fig. 1, cells were exposed to Mβ-CD for 60 min, washed, and recultured in serum-free medium as described previously (7). Afterward, cells were sampled at different times and subjected to cholesterol measurement and Western blot analyses. A 40% reduction in cellular cholesterol was recorded right after the Mβ-CD was washed off the cells. Over the next 24 h, cellular cholesterol was gradually recovered (Fig. 1A). When the cellular Na/K-ATPase was analyzed in the same samples, we observed a similar change in expression of the Na/K-ATPase α1 subunit (Fig. 1B). A 40% down-regulation occurred after Mβ-CD treatment, and the expression recovered after 24 h, indicating that the cellular α1 Na/K-ATPase level was positively correlated to the cholesterol level. To test the specificity of this regulation, we determined expression of another plasma membrane protein, the insulin receptor β subunit, under the same experimental conditions. As depicted in Fig. 1B, changes in cellular cholesterol did not alter expression of the insulin receptor. To further test the specificity, we measured another receptor tyrosine kinase, namely EGF receptor, and observed no difference between the control and Mβ-CD-treated LLC-PK1 cells (data not shown). To verify the effect of Mβ-CD on α1 Na/K-ATPase expression, cells were immunostained and then imaged. As shown in Fig. 1C, Mβ-CD treatment significantly reduced the plasma membrane pool of the α1 subunit. Interestingly, although the majority of α1 Na/K-ATPase resided in the plasma membrane in control LLC-PK1 cells, a significant portion of α1 moved to the cytoplasm, residing in vesicular structures after Mβ-CD treatment, suggesting that decreases in the plasma membrane cholesterol might increase the endocytosis of α1 Na/K-ATPase.
FIGURE 1.
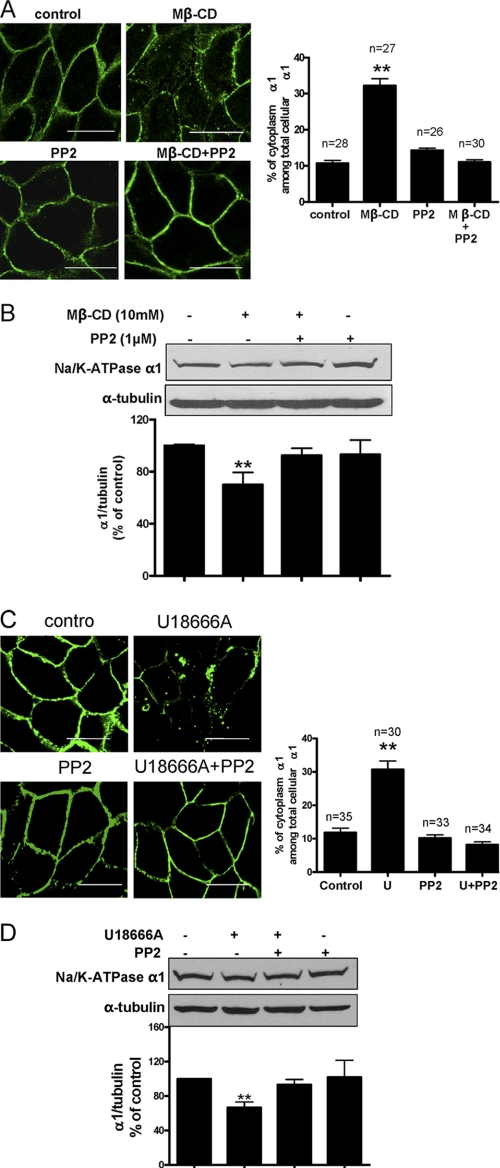
Reduction of membrane cholesterol by Mβ-CD down-regulates α1 Na/K-ATPase. LLC-PK1 cells were treated with 10 mm Mβ-CD in the serum-free medium at 37 °C for 1 h, washed, and then collected after the washed cells were cultured in serum-free medium for 0, 6, and 24 h. A, cholesterol content in the cell lysates from different time points were measured, adjusted to protein level, and compared. B, representative Western blots are shown on the levels of the α1 subunit, the insulin receptor β subunit, and α-tubulin (used as loading control). Quantitative data in A and B are combined from four separate experiments and expressed as mean ± S.E. The calculations are based on paired analyses between control and Mβ-CD at each time point. *, p < 0.05; **, p < 0.01. C, representative confocal images of the cellular distribution of α1 Na/K-ATPase in the non-treated cells (top) and Mβ-CD-treated cells (bottom) are displayed.
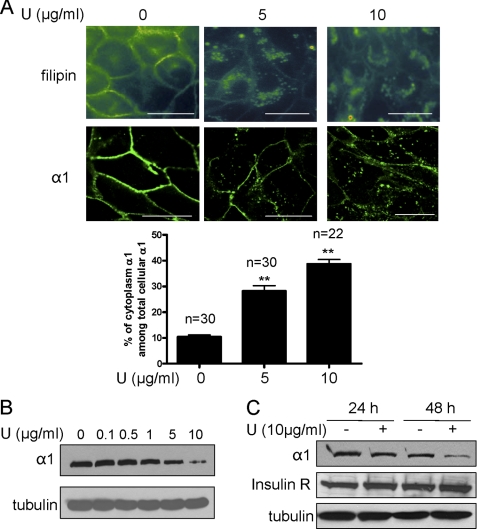
To further establish that it was the plasma membrane cholesterol pool that regulated the expression of the α1 Na/K-ATPase, we treated the serum-starved cells with an intracellular cholesterol trafficking inhibitor, compound U18666A. Consistent with the literature (16, 17), treatment of LLC-PK1 cells with compound U18666A led to redistribution of free cholesterol from the plasma membrane to intracellular compartments, as revealed by filipin staining (Fig. 2A). Consequently, this caused a significant reduction in the plasma membrane α1 Na/K-ATPase. Concomitantly, vesicular accumulation of α1 Na/K-ATPase was readily detectable in compound U18666A-treated cells. These effects were dose-dependent. When the intracellular α1 Na/K-ATPase signal was quantified using Image J software, it amounted to about 10% of the total cellular α1 Na/K-ATPase signal. This pool of α1 Na/K-ATPase increased to 28 and 38% after cells were treated for 24 h with U18666A at doses of 5 and 10 μg/ml, respectively (Fig. 2A). To confirm the regulatory effect of U18666A on the Na/K-ATPase, we analyzed the cell lysates by Western blotting. As depicted in Fig. 2, B and C, U18666A produced a dose- and time-dependent down-regulation of α1 Na/K-ATPase. However, U18666A failed to change the expression of the insulin receptor β subunit (Fig. 2C) and EGF receptor (data not shown). Taken together, the data indicate that plasma membrane cholesterol specifically regulates the expression of α1 Na/K-ATPase in LLC-PK1 cells.
FIGURE 2.
Compound U18666A decreases plasma membrane cholesterol and specifically down-regulates α1 Na/K-ATPase. A, LLC-PK1 cells were treated with different doses of U18666A for 24 h and subjected to filipin (top row of upper panel) and α1 immunostaining (bottom row of upper panel). Representative images show dose-dependent effects of the U18666A compound on cellular distribution of cholesterol and α1 Na/K-ATPase. The intracellular and total α1 Na/K-ATPase signals were quantified by Image J software from randomly selected cells, and the ratio was calculated. Values are expressed as mean ± S.E. The number of the cells used for quantification was indicated as n value above the bar graph (bottom panel). **, p < 0.01 compared with control. Scale bar = 20 μm. U, U18666A. B, representative Western blot analysis from three independent experiments shows the effect of U18666A on the α1 subunit and α-tubulin after cells were treated for 48 h. C, representative Western blot analysis from three independent experiments shows the effect of U18666A on the protein levels of the α1 subunit, the insulin receptor β subunit, and α-tubulin after cells were treated by U18666A for 24 h and 48 h, respectively.
Reduction in the Membrane Cholesterol Stimulates the Endocytosis and Degradation of α1 Na/K-ATPase
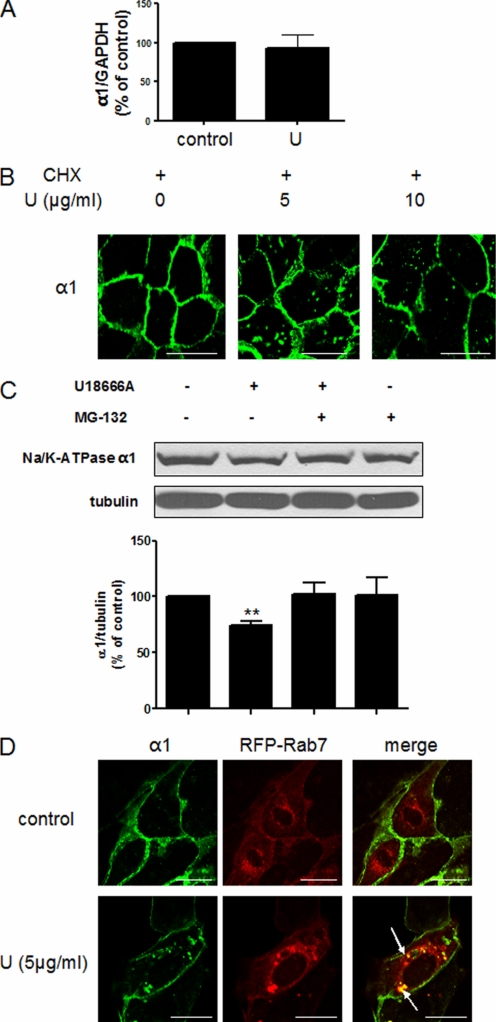
To explore the molecular mechanism underlying the effect of cholesterol-regulated expression of α1 Na/K-ATPase, we performed the following experiments. First, we tested whether cholesterol affects transcriptional regulation of α1 Na/K-ATPase. As depicted in Fig. 3A, quantitative RT-PCR revealed no difference in the α1 Na/K-ATPase mRNA level between control and compound U18666A-treated cells. Second, because both Mβ-CD and compound U18666A increased the accumulation of α1 Na/K-ATPase in intracellular vesicles, we assessed whether cholesterol regulates the endocytosis of existing α1 Na/K-ATPase or exocytotic delivery of the newly synthesized α1 Na/K-ATPase. Cells were treated with a protein synthesis inhibitor, cycloheximide, for 1 h as described previously (13) before being exposed to compound U18666A. As shown in Fig. 3B, blockade of protein de novo synthesis by cycloheximide did not prevent compound U18666A-induced accumulation of α1 Na/K-ATPase in intracellular vesicles. These findings indicate that compound U18666A could stimulate the endocytosis and then degradation of the Na/K-ATPase. It has been reported that α1 Na/K-ATPase is ubiquitinated and degraded in the lysosomes in response to hypoxia (18, 19). To test whether that is also the case here, we first used MG-132, an inhibitor of the ubiquitin-proteasomal pathway (12), in U18666A-treated cells. As shown in Fig. 3C, MG-132 completely blocked U18666A-induced α1 down-regulation. To further test this postulation, we measured whether compound U18666A increased the accumulation of the Na/K-ATPase in late endosomes/lysosomes. To do so, we transfected LLC-PK1 cells with RFP-tagged Rab7, a marker for late endosome/lysosomes (20). As shown in Fig. 3D, the majority of α1 Na/K-ATPase resided in the plasma membrane, and no colocalization between the α1 Na/K-ATPase and Rab7 was detected in control cells. In contrast, compound U18666A treatment led to massive intracellular accumulation of α1 Na/K-ATPase, most of which was clearly colocalized with the Rab7 signals.
FIGURE 3.
Membrane cholesterol reduction leads to the endocytosis and degradation of α1 Na/K-ATPase. A, LLC-PK1 cells were treated with 10 μg/ml U18666A for 24 h. Total RNA was extracted, and quantitative RT-PCR was performed to probe α1 and GAPDH mRNA as described under “Experimental Procedures.” Data were from three independent experiments. U, U18666A. B, LLC-PK1 cells were treated with cycloheximide (10 μg/ml for 1 h) before being exposed to different doses of U18666A for 24 h. Cells were then fixed and immunostained for the Na/K-ATPase α1 subunit. Representative confocal images are shown. CHX, cycloheximide. U, U18666A. C, LLC-PK1 cells were treated with 10 μg/ml U18666A in the presence (+) or absence (-) of 20 μm MG-132 for 24 h. Representative Western blots are shown on the levels of the α1 subunit and α-tubulin. Quantitative data from four separate experiments are shown below and expressed as mean ± S.E. **, p < 0.01 compared with control. D, LLC-PK1 cells were transfected with RFP-Rab7 for 24 h before exposed to 5 μg/ml U18666A for 24 h. Afterward, cells were fixed and immunostained for the α1 subunit. Representative confocal images of α1 staining, RFP-Rab7, and merged figures are shown. The arrows point to the colocalization of α1 and RFP-Rab7. The same experiments were repeated at least three times. Scale bar = 20 μm.
Regulation of α1 Na/K-ATPase by Cholesterol Requires the Activation of Src
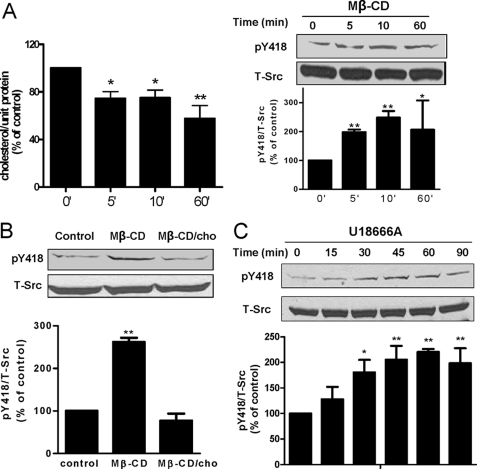
We previously demonstrated that ouabain stimulated the endocytosis of α1 Na/K-ATPase through a Src-dependent pathway (21). To test whether Src kinase is involved in cholesterol-regulated α1 trafficking, we first measured the effect of cholesterol reduction on Src phosphorylation at tyrosine 418, an indication of Src activation (9). As shown in Fig. 4A, Mβ-CD decreased cholesterol and activated Src in a time-dependent manner. These effects were fast. Significant changes were detected in 5 min, which is in accordance with prior observations (11, 22). To be sure that the effect of Mβ-CD on Src is mediated by the reduction in cholesterol, we remeasured Src pY418 after cells were repleted with cholesterol in the presence of Mβ-CD. As depicted in Fig. 4B, addition of cholesterol to Mβ-CD prevented Src activation. Consistently, exposure of cells to U18666A also induced Src activation in a time-dependent manner (Fig. 4C). To assess whether activation of Src is involved in the cholesterol regulation of α1 Na/K-ATPase trafficking, we used Src kinase inhibitor PP2 to block Src activation. As revealed by α1 Na/K-ATPase immunostaining and quantification in Fig. 5A, PP2 abolished Mβ-CD-induced endocytosis of α1 Na/K-ATPase. To verify this, we performed Western blot analyses of cell lysates. The data showed that PP2 prevented Mβ-CD-induced down-regulation of cellular α1 Na/K-ATPase (Fig. 5B). As expected, PP2 was also effective in blocking U18666A-induced α1 Na/K-ATPase endocytosis and degradation (Fig. 5, C and D). Taken together, our results indicate that the plasma membrane cholesterol regulates trafficking of the Na/K-ATPase via an Src-dependent pathway.
FIGURE 4.
Membrane cholesterol reduction activates Src kinase. A, LLC-PK1 cells were treated with 10 mm Mβ-CD for different times. Cholesterol content in the cell lysates from different time points were measured, adjusted to protein level, and compared (left panel). Representative Western blots are shown on the levels of Src-pY418 and c-Src (right panel). Quantitative data from four separate experiments are shown below and expressed as mean ± S.E. *, p < 0.05; **, p < 0.01 compared with control. B, LLC-PK1 cells were treated with 10 mm Mβ-CD or 10 mm Mβ-CD/0.1 mm cholesterol for 10 min. Representative Western blots are shown on Src-pY418 and c-Src. Quantitative data from four separate experiments are shown below and expressed as mean ± S.E. **, p < 0.01 compared with control. C, LLC-PK1 cells were treated with 10 μg/ml U18666A for different times. Representative Western blots are shown on the levels of Src-pY418 and c-Src. Quantitative data from four separate experiments are shown below and expressed as mean ± S.E. *, p < 0.05; **, p < 0.01 compared with control.
FIGURE 5.
Membrane cholesterol-regulated trafficking of α1 Na/K-ATPase is dependent on Src kinase activity. A, LLC-PK1 cells were pretreated with 1 μm PP2 for 1 h before addition of 10 mm Mβ-CD for 3 h. Cells were fixed and immunostained with Na/K-ATPase α1. Representative confocal images of α1 staining are shown on the left. The quantification of the immunostaining results was done as in Fig. 2A, and is shown on the right. The number of the cells used for quantification was indicated as the n value above the bar graph (right panel). **, p < 0.01 compared with control. Scale bar = 20 μm. B, the same treatments were performed as in A, and representative Western blots are shown on the levels of the α1 subunit and α-tubulin. Quantitative data from four separate experiments are shown below and expressed as mean ± S.E. **, p < 0.01 compared with control. C, LLC-PK1 cells were pretreated with 1 μm PP2 and cycloheximide (10 μg/ml for 1 h) before addition of 10 μg/ml U18666A (U) for 24 h. Cells were fixed and immunostained for the Na/K-ATPase α1 subunit. Representative confocal images of α1 staining are shown on the left and quantification shown on the right. **, p < 0.01 compared with control. D, the same treatments were performed as in C, and representative Western blots are shown on the levels of the Na/K-ATPase α1 subunit and α-tubulin. Quantitative data from four separate experiments are shown below and expressed as mean ± S.E. **, p < 0.01 compared with control. Scale bar = 20 μm.
Down-regulation of the Na/K-ATPase α1 Isoform in the Liver and Brain of NPC1 Mice
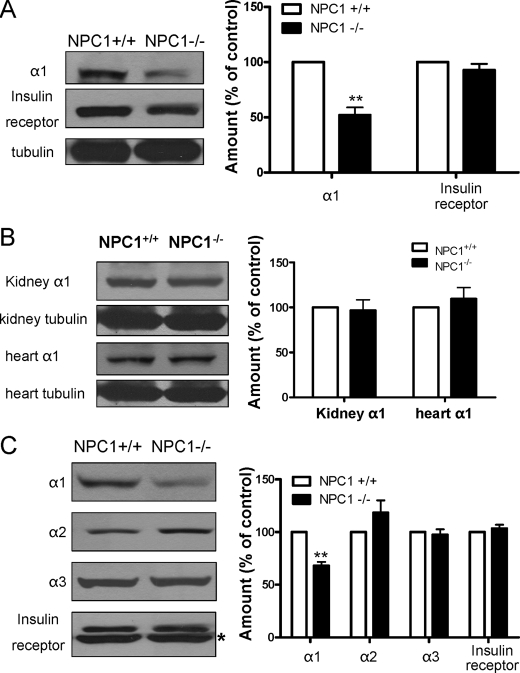
U18666A treatment is known to produce an NPC1-like phenotype in cell cultures (16). NPC1 disease is a cholesterol storage disorder causing prominent neurodegeneration in the central nervous system (23). Peripherally, it also affects the liver and spleen. A mouse model for NPC1 disease, the BALB/c npcnih/npcnih (NPC1−/−) mouse, has been shown to resemble human NPC1 disease (24). Moreover, massive cholesterol accumulation is observed in the NPC1−/− mouse liver. Thus, to verify that abnormal cholesterol metabolism affects expression of the Na/K-ATPase in vivo, we analyzed the α1 Na/K-ATPase content in liver lysates. As depicted in Fig. 6A, expression of α1 Na/K-ATPase was significantly reduced in the liver of NPC1−/− mice. On the other hand, no change in the insulin receptor β subunit was detected. Interestingly, when the α1 Na/K-ATPase content was analyzed in the kidney and heart lysates, two of the less affected organs, no reduction was observed (Fig. 6B). Because the Na/K-ATPase is centrally important for maintaining membrane potential and excitability of neurons, the above findings prompted us to assess whether neuronal expression of the α1 subunit is also reduced in NPC1−/− mice. As depicted in Fig. 6C, significant reduction in α1 Na/K-ATPase, but not in the insulin receptor β subunit, was detected in whole brain lysates in NPC1−/− mice. It is known that neurons express both α1 and α3 isoforms of the Na/K-ATPase, whereas gliocytes express α1 and α2 isoforms (25). When we measured α2 and α3 subunits, we failed to detect significant changes in either α2 or α3 subunits (Fig. 6C). Thus, it appears that the cholesterol trafficking defect in NPC1 disease specifically affects the expression of the α1 subunit in target organs such as the liver and brain.
FIGURE 6.
Down-regulation of the α1 Na/K-ATPase in target organs of BALB/c npcnih/npcnih mice. A, representative Western blots on the Na/K-ATPase α1 subunit, insulin receptor β, and α-tubulin of liver samples from NPC1+/+ and NPC1−/− mice are shown. **, p < 0.01. B, representative Western blots on the Na/K-ATPase α1 subunit and α-tubulin of kidney or heart samples from NPC1+/+ and NPC1−/− mice are shown. C, representative Western blots show the effect of NPC1 knockout on brain Na/K-ATPase α1, α2, and α3 subunit and the insulin receptor β subunit. The asterisk beside the lower bands in the immunoblot results of the insulin receptor β denotes an unknown band. All Western results were quantified from five NPC1+/+ and 5 NPC1−/− mice, normalized to α-tubulin level except for brain proteins, shown in the right panels, and expressed as mean ± S.E. **, p < 0.01.
DISCUSSION
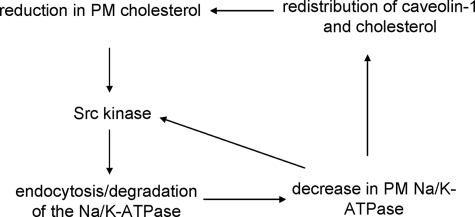
Cholesterol and Na/K-ATPase are two indispensable components of the plasma membrane in mammalian cells. Na/K-ATPase is a key regulator of transmembrane transport processes and a receptor for endogenous cardiotonic steroids (26). The plasma membrane cholesterol is important for many cellular activities, including the formation of lipid rafts and the regulation of ER cholesterol content and, thus, cholesterol synthesis and metabolism (27–29). We have reported that the Na/K-ATPase regulates the content of cholesterol in the plasma membrane through a highly conserved N-terminal caveolin-1 binding motif (6, 8). Alteration in the expression of Na/K-ATPase affects the formation of caveolae and cholesterol synthesis and trafficking in vitro and in vivo. In this study, we demonstrated that the plasma membrane pool of cholesterol regulates membrane trafficking and expression of Na/K-ATPase. A decrease in cholesterol leads to the activation of Src kinase, which promotes the endocytosis of and degradation of α1 Na/K-ATPase. In view of the fact that reduction of α1 Na/K-ATPase activates cellular Src, resulting in a decrease in caveolin-1 and cholesterol in the plasma membrane (6, 8, 15), it appears that cells have acquired a Src-dependent interplay among Na/K-ATPase, cholesterol, and caveolin-1. This functional interplay may establish a highly efficient feed-forward mechanism that detects the change in cellular cholesterol or/and α1 Na/K-ATPase and then alters the structure and function of the plasma membrane (Fig. 7). For example, a decrease in cholesterol could initiate a Src-dependent endocytosis and degradation of α1 Na/K-ATPase, which in turn would reduce the number of Na/K-ATPase in the plasma membrane, causing further activation of Src and, consequently, a further decrease in the membrane cholesterol. Interestingly, activation of Src has been noted in the target organs of NPC1 mice (30) and contributed to neurodegeneration (31). Thus, the proposed feed-forward mechanism could be functional, resulting in a significant decrease in neuronal and hepatic Na/K-ATPase in NPC1 mice. It is important to note that the effect of cholesterol on the activity of Na/K-ATPase has been well documented (32, 33). Moreover, several reports show a decrease in Na/K-ATPase activity in diseases that are associated with abnormal cholesterol metabolism (34, 35).
FIGURE 7.
The proposed model of the Src-dependent interplay among the α1 Na/K-ATPase, caveolin-1, and cholesterol. This interplay establishes a feed-forward signaling mechanism that allows cells to regulate cholesterol distribution and Na/K-ATPase expression.
An unresolved issue in our proposed model is how cholesterol reduction activates Src kinase. Because it is well known that cholesterol reduction results in an increase in caveolin mobility and endocytosis (11, 12, 22, 36), one possibility is that cholesterol reduction attenuates the inhibitory effect of caveolin-1 on Src kinase (37). Moreover, we have demonstrated before that α1 Na/K-ATPase directly interacts and inhibits Src kinase activity (9, 38). Thus, it is conceivable that a reduction in the membrane Na/K-ATPase could further the Src activation (Fig. 7). Undoubtedly, further experiments are required to test our hypothesis and resolve this issue. Finally, it is important to mention that Mβ-CD was also found to reduce the phosphorylation of Src in breast cancer cells (39). Thus, it is possible that cholesterol reduction may have a different effect on the α1 Na/K-ATPase in these cancer cells.
Although it remains to be investigated how reduction of cell surface cholesterol induces the endocytosis and degradation of α1 Na/K-ATPase, it would be of interest to compare the α1 Na/K-ATPase with two well studied membrane proteins, SREB cleavage-activating protein (SCAP) and caveolin-1. SCAP is a cholesterol sensor embedded in the ER membrane. It is in complex with SREBP (sterol regulatory element binding protein). The interaction between the cholesterol and the ER SCAP enables the binding of Insig to the complex, which stabilizes the complex in the ER and prevents the transport of SCAP/SREBP to the Golgi apparatus (28). Similarly, the binding of plasma membrane cholesterol to the α1 Na/K-ATPase could promote the association of α1 Na/K-ATPase to another protein, resulting in the inhibition of budding and endocytosis of α1 Na/K-ATPase. On the other hand, because the Na/K-ATPase is highly enriched in caveolae, it may go through a pathway similar to that of caveolin-1. In this case, removal of cholesterol stimulates Src and other protein kinases, which increases the mobility and endocytosis of caveolin-1 (11, 22). Indeed, we found that the regulatory effect of cholesterol on α1 Na/K-ATPase required the activation of Src kinase (Figs. 4 and 5).
The NPC1 disease is characterized by accumulation of intracellular cholesterol within the late endosome/lysosomes, which is believed to be due to a defect of the cholesterol trafficking between internal membranes and the plasma membrane. The disease is marked by neuronal dysfunction and cell death in the central nervous system (23). However, it is not well understood how these two events are connected. On the other hand, Na/K-ATPase is known to be a major force in maintaining the resting membrane potential and neuron excitability. Recent studies have further demonstrated that it also regulates cell growth (13, 40) and plays a key role in neuronal cell survival/death (41). Thus, our new findings warrant a need of further investigation as to whether the down-regulation of α1 Na/K-ATPase contributes to the pathogenesis of NPC1 disease. Moreover, these studies are also relevant to many other neurodegenerative diseases (e.g. Alzheimer and Parkinson) because deregulation of cholesterol metabolism has been well documented in the central nervous system of these patients.
Peripherally, the liver represents the most affected organ in NPC1−/− mouse. Hepatic enlargement and dysfunction are well documented. Interestingly, although total hepatic cholesterol has increased several-fold in the NPC1−/− liver, cholesterol synthesis and uptake are continuously increased. This abnormality is apparently due to the decrease in cholesterol content in the plasma and ER membranes and the subsequent activation of SREBP (28, 42). Interestingly, we observed a significant decrease in cellular α1 Na/K-ATPase in the NPC1−/− liver. This decrease may be relevant to the abnormal regulation of cholesterol metabolism. We reported that knockdown of hepatic α1 Na/K-ATPase activated SREBP and consequently increased hepatic cholesterol synthesis and uptake. Moreover, it also enlarged the liver (8). These changes are similar to the phenotypic changes observed in the NPC1−/− liver. At the molecular level, it is known that most of the cellular cholesterol localizes and functions in the plasma membrane and that the ER cholesterol pool is controlled by the plasma membrane cholesterol content (29, 43). Thus, we speculate that the Na/K-ATPase/cholesterol interaction could act as a cholesterol sensor in the plasma membrane. Disruption of this interaction would impair the ability of cells to properly regulate intracellular cholesterol trafficking and, consequently, cholesterol synthesis and metabolism. On the other hand, the proper operation of the feed-forward mechanism described above would provide cells with a robust signaling mechanism to activate SREBP pathways and, thus, cholesterol synthesis.
Acknowledgments
We thank Manoranjani P. M. Tillekeratne for excellent technical support, Dr. David R. Giovannucci and Dr. Christian G. Peters for help with the imaging study, and Martha Heck for editing the manuscript.
This work was supported, in whole or in part, by National Institutes of Health Grants HL-36573 and GM 78565.
- NPC
- Niemann-Pick type C
- α1
- Na/K-ATPase α1 subunit
- Mβ-CD
- methyl β-cyclodextrin
- ER
- endoplasmic reticulum
- RFP
- red fluorescence protein
- SREBP
- sterol regulatory element binding protein.
REFERENCES
- 1. Skou J. C. (1957) Biochim. Biophys. Acta 23, 394–401 [DOI] [PubMed] [Google Scholar]
- 2. Sweadner K. J. (1989) Biochim. Biophys. Acta 988, 185–220 [DOI] [PubMed] [Google Scholar]
- 3. Lingrel J. B. (1992) J. Bioenerg. Biomembr. 24, 263–270 [DOI] [PubMed] [Google Scholar]
- 4. Kometiani P., Li J., Gnudi L., Kahn B. B., Askari A., Xie Z. (1998) J. Biol. Chem. 273, 15249–15256 [DOI] [PubMed] [Google Scholar]
- 5. Xie Z., Kometiani P., Liu J., Li J., Shapiro J. I., Askari A. (1999) J. Biol. Chem. 274, 19323–19328 [DOI] [PubMed] [Google Scholar]
- 6. Cai T., Wang H., Chen Y., Liu L., Gunning W. T., Quintas L. E., Xie Z. J. (2008) J. Cell Biol. 182, 1153–1169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang H., Haas M., Liang M., Cai T., Tian J., Li S., Xie Z. (2004) J. Biol. Chem. 279, 17250–17259 [DOI] [PubMed] [Google Scholar]
- 8. Chen Y., Cai T., Wang H., Li Z., Loreaux E., Lingrel J. B., Xie Z. (2009) J. Biol. Chem. 284, 14881–14890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tian J., Cai T., Yuan Z., Wang H., Liu L., Haas M., Maksimova E., Huang X. Y., Xie Z. J. (2006) Mol. Biol. Cell 17, 317–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Uittenbogaard A., Everson W. V., Matveev S. V., Smart E. J. (2002) J. Biol. Chem. 277, 4925–4931 [DOI] [PubMed] [Google Scholar]
- 11. Park E. K., Park M. J., Lee S. H., Li Y. C., Kim J., Lee J. S., Lee J. W., Ye S. K., Park J. W., Kim C. W., Park B. K., Kim Y. N. (2009) J. Pathol. 218, 337–349 [DOI] [PubMed] [Google Scholar]
- 12. Hayer A., Stoeber M., Ritz D., Engel S., Meyer H. H., Helenius A. (2010) J. Cell Biol. 191, 615–629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tian J., Li X., Liang M., Liu L., Xie J. X., Ye Q., Kometiani P., Tillekeratne M., Jin R., Xie Z. (2009) J. Biol. Chem. 284, 14921–14929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kilsdonk E. P., Yancey P. G., Stoudt G. W., Bangerter F. W., Johnson W. J., Phillips M. C., Rothblat G. H. (1995) J. Biol. Chem. 270, 17250–17256 [DOI] [PubMed] [Google Scholar]
- 15. Liang M., Cai T., Tian J., Qu W., Xie Z. J. (2006) J. Biol. Chem. 281, 19709–19719 [DOI] [PubMed] [Google Scholar]
- 16. Lange Y., Ye J., Rigney M., Steck T. L. (2002) J. Lipid Res. 43, 198–204 [PubMed] [Google Scholar]
- 17. Lange Y., Ye J., Steck T. L. (1998) J. Biol. Chem. 273, 18915–18922 [DOI] [PubMed] [Google Scholar]
- 18. Dada L. A., Welch L. C., Zhou G., Ben-Saadon R., Ciechanover A., Sznajder J. I. (2007) Cell. Signal. 19, 1893–1898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lecuona E., Sun H., Vohwinkel C., Ciechanover A., Sznajder J. I. (2009) Am. J. Respir. Cell Mol. Biol. 41, 671–679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhang M., Chen L., Wang S., Wang T. (2009) Biosci Rep. 29, 193–209 [DOI] [PubMed] [Google Scholar]
- 21. Liu J., Kesiry R., Periyasamy S. M., Malhotra D., Xie Z., Shapiro J. I. (2004) Kidney Int. 66, 227–241 [DOI] [PubMed] [Google Scholar]
- 22. Pelkmans L., Zerial M. (2005) Nature 436, 128–133 [DOI] [PubMed] [Google Scholar]
- 23. Sturley S. L., Patterson M. C., Balch W., Liscum L. (2004) Biochim. Biophys. Acta 1685, 83–87 [DOI] [PubMed] [Google Scholar]
- 24. Loftus S. K., Morris J. A., Carstea E. D., Gu J. Z., Cummings C., Brown A., Ellison J., Ohno K., Rosenfeld M. A., Tagle D. A., Pentchev P. G., Pavan W. J. (1997) Science 277, 232–235 [DOI] [PubMed] [Google Scholar]
- 25. Urayama O., Shutt H., Sweadner K. J. (1989) J. Biol. Chem. 264, 8271–8280 [PubMed] [Google Scholar]
- 26. Schoner W., Scheiner-Bobis G. (2007) Am. J. Physiol. Cell Physiol. 293, C509–536 [DOI] [PubMed] [Google Scholar]
- 27. Parton R. G., Hanzal-Bayer M., Hancock J. F. (2006) J. Cell Sci. 119, 787–796 [DOI] [PubMed] [Google Scholar]
- 28. Goldstein J. L., DeBose-Boyd R. A., Brown M. S. (2006) Cell 124, 35–46 [DOI] [PubMed] [Google Scholar]
- 29. Lange Y., Ye J., Rigney M., Steck T. L. (1999) J. Lipid Res. 40, 2264–2270 [PubMed] [Google Scholar]
- 30. Garver W. S., Hossain G. S., Winscott M. M., Heidenreich R. A. (1999) Biochim. Biophys. Acta 1453, 193–206 [DOI] [PubMed] [Google Scholar]
- 31. Khanna S., Roy S., Park H. A., Sen C. K. (2007) J. Biol. Chem. 282, 23482–23490 [DOI] [PubMed] [Google Scholar]
- 32. Cornelius F. (1995) Biochim. Biophys. Acta 1235, 205–212 [DOI] [PubMed] [Google Scholar]
- 33. Cornelius F. (2001) Biochemistry 40, 8842–8851 [DOI] [PubMed] [Google Scholar]
- 34. Chen M., Mason R. P., Tulenko T. N. (1995) Biochim. Biophys. Acta 1272, 101–112 [DOI] [PubMed] [Google Scholar]
- 35. De Luise M., Blackburn G. L., Flier J. S. (1980) N. Engl. J. Med. 303, 1017–1022 [DOI] [PubMed] [Google Scholar]
- 36. Hailstones D., Sleer L. S., Parton R. G., Stanley K. K. (1998) J. Lipid Res. 39, 369–379 [PubMed] [Google Scholar]
- 37. Li S., Couet J., Lisanti M. P. (1996) J. Biol. Chem. 271, 29182–29190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Li Z., Cai T., Tian J., Xie J. X., Zhao X., Liu L., Shapiro J. I., Xie Z. (2009) J. Biol. Chem. 284, 21066–21076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Raghu H., Sodadasu P. K., Malla R. R., Gondi C. S., Estes N., Rao J. S. (2010) BMC Cancer 10, 647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Desfrere L., Karlsson M., Hiyoshi H., Malmersjö S., Nanou E., Estrada M., Miyakawa A., Lagercrantz H., El Manira A., Lal M., Uhlén P. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 2212–2217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yu S. P. (2003) Biochem. Pharmacol. 66, 1601–1609 [DOI] [PubMed] [Google Scholar]
- 42. Garver W. S., Jelinek D., Oyarzo J. N., Flynn J., Zuckerman M., Krishnan K., Chung B. H., Heidenreich R. A. (2007) J. Cell. Biochem. 101, 498–516 [DOI] [PubMed] [Google Scholar]
- 43. Abi-Mosleh L., Infante R. E., Radhakrishnan A., Goldstein J. L., Brown M. S. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 19316–19321 [DOI] [PMC free article] [PubMed] [Google Scholar]