Abstract
Keratinocyte growth factor (KGF) is an epithelial mitogen that has been reported to protect the lungs from a variety of insults. In this study, we tested the hypothesis that KGF augments pulmonary host defense. We found that a single dose of intrapulmonary KGF enhanced the clearance of Escherichia coli or Pseudomonas aeruginosa instilled into the lungs 24 h later. KGF augmented the recruitment, phagocytic activity, and oxidant responses of alveolar macrophages, including lipopolysaccharide-stimulated nitric oxide release and zymosan-induced superoxide production. Less robust alveolar macrophage recruitment and activation was observed in mice treated with intraperitoneal KGF. KGF treatment was associated with increased levels of MIP1γ, LIX, VCAM, IGFBP-6, and GM-CSF in the bronchoalveolar lavage fluid. Of these, only GM-CSF recapitulated in vitro the macrophage activation phenotype seen in the KGF-treated animals. The KGF-stimulated increase in GM-CSF levels in lung tissue and alveolar lining fluid arose from the epithelium, peaked within 1 h, and was associated with STAT5 phosphorylation in alveolar macrophages, consistent with epithelium-driven paracrine activation of macrophage signaling through the KGF receptor/GM-CSF/GM-CSF receptor/JAK-STAT axis. Enhanced bacterial clearance did not occur in response to KGF administration in GM-CSF−/− mice, or in mice treated with a neutralizing antibody to GM-CSF. We conclude that KGF enhances alveolar host defense through GM-CSF-stimulated macrophage activation. KGF administration may constitute a promising therapeutic strategy to augment innate immune defenses in refractory pulmonary infections.
Keywords: Cytokines Induction, Epithelium, Growth Factors, Lung, Macrophage
Introduction
Pneumonia accounts for over one million hospital discharges each year, and for about 5% of all inpatient hospital deaths (1–3). Although antibiotics typically speed recovery in bacterial pneumonias, even the most aggressive available therapies can fail to control advanced infections. The mortality and morbidity of serious respiratory infections could be substantially impacted by the development of broad-spectrum strategies to pharmacologically augment the innate immune defenses of the lung.
Keratinocyte growth factor (KGF)2 is a potent epithelial mitogen and differentiation factor (4) that plays a central role in development and repair of injured epithelial tissues. KGF is produced exclusively by mesenchymal cells, including fibroblasts and smooth muscle cells, and acts only on epithelial cells (4), through ligation of an alternatively spliced FGF-2 tyrosine kinase receptor called FGFR2-IIIb. KGF has been reported to protect the lung from injury due to acid instillation (5), hyperoxia (6), bleomycin (7), and radiation exposure (8, 9), but less is known about KGF effects on defense against infectious challenges. The protective actions of KGF have been linked to stimulation of type II cell proliferation and differentiation, DNA repair, ion transport, and surfactant lipid and protein production (10–15).
This study was performed to determine whether KGF would enhance the clearance of Gram-negative pathogens from the lung. We found that KGF augments pulmonary host defenses to Escherichia coli and Pseudomonas aeruginosa, in part through GM-CSF-dependent macrophage recruitment and activation.
EXPERIMENTAL PROCEDURES
Mouse Models of Infection
C3H/HEN mice were used for P. aeruginosa and E. coli infection models, except in experiments to determine the role of GM-CSF in the KGF effect on host defense, in which GM-CSF−/− mice (gift of J. Whitsett) and strain matched C57BL/6 control mice were employed (16). In all cases, when KGF was administered by the intranasal route, the dose was 5 mg/kg (17), and when administered by the intraperitoneal route, the dose was 1.5 mg/kg (18, 19). After 24 h, the mice were inoculated with 1.5 × 107 E. coli K12 intranasally or 2 × 107 P. aeruginosa (PAO1). At 6 or 16 h post-infection for E. coli and P. aeruginosa, respectively, the lungs were removed, homogenized, and plated on nutrient agar. After overnight incubation, the bacterial colony forming units on the plate were counted. Mice were handled in accordance with approved protocols through the Institutional Animal Care and Use Committee at the University of Cincinnati School of Medicine. All mice were maintained under barrier containment in the vivarium facilities, and appeared healthy and free of infection at the time of the study.
KGF Pretreatment and Bronchoalveolar Lavage (BAL) Preparation
Mice were given a single dose of KGF (kindly provided by Biovitrium Corporation) via the intranasal or intraperitoneal route, and sacrificed at the indicated time points. The lungs were lavaged by five cycles of infusion and aspiration of 1 ml of sterile buffer containing 5 mm Tris (pH 7.4) and 150 mm NaCl. The cells were sedimented by spinning at 400 × g for 5 min at 4 °C and for some experiments the BAL supernatants fluids were concentrated using a 3000 MW cut off spin filter (Centricon). Routine protein concentrations were determined with a bicinchoninic acid protein assay kit (BCA; Pierce Chemical Co.) using bovine serum albumin (BSA) as a standard.
KGF Effects on STAT5 Expression by Immunoblot Analysis
To assess the effects of KGF on STAT5 expression, alveolar macrophages were isolated by BAL and placed in RIPA lysis buffer (Santa Cruz Biotechnology) containing protease inhibitors. STAT5 was immunoprecipitated using anti-STAT5 antibodies (Santa Cruz Biotechnology), and protein A/G plus-agarose (Santa Cruz Biotechnology). After separation on 4–12% SDS-PAGE gels, proteins were transferred to Hybond-C Extra membranes and reacted with anti-STAT5 antibody or anti-phospho-STAT5 antibody (Millipore). Blots were developed using SuperSignal West Femto Maximum Sensitivity Substrate (Pierce) and autoradiography.
Analysis of BAL Cytokines and Chemokines Induced by KGF
Mice were challenged with intratracheal KGF and sacrificed after 1, 6, 24, or 72 h as indicated. The BAL fluid was collected and concentrated as described above. Quantification of cytokine and chemokine levels in BAL fluid or lung homogenates was performed using an inflammatory cytokine immunoblot array (Ray Biotech) as described (20) or, in the case of GM-CSF, by specific ELISA (R & D Systems), according to the manufacturer's instructions.
Assessment of Cellular Recruitment in the Lung
Mice were pretreated with KGF or PBS as a single dose intranasally or daily dose intraperitoneally for 1, 2, or 3 days, and then sacrificed and lavaged with 5 cycles of instillation and aspiration of 1 ml of saline containing 5 mm Tris. The BAL cells were collected by centrifugation and total cells were counted. Differential counts were performed on cytospun specimens. A total of 500 cells were counted on each slide.
Nitrite Accumulation Assay
Alveolar macrophages isolated from KGF or PBS pretreated mice were plated at 2.5 × 105 cells per well in 96-well plates and incubated for 18 h in RPMI with 10% FBS. The cells were then challenged with 1 μg/ml of LPS J5 (Sigma) for 48 h. Nitric oxide (NO) production was assessed by measuring the accumulation of nitrite in the culture medium (20). Briefly, culture medium (50 μl) was mixed with an equal volume of Griess reagent, composed of 1% sulfanilamide, 0.1% naphthalene diamine dihydrochloride, and 25% hydrochloric acid, according to the manufacturer's protocol (Promega). The plate was incubated in the dark for 10 min at room temperature and read in a plate spectrophotometer at 535 nm. Sodium nitrite prepared at concentrations ranging from 1.5 to 100 μm was used to generate a standard curve.
Macrophage Chemiluminescence Assay
Circulating neutrophils were depleted in mice by pretreatment with 200 μg of intraperitoneal RB6 (antimouse-Ly-6G (GR-1), eBioscience) 1 day prior to challenge with KGF or PBS. At 24, 48, and 72 h post-challenge, the animals were euthanized and BAL cells were collected by centrifugation. Equivalent numbers of alveolar macrophages were plated (0.85 × 106) in Hanks' balanced salt solution buffer, and 1 mm luminal (Sigma, A4685) was added for 10 min at constant temperature (37 °C) (21). Zymosan (Sigma Z4250) was added to a final concentration of 0.35 mg/ml and chemiluminescence was monitored continuously for 90 min at 37 °C in a luminometer (22).
Effect of KGF on Macrophage Phagocytosis
Mice were pretreated with intranasal KGF or PBS, and then sacrificed and lavaged 24 h later. The BAL was centrifuged and 1 × 106 cells/well were placed into 6-well plates. The cells were allowed to attach for 1 h at 37 °C in fresh DMEM with 10% FBS and IgG-coated fluorescent Nile Red beads (Spherotech) (5 × 106) were added to each well (23). After incubation at 37 °C for 1 h, the cells were washed, warm DMEM was added, and incubation was continued at 37 °C for 10 min. The cells were detached with trypsin, fixed in 4% paraformaldehyde, and analyzed by flow cytometry or fluorescence microscopy. The phagocytic index was calculated according to the formula: phagocytic index = (% gated) × (geometric mean of fluorescence).
Effect of an Anti-GM-CSF Antibody on KGF-stimulated Bacterial Clearance
C57BL/6 mice were treated intraperitoneally with 100 μg of neutralizing anti-GM-CSF mAb (MP1–22E9, eBioscience) or rat IgG2a isotype control antibody 24 h prior to challenge with intranasal KGF. GM-CSF levels were quantified in lung homogenates by ELISA (R & D Systems). In parallel experiments, the animals treated with 22E9 or IgG2a antibody and KGF as above were inoculated intranasally with 2 × 107 of E. coli. After 6 h, the lungs were harvested, homogenized, and plated, and bacterial colony forming units on the plate were counted.
Isolation of Alveolar Type II Cells and Alveolar Macrophages in Lung
Mice were treated with intranasal KGF or PBS, and sacrificed 1 h later. The lungs were lavaged to obtain alveolar macrophages and alveolar type II cells were isolated by the method of Brody and co-workers (24). Briefly, after lungs were perfused with 10–20 ml of sterile 150 mm NaCl via the pulmonary artery, 3 ml of dispase (BD Biosciences) was rapidly instilled into the airway followed by 0.5 ml of 1% low-melt agarose (warmed to 45 °C). Lungs were immediately cooled with ice for 2 min, incubated in 1 ml of dispase for 45 min at room temperature, and transferred to a culture dish containing 100 units/ml of DNase I (Sigma). The parenchymal tissue was gently teased from the bronchi. Cell suspensions were filtered, collected by centrifugation, and placed on prewashed 100-mm tissue culture plates coated with CD45 and CD32 antibodies (BD Biosciences). After incubation for 90 min at 37 °C in 5% CO2, to remove macrophages and fibroblasts, the type II cells were gently panned from the plate, collected by centrifugation, and counted. The cellular content of GM-CSF for equivalent numbers of freshly isolated type II cells and alveolar macrophages (1 million cells) was assessed by ELISA of cell lysates. For in vitro experiments, type II cells were plated on Cultrex BME (Trevein, MD) and incubated in BEGM (Lonza, catalog number CC-3171) medium containing 5% charcoal-stripped FBS and antibiotics for 72 h with medium changes every 24 h. Isolated alveolar macrophages were plated on plastic tissue culture dishes and incubated with DMEM containing 10% FBS and antibiotics for 24 h. After incubation of the cells with 200 ng/ml of KGF or PBS for 24 h, GM-CSF levels were measured in the supernatants by ELISA.
Statistics
Differences between groups were analyzed by the Student's t test, p values of <0.05 were considered significant.
RESULTS
KGF Enhances the Clearance of Gram-negative Pathogens from the Lungs of Mice
The effect of KGF on the pulmonary clearance of E. coli and P. aeruginosa was tested in mice (25). Twenty-four hours after intranasal instillation of 5 mg/kg of KGF or PBS, C3H/HeN mice were inoculated intranasal with 1.5 × 107 E. coli or 2 × 107 P. aeruginosa. The lungs were harvested 6 h later for E. coli (Fig. 1A) and 16 h later for P. aeruginosa (Fig. 1B), and homogenized and plated on agar. Colony forming units were compared in lung homogenates to determine the effect of KGF on the burden of organisms in the lung. Pretreatment with intranasal KGF resulted in a 6-fold increase in the clearance of E. coli compared with PBS pretreatment at 6 h (0.2 ± 0.05 × 105 colony forming units and 1.22 ± 0.14 × 105 colony forming units, respectively, n = 5 mice per group, p < 0.01)(25). P. aeruginosa (PAO1) was cleared almost 4-fold more rapidly after pretreatment of mice with intranasal KGF compared with PBS (7.16 ± 2.31 colony forming units and 28.18 ± 3.53 × 105 colony forming units, respectively, n = 5 mice per group, p < 0.01). These data indicate that KGF accelerates the clearance of Gram-negative pathogens from the lungs of mice.
FIGURE 1.

KGF enhances bacterial clearance from lung. Wild type C3H/HEN mice were treated with a single dose of intranasal KGF or PBS. After 24 h, 1.5 × 107 E. coli (panel A) or 2 × 107 colony forming units of P. aeruginosa (PAO1) (panel B) were inoculated intranasally. The lungs were harvested after 6 or 16 h, respectively, for homogenization, plating, and colony counting. Results are mean ± S.E. of 5 mice in each group; **, p < 0.01 compared with PBS control.
KGF Treatment Results in Alveolar Macrophage Recruitment and Activation
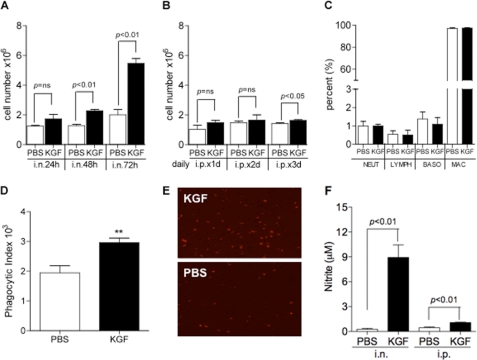
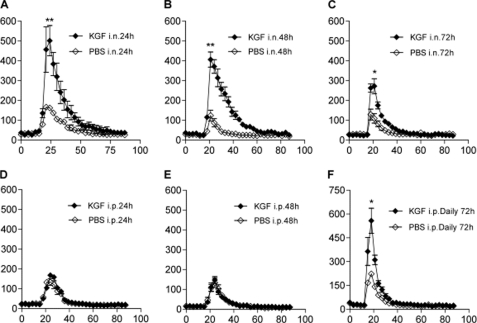
Experiments were performed to determine whether alveolar macrophages contribute to the enhanced microbial clearance induced by KGF. Pretreatment of mice with intranasal KGF increased the number of alveolar cells isolated by BAL 48 and 72 h later (Fig. 2A). Treatment with daily intraperitoneal KGF for 1 or 2 days had no effect on alveolar cell recruitment, at 1.5 (Fig. 2A) or 5 mg/kg (not shown) of intraperitoneal KGF, but there was a small but significant effect on cell numbers from daily intraperitoneal administration for 3 days (Fig. 2B). The BAL cell profile remained largely unchanged by KGF pretreatment by either route of administration, with a >95% mononuclear cell predominance (Fig. 2C). The phagocytic capacity of alveolar macrophages was tested using BAL cells that were collected 24 h after treatment with intranasal KGF or PBS, plated, and exposed to IgG-coated fluorescent beads (Fig. 2D). The phagocytic index determined by FACS was shown to be 1.58 ± 0.21-fold greater for alveolar macrophages isolated from KGF-pretreated mice than for cells from PBS-pretreated mice. A representative fluorescence image showing the differences in bead uptake between macrophages isolated from KGF versus PBS-pretreated mice is shown in Fig. 2E. For measurement of nitric oxide production, alveolar macrophages were isolated by BAL 48 h after intranasal or intraperitoneal exposure of mice to KGF or PBS, plated, and exposed to 1 μg/ml of LPS for 48 h. Nitrite accumulation due to production of nitric oxide by macrophages was measured in the medium using Greiss reagent (Fig. 2F). Intranasal KGF pretreatment enhanced the LPS-stimulated production of nitric oxide from isolated alveolar macrophages ∼34-fold (p < 0.01), compared with PBS pretreatment. There was also a significant increase in nitric oxide production after intraperitoneal KGF administration, but the magnitude of the response was considerably smaller (2.4 ± 0.1-fold, p < 0.01) (Fig. 2F) and no differences were seen at the 5 mg/kg of intraperitoneal administration (not shown). The effect of KGF on the production of superoxide in alveolar macrophages was also assessed. Mice were depleted of neutrophils by intraperitoneal injection of the anti-neutrophil antibody, RB6, and then treated with intranasal KGF 24, 48, or 72 h prior to BAL. For mice treated by the intraperitoneal route, KGF was administered 24, 48, or daily for 3 days prior to BAL. BAL cells were collected by centrifugation, and equivalent numbers of alveolar macrophages were plated. Luminal and zymosan were added sequentially and chemiluminescence was monitored continuously. The RB6 antibody reduced the abundance of neutrophils in the BAL from up to 5% of BAL cells to less than 0.1%, in both the PBS and KGF groups. Intranasal KGF produced an increase in zymosan-induced fluorescence that was almost 3-fold greater than the PBS responses at the 24- and 48-h time points, and more than 2-fold greater than the PBS response at the 72-h time point (Fig. 3, A–C). Intraperitoneal KGF did not produce an increase in superoxide production from isolated alveolar macrophages at 24 or 48 h post-injection, but when given daily for 3 days, resulted in a burst that was comparable with a 3-fold elevation in luminescence levels produced by intranasal dosing at the 24-h time point (Fig. 3, D–F).
FIGURE 2.
Pretreatment with KGF results in alveolar macrophage recruitment and activation. Mice were pretreated with intranasal (panel A) or intraperitoneal (panel B) KGF or PBS, and then sacrificed and lavaged at the indicated time points. BAL cells were collected by centrifugation and counted. Data are mean ± S.E. of 4 mice each group. Panel C, cellular composition of BAL 72 h after treatment with intranasal KGF. NEUT, neutrophil; LYmPH, lymphocyte. D, fluorescent IgG-coated beads were incubated with alveolar macrophages isolated 24 h after intranasal KGF or PBS treatment. Phagocytosis was measured by FACS quantification of fluorescence per cell and expressed as phagocytic index, calculated as: phagocytic index = (% gated) × (geometric mean fluorescence). Data are mean ± S.E. of 4 mice each group, **, p < 0.01 compared with PBS control. E, representative fluorescent photomicrograph of alveolar macrophages from the experiment outlined in D. Panel F, alveolar macrophages isolated from mice that were treated with KGF or PBS intranasally (i.n.) or intraperitoneally (i.p.) for 48 h previously as in panels A and B were plated in 96-well dishes and incubated in DMEM containing 10% FBS at 37 °C for 18 h. The cells were challenged with E. coli J5 LPS (1 μg/ml) for 48 h. Nitric oxide production was assessed by measuring the accumulation of nitrite in the culture medium by the Griess reaction using a spectrophotometer to assess absorption at 535 nm. Data are mean ± S.E., n = 3.
FIGURE 3.
Pretreatment with KGF primes alveolar macrophages for zymosan-stimulated superoxide production. Mice were depleted of neutrophils by intraperitoneal (i.p.) injection of the anti-neutrophil antibody, RB6, and then treated with KGF or PBS by the intranasal (i.n.) or intraperitoneal routes. Alveolar macrophages were isolated by BAL, centrifuged, and plated. Luminol (1 mm) and zymosan (0.35 mg/ml) were added sequentially, and chemiluminescence was monitored continuously. Panels A–C, alveolar macrophages isolated from mice pretreated with a single dose of 5 mg/kg of intranasal KGF or PBS 24, 48, or 72 h prior to BAL. Panels D–F, mice were pretreated with 1.5 mg/kg of intraperitoneal KGF or PBS for 24 or 48 h or daily for 3 days, as indicated. Data are mean ± S.E.; n = 4; *, p < 0.05, or **, p < 0.01 for KGF versus PBS control.
GM-CSF Signaling Is Induced by KGF
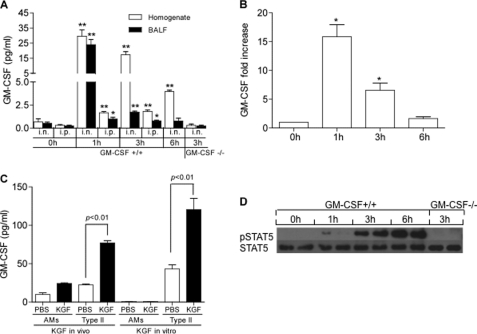
To explore the mechanisms involved in KGF-induced macrophage recruitment and activation, we surveyed the program of BAL cytokine and chemokine expression following KGF administration using multiplex immunoblot arrays (20). We found that MIP1γ, LIX, VCAM-1, and IGFBP-6 were all elevated in BAL 6 h after intratracheal KGF instillation (Fig. 4). There was, at most, an equivocal increase in GM-CSF on the cytokine array. However, because GM-CSF is known to be cleared rapidly (26), we measured GM-CSF expression at multiple time points in the lung homogenate and BAL of mice that had been treated with KGF. There was a 40-fold increase in the GM-CSF protein level in lung homogenates and BAL fluid, which peaked at 1 h (Fig. 5A). At 3 h post-intranasal KGF, GM-CSF levels fell to low levels in BAL fluid but remained elevated in lung homogenates. By 6 h post-intranasal KGF, GM-CSF levels in lung homogenates were less than th of the 1-h peak and the BAL levels had returned to baseline. Intranasal KGF induced a rapid, 16 ± 3-fold increase in whole lung GM-CSF gene expression at 1 h, which persisted at diminished levels through at least 3 h and returned to baseline by 6 h (Fig. 5B). Intraperitoneal KGF produced a similarly rapid but much less robust increase (Fig. 5B). There was no measurable increase in GM-CSF gene (not shown) or protein (Fig. 5D) expression in GM-CSF−/− mice treated with intranasal KGF. Both in vivo and in vitro experimental approaches were employed to determine the source of GM-CSF in the KGF-treated mice. GM-CSF protein levels were measured by ELISA in lysates of alveolar type II cells and alveolar macrophages isolated 1 h post-intranasal challenge. KGF, but not PBS, administration induced an increase in KGF content of isolated type II cells but not alveolar macrophages (Fig. 5C, in vivo). In addition, in the KGF-treated animals, GM-CSF protein levels were approximately three times higher in lysates of type II cells than in lysates of alveolar macrophages. To determine whether GM-CSF expression could be induced in alveolar cells in vitro, alveolar type II cells and alveolar macrophages isolated from unchallenged mice were plated on plastic or Matrigel, respectively, and treated with 200 ng/ml of KGF, in vitro. Baseline GM-CSF expression was detected in the medium of type II cells but not alveolar macrophage cultures, and was augmented ∼3-fold in alveolar type II cells by KGF treatment compared with PBS control (Fig. 5C). Thus, both in vivo and in vitro lines of evidence support the notion that the alveolar epithelium is the primary source of GM-CSF in the KGF-treated animals, and are consistent with the fact that KGF receptors are expressed only on the epithelium. There was a progressive rise in STAT5 phosphorylation in alveolar macrophages isolated from intranasal KGF-pretreated GM-CSF+/+ but not GM-CSF−/− mice, which was easily detectable at 3 h and rose through a 6 h post-challenge (Fig. 5D). Collectively, these data indicate that KGF treatment results in rapid induction of GM-CSF gene expression and release of GM-CSF from alveolar type II cells, ligation of the GM-CSF receptor on alveolar macrophages by secreted GM-CSF, and activation of the JAK/STAT signaling pathway in the phagocytes.
FIGURE 4.
KGF enhances the production of multiple proinflammatory cytokines and chemokines. Side by side inflammatory cytokine immunoblot arrays (Ray Biotech) were performed on BAL fluid isolated from wild type mice unchallenged (left panel) or challenged with intranasal KGF (right panel) 6 h prior. The membranes were developed per the manufacturer's instructions. Test cytokines appear as a vertical doublet, whereas exposure controls are horizontal quartets in the upper right corner and horizontal doublets in the lower left corner. Visual inspection reveals that KGF up-regulates the levels of LIX, VCAM-1, IGFBP-6, and MIP-1γ (representative blots from n = 2 animals). GM-CSF was equivocally elevated.
FIGURE 5.
KGF causes a rapid increase in GM-CSF protein and gene expression in alveolar type II cells and STAT phosphorylation in alveolar macrophages. Mice were given intranasal (i.n.) or intraperitoneal (i.p.) KGF or PBS. At the indicated time points, BAL was performed and lungs were harvested and homogenized. Panel A, GM-CSF levels in lung homogenate and BAL fluid (BALF) of KGF challenged GM-CSF+/+ and GM-CSF−/− mice were measured by ELISA. Data are mean ± S.E.; n = 3–4, **, p < 0.01; *, p < 0.05 for KGF versus 0 h unchallenged control. Panel B, GM-CSF gene expression was measured by real time PCR and compared with the constitutively expressed gene, β-actin. Data are mean ± S.E.; n = 3, *, p < 0.05, for KGF versus 0 h unchallenged control. Panel C, data represent GM-CSF levels in cell lysates of 1 million isolated alveolar macrophages (AMs) or type II cells (type II), harvested from mice 1 h after treatment with intranasal PBS or KGF in vivo. For in vitro experiments, 1 million alveolar macrophages or type II cells isolated from unchallenged mice and plated on plastic or Matrigel, respectively, were incubated with 200 ng/ml of KGF or PBS. After 24 h, the supernatants were harvested and GM-CSF levels were measured. Data are mean ± S.E.; n = 3. Panel D, strain-matched wild type (C57BL/6) and GM-CSF−/− mice were given with intranasal KGF or PBS. At the indicated time points, alveolar macrophages were isolated by BAL and STAT5 was immunoprecipitated from cell lysates using a rabbit polyclonal anti-STAT5 antibody. After SDS-PAGE and transfer to nitrocellulose, membranes were immunoblotted with anti-STAT5 and anti-phospho-STAT5 antibodies.
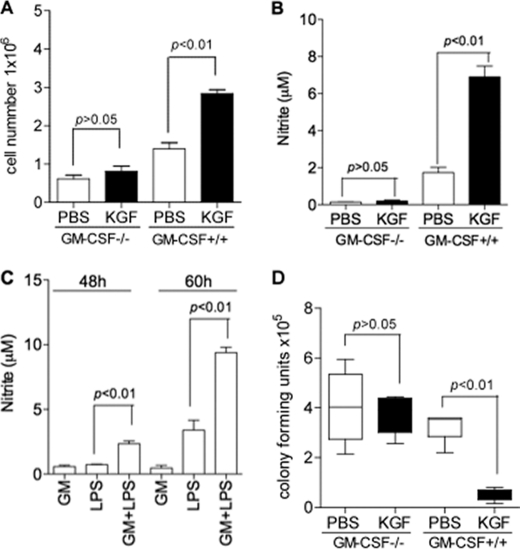
KGF-induced Microbial Clearance, Macrophage Recruitment, and Macrophage Activation Are GM-CSF Dependent
In contrast to the effects of KGF on alveolar macrophage signaling in wild type mice, there was no increase in STAT5 phosphorylation in alveolar macrophages isolated from GM-CSF−/− mice treated with KGF (Fig. 5D). The absence of GM-CSF in these animals also had a marked effect on induction of macrophage recruitment by KGF; intranasal KGF did not increase the number of alveolar macrophages isolated by BAL from GM-CSF−/− mice (Fig. 6A) as it did in strain-matched GM-CSF+/+ mice. Similarly, there was no increase in the LPS-stimulated production of nitric oxide from alveolar macrophages isolated from KGF-pretreated GM-CSF−/− mice (Fig. 6B) as was the case with macrophages from GM-CSF+/+ animals. In vitro exposure of alveolar macrophages isolated from wild type mice to the other soluble cytokines and chemokines that were detected in the BAL of KGF pretreated animals, including MIP1γ, LIX, IGFBP-6, did not enhance LPS-stimulated nitric oxide release, either individually or in combination (data not shown). However, the addition of GM-CSF to cultures of wild type alveolar macrophages primed the phagocytes for LPS-stimulated nitric oxide release, detected after 48 and 60 h in culture (Fig. 6C). In contrast to its effects in GM-CSF+/+ mice, intranasal KGF did not augment the clearance of E. coli from the lungs of GM-CSF−/− mice (Fig. 6D), but intranasal KGF produced a 6.1 ± 0.9-fold increase in bacterial clearance from strain-matched control mice. Finally, a series of experiments was performed to determine whether KGF-induced early clearance of bacteria is mediated by GM-CSF. The experiments outlined in Fig. 1A were repeated with bacterial challenge occurring 1 h after KGF was delivered (Fig. 7). E. coli clearance was enhanced more than 2-fold under these conditions (Fig. 7A). Mice were then treated with a neutralizing antibody to GM-CSF or an isotype control antibody, followed in 24 h by intranasal KGF. At 3 h post-KGF, the level of GM-CSF in lung homogenates of the anti-GM-CSF-treated animals was reduced more than 5-fold compared with animals treated with the control antibody (Fig. 7B). Finally, treatment with the anti-GM-CSF antibody blocked KGF-enhanced E. coli clearance (Fig. 7C). Collectively, these data indicate that GM-CSF mediates the enhanced alveolar macrophage recruitment and activation and augmented bacterial clearance that occurs after KGF administration.
FIGURE 6.
KGF-induced alveolar macrophage recruitment and activation is GM-CSF dependent. Strain matched C57BL/6 wild type or GM-CSF−/− mice were treated with intranasal KGF or PBS, and alveolar macrophages were isolated. Panel A, BAL cells were counted as described in the legend to Fig. 2, A and B. Panel B, nitric oxide release was quantified as outlined in the legend to Fig. 2F. Panel C, in vitro priming of alveolar macrophages for LPS-stimulated NO release with GM-CSF. Alveolar macrophages were isolated from unchallenged C57BL/6 mice and incubated in 96-well dishes for 18 h. The cells were incubated with 0.05 μg/ml of GM-CSF for 2 h and then challenged with E. coli J5 LPS (1 μg/ml) for 48 h. NO production was assessed by measuring the accumulation of nitrite in the culture medium by the Griess reaction using a spectrophotometer (λ = 535 nm). Data are n = 6, **, p < 0.01 for LPS versus GM-CSF + LPS. Panel D, effect of intranasal KGF (5 mg/kg) on bacterial clearance in GM-CSF−/− and C57BL/6 controls mice. The experiment was conducted as outlined in the legend to Fig. 1. Results are mean ± S.E., n = 4 mice each group compared with PBS control.
FIGURE 7.
Early KGF induced bacterial clearance is GM-CSF dependent. Panel A, E. coli (2 × 107 colony forming units) were inoculated into wild type C57BL/6 mice 1 h after a single dose of intranasal KGF (5 mg/kg). Lungs were harvested after 6 h for homogenization, plating, and colony counting. Results are mean ± S.E. of five mice in each group. Panel B, mice were pretreated intraperitoneal (100 μg/mouse) with either anti-GM-CSF mAb 22E9 or IgG2a isotype control 24 h prior to KGF treatment. GM-CSF levels in the lung homogenate were measured by ELISA. Data are mean ± S.E.; n = 4. Panel C, C57BL/6 mice were pretreated intraperitoneal (100 μg/mouse) with either anti-GM-CSF mAb 22E9 or IgG2 isotype control antibody 24 h prior to intranasal KGF administration. One hour later, the mice were challenged intranasally with 2 × 107 colony forming units of E. coli. After 6 h, lungs were harvested, homogenized, and plated for bacterial colony counting. Results are mean ± S.E., n = 5 mice in each group.
DISCUSSION
KGF is an epithelial mitogen and differentiation factor that is known to protect the lung from a variety of insults. There have been only a few reports of potential effects of KGF on pulmonary host defense. We found that KGF indeed enhances the rate of bacterial clearance from the lung through a novel mechanism that is at least partially GM-CSF dependent.
Nature often borrows elements of normal developmental processes to execute repair and host defense functions in the mature organism (27). Examples include the key roles of NFκB pathway proteins both in Drosophila morphogenesis and in inflammation in mammalian organisms. The importance of KGF in lung development is graphically demonstrated by the absence of lungs in mice that overexpress a dominant-negative form of KGF receptor, FGFR2-IIIb, in the pulmonary epithelium (28). Using a similar strategy with a skin-specific promoter, KGF signaling has also been shown to be critical for wound repair in that organ (29). The documented efficacy of KGF in reducing the severity of mucositis following radiation and chemotherapy illustrates the protective potential of KGF for epithelial and mucosal tissues (30). Although the mechanism of KGF action in the amelioration of mucositis is thought to be preservation of epithelial barrier integrity, infection is also central to the pathogenesis of the disease, and we wondered if the antimicrobial actions of KGF might have also contributed to the favorable outcomes in those studies. Reports that KGF is up-regulated in gingival fibroblasts by LPS and inflammatory cytokines (IL1α, IL1β, IL6, TNFα, TGFα, and PDGF-BB) (31–33), and that KGF stimulates the production of antimicrobial peptides and enhances bactericidal activity of skin grafts by more than 500-fold (34) are consistent with a role for the growth factor in the augmentation of innate immune defenses. Viget et al. (17) reported that a single intratracheal instillation of KGF (5 mg/kg) in rats 48 h prior to infection with P. aeruginosa resulted in improved barrier function in vivo and in isolated perfused lungs, increased macrophage and infection-induced neutrophil recruitment, and enhanced bacterial clearance. The mechanism of these effects was not resolved in that study, however.
In the current study, KGF enhanced the clearance of two Gram-negative pathogens from the lungs of mice ∼4–6-fold within 1 or 24 h of administration of the growth factor. To determine the mechanism of augmented pulmonary defense, we assessed the effect of KGF on the antimicrobial functions of alveolar macrophages. The observation that a GM-CSF-dependent increase in bacterial clearance occurs within 1 h of KGF administration directly implicates macrophage activation rather than increases in macrophage recruitment, collectins, or neutrophils in the KGF effect. Treatment of mice with KGF by the respiratory route more than doubled the number of alveolar macrophages in the airspace over a 3-day period, but there were no significant changes in BAL inflammatory cell counts or composition at earlier time points (Fig. 2A), so it is unlikely that recruitment of circulating monocytes explains the early phenotypic changes in the alveolar macrophages associated with enhanced bacterial clearance at 24 h. KGF clearly increased the phagocytic and oxidative tone of alveolar macrophages within this time frame, however. Much less robust effects on inflammatory cell activation were observed with parenteral (intraperitoneal) administration of KGF; but daily dosing of KGF for 3 days produced the same level of superoxide release that was induced by a single dose of intranasal KGF. The KGF-induced macrophage actions were preceded by an increase in the secretion of a number of cytokines and chemokines into the airspace, including GM-CSF. In vitro experiments were performed in which each of these factors, individually and in combination, were tested for the ability to recapitulate the activation phenotype of macrophages isolated from KGF-pretreated animals. Of these, only GM-CSF primed macrophages for nitric oxide production upon exposure to LPS. On this basis, we postulated that GM-CSF was the principal mediator of the macrophage-activating functions induced by KGF.
GM-CSF is a hematopoetic growth factor that is known to be important for pulmonary homeostasis (35). GM-CSF promotes the terminal differentiation and survival of myeloid cells and is expressed by a variety of cells in the lung including activated T cells, macrophages, fibroblasts, and pulmonary epithelial cells (36). GM-CSF plays a central role in surfactant homeostasis and in the terminal differentiation of alveolar macrophages through binding to the GM-CSF receptor on the phagocyte, activation of JAK-STAT signaling, and induction of the transcription factor PU.1 (37). The absence of GM-CSF in gene-targeted mice results in susceptibility to infection with bacterial and fungal organisms, due primarily to deficits in alveolar macrophage phagocytosis, H2O2 production, and killing (36–38). GM-CSF−/− mice also exhibit surfactant accumulation, and increased bacterial dissemination, defective inflammatory mediator responses, and reduced survival despite preserved neutrophil recruitment and function after intratracheal challenge with P. aeruginosa (39).
In our study, the primary source of GM-CSF following KGF induction was the alveolar epithelium, based on the detection of GM-CSF in isolated type II cells but not isolated alveolar macrophages of KGF-pretreated animals and in cultures of KGF challenged alveolar cells isolated from animals that had not been pretreated. GM-CSF levels in BAL and lung homogenate peaked within 1 h of intrapulmonary treatment with KGF. The accelerated kinetics of secretion of GM-CSF is atypical for mechanisms requiring protein synthesis, and suggests the possibility of release from mobilizable, intracellular or cell surface bound stores, as has been described for other growth factors (40). The GM-CSF spike was followed by a trailing, sustained increase in STAT5 phosphorylation in alveolar macrophages. The KGF-induced augmentation of bacterial clearance, and alveolar macrophage STAT5 phosphorylation, recruitment, and nitric oxide response to LPS were all absent in GM-CSF-deficient mice. Although we used young mice (5 weeks) to minimize the effect that accumulation of surfactant may have had on the macrophage phenotype in these animals (16), we cannot exclude the possibility that collateral effects of GM-CSF deficiency may have influenced GM-CSF-dependent macrophage functions. Increased levels of surfactant proteins in these animals may also effect bacterial clearance through direct antimicrobial (25) and phagocyte-dependent mechanisms (41), but if anything would have biased the result in the direction opposite to what was observed.
Collectively, these data are consistent with a model in which KGF stimulates pulmonary innate immunity. KGF enhances the recruitment, oxidant, and killing functions of alveolar macrophages, and accelerates the clearance of Gram-negative pathogens through GM-CSF-dependent mechanisms. We submit that pharmacologic augmentation of macrophage activation through a KGF/FGFR2-IIIb/GM-CSF/GM-CSFR paracrine loop might be exploited to advantage, to enhance host defense functions in the airspace. These findings may have implications for the treatment of patients with pneumonias for which no effective treatment exists (e.g. viral pneumonias) or which fail to respond adequately to available treatments.
Acknowledgment
We appreciate the gift of KGF from Biovitrium, Inc.
This work was supported, in whole or in part, by National Institutes of Health Grant P01AI083222 from the NIAID (to F. X. M.) and a Veterans Affairs Merit grant from the Department of Veteran Affairs (to F. X. M.).
- KGF
- keratinocyte growth factor
- BAL
- bronchoalveolar lavage
- STAT5
- signal transducer and activator of transcription 5
- GM-CSF
- granulocyte-macrophage colony stimulating factor.
REFERENCES
- 1. DeFrancis C.J., Hall M. J. (2005) National Hospital Discharge Survey 2004, www.cdc.gov/nchs/data/ad/ad385 [PubMed] [Google Scholar]
- 2. Anderson R. N., Smith B. L. (2005) Natl. Vital Stat. Rep. 53, 1–89 [PubMed] [Google Scholar]
- 3. Mandell L. A., Wunderink R. G., Anzueto A., Bartlett J. G., Campbell G. D., Dean N. C., Dowell S. F., File T. M., Jr., Musher D. M., Niederman M. S., Torres A., Whitney C. G. (2007) Clin. Infect. Dis. 44, Suppl. 2, S27–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rubin J. S., Osada H., Finch P. W., Taylor W. G., Rudikoff S., Aaronson S. A. (1989) Proc. Natl. Acad. Sci. U.S.A. 86, 802–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yano T., Deterding R. R., Simonet W. S., Shannon J. M., Mason R. J. (1996) Am. J. Respir. Cell Mol. Biol. 15, 433–442 [DOI] [PubMed] [Google Scholar]
- 6. Panos R. J., Bak P. M., Simonet W. S., Rubin J. S., Smith L. J. (1995) J. Clin. Invest. 96, 2026–2033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Deterding R. R., Havill A. M., Yano T., Middleton S. C., Jacoby C. R., Shannon J. M., Simonet W. S., Mason R. J. (1997) Proc. Assoc. Am. Physicians 109, 254–268 [PubMed] [Google Scholar]
- 8. Yi E. S., Williams S. T., Lee H., Malicki D. M., Chin E. M., Yin S., Tarpley J., Ulich T. R. (1996) Am. J. Pathol. 149, 1963–1970 [PMC free article] [PubMed] [Google Scholar]
- 9. Takeoka M., Ward W. F., Pollack H., Kamp D. W., Panos R. J. (1997) Am. J. Physiol. 272, L1174–1180 [DOI] [PubMed] [Google Scholar]
- 10. Chang Y., Edeen K., Lu X., De Leon M., Mason R. J. (2006) Am. J. Respir. Cell Mol. Biol. 35, 268–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chang Y., Wang J., Lu X., Thewke D. P., Mason R. J. (2005) J. Lipid Res. 46, 2624–2635 [DOI] [PubMed] [Google Scholar]
- 12. Mason R. J., Pan T., Edeen K. E., Nielsen L. D., Zhang F., Longphre M., Eckart M. R., Neben S. (2003) J. Clin. Invest. 112, 244–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mason R. J., Gao B., Pan T., Jiang X., Eckart M., Neben S. (2002) Chest 121, 77S. [DOI] [PubMed] [Google Scholar]
- 14. Fehrenbach H., Kasper M., Koslowski R., Pan T., Schuh D., Müller M., Mason R. J. (2000) Histochem. Cell Biol. 114, 49–61 [DOI] [PubMed] [Google Scholar]
- 15. Charafeddine L., D'Angio C. T., Richards J. L., Stripp B. R., Finkelstein J. N., Orlowski C. C., LoMonaco M. B., Paxhia A., Ryan R. M. (1999) Am. J. Physiol. 276, L105–L113 [DOI] [PubMed] [Google Scholar]
- 16. Dranoff G., Crawford A. D., Sadelain M., Ream B., Rashid A., Bronson R. T., Dickersin G. R., Bachurski C. J., Mark E. L., Whitsett J. A., Mulligan R. C. (1994) Science 264, 713–716 [DOI] [PubMed] [Google Scholar]
- 17. Viget N. B., Guery B. P., Ader F., Nevière R., Alfandari S., Creuzy C., Roussel-Delvallez M., Foucher C., Mason C. M., Beaucaire G., Pittet J. F. (2000) Am. J. Physiol. Lung Cell. Mol. Physiol. 279, L1199–1209 [DOI] [PubMed] [Google Scholar]
- 18. Miceli R., Hubert M., Santiago G., Yao D. L., Coleman T. A., Huddleston K. A., Connolly K. (1999) J. Pharmacol. Exp. Ther. 290, 464–471 [PubMed] [Google Scholar]
- 19. Franco-Montoya M. L., Bourbon J. R., Durrmeyer X., Lorotte S., Jarreau P. H., Delacourt C. (2009) Am. J. Physiol. Lung Cell. Mol. Physiol. 297, L965–976 [DOI] [PubMed] [Google Scholar]
- 20. Young L. R., Borchers M. T., Allen H. L., Gibbons R. S., McCormack F. X. (2006) J. Immunol. 176, 4361–4368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Saeed F. A., Castle G. E. (1998) Clin. Diagn. Lab. Immunol. 5, 740–743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kuzmenko A. I., Wu H., Wan S., McCormack F. X. (2005) J. Biol. Chem. 280, 25913–25919 [DOI] [PubMed] [Google Scholar]
- 23. Berclaz P. Y., Shibata Y., Whitsett J. A., Trapnell B. C. (2002) Blood 100, 4193–4200 [DOI] [PubMed] [Google Scholar]
- 24. Corti M., Brody A. R., Harrison J. H. (1996) Am. J. Respir. Cell Mol. Biol. 14, 309–315 [DOI] [PubMed] [Google Scholar]
- 25. Wu H., Kuzmenko A., Wan S., Schaffer L., Weiss A., Fisher J. H., Kim K. S., McCormack F. X. (2003) J. Clin. Invest. 111, 1589–1602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Metcalf D., Nicola N. A., Mifsud S., Di Rago L. (1999) Blood 93, 1579–1585 [PubMed] [Google Scholar]
- 27. Whitsett J. A. (2002) J. Clin. Invest. 109, 565–569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Peters K., Werner S., Liao X., Wert S., Whitsett J., Williams L. (1994) EMBO J. 13, 3296–3301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Li C., Guo H., Xu X., Weinberg W., Deng C. X. (2001) Dev. Dyn. 222, 471–483 [DOI] [PubMed] [Google Scholar]
- 30. Spielberger R., Stiff P., Bensinger W., Gentile T., Weisdorf D., Kewalramani T., Shea T., Yanovich S., Hansen K., Noga S., McCarty J., LeMaistre C. F., Sung E. C., Blazar B. R., Elhardt D., Chen M. G., Emmanouilides C. (2004) N. Engl. J. Med. 351, 2590–2598 [DOI] [PubMed] [Google Scholar]
- 31. Chedid M., Rubin J. S., Csaky K. G., Aaronson S. A. (1994) J. Biol. Chem. 269, 10753–10757 [PubMed] [Google Scholar]
- 32. Brauchle M., Angermeyer K., Hübner G., Werner S. (1994) Oncogene 9, 3199–3204 [PubMed] [Google Scholar]
- 33. Sanale A. R., Firth J. D., Uitto V. J., Putnins E. E. (2002) J. Periodontal. Res. 37, 66–74 [PubMed] [Google Scholar]
- 34. Erdag G., Medalie D. A., Rakhorst H., Krueger G. G., Morgan J. R. (2004) Mol. Ther. 10, 76–85 [DOI] [PubMed] [Google Scholar]
- 35. Stanley E., Lieschke G. J., Grail D., Metcalf D., Hodgson G., Gall J. A., Maher D. W., Cebon J., Sinickas V., Dunn A. R. (1994) Proc. Natl. Acad. Sci. U.S.A. 91, 5592–5596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Paine R., 3rd, Preston A. M., Wilcoxen S., Jin H., Siu B. B., Morris S. B., Reed J. A., Ross G., Whitsett J. A., Beck J. M. (2000) J. Immunol. 164, 2602–2609 [DOI] [PubMed] [Google Scholar]
- 37. Trapnell B. C., Whitsett J. A. (2002) Annu. Rev. Physiol. 64, 775–802 [DOI] [PubMed] [Google Scholar]
- 38. LeVine A. M., Reed J. A., Kurak K. E., Cianciolo E., Whitsett J. A. (1999) J. Clin. Invest. 103, 563–569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ballinger M. N., Paine R., 3rd, Serezani C. H., Aronoff D. M., Choi E. S., Standiford T. J., Toews G. B., Moore B. B. (2006) Am. J. Respir. Cell Mol. Biol. 34, 766–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Grenier A., Chollet-Martin S., Crestani B., Delarche C., El Benna J., Boutten A., Andrieu V., Durand G., Gougerot-Pocidalo M. A., Aubier M., Dehoux M. (2002) Blood 99, 2997–3004 [DOI] [PubMed] [Google Scholar]
- 41. Manz-Keinke H., Plattner H., Schlepper-Schäfer J. (1992) Eur. J. Cell Biol. 57, 95–100 [PubMed] [Google Scholar]