Abstract
Introduction
Receptor tyrosine kinases (RTKs) are validated targets for oncology drug discovery and several RTK antagonists have been approved for the treatment of human malignancies. Nonetheless, the discovery and development of RTK antagonists has lagged behind the discovery and development of agents that target G-protein coupled receptors. In part, this is because it has been difficult to discover analogs of naturally-occurring RTK agonists that function as antagonists.
Areas covered
Here we describe ligands of ErbB receptors that function as partial agonists for these receptors, thereby enabling these ligands to antagonize the activity of full agonists for these receptors. We provide insights into the mechanisms by which these ligands function as antagonists. We discuss how information concerning these mechanisms can be translated into screens for novel small molecule- and antibody-based antagonists of ErbB receptors and how such antagonists hold great potential as targeted cancer chemotherapeutics.
Expert opinion
While there have been a number of important key findings into this field, the identification of the structural basis of ligand functional specificity is still of the greatest importance. While it is true that, with some notable exceptions, peptide hormones and growth factors have not proven to be good platforms for oncology drug discovery; addressing the fundamental issues of antagonistic partial agonists for receptor tyrosine kinases has the potential to steer oncology drug discovery in new directions. Mechanism based approaches are now emerging to enable the discovery of RTK partial agonists that may antagonize both agonist-dependent and –independent RTK signaling and may hold tremendous promise as targeted cancer chemotherapeutics.
Keywords: ErbB/EGF receptors, Ligand-based antagonists, Receptor partial agonists and antagonists, Receptor tyrosine kinases, Targeted cancer chemotherapeutics
1. Introduction
1.1 Introduction to receptor tyrosine kinases and their functional probes
Deregulated signaling by receptor tyrosine kinases (RTKs) plays critical roles in numerous disease states, particularly various human malignancies. Indeed, numerous RTKs are validated targets for oncology drug discovery. Paradigms exist for targeting these proteins, including small molecule tyrosine kinase inhibitors, antagonistic antibodies, and ligand sinks. Nonetheless, the discovery and development of functional probes and lead antagonists of these receptors have occurred at a pace dwarfed by the pace at which molecules that target many G-protein coupled receptors (GPCRs) have been discovered and developed. In part, this disparity reflects the relatively ease in discovering analogs of endogenous GPCR ligands that possess antagonistic activity. However, here we will discuss the discovery of analogs of endogenous RTK ligands that possess partial agonist activity and herald the possibility of clinically-relevant ligand-based RTK antagonists.
1.2. Principles of ligand-induced receptor tyrosine kinase signaling
RTKs are single-pass transmembrane proteins that possess an extracellular ligand-binding motif, an intracellular tyrosine kinase domain, and intracellular tyrosine residues whose phosphorylation creates docking sites for the intracellular signaling effectors that mediate receptor coupling to biological responses. The fundamental effect of agonist binding to RTKs is RTK tyrosine phosphorylation as a consequence of agonist-induced RTK dimerization. There are multiple mechanistic paradigms for agonist-induced RTK dimerization1. (1) A multivalent, monomeric agonist bridges two RTK monomers. (2) A dimer of agonists (frequently monovalent), bridges two RTK monomers. (3) Two monomeric agonists each induce or stabilize a conformational change in an RTK monomer that enables RTK dimerization. A general paradigm for the mechanism by which RTK dimerization promotes RTK tyrosine phosphorylation has yet to be established. It has been postulated that agonist-induced dimerization of the Epidermal Growth Factor (EGF) Receptor extracellular domains induces asymmetric dimerization of the EGF receptor (EGFR) intracellular domains. This causes the tyrosine kinase domain of one of the EGFR monomers to adopt an active conformation and juxtaposes the cytoplasmic tyrosine residues of the other EGFR monomer with that active tyrosine kinase domain. Thus, at least in the case of EGFR and related ErbB family RTKs, agonist binding may not induce autophosphorylation per se; rather, agonist binding causes tyrosine phosphorylation in trans across the receptor dimer2-5. It should be noted that some data indicate that tyrosine phosphorylation is due to autophosphorylation, in a manner somewhat reminiscent of Src family kinase autophosphorylation6-7.
1.3. Common strategies for antagonizing ligand-induced receptor tyrosine kinase signaling
Small molecules and antibodies that target and antagonize RTK signaling have entered clinical practice. Emerging paradigms for targeting RTK signaling include RTK fragments and agonist fragments and analogs. Here we will briefly review these paradigms and highlight the challenges associated with their development into clinical agents.
1.3.1. Small molecule tyrosine kinase inhibitors
Small molecule tyrosine kinase inhibitors (TKIs) target the ATP binding pocket of RTKs. TKIs antagonize RTK coupling to biological responses by inhibiting RTK tyrosine kinase activity and phosphorylation-dependent RTK coupling to signaling effectors. The discovery and development of RTK TKIs has been spurred in part by the success of the Abl/c-Kit TKI imatinib (Gleevec® - Novartis) in treating Philadelphia chromosome-positive Chronic Myelogenous Leukemia and c-Kit-positive Gastrointestinal Stromal Tumors8-15. However, this advance has not translated into widespread successful targeting of RTKs with TKIs, in part due to the frequency of RTK kinase domain mutations that abrogate TKI activity. For example, the EGFR TKIs gefitinib (Iressa™ - Astra-Zeneca) and erlotinib (Tarceva® – Genentech) are effective against only the small fraction of non-small cell lung carcinomas that harbor kinase domain mutations that render the tumor cells dependent on EGFR. Moreover, this efficacy is frequently abrogated by a second site mutation that reduces TKI affinity for the EGFR kinase domain16, 17.
1.3.2. Antibodies
There are numerous therapeutic monoclonal antibodies that target extracellular epitopes of cell surface proteins whose expression is associated with a pathologic state. In some cases these antibodies appear to function primarily by eliciting an immune response specific for the cells that express the targeted cell surface antigen. For example, the monoclonal antibody rituximab (Rituxan® – Genentech) is effective against many B-cell lymphomas by targeting the CD20 antigen, which is overexpressed by these tumor cells18-23. A thorough discussion of this class of agents lies outside the scope of this review.
In addition, there are several antibodies that elicit their therapeutic effects by disrupting RTK signaling. These antibodies can be grouped according to their mechanism of action. These groups include ligand sinks, inhibitors of ligand binding, inhibitors of receptor dimerization, and agents with other mechanisms of action.
1.3.2.1. Ligand sinks
Ligand sinks antagonize RTK signaling by binding the RTK agonist and preventing the agonist from binding to the RTK and stimulating its signaling. One example is the monoclonal antibody bevacizumab (Avastin® – Genentech), which binds to vascular endothelial growth factor (VEGF). This prevents VEGF from binding to the VEGF receptor and prevents VEGF stimulation of VEGF receptor signaling. Bevacizumab is approved as part of combination therapies for the treatment of NCSLC, as well as metastatic breast, kidney, and colorectal cancers24-31.
1.3.2.2. Inhibitors of ligand binding
Other monoclonal antibodies bind to an RTK and prevent agonist binding to the RTK and agonist stimulation of RTK signaling. Theoretically, two mechanisms of action are possible. Monoclonal antibodies could directly compete with agonists for binding to a common or overlapping binding site on the RTK. Cetuximab (Erbitux® - Bristol-Myers Squibb) is an example of this class of agents; it competes with EGF and other EGFR agonists for binding to EGFR, thereby inhibiting agonist-induced EGFR signaling32, 33. Monoclonal antibodies could theoretically inhibit agonist-induced RTK signaling by inducing the RTK to adopt a conformation with lower affinity for agonist (allosteric inhibition). However, the challenges associated with generating such agents may be part of the reason why this mechanistic paradigm has yet to be widely exploited.
1.3.2.3. Inhibitors of receptor dimerization
Pertuzumab (fka Omnitarg) is an antibody specific for ErbB2 (HER2/Neu) RTK that inhibits ErbB2 heterodimerization with other ErbB family receptors, including EGFR and ErbB3 (HER3)34, 35. Because ErbB2 lacks a specific soluble agonist, agonist binding to an ErbB receptor other than ErbB2 and consequent heterodimerization and cross-talk with ErbB2 is a common mechanism by which ErbB2 signaling can be regulated. Clinical trials (MARIANNE - NCT01120184 and CLEOPATRA - NCT00567190) that assess the efficacy of pertuzumab in combination chemotherapy regimens for the treatment of ErbB2-positive breast tumors are ongoing.
1.3.2.4. Other mechanisms of action
Trastuzumab (Herceptin®) is perhaps the most famous therapeutic antibody used in oncology. This antibody is specific for ErbB2 and is used to target breast tumors that overexpress ErbB2. A number of mechanisms, including antibody-dependent cellular cytotoxicity, may account for the antitumor activities of trastuzumab36-39. However, 4D5, the mouse monoclonal antibody from which trastuzumab is derived, stimulates ErbB2 tyrosine phosphorylation and internalization40. This mechanism may also account for some of the antitumor activities displayed by trastuzumab.
1.3.3. Other agents
RTK fragments that include the agonist-binding domain(s) may serve as decoy receptors for agonists (agonist sinks). For example, a recombinant soluble protein containing the extracellular subdomains I-III of ErbB4 antagonizes agonist-induced signaling by ErbB441. Proteins that are not derived from RTKs may also function as agonist sinks. Perhaps the best know is the drosophila Argos protein, which binds to the drosophila EGF homolog Spitz and antagonizes stimulation of drosophila EGFR (DER) signaling by preventing Spitz binding to DER42, 43. Finally, a fragment of an RTK agonist that retains the site of binding to the RTK may competitively antagonize agonist-induced signaling by that RTK. For example, a fragment corresponding to residues 33-42 of murine EGF inhibits EGF stimulation of endothelial cell motility and EGF stimulation of chicken egg angiogenesis44. Nonetheless, these three mechanisms have not been common approaches for developing therapeutic RTK antagonists.
2. Some ligands for ErbB receptor tyrosine kinases function as partial agonists
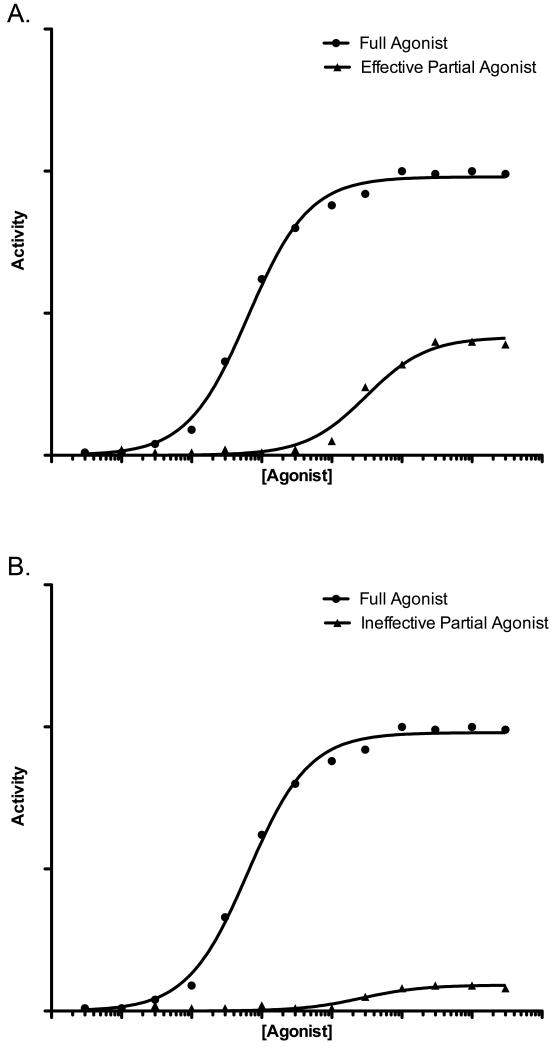
The ErbB receptor signaling network is quite complex and features multiple naturally-occuring peptide growth factor ligands for EGFR, ErbB3, and ErbB4. An emergent attribute of this signaling network is that different EGF family ligands for the same ErbB family receptor are functionally distinct, with some of these ligands functioning as partial agonists and others functioning as full agonists at the same receptor45. For example, a saturating concentration of the EGFR ligand amphiregulin (AREG) stimulates greater EGFR coupling to cell proliferation, motility, and metastatic activities than does a saturating concentration of EGF, another EGFR ligand. Thus, with respect to these outputs, AREG is a full agonist for EGFR whereas EGF is a partial agonist (Figure 1a)45-47.
Figure 1. A saturating concentration of a theoretical full agonist elicits a greater response than a saturating concentration of a theoretical partial agonist.
(A) A theoretical full agonist versus a relatively effective theoretical partial agonist. The activity of these ligands resembles the activity of AREG (full agonist) and EGF (partial agonist). (B) A theoretical full agonist versus a relatively ineffective theoretical partial agonist. The activity of these ligands resembles the activity of wild-type NRG2β (full agonist) and NRG2β/Q43L (partial agonist).
Published and unpublished data indicate that the functional difference appears to be due to the fact that EGF stimulates greater EGFR tyrosine phosphorylation at EGFR Tyr1045 than does AREG, resulting in greater EGFR coupling to the ubiquitin ligase c-Cbl and greater EGFR ubiquitination and more rapid EGFR turnover. Thus, AREG stimulates EGFR signaling of greater duration than does EGF. This appears to permit greater EGFR coupling to phospholipase C gamma (PLCγ) through phosphorylation of EGFR Tyr99245-47. It has been postulated this specificity in ligand-induced EGFR signaling is due to differences in the geometry of the EGFR dimer, resulting in differences in the presentation of tyrosine residues of one monomer to the catalytic domain of the other monomer and differences in the sites of EGFR tyrosine phosphorylation45, 48.
More compelling results have been obtained through analyses of ErbB4 ligands. The ErbB4 ligand Neuregulin 2beta (NRG2β) potently stimulates ErbB4 coupling to cell proliferation, whereas the ErbB4 ligand Neuregulin 2alpha (NRG2α) does not. Dose-response experiments suggest that the failure of NRG2α to stimulate ErbB4 coupling to cell proliferation is not due inadequate affinity of NRG2α for ErbB4. Moreover, the NRG2β/F45K mutant stimulates ErbB4 coupling to cell proliferation, but the NRG2α/K45F mutant still does not. This is despite the fact that the NRG2α/K45F mutant and the NRG2β/F45K mutant have roughly equivalent affinity for ErbB4 and despite the fact that the two ligands stimulate ErbB4 tyrosine phosphorylation with roughly equivalent potency49-51.
A genetic approach can resolve the functional distinction between NRG2α and NRG2β. The NRG2β/Q43L mutant fails to stimulate ErbB4 coupling to cell proliferation, despite the fact that the Q43L mutation does not markedly reduce the affinity of NRG2β for ErbB4 and does not markedly reduce the potency of NRG2β stimulation of ErbB4 tyrosine phosphorylation. Moreover, the corresponding NRG2α/L43Q mutant stimulates ErbB4 coupling to cell proliferation, despite the fact that the L43Q mutation does not markedly increase the affinity of NRG2α for ErbB4 and does not markedly increase the potency of NRG2α stimulation of ErbB4 tyrosine phosphorylation52. Thus, with respect to ErbB4 coupling to cell proliferation, wild-type NRG2α and the NRG2β/Q43L mutant function as very weak partial agonists at ErbB4 whereas wild-type NRG2β and the NRG2α/L43Q mutant function as full agonists (Figure 1b).
Unpublished data indicate that NRG2α stimulates much less ErbB4 coupling to Akt (Protein Kinase B) phosphorylation than does NRG2β. Furthermore the signaling pathway comprised in part by phosphoinositide 3-kinase (PI3K) and Akt is required for NRG2β stimulation of ErbB4 coupling to cell proliferation. Given the paradigm for ligand-specific EGFR signaling, it seems reasonable to postulate that ErbB4 full agonists stimulate greater ErbB4 tyrosine phosphorylation on a site that is coupled to the PI3K/Akt pathway than do ErbB4 partial agonists. Another possibility is that ErbB4 partial agonists stimulate greater ErbB4 tyrosine phsophorylation on a site that is coupled to ErbB4 ubiquination and/or turnover than do ErbB4 full agonists. Experimentation is required to address these two possibilities.
3. ErbB receptor partial agonists function antagonize ErbB receptor signaling
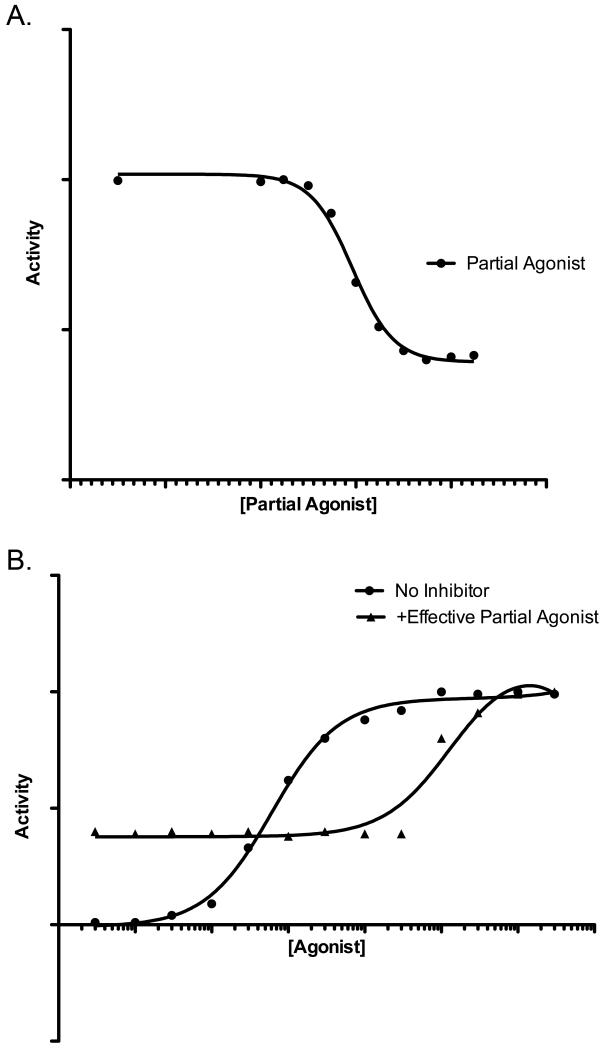
The full and partial agonists for a given ErbB receptor share a common binding site on that receptor. Thus, full and partial agonists are expected to compete with each other for receptor binding and partial agonists are expected to function as competitive antagonists of agonist-induced ErbB receptor signaling. Indeed, preliminary data indicate that increasing concentrations of EGF partially antagonize stimulation of EGFR coupling to cell proliferation by a fixed concentration of AREG (as in Figure 2a). Because EGF possesses some ability to stimulate EGFR coupling to cell proliferation, EGF does not fully antagonize EGFR coupling to cell proliferation. This effect is mediated by EGFR, as increasing concentrations of AREG overcome partial antagonism of EGFR signaling by a fixed concentration of EGF; the rightward shift of the AREG dose response curve in the presence of a fixed concentration of EGF indicates that EGF competitively antagonizes AREG stimulation of EGFR signaling, thereby reducing the potency (but not efficacy) of AREG (as in Figure 2b).
Figure 2. A theoretical partial agonist can antagonize the activity of a theoretical full agonist.
The activity of these ligands resembles the activity of AREG (full agonist) and EGF (partial agonist). (A) Increasing concentrations of a relatively effective theoretical partial agonist inhibit the activity of a fixed concentration of a theoretical full agonist. (B) The antagonistic effects of a fixed concentration of a relatively effective theoretical partial agonist can be overcome by increasing concentrations of a theoretical full agonist. The resulting rightward shift in the full agonist dose response curve in the presence of the partial agonist is indicative of competitive inhibition of full agonist activity by the partial agonist.
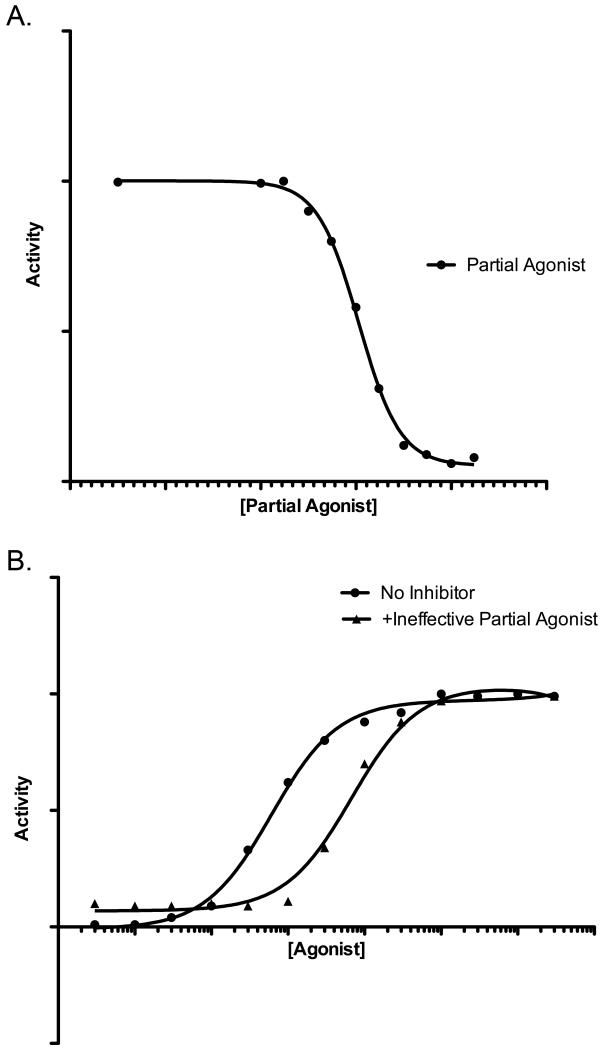
Preliminary data reveal that ErbB4 ligands tell a more compelling story. The NRG2β/Q43L mutant fully antagonizes stimulation of ErbB4 coupling to cell proliferation by a fixed concentration of NRG2β (as in Figure 3a). This effect is mediated by ErbB4, as increasing concentrations of NRG2β overcome antagonism of ErbB4 signaling by a fixed concentration of NRG2β/Q43L; the rightward shift of the NRG2β dose response curve in the presence of a fixed concentration of NRG2β/Q43L indicates that NRG2β/Q43L competitively antagonizes NRG2βstimulation of ErbB4 signaling, thereby reducing the potency (but not efficacy) of NRG2β (as in Figure 3b). NRG2β is also an agonist for EGFR and the ErbB2/ErbB3 heterodimer50, 53. Consequently, it is not entirely surprising that NRG2β/Q43L competitively antagonizes agonist stimulation of EGFR signaling and agonist stimulation of ErbB2/ErbB3 signaling. Thus, NRG2β/Q43L functions as a pan-ErbB receptor antagonist.
Figure 3. A relatively ineffective theoretical partial agonist can have a profound effect on the activity of a theoretical full agonist.
The activity of these ligands resembles the activity of wild-type NRG2β (full agonist) and NRG2β/Q43L (partial agonist). (A) Increasing concentrations of a relatively ineffective theoretical partial agonist almost completely abrogate the activity of a fixed concentration of a theoretical full agonist. (B) The antagonistic effects of a fixed concentration of a relatively effective theoretical partial agonist can be overcome by increasing concentrations of a theoretical full agonist. The resulting rightward shift in the full agonist dose response curve in the presence of the partial agonist is indicative of competitive inhibition of full agonist activity by the partial agonist.
Preliminary data indicate two additional findings of some note. Preincubation of cells that express EGFR with NRG2β/Q43L irreversibly partially blocks EGF binding to EGFR, suggesting that NRG2β/Q43L antagonizes EGFR signaling in part by stimulating rapid EGFR ubiquitination and turnover. Consistent with this hypothesis, expression of EGF in cells irreversibly blocks AREG stimulation of EGFR coupling to cell proliferation.
4. Conclusion
Some naturally-occurring ErbB family receptor peptide growth factor ligands function as partial agonists for their cognate receptor. Partial and full agonism are mutable; ligand mutations can convert partial agonists into full agonists and vice versa. The mechanistic basis for the difference in efficacy displayed by full and partial ErbB receptor agonists appears to be differences in the individual sites of receptor tyrosine phosphorylation. Thus, screens based on the absence or presence of particular sites of RTK tyrosine phosphorylation could be used to identify novel RTK partial agonists that possess RTK antagonist activities.
5. Expert Opinion
5.1. Key findings and weaknesses
One key finding is that some naturally-occurring ErbB receptor ligands function as a partial agonist for their cognate receptor(s). The rationale for the existence of full and partial agonists remains to be elucidated. Partial agonists may allow for spatiotemporal fine-tuning of ErbB receptor signaling. Partial agonists may also serve to prevent pathologic levels of ErbB receptor signaling.
Another key finding is that functional specificity of ligands for a given ErbB receptor appears to reflect differences in the sites of tyrosine phosphorylation, resulting in differences in ErbB receptor coupling to effectors and/or differences in ErbB receptor turnover. However, in most cases, the mechanism underlying ligand functional specificity has yet to be definitively established or adequately explored. In particular, identifying the structural basis of ligand functional specificity is of paramount importance, as such information will yield new insights into the development of partial agonists with therapeutic potential.
Partial agonists for a given ErbB receptor appear to antagonize the activity of full agonists at that ErbB receptor. In part, the partial agonists competitively antagonize the activity of the full agonists, as an increasing concentration of agonist can overcome the antagonistic activity of a fixed concentration of a partial antagonist. However, these experiments have been performed using heterologous, artificial systems; antagonism of ErbB receptor signaling in tumor cells by partial agonists has yet to be observed.
Finally, preincubation of target cells with a partial agonist or expression of a partial agonist by target cells results in irreversible antagonism of agonist-induced ErbB receptor signaling. Presumably this irreversible inhibition reflects partial agonist stimulation of receptor ubiquitination and/or turnover. However, these mechanistic details have yet to be definitively established.
5.2. Future topics of interest and potential of this research
With some notable exceptions, peptide hormones and growth factors have not proven to be good platforms for oncology drug discovery. Nonetheless, once some fundamental issues are addressed, antagonistic partial agonists for receptor tyrosine kinases could drive oncology drug discovery in new directions.
First of all, the antagonistic activity of ErbB receptor partial agonists in human tumor cell lines must be established. It is anticipated that partial agonists will antagonize the effects of soluble full agonists added to the culture medium. However, it is not apparent whether partial agonists will antagonize full agonist/receptor autocrine loops. Given that these autocrine loops may feature receptor signaling prior to the appearance of the receptor at the cell surface, it is likely that soluble partial agonists will not disrupt autocrine signaling but expression of the partial agonists by the target cells will disrupt autocrine signaling. Moreover, it is not apparent whether partial agonists will antagonize ligand-independent receptor signaling. Given that partial agonists may function by causing receptor ubiquitination and/or turnover, it is possible that partial agonists may antagonize pathologic, ligand-independent receptor signaling caused by overexpression or by those activating mutations of the receptor that do not disrupt antagonist binding54. However, ErbB receptor truncation/deletion mutants that feature reduced agonist binding (such as EGFR vIII54) are predicted to be refractory to the antagonistic action of ErbB receptor partial agonists.
Next, the mechanism by which partial agonists antagonize agonist-induced (or agonist-independent) ErbB receptor signaling must be definitively established. A focal point will be the identification of agonist- and antagonist-specific sites of receptor tyrosine phosphorylation and the validation of their relevance to ErbB receptor coupling. As stated elsewhere, two hypotheses will be explored. (1) Agonists stimulate greater phosphorylation of tyrosine residues that enable receptor coupling to mitogenic and other pro-malignant responses than do antagonists. (2) Antagonists stimulate greater phosphorylation of tyrosine residues that trigger receptor ubiquitination and/or turnover than do agonists.
The identification of agonist- and antagonist-specific sites of ErbB receptor tyrosine phosphorylation will permit the development of screening strategies for novel ErbB receptor antagonists. It may be possible that such screening strategies will take advantage of the numerous commercial antibodies specific for individual sites of EGFR and ErbB2 tyrosine phosphorylation. However, in many cases it may be necessary to develop novel tools for assessing phosphorylation of specific ErbB receptor tyrosine residues.
The approaches could be deployed to identify novel mutants of naturally-occuring ligands that possess partial agonist and antagonist activities. However, as noted elsewhere, efforts to exploit peptide growth factors and hormones as platforms for oncology drug discovery have not been particularly successful. Part of the reason may be due to the pleiotropic effects of peptide growth factors and hormones, particularly when administered systemically. Thus, screens for small molecule and antibody partial agonists (that possess antagonistic activity) may yield molecules with greater therapeutic potential than low-efficacy naturally-occurring peptide growth factors or growth factor mutants. The fact that trastuzumab possesses partial agonist activity38, is validation for such a screening strategy.
Targeting the extracellular domain with partial agonists that possess antagonistic activities may be a more effective general paradigm that targeting the intracellular tyrosine kinase domain with small molecule, ATP-competitive inhibitors. Partial agonists do not need to need to fulfill the design constraints necessary to ensure cell penetrance. Moreover, the fact that partial agonists are likely to share the same binding sites on receptor tyrosine kinases as the naturally-occurring RTK agonists suggests that mutations that abrogate the binding of partial agonists will not be selected for, as these mutations are likely to also disrupt the binding of the naturally occurring agonists. Indeed, there have been no descriptions of acquired ErbB2 mutations that disrupt the binding of the trastuzumab partial agonist; likewise, there have been no descriptions of acquired EGFR mutations that disrupt the binding of cetuximab, which shares its binding site on EGFR with the naturally-occurring EGFR agonists. In contrast, there are numerous examples of acquired tyrosine kinase domain mutations that disrupt the activity of small molecule tyrosine kinase inhibitors.
Finally, the partial agonist/antagonist paradigm may be generally applicable to all RTKs. Screening for competitive antibody or small molecule partial agonists that function as antagonists would be performed in two steps. The first would be to assess whether candidate molecules inhibit the binding of naturally-occurring agonists to the RTK. The second would be to assess whether candidate molecules fail to stimulate RTK phosphorylation on those tyrosine residues that enable RTK coupling to a malignant phenotype. Screening for irreversible antibody or small molecule partial agonists that function as antagonists would also be performed in two steps. Again, the first would be to assess whether candidate molecules inhibit the binding of naturally-occurring agonists to the RTK. The second would be to assess whether candidate molecules stimulate RTK phosphorylation on those tyrosine residues that enable RTK ubiquitination and/or turnover.
Article highlights.
-
-
Many receptor tyrosine kinases (RTKs), including the vascular endothelial growth factor receptor (VEGFR), the epidermal growth factor receptor (EGFR/ErbB2), and ErbB2 (HER2/Neu), are validated targets for oncology drug discovery.
-
-
Existing strategies for targeting RTKs include antibodies that function as ligand sinks, antibodies that bind to an RTK and antagonize ligand binding to that RTK, antibodies that inhibit RTK dimerization and signaling, antibodies that may function as RTK partial agonists, and small molecule tyrosine kinase inhibitors.
-
-
Some naturally-occurring ligands for ErbB receptor tyrosine kinases possess less efficacy (intrinsic activity) than others. These partial agonists can competitively inhibit (antagonize) the activity of more efficacious agonists at the same receptor. In the case of partial agonists that stimulate ErbB receptor turnover, the antagonistic effects may be irreversible.
-
-
Some cases of differences in ErbB receptor ligand efficacy appear to be due to differences in the sites of ErbB receptor tyrosine phosphorylation and differences in ErbB receptor coupling to signaling effectors and biological responses. Generalization of this paradigm to other RTKs may lead to straightforward strategies for generating novel ligand-based small molecule or antibody ErbB receptor antagonists.
Footnotes
Declaration of Interest:
DJ Riese II is supported by an NIH grant R01CA114209.
References
- 1.Lemmon MA, Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2010 Jun 25;141(7):1117–34. doi: 10.1016/j.cell.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lemmon MA. Ligand-induced ErbB receptor dimerization. Exp Cell Res. 2009 Feb 15;315(4):638–48. doi: 10.1016/j.yexcr.2008.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jura N, Endres NF, Engel K, Deindl S, Das R, Lamers MH, et al. Mechanism for activation of the EGF receptor catalytic domain by the juxtamembrane segment. Cell. 2009 Jun 26;137(7):1293–307. doi: 10.1016/j.cell.2009.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang X, Gureasko J, Shen K, Cole PA, Kuriyan J. An allosteric mechanism for activation of the kinase domain of epidermal growth factor receptor. Cell. 2006 Jun 16;125(6):1137–49. doi: 10.1016/j.cell.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 5.Hubbard SR. EGF receptor activation: push comes to shove. Cell. 2006 Jun 16;125(6):1029–31. doi: 10.1016/j.cell.2006.05.028. [DOI] [PubMed] [Google Scholar]
- 6.Landau M, Fleishman SJ, Ben-Tal N. A putative mechanism for downregulation of the catalytic activity of the EGF receptor via direct contact between its kinase and C-terminal domains. Structure. 2004 Dec;12(12):2265–75. doi: 10.1016/j.str.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 7.Aifa S, Miled N, Frikha F, Aniba MR, Svensson SP, Rebai A. Electrostatic interactions of peptides flanking the tyrosine kinase domain in the epidermal growth factor receptor provides a model for intracellular dimerization and autophosphorylation. Proteins. 2006 Mar 1;62(4):1036–43. doi: 10.1002/prot.20780. [DOI] [PubMed] [Google Scholar]
- 8.Waller CF. Imatinib mesylate. Recent Results Cancer Res. 2010;184:3–20. doi: 10.1007/978-3-642-01222-8_1. [DOI] [PubMed] [Google Scholar]
- 9.Stegmeier F, Warmuth M, Sellers WR, Dorsch M. Targeted cancer therapies in the twenty-first century: lessons from imatinib. Clin Pharmacol Ther. 2010 May;87(5):543–52. doi: 10.1038/clpt.2009.297. [DOI] [PubMed] [Google Scholar]
- 10.Shi F, Telesco SE, Liu Y, Radhakrishnan R, Lemmon MA. ErbB3/HER3 intracellular domain is competent to bind ATP and catalyze autophosphorylation. Proc Natl Acad Sci U S A. 2010 Apr 27;107(17):7692–7. doi: 10.1073/pnas.1002753107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pierotti MA, Negri T, Tamborini E, Perrone F, Pricl S, Pilotti S. Targeted therapies: the rare cancer paradigm. Mol Oncol. 2010 Feb;4(1):19–37. doi: 10.1016/j.molonc.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mena AC, Pulido EG, Guillen-Ponce C. Understanding the molecular-based mechanism of action of the tyrosine kinase inhibitor: sunitinib. Anticancer Drugs. 2010 Jan;21(Suppl 1):S3–11. doi: 10.1097/01.cad.0000361534.44052.c5. [DOI] [PubMed] [Google Scholar]
- 13.Bayraktar UD, Bayraktar S, Rocha-Lima CM. Molecular basis and management of gastrointestinal stromal tumors. World J Gastroenterol. 2010 Jun 14;16(22):2726–34. doi: 10.3748/wjg.v16.i22.2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.An X, Tiwari AK, Sun Y, Ding PR, Ashby CR, Jr., Chen ZS. BCR-ABL tyrosine kinase inhibitors in the treatment of Philadelphia chromosome positive chronic myeloid leukemia: a review. Leuk Res. 2010 Oct;34(10):1255–68. doi: 10.1016/j.leukres.2010.04.016. [DOI] [PubMed] [Google Scholar]
- 15.Agrawal M, Garg RJ, Cortes J, Quintas-Cardama A. Tyrosine kinase inhibitors: the first5decade. Curr Hematol Malig Rep. 2010 Apr;5(2):70–80. doi: 10.1007/s11899-010-0045-y. [DOI] [PubMed] [Google Scholar]
- 16.De Luca A, Normanno N. Predictive biomarkers to tyrosine kinase inhibitors for the epidermal growth factor receptor in non-small-cell lung cancer. Curr Drug Targets. 2010 Jul;11(7):851–64. doi: 10.2174/138945010791320773. [DOI] [PubMed] [Google Scholar]
- 17.Gazdar AF. Epidermal growth factor receptor inhibition in lung cancer: the evolving role of individualized therapy. Cancer Metastasis Rev. 2010 Mar;29(1):37–48. doi: 10.1007/s10555-010-9201-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beers SA, Chan CH, French RR, Cragg MS, Glennie MJ. CD20 as a target for therapeutic type I and II monoclonal antibodies. Semin Hematol. 2010 Apr;47(2):107–14. doi: 10.1053/j.seminhematol.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 19.Capietto AH, Keirallah S, Gross E, Dauguet N, Laprevotte E, Jean C, et al. Emerging concepts for the treatment of hematological malignancies with therapeutic monoclonal antibodies. Curr Drug Targets. 2010 Jul;11(7):790–800. doi: 10.2174/138945010791320845. [DOI] [PubMed] [Google Scholar]
- 20.Cillessen SA, Meijer CJ, Notoya M, Ossenkoppele GJ, Oudejans JJ. Molecular targeted therapies for diffuse large B-cell lymphoma based on apoptosis profiles. J Pathol. 2010 Apr;220(5):509–20. doi: 10.1002/path.2670. [DOI] [PubMed] [Google Scholar]
- 21.Czuczman MS, Gregory SA. The future of CD20 monoclonal antibody therapy in B-cell malignancies. Leuk Lymphoma. 2010 Jun;51(6):983–94. doi: 10.3109/10428191003717746. [DOI] [PubMed] [Google Scholar]
- 22.Hagemeister F. Rituximab for the treatment of non-Hodgkin’s lymphoma and chronic lymphocytic leukaemia. Drugs. 2010 Feb 12;70(3):261–72. doi: 10.2165/11532180-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 23.Weiner GJ. Rituximab: mechanism of action. Semin Hematol. 2010 Apr;47(2):115–23. doi: 10.1053/j.seminhematol.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bossung V, Harbeck N. Angiogenesis inhibitors in the management of breast cancer. Curr Opin Obstet Gynecol. 2010 Feb;22(1):79–86. doi: 10.1097/GCO.0b013e328334e462. [DOI] [PubMed] [Google Scholar]
- 25.Greenberg S, Rugo HS. Triple-negative breast cancer: role of antiangiogenic agents. Cancer J. 2010 Jan-Feb;16(1):33–8. doi: 10.1097/PPO.0b013e3181d38514. [DOI] [PubMed] [Google Scholar]
- 26.Korpanty G, Smyth E, Sullivan LA, Brekken RA, Carney DN. Antiangiogenic therapy in lung cancer: focus on vascular endothelial growth factor pathway. Exp Biol Med (Maywood) 2010 Jan;235(1):3–9. doi: 10.1258/ebm.2009.009191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koutras AK, Fountzilas G, Makatsoris T, Peroukides S, Kalofonos HP. Bevacizumab in the treatment of breast cancer. Cancer Treat Rev. 2010 Feb;36(1):75–82. doi: 10.1016/j.ctrv.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 28.Loupakis F, Bocci G, Pasqualetti G, Fornaro L, Salvatore L, Cremolini C, et al. Targeting vascular endothelial growth factor pathway in first-line treatment of metastatic colorectal cancer: state-of-the-art and future perspectives in clinical and molecular selection of patients. Curr Cancer Drug Targets. 2010 Feb 1;10(1):37–45. doi: 10.2174/156800910790980179. [DOI] [PubMed] [Google Scholar]
- 29.McDermott DF, George DJ. Bevacizumab as a treatment option in advanced renal cell carcinoma: an analysis and interpretation of clinical trial data. Cancer Treat Rev. 2010 May;36(3):216–23. doi: 10.1016/j.ctrv.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 30.Ramalingam SS, Belani CP. Antiangiogenic agents in the treatment of nonsmall cell lung cancer: reality and hope. Curr Opin Oncol. 2010 Mar;22(2):79–85. doi: 10.1097/CCO.0b013e328335a583. [DOI] [PubMed] [Google Scholar]
- 31.Welch S, Spithoff K, Rumble RB, Maroun J. Bevacizumab combined with chemotherapy for patients with advanced colorectal cancer: a systematic review. Ann Oncol. 2010 Jun;21(6):1152–62. doi: 10.1093/annonc/mdp533. [DOI] [PubMed] [Google Scholar]
- 32.Ruzzo A, Graziano F, Canestrari E, Magnani M. Molecular predictors of efficacy to anti-EGFR agents in colorectal cancer patients. Curr Cancer Drug Targets. 2010 Feb 1;10(1):68–79. doi: 10.2174/156800910790980205. [DOI] [PubMed] [Google Scholar]
- 33.Vincenzi B, Zoccoli A, Pantano F, Venditti O, Galluzzo S. Cetuximab: from bench to bedside. Curr Cancer Drug Targets. 2010 Feb 1;10(1):80–95. doi: 10.2174/156800910790980241. [DOI] [PubMed] [Google Scholar]
- 34.Kristjansdottir K, Dizon D. HER-dimerization inhibitors: evaluating pertuzumab in women’s cancers. Expert Opin Biol Ther. 2010 Feb;10(2):243–50. doi: 10.1517/14712590903514090. [DOI] [PubMed] [Google Scholar]
- 35.Langdon SP, Faratian D, Nagumo Y, Mullen P, Harrison DJ. Pertuzumab for the treatment of ovarian cancer. Expert Opin Biol Ther. 2010 Jul;10(7):1113–20. doi: 10.1517/14712598.2010.487062. [DOI] [PubMed] [Google Scholar]
- 36.Barros FF, Powe DG, Ellis IO, Green AR. Understanding the HER family in breast cancer: interaction with ligands, dimerization and treatments. Histopathology. 2010 Apr;56(5):560–72. doi: 10.1111/j.1365-2559.2010.03494.x. [DOI] [PubMed] [Google Scholar]
- 37.Brufsky A. Trastuzumab-based therapy for patients with HER2-positive breast cancer: from early scientific development to foundation of care. Am J Clin Oncol. 2010 Apr;33(2):186–95. doi: 10.1097/COC.0b013e318191bfb0. [DOI] [PubMed] [Google Scholar]
- 38.Esteva FJ, Yu D, Hung MC, Hortobagyi GN. Molecular predictors of response to trastuzumab and lapatinib in breast cancer. Nat Rev Clin Oncol. 2010 Feb;7(2):98–107. doi: 10.1038/nrclinonc.2009.216. [DOI] [PubMed] [Google Scholar]
- 39.Tagliabue E, Balsari A, Campiglio M, Pupa SM. HER2 as a target for breast cancer therapy. Expert Opin Biol Ther. 2010 May;10(5):711–24. doi: 10.1517/14712591003689972. [DOI] [PubMed] [Google Scholar]
- 40.Hudziak RM, Lewis GD, Winget M, Fendly BM, Shepard HM, Ullrich A. p185HER2 monoclonal antibody has antiproliferative effects in vitro and sensitizes human breast tumor cells to tumor necrosis factor. Mol Cell Biol. 1989 Mar;9(3):1165–72. doi: 10.1128/mcb.9.3.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gilmore JL, Riese DJ., 2nd. secErbB4-26/549 antagonizes ligand-induced ErbB4 tyrosine phosphorylation. Oncol Res. 2004;14(11-12):589–602. doi: 10.3727/0965040042707907. [DOI] [PubMed] [Google Scholar]
- 42.Klein DE, Nappi VM, Reeves GT, Shvartsman SY, Lemmon MA. Argos inhibits epidermal growth factor receptor signalling by ligand sequestration. Nature. 2004 Aug 26;430(7003):1040–4. doi: 10.1038/nature02840. [DOI] [PubMed] [Google Scholar]
- 43.Klein DE, Stayrook SE, Shi F, Narayan K, Lemmon MA. Structural basis for EGFR ligand sequestration by Argos. Nature. 2008 Jun 26;453(7199):1271–5. doi: 10.1038/nature06978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nelson J, Allen WE, Scott WN, Bailie JR, Walker B, McFerran NV, et al. Murine epidermal growth factor (EGF) fragment (33-42) inhibits both EGF- and laminin-dependent endothelial cell motility and angiogenesis. Cancer Res. 1995 Sep 1;55(17):3772–6. [PubMed] [Google Scholar]
- 45.Wilson KJ, Gilmore JL, Foley J, Lemmon MA, Riese DJ., 2nd. Functional selectivity of EGF family peptide growth factors: implications for cancer. Pharmacol Ther. 2009 Apr;122(1):1–8. doi: 10.1016/j.pharmthera.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gilmore JL, Scott JA, Bouizar Z, Robling A, Pitfield SE, Riese DJ, 2nd, et al. Amphiregulin-EGFR signaling regulates PTHrP gene expression in breast cancer cells. Breast Cancer Res Treat. 2008 Aug;110(3):493–505. doi: 10.1007/s10549-007-9748-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Willmarth NE, Baillo A, Dziubinski ML, Wilson K, Riese DJ, 2nd, Ethier SP. Altered EGFR localization and degradation in human breast cancer cells with an amphiregulin/EGFR autocrine loop. Cell Signal. 2009 Feb;21(2):212–9. doi: 10.1016/j.cellsig.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Alvarado D, Klein DE, Lemmon MA. Structural basis for negative cooperativity in growth factor binding to an EGF receptor. Cell. 2010 Aug 20;142(4):568–79. doi: 10.1016/j.cell.2010.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hobbs SS, Cameron EM, Hammer RP, Le AT, Gallo RM, Blommel EN, et al. Five carboxyl-terminal residues of neuregulin2 are critical for stimulation of signaling by the ErbB4 receptor tyrosine kinase. Oncogene. 2004 Jan 29;23(4):883–93. doi: 10.1038/sj.onc.1207250. [DOI] [PubMed] [Google Scholar]
- 50.Hobbs SS, Coffing SL, Le AT, Cameron EM, Williams EE, Andrew M, et al. Neuregulin isoforms exhibit distinct patterns of ErbB family receptor activation. Oncogene. 2002 Dec 5;21(55):8442–52. doi: 10.1038/sj.onc.1205960. [DOI] [PubMed] [Google Scholar]
- 51.Hobbs SS, Gallo RM, Riese DJ., Jr. Phe45 of NRG2beta is critical for the affinity of NRG2beta for ErbB4 and for potent stimulation of ErbB4 signaling by NRG2beta*. Growth Factors. 2005 Dec;23(4):273–83. doi: 10.1080/08977190500199345. [DOI] [PubMed] [Google Scholar]
- 52.Wilson KJ, Mill CP, Cameron EM, Hobbs SS, Hammer RP, Riese DJ., 2nd. Inter-conversion of neuregulin2 full and partial agonists for ErbB4. Biochem Biophys Res Commun. 2007 Dec 14;364(2):351–7. doi: 10.1016/j.bbrc.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gilmore JL, Gallo RM, Riese DJ., 2nd. The epidermal growth factor receptor (EGFR)-S442F mutant displays increased affinity for neuregulin-2beta and agonist-independent coupling with downstream signalling events. Biochem J. 2006 May 15;396(1):79–88. doi: 10.1042/BJ20051687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Riese DJ, 2nd, Gallo RM, Settleman J. Mutational activation of ErbB family receptor tyrosine kinases: insights into mechanisms of signal transduction and tumorigenesis. Bioessays. 2007 Jun;29(6):558–65. doi: 10.1002/bies.20582. [DOI] [PMC free article] [PubMed] [Google Scholar]