Abstract
The reaction of the phosphonium alkylidene [(H2IMes)RuCl2=CHP(Cy)3)]+ BF4– with propene, 1-butene, and 1-hexene at –45 °C affords various substituted, metathesis-active ruthenacycles. These metallacycles were found to equilibrate over extended reaction times in response to decreases in ethylene concentrations, which favored increased populations of α-monosubstituted and α,α’-disubstituted (both cis and trans) ruthenacycles. On an NMR timescale, rapid chemical exchange was found to preferentially occur between the β-hydrogens of the cis and trans stereoisomers prior to olefin exchange. Exchange on an NMR timescale was also observed between the α- and β-methylene groups of the monosubstituted ruthenacycle (H2IMes)Cl2Ru(CHRCH2CH2) (R = CH3, CH2CH3, (CH2)3CH3). EXSY NMR experiments at –87 °C were used to determine the activation energies for both of these exchange processes. In addition, new methods have been developed for the direct preparation of metathesis-active ruthenacyclobutanes via the protonolysis of dichloro(1,3-bis(2,4,6-trimethylphenyl)-2-imidazolidinylidene)(benzylidene) bis(pyridine)ruthenium(II) and its 3-bromopyridine analog. Using either trifluoroacetic acid or silica-bound toluenesulfonic acid as the proton source, the ethylene-derived ruthenacyclobutane (H2IMes)Cl2Ru(CH2CH2CH2) was observed in up to 98% yield via NMR at –40 °C. On the basis of these studies, mechanisms accounting for the positional and stereochemical exchange within ruthenacyclobutanes are proposed, as well as the implications of these dynamics towards olefin metathesis catalyst and reaction design are described.
Introduction
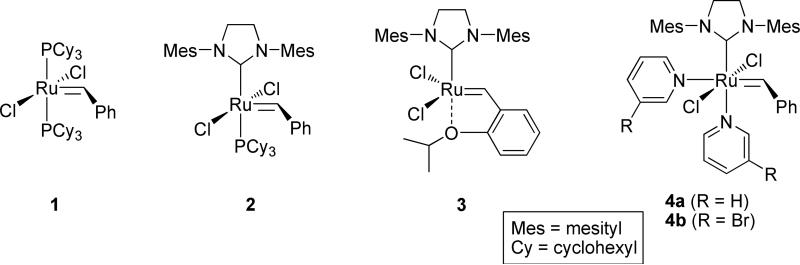
Olefin metathesis is a powerful method for the clean, reliable, and efficient construction of carbon-carbon bonds, a goal that is ubiquitous to many areas of synthetic, polymer, and materials chemistry.1 Olefin metathesis has also garnered recent interest due to its potential in green chemistry applications.2 In particular, the commercially-available ruthenium olefin metathesis catalysts 1-4 have been extensively used in organic and polymer chemistry due to their robustness, high activity, and functional group tolerance (Figure 1).1a,3
Figure 1.
Commercially-available metathesis catalysts.
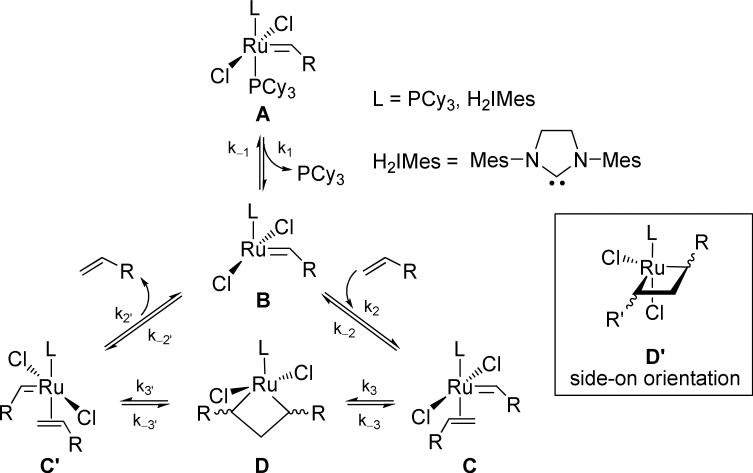
The mechanism of ruthenium-catalyzed olefin metathesis has been extensively studied,4 with the principal steps corresponding to those in the mechanism initially proposed by Hérisson and Chauvin in 1971.5 Starting from an initial 16-electron catalyst precursor (A, Scheme 1), the reversible loss of a ligand, such as a phosphine, trans to L affords the 14-electron alkylidene intermediate B, which can then proceed to bind substrate olefin to produce the square pyramidal, π-complex C. A formal [2+2] cycloaddition then forms metallayclobutane D, which can then undergo a [2+2] cycloreversion to either reform C or productively generate complex C’ (shown as degenerate in Scheme 1). Dissociation of the product olefin completes the cycle.
Scheme 1.
Proposed mechanism for olefin metathesis by ruthenium carbene complexes.
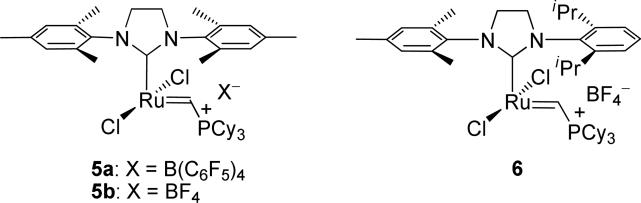
The structures of the various intermediates of ruthenium-catalyzed olefin metathesis have been established via 1H-NMR studies,6,7 X-ray crystallographic data of isolable complexes,8 and computational investigation.6a,9 In particular, the geometry and stereochemical orientation about the short-lived ruthenacyclobutane complex D has been of key interest for the rational design of enantioselective and E/Z diastereoselective olefin metathesis catalysts. Indirect and computational evidence had suggested the viability of either a bottom-face8c,9g,j (D) or side-on (D’)6,8a orientation of the metallacycle. The direct observation of metathesis-active ruthenacycles proved elusive until Romero and Piers employed catalyst 5 (Figure 2) in the degenerate metathesis of ethylene at low temperatures (–50 °C).7e Catalyst 5, which can be generated via the protonation of a carbide precursor originally reported by Heppert,10 can be viewed as a “pre-initiated” 14-electron complex, thereby allowing direct access to the catalytically-active complex B. Complex 5 also has the additional feature that the vinyl trialkylphosphonium salt is not a metathesis-active olefin substrate, thereby greatly simplifying metallacycle preparation and analysis. Upon reaction with ethylene, the bottom-face ruthenacycle D was readily observed by low-temperature NMR. In our laboratories, we later confirmed the proposed trigonal-bipyramidal geometry by using the asymmetrically-substituted catalyst 6.7d In this case, the Ru-NHC (N-heterocyclic carbene) ligand rotation was sufficiently slow on an NMR timescale at –40 °C that we were able to observe the preferred stereochemical orientation of the ruthenacyclobutane Ru(CH2)3 ring to lie beneath the NHC ligand such that the planes of each were co-incident. Additionally, we found ethylene-derived ruthenacyclobutanes to be dynamic structures that proceed through a series of nonproductive metallacycle formations/cycloreversions prior to product dissociation; EXSY spectroscopy measurements were utilized to determine the rate of degenerate exchange between the α- and β-methylene groups to be 26 ± 2 s–1 at –40 °C, corresponding to an activation energy of exchange of ΔG≠233K = 12.18 ± 0.04 kcal/mol. These measurements were later independently confirmed.7c
Figure 2.
Isolable, 14-electron olefin metathesis catalysts.
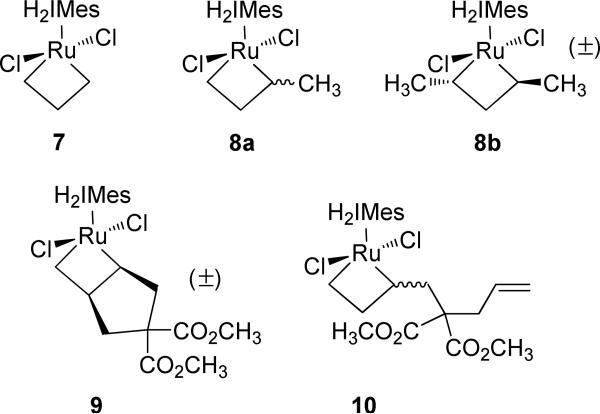
While many insights can be gained from the study of ethylene-derived ruthenacycles, the structural and stereochemical orientations of substituted metallacycles have a greater potential to provide critical information regarding overall reaction behavior with regard to modes of stereocontrol. In 2006, we reported the first examples of substituted, metathesis-active ruthenacyclobutanes derived from propene.7d In the cross-metathesis (CM) reaction of propene, 2-butene and ethylene are generated as reaction products. Consequently, starting from catalyst 5, the ethylene-derived metallacycle 7 and substituted metallacycles 8a and 8b (Figure 3) were observed in a 59:38:3 ratio (76% combined yield) via 1H-NMR at –95 °C after a three-hour time period. Similar to metallacycle 7, metallacycles 8a-b were also found to adopt a bottom-face orientation. This work was subsequently followed by detailed studies by Piers and coworkers, wherein metallacycles 9 and 10 were observed by introducing metallacycle 7 to the ring-closing metathesis (RCM) product dimethyl cyclopent-3-ene-1,1-dicarboxylate.7a,b
Figure 3.
Substituted, metathesis-active ruthenacycles reported to date.
To date, metallacycles 8-10 have afforded valuable information regarding the preferred stereochemical orientation of metathesis-active ruthenacyclobutanes. However, it is important to note that 8a and 10 are monosubstituted species, and metallacycle 8b was observed in too small a concentration to be definitively useful. In addition, the constrained α,β stereochemistry of ruthenacycle 9 does not allow for exchange dynamics within the metallacycle to be readily observed. In an attempt to acquire greater information regarding the dynamics of ruthenacyclobutanes, we have reinvestigated the reaction of catalyst 5 with various terminal olefins. In addition, we have developed a more accessible model system for analysis through the direct formation of ruthenacyclobutanes from commercially-available catalysts. Herein, we report the preparation of cis and trans α,α’-disubstituted and α-monosubstituted ruthenacyclobutanes derived from propene, 1-butene, and 1-hexene. The cis α,α’-disubstituted metallacycles are hereto unreported, and our present study into the dynamics of these systems has revealed previously unobserved mechanistic features regarding the olefin metathesis reaction. In addition, we report that ruthenacyclobutanes can be directly generated from the commercially-available bispyridyl catalysts 4a and 4b.11,12 From this work, a better understanding as to the effect of ethylene on ruthenacyclobutane distributions in olefin metathesis has emerged.
Results and Discussion
Generation of α,α’-Disubstituted and α-Monosubstituted CM Ruthenacycles
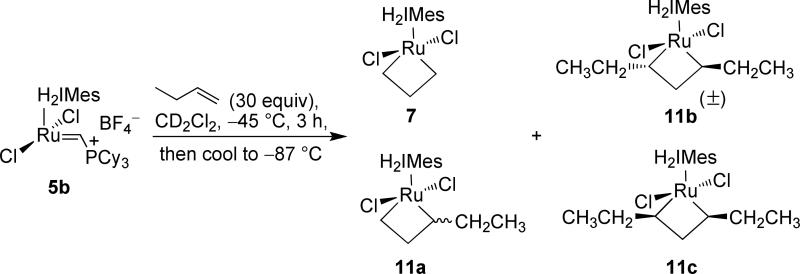
Catalyst 5b was prepared according to literature procedures.7d,e,10 Upon exposure of 5b to 1-butene at –45 °C, complete conversion of the starting catalyst was observed after 3 h (Scheme 2). Excess 1-butene (30 equiv) was used to minimize catalyst decomposition and favor metallacycle formation.131H-NMR analysis at –87 °C revealed a combined metallacycle yield of 87% relative to anthracene as the internal standard. In behavior similar to that of the propene-derived metallacycles previously reported,7d conversion to three metallacycles was observed: 7 (60%), 11a (39%), and 11b (~1%), respectively. The β-hydrogen region is shown in Figure 4a. The prevalence of metallacycle 7 is consistent with previous results that suggest that the ethylene-derived ruthenacycle is more thermodynamically stable than its substituted derivatives.7 Analogous to other reported metathesis-active ruthenacycles, the chemical shifts for the Hβ protons of 11a and 11b are significantly upfield of those observed for Hα. This feature has also been observed in metathesis-active, early-metal tungsten-14 and titanacyclobutanes,15 and is generally attributed to Cα-Cβ agostic interactions stabilizing the electron-deficient, 14-electron ruthenacyclobutane structure.16,7c Computed structures support this assessment.9c,h,i
Scheme 2.
Reaction of 5b with 1-butene.
Figure 4.
500 MHz 1H NMR (–87 °C) spectra of (a) the reaction of 5b with 1-butene after 3 h: 7:11a:11b = 60:39:1; and (b) the reaction of 5b with 1-butene after 72 h: 7:11a:11b:11c = 1:24:52:24 (β-H region).
All of these spectral data correlated with previous experiments. However, we were intrigued by the presence of small resonances in the upfield region of the proton NMR spectrum at –1.72 and –3.20 ppm (Figure 4a). Thinking that these might be due to other, rapidly-exchanging metallacycle species, the same reaction was set up in a diethyl ether-d10/tetrahydrofuran-d8 (2:3) solvent mixture and studied at –115 °C in an attempt to further resolve the resonances in the NMR spectrum. Unfortunately, all efforts to investigate reaction mixtures at lower temperatures failed to resolve any additional ruthenacyclobutanes. Fortuitously, during these experiments, we had retained the original butene reaction, storing it at –78 °C until further analysis. Investigation 72 h after reaction initiation revealed not the original metallacycle distribution found in Figure 4a, but a new one (Figure 4b). While the combined yield of metallacycle species was relatively unchanged, the ethylene-derived metallacycle 7 now comprised only about 1% of the metallacycle yield, with the mixture now being enriched in ruthenacycles 11a (24%), 11b (52%), and a new metallacycle 11c (24%), which we attribute to the cis α,α’-disubstituted ruthenacycle, the β-hydrogens of which corresponding to the former trace resonances at –1.72 and –3.20 ppm. Instead of 7, the trans α,α’-disubstituted ruthenacycle 11b was now the major species, the proton resonances of which were assigned via 2D-COSY, 1H-13C HSQC, and 2D-NOESY NMR experiments to reside at 7.75 ppm (Hα, 2H, br) and –2.41 ppm (Hβ, 2H, d, 1JC-H = 7.9 Hz).17 The 13C NMR shifts of 11b were assigned at 123.0 and 14.0 ppm for the α and β positions, respectively (δCα – δCβ = 109 ppm). For the cis α,α’-disubstituted ruthenacycle 11c, the β-hydrogen syn to the two α-hydrogens was assigned as the upfield triplet at –3.20 ppm (1H, 1JC-H = 7.9 Hz); the β-hydrogen syn to the two ethyls was assigned the resonance at –1.72 ppm. Analysis via 1H-13C HSQC confirmed that both protons reside on the same carbon (δ13C = 12.6 ppm). The α-proton resonances of 11c were assigned as a broad peak in the 1H NMR at 7.08 ppm; the carbon resonance was assigned at 121.1 ppm (δCα – δCβ = 108.5 ppm). Again, these dramatic chemical shift differences between the α and β positions can be associated with a trigonal bipyramidal structure that possesses a M→ Cβ interaction.7,18 Ruthenacycle 11c represents the first reported example of a cis-substituted, metathesis-active ruthenacyclobutane existing in equilibrium with its trans counterpart. As predicted by computation1,9 and conventional wisdom, the trans-substituted 11b was observed in a factor of ca. 2 over the cis-substituted 11c. The shifting equilibrium of metallacycle composition over extended reaction times can be largely attributed to the concentration of ethylene in the reaction mixture. At the three-hour time point, where the ethylene-derived 7 comprised 60% of the metallacycle mixture, the 1-butene:ethylene ratio was determined to be 86:14. However, at 72 h, the 1-butene:ethylene ratio was measured to be approximately 99:1.
Ethylene removal from an olefin metathesis reaction is known to provide a powerful entropic driving force towards improving reaction yields.1 Lee and coworkers have observed that ethylene is more poorly soluble than one would anticipate in organic solvents.19 Coupled with the common protocol of conducting olefin metathesis reactions at elevated temperatures under an atmosphere of nitrogen or argon, ethylene volatilization can be rapidly and irreversibly achieved. In our case, the butene reactions described were conducted in sealed NMR tubes at low temperatures: sparging was not employed. However, at –78 °C, 1-butene (bp –6.3 °C) and the product 3-hexenes (bp 66-68 °C) are liquids, while ethylene (bp –103.7 °C) remains a gas that can freely diffuse out of the reaction mixture. Further facilitating this process was the fact that, due to the liquefaction of the majority of the gaseous components added to the reaction, the NMR tube headspace existed under reduced pressure at the temperatures investigated.
To investigate the possibility of a similar shifting equilibration occurring in the presence of propene, an analogous reaction with 5b was set up according to the conditions described above. After three hours at –45 °C, cooling the reaction mixture to –87 °C revealed a 76% combined yield of metallacycle species, with a ratio of 7:8a:8b = 59:38:3, which was similar to our previously reported results.7d The ratio of propene:ethylene in solution was determined to be 95:5 at this time. Reaction analysis at 72 h revealed that the relative amount of ethylene in solution had diminished slightly (propene:ethylene 96:4), with the amount of the ethylene-derived ruthenacycle 7 concomitantly diminishing to 38% of the overall metallacycle mixture. In addition to increased amounts of 8a (50%) and 8b (9%), the cis α,α’-disubstituted ruthenacycle 8c was now visible (3%). It is important to note that in one instance, when the experiment was conducted under the same conditions, the propene:ethylene ratio in solution was measured to be 97:3 via 1H NMR after 72 h at –78 °C. In this case, the metallacycle composition observed was: 7 (23%), 8a (63%), 8b (9%), and 8c (4%). These results are highlighted to illustrate how time and changes in olefin composition can dramatically affect the metallacycle population observed. Similar to the reactions with 1-butene, the concentration of the trans α,α’-disubstituted stereoisomer in all propene reactions was observed over the cis by a factor of three. The reactions with propene proved to be less robust than those with 1-butene: at the 72 h time point, the reaction yields had generally diminished from 76% (at 3 h) to approximately 64%.
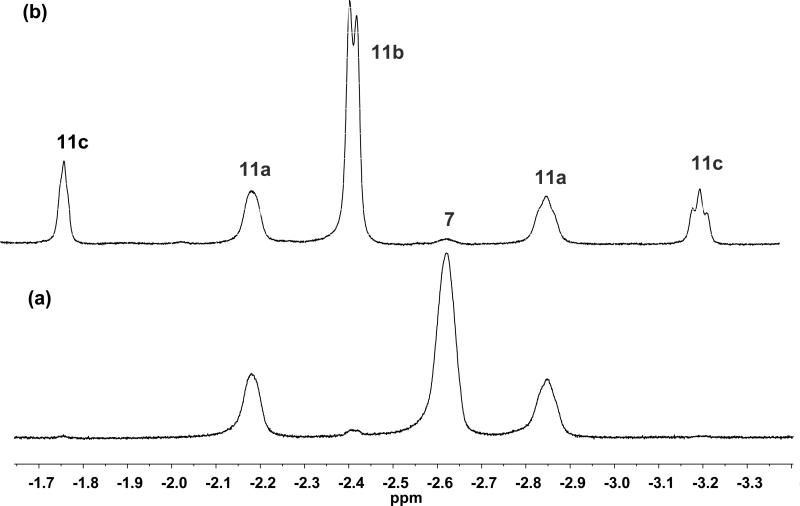
To investigate less-volatile olefins, 1-hexene (30 equiv) was directly added to a dichloromethane-d2 solution of 5b (0.035 M) at –78 °C under an atmosphere of argon. After addition, the reaction was warmed to –45 °C for 3 h, after which analysis via NMR spectroscopy at –87 °C revealed the complete conversion of 5b and a combined yield of metallacycle species of 86% (Figure 5). Unexpectedly, unlike the reactions with 1-butene and propene, no extended times were required to observe the cis α,α’-disubstituted 12c; the 1-hexene:ethylene ratio in solution at 3 h was determined to be ~99:1. Two additional, identical experiments afforded equivalent results. The concentration of the trans stereoisomer 12b was observed over the cis by a factor of 2.5. The NMR chemical shift and coupling constant data were found to correlate with those of metallacycles 8a-c and 11a-c.17 Monitoring over extended reaction times led to no further metallacycle equilibration—only slow decomposition was observed.
Figure 5.
500 MHz 1H NMR (–87 °C) of the reaction of 5b with 1-hexene after 3 h (β-H region).
These studies reveal how minute changes in olefin concentrations can dramatically affect metallacycle, and hence product, composition within an olefin metathesis reaction. It is important to note that no β-substituted metallacyles were observed in our studies: in the absence of ethylene to enable the formation of metallaycles with an unsubstituted β-methylene moiety, metallacycles were not observed. For example, no metallacycles were observed when 3-hexene (the 1-butene cross-metathesis product; 30 equiv) was added to a solution of 7 at –45 °C. In addition, directly adding 3-hexene to a solution of 5b in dichloromethane-d2 or diethyl ether-d10/tetrahydrofuran-d8 (an ethylene-free reaction) resulted in no observable β-substituted metallacycles, even at temperatures as low as –115 °C. While β-substituted ruthenacyclobutanes must exist to effect productive cross-metathesis reactions, these current conditions did not allow for their observation.
Metallacycle Dynamics
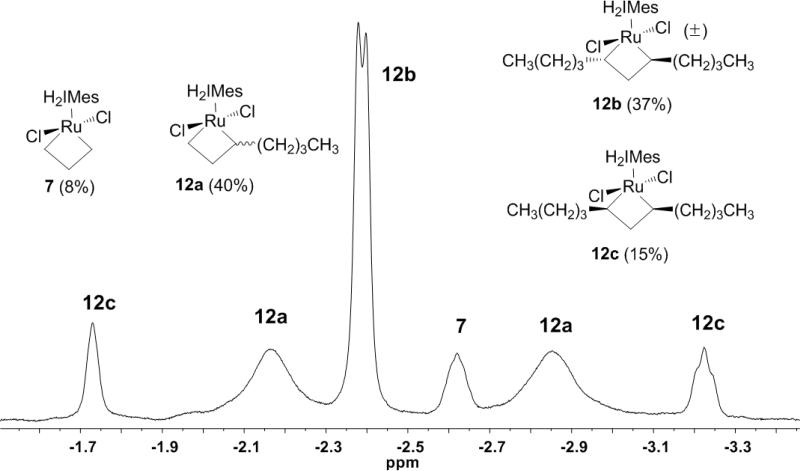
In 2006, we reported the use of EXSY NMR to analyze the dynamics of exchange between the α- and β-methylene groups of 7 (ΔG≠233K = 12.18 ± 0.04 kcal/mol).7d Similar exchange behavior was recently reported between the α- and β-protons in the ring-closing metathesis-(RCM) derived metallacycle 9.7a In our experiments, exchange cross-peaks were clearly visible in the ROESY-2D NMR spectra of 8a, 11a, and 12b, wherein resonances in phase with the diagonal are indicative of chemical exchange.20 For example, a section of the ROESY-2D NMR (–80 °C) for the reaction of 5b with 1-butene at 72 h is depicted in Figure 6. In this spectrum, the cross-peak at (7.78, –2.40, yellow) is indicative of a legitimate NOE interaction between the α- and β- hydrogens of the trans α,α’-disubstituted 11b, of which no chemical exchange would be anticipated. However, the cross-peaks at (6.65, –2.84, blue) and (6.09, –2.17, blue) clearly show positional exchange occurring on an NMR timescale at –80 °C. Using EXSY experiments6c,21,22 derived from the corresponding NOESY-2D data (186 K, mix = 35 ms), the rate of exchange between the α- and β-methylenes of 11a was determined to be 6.7 ± 0.5 s–1, corresponding to a G≠186K = 9.99 ± 0.03 kcal/mol. Unfortunately, while similar cross-peaks were visible for the propene and 1-hexene reactions,17 poor baseline separation prevented the application of EXSY experiments to obtain these data.
Figure 6.
500 MHz ROESY-2D NMR (–80 °C; region of α-β methylene exchange) of the reaction of 5b with 1-butene after 72 h.
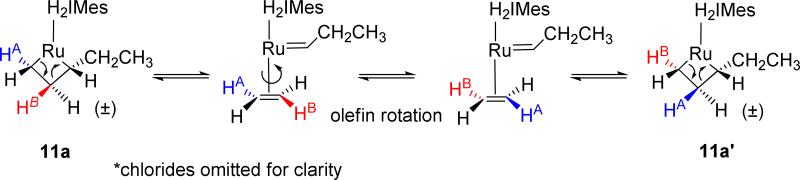
Of note in these experiments was the preference of one β-proton to selectively exchange with a specific α-proton. One can envision this pathway occurring via the route depicted in Scheme 3. Starting from the monosubstituted metallacycle 11a, cycloreversion results in the formation of the olefin π complex. Olefin rotation, followed by [2+2] cycloaddition forms metallacycle 11a’, where the α and β positions are exchanged, thereby explaining the positional exchange observed via ROESY-2D and NOESY-2D NMR. The presence of these cross-peaks also carries the strong implication that metallacycle cycloreversion with NHC-ligated ruthenium catalysts is kinetically driven to proceed via a propagating alkylidene, rather than a propagating methylidene,1 a feature that is known to have beneficial effects on catalyst robustness.1,13
Scheme 3.
Proposed exchange pathway between the α and β methylene protons of mono- α-substituted metallacycles.
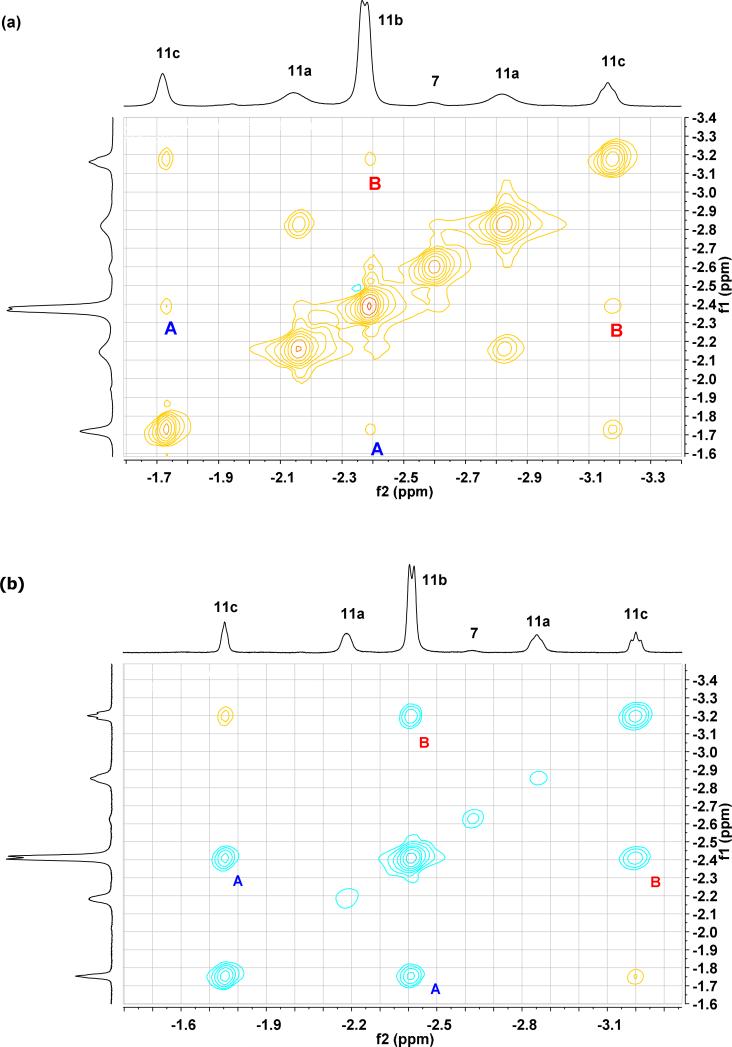
More intriguing exchange behavior was observed in the β-hydrogen region of the NOESY-2D and ROESY-2D spectra for these metallacycles. In the reaction of 5b with 1-butene at –80 °C, a standard NOE interaction was observed between the geminal β-protons of 11c (Figure 7). However, to our surprise, exchange cross-peaks were clearly visible between the β-protons of the trans and cis ruthenacycles! These results indicate that not only is olefin rotation possible between degenerate cycloadditions/reversions at –80 °C, but stereoisomerization is as well. Hypothetical mechanisms for trans-cis exchange are depicted in Scheme 4.
Figure 7.
Chemical exchange between the β-protons of 11b and 11c in the 500 MHz (a) NOESY-2D NMR and (b) ROESY-2D NMR spectra (–80 °C; upfield region).
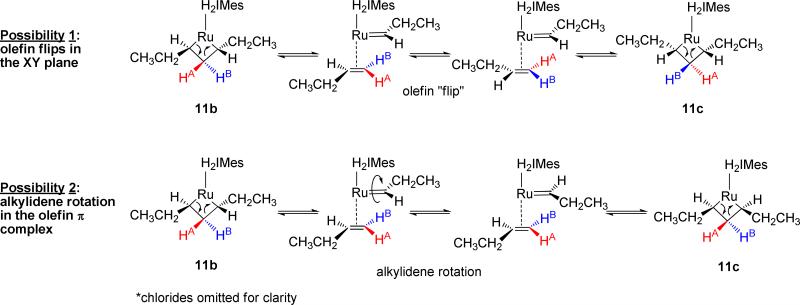
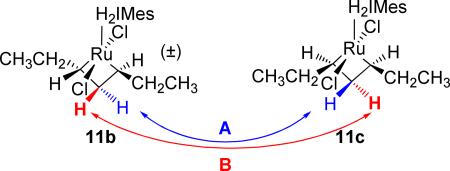
Scheme 4.
Mechanistic possibilities for the exchange between the β protons of 11b and 11c.
One possibility is that, subsequent to the cycloreversion of 11b to form the olefin π complex, the olefin “flips” in the XY plane to interconvert HA and HB. However, a more likely scenario for this interconversion is alkylidene rotation within the π complex. Such rotations have been observed to occur quite freely,23,9d and have been observed to occur about the Ru=CH2 of olefin methylidene complexes as low as –64 °C.7a EXSY experiments were performed at –87 °C (186 K) to determine the rate of exchange between the cis and trans stereoisomers of metallacycles 8, 11, and 12 (Table 1). The rate constants obtained incorporate the rates of metallacycle cycloreversion, rotation, and metallacycle formation to interconvert the two species. Given that propene, 1-butene, and 1-hexene are all Type I24 olefins for cross-metathesis that possess similar steric properties, it is not surprising that these results were found to correlate with each other. The low energies required for this facile exchange process have serious implications to the design of diastereoselective metathesis catalysts: the possible impact of rapid, stereochemical exchange prior to product dissociation will now have to be taken into account, particularly when attempting to prepare the less thermodynamically-favored Z stereoisomers.
Table 1.
Results of EXSY experiments detailing the cis-trans exchange of alkyl-substituted metallacycles at –87 °C (186 K).
| olefin | kcis→trans (s–1) | ΔG≠cis→trans (kcal/mol) | ktrans→cis (s–1) | ΔG≠trans→cis (kcal/mol) | |
|---|---|---|---|---|---|
| 8b ⇆ 8c | propene | 0.76 ± 0.06 | 10.80 ± 0.03 | 0.19 ± 0.02 | 11.31 ± 0.04 |
| 11b ⇆ 11c | 1-butene | 0.74 ± 0.06 | 10.81 ± 0.03 | 0.17 ± 0.01 | 11.35 ± 0.03 |
| 12b ⇆ 12c | 1-hexene | 0.9 ± 0.1 | 10.7 ± 0.1 | 0.18 ± 0.02 | 11.33 ± 0.08 |
While intramolecular exchange was readily visible via NMR, intermolecular exchange between the metallacycle protons and free olefin in these cross-metathesis reactions proved to be sufficiently slow that they were not observed via NMR at the temperatures investigated. Supporting this assessment, in the case of the RCM reaction involving 9, Piers and coworkers measured a dissociative rate constant of (2.5 × 0.5) × 10–4 s–1 (209 K) for the dissociation of cyclopentene derivative.7a We were, however, able to qualitatively observe exchange with free olefin occurring at temperatures as low as –95 °C: upon combining solutions of 7 with propene (15-35 equiv) in dichloromethane-d2, the resonances of 7 were observed to rapidly diminish and resonances corresponding to metallacycles 8 were observed. Attempts were made to measure the rate of this exchange utilizing propene-d6 and observing the disappearance of 7; however, similar to what had previously been observed by Piers,7c the degenerate exchange occurring within the system and the thermodynamic preference for 7 sufficiently complicated the results to prevent definitive, reproducible kinetic measurements.
Ruthenium Metallacycles from Readily Available Catalysts
The observation of metathesis-active ruthenacyclobutane intermediates has traditionally been hindered by the ligand that must be released in the initiation step of the reaction.4 In general, only a small percentage of the catalyst precursor added to an olefin metathesis reaction will initiate and be active at any one time. For example, in the case of catalyst 2, the initiation rate constant (kobs) associated with phosphine dissociation was measured to be only (4.6 ± 0.4) × 10–4 s–1 at 35 °C.4b The use of weaker, more labile donors, such as those in 4a and 4b, have recently enabled the characterization of olefin-carbene complexes.6 However, these catalysts have not been observed to initiate at temperatures below –20 °C. As mentioned previously, complexes 5a and 5b can be viewed as “pre-initiated,” thereby allowing for facile access into the catalytic cycle at the temperatures required to observe metallacycle formation.7e Despite the utility of these catalysts, the preparation of catalysts 5a,b requires a multi-step synthetic route that requires the use of costly reagents.7d,e In addition, the vinyl trialkylphosphonium salt formed upon the reaction of 5 presents a less relevant model in comparison to the styrene formed from the commercially-available benzylidene catalysts. To circumvent these problems, we envisioned the use of ligand scavengers in the presence of olefin at low temperatures to directly access metallacycles, such as 7, from commercially-available catalysts (e.g. 2-4).
Phosphine scavengers25 in olefin metathesis chemistry have previously been used to either accelerate reactions4e or facilitate the preparation of alternative catalysts, such as 3.26 In particular, copper (I) chloride (CuCl) initially looked promising as a potential ligand scavenger to effect catalyst initiation and subsequent metallacycle formation. Unfortunately, upon exposure of 2 to CuCl (1-4 equiv) at –40 °C in the presence of an atmosphere of ethylene, insufficient initiation occurred to visualize 7. Reasoning that the more-labile pyridyl ligands of 4a or 4b might be more readily removed, we next attempted to effect initiation via the protonolysis of 4a.
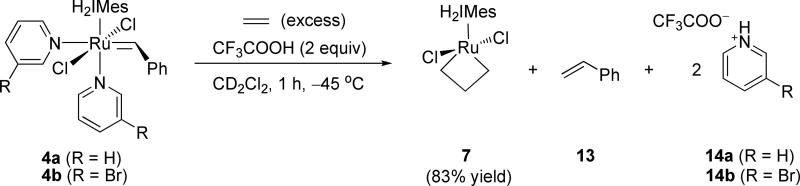
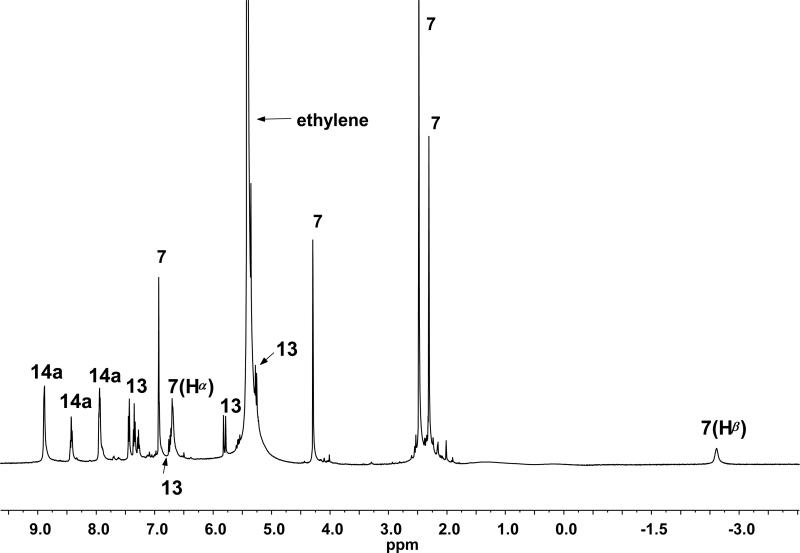
In our first attempt, trifluoroacetic acid (2 equiv) was directly added to a solution of 4a (0.17 M) in dichloromethane-d2 under an atmosphere of ethylene at –78 °C. The reaction was then warmed to –40 °C and observed via NMR. Within 5 minutes, 90% conversion of 4a was observed relative to anthracene as the internal standard. A 32% NMR yield of the ethylene-derived metallacycle 7 was obtained, along with a 28% yield of an unidentified alkylidene species. By 30 minutes, the metallacycle had somewhat decomposed to 29% of [4a]0, and complete decomposition occurred within 24 h. In contrast, we have observed solutions of 7 derived from 5b in dichloromethane-d2 to be stable at –40 °C for periods up to 1.5 weeks. Thinking that protonation might be sufficiently slow relative to TFA-promoted decomposition, we next modified the experimental procedure to add the acid more slowly via syringe pump (Scheme 5). Gratifyingly, the addition of a solution of TFA (2.0 equiv) slowly over a 1-hour time period to a solution of 4a in dichloromethane-d2 at –45 °C afforded 7 in 83% yield via NMR (Scheme 5). Equivalent results were obtained when the commercially-available 4b was employed in place of 4a under identical conditions. Due to an excess of ethylene in the reaction mixture, (~17 equiv), concentrations of ruthenium benzylidene were expected to be negligible, consistent with the observation of free styrene (13) in the reaction mixture (Figure 8). To prepare 1H NMR samples for analysis, reaction mixtures were cannulated at –78 °C away from any pyridyl trifluoroacetate (14) solids present in the reaction flask into screw-cap NMR tubes and then placed under an atmosphere of ethylene. It is important to note that salt removal was not complete, as resonances corresponding to 14 were still clearly visible via NMR.
Scheme 5.
Protonolysis of 4a and 4b with TFA.
Figure 8.
1H NMR (500 MHz, –40 °C) of reaction of 4a with TFA (2 equiv) after 1 h.
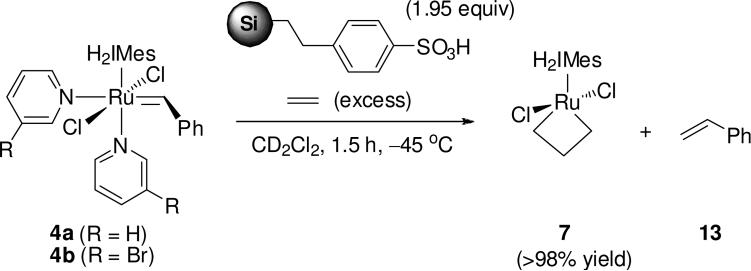
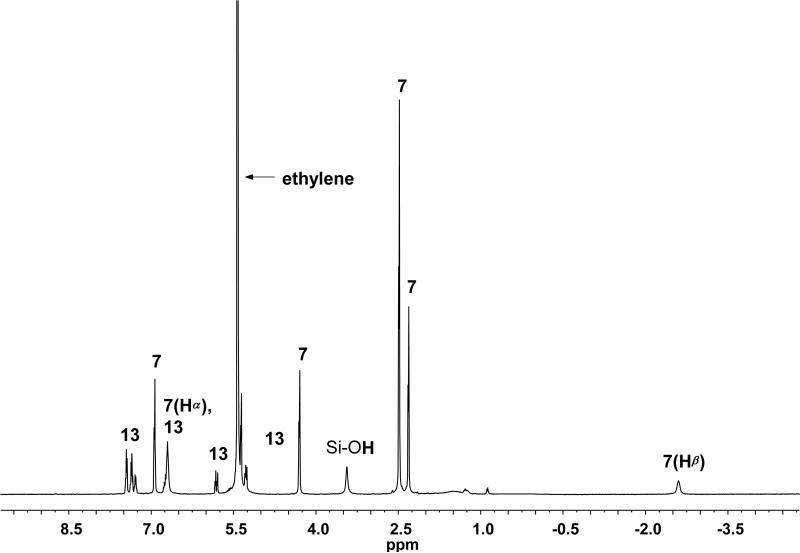
In an effort to obtain cleaner reaction spectra and potentially boost product yields, we next turned to using scavenger resins in place of TFA. Silica-bound toluenesulfonic acid (Si-TsOH), a commercially-available amine scavenger,27 was selected for this purpose. After several attempts, the best results were achieved via the direct addition of Si-TsOH (1.95 equiv) to a solution of either 4a or 4b (0.014 M) in dichloromethane-d2 under an atmosphere of ethylene at –45 °C (Scheme 6). After stirring for 1.5 h, cannula transfer into a screw-cap NMR tube and analysis via 1H NMR (500 MHz, –40 °C) revealed 7 in greater than 98% yield (Figure 9). Not only was the use of Si-TsOH higher-yielding and more procedurally simple than the use of TFA, the stability of 7 under these conditions was found to be comparable to that in reactions of 5b with ethylene. In addition, the use of a scavenger resin proved effective at pyridine removal: in addition to 7 and styrene, only a broad singlet at δ 3.43 ppm, corresponding to residual silica (Si-OH) in the reaction mixture, was visible. Again, the presence of excess ethylene (~16-17 equiv) ensured that benzylidene complex concentrations were negligible.
Scheme 6.
Protonolysis of 4a and 4b with silica-bound toluenesulfonic acid.
Figure 9.
1H NMR (500 MHz, –40 °C) of reaction of 4a with Si-TsOH (1.95 equiv) after 1.5 h.
These experiments highlight that metathesis-active ruthenacyclobutanes can be effectively prepared starting from readily-available catalysts. However, translating this method to the preparation of substituted metallacycles, such as 8, 11, and 12, has thus far proven difficult. For example, the direct addition of TFA to a NMR tube of 4a and 1-hexene (30 equiv) at low temperatures afforded a combined metallacycle yield of 19% (7:12a:12b:12c = 1:18:60:21), with the remainder of the starting material decomposing to unknown reaction products. The slow addition of TFA or the use of Si-TsOH, methods that led to the higher yields observed with ethylene, require vigorous reaction agitation in the presence of olefin. When set up in sealed reaction flasks equipped with stirbars, judging qualitatively by reaction color, reactions of 4a and Si-TsOH with propene, 1-butene, and 1-hexene appeared to have worked. Unfortunately, unlike the more robust metallacycle 7, cannula transfer disrupted the delicate balance of olefins in the reaction mixture and led to rapid decomposition prior to NMR analysis.
While this method cannot yet be extended to the formation of substituted ruthenacycles, these experiments have additional merit beyond serving as a mechanistic model. Catalysts 5a and 5b are amongst the fastest NHC ruthenium catalysts known.7c,4 However, the efficacy of these catalysts is somewhat limited with sterically-hindered substrates due to the bulk of the phosphonium carbene. Much like the use of CuCl to increase the rate of initiation of phosphine-based catalysts,4e our NMR results suggest that the addition of a small amount of Si-TsOH to reactions employing 4a or 4b might serve a similar purpose more effectively.
Conclusions
In summary, metathesis-active cis α,α’-substituted ruthenacyclobutanes have been observed for the first time. These dynamic structures were found to proceed through a series of nonproductive metallacycle formations/cycloreversions at –87 °C prior to olefin exchange. In this, the β-hydrogens of the cis ruthenacycles were found to undergo rapid chemical exchange with their trans analogs, with the concentration of trans being favored over the cis by as much as a factor of three. The presence of cis-trans exchange hints that alkylidene rotation is likely occurring several times prior to product dissociation, which implies that alkylidene dynamics, in addition to metallacycle stability, play a role in the stereochemical outcome of an olefin metathesis reaction. Diastereocontrol remains a significant goal in olefin metathesis. Fortunately, directing the stereochemistry of both the ruthenacycle and alkylidene are not necessarily mutually exclusive. For example, catalyst steric modifications, such as the introduction of a new X-type ligand, could potentially direct both. It can be envisioned that the systematic measurement of ruthenacycle cis-trans exchange could serve as a future probe into the effects of catalyst structure on stereochemical dynamics, thereby facilitating rational catalyst design.
In addition to cis-trans exchange, the chemical exchange between the α and β methylene groups of monosubstituted metallacycles, such as 11a, implies that metallacycle cycloreversion with NHC-ligated ruthenium catalysts is favored to proceed via a propagating alkylidene, rather than a propagating methylidene. Given that favoring a propagating alkylidene is known to play a role in catalyst stability, performing similar measurements on new catalyst analogs could provide information regarding their anticipated robustness. Such information would be of particular use in evaluating the potential of a ruthenium catalyst for commercialized processes.
Lastly, we have demonstrated that metathesis-active ruthenacyclobutanes can be directly prepared from the commercially-available catalysts 4a and 4b. In particular, when silica-bound toluenesulfonic acid was used as the proton source, the reaction of 4a and 4b in the presence of excess ethylene led to the formation of ruthenacyclobutane 7 in near-quantitative yield. These experiments highlight that metallacycle preparation and observation can extend beyond the use of model systems. In addition, the high yields attained indicate that silica-bound toluenesulfonic acid could serve a more generalized role in facilitating bispyridyl catalyst initiation. Ultimately, these studies form a clearer picture into the inner workings of this remarkable reaction.
Supplementary Material
Acknowledgement
The authors wish to thank Mr. Ian Tonks, Mr. Edward C. Weintrob, and Professor John Bercaw for the use of the Schlenk manifold used in the quantitated gas addition experiments. Additional thanks goes to Mr. James Luchi and Dr. Daniel Levin of Norac Pharma for the generous donation of the silica-bound toluenesulfonic acid used in these experiments. Materia Inc. is gratefully acknowledged for the generous donation of catalyst. Professors J. M. O'Connor and Charles Perrin of U.C. San Diego and B. Scott Williams and John Carl Olsen of JSD are acknowledged for invaluable research discussion. Funding for AGW and GB was provided by Claremont McKenna, Scripps, and Pitzer Colleges. JSD NMR instrumentation funding was provided by NSF (CHE 0922393); Caltech NMR instrumentation funding was provided by NIH (NIH RR027690). Additional funding for RHG was provided by NIH (5R01GM031332).
Footnotes
Supporting Information Available: Additional experimental procedures, as well as NMR spectra and characterization data, are available free of charge via the Internet at http://pubs.acs.org.
References
- 1.a Grubbs RH, editor. Handbook of Metathesis. 1-3. Weinheim, Germany; Wiley-VCH: 2003. [Google Scholar]; b Ivin KJ, Mol JC. Olefin Metathesis and Metathesis Polymerization. Academic Press; San Diego, CA: 1997. [Google Scholar]
- 2.Dragutan V, Demonceau A, Dragutan I, Finkelshtein ES, editors. Green Metathesis Chemistry: Great Challenges in Synthesis, Catalysis and Nanotechnology (NATO Science for Peace and Security Series A: Chemistry and Biology) Springer; Dordrecht, The Netherlands: 2009. [Google Scholar]
- 3.a Imamogammalu Y, editor. Metathesis Polymerization of Olefins and Polymerization of Alkynes (NATO Science Series C) Springer; Dordrecht, the Netherlands: 2009. [Google Scholar]; b Grubbs RH. Tetrahedron. 2004;60:7117–7140. [Google Scholar]; c Trnka TM, Grubbs RH. Acc. Chem. Res. 2001;34:18–29. doi: 10.1021/ar000114f. [DOI] [PubMed] [Google Scholar]
- 4.a Sanford MS, Love JA, Grubbs RH. J. Am. Chem. Soc. 2001;123:6543–6554. doi: 10.1021/ja010624k. [DOI] [PubMed] [Google Scholar]; b Sanford MS, Ulman M, Grubbs RH. J. Am. Chem. Soc. 2001;123:749–750. doi: 10.1021/ja003582t. [DOI] [PubMed] [Google Scholar]; c Kingsbury JS, Harrity JP, Bonitaebus PJ, Hoveyda AH. J. Am. Chem. Soc. 1999;121:791–799. [Google Scholar]; d Ulman R, Grubbs RH. Organometallics. 1998;17:2484–2489. [Google Scholar]; e Dias EL, Nguyen SB, Grubbs RH. J. Am. Chem. Soc. 1997;119:3587–3897. [Google Scholar]
- 5.Hérisson JL, Chauvin Y. Makromol. Chem. 1971;141:161–176. [Google Scholar]
- 6.a Stewart IC, Benitez D, O'Leary DJ, Tkatchouk E, Day MW, Goddard WA, III, Grubbs RH. J. Am. Chem. Soc. 2009;131:10269–10278. [Google Scholar]; b Anderson DR, O'Leary DJ, Grubbs RH. Chem. Eur. J. 2008;14:7536–7544. doi: 10.1002/chem.200701362. [DOI] [PubMed] [Google Scholar]; c Anderson DR, Hickstein DD, O'Leary DJ, Grubbs RH. J. Am. Chem. Soc. 2006;128:8386–8387. doi: 10.1021/ja0618090. [DOI] [PubMed] [Google Scholar]
- 7.a van der Eide EF, Piers WE. Nature Chemistry. 2010;2:571–576. doi: 10.1038/nchem.653. [DOI] [PubMed] [Google Scholar]; b van der Eide EF, Romero PE, Piers WE. J. Am. Chem. Soc. 2008;130:4485–4491. doi: 10.1021/ja710364e. [DOI] [PubMed] [Google Scholar]; c Romero PE, Piers WE. J. Am. Chem. Soc. 2007;129:1698–1704. doi: 10.1021/ja0675245. [DOI] [PubMed] [Google Scholar]; d Wenzel AG, Grubbs RH. J. Am. Chem. Soc. 2006;128:16048–16049. doi: 10.1021/ja0666598. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Romero PE, Piers WE. J. Am. Chem. Soc. 2005;127:5032–5033. doi: 10.1021/ja042259d. [DOI] [PubMed] [Google Scholar]
- 8.a Trnka TM, Day MW, Grubbs RH. Organometallics. 2001;20:3845–3847. [Google Scholar]; b Sanford MS, Henling LM, Day MW, Grubbs RH. Angew. Chem. Int. Ed. 2000;39:3451–3453. doi: 10.1002/1521-3773(20001002)39:19<3451::aid-anie3451>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]; c Tallarico JA, Bonitatebus PJ, Snapper ML. J. Am. Chem. Soc. 1997;119:7157–7158. [Google Scholar]
- 9.a Benitez D, Tkatchouk E, Goddard WA. Organometallics. 2009;28:2643–2645. [Google Scholar]; b Benitez D, Tkatchouk E, Goddard WA., III Chem. Commun. 2008:6194–6196. doi: 10.1039/b815665d. [DOI] [PubMed] [Google Scholar]; c Rowley CN, van der Eide EF, Piers WE, Woo TK. Organometallics. 2008;27:6043–6045. [Google Scholar]; d Naumov S, Buchmeiser MR. J. Phys. Org. Chem. 2008;21:963–970. [Google Scholar]; e Correa A, Cavallo L. J. Am. Chem. Soc. 2006;128:13352–13353. doi: 10.1021/ja064924j. [DOI] [PubMed] [Google Scholar]; f Benitez D, Goddard WA., III J. Am. Chem. Soc. 2005;127:12218–12219. doi: 10.1021/ja051796a. [DOI] [PubMed] [Google Scholar]; g Straub BF. Angew. Chem., Int. Ed. 2005;44:5974–5978. doi: 10.1002/anie.200501114. [DOI] [PubMed] [Google Scholar]; h Suresh CH, Baik M–H. J. Chem. Soc., Dalton Trans. 2005:2982–2984. doi: 10.1039/b508192k. [DOI] [PubMed] [Google Scholar]; i Suresh CH, Koga N. Organometallics. 2004;23:76–80. [Google Scholar]; j Adlhart C, Chen P. J. Am. Chem. Soc. 2004;126:3496–3510. doi: 10.1021/ja0305757. [DOI] [PubMed] [Google Scholar]; k Cavallo L. J. Am. Chem. Soc. 2002;124:8965–8973. doi: 10.1021/ja016772s. [DOI] [PubMed] [Google Scholar]
- 10.Carlson RG, Gile MA, Heppert JA, Mason MH, Powell DR, Vander Velde D, Vilain JM. J. Am. Chem. Soc. 2002;124:1580–1581. doi: 10.1021/ja017088g. [DOI] [PubMed] [Google Scholar]
- 11.Love JA, Morgan JP, Trnka TM, Grubbs RH. Angew. Chem. Int. Ed. 2002;41:4035–4037. doi: 10.1002/1521-3773(20021104)41:21<4035::AID-ANIE4035>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 12.Dichloro[1,3-bis(2,4,6-trimethylphenyl)-2-imidazolidinylidene](benzylidene)bis(3-bromopyridine)ruthenium(II) (4b) is commercially available from Sigma-Aldrich: item 682330 ($60/100 mg, 12/26/2010).
- 13.a Hong SH, Wenzel AG, Salguero TT, Day MW, Grubbs RH. J. Am. Chem. Soc. 2007;129:7961–7968. doi: 10.1021/ja0713577. [DOI] [PubMed] [Google Scholar]; b Janse van Rensburg W, Steynberg PJ, Meyer WH, Kirk MM, Forman GS. J. Am. Chem. Soc. 2004;126:14332–14333. doi: 10.1021/ja0453174. [DOI] [PubMed] [Google Scholar]
- 14.a Blanc F, Berthoud R, Copéret C, Lesage A, Emsley L, Singh R, Kreickmann T, Schrock RR. PNAS. 2008;105:12123–12127. doi: 10.1073/pnas.0802147105. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Jiang AJ, Simpson JH, Müller P, Schrock RR. J. Am. Chem. Soc. 2009;131:7770–7780. doi: 10.1021/ja9012694. [DOI] [PubMed] [Google Scholar]; c Flook MM, Jiang AJ, Schrock RR, Müller P, Hoveyda AH. J. Am. Chem. Soc. 2009;131:7962–7963. doi: 10.1021/ja902738u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.a Meinhart JD, Anslyn EV, Grubbs RH. Organometallics. 1989;8:583–589. [Google Scholar]; b Hawkins JM, Grubbs RH. J. Am. Chem. Soc. 1988;110:2821–2823. [Google Scholar]; c Howard TR, Lee JB, Grubbs RH. J. Am. Chem. Soc. 1980;102:6878–6880. [Google Scholar]
- 16.For a discussion on the role of agostic interactions in the inhibition of decomposition in catalytic cycles, see: Harvey BG, Mayne CL, Arif AM, Ernst RD. J. Am. Chem. Soc. 2005;127:16426–16435. doi: 10.1021/ja052287b.; Grubbs RH, Coates GW. Acc. Chem. Res. 1996;29:85–93.; Piers WE, Bercaw JE. J. Am. Chem. Soc. 1990;112:9406–9407.
- 17.Detailed spectral information can be found in the Experimental and Supporting Information sections.
- 18.Feldman J, Schrock RR. Prog. Inorg. Chem. 1991;39:1–6. [Google Scholar]
- 19.Lee L–S, Ou H–J, Hsu H–L. Fluid Phase Equilib. 2005;231:221–230. [Google Scholar]
- 20.Neuhaus D, Williamson MP. The Nuclear Overhauser Effect in Structural and Conformational Analysis. 2nd Ed. Wiley-VCH; New York: 2000. pp. 185–187. [Google Scholar]
- 21.Perrin CL, Dwyer TJ, Ramachandran R, Knight CTG, Kirkpatrick RJ, Oldfield E. Chem. Rev. J. Magn. Res. 1990;1985;9065:935–967. 136–141. [Google Scholar]
- 22.The EXSYCALC software package and instructions for crosspeak analysis can be obtained, free of charge, at: http://www.mestrelab.com
- 23.a Keitz BK, Grubbs RH. Organometallics. 2010;29:403–408. doi: 10.1021/om900864r. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Sliwa P, Handzlik J. Chem. Phys. Lett. 2010;493:273–278. [Google Scholar]
- 24.a Wenzel AG, Chatterjee AK, Grubbs RH. Olefin Cross-Metathesis. In: Ojima I, Hiyama T, editors. Comprehensive Organometallic Chemistry III: Review of the Literature 1993-2005. Vol. 11. Elsevier Ltd.; Oxford, U. K.: p. 2006. [Google Scholar]; b Chatterjee AK. Olefin Cross-Metathesis. In: Grubbs RH, editor. Handbook of Metathesis. 1st ed. Vol. 2. Wiley-VCH; Weinheim, Germany: 2002. pp. 246–295. [Google Scholar]
- 25.a Zhao X, Ivanova N, Hadzovic A, Zimmer–De Iuliis M, Lough AJ, Morris RH. Organometallics. 2008;27:503–508. [Google Scholar]; b Czelusniak I, Heywood JD, Kenwright AM, Khosravi E. J. Mol. Catal. A: Chemical. 2008;280:29–34. [Google Scholar]; c Kuhn P, Semeril D, Matt D, Chetcuti MJ, Lutz P, Qihui C, Yu J, Huang J. Dalton Trans. Organometallics. 2007;2007;26:515–528. 617–625. doi: 10.1039/b615259g. [DOI] [PubMed] [Google Scholar]; d Bailey PJ, Melchionna M, Parsons S. Organometallics. 2007;26:128–135. [Google Scholar]; e Frieman BA. 2006 [Google Scholar]; f Krause JO, Lubbad S, Nuyken O, Buchmeiser MR, Ivanov SA. Adv. Synth. Catal. 2003;8:996–1004. [Google Scholar]; g Nichiporuk RV, Medniknov EG, Dahl LF. J. Chem. Soc., Dalton Trans. 2002:4116–4127. [Google Scholar]; h Connon SJ, Dunne AM, Blechert S. Angew. Chem., Int. Ed. 2002;41:3835–3838. doi: 10.1002/1521-3773(20021018)41:20<3835::AID-ANIE3835>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]; i Wang C, Friedrich S, Younkin TR, Li RT, Grubbs RH, Bansleben DA, Day MW. Organometallics. 1998;17:3149–3151. [Google Scholar]; j Van der Velden JWA, Bour JJ, Bosman WP, Noordik JH. J. Chem. Soc., Chem. Commun. 1981:1218–1219. [Google Scholar]
- 26.Garber SB, Kingsbury JS, Gray BL, Hoveyda AH. J. Am. Chem. Soc. 2000;121:8168–8179. [Google Scholar]
- 27.The silica-bound toluenesulfonic acid (0.67 mmol/g) used in this experiment was purchased from Silicycle: www.silicycle.com item R60530B-10G.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.