SUMMARY
The terrestrial slug Arion subfuscus secretes a glue that is a dilute gel with remarkable adhesive and cohesive strength. The function of this glue depends on metals, raising the possibility that metal-catalyzed oxidation plays a role. The extent and time course of protein oxidation was measured by immunoblotting to detect the resulting carbonyl groups. Several proteins, particularly one with a relative molecular mass (Mr) of 165×103, were heavily oxidized. Of the proteins known to distinguish the glue from non-adhesive mucus, only specific size variants were oxidized. The oxidation appears to occur within the first few seconds of secretion. Although carbonyls were detected by 2,4-dinitrophenylhydrazine (DNPH) in denatured proteins, they were not easily detected in the native state. The presence of reversible cross-links derived from carbonyls was tested for by treatment with sodium borohydride, which would reduce uncross-linked carbonyls to alcohols, but stabilize imine bonds formed by carbonyls and thus lead to less soluble complexes. Consistent with imine bond formation, sodium borohydride led to a 20–35% decrease in the amount of soluble protein with a Mr of 40–165 (×103) without changing the carbonyl content per protein. In contrast, the nucleophile hydroxylamine, which would competitively disrupt imine bonds, increased protein solubility in the glue. Finally, the primary amine groups on a protein with a Mr of 15×103 were not accessible to acid anhydrides. The results suggest that cross-links between aldehydes and primary amines contribute to the cohesive strength of the glue.
KEY WORDS: adhesion, glue, gel, oxidation, metal-catalyzed, gastropod, slug, Arion subfuscus
INTRODUCTION
The gel-based adhesives of mollusks have unusual material properties. They adhere strongly to a wide variety of surfaces and have substantial elasticity and stiffness, yet contain almost 97% water by mass. The combination of adhesive and cohesive strength with high flexibility in a dilute gel makes them intriguing biomaterials. This is particularly true because most common non-covalent bonds that could participate in adhesion are degraded in the presence of water.
A possible explanation for these unusual properties lies in the presence of different metals in the glue. Terrestrial slug glue contains significant amounts of zinc, iron, copper and manganese (Werneke et al., 2007). The presence of metals is essential to the glue; metal removal prevents the gel-stiffening activity of the primary cross-linking proteins (Werneke et al., 2007), and disrupts the integrity of the gel (Smith et al., 2009). A major question now is the mechanism by which the metals cross-link the glue.
Metals have attracted recent interest for their ability to form strong, stable cross-links in aqueous systems. Metal ions can play an important role in strengthening biomaterials, and they can act on polymers in a variety of ways. They can link polymers together directly. This can occur when different amino acid side chains simultaneously coordinate the metals (Lichtenegger et al., 2008). Such interactions help link together the collagenous threads in mussel byssal fibers (Coyne et al., 1997), strengthen the cuticle covering the threads (Zeng et al., 2010) and join adhesive plaque proteins to the threads (Zhao and Waite, 2006). Similarly, the hardness of nereid worm jaws depends on histidine-rich proteins chelating zinc via coordinate covalent bonds (Lichtenegger et al., 2003; Broomell et al., 2006). Another direct cross-linking mechanism occurs in sabellarid tube worm cement, which solidifies in part because of the formation of insoluble complexes between calcium and phosphate groups on proteins (Stewart et al., 2004; Sagert et al., 2006; Sun et al., 2007).
In addition, redox active metals such as iron and copper can catalyze the oxidation of specific amino acid side chains, creating reactive groups that go on to form cross-links. The amino acid 3,4dihydroxyphenylalanine (DOPA) can be oxidized into dopaquinone by the copper-containing enzyme catecholoxidase, and then it can react with a variety of substrates (Sagert et al., 2006). Quinone-tanning in general has been implicated in strengthening a wide variety of materials from egg cases to cements to arthropod cuticles (Waite, 1990). In addition, Sever et al. (Sever et al., 2004) have provided evidence that iron bound to DOPA can catalyze the formation of organic radicals, which could then react with a variety of substrates. In connective tissues, a major step in collagen and elastin fiber cross-linking is the metal-catalyzed oxidation of lysine residues by the enzyme lysyl oxidase (Tanzer, 1973; Smith-Mungo and Kagan, 1998). This creates a carbonyl group, specifically an aldehyde, at the end of a flexible chain. This makes an excellent cross-linking site (Stadtman and Oliver, 1991). Two such aldehydes can join to form an aldol condensation product, or an aldehyde can react with the primary amine of lysine to form a Schiff base (Tanzer, 1973). These reactions are reversible. Further reactions can occur to create an array of different, more stable cross-links (Tanzer, 1973). In addition to controlling connective tissue mechanics, metal-catalyzed oxidation of proteins also widely occurs in a non-controlled way and has been implicated in tissue degradation during aging (Stadtman and Oliver, 1991). Arginine and proline can be oxidized in addition to lysine, as well as some other amino acid side chains (Requena et al., 2001; Berlett and Stadtman, 1997). Thus, metal-catalyzed oxidation is a common event with substantial mechanical implications.
The presence of iron bound to the primary gel-stiffening protein in slug glue suggests a possible oxidative role (Werneke et al., 2007). This would be a significant finding, as protein oxidation is a common biochemical event, but protein oxidation involving amino acids other than DOPA has not been studied in biological adhesives. Its possible contribution to the strength of adhesive gels suggests a broader role of oxidation in controlling material properties. This may represent a relatively simple yet powerful stiffening method. Thus, this paper investigated the hypothesis that oxidative cross-linking occurs in the glue of the slug Arion subfuscus. To test this, we first determined whether any of the proteins in the glue were significantly oxidized. We then tested whether the resulting carbonyl groups formed cross-links.
MATERIALS AND METHODS
Experimental animal
The terrestrial slug Arion subfuscus (Draparnaud 1805) and its defensive secretions were analyzed. Each slug was collected locally (Ithaca, NY, USA); the glue was collected on the same day and stored at –80°C, as described by Werneke et al. (Werneke et al., 2007). The gel is released as a viscous secretion that quickly sets to form an elastic mass, without dehydration. This fully set sample was used in all experiments unless otherwise noted. The glue contains several prominent proteins, which will be identified by the abbreviation asmp (Arion subfuscus mucus protein) followed by the relative molecular mass (Mr; ×10–3). Hence the primary glue protein will be referred to as asmp-15.
Immunoblotting to detect protein oxidation
The proteins in the samples were separated by discontinuous sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and then blotted onto membranes for immunostaining. The gels were 12.5% total acrylamide, and the methods of Hames (Hames, 1990) were used. Glue samples were dissolved in 20 mmol l–1 Tris-Cl, 10 mmol l–1 ethylenediaminetetraacetic acid (EDTA) (pH 8, 50 mg glue per ml buffer) using a rotor-stator homogenizer (PRO-200, PRO Scientific, Oxford, CT, USA). Samples were then sonicated for 5–10 s to break up large polysaccharides. They were then combined with an equal volume of SDS-PAGE sample buffer (0.125 mol l–1 Tris-Cl, pH 6.8, 4% SDS, 10% 2-mercaptoethanol, 1.6 mol l–1 urea). Samples were also collected directly into 3 mmol l–1 deferoxamine mesylate with 10 mmol l–1 EDTA as described by Werneke et al. (Werneke et al., 2007) so that metals would be removed before the glue could set. For comparison, a protein extract from the body wall of the slug was similarly analyzed. The head and internal organs were removed, leaving the outer body wall of the dorsal surface and foot. Approximately 25 mg body wall per ml buffer was used. The frozen body wall was homogenized and the homogenate was directly mixed with SDS-PAGE sample buffer.
A positive control for protein oxidation was created using the methods of Robinson et al. (Robinson et al., 1999). Bovine serum albumin (BSA, 1 mg ml–1) was mixed with equal volumes of hydrogen peroxide (1 mg ml–1) and ferrous sulfate (1 mmol l–1). It was incubated at room temperature for 10 min and then precipitated with an equal volume of 20% trichloroacetic acid (TCA). The precipitate was collected at low speed in a clinical centrifuge, washed with 20% TCA and then redissolved in the Tris-EDTA buffer.
Proteins were electroblotted onto polyvinylidene fluoride (PVDF) membranes in Towbin buffer (25 mmol l–1 Tris-Cl, 192 mmol l–1 glycine, 0.1% SDS and 20% methanol) using a semi-dry apparatus. For each experiment, duplicate gels were blotted, with one blot stained for overall protein concentration and the other immunostained for carbonyl groups. Proteins were stained with Coomassie Blue R-250 (0.02% in 40% methanol and 5% acetic acid) for 30 s and then destained in 40% methanol for 1 min. Duplicate membranes were immunostained following the methods of Robinson et al. (Robinson et al., 1999) to detect carbonyls; these are primarily aldehydes resulting from the oxidation of lysine or several other amino acids. Briefly, the staining procedure involves derivatization of carbonyl groups with 2,4-dinitrophenylhydrazine (DNPH) in an acid environment, followed by immunostaining for DNPH. Aside from a few minor changes in the washing steps, the following modifications to Robinson et al.'s procedure were made. As recommended by Wang and Powell (Wang and Powell, 2010), DNPH was dissolved in concentrated trifluoroacetic acid (TFA) instead of hydrochloric acid and then diluted to 10 mmol l–1 DNPH, 10% TFA. This solution was typically prepared fresh. The primary antibody (anti-DNP, produced in rabbit, Sigma-Aldrich, St Louis, MO, USA) was diluted 1:10,000 and incubated for 1 h at room temperature. The secondary antibody (anti-rabbit peroxidase conjugate, produced in goat, Sigma-Aldrich) was also used at 1:10,000 for 1 h at room temperature.
Antibody binding was detected using a chemi-luminescent kit (ECL Plus, GE Healthcare, Piscataway, NJ, USA) and photographed on a ChemiDoc gel imaging system (Bio-Rad, Hercules, CA, USA). This system was also used to photograph Coomassie-Blue-stained duplicate blots. Quantification of blots was performed using the software accompanying the system (Quantity One, BioRad). This method has been shown to be reliable for measuring relative carbonyl content, though it is only semi-quantitative (Shacter et al., 1994). Unless otherwise noted, five to six independent samples were tested in each experiment. All measures of oxidation were normalized for the amount of protein on the duplicate blot (anti-DNPH signal intensity divided by Coomassie Blue signal intensity for each protein).
Accessibility of carbonyls
As a preliminary test for the formation of cross-links from carbonyl groups, the accessibility of the carbonyls in the native glue was assessed relative to the denatured state. The denaturing conditions of SDS-PAGE can disrupt reversible cross-links formed from carbonyls, rendering the carbonyls accessible for detection. If the sample is not denatured first, the carbonyls that have formed cross-links will be effectively inaccessible to DNPH. Proteins were separated by gel filtration (Sephacryl S-400, GE Healthcare) as described by Smith et al. (Smith et al., 2009), using 20 mmol l–1 Tris, 10 mmol l–1 EDTA (pH 8) as a column buffer. Each protein-containing fraction from the column was divided in two; half was heat denatured in 1% SDS and the other half was left untreated. Samples of the denatured and native fractions were then spotted onto PVDF (5 μl) and immunoblotted to detect carbonyls. The immunostaining in this experiment was carried out using the OxiSelect Protein Carbonyl Immunoblot Kit (Cell Biolabs, San Diego, CA, USA). All other experiments used the previously described immunostaining procedure. The remainder of each fraction was concentrated on a Speed-Vac (Savant, Thermo Scientific, Waltham, MA, USA) and assayed by SDS-PAGE. Three different samples were analyzed in this way.
Experimental analysis to test for carbonyl-derived cross-links
Two experiments were performed to test for carbonyl-derived cross-links. It was hypothesized that a primary cross-linking mechanism would be the reaction between a carbonyl and a primary amine to form an imine bond (C=N), in this case a Schiff base. If this occurs, treatment with the reducing agent sodium borohydride would stabilize the bond and lead to reduced solubility and larger aggregates that would not show up on SDS-PAGE. If this cross-link is not present, however, borohydride treatment will reduce the carbonyls to alcohols instead (Tanzer, 1973; Robins, 1982). In contrast, treatment with the nucleophile hydroxylamine would lead to dissociation of this cross-link, with the hydroxylamine displacing the other primary amine. This would lead to increased solubility of the glue and a decrease in carbonyl content.
Sodium borohydride treatment was performed using the approach of Robinson et al. (Robinson et al., 1999). Blots of treated samples were compared with untreated controls from the same sample. A stock solution of 200 mmol l–1 sodium borohydride (NaBH4) in 100 mmol l–1 sodium hydroxide was made up fresh immediately before each experiment. This was then mixed with the glue to a final concentration of 20 mmol l–1 NaBH4 and incubated for 1 h at 37°C. The pH of the glue sample after adding the borohydride solution was typically 8.8. A range of concentrations (10–100 mmol l–1), temperatures and durations were tested, but those recommended by Robinson et al. (Robinson et al., 1999) were most effective at reducing the control protein while minimizing non-specific effects.
Hydroxylamine was used at a final concentration of 16 mmol l–1 in 10 mmol l–1 sodium phosphate, 1 mmol l–1 EDTA (pH 8). Samples were incubated for 15 min at room temperature. After each treatment, samples were centrifuged at 14,000 g for 10 min to remove any precipitate.
To test for changes in oxidation and possible bond formation over time, samples were collected into liquid nitrogen at time intervals. Samples were collected by wiping the back of the slug with a thin plastic strip to initiate secretion and collect the glue, which was then immediately dropped into liquid nitrogen. In some experiments, samples were allowed to set for 5, 10 or 60 s after secretion before freezing. Frozen samples were scraped into chilled SDS-PAGE sample buffer on ice, then homogenized and heat denatured. In some cases, samples that were frozen before they had a chance to set were scraped into chilled 20 mmol l–1 NaBH4 on ice, immediately homogenized and then incubated for 1 h at 37°C before denaturation in SDS-PAGE sample buffer. Because of the need for careful rapid handling, samples were not split to provide duplicates for the different treatments. Thus, the borohydride-reduced samples came from different slugs than the untreated samples. All samples were compared with normal glue samples that had been allowed to set fully before treatment. Duplicate blots of all samples were stained for protein and carbonyls as described previously (see Immunoblotting to detect protein oxidation). In addition, samples that had been collected directly into 10 mmol l–1 EDTA as described by Werneke et al. (Werneke et al., 2007), and had thus not been able to set, were also assayed.
Modification of primary amines
Because primary amines are common nucleophiles that would readily link with carbonyl groups, the accessibility of primary amines in the glue was measured. These are typically found on lysine side chains. An unusually low accessibility of primary amines might indicate their participation in cross-links. The number of accessible primary amines was estimated by modifying them with acid anhydrides followed by a mobility shift assay on SDS-PAGE. Citraconic anhydride is a potent and reversible reagent for binding to primary amino groups (Dixon and Perham, 1968; Palacian et al., 1990). Each citraconate group adds a known mass (112 Da) to a protein such that if 10% of a protein's amino acids carry a primary amine (i.e. lysine), then its mass would increase by roughly 10% upon citraconylation. Although this change in mass might not correspond directly to a change in migration rate on SDS-PAGE, the gel shift provides a useful estimate.
Citraconylation was carried out on glue dissolved in 10 mmol l–1 EDTA (4 μl of citraconic anhydride per ml sample). Samples were incubated for 1 h at room temperature, maintaining a constant pH. A range of pH values from 6–10 was tested, using NaOH to adjust the pH. The modifications are reported to be more stable at slightly alkaline pH (8–9) (Palacian et al., 1990), but modifications of slug glue samples that were detectable by a gel shift assay occurred much more consistently at pH 6. Thus, quantitative citraconylation experiments were carried out at pH 6. Three different samples were compared. In addition, acetic anhydride was tested on all samples. This reacts similarly, but adds significantly less mass.
Amino acid analyses were performed on either whole glue samples or selected individual proteins separated by SDS-PAGE, transferred to PVDF and excised from the membrane. The proteins were hydrolyzed overnight at 110°C under vacuum in 6 mol l–1 HCl with 5% phenol, and then analyzed on a Beckman System 6300 Auto Analyzer (Beckman Coulter, Brea, CA, USA). Blanks were taken from adjacent regions on the membrane outside the lanes containing protein. Typically two replicates were performed for each protein, often from different samples.
RESULTS
Extent of protein oxidation in A. subfuscus glue
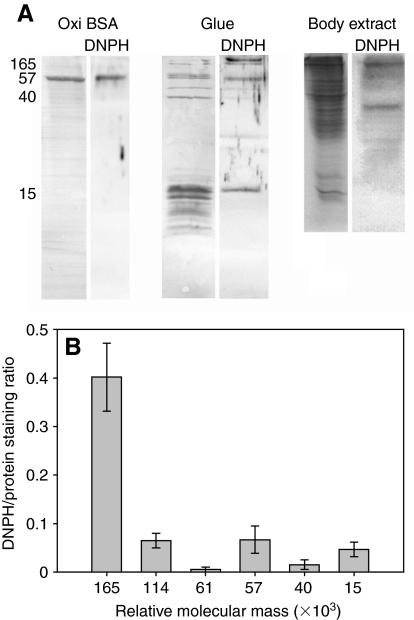
The proteins in the glue secreted by A. subfuscus were strongly oxidized (Fig. 1). Asmp-165 was most strongly oxidized, typically equal to or greater than the positive control (BSA oxidized with ferrous iron and hydrogen peroxide). Note that the positive control used in these experiments gave a signal that was much stronger than a similar commercially available control (Oxiselect protein carbonyl immunostaining kit). A number of the other proteins were also oxidized, notably asmp-114, asmp-57 and asmp-15. Negative controls without DNPH showed no staining. The group of proteins with a Mr of ∼15×103 are characteristic of the adhesive form of mucus, and they cause gel stiffening (Pawlicki et al., 2004), so it is intriguing that only one member of this group stained for carbonyls. The staining of this protein was variable; in some samples it stained only faintly. Similarly, asmp-57 and asmp-61 also characterize the adhesive form of mucus (Pawlicki et al., 2004), but only asmp-57 was consistently oxidized; asmp-61 only occasionally showed oxidation, but sometimes it was strongly oxidized. Results from quantifying the area of the bands instead of their peak intensity gave similar results overall, though peak area tended to underestimate the oxidation of asmp-15 as the program had difficulty separating it from neighboring peaks. The staining was specific, as whole body extracts showed only a few proteins that were oxidized (Fig. 1A). Interestingly, none of the oxidized proteins in the glue had an oxidized counterpart at the same relative mobility in the whole body extract. Unless their relative molecular mass changes significantly during or after secretion, they appear not to be oxidized before secretion.
Fig. 1.
The extent of oxidation of the proteins in Arion subfuscus glue, based on anti-DNPH immuno-staining for carbonyl groups. (A) Sample of oxidized BSA (positive control), glue and whole body extract. Left lanes show each blot stained for total protein content using Coomassie Blue R-250. Right lanes (DNPH) show the corresponding anti-DNPH immune blots. The numbers on the left show relative molecular mass (×103). (B) Quantification of the extent of oxidation of selected proteins in the glue based on signal intensity. Values are the ratios of staining intensity of the immunostain relative to the staining intensity of Coomassie Blue for that protein on a duplicate blot (mean ± s.e.m., N=6). Oxidized BSA (positive control) had a DNPH/protein staining ratio of 0.37±0.04.
Accessibility of carbonyl groups
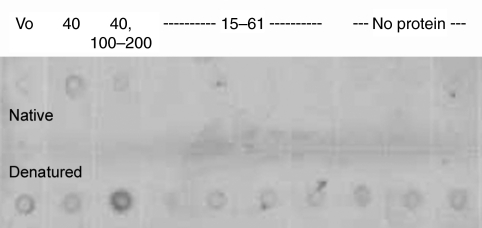
The oxidation of the proteins was not readily detectable in their native state. When proteins were separated by gel filtration and spotted directly onto a membrane without denaturation, most did not stain for the presence of carbonyls, though there was some staining centered around the fractions containing asmp-40 [which eluted as a giant complex, as seen by Smith et al. (Smith et al., 2009)] (Fig. 2). There was no detectable staining in the fractions containing asmp-15, despite containing the largest amount of protein. When the samples were treated with SDS, fractions containing asmp-114 and asmp-165 stained much more strongly, suggesting that the carbonyl groups became more accessible to DNPH. This was also true to a lesser extent for the void volume fraction, which likely contains very large carbohydrates. However, SDS denaturation did not lead to increased staining of fractions containing asmp-15. There was uniform light staining of all the other SDS-treated fractions, which may be due in part to contaminants in the SDS, as it was equally present in samples that appear to contain little to no protein. The fractions that eluted after asmp-15 contained no protein detectable by SDS-PAGE, but they did contain a yellow pigment and, in some other experiments, proteins with a Mr <10×103. These would not show up on the 12.5% gels used in this experiment.
Fig. 2.
DNPH immunostained dot blot of proteins separated by gel filtration. The top row shows proteins in their native state, while the bottom row shows the same samples denatured before adding to the membrane. The numbers across the top show the relative molecular mass (×103) of the proteins in each fraction. The first spot contains the void volume fraction (Vo), which may contain giant polysaccharides and some protein. The second spot contains asmp-40, which elutes as a giant complex near the void volume. The third spot contains primarily asmp-114 and asmp-165, with a small amount of asmp-40. The fourth through seventh spots contain asmp-15, asmp-57 and asmp-61, with the proteins with a Mr of ∼15×103 making up the bulk of the material. The remaining spots contained no proteins that were visible on SDS-PAGE, though there was some material, as indicated by a yellow color in the samples.
Experimental analysis to test for carbonyl-derived cross-links
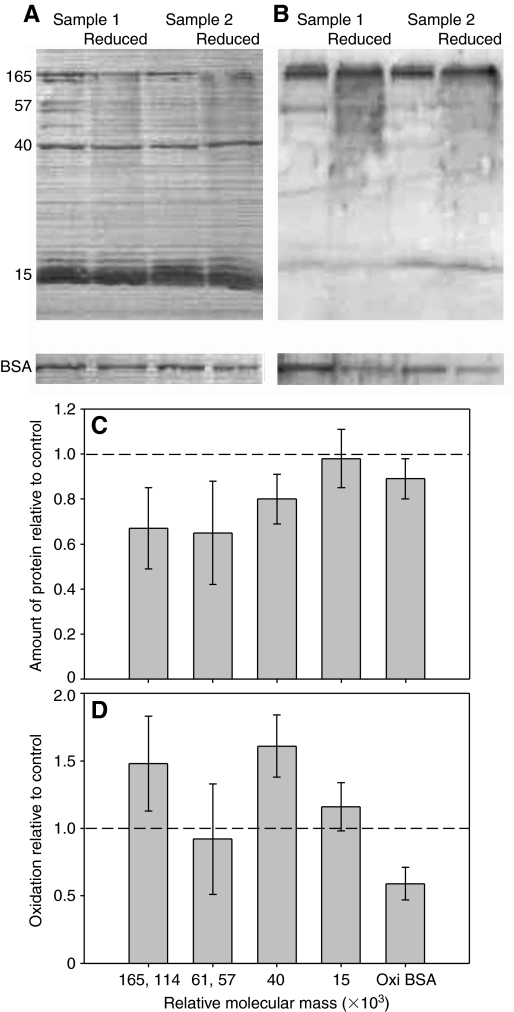
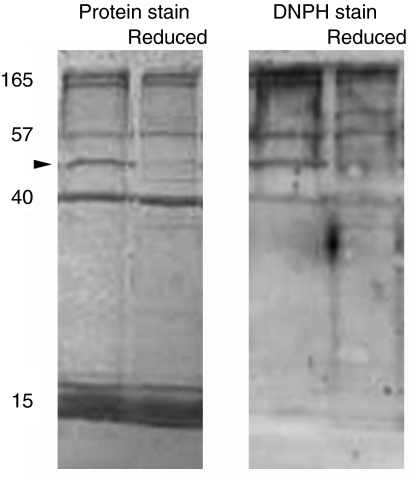
The primary effect of sodium borohydride on the glue was to cause a decrease in the amount of soluble protein visible on SDS-PAGE (Fig. 3A,C). The amount of higher molecular weight proteins (Mr=57–165×103) decreased by approximately one-third, whereas asmp-40 decreased by ∼20%. In contrast, the group of proteins including asmp-15 was not noticeably affected. Also, sodium borohydride did not detectably decrease the extent of oxidation of the proteins in the glue (Fig. 3B,D). The variation in the latter results was relatively high, which was likely due to two reasons. First, there was increased background staining in the anti-DNPH immunoblots of all borohydride-treated samples. Second, the decrease in soluble protein in borohydride-treated samples led to fainter bands, making precise quantification more difficult. Also, in several different samples, asmp-40 had no detectable anti-DNPH staining in the control, so changes in the extent of oxidation of this protein could only be quantified in two of the five samples. Overall, though, it is clear that there was no significant loss of carbonyl groups when protein concentration was accounted for. In contrast, sodium borohydride was effective at reducing the control protein, consistently decreasing the carbonyl content of the oxidized BSA by nearly half. Higher concentrations of sodium borohydride caused greater loss of soluble protein (except those in the group including asmp-15), but they still did not lead to reduction of the carbonyl content of the proteins in the glue (data not shown).
Fig. 3.
The effect of sodium borohydride on the proteins in A. subfuscus glue. (A) Coomassie-Blue-stained blot showing the proteins present in the glue, and (B) anti-DNPH immunostained duplicate blot. Two different samples are shown; for each, the left lane is untreated whereas the right lane was reduced with borohydride. Oxidized BSA controls treated in the same way are shown at the bottom. The numbers on the left show relative molecular mass (×103). (C) Amount of soluble protein in borohydride-treated samples relative to untreated controls for selected proteins. (D) Extent of oxidation of selected proteins from borohydride-treated samples relative to their untreated counterpart. Some proteins were grouped together because they were difficult to quantify separately. The dashed lines in C and D correspond to the y-value if there was no difference between the control and treatment. Values are means ± s.e.m. (N=5).
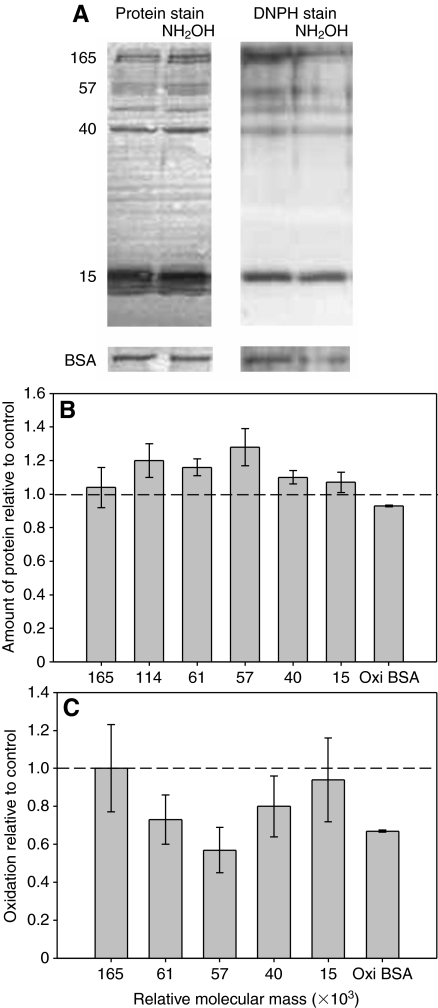
Hydroxylamine caused an increase in the amount of soluble protein and a decrease in the extent of oxidation of many of the proteins (Fig. 4). The increase in protein concentration was typically in the range of 10–20% for the proteins in the glue, whereas BSA was slightly decreased if at all. After adjusting for the amount of protein present, the decrease in oxidation of asmp-40, asmp-57 and asmp-61 was ∼20–40% on average. This was comparable to the effect that hydroxylamine had on oxidized BSA. Asmp-15 and asmp-165 were not affected on average, though this was primarily due to one sample; all the other samples showed a decrease in oxidation similar to the other proteins. Also, because of the variability in carbonyl staining of asmp-15 and asmp-61, changes in the extent of their oxidation could only be quantified in three of the five samples.
Fig. 4.
The effect of hydroxylamine on the proteins in A. subfuscus glue. (A) The left two lanes show the Coomassie-Blue-stained blot, and the right two lanes show the DNPH immunostained duplicate blot. For each pair, the left lane is untreated and the right lane is treated with hydroxylamine (NH2OH). Oxidized BSA controls treated in the same way are shown at the bottom. The numbers on the left show relative molecular mass (×103). (B) Amount of soluble protein in hydroxylamine-treated samples relative to untreated controls for selected proteins. (C) Extent of oxidation of selected proteins from hydroxylamine-treated samples relative to their untreated counterpart. Note that asmp-114 is not included in this graph because its peak could not be easily distinguished from the strong signal for asmp-165. The dashed lines in B and C correspond to the y-value if there was no difference between the control and treatment. Values are means ± s.e.m. (N=5).
Changes over the course of glue setting
The extent of oxidation of samples that were frozen immediately upon secretion was not noticeably different from that of mature glue samples (Fig. 5). Although Fig. 5 shows little staining of asmp-15, this was not consistent, as it stained well in other samples. Similarly, no clear differences were apparent among samples frozen 2–60 s after secretion (data not shown). During this time, the glue set from a viscous liquid to a firmly elastic gel. Similarly, samples that were collected directly into 10 mmol l–1 EDTA were just as oxidized as samples from mature glue (data not shown). As previously noted, none of the oxidized proteins in the glue have corresponding oxidized counterparts at the same relative mobility in the whole body extract (Fig. 1A). Together these results suggest that oxidation occurs less than 2 s after secretion.
Fig. 5.
Analysis of A. subfuscus glue that was frozen before setting. The left two lanes show the Coomassie-Blue-stained blot, and the right two lanes show the DNPH immunostained duplicate blot. For each pair, the left side is the control and the right side is reduced with NaBH4. In this procedure, the control and reduced samples are not from the same animal. The arrowhead indicates asmp-44, which is prominent in immediately frozen samples. The numbers on the left show relative molecular mass (×103).
Another significant finding was that there is a protein with a Mr of 44×103 (asmp-44) that was present in substantially greater quantities in unset glue that was frozen immediately compared with mature glue (Fig. 5). The change in the relative abundance of this protein was not quantified because the need for rapid sampling made it difficult to control for the amount of glue collected in each swipe. Nevertheless, in all immediately frozen samples, it was a prominent band on SDS-PAGE, whereas it was always one of the more faint bands from mature glue. All the other proteins were present in roughly similar amounts in the unset and mature glue, and at the same relative molecular mass. Finally, as with the mature glue, sodium borohydride appeared unable to reduce the carbonyl content of the proteins (Fig. 5). The comparison was not quantified, nor was the possible loss of soluble protein, as the reduced and non-reduced samples came from different animals. However, borohydride treatment had a particularly striking effect on asmp-44. This protein was almost absent in borohydride-treated samples.
Accessibility of primary amines
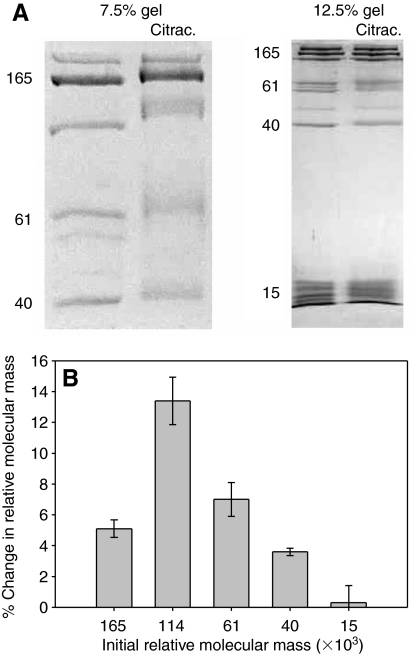
The glue as a whole had a significant lysine content (5.5 mol%), and asmp-15 was particularly enriched in lysine (8–9%). Nevertheless, the proteins in the glue were only modified consistently by citraconic anhydride at pH 6. Furthermore, even at pH 6 there was no change in apparent mass of the proteins in the group that included asmp-15 (Fig. 6). Treatment with acetic anhydride gave similar results. Although there appeared to be significant variability in the results for asmp-15, this was due to distortions that were more common at the bottom of the lane, such as uneven bands. The only effect of the citraconic anhydride on the proteins in this range was to cause the bands to blur and spread out somewhat. This could be a result of partial modification of some of the proteins. It should be noted that in a few of the preliminary experiments, this protein did show some modification, but it was not repeatable.
Fig. 6.
The effect of citraconylation at pH 6 on the relative molecular mass of the proteins in A. subfuscus glue. (A) Coomassie-Blue-stained SDS-PAGE. A low- and a high-percentage gel are shown (7.5 and 12.5%, respectively). For each pair, the left lane is the control and the right lane is treated with citraconic anhydride (citrac.) to modify primary amines. The numbers on the left of each show relative molecular mass (×103). (B) Percent change in relative molecular mass of selected proteins after citraconylation (mean ± s.e.m., N=3).
The amino acid analysis showed no evidence of collagen proteins; there was no hydroxyproline, and the overall glycine content of the glue was 9.2%, which was roughly similar among the proteins tested. In addition, there was no DOPA. Three of the prominent bands at Mr values of ∼15×103 were analyzed, and all had strikingly similar amino acid compositions.
DISCUSSION
The experimental results are consistent with the hypothesis that oxidative cross-linking occurs in slug glue. A number of proteins in A. subfuscus glue are substantially oxidized (Fig. 1). The extent of oxidation suggests that it plays a significant functional role. The carbonyls that are formed could readily react with each other and with primary amines to create reversible cross-links. When carbonyls react with primary amines, the result is an imine bond, in this case a Schiff base. The presence of such cross-links in slug glue is suggested by the inaccessibility of carbonyls in the native state (Fig. 2). They are only detected after denaturing in SDS, which would presumably dissociate these reversible cross-links. More importantly, sodium borohydride treatment caused a decrease in the amount of soluble protein seen on SDS-PAGE (Fig. 3A,C), specifically those with Mr ≥40×103. This is what was expected if imine bonds were present, as they would be stabilized by reduction. This stabilization would lead to undissociable aggregates that would not appear in SDS-PAGE. Such aggregates may have contributed to the increased background that was seen. Alternatively, if the hypothesized bonds had not been present, borohydride would have reduced the carbonyls in the glue to alcohols instead. Borohydride treatment is a potent, commonly used method of converting protein carbonyl groups to alcohols (Robinson et al., 1999). The treatment was strong enough to reduce the carbonyls in the positive control by almost half. Nevertheless, it did not cause a noticeable loss of carbonyls from the proteins in the glue (Fig. 3B,D). The effect of hydroxylamine (Fig. 4) is also consistent with the presence of these bonds, as hydroxylamine would lead to dissociation of the bond and modification of the carbonyl, as was seen. Finally, the difficulty in modifying the primary amines, particularly those on asmp-15, suggests that they may participate in cross-links.
Differences in oxidation among proteins in the glue
It is intriguing that there was variation in the extent of oxidation. Although immuno-blotting methods are only partially quantitative for measuring carbonyl groups, they can give a clear indication of the relative extent of oxidation (Shacter et al., 1994). The differences among proteins were clear and consistent, and they represent substantial differences in the number of carbonyl groups per protein. A strongly oxidized protein can have a seven- to 14-fold increase in the amount of glutamic semialdehydes (a common result of metal-catalyzed oxidation) relative to a lightly oxidized protein (Requena et al., 2001). Thus, a strongly oxidized protein would clearly have a substantial number of carbonyl groups that could form cross-links. Given that asmp-165 stands out in being most strongly oxidized, it is worth noting that it is a good size for forming the primary backbone of a gel; Ledward found that proteins with Mr between 100×103 and 200×103 form the strongest gels in gelatin (Ledward, 2000).
The proteins with Mr of ∼15×103 are interesting as they are characteristic of A. subfuscus glue and have been implicated in gel stiffening. The fact that only one of these proteins was oxidized likely indicates that the protein plays a somewhat different role in that process. Explaining why this one protein in the group is oxidized may help a great deal in understanding their role in cross-linking. The proteins with Mr of ∼15×103 may be related, which also makes the difference in oxidation notable. The proteins in this region have similar amino acid compositions, and they respond similarly to different treatments. They could be different size variants of a specific protein, they could represent different degrees of post-translational modification or they could reflect the effect of proteolytic cleavage. There do not appear to be any differences in the amount or relative molecular mass of these proteins in samples from unset glue and mature glue, suggesting that there is no proteolytic cleavage involved in maturation, unlike in barnacle cement, where proteolytic cleavage appears to lead to the loss of some proteins because of their incorporation into larger complexes (Dickinson et al., 2009). If there is a modification, it likely occurs before secretion.
An important remaining question is when the oxidation occurs. The extent of oxidation is comparable in glue immediately frozen upon collection and glue that has had several minutes to set. However, there do not appear to be any comparable oxidized proteins in extracts of the slug's body. This suggests that oxidation occurs immediately before secretion, or within the next second or two. It will also be important to determine how the oxidation is catalyzed. The relationship between the proteins with Mr of ∼15×103 and iron (Werneke et al., 2007) is suggestive, but further work needs to be performed to identify whether any of the proteins in this range have catalytic activity.
Differences among proteins in contribution to cross-linking
The differential effect of borohydride on protein solubility is worth noting. The more heavily oxidized proteins such as asmp-165, asmp-114 and asmp-57 seem to be the most strongly affected, suggesting that they form the main cross-links as a result of oxidation. The effect on asmp-44, which is characteristic of freshly secreted glue, was even more striking, as it disappeared after borohydride treatment. It is possible that this loss is a normal part of maturation, which the borohydride fails to block, but this protein is normally present in small quantities in the mature glue, whereas it is almost completely absent after borohydride treatment of the unset glue. It is also not greatly affected by borohydride once the glue has matured. Also striking is the insensitivity of the proteins with Mr of ∼15×103. These were not affected by borohydride or by citraconylation. This suggests that they participate differently in cross-links.
The possibility that primary amines contribute to cross-links is consistent with the citraconylation experiments (Fig. 6). The proteins in the range of 15×103 Mr have a large number of primary amines when hydrolyzed, but they were not detectably modified by citraconic anhydride in their native state. All the other proteins were strongly modified at pH 6, creating a mobility shift in SDS-PAGE of a magnitude typical for roughly 3–10% lysine content. The inaccessibility of the primary amines on the group of proteins including asmp-15 is consistent with their possible participation in cross-links. Notably, none of the proteins in the glue were consistently modified at slightly alkaline pH, despite the fact that alkaline pH is the standard condition for such treatments and typically leads to more stable modification (Palacian et al., 1990). This is why the tests were run at a pH of 6. The difference between asmp-15 and the other proteins may reflect differences in the dissociation of cross-links at different pH values. The fact that the group of proteins including asmp-15 is more resistant to modification than the other proteins may mean that they form different cross-links, as suggested above.
The role of oxidation in the context of other cross-linking mechanisms
Because of the extent of oxidation, and the evidence that the resulting carbonyl groups participate in cross-links, protein oxidation may play an important role in holding the glue together. However, it is not the only mechanism. It will be interesting to determine the relative contribution of protein oxidation to gel mechanics, as there are clearly multiple cross-linking mechanisms that are active. The fact that EDTA disrupts the mature glue strongly suggests that there are direct metal-based cross-links. There is a substantial amount of zinc in the glue, and zinc often forms cross-links in biomaterials (Lichtenegger et al., 2008). In addition, there are likely to be significant amounts of other common divalent ions such as calcium and magnesium, which may also contribute to direct cross-links. Finally, asmp-40 forms large complexes that are insensitive to EDTA (Smith et al., 2009) and do not show a high level of oxidation. These may be cross-linked in a different way.
The different mechanisms may contribute to different aspects of mechanics; for example, coordinate bonds where metals bind directly to ligands on polymers are useful because they are strong but can break and reform easily, thus allowing energy dissipation and healing (Lichtenegger et al., 2008). Additionally, the mechanisms may work together, as is the case for tubeworm cement (Stewart et al., 2004). In this case, interactions between calcium and phosphate bring polymers together by triggering complex coacervation, where polymers coalesce locally leaving fluid-filled spaces. This creates a foam-like material. After coacervation, DOPA-based cross-linking occurs between the polymers in order to tan the cement and make it more permanent. Slug glue may involve similar stages of bringing polymers together in a network to make a gel, followed by oxidative cross-linking to further stabilize the gel. Finally, it is important to remember that metals and oxidation might also contribute to adhesion, and not just to the cross-linking of polymers to create cohesive strength.
These results suggest that protein oxidation may be a generally useful way to cross-link gels. Metal-catalyzed oxidations involving DOPA and other phenolic molecules have already been shown to contribute in a number of biomaterials (Waite, 1990). It is known that carbonyl formation occurs with other amino acids as well. Given how common metal-catalyzed oxidations are, and the widespread presence of primary amines on protein side chains, it is possible that many biomaterials are strengthened by interactions between carbonyls and primary amines. These interactions play a central role in cross-linking collagen and elastin. Such a potent and relatively simple mechanism may contribute to cross-linking in other materials as well. In addition, it provides an alternative means of strengthening synthetic bioadhesives. Already, several groups have successfully used the interaction between carbonyls and primary amines to create stiffer synthetic gels using DOPA quinone (Kumar et al., 2000; Chen et al., 2003); it may be possible to use other oxidized amino acids as well.
LIST OF ABBREVIATIONS
- BSA
bovine serum albumin
- DNPH
2,4-dinitrophenylhydrazine
- DOPA
dihydroxyphenylalanine
- EDTA
ethylenediaminetetraacetic acid
- NaBH4
sodium borohydride
- PVDF
polyvinylidene fluoride
- SDS-PAGE
sodium dodecyl sulphate polyacrylamide gel electrophoresis
- TCA
trichloroacetic acid
- TFA
trifluoroacetic acid
FOOTNOTES
This work was supported by grant no. 1 R15 EB006001-01 from the National Institutes of Health (NIH) and a summer research grant from Ithaca College. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of NIH. We thank S. Ulrich for helpful suggestions and discussions. Deposited in PMC for release after 12 months.
REFERENCES
- Berlett B. S., Stadtman E. R. (1997). Protein oxidation in aging, disease, and oxidative stress. J. Biol. Chem. 272, 20313-20316 [DOI] [PubMed] [Google Scholar]
- Broomell C. C., Mattoni M. A., Zok F. W., Waite J. H. (2006). Critical role of zinc in hardening of Nereis jaws. J. Exp. Biol. 209, 3219-3225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T., Embree H. D., Brown E. M., Taylor M. M., Payne G. F. (2003). Enzyme-catalyzed gel formation of gelatin and chitosan: potential for in situ applications. Biomaterials 24, 2831-2841 [DOI] [PubMed] [Google Scholar]
- Coyne K. J., Qin X. X., Waite J. H. (1997). Extensible collagen in mussel byssus: a natural block copolymer. Science 277, 1830-1832 [DOI] [PubMed] [Google Scholar]
- Dickinson G. H., Vega I. E., Wahl K. J., Orihuela B., Beyley V., Rodriquez E. N., Everett R. K., Bonaventura J., Rittschof D. (2009). Barnacle cement: a polymerization model based on evolutionary concepts. J. Exp. Biol. 212, 3499-3510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon H. B. F., Perham R. N. (1968). Reversible blocking of amino groups with citraconic anhydride. Biochem. J. 109, 312-314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hames B. D. (1990). One dimensional polyacrylamide gel electrophoresis. In Gel Electrophoresis of Proteins: A Practical Approach (ed. Hames B. D., Rickwood D.), pp. 1-147 Oxford: IRL Press; [Google Scholar]
- Kumar G., Bristow J. F., Smith P. J., Payne G. F. (2000). Enzymatic gelation of the natural polymer chitosan. Polymer 41, 2157-2168 [Google Scholar]
- Ledward D. A. (2000). Gelatin. In Handbook of Hydrocolloids (ed. Phillips G. O., Williams P. A.), pp. 67-86 Cambridge: Woodhead Publishing Limited; [Google Scholar]
- Lichtenegger H. C., Schoberl T., Ruokolainen J. T., Cross J. O., Heald S. M., Birkedal H., Waite J. H., Stucky G. D. (2003). Zinc and mechanical prowess in the jaws of Nereis, a marine worm. Proc. Natl. Acad. Sci. USA 100, 9144-9149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenegger H. C., Birkedal H., Waite J. H. (2008). Heavy metals in the jaws of invertebrates. Met. Ions Life Sci. 4, 295-325 [Google Scholar]
- Palacian E., Gonzalez P. J., Pineiro M., Hernandez F. (1990). Dicarboxylic acid anhydrides as dissociating agents of protein-containing structures. Mol. Cell. Biochem. 97, 101-111 [DOI] [PubMed] [Google Scholar]
- Pawlicki J. M., Pease L. B., Pierce C. M., Startz T. P., Zhang Y., Smith A. M. (2004). The effect of molluscan glue proteins on gel mechanics. J. Exp. Biol. 207, 1127-1135 [DOI] [PubMed] [Google Scholar]
- Requena J. R., Chao C.-C., Levine R. L., Stadtman E. R. (2001). Glutamic and aminoadipic semialdehydes are the main carbonyl products of metal-catalyzed oxidation of proteins. Proc. Natl. Acad. Sci. USA 98, 69-74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robins S. P. (1982). Analysis of the crosslinking components in collagen and elastin. Methods Biochem. Anal. 28, 329-379 [DOI] [PubMed] [Google Scholar]
- Robinson C. E., Keshavarzian A., Pasco D. S., Frommel T. O., Winship D. H., Holmes E. W. (1999). Determination of protein carbonyl groups by immunoblotting. Anal. Biochem. 266, 48-57 [DOI] [PubMed] [Google Scholar]
- Sagert J., Sun C., Waite J. H. (2006). Chemical subtleties of mussel and polychaete holdfasts. In Biological Adhesives (ed. Smith A. M., Callow J. A.), pp. 125-143 Berlin: Springer; [Google Scholar]
- Sever M. J., Weisser J. T., Monahan J., Srinivasan S., Wilker J. J. (2004). Metal-mediated cross-linking in the generation of a marine-mussel adhesive. Angew. Chem. Int. Ed. Engl. 43, 448-450 [DOI] [PubMed] [Google Scholar]
- Shacter E., Williams J. A., Lim M., Levine R. L. (1994). Differential susceptibility of plasma proteins to oxidative modification: examination by western blot immunoassay. Free Radic. Biol. Med. 17, 429-437 [DOI] [PubMed] [Google Scholar]
- Smith A. M., Robinson T. M., Salt M. D., Hamilton K. S., Silvia B. E., Blasiak R. (2009). Robust cross-links in molluscan adhesive gels: testing for contributions from hydrophobic and electrostatic interactions. Comp. Biochem. Physiol. 152B, 110-117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith-Mungo L. I., Kagan H. M. (1998). Lysyl oxidase: properties, regulation and multiple functions in biology. Matrix Biol. 16, 387-398 [DOI] [PubMed] [Google Scholar]
- Stadtman E. R., Oliver C. N. (1991). Metal-catalyzed oxidation of proteins: physiological consequences. J. Biol. Chem. 266, 2005-2008 [PubMed] [Google Scholar]
- Stewart R. J., Weaver J. C., Morse D. E., Waite J. H. (2004). The tube cement of Phragmatopoma californica: a solid foam. J. Exp. Biol. 207, 4727-4734 [DOI] [PubMed] [Google Scholar]
- Sun C. J., Fantner G. E., Adams J., Hansma P. K., Waite J. H. (2007). The role of calcium and magnesium in the concrete tubes of the sandcastle worm. J. Exp. Biol. 210, 1481-1488 [DOI] [PubMed] [Google Scholar]
- Tanzer M. L. (1973). Cross-linking of collagen. Science 180, 561-566 [DOI] [PubMed] [Google Scholar]
- Waite J. H. (1990). The phylogeny and chemical diversity of quinone-tanned glues and varnishes. Comp. Biochem. Physiol. 97B, 19-29 [DOI] [PubMed] [Google Scholar]
- Wang P., Powell S. R. (2010). Decreased sensitivity associated with an altered formulation of a commercially available kit for detection of protein carbonyls. Free Radic. Biol. Med. 49, 119-121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werneke S. W., Swann C., Farquharson L. A., Hamilton K. S., Smith A. M. (2007). The role of metals in molluscan adhesive gels. J. Exp. Biol. 210, 2137-2145 [DOI] [PubMed] [Google Scholar]
- Zeng H., Hwang D. S., Israelachvili J. N., Waite J. H. (2010). Strong reversible Fe3+-mediated bridging between dopa-containing protein films in water. Proc. Natl. Acad. Sci. USA 107, 12850-12853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H., Waite J. H. (2006). Proteins in load-bearing junctions: the histidine-rich metal-binding protein of mussel byssus. Biochemistry 45, 14223-14231 [DOI] [PMC free article] [PubMed] [Google Scholar]