Abstract
Background Medically unexplained symptoms (MUS) are common in primary health care. Both patients and doctors are burdened with the symptoms that negatively affect patients' quality of life. General practitioners (GPs) often face difficulties when giving patients legitimate and convincing explanations for their symptoms. This explanation is important for reassuring patients and for maintaining a good doctor–patient communication and relationship.
Objective To provide an overview of explanatory models for MUS.
Study design We performed a systematic search of reviews in PsycINFO and PubMed about explanatory models of MUS. We performed a qualitative analysis of the data according to the principles of constant comparative analysis to identify specific explanatory models.
Results We distinguished nine specific explanatory models of MUS in the literature: somatosensory amplification, sensitisation, sensitivity, immune system sensitisation, endocrine dysregulation, signal filter model, illness behaviour model, autonomous nervous system dysfunction and abnormal proprioception. The nine different explanatory models focus on different domains, including somatic causes, perception, illness behaviour and predisposition. We also found one meta‐model, which incorporates these four domains: the cognitive behavioural therapy model.
Conclusion Although GPs often face difficulties when providing explanations to patients with MUS, there are multiple explanatory models in the scientific literature that may be of use in daily medical practice.
Keywords: explanatory models, medically unexplained symptoms, primary health care
Introduction
Medically unexplained symptoms (MUS) have a high prevalence in health care. Physical symptoms such as headache, backache, pain in muscles and joints and fatigue are common. In the general population two‐thirds of men and four‐fifths of women report at least one of these complaints in the previous two weeks.1 In about 25–50% of symptoms seen in primary health care, no evidence can be found for any physical disease.2,3 In specialist care these percentages are even higher, ranging from 30 to 70%.4,5
MUS can become chronic. Patients with persistent MUS are at risk for extensive investigations and referrals, therefore becoming a great burden on health care.6,7 Doctors and patients are both burdened by the phenomenon of symptoms without disease. Bodily symptoms with unknown physical pathology have a great impact on patient functioning. Such patients suffer greatly from the symptoms and their quality of life is negatively affected.8,9
Unexplained physical symptoms are often confusing for both doctor and patient.10,11 Many general practitioners (GPs) feel powerless and irritated when patients repeatedly visit their practice with these symptoms.12 Patients often feel disbelieved and not taken seriously by their doctors.13 Although it is often suggested that GPs are pressured by patients with MUS to deliver somatic interventions, Ring et al point out that patients with MUS request somatic interventions less often than physicians offer them.14 Moreover, patients seek emotional support and a legitimate and convincing explanation for their symptoms.15–17
GPs recognise the importance of explaining the diagnosis of MUS adequately to patients with persistent MUS. However, they often face difficulties in explaining the nature of the symptoms during clinical encounters with these patients.18 Therefore, we searched and analysed the literature for explanatory models for MUS. Providing an overview of such models can improve the knowledge and communication of GPs, thus enhancing the quality of care for patients with MUS.
Methods
Data sources and search strategy
We performed a qualitative analysis of systematic and narrative reviews on the topic of medically unexplained symptoms using the databases PubMed and PsycINFO. We decided to search for reviews, as in this type of article views of MUS and explanatory models are frequently discussed. Our search strategy consisted of two search strings which we combined with the Boolean operator AND. The first string contained keywords relating to MUS, combined with the Boolean operator OR. The second string of our search strategy contained terms for explanatory models, combined with OR (see Figure 1). This search string was limited to reviews, the English and Dutch languages, articles published in the last five years, and age over 18 years. We limited our search strategy to articles published in the last five years as most articles about explanatory models of MUS published before 2005 have been reviewed in more recent reviews.
Figure 1.
Search strategy
We tested the accuracy of our search strategy by checking whether or not five key papers on explanatory models in MUS were included in the results.
Study selection
Two researchers (JvR, ToH) independently performed inclusion and exclusion of articles, studying title and abstract. In case of doubt they consulted the full paper. Disagreements on inclusion were discussed in a consensus meeting. All disagreements were easily resolved. We calculated inter‐rater agreement for inclusion with kappa statistics.19
We excluded studies that focused primarily on patients suffering from single‐symptom unexplained disorder (tension headaches, dysmenorrhoea) and distinctive functional somatic syndromes (irritable bowel syndrome, chronic fatigue syndrome) because we were interested in explanatory models of undifferentiated MUS in the literature. We focused on undifferentiated MUS as we assume that these are more difficult to explain than single symptom unexplained disorders and distinctive functional syndromes.20 We also excluded studies that focused primarily on patients with medical or psychiatric disease (except somatoform disorders). Studies on children and adolescents (age less than 18 years) and studies on specific groups of patients such as refugees, street prostitutes etc. were also excluded.
Data analysis
We analysed the included reviews for explanatory models describing the cause of MUS. The publications were fully entered into a computer database (Atlas.ti) suitable for qualitative processing. The collection and analysis of data from the included reviews was performed both parallel and cyclic, thus mutually influencing each other. First, two researchers (JvR and ToH) independently read the articles in which many different models were assembled, to develop a coding scheme of explanatory models. Initial coding was discussed to seek agreement on content. The coding was improved, adjusted, explicated and specified by applying the constant comparative method.21 One researcher (JvR) thematically coded the included articles in Atlas.ti according to the final coding scheme.
Results
We retrieved 710 articles from the search in the electronic databases (480 from PubMed and 230 from PsycINFO). Sixty‐five papers were duplicates. After two independent researchers screened title and abstract by, 24 papers fulfilled the inclusion criteria (Figure 2). The inter‐rater agreement (kappa) was 0.65 (95% CI: 0.51–0.79), which was considered ‘good’. Two articles were not available in the Netherlands and were therefore excluded. After reading the full text, 19 out of 22 articles were included in our study.13,22–39 The three articles that were excluded reported on therapy/diagnosis or somatic disease and one turned out to be a review of a book.
Figure 2.
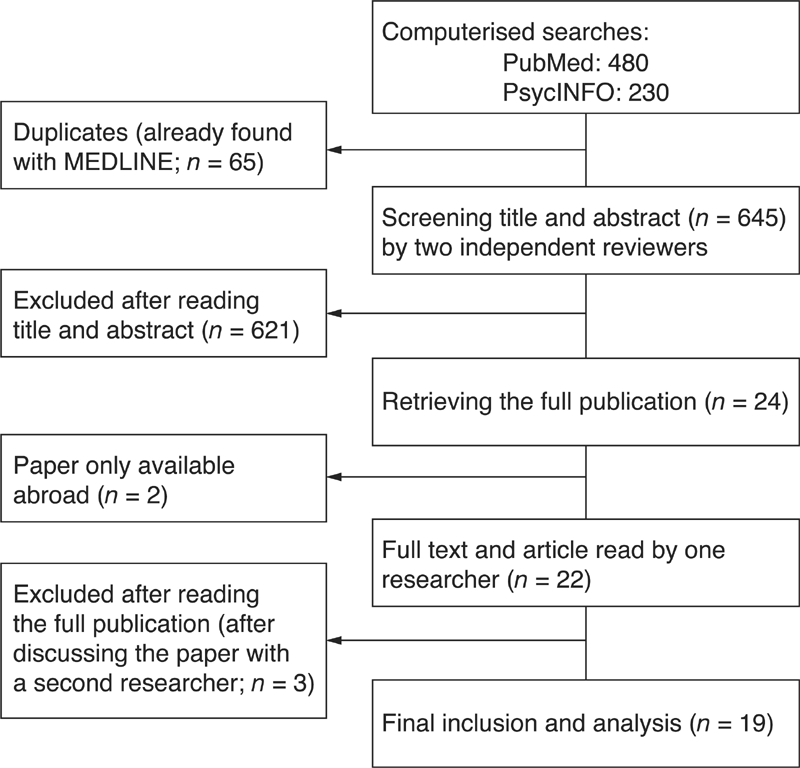
Selection of studies
We could distinguish nine different explanatory models (somatosensory amplification, sensitisation, sensitivity, immune system sensitisation, endocrine dysregulation, signal filter model, illness behaviour model, autonomous nervous system dysfunction and abnormal proprioception) and one meta‐model (the cognitive behavioural therapy model) that contains components of these nine different explanatory models. Each model is described, including citations and comments from the reviews.
Explanatory models
A. Somatosensory amplification theory
The process described as somatosensory amplification suggests that a physical sensation arises and that, as a consequence, patients focus their attention on this sensation. They develop certain cognitions and attributions which further amplify the perception of these physical signals. This amplification results in a vicious circle in a way that symptoms are reinforced by patients' thoughts and concerns. As a result patients with MUS experience a range of feelings as more severe, more damaging and more alarming.
‘The strength of this model is its simple formulation, and it can even be used to explain the disorder to patients. The basic mechanisms used in this model, such as attention, perception, and attribution processes have some empirical validation, although the model neglects many other well‐validated factors, or offers only indirect explanations for them.’ (p. 837)22
‘Petrie and Weinman (2003) have called for more attention to be given to symptom appraisal and we would widen this by calling for more attention to attention in general. The theoretical literature and some of the empirical literature supports this mechanism as being an important part of the cycle maintaining MUS.’ (p. 791)23
‘Amplification has, in general, been found to be related to reporting of somatic symptoms. However, there are conflicting reports on whether this is an independent effect or whether this is mediated by such factors as anxiety, depression and negative affect/neuroticism. Findings suggest that somatosensory amplification can only partially account for somatisation, and that other mechanisms may also be important in this process.’ (p. 28)26
B. Sensitisation theory
Sensitisation means having an enhanced somatic response to sensations as a result of former experiences of these sensations. In patients with MUS, repeated experiences of pain and symptoms can lead to memory traces at a neuronal level which increase sensitivity for future stimulation. This could result in normal benign stimuli being perceived as pain. A patient's body reacts stronger to stimuli when it has become more sensitive by earlier and repetitive encounters. The process of sensitisation has, besides a neural and sensory part, also a psychological component. In MUS in general, a larger memory complex may play a role. Experiencing a single symptom would not only sensitise this sensation, but would also activate a wider memory trace. This in turn, can result in the experience of other physical symptoms. Therefore, sensitisation may cause a wide range of symptoms. Furthermore, expectations also play a role in further sensitisation.
‘The development of symptom memories can be associated with cerebral restructuring. This has been shown for single pain symptoms, where already 24 hours of pain perception can cause neuronal reorganisation (neural plasticity) that will facilitate and intensify further symptom perceptions (Arnstein, 1997). For the phenomenon of multiple physical complaints, a general symptom memory matrix can be postulated.’ (p. 830)22
‘The repeated perception of physical signals in combination with uncertainty about the origin of the sensations can hinder the habituation that would ordinarily be expected.’ (p. 1000)27
C. Sensitivity theory
This theory suggests that some individuals are more vulnerable to develop MUS. This vulnerability can be based on personality traits, such as negative affect and neuroticism. Furthermore, patients with MUS seem to have difficulty in experiencing the relationship between bodily signals and emotions and thoughts. Catastrophic thinking may also play a part in the vulnerability of pain in these patients. There is little evidence for genetic influences, but many researchers suggest that early childhood experiences, such as abuse, insecure attachment and parental influence, play an important role in the development of MUS.
‘Viewing the MUS from the perspective of underlying developmental influences that affect the function of a variety of organs based on familial (genetic and environmental) predispositions rather than from traditional viewpoint of isolated organ‐originated diseases has at least two important implications. First, it provides a more parsimonious explanation for many findings that have been quite difficult to account for … Second, and more importantly, it invites investigation of new areas of therapy that may otherwise escape consideration.’ (p. 142)28
‘Studies within the framework of attachment theory have provided clear evidence that insecure attachment patterns, and in particular an insecure dismissing attachment pattern, are associated with an avoidant style of affect regulation.’ (p. 21)29
D. Immune system sensitisation theory
The brain has a cytokine system that reacts to the immune system. It monitors danger in parts of our body and coordinates the responses to these threats. The brain cytokine system is activated by the immune system and mediates the subjective, behavioural and physiological components of sickness, in a reversible way. It can be sensitised in response to activation during early stages of development, repetitive stimulation or prior exposure to immunological stimuli. The brain cytokine system, when sensitised, reacts very fast and is less likely to shut down after eliminating the initial stimulus. Furthermore the brain cytokine system can be triggered by non‐immunological stimuli. In patients with MUS, a chronic immune activation with production of cytokines can act as a motivation for the brain to change priorities in face of the presented threat (such as stress or trauma) resulting in a feeling of being sick.
‘The brain cytokine system also plays a key role in the experience of pain that is associated with danger, to the point that it has been proposed that pain is actually the main determinant of sickness behaviour rather than just a component of it (Watkins and Mayer, 2000).’ (p. 951)30
‘The main medical implication of this view is that many somatisation symptoms including depressed mood, fatigue, and pain may represent the expression of a previously sensitised brain cytokine system that is reactivated by infectious or noninfectious trauma.’ (p. 853)31
‘A growing body of evidence suggests that patho‐physiological processes explain some of the aspects of illness behaviour that are typically viewed as psychological in origin. The experience of general malaise or feeling sick has a physiological basis, mediated by centrally acting proinflammatory cytokines such as interleukin and tumour necrosis factor.’ (p. 56)32
E. Endocrine dysregulation theory
In the hypothalamus pituitary adrenal (HPA) axis, feedback loops exist to regulate the body's response to acute and chronic stress. Dysregulation of this axis has been found in patients with MUS. One interpretation is that prolonged activation has led to a ‘burnout’ response and a down regulation of HPA activity in MUS. Another suggestion is that hypocortisolism may in fact be a protective response of the body. Hypercortisolism has been found in patients with MUS. Early traumata during pregnancy or childhood can have long lasting effects on the stress sensitivity of the HPA axis which may be associated with increased prevalence of MUS.
‘The link so far found between central nervous system processes, such as the HPA axis, and immunological processes are intriguing but far from conclusive; the causal relationships are unclear, as are the nature of the change in these systems in different conditions at different stages. There is however already sufficient data to propose hypotheses about some of the important links, for example, between life events, HPA axis and immune functioning, that could be tested in prospective studies.’ (p. 791)23
‘We can conclude that the relevance of the HPA‐axis for the somatisation syndrome is still unclear. HPA‐activity definitely plays a role; however, this role might be unspecific, course depending, and multi‐directional.’ (p. 998)27
F. Signal filter theory
There is a permanent sensory stimulation from the body sending information to the brain. In healthy individuals, however, this ‘sensory noise’ is filtered, in order to ensure that the brain is not overstimulated by information from physiological processes. In patients with MUS ‘faulty filtering' leads to the inability of these patients to differentiate between information from physiological process (produced by the body) and information from pathophysiological processes (produced externally). Patients with MUS experience both types of information. Therefore, the number of physical sensations experienced by these patients is increased.
‘The perception‐filtering‐model is in line with the findings on the relevance of memory processes and expectation, two empirically well‐founded mechanisms not directly included in the other models. Further strength of this model is the close relationship to the neuronal process of perception. Therefore they offer a link between psychological and psychobiological findings on MUS.’ (p. 837)22
‘The effect of distraction on pain perception was demonstrated by Bantick et al, who found that distraction leads to reduced activity in pain‐associated centers (Bantick et al, 2002), again supporting a signal‐filter‐model as presented.’ (p. 999)27
G. Illness behaviour theory
This theory hypothesises that patients' beliefs influence their behaviour. This behaviour can in turn affect physiology and symptoms, resulting in a vicious circle and maintaining symptoms. Avoidance of physical, social or mental activity can result in more symptoms. For example, when a patient with chronic fatigue believes she will get more tired by doing sports, she will stop all physical activity. This may result in an increase of bodily attention and physical deconditioning, ending in more awareness and susceptibility of physical symptoms. Therefore symptoms can be sustained because of patients' behaviour.
‘There is actually relatively little literature concerning illness responses, despite a clinically prevalent belief that “all or nothing coping” and avoidance behaviours are important in the onset and perpetuation of syndromes such as CFS. More longitudinal work of this nature is needed to clarify the role of behaviour in the development of MUS.’ (p. 787)23
‘Behavioral aspects are also important in operant conditioning of illness behavior, confirmation of health attitudes, and the development of physical deconditioning. While these aspects could be of major importance for this patient group, their role has been insufficiently investigated in scientific trials.’ (p. 836)22
‘Cognitive, emotional and behavioural factors have the capacity to relieve symptoms and even change the brain.’ (p. 994)33
H. Autonomic nervous system dysfunction theory
Autonomic nervous system (ANS) dysfunction is a potential mechanism connecting psychosocial stress to MUS. In healthy controls, the change from attention tasks to rest periods is associated with a substantial decrease in heart rate activity (‘recovery response’). This reduction of physiological activity after mentally distressing tasks is not present in patients with MUS. It is hypothesised that this is a result of a parasympathetic nerve system dysfunction, resulting in a long lasting increased heart rate and stress burden in these patients.
‘To summarize the results on autonomic physiological activity, we can conclude that only few studies have addressed this question so far. Only small differences have been found, although there is some consistency indicating the involvement of the cardiovascular system.’ (p. 998)27
‘We conclude that current available evidence is not adequate to firmly reject or accept a role of ANS dysfunction in functional somatic disorders and it would therefore be misleading to provide a definitive summary estimate.’ (p. 108)34
I. Abnormal proprioception theory
Increased or abnormal proprioception can be a cause of physical symptoms in patients with MUS. It is suggested that patients with MUS demonstrate more exact and sensitive perception of their body than healthy individuals. In patients with MUS, minimal changes in muscle tension would lead to an enhanced feeling of abnormality. Therefore, benign physiological sensations (small changes in their body) can be interpreted as signs of a physical disease.
‘If patients with MUS perceive physical sensations more precisely, this could lead to increased likelihoods of perceiving even minor physical symptoms, although these differences could also be due to higher distraction by external stimuli in healthy controls.’ (p. 828)22
J. Cognitive behavioural therapy model
This meta‐model proposes that the cause of MUS is a self‐perpetuating multi‐factorial cycle, with interaction of different factors in several domains. This model provides a framework to incorporate patients' own personal perpetuating factors as well as predisposing and precipitating factors. Each factor can result in physical symptoms and/or distress. Doctor and patient together have to search for the patient's personal circumstances that might contribute to the distress. Furthermore, this meta‐model incorporates processes from at least five different theories described above: sensitivity, sensitisation, somatosensory amplification, endocrine dysregulation and the illness behaviour model.
‘This is the explicit purpose of the CBT assessment: to form a coherent multi‐factorial case conceptualization that forms the rationale for treatment.’ (p. 789)23
‘The biopsychosocial perspective becomes increasingly sophisticated, thus allowing the formation of a tight chain of findings from psychology to specific disease processes playing a role in the etiology and maintenance of illness conditions.’ (p. 182)39
‘As such the autopoietic explanation of MUS as proposed by the CBT model both fits the current data and could form a theoretically coherent basis for further research. More generally, the research bears out the over‐arching CBT hypothesis that the autopoietic interaction of distinct but linked systems could serve to produce physical symptoms in the absence of physical pathology.’ (p. 789)23
Discussion
Summary of main findings
This review illustrates a considerable number of explanatory models of MUS, grounded in the scientific literature. We could distinguish nine different explanatory models of MUS in the literature: somatosensory amplification, sensitisation, sensitivity, immune system sensitisation, endocrine dysregulation, signal filter model, illness behaviour model, autonomous nervous system dysfunction and abnormal proprioception. Furthermore, we found one meta‐model, the cognitive behavioural therapy model.
Some of the models aim at a physical explanation, such as the immune system sensitisation theory, the endocrine dysregulation theory, the autonomic nervous system dysfunction theory and the abnormal proprioception theory. Other models aim at a psychological explanation, such as the somatosensory amplification theory and the sensitivity theory. And some models combine a physical and psychological explanation, such as the sensitisation theory, the signal filter theory and the illness behaviour model.
The nine different explanatory models seek an explanation in different domains, including somatic causes, perception, illness behaviour and predisposition. The meta‐model integrates these four domains.
Medical explanations in clinical practice
Current medical training focuses on acting (diagnosing and treating patients) instead of listening, explaining and reflecting. Several studies pointed out that patients seek legitimacy for their symptoms.16,40–42 They want to feel that the doctor accepts that the symptoms are real and warrant the doctor's attention.25 Therefore, good and relevant doctor consultation skills, including explaining symptoms, are needed. Plenty of doctors think in terms of action and reaction, while the explanation of symptoms in itself might be the most important intervention for patients with MUS.43 Such explanations might prevent patients from extending or elaborating symptoms and doctors from providing investigations or somatic treatment.3 Explanation as a consultation skill in its own right is rarely addressed in the literature and in teaching programmes. As education on explaining and explanatory models is limited in today's clinical education programmes, medical students and GPs have little knowledge of theories and models which they can use during consultation. This might explain part of the difficulties GPs experience in giving an adequate and tangible explanation to patients with MUS. However, GPs indicate that they build their own explanatory models of medically unexplained symptoms based on their experience in daily practice.44 Furthermore, building acceptable and effective (i.e. reassuring) explanations together with the patient needs a mutual understanding of patients' beliefs, concerns and expectations regarding their symptoms.45,46 Knowledge of explanatory models of MUS, together with this mutual understanding and daily practice experience can facilitate doctor–patient communication and strengthen the doctor's relationship with these patients. Furthermore, mutual understanding between GP and individual patients on the aetiology of MUS might result in greater reassurance, patientsatisfaction and commitment to the proposed interventions.25
Strengths and limitations of this study
In this qualitative analysis of the literature, we used an extensive and systematic search strategy to identify relevant reviews. Including the full text papers and having them coded by two independent researchers added rigour to our study. Moreover, we had good inter‐rater agreement for inclusion and exclusion.
By using a cyclical way of analysing data, we were able to focus and explore explanatory models in depth.47 Entering the full text of included studies into Atlas.ti and using the constant comparative method to code and reorganise data strengthened our findings.
We limited our literature search to the past five years. It seems, however, that we have captured most explanatory models in the literature as the reviews included in our study also discussed and summarised explanatory models described in earlier literature. Although across cultures many systems of medicine provide sociosomatic explanations linking problems in family and community with bodily distress, we did not find culturally based explanatory models in our literature search.48
A qualitative analysis of the literature is not as objective as a meta‐analysis. However, we were able to summarise the range of explanatory models grounded in the current scientific literature. As studying the scientific evidence of the different models was not the goal of our study, we are not able to draw conclusions on the degree of evidence of the explanatory models found in the literature.
Implications for future practice and research
This review illustrates quite a number of different explanatory models of MUS described in the literature. Most theories are based on symptom perception, somatic causes, illness behaviour and predisposition. On the other hand, more progress has to be made towards a fuller understanding of the complex aetiology of MUS.
Further studies using in‐depth interviews with GPs may reveal new explanatory models based on experiences in daily medical practice. This qualitative analysis of the literature examines explanatory models of MUS and not the usefulness of these models in clinical practice. Therefore, new research has to clarify the usefulness of the different explanatory models in daily practice. In addition, studies using a mixed method methodology have to point out patient preferences and the effectiveness of the explanatory models individually in family practice.
As persistent MUS are present in all medical specialties, these explanatory models should be integrated in the educational programs of all medical doctors in order to improve the quality of care for patients with persistent MUS.
Contributor Information
J van Ravenzwaaij, Family Physician.
TC olde Hartman, PhD Student and Family Physician.
H van Ravesteijn, Psychiatry Resident and PhD Student.
R Eveleigh, PhD Student and Family Physician Resident.
E van Rijswijk, Family Physician Resident.
PLBJ Lucassen, Family Physician, Department of Primary and Community Medicine, Radboud University Nijmegen Medical Centre, Nijmegen, The Netherlands.
REFERENCES
- 1.Houtveen JH. De dokter kan niets vinden. Amsterdam: Uitgeverij Bert Bakker, 2009 [Google Scholar]
- 2.Peveler R, Kilkenny L, Kinmonth AL. Medically unexplained physical symptoms in primary care: a comparison of self‐report screening questionnaires and clinical opinion. Journal of Psychosomatic Research 1997;42:245–52 [DOI] [PubMed] [Google Scholar]
- 3.Ring A, Dowrick CF, Humphris GM, Davies J, Salmon P. The somatising effect of clinical consultation: what patients and doctors say and do not say when patients present medically unexplained physical symptoms. Social Science and Medicine 2005;61:1505–15 [DOI] [PubMed] [Google Scholar]
- 4.Nimnuan C, Hotopf M, Wessely S. Medically unexplained symptoms: an epidemiological study in seven specialities. Journal of Psychosomatic Research 2001;51:361–7 [DOI] [PubMed] [Google Scholar]
- 5.Reid S, Wessely S, Crayford T, Hotopf M. Medically unexplained symptoms in frequent attenders of secondary health care: retrospective cohort study. BMJ 2001;322:767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kroenke K, Mangelsdorff AD. Common symptoms in ambulatory care: incidence, evaluation, therapy, and outcome. American Journal of Medicine 1989;86:262–6 [DOI] [PubMed] [Google Scholar]
- 7.Barsky AJ, Borus JF. Somatization and medicalization in the era of managed care. Journal of the American Medical Association 1995;274:1931–4 [PubMed] [Google Scholar]
- 8.Smith GR, Jr, Monson RA, Ray DC. Patients with multiple unexplained symptoms. Their characteristics, functional health, and health care utilization. Archives of Internal Medicine 1986;146:69–72 [PubMed] [Google Scholar]
- 9.de Waal MW, Arnold IA, Eekhof JA, van Hemert AM. Somatoform disorders in general practice: prevalence, functional impairment and comorbidity with anxiety and depressive disorders. British Journal of Psychiatry 2004;184:470–6 [DOI] [PubMed] [Google Scholar]
- 10.Banks J, Prior L. Doing things with illness. The micro politics of the CFS clinic. Social Science and Medicine 2001;52:11–23 [DOI] [PubMed] [Google Scholar]
- 11.Verhaak PF, Meijer SA, Visser AP, Wolters G. Persistent presentation of medically unexplained symptoms in general practice. Family Practice 2006;23:414–20 [DOI] [PubMed] [Google Scholar]
- 12.Mathers N, Jones N, Hannay D. Heartsink patients: a study of their general practitioners. British Journal of General Practice 1995;45:293–6 [PMC free article] [PubMed] [Google Scholar]
- 13.Hatcher S, Arroll B. Assessment and management of medically unexplained symptoms. BMJ 2008;336:1124–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barsky AJ, Ettner SL, Horsky J, Bates DW. Resource utilization of patients with hypochondriacal health anxiety and somatization. Medical Care 2001;39:705–15 [DOI] [PubMed] [Google Scholar]
- 15.Ring A, Dowrick C, Humphris G, Salmon P. Do patients with unexplained physical symptoms pressurise general practitioners for somatic treatment? A qualitative study. BMJ 2004;328:1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peters S, Stanley I, Rose M, Salmon P. Patients with medically unexplained symptoms: sources of patients' authority and implications for demands on medical care. Social Science and Medicine 1998;46:559–65 [DOI] [PubMed] [Google Scholar]
- 17.Salmon P, Ring A, Dowrick CF, Humphris GM. What do general practice patients want when they present medically unexplained symptoms, and why do their doctors feel pressurized? Journal of Psychosomatic Research 2005;59:255–60 [DOI] [PubMed] [Google Scholar]
- 18.olde Hartman TC, Hassink‐Franke LJ, Lucassen PL, van Spaendonck KP, van Weel C. Explanation and relations. How do general practitioners deal with patients with persistent medically unexplained symptoms: a focus group study. BMC Family Practice 2009;10:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cohen J. A coefficient of agreement for nominal scales. Educational and Psychological Measurement 1960;20:37–46 [Google Scholar]
- 20.olde Hartman TC, Borghuis MS, Lucassen PL, van de Laar FA, Speckens AE, van Weel C. Medically unexplained symptoms, somatisation disorder and hypochondriasis: course and prognosis. A systematic review. Journal of Psychosomatic Research 2009;66:363–77 [DOI] [PubMed] [Google Scholar]
- 21.Glaser BG, Strauss AL. The Discovery of Grounded Theory. Chigago: Aldine, 1967 [Google Scholar]
- 22.Rief W, Broadbent E. Explaining medically unexplained symptoms – models and mechanisms. Clinical Psychology Review 2007;27:821–41 [DOI] [PubMed] [Google Scholar]
- 23.Deary V, Chalder T, Sharpe M. The cognitive behavioural model of medically unexplained symptoms: a theoretical and empirical review. Clinical Psychology Review 2007;27:781–97 [DOI] [PubMed] [Google Scholar]
- 24.Henningsen P, Zipfel S, Herzog W. Management of functional somatic syndromes. The Lancet 2007;369:946–55 [DOI] [PubMed] [Google Scholar]
- 25.Salmon P. Conflict, collusion or collaboration in consultations about medically unexplained symptoms: the need for a curriculum of medical explanation. Patient Education and Counseling 2007;67:246–54 [DOI] [PubMed] [Google Scholar]
- 26.Duddu V, Isaac MK, Chaturvedi SK. Somatization, somatosensory amplification, attribution styles and illness behaviour: a review. International Review of Psychiatry 2006;18:25–33 [DOI] [PubMed] [Google Scholar]
- 27.Rief W, Barsky AJ. Psychobiological perspectives on somatoform disorders. Psychoneuroendocrinology 2005;30:996–1002 [DOI] [PubMed] [Google Scholar]
- 28.Buffington CA. Developmental influences on medically unexplained symptoms. Psychotherapy and Psychosomatics 2009;78:139–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Waller E, Scheidt CE. Somatoform disorders as disorders of affect regulation: a development perspective. International Review of Psychiatry 2006;18:13–24 [DOI] [PubMed] [Google Scholar]
- 30.Dantzer R. Somatization: a psychoneuroimmune perspective. Psychoneuroendocrinology 2005;30:947–52 [DOI] [PubMed] [Google Scholar]
- 31.Dimsdale JE, Dantzer R. A biological substrate for somatoform disorders: importance of pathophysiology. Psychosomatic Medicine 2007;69:850–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kirmayer LJ, Looper KJ. Abnormal illness behaviour: physiological, psychological and social dimensions of coping with distress. Current Opinion in Psychiatry 2006;19:54–60 [DOI] [PubMed] [Google Scholar]
- 33.Wilhelmsen I. Biological sensitisation and psychological amplification: gateways to subjective health complaints and somatoform disorders. Psychoneuroendocrinology 2005;30:990–5 [DOI] [PubMed] [Google Scholar]
- 34.Tak LM, Riese H, de Bock GH, Manoharan A, Kok IC, Rosmalen JG. As good as it gets? Ameta‐analysis and systematic review of methodological quality of heart rate variability studies in functional somatic disorders. Biological Psychology 2009;82:101–10 [DOI] [PubMed] [Google Scholar]
- 35.Spaans JA, Veselka L, Luyten P, Buhring ME. (Bodily aspects of mentalisation: a therapeutic focus in the treatment of patients with severe medically unexplained symptoms.) Tijdschrift voor Psychiatrie 2009;51:239–48 [PubMed] [Google Scholar]
- 36.Garcia‐Campayo J, Fayed N, Serrano‐Blanco A, Roca M. Brain dysfunction behind functional symptoms: neuroimaging and somatoform, conversive, and dissociative disorders. Current Opinion in Psychiatry 2009;22:224–31 [DOI] [PubMed] [Google Scholar]
- 37.Maes M. Inflammatory and oxidative and nitrosative stress pathways underpinning chronic fatigue, somatization and psychosomatic symptoms. Current Opinion in Psychiatry 2009;22:75–83 [DOI] [PubMed] [Google Scholar]
- 38.Roelofs K, Spinhoven P. Trauma and medically unexplained symptoms towards an integration of cognitive and neuro‐biological accounts. Clinical Psychology Review 2007;27:798–820 [DOI] [PubMed] [Google Scholar]
- 39.Nater UM, Gaab J, Rief W, Ehlert U. Recent trends in behavioral medicine. Current Opinion in Psychiatry 2006;19:180–3 [DOI] [PubMed] [Google Scholar]
- 40.Werner A, Malterud K. It is hard work behaving as a credible patient: encounters between women with chronic pain and their doctors. Social Science and Medicine 2003;57:1409–19 [DOI] [PubMed] [Google Scholar]
- 41.Chew CA, May CR. The benefits of back pain. Family Practice 1997;14:461–5 [DOI] [PubMed] [Google Scholar]
- 42.Nettleton S. ‘I just want permission to be ill’: towards a sociology of medically unexplained symptoms. Social Science and Medicine 2005;62:1167–78 [DOI] [PubMed] [Google Scholar]
- 43.Heijmans M. How to Manage Medically Unexplained Symptoms in Primary Care? A qualitative analysis of reviews and scientific editorials. 2010. Unpublished work [DOI] [PubMed] [Google Scholar]
- 44.van Ravenzwaaij J. Explaining Medically Unexplained Symptoms. A qualitative literature study supported by interviews with general practitioners. 2010. Unpublished work [Google Scholar]
- 45.Balint M. The Doctor, His Patient and the Illness. Edinburgh: Churchill Livingstone, 2000 [Google Scholar]
- 46.Dowrick CF, Ring A, Humphris GM, Salmon P. Normalisation of unexplained symptoms by general practitioners: a functional typology. British Journal of General Practice 2004;54:165–70 [PMC free article] [PubMed] [Google Scholar]
- 47.Britten N, Jones R, Murphy E, Stacy R. Qualitative research methods in general practice and primary care. Family Practice 1995;12:104–14 [DOI] [PubMed] [Google Scholar]
- 48.Kirmayer LJ, Groleau D, Looper KJ, Dao MD. Explaining medically unexplained symptoms. Canadian Journal of Psychiatry 2004;49:663–72 [DOI] [PubMed] [Google Scholar]
CONFLICTS OF INTEREST
None.



