Abstract
Declining cell-mediated immunity to varicella zoster virus (VZV) in elderly individuals results in virus reactivation manifest by zoster (shingles) and postherpetic neuralgia (PHN). To prevent virus reactivation, a new VZV vaccine (Zostavax, Merck) that boosts cell-mediated immunity to VZV was developed. The 3-year Shingles Prevention Study showed that Zostavax significantly reduced burden of disease due to zoster and PHN. Despite its cost-effectiveness for adults ages 65 to 75 years, as determined in the US, Canada and UK, less than 2% of immunocompetent adults over age 60 years in the US were immunized in 2007. This was due to a combination of lack of patient awareness of the vaccine, physicians’ uncertainty about the duration of protection, and different cost-sharing plans for immunization. Nevertheless, zoster vaccine is safe, effective, and highly recommended for immunization of immunocompetent individuals over age 60 years with no history of recent zoster.
Keywords: zoster, shingles, immunization, postherpetic neuralgia
Introduction
Varicella zoster virus (VZV), an exclusively human neurotropic alphaherpesvirus, causes varicella (chickenpox), after which virus becomes latent in cranial nerve ganglia, dorsal root ganglia and autonomic ganglia along the entire neuraxis. Years later, as cell-mediated immunity to VZV declines with age or with immunosuppression in organ transplant recipients and patients with cancer or AIDS, VZV reactivates to cause zoster (shingles), often followed by chronic pain (postherpetic neuralgia or PHN), vasculopathy, meningoencephalitis, myelopathy, cerebellitis, and various ocular disorders (Fig. 1). VZV reactivation can also produce radicular pain without rash (zoster sine herpete), and it is now clear that all of the known neurological and ocular complications of VZV reactivation can occur without rash.
Fig. 1.
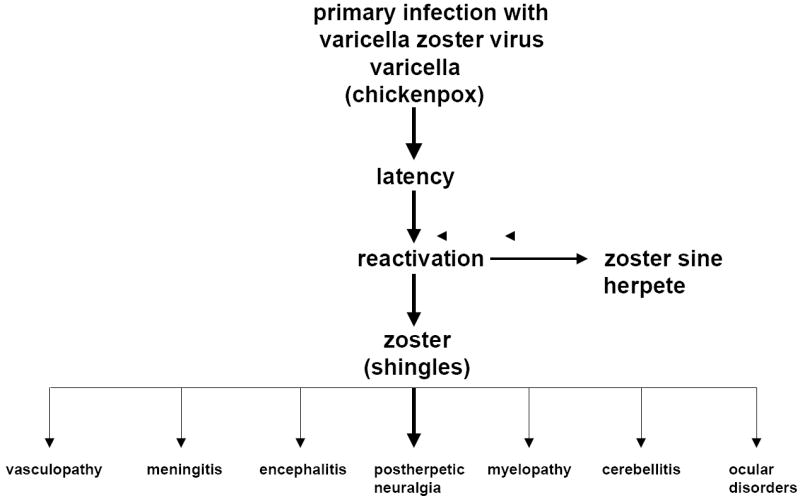
The neurological complications of varicella zoster virus reactivation.
To prevent any and all of the neurological complications produced by VZV reactivation, a new VZV vaccine (Zostavax, Merck) was developed. The preventive effect of zoster vaccine rests in its ability to boost host cell-mediated immunity to VZV. Here, we describe the protean neurological complications of VZV reactivation and cite the clinical evidence for the effectiveness of zoster vaccine, important features regarding its clinical usefulness, adverse effects, and cost-effectiveness.
Neurological complications of VZV reactivation
Herpes zoster
Zoster is common, with nearly 1,000,000 individuals in the United States affected annually. The incidence of zoster is 5 to 6.5 per 1,000 individuals at age 60 years, increasing to 8 to 11 per 1,000 at age 70 years [1]. Unlike varicella (chickenpox), which occurs primarily in the spring, there is no seasonal predilection for zoster. Zoster in young adults may be the first manifestation of HIV infection [2]. Interestingly, chickenpox in infancy predisposes to zoster earlier in life [3]. Zoster is characterized by dermatomal distribution pain and rash. VZV is highly infectious and transmission occurs by direct contact with skin lesions or by respiratory aerosols. Immunosuppression increases the risk of disseminated zoster [4]. In most patients, the disappearance of skin lesions is accompanied by decreased pain and complete resolution of pain in 4 to 6 weeks. Magnetic resonance imaging (MRI) has shown enhancement of ganglia and affected nerve roots [5]. Because VZV becomes latent in ganglia along the entire neuraxis, zoster can develop anywhere on the body. Zoster can affect any cranial [6-17] or spinal nerves at all levels. Zoster paresis (zoster with lower motor neuron type weakness) occurs in the arm, leg [18, 19], diaphragm [20] or abdominal muscles [21]. Subclinical VZV reactivation (without pain or rash) has been demonstrated in astronauts [22], with shedding of infectious virus [23]. Cardinal pathological features of zoster are inflammation and hemorrhagic necrosis with associated neuritis, localized leptomeningitis, unilateral segmental poliomyelitis, and degeneration of related motor and sensory roots [24, 25]. Demyelination occurs in areas with mononuclear cell (MNC) infiltration and microglial proliferation. Intranuclear inclusions, viral antigen and herpesvirus particles have been detected in acutely infected ganglia [26-29]. Antiviral drugs (e.g., valacyclovir, 1 g three times daily for 7-10 days) speed healing of rash and shorten the duration of acute pain. In immunocompromised patients, intravenous acyclovir (5-10 mg/kg three times per day for 5-7 days) is recommended.
Postherpetic neuralgia (PHN)
PHN is characterized by constant, severe, stabbing or burning dysesthetic pain that persists for at least 3 months and sometimes years after resolution of rash. About 40% of zoster patients over age 60 years experience PHN [30, 31]. The cause and pathogenesis of PHN are unknown. Two non-mutually exclusive theories are that: (1) excitability of ganglionic or even spinal cord neurons is altered; and (2) persistent or low-grade productive virus infection exists in ganglia. In a study by Watson et al. [32], who detailed the pathology in a case of severe thoracic PHN for 5 years, the most striking finding was atrophy of the dorsal horn over five segments, with only one ganglion affected by fibrosis and cellular loss and with only the roots at that level involved. Further studies in three PHN cases with severe pain [33] again revealed such dorsal root atrophy and ganglionic changes but also marked inflammation in the dorsal horn in one acute case and loss of axons and myelin in the sensory root and peripheral nerve. The finding of inflammatory changes at multiple levels bilaterally affecting roots, ganglia and nerves raised the possibility of ongoing generalized inflammation as a pathogenetic mechanism in some cases. Further support for the concept that PHN is produced by low-level ganglionitis comes from the detection of VZV DNA and proteins in blood MNCs of many patients with PHN [34-36], and from the favorable response of some PHN patients to antiviral treatment [37-39]. In a prospective, open-label phase I/II clinical trial, 15 patients with moderate to severe PHN were treated with intravenous acyclovir for 2 weeks, followed by oral valacyclovir for 1 month; 8 of 15 (53%) patients reported improvement of pain [39].
VZV vasculopathy
VZV vasculopathy results from productive virus infection in large and/or small cerebral arteries. Patients present with headache, fever, mental status changes, transient ischemic attacks (TIAs) and/or focal deficit (stroke). The clinical spectrum includes aneurysms [40] and hemorrhage, arterial ectasia and dissection [41]. More than one-third of cases of VZV vasculopathy occur without rash [42]. The cerebrospinal fluid (CSF) often contains a mononuclear pleocytosis and oligoclonal bands; the oligoclonal IgG is antibody directed against VZV [43]. Brain imaging usually reveals ischemic and/or hemorrhagic infarcts, more deep-seated than cortical lesions and particularly at gray-white matter junctions, a clue to diagnosis. Cerebral angiography may show focal arterial stenosis or occlusion. Macroscopically, lesions at gray-white matter junctions predominate. Virus is present in affected cerebral arteries as evidenced by the presence of multinucleated giant cells, Cowdry A inclusion bodies, herpes virus particles seen by electron microscopy, VZV DNA and VZV antigen. In chronic cases, virus is also found in areas of infarction, usually close to arteries and veins.
Two recent studies revealed an increased risk of stroke after zoster. Analysis of 7760 patients who had been treated for zoster showed that the risk of stroke was 31% higher within a year after zoster and approximately 4-fold higher in patients with herpes zoster ophthalmicus (HZO) versus the comparison cohort of 23,380 controls [44]. Similarly, Lin et al. [45] found that the risk of stroke in 658 patients with HZO as compared with that in 1,974 controls was 4.52-fold higher. These studies are important because the aging population is rapidly increasing. Stroke can now be added to the list of other serious complications of HZO such as keratitis and PHN.
Confirmation of VZV vasculopathy requires virological analysis to detect amplifiable VZV DNA or anti-VZV IgG antibodies or both in the CSF. The CSF does not always contain PCR-amplifiable VZV DNA, but does contain anti-VZV IgG [46]. The detection of anti-VZV IgG, but not VZV DNA, likely reflects the chronic, protracted course of disease. Testing for both VZV DNA and anti-VZV IgG must be done, and only negative findings in both can exclude the diagnosis of VZV vasculopathy. Also, since VZV vasculopathy can occur without rash, all vasculopathies of unknown etiology should be evaluated for VZV. Rapid diagnosis of VZV vasculopathy is important since the mortality without treatment is 25% [47], while treatment with intravenous acyclovir, even after neurologic disease has been present for months, can be curative [48].
VZV meningitis, cerebellitis and meningoradoencephalitis
VZV may also present as meningitis or a meningoencephalitis. Many reported cases of VZV encephalitis may actually be VZV vasculopathy [49]. Recent reports of VZV meningitis [50, 51], meningoradiculitis [52] and cerebellitis (gait ataxia and tremor predominated) [53, 54], all in the absence of rash and confirmed by the detection of VZV DNA and anti-VZV antibody in CSF, revealed that VZV is not an uncommon cause of aseptic meningitis.
VZV myelopathy
VZV myelopathy presents in various ways. One form is a self-limiting, monophasic spastic paraparesis, with or without sensory features and sphincter problems. This so-called post-infectious myelitis usually occurs in immunocompetent patients, days to weeks after acute varicella or zoster. Its pathogenesis is unknown. The CSF usually contains a mild mononuclear pleocytosis, with a normal or slightly elevated protein. Steroids are used to treat these patients [55], although some improve spontaneously [56]. Rarely, VZV myelitis recurs, even in immunocompetent patients [57].
VZV myelopathy may also present as an insidious, progressive and sometimes fatal myelitis, mostly in immunocompromised individuals. Indeed, AIDS has commonly and increasingly become associated with VZV myelitis. MRI reveals longitudinal serpiginous enhancing lesions. Diagnosis is confirmed by the presence of VZV DNA or anti-VZV IgG or both in CSF [57]. Pathological and virological analyses of the spinal cord from fatal cases have shown frank invasion of VZV in the parenchyma [58] and in some instances, spread of virus to adjacent nerve roots [59]. Not surprisingly, some patients respond favorably to antiviral therapy [60-62]. Importantly, VZV myelitis may develop without rash. Early diagnosis and aggressive treatment with intravenous acyclovir has been helpful, even in immunocompromised patients [60]. The benefit of steroids in addition to antiviral agents is unknown.
VZV can also produce spinal cord infarction, identified by diffusion-weighted MRI and confirmed virologically [63]. Thus, VZV vasculopathy can cause stroke in the spinal cord as well as in the brain.
VZV retinal necrosis
VZV produces multiple ocular disorders, including both acute retinal necrosis (ARN) and progressive outer retinal necrosis (PORN). ARN develops in both immunocompetent and immunocompromised hosts. Patients present with periorbital pain and floaters with hazy vision and loss of peripheral vision. Treatment is typically intravenous acyclovir, steroids and aspirin followed by oral acyclovir [64]. Intravitreal injections of foscarnet and oral acyclovir have been used in early, milder cases. Although PORN can be caused by herpes simplex virus and cytomegalovirus, most cases are produced by VZV, primarily in AIDS patients with CD4+ counts typically less than 10 cells/mm3 of blood [65], as well as in other immunosuppressed individuals [66]. PORN may be preceded by retrobulbar optic neuritis and aseptic meningitis [67], central retinal artery occlusion or ophthalmic-distribution zoster [68], and may occur together with multifocal vasculopathy or myelitis. Patients present with sudden painless loss of vision, floaters and constricted visual fields with resultant retinal detachment. Multifocal, discrete opacified lesions begin in the outer retinal layers peripherally and/or posterior pole; only late in disease are inner retinal layers involved. Diffuse retinal hemorrhages and whitening with macular involvement bilaterally are characteristic findings. Treatment with intravenous acyclovir has given poor or inconsistent results [69], and even when acyclovir helped, VZV retinopathy recurred when drug was tapered or stopped. PORN patients treated with a combination of ganciclovir and foscarnet or with ganciclovir alone had a better final visual acuity than those treated with acyclovir or foscarnet [70]. The best treatment for PORN in AIDS patients may be prevention with HAART, which appears to decrease its incidence [71].
Zoster sine herpete
Zoster sine herpete (radicular pain without rash) is due to reactivation of VZV [72], a concept first supported by the description of dermatomal distribution radicular pain in areas distinct from pain with rash in zoster patients [73]. Currently, most clinicians regard zoster sine herpete exclusively as the rare occurrence of chronic radicular pain without rash. Other causes of chronic radicular pain include diabetes, lymphoma, cancer and sarcoidosis. Virological confirmation of zoster sine herpete is best provided by detection of VZV DNA in CSF. In recent years, the detection of VZV DNA and anti-VZV IgG antibody in patients with meningoencephalitis, vasculopathy, myelitis, cerebellar ataxia and polyneuritis cranialis, all without rash, has expanded the spectrum of neurological disease produced by VZV in the absence of rash. Prevalence estimates of VZV-induced pathology without rash await virological analysis of additional patients with prolonged radicular pain or other neurological symptoms and signs. Analyses should include tests for anti-VZV IgG, anti-VZV IgM and PCR-amplifiable VZV DNA in CSF, anti-VZV IgM in serum, as well as examination of blood MNCs for VZV DNA.
VZV genome
The VZV genome contains at least 70 genes, nearly all of which have homologs in herpes simplex virus (HSV). The complete sequence of the VZV genome was determined by Davison and Scott [74]. The prototype strain, VZV Dumas, is 124,884 base pairs in length. The genome consists of a unique long region bounded by terminal and internal repeat sequences, and a unique short region bounded by internal short and terminal short repeat sequences. Strain differences have been identified by variation in the length of the repeat regions. The genome is linear in virions and circular in infected cells. Genotyping by restriction enzyme analysis and single nucleotide polymorphisms has proven that the virus causing varicella is identical to that which later reactivates as zoster in the same person. Furthermore, these technologies have shown that reinfection, which is mostly asymptomatic, may also occur and that the second virus may establish latency and reactivate [75].
Viral pathogenesis
VZV is the only human herpesvirus that spreads by the respiratory route from skin lesions of individuals with varicella or zoster [76]. Spread from the respiratory tract is also possible, but varicella is not usually accompanied by coughing and sneezing which propel infectious droplets into the air [77]. During primary infection, VZV disseminates to multiple sites resulting in vesicles on an erythematous base all over the skin. VZV-infected tonsillar CD4+ T cells transport virus from lymph nodes to skin during primary viremia. VZV-infected T cells also transfer virus to neurons in ganglia. Dendritic cells of the respiratory mucosa appear to be the first cells to encounter VZV, and these cells transport virus to draining lymph nodes. The incubation period is long, often up to 21 days before skin lesions occur. Varicella can be acquired from patients with zoster. Zoster occurs only after virus reactivation, thus it is not acquired from other patients with zoster or varicella. Reactivation allows VZV to transmit infection to a younger generation. Thus, VZV, a virus without any animal reservoir, can perpetuate itself.
VZV latency
VZV establishes latency in neurons of human ganglia where the virus genome is most likely maintained as a circular episome bound to histones. There is considerable variability among individuals in the number of latent VZV DNA copies. Expression of VZV genes during latency is restricted and regulated epigenetically. Of the VZV open reading frames (ORFs) that have been analyzed for transcription during latency using cDNA sequencing, only ORF 21, 29, 62, 63 and 66 have been detected. VZV ORF 63 is the most frequently and abundantly transcribed VZV gene detected in latently-infected human ganglia, suggesting a critical role for this gene in maintaining a latent state. Other transcripts have been detected inconsistently, suggesting that these genes either play secondary roles in latency or possibly reflect subclinical VZV reactivation. Deep sequencing of RNA extracted from human trigeminal ganglia positive for both VZV and HSV-1 DNA revealed several microRNAs mapping to the HSV genome, but not VZV-specific microRNAs [78].
VZV-specific immunity
Primary VZV infection that induces varicella is followed by the production of VZV-specific antibody and VZV-specific T cell mediated immunity. Antibodies to VZV are detected throughout life. The serum of individuals 60 to 94 years of age contains a variable presence of VZV-specific antibodies to VZV glycoproteins I-IV and to three nonglycosylated proteins; antibodies to VZV are also present in some elderly individuals with no history of varicella or zoster, indicative of subclinical infection [34]. Increased levels of antibody to VZV do not confer protection against zoster or PHN. In fact, increased levels of antibody to VZV after the onset of zoster are associated with more severe disease and a greater risk of PHN, perhaps because they reflect more extensive VZV replication [79].
Recovery from varicella is associated with the development of VZV-specific T cell-mediated immunity [80]. The cell-mediated immune response to VZV is detected within 1 to 2 weeks after disappearance of rash, and consists of CD4 and CD8 effector and memory T cells. T-cell immunity to VZV is more important than the antibody response, as determined in studies of natural VZV infection in humans with specific immune deficiencies. For example, patients with agammaglobulinemia are unable to produce VZV-specific antibodies but are protected against second episodes of varicella because they are able to mount a VZV-specific T cell-mediated immune response [81]. Individuals with T cell-immune deficiency disorders have more severe VZV-specific disease than do normal hosts [82]. Furthermore, immune suppression is associated with a significant increase in the incidence of zoster [83]. Even in human stem cell recipients who received inactivated VZV vaccine, protection correlated with VZV-specific T cell-mediated immunity but not with anti-VZV antibody [84].
VZV-specific T cell-mediated immunity maintains VZV in a latent state in ganglia. The immune response can subsequently be boosted by subclinical reactivation of latent virus or environmental exposure to virus. Most important, the incidence of zoster increases with age as VZV-specific T cell-mediated immunity declines. The age-related decline in VZV-specific T-cell immunity is evident during the first 3 years after varicella, when the frequency of VZV-specific memory CD4 T cells already decreases [85] and after mid-adult life, the intensity and quality of antigenic stimulation provided by re-exposure and asymptomatic reactivation are not sufficient to maintain VZV-specific T cell immunity. Furthermore, a comparison of the cell-mediated immune response to VZV antigen in vitro in young adults and individuals over age 60 years revealed 5-fold fewer CD4 cells that produce interferon-gamma or interleukin-4 and -5, as well as fewer CD4 early effectors and CD8 effector memory cells in the older age group [86].
Recognition of the essential role of cell-mediated immunity to VZV for protection against and recovery from varicella and zoster led to studies designed to boost the cell-mediated immune response to VZV by immunization of elderly adults. Demonstration of the ability of zoster vaccine to reverse VZV-specific T cell deficiencies present before immunization was followed by the Shingles Prevention Study (described below).
VZV vaccine
There are two VZV vaccines, both of which contain live attenuated virus. The first to be developed was the varicella (Oka strain) vaccine (Varivax, Merck), which has been used to prevent chickenpox in children in Japan since 1975 and in the US since 1996. Varivax generates VZV-specific humoral and cell-mediated immune responses, particularly CD8 T cells [87], and the memory cell response that occurs after vaccination protects from re-exposure to VZV. The second vaccine (Zostavax, Merck) was developed to prevent zoster (shingles) by boosting a waning cell-mediated immune response to VZV with advancing age. In the United States, Zostavax was licensed by the FDA in May 2006, provisionally recommended by the Center for Disease Control Advisory Committee on Immunization Practices for vaccination of persons aged ≤60 years in October 2006, and the recommendation became formalized upon publication in June 2008. The only difference between the two vaccines is that Zostavax contains 19,400 pfu per dose, 14-fold more virions than the varicella vaccine.
Zoster vaccine
The most widely used measure of the cell-mediated immune response to VZV in elderly individuals before and after receiving zoster vaccines has been the responder-cell frequency [88]. When administered to people over age 60 years, zoster vaccine boosted VZV-specific T cell-mediated immunity, inducing increased numbers of CD4 and CD8 cells, CD4 and CD8 effector memory T cells, and CD8 early-effector T cells; the half-life of the boost in T cell immunity to VZV is at least 5 years [89]. Zoster vaccine also boosts VZV-specific immunity in adults with a history of zoster before vaccination or with chronic illness, and can be administered in these populations.
Zostavax is injected subcutaneously and stored frozen. The vaccine is indicated for the prevention of zoster in individuals 60 years of age and older; it is not indicated for the treatment of zoster or PHN, or for women of child-bearing age. Administration is also not recommended for individuals with a history of an immunodeficiency state, including leukemia, lymphoma, malignant neoplasm infecting the bone marrow or lymphatic system, or AIDS, and should not be administered to pregnant women.
The critical trial of the licensed zoster vaccine (Shingles Prevention Study, SPS) was a placebo-controlled, double-blind trial in which more than 38,000 adults over 60 years of age were randomized to receive either zoster vaccine or placebo. All subjects were monitored for zoster, with endpoints including burden of illness due to zoster and zoster-associated pain, as well as incidence of clinically significant PHN. Subjects received a single-dose of Zostavax (n = 19,270) or placebo (n = 19,276). Ratio distribution across both vaccination groups was similar: White (95%), Black (2%), Hispanic (1%) and other (1%) in both groups. The gender distribution was 59% male and 41% female in both groups. The most common side effects reported by vaccinated participants were redness, pain, itching, swelling, warmth or bruising at the vaccination site and sometimes headache. Varicella-like rashes at the injection site were more common in zoster vaccinees than in placebo recipients (6.4% versus 0.1%; p < 0.05).
After a mean follow-up of 3 years, the SPS found that Zostavax vaccine significantly reduced the incidence of zoster by 51%. Moreover, subjects in the immunization group who developed zoster reported significantly less pain and discomfort, and fewer PHN cases were detected in these subjects (an overall 61% lower burden of disease) than those in the placebo group. Table 1 shows adthat during the first 42 days after immunization, varicella-like rashes at the injection site were more common in vaccine recipients than in placebo recipients (0.1% vs. 0.04%, p<,0.05); compared to placebo recipients, the vaccine group also had more erythema (36% vs. 7%), localized pain or tendeness (35% vs. 9%), swelling (25% vs. 5%) and itching (7% vs. 15) (p<0.05 for all four comparisons). Importantly, no significant differences were seen between zoster vaccine and placebo groups in the incidence of vaccine-related serious adverse events (both <0.1%).
Table 1.
Adverse events caused by Zostavax
| Type of event | % |
|---|---|
| Injection site (N = 3345) | |
| Erythema | 34 |
| Pain/tenderness | 33 |
| Swelling | 25 |
| Hematoma | 1.4 |
| Pruritus | 6.6 |
| Warmth | 1.5 |
| Symptoms | |
| Headache | 1.4 |
Baseline immunologic measurements from the SPS confirmed that VZV-specific T cell immunity measured by responder cell frequency, as well as an ELISPOT assay, declined continuously with advancing age [90]. ELISPOT responses peaked at 2 weeks after immunization and at 6 weeks, were approximately 2-fold higher in immunized recipients than in placebo recipients [91]. All values fell during the first year after immunization, but remained approximately 50% higher than pre-immunization levels for the 3-year study period. Importantly, the boost in VZV-specific T cell-mediated immunity was similar to that developing after naturally occurring zoster [79]. The magnitude of the boost in cell-mediated immunity to VZV was greatest in younger subjects, consistent with the greater efficacy of vaccine in preventing zoster in adults of age 60 to 69 years compared to those older than 70 years. The memory responses elicited in older subjects is similar to that seen after vaccination of such individuals with pneumococcal and influenza vaccines. Zoster vaccine was well-tolerated when administered concomitantly or sequentially with an inactivated influenza vaccine or pneumococcal vaccine.
Importantly, in immunized individuals who developed zoster, high VZV-specific cell-mediated immune responses were associated with reduced zoster severity and a lower frequency of PHN than in participants with lower VZV-specific cell-mediated responses, whereas a high humoral response was associated with increased severity of zoster and a higher frequency of PHN than in participants with a lower humoral immune response [79].
The Centers for Disease Control and Prevention (CDC) Advisory Committee on Immunization Practices recommends zoster vaccine for all persons over age 60 years who have no prior indications, including a previous episode of zoster or chronic medical conditions. Although zoster vaccine is not recommended for immunocompromised individuals, it appears that the current zoster vaccine could be safely administered to several groups of moderately immunocompromised adult patients, such as VZV-seropositive HIV-infected patients with CD4 T cell counts greater than 200 cells/ml, or even to patients with rheumatoid arthritis or psoriasis and receiving moderate doses of methotrexate, steroids or tumor necrosis factor inhibitors [92]. The currently licensed zoster vaccine remains to be tested in populations of moderately immunocompromised patients. Higher titer zoster vaccines also await testing for safety and immunogenicity. Note that heat-inactivated VZV vaccine has been safely administered to autologous bone marrow transplant recipients, in whom an accelerated recovery of cell-mediated immunity to VZV and a reduced incidence of zoster were observed [84, 93].
Several important questions regarding vaccines remain to be addressed. How many years will the current zoster vaccine maintain immunity to prevent zoster? Is zoster vaccine safe for immunocompromised individuals? Will a killed VZV vaccine produce a significant increase in cell-mediated immunity to VZV? Should multiple vaccinations of the elderly be considered for every decade of life after age 60 years? Should zoster vaccine be refined to include epitopes that induce cell-mediated immunity to VZV?
Cost of zoster and cost-effectiveness of vaccine use
Epidemiologic analysis of the disease burden of zoster in pre-vaccine Taiwan [94] revealed an overall incidence of zoster of 4.97 cases per 1000 people, with women having a significantly higher incidence than men. The incidence increased stepwise with age. The average cost of one case of zoster was about $204 per person for those 80 years or older. Zoster-related hospitalizations and medical cost per patient increased with age. Overall, approximately two-thirds of Taiwanese zoster cases occurred in adults older than 40 years, and about one-third of the population would develop zoster in their lifetime (lifetime risk of 32.2%). On the whole, zoster is a prevalent and costly condition.
Several studies have evaluated the cost-effectiveness of the use of vaccine to prevent zoster and PHN. A decision model to study the cost-effectiveness of vaccine in older adults suggested that immunization would increase quality-adjusted life-years (QALYs) compared to no immunization [95]. Cost-effectiveness of the zoster vaccine varied substantially with patient age and often exceeded $100,000 per QALY saved, and it was recommended that age should be considered in vaccine recommendation [96; see below]. Another study of the cost-effectiveness of zoster immunization in Canada, a country with a population of 30 million people, estimated that there are 130,000 new cases of zoster, 17,000 cases of PHN and 20 deaths [97]. Vaccinating 65-year-old individuals (zoster efficacy = 63%, PHN efficacy = 67%, cost/course = $150) is estimated to cost $33,000 per QALY-gained. Assuming a cost per course of zoster immunization of $150, a probabilistic sensitivity analysis suggested that immunizing between 65 and 75 years of age will likely yield cost-effectiveness ratios below $40,000 per QALY gained, while immunizing adults older than 74 years will yield ratios less than $70,000 per QALY gained. Thus, immunizing adults age 65 to 75 years is likely cost-effective and a judicious use of healthcare resources. In a separate analysis of immunization at ages 65 to 75 years, the same conclusion was reached for England and Wales; An additional conclusion was that a booster dose at a later age was unlikely to be cost-effective [97, 98]. A United States study, also based on a price of $150 for zoster vaccine, estimated that use of vaccine for immunocompetent adults aged 60 years and older was cost-effective; the cost-effectiveness ratios ranged from $16,229 to $27,609 per QALY gained [99]. The studies in England, Wales, United States and Canada all used statistical models that track individuals through various alternative health states after immunization (for example, no herpes zoster, herpes zoster, PHN, death). Studies calculated health care costs based on estimates from societal or health care payer perspectives. Assumptions regarding age-specific incidence of zoster and PHN were derived from epidemiologic studies, while vaccine efficacy against those disorders was based on the SPS for different ages over a mean 3 years of follow-up. In November 2010, a call to three hospital pharmacies in Denver, Colorado, revealed prices of $115-$220 to receive zoster vaccine. Thus, the studies above may overestimate cost-effectiveness if vaccine price is routinely greater than $150.
In addition to questions about cost-effectiveness, insurance coverage and related logistical concerns pose additional challenges to the widespread use of zoster vaccine. While all Medicare Part D plans cover the zoster vaccine, the amount of cost-sharing (i.e., patient out-of-pocket cost) for immunization varies. Essentially, the zoster vaccine is treated like a prescription drug, with varying co-payments depending on the patient’s drug plan. A national survey of primary care physicians regarding zoster and the zoster vaccine [100] revealed that while the physicians perceived a high level of burden from zoster and PHN and generally favored use of zoster vaccine, some physicians expressed concern regarding the uncertainty of protection duration, the need to store the vaccine in a freezer, and the potential unwillingness of patients to pay for the vaccine if it was not covered by insurance. Thus many primary care physicians are not proactive in recommending zoster immunization.
In 2007, one year after zoster vaccine was licensed and recommended by the Advisory Committee on Immunization Practices for persons age 60 years and older, less than 2% of the age group was immunized in the US. This was due to a combination of lack of patient awareness that a vaccine exists, physicians’ uncertainty about the duration of protection, and different cost-sharing plans for immunization [101]. This is disappointing. Zoster vaccine should be universally administered to all individuals over age 60 years. While routine immunization is not currently recommended for people age 50 to 59 years because of lack of efficacy data and cost-effectiveness information in this group, immunization of this population should be considered since about 19% of zoster occurs between ages 50 and 59 years [102].
Conclusions
Zoster (shingles), PHN, and other serious neurological and ocular disorders result from reactivation of VZV, primarily in elderly individuals. Immunization of men and women over age 60 years with Zostavax, a live attenuated VZV vaccine that boosts a naturally declining cell-mediated immunity to VZV with age, significantly reduces the burden of disease due to zoster and PHN. Despite its cost-effectiveness for adults ages 65 to 75 years, as determined by decision models in multiple large countries, less than 10% of immunocompetent adults over age 60 years in the US have been immunized. Although physicians are unsure about the duration of protection and how different cost-sharing plans affect consumer utilization, zoster vaccine is safe, effective, and highly recommended for immunization of immunocompetent individuals over age 60 years with no history of recent zoster.
Acknowledgments
I thank Dr. Adam Gilden Tsai for helpful analysis of data regarding the cost-effectiveness of zoster vaccine, Marina Hoffman for editorial assistance and Cathy Allen for manuscript preparation. This work was supported by the National Institutes of Health grants AG032958 and AG006127.
Footnotes
Conflict of interest statement No conflict of interest to declare.
References
- 1.Donahue JG, Choo PW, Manson JE, Platt R. The incidence of herpes zoster. Arch Intern Med. 1995;155:1605–9. [PubMed] [Google Scholar]
- 2.Leppard B, Naburi AE. Herpes zoster: an early manifestation of HIV infection. Afr Health. 1998;21:5–6. [PubMed] [Google Scholar]
- 3.Kakourou T, Theodoridou M, Mostrou G, Syriopoulou V, Papadogeorgaki H, Constantopoulos A. Herpes zoster in children. J Am Acad Dermatol. 1998;39:207–10. doi: 10.1016/s0190-9622(98)70076-3. [DOI] [PubMed] [Google Scholar]
- 4.Gallagher JG, Merigan TC. Prolonged herpes-zoster infection associated with immunosuppressive therapy. Ann Intern Med. 1979;91:842–46. doi: 10.7326/0003-4819-91-6-842. [DOI] [PubMed] [Google Scholar]
- 5.Blumenthal DT, Salzman KL, Baringer JR, Forghani B, Gilden DH. MRI abnormalities in chronic active varicella zoster infection. Neurology. 2004;63:1538–9. doi: 10.1212/01.wnl.0000141855.67420.73. [DOI] [PubMed] [Google Scholar]
- 6.Carroll WM, Mastaglia FL. Optic neuropathy and ophthalmoplegia in herpes zoster oticus. Neurology. 1979;29:726–9. doi: 10.1212/wnl.29.5.726. [DOI] [PubMed] [Google Scholar]
- 7.Garty B-Z, Dinari G, Sarnat H, Cohen S, Nitzan M. Tooth exfoliation and osteonecrosis of the maxilla after trigeminal herpes zoster. J Pediatr. 1985;106:71–3. doi: 10.1016/s0022-3476(85)80469-8. [DOI] [PubMed] [Google Scholar]
- 8.Manz HJ, Canter HG, Melton J. Trigeminal herpes zoster causing mandibular osteonecrosis and spontaneous tooth exfoliation. South Med J. 1986;79:1026–8. doi: 10.1097/00007611-198608000-00028. [DOI] [PubMed] [Google Scholar]
- 9.Robillard RB, Hilsinger RL, Jr, Adour KK. Ramsay Hunt facial paralysis: clinical analyses of 185 patients. Otolaryngol Head Neck Surg. 1986;95:292–7. doi: 10.1177/01945998860953P105. [DOI] [PubMed] [Google Scholar]
- 10.Asnis DS, Micic L, Giaccio D. Ramsay Hunt syndrome presenting as a cranial polyneuropathy. Cutis. 1996;57:421–4. [PubMed] [Google Scholar]
- 11.Archambault P, Wise JS, Rosen J, Polomeno RC, Auger N. Herpes zoster ophthalmoplegia. Report of six cases. J Clin Neuroophthalmol. 1998;8:185–93. [PubMed] [Google Scholar]
- 12.Meenken C, van den Horn GJ, de Smet MD, van der Meer JT. Optic neuritis heralding varicella zoster virus retinitis in a patient with acquired immunodeficiency syndrome. Ann Neurol. 1998;43:534–6. doi: 10.1002/ana.410430420. [DOI] [PubMed] [Google Scholar]
- 13.Sodhi PK, Goel JL. Presentations of cranial nerve involvement in two patients with herpes zoster ophthalmicus. J Commun Dis. 2001;33:130–5. [PubMed] [Google Scholar]
- 14.Sweeney CJ, Gilden DH. Ramsay Hunt syndrome. J Neurol Neurosurg Psychiatry. 2001;71:149–54. doi: 10.1136/jnnp.71.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Volvoikar P, Patil S, Dinkar A. Tooth exfoliation, osteonecrosis and neuralgia following herpes zoster of trigeminal nerve. Indian J Dent Res. 2002;13:11–4. [PubMed] [Google Scholar]
- 16.Furuta Y, Ohtani F, Aizawa H, Fukuda S, Kawabata H, Bergström T. Varicella-zoster virus reactivation is an important cause of acute peripheral facial paralysis in children. Pediatr Infect Dis J. 2005;24:97–101. doi: 10.1097/01.inf.0000151032.16639.9c. [DOI] [PubMed] [Google Scholar]
- 17.Karmon Y, Gadath N. Delayed oculomotor nerve palsy after bilateral cervical zoster in an immunocompetent patient. Neurology. 2005;65:170. doi: 10.1212/01.wnl.0000167287.02490.76. [DOI] [PubMed] [Google Scholar]
- 18.Merchet MP, Gruener G. Segmental zoster paresis of limbs. Electromyogr Clin Neurophysiol. 1996;36:369–75. [PubMed] [Google Scholar]
- 19.Yoleri O, Olmez N, Oztura I, Sengül I, Günaydin R, Memis A. Segmental zoster paresis of the upper extremity: a case report. Arch Phys Med Rehabil. 2005;86:1492–4. doi: 10.1016/j.apmr.2004.09.032. [DOI] [PubMed] [Google Scholar]
- 20.Anderson JP, Keal EE. Cervical herpes zoster and diaphragmatic paralysis. Br J Dis Chest. 1969;63:222–6. doi: 10.1016/s0007-0971(69)80022-7. [DOI] [PubMed] [Google Scholar]
- 21.Tjandra J, Mansel RE. Segmental abdominal herpes zoster paresis. Aust N Z J Surg. 1986;56:807–8. doi: 10.1111/j.1445-2197.1986.tb02331.x. [DOI] [PubMed] [Google Scholar]
- 22.Mehta SK, Cohrs RJ, Forghani B, Zerbe G, Gilden DH, Pierson DL. Stress-induced subclinical reactivation of varicella zoster virus in astronauts. J Med Virol. 2004;72:174–9. doi: 10.1002/jmv.10555. [DOI] [PubMed] [Google Scholar]
- 23.Cohrs RJ, Mehta SK, Schmid DS, Gilden DH, Pierson DL. Asymptomatic reactivation and shed of infectious varicella zoster virus in astronauts. J Med Virol. 2008;80:1116–22. doi: 10.1002/jmv.21173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Head H, Campbell AW. The pathology of herpes zoster and its bearing on sensory localization. Brain. 1900;23:353–523. doi: 10.1002/(sici)1099-1654(199709)7:3<131::aid-rmv198>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 25.Denny-Brown D, Adams RD, Fitzgerald PJ. Pathologic features of herpes zoster: A note on “geniculate herpes”. Arch Neurol Psychiatry. 1944;51:216–31. [Google Scholar]
- 26.Cheatham WJ, Dolan TF, Jr, Dower JC, Weller TH. Varicella: report on two fatal cases with necropsy, virus isolation, and serologic studies. Am J Pathol. 1956;32:1015–35. [PMC free article] [PubMed] [Google Scholar]
- 27.Esiri MM, Tomlinson AH. Herpes zoster: demonstration of virus in trigeminal nerve and ganglion by immunofluorescence and electron microscopy. J Neurol Sci. 1972;15:35–48. doi: 10.1016/0022-510x(72)90120-7. [DOI] [PubMed] [Google Scholar]
- 28.Ghatak NR, Zimmerman HM. Spinal ganglion in herpes zoster. Arch Pathol. 1973;95:411–5. [PubMed] [Google Scholar]
- 29.Nagashima K, Nakazawa M, Endo H. Pathology of the human spinal ganglia in varicella-zoster virus infection. Acta Neuropathol. 1975;33:105–17. doi: 10.1007/BF00687537. [DOI] [PubMed] [Google Scholar]
- 30.Rogers RS, III, Tindall JP. Herpes zoster in the elderly. Postgrad Med. 1971;50:153–7. doi: 10.1080/00325481.1971.11697705. [DOI] [PubMed] [Google Scholar]
- 31.Bowsher D. The effects of pre-emptive treatment of postherpetic neuralgia with amitriptyline: a randomized, double-blind, placebo-controlled trial. J Pain Symptom Manage. 1997;13:327–31. doi: 10.1016/s0885-3924(97)00077-8. [DOI] [PubMed] [Google Scholar]
- 32.Watson CPN, Morshead C, Van der Kooy D, Deck JH, Evans RJ. Postherpetic neuralgia: post-mortem analysis of a case. Pain. 1988;34:129–38. doi: 10.1016/0304-3959(88)90158-3. [DOI] [PubMed] [Google Scholar]
- 33.Watson CPN, Deck JH, Morshead C, Van der Kooy D, Evans RJ. Postherpetic neuralgia: further post-mortem studies of cases with and without pain. Pain. 1991;44:105–17. doi: 10.1016/0304-3959(91)90124-G. [DOI] [PubMed] [Google Scholar]
- 34.Vafai A, Wellish M, Gilden DH. Expression of varicella-zoster virus in blood mononuclear cells of patients with postherpetic neuralgia. Proc Natl Acad Sci USA. 1988;85:2767–70. doi: 10.1073/pnas.85.8.2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Devlin ME, Gilden DH, Mahalingam R, Dueland AN, Cohrs R. Peripheral blood mononuclear cells of the elderly contain varicella-zoster virus DNA. J Infect Dis. 1992;165:619–22. doi: 10.1093/infdis/165.4.619. [DOI] [PubMed] [Google Scholar]
- 36.Mahalingam R, Wellish M, Brucklier J, Gilden DH. Persistence of varicella-zoster virus DNA in elderly patients with postherpetic neuralgia. J Neurovirol. 1995;1:130–3. doi: 10.3109/13550289509111018. [DOI] [PubMed] [Google Scholar]
- 37.Terada K, Niizuma T, Kawano S, Kataoka N, Akisada T, Orita Y. Detection of varicella-zoster virus DNA in peripheral mononuclear cells from patients with Ramsay Hunt syndrome or zoster sine herpete. J Med Virol. 1998;56:359–63. [PubMed] [Google Scholar]
- 38.Gilden DH, Cohrs RJ, Hayward AR, Wellish M, Mahalingam R. Chronic varicella zoster virus ganglionitis – a possible cause of postherpetic neuralgia. J Neurovirol. 2003;9:404–7. doi: 10.1080/13550280390201722. [DOI] [PubMed] [Google Scholar]
- 39.Quan D, Hammack BN, Kittelson J, Gilden DH. Improvement of postherpetic neuralgia after treatment with intravenous acyclovir followed by oral valacyclovir. Arch Neurol. 2006;63:940–2. doi: 10.1001/archneur.63.7.noc60049. [DOI] [PubMed] [Google Scholar]
- 40.O’Donohue JM, Enzmann DR. Mycotic aneurysm in angiitis associated with herpes zoster ophthalmicus. Am J Neuroradiol. 1987;8:615–9. [PMC free article] [PubMed] [Google Scholar]
- 41.Bhayani N, Ranade P, Clark NM, McGuinn M. Varicella-zoster virus and cerebral aneurysm: case report and review of the literature. Clin Infect Dis. 2008;47:e1–3. doi: 10.1086/588842. [DOI] [PubMed] [Google Scholar]
- 42.Nagel MA, Cohrs RJ, Mahalingam R, et al. The varicella zoster vasculopathies: clinical, CSF, imaging, and virologic features. Neurology. 2008;70:853–60. doi: 10.1212/01.wnl.0000304747.38502.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Burgoon MP, Hammack BN, Owens GP, Maybach AL, Eikelenboom MJ, Gilden DH. Oligoclonal immunoglobulins in cerebrospinal fluid during varicella zoster virus (VZV) vasculopathy are directed against VZV. Ann Neurol. 2003;54:459–63. doi: 10.1002/ana.10685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kang JH, Ho JD, Chen YH, Lin HC. Increased risk of stroke after a herpes zoster attack: a population-based follow-up study. Stroke. 2009;40:3443–8. doi: 10.1161/STROKEAHA.109.562017. [DOI] [PubMed] [Google Scholar]
- 45.Lin H-C, Chien C-W, Ho J-D. Herpes zoster ophthalmicus and the risk of stroke: a population-based follow-up study. Neurology. 2010;74:792–7. doi: 10.1212/WNL.0b013e3181d31e5c. [DOI] [PubMed] [Google Scholar]
- 46.Nagel MA, Forghani B, Mahalingam R, et al. The value of detecting anti-VZV IgG antibody in CSF to diagnose VZV vasculopathy. Neurology. 2007;68:1069–73. doi: 10.1212/01.wnl.0000258549.13334.16. [DOI] [PubMed] [Google Scholar]
- 47.Hilt DC, Buchholz D, Krumholz A, Weiss H, Wolinsky JS. Herpes zoster ophthalmicus and delayed contralateral hemiparesis caused by cerebral angiitis: diagnosis and management approaches. Ann Neurol. 1983;14:543–53. doi: 10.1002/ana.410140509. [DOI] [PubMed] [Google Scholar]
- 48.Gilden DH, Lipton HL, Wolf JS, Akenbrandt W, Smith JE, Mahalingam R, Forghani B. Two patients with unusual forms of varicella-zoster virus vasculopathy. N Eng J Med. 2002;347:1500–3. doi: 10.1056/NEJMoa020841. [DOI] [PubMed] [Google Scholar]
- 49.Gilden DH. Varicella zoster virus vasculopathy and disseminated encephalomyelitis. J Neurol Sci. 2002;195:99–101. doi: 10.1016/s0022-510x(02)00021-7. [DOI] [PubMed] [Google Scholar]
- 50.Habib AA, Gilden D, Schmid DS, Safdieh JE. Varicella zoster virus meningitis with hypoglycorrhachia in the absence of rash and in an immunocompetent woman. J Neurovirol. 2009;15:206–8. doi: 10.1080/13550280902725550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Klein NC, McDermott B, Cunha BA. Varicella-zoster virus meningoencephalitis in an immunocompetent patient without a rash. Scand J Infect Dis. 2010;42:631–3. doi: 10.3109/00365540903510716. [DOI] [PubMed] [Google Scholar]
- 52.Gunson RN, Aitken C, Gilden D. A woman with acute headache and sacral dermatomal numbness. J Clin Virol. 2011 doi: 10.1016/j.jcv.2010.11.001. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Moses H, Nagel MA, Gilden DH. Acute cerebellar ataxia in a 41 year old woman. Lancet Neurol. 2006;5:984–8. doi: 10.1016/S1474-4422(06)70601-9. [DOI] [PubMed] [Google Scholar]
- 54.Ratzka P, Schlachetzki JC, Bähr M, Nau R. Varicella zoster virus cerebellitis in a 66-year-old patient without herpes zoster. Lancet. 2006;367:182. doi: 10.1016/S0140-6736(06)67967-1. [DOI] [PubMed] [Google Scholar]
- 55.Pina MA, Ara JR, Capablo JL, Omeñaca M. Myelitis and optic neuritis caused by varicella. Rev Neurol. 1997;25:1575–6. [PubMed] [Google Scholar]
- 56.Celik Y, Tabak F, Mert A, Celik AD, Aktuğlu Y. Transverse myelitis caused by varicella. Clin Neurol Neurosurg. 2001;103:260–1. doi: 10.1016/s0303-8467(01)00166-4. [DOI] [PubMed] [Google Scholar]
- 57.Gilden DH, Beinlich BR, Rubinstien EM, Stommel E, Swenson R, Rubinstein D, Mahalingam R. Varicella-zoster virus myelitis: an expanding spectrum. Neurology. 1994;44:1818–23. doi: 10.1212/wnl.44.10.1818. [DOI] [PubMed] [Google Scholar]
- 58.Kleinschmidt-DeMasters BK, Gilden DH. Varicella-zoster virus infections of the nervous system: clinical and pathologic correlates. Arch Pathol Lab Med. 2001;125:770–80. doi: 10.5858/2001-125-0770-VZVIOT. [DOI] [PubMed] [Google Scholar]
- 59.Devinsky O, Cho ES, Petito CK, Price RW. Herpes zoster myelitis. Brain. 1991;114:1181–96. doi: 10.1093/brain/114.3.1181. [DOI] [PubMed] [Google Scholar]
- 60.de Silva SM, Mark AS, Gilden DH, Mahalingam R, Balish M, Sandbrink F, Houff S. Zoster myelitis: improvement with antiviral therapy in two cases. Neurology. 1996;47:929–31. doi: 10.1212/wnl.47.4.929. [DOI] [PubMed] [Google Scholar]
- 61.Chua HC, Tjia H, Sitoh YY. Concurrent myelitis and Guillain-Barre syndrome after varicella infection. Int J Clin Pract. 2001;55:643–4. [PubMed] [Google Scholar]
- 62.Schvoerer E, Frechin V, Warter A, Gasser B, Jouin H, Gut JP, Stoll-Keller F. Persistent multiple pulmonary nodules in a nonimmunocompromised woman after varicella-related myelitis treated with acyclovir. J Clin Microbiol. 2003;41:4904–5. doi: 10.1128/JCM.41.10.4904-4905.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Orme HT, Smith G, Nagel MA, Bert RJ, Mickelson TS, Gilden DH. VZV spinal cord infarction identified by diffusion-weighted magnetic resonance imaging (DWI) Neurology. 2007;69:398–400. doi: 10.1212/01.wnl.0000266390.27177.7b. [DOI] [PubMed] [Google Scholar]
- 64.Bonfioli AA, Eller AW. Acute retinal necrosis. Semin Ophthalmol. 2005;20:155–60. doi: 10.1080/08820530500232027. [DOI] [PubMed] [Google Scholar]
- 65.Guex-Crosier Y, Rochat C, Herbort CP. Necrotizing herpetic retinopathies. A spectrum of herpes virus-induced diseases determined by the immune state of the host. Ocul Immunol Inflamm. 1997;5:259–65. doi: 10.3109/09273949709085066. [DOI] [PubMed] [Google Scholar]
- 66.Lewis JM, Nagae Y, Tano Y. Progressive outer retinal necrosis after bone marrow transplantation. Am J Ophthalmol. 1996;122:892–5. doi: 10.1016/s0002-9394(14)70391-5. [DOI] [PubMed] [Google Scholar]
- 67.Franco-Paredes C, Bellehemeur T, Merchant A, Sanghi P, DiazGranados C, Rimland D. Aseptic meningitis and optic neuritis preceding varicella-zoster progressive outer retinal necrosis in a patient with AIDS. AIDS. 2002;16:1045–9. doi: 10.1097/00002030-200205030-00011. [DOI] [PubMed] [Google Scholar]
- 68.Menerath JM, Gerard M, Laurichesse H, Goldschmidt P, Peigue-Lafeuille H, Rozenberg F, Beytout J. Bilateral acute retinal necrosis in a patient with acquired immunodeficiency syndrome. J Fr Ophtalmol. 1995;18:625–33. [PubMed] [Google Scholar]
- 69.Johnston WH, Holland GN, Engstrom RE, Jr, Rimmer S. Recurrence of presumed varicella-zoster virus retinopathy in patients with acquired immunodeficiency syndrome. Am J Ophthalmol. 1993;116:42–50. doi: 10.1016/s0002-9394(14)71742-8. [DOI] [PubMed] [Google Scholar]
- 70.Moorthy RS, Weinberg DV, Teich SA, et al. Management of varicella zoster virus retinitis in AIDS. Br J Ophthalmol. 1997;81:189–94. doi: 10.1136/bjo.81.3.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Austin RB. Progressive outer retinal necrosis syndrome: a comprehensive review of its clinical presentation, relationship to immune system status, and management. Clin Eye Vis Care. 2000;12:119–29. doi: 10.1016/s0953-4431(00)00052-7. [DOI] [PubMed] [Google Scholar]
- 72.Gilden DH, Wright RR, Schneck SA, Gwaltney JM, Jr, Mahalingam R. Zoster sine herpete, a clinical variant. Ann Neurol. 1994;35:530–3. doi: 10.1002/ana.410350505. [DOI] [PubMed] [Google Scholar]
- 73.Lewis GW. Zoster sine herpete. Br Med J. 1958;34:418–21. doi: 10.1136/bmj.2.5093.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Davison AJ, Scott JE. The complete DNA sequence of varicella-zoster virus. J Gen Virol. 1986;67:1759–1816. doi: 10.1099/0022-1317-67-9-1759. [DOI] [PubMed] [Google Scholar]
- 75.Breuer J. VZV molecular epidemiology. Curr Top Microbiol Immunol. 2010;342:15–42. doi: 10.1007/82_2010_9. [DOI] [PubMed] [Google Scholar]
- 76.Breuer J, Whitley R. Varicella zoster virus: natural history and current therapies of varicella and herpes zoster. Herpes. 2007;14(Suppl 2):25–9. [PubMed] [Google Scholar]
- 77.Gershon AA, Arvin AM, Levin MJ, Seward JF, Schmid DS. Varicella vaccine in the United States: a decade of prevention and the way forward. J Infect Dis. 2008;197(Suppl 2):S39–40. doi: 10.1086/522165. [DOI] [PubMed] [Google Scholar]
- 78.Umbach JL, Nagel MA, Cohrs RJ, Gilden DH, Cullen BR. Analysis of human α-herpesvirus microRNA expression in latently infected human trigeminal ganglia. J Virol. 2009;83:10677–83. doi: 10.1128/JVI.01185-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Weinberg A, Zhang JH, Oxman MN, et al. Varicella-zoster virus-specific immune responses to herpes zoster in elderly participants in a trial of a clinically effective zoster vaccine. J Infect Dis. 2009;200:1068–77. doi: 10.1086/605611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Arvin AM, Koropchak CM, Williams BR, Grumet FC, Foung SK. Early immune response in healthy and immunocompromised subjects with primary varicella-zoster virus infection. J Infect Dis. 1986;154:422–9. doi: 10.1093/infdis/154.3.422. [DOI] [PubMed] [Google Scholar]
- 81.Good RA, Zak SJ. Disturbances in gamma globulin synthesis as experiments of nature. Pediatrics. 1956;18:109–49. [PubMed] [Google Scholar]
- 82.Gershon AA, Steinberg SP. Cellular and humoral immune responses to varicella-zoster virus in immunocompromised patients during and after varicella-zoster infections. Infect Immun. 1979;26:170–4. doi: 10.1128/iai.25.1.170-174.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Buchbinder SP, Katz MH, Hessol NA, Liu JY, O’Malley PM, Underwood R, Holmberg SD. Herpes oster and human immunodeficiency virus infection. J Infect Dis. 1992;166:1153–6. doi: 10.1093/infdis/166.5.1153. [DOI] [PubMed] [Google Scholar]
- 84.Hata A, Asanuma H, Rinki M, Sharp M, Wong RM, Blume K, Arvin AM. Use of an inactivated varicella vaccine in recipients of hematopoietic-cell transplants. N Engl J Med. 2002;347:26–34. doi: 10.1056/NEJMoa013441. [DOI] [PubMed] [Google Scholar]
- 85.Burke BL, Steele RW, Beard Ow, Wood JS, Cain TD, Marmer DJ. Immune responses to varicella-zoster in the aged. Arch Intern Med. 1982;142:291–3. [PubMed] [Google Scholar]
- 86.Patterson-Bartlett J, Levin MJ, Lang N, Schödel FP, Vessey R, Weinberg A. Phenotypic and functional characterization of ex vivo T cell responses to the live attenuated herpes zoster vaccine. Vaccine. 2007;25:7087–93. doi: 10.1016/j.vaccine.2007.07.051. [DOI] [PubMed] [Google Scholar]
- 87.Frey CR, Sharp MA, Min AS, Schmid DS, Loparev V, Arvin AM. Identification of CD8+ T cell epitopes in the immediate early 62 protein (IE62) of varicella-zoster virus, and evaluation of frequency of CD8+ T cell response to IE62, by use of IE62 peptides after varicella vaccination. J Infect Dis. 2003;182:40–52. doi: 10.1086/375828. [DOI] [PubMed] [Google Scholar]
- 88.Hayward AR, Zerbe GO, Levin MJ. Clinical application of responder cell frequency estimates with four years of follow up. J Immunol Methods. 1994;170:27–36. doi: 10.1016/0022-1759(94)90242-9. [DOI] [PubMed] [Google Scholar]
- 89.Levin MJ, Barber D, Goldblatt E, et al. Use of a live attenuated varicella vaccine to boost varicella-specific immune responses in seropositive people 55 years of age and older: duration of booster effect. J Infect Dis. 1998;178(Suppl 1):S109–12. doi: 10.1086/514264. [DOI] [PubMed] [Google Scholar]
- 90.Levin MJ, Oxman MN, Zhang JH, et al. VZV-specific immune responses in elderly recipients of herpes zoster vaccine. J Infect Dis. 2008;197:825–35. doi: 10.1086/528696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Weinberg A, Levin MJ. VZV T cell-mediated immunity. Curr Top Microbiol Immunol. 2010;342:341–57. doi: 10.1007/82_2010_31. [DOI] [PubMed] [Google Scholar]
- 92.Harpaz R, Ortega-Sanchez IR, Seward JF. Prevention of herpes zoster: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2008;57(RR-5):1–30. [PubMed] [Google Scholar]
- 93.Redman RL, Nader S, Zerboni L, Liu C, Wong RM, Brown BW, Arvin AM. Early reconstitution of immunity and decreased severity of herpes zoster in bone marrow transplant recipients immunized with inactivated varicella vaccine. J Infect Dis. 1997;176:578–85. doi: 10.1086/514077. [DOI] [PubMed] [Google Scholar]
- 94.Lin Y-H, Huang L-M, Chang I-S, Tsai F-Y, Lu C-Y, Shao P-L, Chang L-Y. Disease burden and epidemiology of herpes zoster in pre-vaccine Taiwan. Vaccine. 2010;28:1217–20. doi: 10.1016/j.vaccine.2009.11.029. [DOI] [PubMed] [Google Scholar]
- 95.Hornberg J, Robertus K. Cost-effectiveness of a vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. Ann Intern Med. 2006;145:386–7. doi: 10.7326/0003-4819-145-5-200609050-00004. [DOI] [PubMed] [Google Scholar]
- 96.Rothberg MB, Virapongse Am, Smith KJ. Cost-effectiveness of a vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. Clin Infect Dis. 2007;44:1280–8. doi: 10.1086/514342. [DOI] [PubMed] [Google Scholar]
- 97.Brisson M, Pellissier JM, Camden S, Quach C, De Wals P. The potential cost-effectiveness of vaccination against herpes zoster and post-herpetic neuralgia. Hum Vaccin. 2008;4:238–45. doi: 10.4161/hv.4.3.5686. [DOI] [PubMed] [Google Scholar]
- 98.van Hoek AJ, Gay N, Melegaro A, Opstelten W, Edmunds WJ. Estimating the cost-effectiveness of vaccination against herpes zoster in England and Wales. Vaccine. 2009;27:1454–67. doi: 10.1016/j.vaccine.2008.12.024. [DOI] [PubMed] [Google Scholar]
- 99.Pellissier JM, Brisson M, Levin MJ. Evaluation of the cost-effectiveness in the United States of a vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. Vaccine. 2007;25:8326–37. doi: 10.1016/j.vaccine.2007.09.066. [DOI] [PubMed] [Google Scholar]
- 100.Hurley LP, Harpaz R, Daley MF, et al. National survey of primary care physicians regarding herpes zoster and the herpes zoster vaccine. J Infect Dis. 2008;197(Suppl 2):S216–23. doi: 10.1086/522153. [DOI] [PubMed] [Google Scholar]
- 101.Lu PJ, Euler GL, Jumaan AO, Harpaz R. Herpes zoster vaccination among adults aged 60 years or older in the United States, 2007: uptake of the first new vaccine to target seniors. Vaccine. 2009;27:882–7. doi: 10.1016/j.vaccine.2008.11.077. [DOI] [PubMed] [Google Scholar]
- 102.Hope-Simpson RE. The nature of herpes zoster: a long-term study and a new hypothesis. Proc R Soc Med. 1965;58:9–20. [PMC free article] [PubMed] [Google Scholar]


