Abstract
Summary
Mesenchymal stem cells (MSCs) and their stromal progeny may be considered powerful regulatory cells, a sort of dendritic cell counterpart, which influence all the main immune effectors and functional roles in vivo, as well as potential applications in the treatment of a number of human immunological diseases. By choosing MSC tissue origin, cell dose, administration route, and treatment schedule, all the potential side effects related to MSC use, including tumor growth enhancement, have to be well considered to maximize the benefits of MSC-depen-dent immune regulation without significant risks for the patients.
Key Words: Mesenchymal stem cells, Cell therapy, Immunomodulation, Graft-versus-host disease
Abstract
Zusammenfassung
Mesenchymale Stammzellen (MSC) sowie davon abstammende Stromazellen können als leistungsstarke regulatorische Zellen (eine Art Gegenstück zu den dendritischen Zellen) betrachtet werden, die in vivo Auswirkungen auf die wichtigsten Immuneffektoren und Funktionen haben sowie die potentielle Anwendung in der Behandlung einer Reihe von immunologischen Erkrankungen beim Menschen beeinflussen. Bei der Auswahl des MSC-Ursprungsgewebes, der Zelldosis, der Art der Verabreichung sowie des Behandlungsplans müssen alle potentiellen Nebenwirkungen einer MSC-Gabe (inklusive Förderung des Tumorwachstums) gut bedacht werden, um die MSC-abhängige Immunregulierung ohne maßgebliche Risiken für die Patienten maximal nutzen zu können.
Introduction
The close link between hematopoiesis/lymphopoiesis and stromal cell compartment has been well known for many years. In bone marrow, the hematopoietic stem cell (HSC) niche represents a peculiar microenviroment that ensures the balance between proliferation and self-renewal of HSCs to maintain long-term hematopoiesis [1, 2]. Subendothelial stromal cell precursors, which give rise to stromal cell components (reticular cells, fibroblasts, adipocytes, and osteoblasts) and express the typical immunophenotypic pattern of mesenchymal stem cells (MSCs) including specific molecules such as CD146, have been recently shown in bone marrow [3]. MSCs are multipotent non-hematopoietic progenitor cells capable of differentiating not only into various tissues of mesodermal origin (fibroblasts, osteocytes, adipocytes, and chondrocytes), but also into tissues of endodermal and neuroecto-dermal lineages, including hepatocytes [4], epithelia [5], and neurons [6, 7]. MSCs constitutively secrete regulatory molecules and cytokines, most of which are shared also by stromal cells, which enhance the proliferation/differentiation of hematopoietic stem/progenitor cells as well as of mature lymphoid cells, such as macrophage-colony stimulating factor (M-CSF), Flt-3L, stem cell factor (SCF), interleukin (IL)-6, IL-7, IL-8, IL-11, IL-12, IL-14, and IL-15 [8, 9, 10]. Upon IL-1α stimulation, MSCs can produce IL-1α itself, leukemia inhibitory factor (LIF), granulocyte(G)-CSF, and granulocyte-macrophage(GM)-CSF [10]. In addition, there is strong evidence that MSCs and their stromal progeny play a fundamental role in lymphopoiesis [11]. After bone marrow transplantation, stromal cells appear to migrate to the thymus where they participate in the positive selection of thymocytes [12, 13]. Furthermore, in the absence of the thymus, the majority of T cells adhering to bone marrow stroma display an immature phenotype [14, 15]. B cell development also requires close association of B cell progenitors with stromal cells [16, 17] through the interaction between pre-B cell receptor (pre-BCR) and its stromal ligand Galectin-1 [18], which is essential for pre-B-cell survival, proliferation, and differentiation [19]. Stromal cell precursors with the immunophenotype and multilineage differentiation potential of MSCs are present also in adult lymphoid tissues, such as lymph nodes [19], spleen, and thymus [20]. Finally, co-transplantation of human ex vivo-expanded MSCs together with HSCs supports hematopoietic and lymphoid recovery in animal models [21, 22, 23, 24] and in humans [25, 26, 27]. More recently, MSC regulatory activity on a large number of effector cells of adaptive and innate immunity has been extensively characterized, including CD4+ and CD8+ T cells [28, 29, 30, 31, 32, 33, 34, 35, 36], B cells [28, 37], natural killer (NK) cells [28, 30, 38, 39], monocyte-derived dendritic cells (DCs) [40, 41, 42, 43], and neutrophils [44]. The interaction with MSCs leads to lymphocyte [32] and DC [45] anergy due to early proliferation arrest, and it inhibits apoptosis of resting and activated neutrophils [44]. MSCs may suppress immune reactions in vitro and in vivo in a major histocompatibility complex (MHC)-independent manner [28, 29, 30, 46]. Interestingly, the immune regulatory properties are expressed not only by bone marrow MSCs, but also by MSCs derived from other tissues, including fat [47], thymus, and spleen [20]. Moreover, MSCs differentiated into fibroblasts, adipocytes, and osteoblasts [48, 49, 50, 51] retain similar functions.
At present, there is no unique and hierarchically prevalent mechanism responsible for MSC immune regulation, but there is a redundant panel of mechanisms that suggests the in vivo relevance of immune regulation by the stromal cell compartment. Some contradictory results have been produced by different groups, probably due to different experimental factors related to MSC origin, culture conditions, and lymphocyte subset and activation state. On the whole, these data suggest that both soluble factors and cell-cell contact are involved. MSC regulatory effects are operational in vivo, as MSC infusion can significantly prolong the survival of MHC-mis-matched skin grafts in baboons [46], lower the incidence and cure the refractoriness to treatment of graft-versus-host disease (GvHD) after allogeneic HSC transplantation in humans [52], and improve experimental autoimmune encephalo-myelitis (EAE) in mice [53]. Thus, it is important to know the kinetics, mechanisms, and administration modalities of MSC-based immune therapies to achieve clinical benefit with no or only a small number of potential side effects.
Immunogenicity of Mesenchymal Stem Cells
MSCs are unable to induce significant alloreactivity [30]. Human MSCs express a low-intermediate level of HLA class I and LFA-3, and they do not express the co-stimulatory molecules CD80 (B7–1), CD86 (B7–2), CD40, or CD40L, even after IFN-γ stimulation [28, 48, 54] which in turn may induce HLA class II molecule up-regulation [28]. MSCs may escape not only the recognition by alloreactive T cells [30] but also the cell-specific lysis by CD8+ cytotoxic cells [55] and freshly isolated NK cells [34]. By contrast, activated NK cells are capable to lyse MSCs efficiently [38]. Moreover, MSCs exogenously loaded with the relevant MHC class I peptide epitopes still remain resistant to lysis [56]. At a low MSC/effector ratio (1:0.2 to 1:0.01), the number of proliferating T cells increases after 96 h of mixed lymphocyte reaction (MLR), thus suggesting that the balance between T cell suppression and activation by MSCs may be finely tuned [57]. Alternatively, the enhancement of T cell survival could derive from the MSC-mediated inhibition of T cell apoptosis through the T cell receptor (TCR) engagement [58].
At basal conditions, human MSCs do not express surface MHC class II molecules, although some intracellular deposits of class II alloantigens are present [59, 60]. In the presence of low IFN-γ levels, MSCs express MHC-class II molecules and acquire phagocytic function and antigen presentation activity to CD4+ T cells [61, 62]. High IFN-γ levels down-regulate the expression of MHC class II molecules by MSCs, thus leading to a shift in MSC function to immunosuppression [61]. MSC number in culture and transforming growth factor (TGF)- β signaling regulate in an opposite fashion the IFN-γ-driven MHC class II expression by acting on CIITA expression [63]. Transplanted allogeneic, MHC-mismatched MSCs fail to induce specific rejection, thus engrafting in adult rodent, porcine, and baboon experimental models [46, 64, 65]. Engraftment of allogeneic MSCs in immune-compromised hosts or inside immune-privileged sites have been shown in animals and in humans [52, 66, 67, 68]. Xenogenic transplantation (mouse MSCs into rats) may induce immunological tolerance [69]. By contrast, allogeneic MSC transplantion into hosts with intact immune system may determine MSC rejection [70, 71, 72]. Some data show that infusion of allogeneic MSCs can prime naive T cells in immunocompetent mice [72]. Moreover, intra-coronary injection of adult human MSCs into rat myocardium is associated with rejection and macrophage infiltration [73]. Thus, allogeneic or xenogeneic MSC transplantation may lead to MSC rejection; this should always be considered in the clinical setting.
Mesenchymal Stem Cells and T Lymphocytes
T cell proliferation, activation, and effector functions may be affected by MSCs in vitro [31] and in vivo [46]. Inhibition of T cell proliferation by MSCs occurs not only when T cells are triggered by non-specific stimuli such as allogeneic peripheral blood lymphocytes, DCs, or mitogens such as phytohemagglutinin (PHA) or IL-2 [31], but also when T cells are activated by their specific antigen [30]. Similarly, T cell-mediated IFN-γ production [28, 29, 30, 32, 33] and cytotoxic activity [30, 55] may be inhibited. Proliferation of CD4+ and CD8+ T cells is equally inhibited by MSCs [28, 30, 31]. This effect does not seem to be related either to the lack of activation or the induction of apoptosis [28, 30, 32]. In fact, in T cell/MSC co-culture, the number of T cells expressing early activation markers, i.e. CD25 and CD69, is not affected [28] although some data are contradictory [48, 54, 55, 74, 75]. CD8+ T cell-mediated lysis is suppressed by MSCs if they are added at the beginning of the mixed lymphocyte culture [34], but not when T cells are already in the cytotoxic phase [55, 60, 76], thus suggesting that it is the generation of activated lytic effector cells that is affected, rather than the lytic effector phase. MSCs interfere with naive CD4+ T cell differentiation into T helper (Th)-1 effector cells by decreasing the amount of IFN-γ produced. By contrast, MSCs may induce a Th-2 shift by increasing the production of IL-4 [43]. Both naive and memory T cells can be inhibited by MSCs [30]. In a mouse model, IFN-γ production by T cells may be restored after MSC removal from culture [30]; by contrast, T cell proliferation is irreversibly abrogated by cyclin D2 inhibition, thus suggesting a mechanism of T cell arrest anergy in the early G1 phase of the cell cycle [32]. This anergic state is only partially reverted by exogenous IL-2 [32]. Other studies with human MSCs show that T cell unresponsiveness is transient and may be restored by MSC removal [59].
The presence of CD4+/CD25+ T cells is not required for the anti-proliferative effect of MSC on T-cells [30]; however, MSCs may induce the expansion of these regulatory T cells [43, 76]. The addition of CD4+, but also CD8+ regulatory T lymphocytes, obtained from lymphocyte/MSC co-cultures, may inhibit mixed lymphocyte reactions and T cell activation by alloantigen, PHA, and CD3-triggering [77]. MSC-induced suppression of T cell proliferation does not require MHC restriction, but it may be mediated also by allogeneic MSCs [29] in a dose-dependent and antigen-independent manner [30, 49]. The optimal ratio between MSCs and responder T cells is quite variable, from 1:10,030 to 1:142, depending on the MSC model (human or animal), the culture conditions, and the origin and purity of MSCs, but most studies show that the maximum inhibitory effect normally occurs at a 1:10 ratio [28, 30, 31, 43]. It is difficult to assess if these ratios are reached inside the tissues, but it is not unlikely; in addition, the persistence of the immune regulatory properties in MSC-derived tissue stremal cells [48, 49, 50, 51] would suggest that this phenomenon may also have a physiological role in vivo.
MSC may inhibit the apoptosis of proliferating thymocytes cultured in the absence of trophic factors and resting T cells [50, 58]. Moreover, MSCs may rescue from activation-induced cell death (AICD) T cells over-stimulated by TCR engagement, through a down-regulation of Fas receptor and Fas ligand [58]. MSC-induced immunosuppression is due to both soluble factors and cell-cell contact, but the latter mechanism is prevalent in rodent MSCs [28, 29, 30, 31, 32, 33, 34, 35, 36]. Most of the inhibitory soluble factors are not constitutively secreted by MSCs, but they can be induced by the interaction between activated effector cells and MSCs. A broad range of factors is involved in the immune regulation induced by MSCs, including IFN-γ [20, 28], IL-1β [74], TGF-β1 [31, 35, 49], indoleamine 2,3-dioxygenase (IDO) [20, 28, 33], IL-6 [78, 79], IL-10 [40, 41], prostaglandin E2 (PGE2) [43], hepatocyte growth factor (HGF) [31], tumor necrosis factor(TNF)-α [42, 59, 60, 80], nitric oxide (NO) [81], heme oxygenase-1 (OH-1) [82], HLA-G5 [83, 84], and other unidentified factors. This probably reflects the redundancy of the MSCs immune regulatory mechanisms. It is interesting that cytokines favoring the immune responses, such as IFN- γ produced by activated T lymphocytes or NK cells, may promote immune modulation by MSCs which in turn suppress T or NK cell proliferation. This effect is related, at least in part, to the enhancement of the IDO activity [28, 85]. Moreover, tryptophan depletion associated with IDO activity results in cell cycle arrest of activated T cells [86]. However, human IFN-γ receptor 1(R1)-deficient MSCs do not elicit IDO transcription, despite the preservation of immune regulation [87]. Following interaction with effector cells, MSCs secrete TGF-β1 which is partially responsible for T cell inhibition. When anti-TGF-β1 and anti-HGF are simultaneously added, T cell proliferation can be restored at values comparable to those detected in absence of MSCs [31]. IL-6 may inhibit both T cell proliferation and apoptosis. The addition of a neutralizing anti-IL-6 antibody, however, restores partially the proliferation of T cells, but it abrogates most of the protection from apoptosis [78, 79]. MSCs constitutively express and up-regulate both COX-1 and COX-2 which both increase PGE2 production. PGE2 inhibitors lower MSC-mediated immune modulation, but only when lymphocytes are stimulated with PHA, not in a mixed lymphocyte reaction. TNF-α may enhance as much as 100-fold the production of immunosuppressive prostaglandins by MSCs [42, 59, 60]. However, in a mouse model of rheumatoid arthritis, the addition of TNFα may revert the inhibition of proliferation of allogeneic T cells induced by MSCs [80]. The production of NO and OH-1 appears to be involved, in part through Stat5 inhibition, in the suppression of T cells by MSCs [81, 82]. Following cell-cell contact with T cells, MSCs can secrete the soluble isoform of HLA-G5; this molecule seems to mediate, at least in part, the expansion of functional CD4+ CD25high FoxP3+ regulatory T cells [83].
Recently, a new mechanism affecting the immunosuppressive activity of MSCs has been described. MSCs were found to express some Toll-like receptors, such as (TLR) 1, TLR3, TLR4, and TLR5. The triggering of TLR3 and TLR4 by their natural ligands may suppress MSC immune regulatory activity, thus suggesting that T cell responses may arise efficiently during infections, leading to pathogen elimination [88].
Mesenchymal Stem Cells and NK Cells
In vitro studies on the interactions between NK cells and MSCs are of potential interest for cancer immunotherapy involving NK cells [89], as well as for GvHD treatment and prevention [52]. Anti-cancer activity of NK cells may be hampered not only by the difficulty of penetrating inside the tumor mass [90], but also by the interactions with the tumor-associated stroma which is of MSC-origin and retains similar immune regulatory properties [48, 50, 91].
The mechanisms underlying MSC-mediated NK cell modulation have been partially unraveled. MSCs may inhibit both IL-2- and IL-15-induced NK proliferation [28, 38]. Soluble factors or cell-cell contact mediate different effects depending on the experimental settings. Thus, IFN-γ secretion following IL-2-mediated NK stimulation is responsible for the inhibition of NK proliferation by MSCs [28]; on the other hand, MSC-dependent inhibition of IL-15-activated NK cells requires both cell-cell contact and soluble factors, such as TGF-β1 and PGE2, that are produced during MSC/NK co-culture [38]. The influence of MSCs on cytotoxicity of freshly isolated NK cells is still controversial. In some studies with freshly isolated NK cells, no MSC-mediated modulation of cytotoxicity has been observed towards HLA class I-negative targets (K562 cell line), whereas MSCs may impair the cytolytic activity against HLA class I-positive targets [38]. In other experience, MSCs not only inhibit the cytokine-induced proliferation of freshly isolated NK cells, but also prevent their effector functions and cytokine production against HLA class I-positive, as well as class I-negative target cells (SKNBE and HTLA-30 cell lines) [92]. Thus, MSC suppression of NK cytolitic activities may be stronger against HLA class I-negative targets expressing a limited number of ligands for different NK receptors [93]. Instead, when considering IL-15-activated NK, the suppressive effect of MSCs on NK cytotoxicity depends on culture time. In fact, short-term co-culture of IL-15-stimulated NK cells and MSCs leads to the inhibition of NK cytolytic activity against both the HLA class I-negative and -positive cells. This phenomenon is associated with the reduction of IL-15-induced cytokines, such as IFN-γ, IL-10, and TNF-α, and it requires cell-cell contact [39]. Similar results have been obtained with longtime co-culture of IL-2-activated NK cells with MSCs, leading to the decrease of killing against the HLA class I-negative K562 cell line [28]. Taken together, these data show that MSCs may inhibit NK functions against HLA class I-negative and -positive targets which, in turn, become less susceptible to NK attacks. The suppression of NK lytic activity and IFN-γ secretion has been related to the release by MSCs of HLA-G5, a soluble isoform of non-classical HLA class I usually expressed in a few healthy tissues such as cytotrophoblasts [94], but is also involved in tumor-driven immune escape [95], and IDO activity [92].
Although most studies based on freshly isolated NK cells support the concept of a sort of MSC immunoprivilege [34], there is also evidence of MSC susceptibility to NK-mediated killing when activated NK cells are employed [38, 39, 92]. In fact, MSCs express some ligands for NK receptors, such as NKp30, NKG2D, and DNAM-1 KK, which make themselves suitable to be lysed [39]. After IL-2 activation, NK cells may lyse MSCs in both autologous or allogeneic settings [95]. However, this phenomenon may be partially prevented by IFN-γ which up-regulates the expression of HLA I molecules by MSCs [95]; in addition, MSCs may inhibit the surface expression of NKp30 and NKG2D, as well as NKp44-activating receptor, thus impairing NK effector functions [92].
Mesenchymal Stem Cells and Dendritic Cells
DCs are the cells mostly specialized in the uptake, transport, and presentation of antigens. Depending on their activation and maturation stage, DCs may act in the primary immune responses as either inducers of T cell immunity or mediators of T cell tolerance [96]. The interactions between DCs and MSCs have been investigated in several studies to assess whether MSCs may alter DC functions and contribute to the generation of tolerogenic antigen-presenting cells (APCs). The presentation of alloantigens by APCs to T cells leads to T cell activation and proliferation which are both inhibited by the presence of MSCs [46, 30, 31]; this evidence raises the question whether the immune regulation by MSCs on T cell functions involves, directly or indirectly, also the role of DCs. In the presence of MSCs, CD14+ monocytes induced to differentiate into fully mature DCs with endotoxin, IL-4, G-CSF, and TRL stimulation, retain high CD14 expression without expressing at high levels CD1a, CD40, CD80, CD83, CD86, and HLA-DR which are all necessary to induce efficient T cell responses [40, 41]. Moreover, MSCs may partially hamper immature (such as endocytic activity) and mature DC functions (such as IL-12 secretion), thus lowering DC ability to induce lymphocyte proliferation [41]. The use of CD14+ monocytes as APCs in MSC/CD4+ or CD8+ T cell co-cultures may prevent T cell proliferation and IFN-γ secretion in a dose-dependent fashion, thus confirming that tolerogenic immature APCs persist instead of mature DCs [40, 41]. APCs generated in the presence of MSCs express low levels of proinflammatory molecules (IL-12, TNF-α, MHC class II), and high levels of IL-1β and IL-10, the anti-inflammatory molecule inducing T cell un-responsiveness, although CD86 expression is similar to normal controls without MSCs [42]. MSCs may determine the shift from the DC1 to the DC2 signaling pathway as suggested by the decrease of TNF-α secretion by activated DCs that leads to the reduction of IFN-γ-producing Th1 cells [43, 76]; on the other hand, MSCs may induce DCs to secrete IL-10 that, in turn, may favor IL-4-producing Th2 cells and increase the proportion of regulatory T cells [43]. MSCs, when added to GM-CSF- and IL-4-stimulated peripheral blood monocytes, may impair their differentiation into DCs by determining division arrest anergy, with complete loss of the ability of activating T cells [45]. Interestingly, MSC effect on monocyte differentiation appears stronger than that exerted by fibroblasts [97] as shown by the lower MSC/monocytes ratio employed and by the lack of functional recovery by supplementing TNF-α to the initial co-culture [41]. In addition, the development of Langerhans cells from CD34+ cells using GM-CSF and TNF-α is not impaired by MSC presence at the beginning of the differentiation culture. Instead, MSCs make DCs maintain an intermediate CD14+ CD1a− phenotype without expressing CD1a, which is typical of dermal/interstitial DCs, even after MSC removal. Soluble factors secreted by MSCs, such as IL-6 and M-CSF [72, 79], as well as cell-cell contact [98] may mediate MSC modulation of DC maturation.
Mesenchymal Stem Cells and B Cells
MSCs may affect B cell proliferation, apoptosis, immunoglobulin (Ig) production, and chemotaxis. Mouse B cell proliferation, induced by either anti-CD40 monoclonal antibody and IL-432 or pokeweed mitogen [99], is inhibited by MSC co-culture. The results are similar with B cells stimulated with endotoxin and obtained from BXBS mice, the animal model for human systemic lupus erythematosus (SLE) [100]. Human B cell proliferation, induced by CpG, rCD40L, anti-Ig antibodies, IL-2, and IL-5, is inhibited by MSC co-culture [28, 37], but the maximum effect is observed at the B cell/MSC ratio of 1:1 of which it is unknown if it can be reached inside the tissues in vivo [37]. In the presence of CpG DSP30F ODN and allogenic T cell-depleted peripheral blood mononuclear cells, human MSCs do not affect B cell proliferation, unless IFN-γ, a typical T cell-dependent B cell-promoting cytokine, is added. B cell response to pokeweed mitogen is completely suppressed by cognate contact with MSCs, and partially by soluble factors present in the supernatant [99]. The addition of blocking antibodies against the molecules of the programmed death pathway (PD-1, PD-L1, and PD-L2) [101] may restore about 30% of B cell proliferation [99]. However, MSCs may also have MHC-independent, anti-apoptotic effects [78]. MSC-dependent inhibition of human B cell proliferation appears to be due to the arrest of the cell cycle in the G0/G1 phases, rather than to the induction of apoptosis [37].
MSCs may also modulate Ig secretion, both in vitro and in vivo. MSC/B cell co-culture at a 1:10 ratio leads to stimulation of IgG secretion when both kinds of cells are in contact, but to a significant reduction in IgG production in transwell experiments [102]. Moreover, MSCs may lower IgG production induced by endotoxin and herpes viruses, but not in the case of weak responses [102]. MSCs may also inhibit IgM, IgG, and IgA production, but at higher MSC/B cell ratios (1:1) [37]. This phenomenon has also been described with BXSB hyper-reactive B cells [100]. The modulation of Ig secretion by MSCs has been reported in in vivo models. In experimental EAE-affected mice, the production of antigen-specific Igs has been measured after MSC infusion which led to a significant decrease in both total antigen-specific IgG and IgG subclasses [103]. In addition, MSCs may modify the chemokine receptor expression by B cells: significant decrease of CXCR4, CXCR5, and CCR7 expression, as well as inhibition of chemotaxis by CXCL12 and CXCL13 have been observed at a 1:1 MSC/B cell ratio [37].
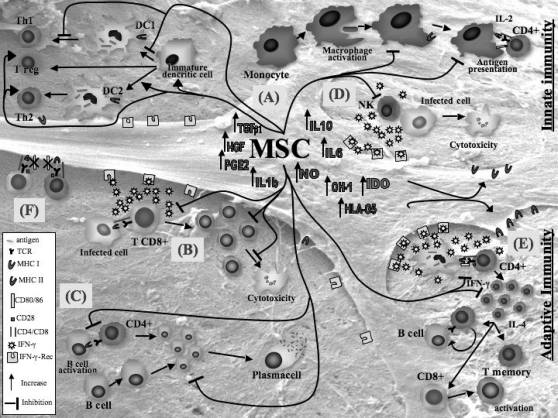
All these variable results reflect different experimental conditions. The proliferative stimuli can be T-independent [28, 78] or T-dependent [32, 99], specific [37] or not specific [99]. The effects of MSCs on B cells are dose-dependent, but the MSC/B cell ratios at which these effects have been observed may vary according to culture conditions. Most results have been observed at a 1:1 ratio [37], but recent studies suggest that lower ratios, such as 1:10 [100, 102] and 1:30 [78] are still effective. Figure 1 shows some of the mechanisms discussed here.
Fig. 1.
Regulatory effect of MSCs on immune effector cells. (A) MSCs delay maturation of monocytes and dendritic cell (DC) precursors, decrease TNF-α and IL-12 secretion by DCs type 1 (DC1), thus inhibiting T-helper cell type 1 (Thl), and increase IL10 secretion by endo-toxin-stimulated DCs type 2 (DC2), thus increasing regulatory T cells. (B) MSCs inhibit the development of cy-totoxic T cells and their IFN-γ production. (C) MSCs decrease proliferation and immuno-globulin secretion by B cells. (D) MSCs decrease cytotoxycity and IFN-γ production by NK cells. (E) MSCs decrease IFN-γ secretion by Thl and increase IL-4 secretion by Th2. Several factors produced by MSCs have been suggested to inhibit immune effector cells, such as indoleamine 2.3-dioxygenase (IDO), prostaglandin E2 (PGE2), transforming growth factor-β1 (TGFβ1), hepatocyte growth factor (HGF), IL-6, IL-10, IL-1β, TNF-α, HLA-G5, nitric oxide (NO), and heme oxygenase-1 (OH-1). (F) Cell-cell contact: the absence of co-stimulatory molecules, such as CD80 and CD86, may contribute to the immunomodulatory effect of MSCs.
In vivo Immune Modulation by Mesenchymal Stem Cells
Animal Models
A large number of animal models have been used to evaluate in vivo MSC immune regulatory properties related to allore-active responses in tissue transplantation and autoimmunity. The evidence of the induction of immunological tolerance by MSCs derives from preliminary data in a mouse model in which allogeneic or xenogeneic (rat) HSCs were transplanted with their marrow microenvironment under the kidney capsule. In these conditions, the authors observed the decrease of GvHD incidence and the onset of immunological tolerance towards xenogeneic rat HSCs23. Subsequently, long-term donorspecific hyporesponsiveness, in absence of cytotoreductive conditioning regimens, and acceptance of donor skin grafts have been achieved [66]. Similarly, fetal sheep co-transplanted in utero with allogeneic HSCs and human MSCs, show increased levels of engraftment and shorter periods of hematopoietic reconstitution [104]. With a single dose of allogeneic MSCs derived from baboon bone marrow, only a modest, although significant, prolongation of skin graft survival is observed [46]. Similar results have been achieved with the systemic infusion of ex vivo-expanded adipose derived MSCs (ADAS) which may control lethal GvHD in mice transplanted with haploidentical HSC grafts [105]; this phenomenon may occur only in the early phases after MSC transplantation so that repeated MSC infusions are required to ameliorate GvHD [105]. This might explain why the infusion of a single MSC dose in allogeneic bone marrow transplantation does not affect the incidence and severity of GvHD in mice [71].
The mechanism behind the enhanced engraftment in vivo is still unknown. At present, it is unclear whether the immunomodulatory properties of MSCs are independent from the engraftment-enhancing effect of these cells. One possibility is that cytokine release by MSCs may promote either homing or proliferation of HSCs, without requiring either MSC persistency inside the bone marrow or the interaction with other cell types, including immune effector cells. In fact, human culture-expanded MSCs, co-transplanted with human cord blood CD34+ cells in irradiated NOD/SCID mice, are not detected inside the bone marrow at 6 weeks after transplantation despite the improvement of hematological recovery, as compared to controls without MSCs [106]. Conversely, other studies have shown that human MSCs can be detected inside the mouse bone marrow microenvironment after 4–10 weeks from transplantation; these cells appear to be involved in the maintenance of human hematopoiesis via both soluble factors and contact with hematopoietic cells [24, 107]. However, MSC infusion is not always followed by their stable engraftment and function. In vivo studies have shown that the administration of allogeneic MSCs into an MHC-mismatched host may result in their rejection [72]. Moreover, co-infusion of allogeneic MSCs in mice receiving allogeneic bone marrow transplantation may not prevent bone marrow rejection as efficiently as in mice infused with autologous MSCs [70].
MSC-based immune modulation is considered a potential novel strategy for autoimmunity. It has been shown that mouse MSCs may improve EAE, a model of human multiple sclerosis, through the induction of peripheral T cell tolerance against the pathogenic antigen; however, MSCs seem to be effective only at the disease onset and peak, but not after disease stabilization [53, 108]. In a mouse model of rheumatoid arthritis (DBA/1 mice immunized with type II collagen in Freund's adjuvant), a single injection of MSCs prevents the occurrence of severe, irreversible bone and cartilage damages, by inducing T cell hyporesponsiveness and modulation of inflammatory cytokines, such as TNF-α [80, 109]. MSCs may also provide protective effects in rat models of kidney and myocardial injury, based on ischemia/reperfusion processes, by secreting soluble immune-modulating factors [110] or by inhibiting the release of pro-inflammatory mediators [111]. Similarly, the infusion of MSCs in an experimental rat model of glomeru-lonephritis accelerates the glomerular healing, probably through the release of growth factors [112]. In mice with type 1 diabetes, it has been recently shown that MSCs and bone marrow cells may have a synergistic effect, by supporting the regeneration of recipient-derived pancreatic insulin-secreting cells and by inhibiting T cell-mediated immune responses against newly formed beta-cells [113].
Clinical Experience
Because of their immunosuppressive properties, MSCs are considered a potential strategy to prevent graft rejection and GvHD. Infusion of MSCs in autologous or allogeneic hema-topoietic stem cell transplantation may accelerate hematopoietic recovery and reduce the risk of graft failure and the incidence of acute GvHD [24, 25, 26, 27, 114. 115, 116], even in the absence of long-term persistence after successful allogeneic stem cells transplantation [117]. To assess the safety of ex vivo-expanded MSCs, a phase I trial has been designed with 15 volunteers with hematological malignancies in complete remission. Patients received intravenous autologous MSCs at different doses, but no adverse effects have been reported [114]. Similarly, 28 patients with advanced breast cancer received autologous hematopoietic stem cell transplantation with ex vivo-expanded MSCs at the dose of 1–2.2 × 106/kg body weight, thus achieving faster hematopoietic recovery with no toxicity [25]. Autologous MSCs are not always available for autologous transplantation as high-dose chemotherapy before hematopoietic stem cell transplantation may damage the bone marrow stroma [118]. Allogeneic MSCs derived from healthy donors may be an alternative choice, at least for immunocompromized patients. In a multicenter clinical trial, 46 patients with heterogeneous hematological malignancies have been co-infused, after a myeloablative conditioning regimen, with HSCs and bone marrow culture-expanded MSCs, both derived from HLA-identical sibling donors [26]. MSCs (1–5 × 106/kg body weight) were infused 4 h before HSCs with neither adverse events nor ectopic bone and cartilage formation, nor increase of the incidence or severity of GvHD. However, hematopoietic recovery and prevention of graft rejection were similar to controls [26]. Similarly, 7 patients of different ages, diagnosis, and disease status were treated with allogeneic (3 cases) or haploidentical (4 cases) MSCs together with HSCs. A neutrophil count of > 0.5 × 109/l and a platelets count of > 30 × 109/l were both achieved after a median of 12 days. Acute GvHD grade 0–I occurred in 5 patients, and grade II acute GvHD in the 2 other patients, evolving into chronic GvHD in 1 patient [27]. Comparable results have been obtained in an European phase I-II study: 14 children received haploidentical HSC grafts in combination with expanded MSCs derived from donor bone marrow. Faster leukocyte recovery was observed, with no immediate adverse effects [115]. Allogeneic MSCs have been used also for the treatment of severe idiopathic aplastic anemia refractory to conventional treatment and not eligible for allogeneic HSC transplantation [116]. One patient was infused twice with allogeneic MSCs (2 × 106 and 6 × 106 MSCs/kg body weight), without any conditioning regimen. No signs of hematopoietic recovery were evident 34 days after the second infusion, and the patient died of invasive fungal infection. However, donor MSC engraftment was detected in the endostium by bone marrow biopsy, but not in the marrow aspirates, thus suggesting that transplanted MSCs are primarily located in the bone and can be transplanted across HLA barriers [116]. Third-party (mother's), haploidentical MSCs were used to treat severe, refractory, grade IV acute GvHD of the gut and liver in a 9-year-old boy, at the dose of 2 × 106 MSCs/kg body weight. No toxicity after MSC infusion, rapid disappearance of symptoms, and strong immunosuppression in vivo were observed [52]. At present, there are different phase II clinical trials running to study the optimal MSC dose and administration schedules that have the best efficiency in preventing or treating GvHD following allogeneic HSC. Similar approaches are used with adipose-derived MSCs which are effective in controlling GvHD [105], as well as inflammatory damages following tissue irradiation [119].
In the future, a large number of autoimmune diseases could benefit from the use of MSCs, such as rheumatoid arthritis, multiple sclerosis, and type I diabetes, as shown by the in vivo studies in animal models [53, 80, 108, 109, 113]. Similarly, MSCs could be used for tissue regeneration in some genetic diseases, such as osteogenesis imperfecta [120], and for wound healing [121].
Safety Concerns for in vivo Use of MSCs
Overall, data show the lack of significant acute or chronic toxicity following systemic MSC administration, the wide tissue distribution of infused MSCs, their long-lasting survival (over 1 year) [122], and the absence of ectopic tissue formation during a follow-up of 3 years [27]. Still, open questions are the tumorigenic potential of MSC infusion and the MSC-mediated support of developing cancers by preventing specific anti-cancer immune responses. In vitro cultured human bone marrow-derived MSCs show a normal karyotype before transplantation into the patients [123], they are not susceptible to malignant transformation after long-term in vitro culture until senescence or passage [25], and they do not exhibit telomere maintenance mechanisms [124]. However, MSCs co-infused with bone marrow into irradiated allogeneic recipients mice may develop sarcoma and display cytogenetic abnormalities [125].
MSCs are capable of homing to the sites of injury, and they may therefore provide site-specific and local immune regulation. However, MSCs may also promote tumor growth and prevent the rejection of allogeneic tumor cells [19, 20, 90, 91, 95, 126, 127, 128, 129, 130, 131]. MSCs, infused either systemically or subcutaneously inside growing B16 melanoma cells, determine the enhancement of tumor formation [131]. MSCs within tumor stroma favor breast cancer metastases in mice bearing subcutaneously MCF7/Ras or MDA-MB-231 human breast cancer xenografts [126]. In vitro studies suggest that molecules belonging to the epidermal growth factor family may play a role in the expansion and differentiation of stromal cell precursors inside tumors [132]. More importantly, MSCs may inhibit in vitro and in vivo the specific anti-tumor immune response against Sp6 plasmacytoma in Balb/c mice previously immunized and refractory to tumor development [20]. On the other hand, some studies have shown that MSCs may inhibit tumor growth in mouse [131] and rat [127, 128] models. Similarly, human MSCs exhibit a dose-dependent anti-proliferative activity on different tumor cells lines of hematopoietic and non-hematopoietic origin, producing the transient arrest of tumor cells in the G1 phase of the cell cycle, which disappears after MSC removal [129]. However, when tumor cells are co-injected with MSCs into NOD-SCID mice, tumor engraftment and growth are favored [129]. Therefore, the clinical use of large doses of MSCs must always consider the potential side effects in terms of tumor development. However, because of their preferential migration to sites of tumor growth, MSCs may be used as vehicles for specific and precise anti-cancer drugs delivery, such as IFN-β [91, 127] and NK4130, with a small number of side effects.
Conclusion
MSCs and their stromal progeny may be considered powerful regulatory cells, a sort of DC counterpart, which influence all the main immune effectors and functional roles in vivo, as well as potential applications in the treatment of a number of human immunological diseases. By choosing MSC tissue origin, cell dose, administration route, and treatment schedule, all the potential side effects related to MSC use, including tumor growth enhancement, have to be well considered to maximize the benefits of MSC-dependent immune regulation without significant risks for the patients
References
- 1.Dazzi F, Ramasamy R, Glennie S, Jones SP, Roberts I. The role of mesenchymal stem cells in haemopoiesis. Blood Rev. 2006;20:161–171. doi: 10.1016/j.blre.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 2.Campioni D, Moretti S, Ferrari L, Puntutieri M, Castoldi GL, Lanza F. Immunophenotypic heterogeneity of bone marrow-derived mesenchymal stromal cells from patients with hematological disorders: correlation with bone marrow microenviroment. Haematologica. 2006;91:364–368. [PubMed] [Google Scholar]
- 3.Sacchetti B, Funari A, Michienzi S, Di Cesare S, Piersanti S, Saggio I, Tagliafico E, Ferrari S, Robey PG, Riminucci M, Bianco P. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell. 2007;131:324–336. doi: 10.1016/j.cell.2007.08.025. [DOI] [PubMed] [Google Scholar]
- 4.Sato Y, Araki H, Kato J, Nakamura K, Kawano Y, Kobune M, Sato T, Miyanishi K, Takayama T, Takahashi M, Takimoto R, Iyama S, Matsunaga T, Ohtani S, Matsuura A, Hamada H, Niitsu Y. Human mesenchymal stem cells xenografted directly to rat liver are differentiated into human hepatocytes without fusion. Blood. 2005;106:756–763. doi: 10.1182/blood-2005-02-0572. [DOI] [PubMed] [Google Scholar]
- 5.Kotton DN, Ma BY, Cardoso WV, Sanderson EA, Summer RS, Williams MC, Fine A. Bone marrow-derived cells as progenitors of lung alveolar epithelium. Development. 2001;128:5181–5188. doi: 10.1242/dev.128.24.5181. [DOI] [PubMed] [Google Scholar]
- 6.Woodbury D, Schwarz EJ, Prockop DJ, Black IB. Adult rat and human bone marrow stromal cells differentiate into neurons. J Neurosci Res. 2000;61:364–370. doi: 10.1002/1097-4547(20000815)61:4<364::AID-JNR2>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 7.Krampera M, Marconi S, Pasini A, Galiè M, Mosna F, Tinelli M, Lovato L, Anghileri E, Andreini A, Pizzolo G, Sbarbati A, Bonetti B. Induction of neural-like differentiation in human mesenchymal stem cells derived from bone marrow, fat, spleen and thymus. Bone. 2007;40:382–390. doi: 10.1016/j.bone.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 8.Haynesworth SE, Baber MA, Caplan AI. Cytokine expression by human marrow-derived mesenchymal progenitor cells in vitro: effects of dexamethasone and IL-1 alpha. J Cell Physiol. 1996;166:585–592. doi: 10.1002/(SICI)1097-4652(199603)166:3<585::AID-JCP13>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 9.Majumdar MK, Thiede MA, Haynesworth SE, Bruder SP, Gerson SL. Human marrow-derived mesenchymal stem cells (MSCs) express hematopoietic cytokines and support long-term hemato-poiesis when differentiated toward stromal and osteogenic lineages. J Hematother Stem Cell Res. 2000;9:841–848. doi: 10.1089/152581600750062264. [DOI] [PubMed] [Google Scholar]
- 10.Banwell M, Partington KM, Jenkinson EJ, Anderson G. Studies on the role of IL-7 presentation by mesenchymal fibroblasts during early thymocyte development. Eur J Immunol. 2000;30:2125–2129. doi: 10.1002/1521-4141(2000)30:8<2125::AID-IMMU2125>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 11.Wilson A, Trumpp A. Bone-marrow haematopoietic-stem cell niches. Nat Rev Immunol. 2006;6:93–106. doi: 10.1038/nri1779. [DOI] [PubMed] [Google Scholar]
- 12.Dejbakhsh-Jones S, Jerabek L, Weissman IL, Strober S. Extrathymic maturation of alpha beta T cells from hemopoietic stem cells. J Immunol. 1995;155:3338–3344. [PubMed] [Google Scholar]
- 13.Barda-Saad M, Rozenszajn LA, Globerson A, Zhang AS, Zipori D. Selective adhesion of immature thymocytes to bone marrow stromal cells: relevance to T cell lymphopoiesis. Exp Hematol. 1996;24:386–391. [PubMed] [Google Scholar]
- 14.Li Y, Hisha H, Inaba M, Lian Z, Yu C, Kawamura M, Yamamoto Y, Nishio N, Toki J, Fan H, Ikehara S. Evidence for migration of donor bone marrow stromal cells into recipient thymus after bone marrow transplantation plus bone grafts: a role of stromal cells in positive selection. Exp Hematol. 2000;28:950–960. doi: 10.1016/s0301-472x(00)00483-5. [DOI] [PubMed] [Google Scholar]
- 15.Suniara RK, Jenkinson EJ, Owen JJ. An essential role for thymic mesenchyme in early T cell development. J Exp Med. 2000;191:1051–1056. doi: 10.1084/jem.191.6.1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kierney PC, Dorshkind K. B lymphocyte precursors and myeloid progenitors survive in diffusion chamber cultures but B cell differentiation requires close association with stromal cells. Blood. 1987;70:1418–1424. [PubMed] [Google Scholar]
- 17.Kurosaka D, LeBien TW, Pribyl JA. Comparative studies of different stromal cell microenvironments in support of human B-cell development. Exp Hematol. 1999;27:1271–128. doi: 10.1016/s0301-472x(99)00067-3. [DOI] [PubMed] [Google Scholar]
- 18.Bradl H, Wittmann J, Milius D, Vettermann C, Jäck HM. Interaction of murine precursor B cell receptor with stroma cells is controlled by the unique tail of lambda 5 and stroma cell-associated heparan sulphate. J Immunol. 2003;171:2338–2348. doi: 10.4049/jimmunol.171.5.2338. [DOI] [PubMed] [Google Scholar]
- 19.Amé-Thomas P, Maby-El Hajjami H, Monvoisin C, Jean R, Monnier D, Caulet-Maugendre S, Guillau-deux T, Lamy T, Fest T, Tarte K. Human mesenchymal stem cells isolated from bone marrow and lymphoid organs support tumor B-cell growth: role of stromal cells in follicular lymphoma pathogenesis. Blood. 2007;109:693–702. doi: 10.1182/blood-2006-05-020800. [DOI] [PubMed] [Google Scholar]
- 20.Krampera M, Sartoris S, Liotta F, Pasini A, Angeli R, Cosmi L, Andreini A, Mosna F, Bonetti B, Rebellato E, Testi MG, Frosali F, Pizzolo G, Tridente G, Maggi E, Romagnani S, Annunziato F. Immune regulation by mesenchymal stem cells derived from adult spleen and thymus. Stem Cells Dev. 2007;16:797–810. doi: 10.1089/scd.2007.0024. [DOI] [PubMed] [Google Scholar]
- 21.Maitra B, Szekely E, Gjini K, Laughlin MJ, Dennis J, Haynesworth SE, Koç ON. Human mesenchymal stem cells support unrelated donor hematopoietic stem cells and suppress T-cell activation. Bone Marrow Transplant. 2004;33:597–604. doi: 10.1038/sj.bmt.1704400. [DOI] [PubMed] [Google Scholar]
- 22.Angelopoulou M, Novelli E, Grove JE, Rinder HM, Civin C, Cheng L, Krause DS. Cotransplantation of human mesenchymal stem cells enhances human myelopoiesis and megakaryocytopoiesis in NOD/SCID mice. Exp Hematol. 2003;31:413–420. doi: 10.1016/s0301-472x(03)00042-0. [DOI] [PubMed] [Google Scholar]
- 23.Gurevich O. Transplantation of allogeneic or xenogeneic bone marrow within the donor stromal microenvironment. Transplantation. 1999;68:1362–1368. doi: 10.1097/00007890-199911150-00024. [DOI] [PubMed] [Google Scholar]
- 24.Muguruma Y, Yahata T, Miyatake H, Sato T, Uno T, Itoh J, Kato S, Ito M, Hotta T, Ando K. Reconstitution of the functional human hematopoietic microenvironment derived from human mesenchymal stem cells in the murine bone marrow compartment. Blood. 2006;107:1878–1887. doi: 10.1182/blood-2005-06-2211. [DOI] [PubMed] [Google Scholar]
- 25.Koc ON, Gerson SL, Cooper BW, Dyhouse SM, Haynesworth SE, Caplan AI, Lazarus HM. Rapid hematopoietic recovery after coinfusion of auto-logous-blood stem cells and culture-expanded marrow mesenchymal stem cells in advanced breast cancer patients receiving high-dose chemotherapy. J Clin Oncol. 2000;18:307–316. doi: 10.1200/JCO.2000.18.2.307. [DOI] [PubMed] [Google Scholar]
- 26.Lazarus HM, Koc ON, Devine SM, Curtin P, Maziarz RT, Holland HK, Shpall EJ, McCarthy P, Atkinson K, Cooper BW, Gerson SL, Laughlin MJ, Loberiza FR, Jr, Moseley AB, Bacigalupo A. Cotransplantation of HLA-identical sibling culture-expanded mesenchymal stem cells and hematopoietic stem cells in hematologic malignancy patients. Biol Blood Marrow Transplant. 2005;11:389–398. doi: 10.1016/j.bbmt.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 27.Le Blanc K, Samuelsson H, Gustafsson B, Remberger M, Sundberg B, Arvidson J, Ljungman P, Lönnies H, Nava S, Ringdén O. Transplantation of mesenchymal stem cells to enhance engraftment of hematopoietic stem cells. Leukemia. 2007;21:1733–1738. doi: 10.1038/sj.leu.2404777. [DOI] [PubMed] [Google Scholar]
- 28.Krampera M, Cosmi L, Angeli R, Pasini A, Liotta F, Andreini A, Santarlasci V, Mazzinghi B, Pizzolo G, Vinante F, Romagnani P, Maggi E, Romagnani S, Annunziato F. Role of the IFN-g in the immuno-modulatory activity of human mesenchymal stem cells. Stem Cells. 2005;24:386–398. doi: 10.1634/stemcells.2005-0008. [DOI] [PubMed] [Google Scholar]
- 29.Le Blanc K, Tammik L, Sundberg B, Haynesworth SE, Ringden O. Mesenchymal stem cells inhibit and stimulate mixed lymphocyte cultures and mitogenic responses independently of the major histocompatibility complex. Scand J Immunol. 2003;57:11–20. doi: 10.1046/j.1365-3083.2003.01176.x. [DOI] [PubMed] [Google Scholar]
- 30.Krampera M, Glennie S, Dyson J, Scott D, Laylor R, Simpson E, Dazzi F. Bone marrow mesenchymal stem cells inhibit the response of naïve and memory antigen-specific T cells to their cognate peptide. Blood. 2003;101:3722–3729. doi: 10.1182/blood-2002-07-2104. [DOI] [PubMed] [Google Scholar]
- 31.Di Nicola M, Carlo-Stella C, Magni M, Milanesi M, Longoni PD, Matteucci P, Grisanti S, Gianni AM. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002;99:3838–3843. doi: 10.1182/blood.v99.10.3838. [DOI] [PubMed] [Google Scholar]
- 32.Glennie S, Soeiro I, Dyson PJ, Lam EW, Dazzi F. Bone marrow mesenchymal stem cells induce division arrest anergy of activated T cells. Blood. 2005;105:2821–2827. doi: 10.1182/blood-2004-09-3696. [DOI] [PubMed] [Google Scholar]
- 33.Meisel R, Zibert A, Laryea M, Gobel U, Daubener W, Dilloo D. Human bone marrow stromal cells inhibit allogeneic T-cell responses by indoleamine 2.3-dioxygenase mediated tryptophan degradation. Blood. 2004;103:4619–4621. doi: 10.1182/blood-2003-11-3909. [DOI] [PubMed] [Google Scholar]
- 34.Rasmusson I, Ringden O, Sundberg B, Le Blanc K. Mesenchymal stem cells inhibit the formation of cytotoxic T lymphocytes, but not activated cytotoxic T lymphocytes or natural killer cells. Transplantation. 2003;76:1208–1213. doi: 10.1097/01.TP.0000082540.43730.80. [DOI] [PubMed] [Google Scholar]
- 35.Tse WT, Pendleton JD, Beyer WM, Egalka MC, Guinan EC. Suppression of allogeneic T-cell proliferation by human marrow stromal cells: implications in transplantation. Transplantation. 2003;75:389–397. doi: 10.1097/01.TP.0000045055.63901.A9. [DOI] [PubMed] [Google Scholar]
- 36.Rasmusson I, Ringden O, Sundberg B, Le Blanc K. Mesenchymal stem cell inhibit lymphocytes proliferation by mitogens and alloantigens by different mechanisms. Exp. Cell Res. 2005;305:33–41. doi: 10.1016/j.yexcr.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 37.Corcione A, Benvenuto F, Ferretti E, Giunti D, Cappiello V, Cazzanti F, Risso M, Gualandi F, Mancardi GL, Pistoia V, Uccelli A. Human mesenchymal stem cells modulate B-cell functions. Blood. 2006;107:367–372. doi: 10.1182/blood-2005-07-2657. [DOI] [PubMed] [Google Scholar]
- 38.Sotiropoulou PA, Perez SA, Gritzapis AD, Baxe-vanis CN, Papamichail M. Interactions between human mesenchymal stem cells and natural killer cells. Stem Cells. 2006;24:74–85. doi: 10.1634/stemcells.2004-0359. [DOI] [PubMed] [Google Scholar]
- 39.Spaggiari GM, Capobianco A, Becchetti S, Mingari MC, Moretta L. Mesenchymal stem cell (MSC)/natural killer (NK) cell interactions: evidence that activated NK cells are capable of killing MSC while MSC can inhibit IL-2-induced NK cell proliferation. Blood. 2006;107:1484–1490. doi: 10.1182/blood-2005-07-2775. [DOI] [PubMed] [Google Scholar]
- 40.Zhang W, Ge W, Li C, You S, Liao L, Han Q, Deng W, Zhao RC. Effects of mesenchymal stem cells on differentiation, maturation, and function of human monocyte-derived dendritic cells. Stem Cells Dev. 2004;13:263–271. doi: 10.1089/154732804323099190. [DOI] [PubMed] [Google Scholar]
- 41.Jiang XX, Zhang Y, Liu B, Zhang SX, Wu Y, Yu XD, Mao N. Human mesenchymal stem cells inhibit differentiation and function of monocyte-derived dendritic cells. Blood. 2005;105:4120–4126. doi: 10.1182/blood-2004-02-0586. [DOI] [PubMed] [Google Scholar]
- 42.Beyth S, Borovsky Z, Mevorach D, Liebergall M, Gazit Z, Aslan H, Galun E, Rachmilewitz J. Human mesenchymal stem cells alter antigen-presenting cell maturation and induce T-cell unresponsiveness. Blood. 2005;105:2214–2219. doi: 10.1182/blood-2004-07-2921. [DOI] [PubMed] [Google Scholar]
- 43.Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2004;105:1815–1822. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- 44.Raffaghello L, Bianchi G, Bertolotto M, Montecucco F, Busca A, Dallegri, Ottonello L, Pistoia V. Human mesenchymal stem cells inhibit neutrophil apoptosis: a model for neutrophil preservation in the bone marrow niche. Stem Cells. 2008;26:151–162. doi: 10.1634/stemcells.2007-0416. [DOI] [PubMed] [Google Scholar]
- 45.Ramasamy K, Fazekasova H, Lam EW, Soeiro I, Lombardi G, Dazzi F. Mesenchymal stem cells inhibit dendritic cell differentiation and function by preventing entry into the cell cycle. Transplantation. 2007;83:71–76. doi: 10.1097/01.tp.0000244572.24780.54. [DOI] [PubMed] [Google Scholar]
- 46.Bartholomew A, Sturgeon C, Siatskas M, Ferrer K, McIntosh K, Patil S, Hardy W, Devine S, Ucker D, Deans R, Moseley A, Hoffman R. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol. 2002;30:42–48. doi: 10.1016/s0301-472x(01)00769-x. [DOI] [PubMed] [Google Scholar]
- 47.Puissant B, Barreau C, Bourin P, Clavel C, Corre J, Bousquet C, Taureau C, Cousin B, Abbal M, Laharrague P, Penicaud L, Casteilla L, Blancher A. Immunomodulatory effect of human adipose tissue-derived adult stem cells: comparison with bone marrow mesenchymal stem cells. Br J Haematol. 2005;129:118–129. doi: 10.1111/j.1365-2141.2005.05409.x. [DOI] [PubMed] [Google Scholar]
- 48.Le Blanc K, Tammik C, Rosendahl K, Zetterberg E, Ringden O. HLA expression and immunologic properties of differentiated and undifferentiated mesenchymal stem cells. Exp Hematol. 2003;31:890–896. doi: 10.1016/s0301-472x(03)00110-3. [DOI] [PubMed] [Google Scholar]
- 49.Liu H, Kemeny DM, Heng BC, Ouyang HW, Melendez AJ, Cao T. The immunogenicity and immunomodulatory function of osteogenic cells differentiated from mesenchymal stem cells. J Immunol. 2006;176:2864–2871. doi: 10.4049/jimmunol.176.5.2864. [DOI] [PubMed] [Google Scholar]
- 50.Jones S, Horwood N, Cope A, Dazzi F. The anti-proliferative effect of mesenchymal stem cells is a fundamental property shared by all stromal cells. J Immunol. 2007;179:2824–2831. doi: 10.4049/jimmunol.179.5.2824. [DOI] [PubMed] [Google Scholar]
- 51.Haniffa MA, Wang XN, Holtick U, Rae M, Isaacs JD, Dickinson AM, Hilkens CM, Collin MP. Adult human fibroblasts are potent immunoregulatory cells and functionally equivalent to mesenchymal stem cells. J Immunol. 2007;179:1595–1604. doi: 10.4049/jimmunol.179.3.1595. [DOI] [PubMed] [Google Scholar]
- 52.Le Blanc K, Rasmusson I, Sundberg B, Gother-strom C, Hassan M, Uzunel M, Ringden O. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004;363:1439–1441. doi: 10.1016/S0140-6736(04)16104-7. [DOI] [PubMed] [Google Scholar]
- 53.Zappia E, Casazza S, Pedemonte E, Benvenuto F. Bonanni I, Gerdoni E, Giunti D, Ceravolo A, Cazzanti F, Frassoni F, Mancardi G, Uccelli A: Mesenchymal stem cells ameliorate experimental autoimmune encephalomyelitis inducing T cell anergy. Blood. 2005;106:1755–1761. doi: 10.1182/blood-2005-04-1496. [DOI] [PubMed] [Google Scholar]
- 54.Le Blanc K. Immunomodulatory effects of fetal and adult mesenchymal stem cells. Cytotherapy. 2003;5:485–489. doi: 10.1080/14653240310003611. [DOI] [PubMed] [Google Scholar]
- 55.Angoulvant D, Qerc A, Benchalal S, Galambrun C, Farre A, Bertrand Y, Eljaafari A. Human mesenchymal stem cells suppress induction of cytotoxic response to alloantigens. Biorheology. 2004;41:469–476. [PubMed] [Google Scholar]
- 56.Rasmusson I, Uhlin M, Le Blanc K, Levitsky V. Mesenchymal stem cells fail to trigger effector functions of cytotoxic T lymphocytes. J Leukoc Biol. 2007;82:887–893. doi: 10.1189/jlb.0307140. [DOI] [PubMed] [Google Scholar]
- 57.Fang L, Lange C, Engel M, Zander AR, Fehse B. Sensitive balance of suppressing and activating effects of mesenchymal stem cells on T-cell proliferation. Transplantation. 2006;82:1370–1373. doi: 10.1097/01.tp.0000232450.62408.f9. [DOI] [PubMed] [Google Scholar]
- 58.Benvenuto F, Ferrari S, Gerdoni E, Gualandi F, Frassoni F, Vito Pistoia V, Mancardi G, Uccelli A. Human mesenchymal stem cell promote survival of T cells in a quiescent state. Stem Cells. 2007;25:1753–1760. doi: 10.1634/stemcells.2007-0068. [DOI] [PubMed] [Google Scholar]
- 59.Klyushnenkova E, Mosca JD, Zernetkina V, Majumdar MK, Beggs KJ, Simonetti DW, Deans RJ, Mclntosh KR. T cell responses to allogeneic human mesenchymal stem cells: immunogenicity, tolerance, and suppression. J Biomed Sci. 2005;12:47–57. doi: 10.1007/s11373-004-8183-7. [DOI] [PubMed] [Google Scholar]
- 60.Potian JA, Aviv H, Ponzio NM, Harrison JS, Rameshwar P. Veto-like activity of mesenchymal stem cells: functional discrimination between cellular responses to alloantigens and recall antigens. J Immunol. 2003;171:3426–3434. doi: 10.4049/jimmunol.171.7.3426. [DOI] [PubMed] [Google Scholar]
- 61.Stagg J, Pommey S, Eliopoulos N, Galipeau J. Interferon- -stimulated marrow stromal cells: a new type of non-hematopoietic antigen-presenting cell. Blood. 2006;107:2570–2577. doi: 10.1182/blood-2005-07-2793. [DOI] [PubMed] [Google Scholar]
- 62.Chan JL, Tang KC, Patel AP, Bonilla LM, Pierobon N, Ponzio NM, Rameshwar P. Antigen-presenting property of mesenchymal stem cells occurs during a narrow window at low levels of interferon-gamma. Blood. 2006;107:4817–4824. doi: 10.1182/blood-2006-01-0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Romieu-Mourez R, François M, Boivin MN, Stagg J, Galipeau J. Regulation of MHC class II expression and antigen processing in murine and human mesenchymal stromal cells by IFN-gamma, TGF-beta, and cell density. J Immunol. 2007;179:1549–1558. doi: 10.4049/jimmunol.179.3.1549. [DOI] [PubMed] [Google Scholar]
- 64.Inoue S, Popp FC, Koehl GE, Piso P, Schlitt HI Geissler EK, Dahlke MH. Immunomodulatory effects of mesenchymal stem cells in a rat organ transplant model. Transplantation. 2006;81:1589–1595. doi: 10.1097/01.tp.0000209919.90630.7b. [DOI] [PubMed] [Google Scholar]
- 65.Devine SM, Cobbs C, Jennings M, Bartholomew A, Hoffman R. Mesenchymal stem cells distribute to a wide range of tissues following systemic infusion into nonhuman primates. Blood. 2003;101:2999–3001. doi: 10.1182/blood-2002-06-1830. [DOI] [PubMed] [Google Scholar]
- 66.Bingaman AW, Waitze SY, Alexander DZ, Cho HR, Lin A, Tucker-Burden C, Cowan SR, Pearson TC, Larsen CP. Transplantation of the bone marrow microenvironment leads to hematopoietic chimerism without cytoreductive conditioning. Transplantation. 2000;69:2491–2496. doi: 10.1097/00007890-200006270-00006. [DOI] [PubMed] [Google Scholar]
- 67.Anklesaria P, Kase K, Glowacki J, Holland CA, Sakakeeny MA, Wright JA, FitzGerald TJ, Lee CY, Greenberger JS. Engraftment of a clonal bone marrow stromal cell line in vivo stimulates hematopoietic recovery from total body irradiation. Proc Natl Acad Sci USA. 1987;84:7681–7685. doi: 10.1073/pnas.84.21.7681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lee ST, Jang JH, Cheong JW, Kim JS, Maemg HY, Hahn JS, Ko YW, Min YH. Treatment of high-risk acute myelogenous leukaemia by myeloablative chemoradiotherapy followed by co-infusion of T cell-depleted haematopoietic stem cells and culture-expanded marrow mesenchymal stem cells from a related donor with one fully mismatched human leucocyte antigen haplotype. Br J Haematol. 2002;118:1128–1131. doi: 10.1046/j.1365-2141.2002.03767.x. [DOI] [PubMed] [Google Scholar]
- 69.Saito T, Kuang JQ, Bittira B, Al-Khaldi A, Chiu RC. Xenotransplant cardiac chimera: immune tolerance of adult stem cells. Ann Thorac Surg. 2002;74:19–24. doi: 10.1016/s0003-4975(02)03591-9. [DOI] [PubMed] [Google Scholar]
- 70.Eliopoulos N, Stagg J, Lejeune L, Pommey S, Galipeau J. Allogeneic marrow stromal cells are immune rejected by MHC class I and II mismatched recipient mice. Blood. 2005;106:4057–4065. doi: 10.1182/blood-2005-03-1004. [DOI] [PubMed] [Google Scholar]
- 71.Sudres M, Norol F, Trenado A, Grégoire S, Charlotte F, Levacher B, Lataillade JJ, Bourin P, Holy X, Vernant JP, Klatzmann D, Cohen JL. Bone marrow mesenchymal stem cells suppress lymphocyte proliferation in vitro but fail to prevent graft-versus-host disease in mice. J Immunol. 2006;176:7761–7767. doi: 10.4049/jimmunol.176.12.7761. [DOI] [PubMed] [Google Scholar]
- 72.Nauta AJ, Westerhuis G, Kruisselbrink AB, Lurvink EG, Willemze R, Fibbe WE. Donor-derived mesenchymal stem cells are immunogenic in an allogeneic host and stimulate donor graft rejection in a non myeloablative setting. Blood. 2006;108:2114–2120. doi: 10.1182/blood-2005-11-011650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Grinnemo KH, Månsson A, Dellgren G, Klingberg D, Wardell E, Drvota V, Tammik C, Holgersson J, Ringdén O, Sylvén C, Le Blanc K. Xenoreactivity and engraftment of human mesenchymal stem cells transplanted into infarcted rat myocardium. J Thorac Cardiovasc Surg. 2004;127:1293–1300. doi: 10.1016/j.jtcvs.2003.07.037. [DOI] [PubMed] [Google Scholar]
- 74.Groh ME, Maitra B, Szekely E, Koc ON. Human mesenchymal stem cells require monocyte-medi-ated activation to suppress alloreactive T cells. Exp Hematol. 2005;33:928–934. doi: 10.1016/j.exphem.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 75.Le Blanc K, Rasmusson I, Götherström C, Seidel C, Sundberg B, Sundin M, Rosendahl K, Tammik C, Ringdén O. Mesenchymal stem cells inhibit the expression of CD25 (interleukin-2 receptor) and CD38 on phytohaemagglutinin-activated lymphocytes. Scand J Immunol. 2004;60:307–315. doi: 10.1111/j.0300-9475.2004.01483.x. [DOI] [PubMed] [Google Scholar]
- 76.Maccario R, Moretta A, Cometa A, Montagna D, Comoli P, Locatelli F, Podestà M, Frassoni F. Interaction of human mesenchymal stem cells with cells involved in alloantigenspecific immune response favors the differentiation of CD4+ T-cell subsets expressing a regulatory/suppressive phenotype. Haematologica. 2005;90:516–525. [PubMed] [Google Scholar]
- 77.Prevosto C, Zancolli M, Canevali P, Zocchi MR, Poggi A. Generation of CD4+ or CD8+ regulatory T cells upon mesenchymal stem cell-lymphocyte interaction. Haematologica. 2007;92:881–888. doi: 10.3324/haematol.11240. [DOI] [PubMed] [Google Scholar]
- 78.Xu G, Zhang Y, Zhang L, Ren G, Shi Y. The role of IL-6 in inhibition of lymphocyte apoptosis by mesenchymal stem cells. Biochem Biophys Res Commun. 2007;361:745–750. doi: 10.1016/j.bbrc.2007.07.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Djouad F, Charbonnier LM, Bouffi C, Louis-Plence P, Bony C, Apparailly F, Cantos C, Jorgensen C, Noël D. Mesenchymal stem cells inhibit the differentiation of dendritic cells through an interleukin-6-dependent mechanism. Stem Cells. 2007;25:2025–2032. doi: 10.1634/stemcells.2006-0548. [DOI] [PubMed] [Google Scholar]
- 80.Djouad F, Fritz V, Apparailly F, Louis-Plence P, Bony C, Sany J, Jorgensen C, Noël D. Reversal of the immunosuppressive properties of mesenchymal stem cells by tumor necrosis factor alpha in collagen-induced arthritis. Arthritis Rheum. 2005;52:1595–1603. doi: 10.1002/art.21012. [DOI] [PubMed] [Google Scholar]
- 81.Sato K, Ozaki K, Oh I, Meguro A, Hatanaka K, Nagai T, Muroi K, Ozawa K. Nitric oxide plays a critical role in suppression of T-cell proliferation by mesenchymal stem cells. Blood. 2007;109:228–234. doi: 10.1182/blood-2006-02-002246. [DOI] [PubMed] [Google Scholar]
- 82.Cbannes D, Hill M, Merieau E, Rossignol J, Brion R, Soulillou JP, Anegon I, Cuturi MC. A role for heme oxygenase-1 in the immunosuppressive effect of adult rat and human mesenchymal stem cells. Blood. 2007;110:3691–3694. doi: 10.1182/blood-2007-02-075481. [DOI] [PubMed] [Google Scholar]
- 83.Selmani Z, Naji A, Zidi I, Favier B, Gaiffe E, Obert L, Borg C, Saas P, Tiberghien P, Rouas-Freiss N, Carosella ED, Deschaseaux F. HLA-G5 secretion by human mesenchymal stem cells is required to suppress T-lymphocyte and NK function and to induce CD4+CD25highFOXP3+ regulatory T cells. Stem Cells. 2008;26:212–222. doi: 10.1634/stemcells.2007-0554. [DOI] [PubMed] [Google Scholar]
- 84.Oh I, Ozaki K, Sato K, Meguro A, Tatara R, Hatanaka K, Nagai T, Muroi K, Ozawa K. Inter-feron-gamma and NF-kappaB mediate nitric oxide production by mesenchymal stromal cells. Biochem Biophys Res Commun. 2007;355:956–962. doi: 10.1016/j.bbrc.2007.02.054. [DOI] [PubMed] [Google Scholar]
- 85.Chang CJ, Yen ML, Chen YC, Chien CC, Huang HI, Bai CH, Yen BL. Placenta-derived multipotent cells exhibit immunosuppressive properties that are enhanced in the presence of interferon-gamma. Stem Cells. 2006;24:2466–2477. doi: 10.1634/stemcells.2006-0071. [DOI] [PubMed] [Google Scholar]
- 86.Munn DH, Shafizadeh E, Attwood JT, Bondarev I, Pashine A, Mellor AL. Inhibition of T cell proliferation by macrophage tryptophan catabolism. J Exp Med. 1999;189:1363–1372. doi: 10.1084/jem.189.9.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gieseke F, Schütt B, Viebahn S, Koscielniak E, Friedrich W, Handgretinger R, Müller I. Human multipotent mesenchymal stromal cells inhibit proliferation of PBMCs independently of IFNgam-maR1 signaling and IDO expression. Blood. 2007;110:2197–2200. doi: 10.1182/blood-2007-04-083162. [DOI] [PubMed] [Google Scholar]
- 88.Liotta F, Angeli R, Cosmi L, Filì L, Manuelli C, Frosali F, Mazzinghi B, Maggi L, Pasini A, Lisi V, Santarlasci V, Consoloni L, Angelotti ML, Romagnani P, Parronchi P, Krampera M, Maggi E, Romagnani S, Annunziato F. TLR3 and TLR4 are expressed by human bone marrow-derived mesenchymal stem cells and can inhibit their T-cell modulatory activity by impairing notch signalling. Stem Cells. 2008;26:279–289. doi: 10.1634/stemcells.2007-0454. [DOI] [PubMed] [Google Scholar]
- 89.Ljunggren HG, Malmberg KJ. Prospects for the use of NK cells in immunotherapy of human cancer. Nat Rev Immunol. 2007;7:329–339. doi: 10.1038/nri2073. [DOI] [PubMed] [Google Scholar]
- 90.Albertsson PA, Basse PH, Hokland M, Goldfarb RH, Nagelkerke JF, Nannmark U, Kuppen PJ. NK cells and the tumour microenvironment: implications for NK-cell function and anti-tumour activity. Trends Immunol. 2003;24:603–609. doi: 10.1016/j.it.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 91.Studeny M, Marini FC, Dembinski JL, Zompetta C, Cabreira-Hansen M, Bekele BN, Champlin RE, Andreeff M. Mesenchymal stem cells: potential precursors for tumor stroma and targeted-delivery vehicles for anticancer agents. J Natl Cancer Inst. 2004;96:1593–1603. doi: 10.1093/jnci/djh299. [DOI] [PubMed] [Google Scholar]
- 92.Spaggiari GM, Capobianco A, Abdelrazik H, Becchetti F, Zingari MC, Moretta L. Mesenchymal stem cells inhibit natural killer cell proliferation, cytotoxicity production: role of indoleamine 2.3-dioxygenase and prostaglandin E2. Blood. 2008;111:1327–1333. doi: 10.1182/blood-2007-02-074997. [DOI] [PubMed] [Google Scholar]
- 93.Bottino C, Castriconi R, Moretta L, Moretta A. Cellular ligands of activating NK receptors. Trends Immunol. 2005;26:221–226. doi: 10.1016/j.it.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 94.Carosella ED, Moreau P, Le Maoult J, Le Discorde M, Dausset J, Rouas-Freiss N. HLA-G molecules: from maternal-fetal tolerance to tissue acceptance. Adv Immunol. 2003;81:199–252. doi: 10.1016/s0065-2776(03)81006-4. [DOI] [PubMed] [Google Scholar]
- 95.Rouas-Freiss N, Moreau P, Menier C, Lemaoult J, Carosella ED. Expression of tolerogenic HLA-G molecules in cancer prevents antitumor responses. Semin Cancer Biol. 2007;17:413–421. doi: 10.1016/j.semcancer.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 95a.Poggi A, Prevosto C, Massaro AM, Negrini S, Urbani S, Pierri I, Saccardi R, Gobbi M, Zocchi MR. Interaction between human NK cells and bone marrow stromal cells induces NK cell triggering: role of NKp30 and NKG2D receptors. J Immunol. 2005;175:6352–6360. doi: 10.4049/jimmunol.175.10.6352. [DOI] [PubMed] [Google Scholar]
- 96.Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, Pulendran B, Palucka K. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 97.Chomarat P, Banchereau J, Davoust J, Palucka AK. IL-6 switches the differentiation of monocytes from dendritic cells to macrophages. Nat Immunol. 2000;1:510–514. doi: 10.1038/82763. [DOI] [PubMed] [Google Scholar]
- 98.Gur-Wahnon D, Borovsky Z, Beyth S, Liebergall M, Rachmilewitz J. Contact-dependent induction of regulatory antigen-presenting cells by human mesenchymal stem cells is mediated via STAT3 signaling. Exp Hematol. 2007;35:426–433. doi: 10.1016/j.exphem.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 99.Augello A, Tasso R, Negrini SM, Amateis A, Indiveri F, Cancedda R, Pennesi G. Bone Marrow mesenchymal progenitors cells inhibit lymphocyte proliferation by activation of the programmed death 1 pathway. Eur J Immunol. 2005;35:1482–1490. doi: 10.1002/eji.200425405. [DOI] [PubMed] [Google Scholar]
- 100.Deng W, Han Q, Liao L, You S, Deng H, Zhao RC. Effects of allogeneic bone marrow-derived mesenchymal stem cells on T and B lymphocytes from BXSB mice. DNA Cell Biol. 2005;24:458–463. doi: 10.1089/dna.2005.24.458. [DOI] [PubMed] [Google Scholar]
- 101.Okazaky T, Wang J. PD-1/PD-L pathway and autoimmunity. Autoimmunity. 2005;38:353–357. doi: 10.1080/08916930500124072. [DOI] [PubMed] [Google Scholar]
- 102.Rasmusson I, Le Blanc K, Sundberg B, Ringden O. Mesenchymal stem cells stimulate antibody secretion in human B cells. Scand J Immunol. 2007;65:336–343. doi: 10.1111/j.1365-3083.2007.01905.x. [DOI] [PubMed] [Google Scholar]
- 103.Gerdoni E, Gallo B, Casazza S, Musio S, Bonanni I, Pedemonte E, Mantegazza R, Frassoni F, Man-cardi G, Pedotti R, Uccelli A. Mesenchymal stem cells effectively modulate pathogenic immune response in experimental autoimmune encepha-lomyelitis. Ann Neurol. 2007;61:219–227. doi: 10.1002/ana.21076. [DOI] [PubMed] [Google Scholar]
- 104.Liechty KW, MacKenzie TC, Shaaban AF, Radu A, Moseley AM, Deans R, Marshak DR, Flake AW. Human mesenchymal stem cells engraft and demonstrate site-specific differentiation after in utero transplantation in sheep. Nat Med. 2000;6:1282–1286. doi: 10.1038/81395. [DOI] [PubMed] [Google Scholar]
- 105.Yañez R, Lamana ML, García-Castro J, Colmenero I, Ramírez M, Bueren JA. Adipose tissue-derived mesenchymal stem cells have in vivo immunosuppressive properties applicable for the control of the graft-versus-host disease. Stem Cells. 2006;24:2582–2591. doi: 10.1634/stemcells.2006-0228. [DOI] [PubMed] [Google Scholar]
- 106.in't Anker PS, Noor WA, Kruisselbrink AB, Scherjon SA, Beekhuizen W, Willemze R, Kanhai HH, Fibbe WE. Nonexpanded primari lung and bone marrow-derived mesenchymal cells promote the engraftment of umbilical cord blood-derived CD34+ cells in NOD/SCID mice. Exp Hematol. 2003;31:881–889. doi: 10.1016/s0301-472x(03)00202-9. [DOI] [PubMed] [Google Scholar]
- 107.Bensidhoum M, Chapel A, Francois S, Demarquay C, Mazurier C, Fouillard L, Bouchet S, Bertho JM, Gourmelon P, Aigueperse J, Charbord P, Gorin NC, Thierry D, Lopez M. Homing of in vitro expanded Stro-1- or Stro-1+ human mesenchymal stem cells into the NOD/SCID mouse and their role in supporting human CD34 cell engraftment. Blood. 2004;103:3313–3319. doi: 10.1182/blood-2003-04-1121. [DOI] [PubMed] [Google Scholar]
- 108.Zhang J, Li Y, Chen J, Cui Y, Lu M, Elias SB, Mitchell JB, Hammill L, Vanguri P, Chopp M. Human bone marrow stromal cell treatment improves neurological functional recovery in EAE mice. Exp Neurol. 2005;195:16–26. doi: 10.1016/j.expneurol.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 109.Augello A, Tasso R, Negrini SM, Cancedda R, Pennesi G. Cell therapy using allogeneic bone marrow mesenchymal stem cells prevents tissue damage in collagen-induced arthritis. Arthritis Rheum. 2007;56:1175–86. doi: 10.1002/art.22511. [DOI] [PubMed] [Google Scholar]
- 110.Tögel F, Hu Z, Weiss K, Isaac J, Lange C, Westenfelder C. Administered mesenchymal stem cells protect against ischemic acute renal failure through differentiation-indipendent mechanisms. Am J Physiol Renal Physiol. 2005;289:F31–F42. doi: 10.1152/ajprenal.00007.2005. [DOI] [PubMed] [Google Scholar]
- 111.Markel TA, Wairiuko GM, Lahm T, Crisostomo PR, Wang M, Herring CM, Meldrum DR. Stem cells improve right ventricular functional recovery after acute pressure overload and ischemia reper-fusion injury. J Surg Res. 2007;141:241–224. doi: 10.1016/j.jss.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 112.Kunter U, Rong S, Djuric Z, Boor P, Müller-Newen G, Yu D, Floege J. Transplanted mesenchymal stem cells accelerate glomerular healing in experimental glomerulonephritis. J Am Soc Nephrol. 2006;17:2202–2212. doi: 10.1681/ASN.2005080815. [DOI] [PubMed] [Google Scholar]
- 113.Urban VS, Kiss J, Kovacs J, Gócza E, Vas V, Monostori E, Uher F. Mesenchymal stem cells cooperate with bone marrow cells in therapy of diabetes. Stem Cells. 2008;26:244–253. doi: 10.1634/stemcells.2007-0267. [DOI] [PubMed] [Google Scholar]
- 114.Lazarus HM, Haynesworth SE, Gerson SL, Rosenthal NS, Caplan AI. Ex vivo expansion and subsequent infusion of human bone marrow-derived stromal progenitors cells (mesenchymal progenitor cells): implications for therapeutic use. Bone Marrow Transplant. 1995;16:557–564. [PubMed] [Google Scholar]
- 115.Ball LM, Bernardo ME, Roelofs H, Lankester A, Cometa A, Egeler RM, Locatelli F, Fibbe WE. Cotransplantation of ex vivo-expanded mesenchymal stem cells accelerates lymphocyte recovery and may reduce the risk of graft failure in haploidentical hematopoietic stem-cell transplantation. Blood. 2007;110:2764–2767. doi: 10.1182/blood-2007-04-087056. [DOI] [PubMed] [Google Scholar]
- 116.Fouillard L, Bensidhoum M, Bories D, Bonte H, Lopez M, Moseley AM, Smith A, Lesage S, Beaujean F, Thierry D, Gourmelon P, Najman A, Gorin NC. Engraftment of allogeneic mesenchymal stem cells in the bone marrow of a patient with severe idiopathic aplastic anemia improves stroma. Leukemia. 2003;17:474–476. doi: 10.1038/sj.leu.2402786. [DOI] [PubMed] [Google Scholar]
- 117.Awaya N, Rupert K, Bryant E, Torok-Storb B. Failure of adult marrow-derived stem cells to generate marrow stroma after successful hematopoietic stem cells transplantation. Exp Hemat. 2002;30:937–942. doi: 10.1016/s0301-472x(02)00821-4. [DOI] [PubMed] [Google Scholar]
- 118.Carlo-Stella C, Tabilio A, Regazzi E, Garau D, La Tagliata R, Trasarti S, Andrizzi C, Vignetti M, Meloni G. Effect of chemotherapy for acute myelogeneous leukemia on hemopoietic and fibroblast marrow progenitors. Bone Marrow Transplant. 1997;20:465–471. doi: 10.1038/sj.bmt.1700916. [DOI] [PubMed] [Google Scholar]
- 119.Rigotti G, Marchi A, Galiè M, Baroni G, Benati D, Krampera M, Pasini A, Sbarbati A. Clinical treatment of radiotherapy tissue damage by lipoaspirate transplant: a healing process mediated by adipose-derived adult stem cells. Plast Reconstr Surg. 2007;119:1409–1422. doi: 10.1097/01.prs.0000256047.47909.71. [DOI] [PubMed] [Google Scholar]
- 120.Horwitz EM, Gordon PL, Koo WK, Marx JC, Neel MD, McNall RY, Muul L, Hofmann T. Isolated allogeneic bone marrow-derived mesenchymal cells engraft and stimulate growth in children with osteogenesis imperfecta: implications for cell therapy of bone. Proc Natl Acad Sci U S A. 2002;99:8932–8937. doi: 10.1073/pnas.132252399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wu Y, Chen L, Scott PG, Tredget EE. Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis. Stem Cells. 2007;25:2648–2659. doi: 10.1634/stemcells.2007-0226. [DOI] [PubMed] [Google Scholar]
- 122.Devine SM, Bartholomew AM, Mahmud N, Nelson M, Patil S, Hardy W, Sturgeon C, Hewett T, Chung T, Stock W, Sher D, Weissman S, Ferrer K, Mosca J, Deans R, Moseley A, Hoffman R. Mesenchymal stem cells are capable of homing to the bone marrow of non-human primates following systemic infusion. Exp Hematol. 2001;29:244–255. doi: 10.1016/s0301-472x(00)00635-4. [DOI] [PubMed] [Google Scholar]
- 123.Zhang ZX, Guan LX, Zhang K, Wang S, Cao PC, Wang YH, Wang Z, Dai LJ. Cytogenetic analysis of human bone marrow-derived mesenchymal stem cells passaged in vitro. Cell Biol Int. 2007;31:645–648. doi: 10.1016/j.cellbi.2006.11.025. [DOI] [PubMed] [Google Scholar]
- 124.Bernardo ME, Zaffaroni N, Novara F, Cometa AM, Avanzini MA, Moretta A, Montagna D, Maccario R, Villa R, Daidone MG, Zuffardi O, Locatelli F. Human bone marrow derived mesenchymal stem cells do not undergo transformation after long-term in vitro culture and do not exhibit telomere maintenance mechanisms. Cancer Res. 2007;67:9142–9149. doi: 10.1158/0008-5472.CAN-06-4690. [DOI] [PubMed] [Google Scholar]
- 125.Tolar J, Nauta AJ, Osborn MJ, Panoskaltsis Mortali A, McElmurry RT, Bell S, Xia L, Zhou N Riddle M, Schroeder TM, Westendorf JJ, Mclvor RS, Hogendoorn PC, Szuhai K, Oseth L, Hirsch B, Yant SR, Kay MA, Peister A, Prockop DJ, Fibbe WE, Blazar BR. Sarcoma derived from cultured mesenchymal stem cells. Stem Cells. 2007;25:371–379. doi: 10.1634/stemcells.2005-0620. [DOI] [PubMed] [Google Scholar]
- 126.Karnoub AE, Dash AB, Vo AP, Sullivan A, Brooks MW, Bell GW, Richardson AL, Polyak K, Tubo R, Weinberg RA. Mesenchymal stem cells within tumour promote breast cancer metastasis. Nature. 2007;449:557–565. doi: 10.1038/nature06188. [DOI] [PubMed] [Google Scholar]
- 127.Nakamura K, Ito Y, Kawano Y, Kurozumi K, Kobune M, Tsuda H, Bizen A, Honmou O, Niitsu Y, Hamada H. Antitumor effect of genetically engineered mesenchymal stem cells in a rat glioma model. Gene Ther. 2004;11:1155–1164. doi: 10.1038/sj.gt.3302276. [DOI] [PubMed] [Google Scholar]
- 128.Ohlsson LB, Varas L, Kjellman C, Edvardsen K, Lindvall M. Mesenchymal progenitor cell-mediated inhibition of tumor growth in vivo and in vitro in gelatin matrix. Exp Mol Pathol. 2003;75:248–225. doi: 10.1016/j.yexmp.2003.06.001. [DOI] [PubMed] [Google Scholar]
- 129.Ramasamy R, Lam EW, Soeiro I, Tisato V, Bonnet D, Dazzi F. Mesenchymal stem cells inhibit proliferation and apoptosis of tumor cells: impact on in vivo tumor growth. Leukemia. 2007;21:304–310. doi: 10.1038/sj.leu.2404489. [DOI] [PubMed] [Google Scholar]
- 130.Kanehira M, Xin H, Hoshino K, Maemondo M, Mizuguchi H, Hayakawa T, Matsumoto K, Nakamura T, Nukiwa T, Saijo Y. Targeted delivery of NK4 to multiple lung tumors by bone marrow-derived mesenchymal stem cells. Cancer Gene Ther. 2007;14:894–903. doi: 10.1038/sj.cgt.7701079. [DOI] [PubMed] [Google Scholar]
- 131.Maestroni GJ, Hertens E, Galli P. Factor(s) from nonmacrophage bone marrow stromal cells inhibit Lewis lung carcinoma and B16 melanoma growth in mice. Cell Mol Life Sci. 1999;55:663–667. doi: 10.1007/s000180050322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Krampera M, Pasini A, Rigo A, Scupoli MT, Tecchio C, Malpeli G, Scarpa A, Dazzi F, Pizzolo G, Vinante F. HB-EGF/HER-1 signalling in bone marrow mesenchymal stem cells: inducing cell expansion and reversibly preventing multi-lineage differentiation. Blood. 2005;106:59–66. doi: 10.1182/blood-2004-09-3645. [DOI] [PubMed] [Google Scholar]